Measles
Wilbert H. Mason, Hayley A. Gans
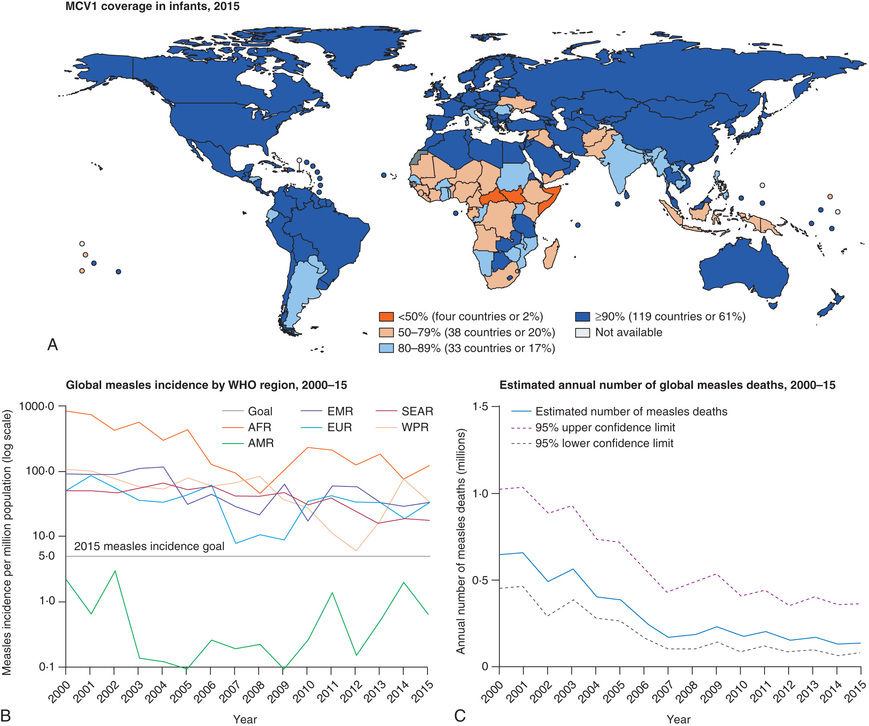
Measles is highly contagious, but endemic transmission has been interrupted in the United States as a result of widespread vaccination; indigenous or imported cases have occasionally resulted in epidemics in the United States in unimmunized or partially immunized American or foreign-born children (adopted children, refugees, returning tourists). In some areas of the world, measles remains a serious threat to children (Fig. 273.1 ).
Etiology
Measles virus is a single-stranded, lipid-enveloped RNA virus in the family Paramyxoviridae and genus Morbillivirus. Other members of the genus Morbillivirus affect a variety of mammals, such as rinderpest virus in cattle and distemper virus in dogs, but humans are the only host of measles virus. Of the 6 major structural proteins of measles virus, the 2 most important in terms of induction of immunity are the hemagglutinin (H) protein and the fusion (F) protein. The neutralizing antibodies are directed against the H protein, and antibodies to the F protein limit proliferation of the virus during infection. Small variations in genetic composition have also been identified that result in no effect on protective immunity but provide molecular markers that can distinguish between viral types. Related genotypes have been grouped by clades, and the World Health Organization recognizes 8 clades, A-H, and 23 genotypes. These markers have been useful in the evaluation of endemic and epidemic spread of measles.
Epidemiology
The measles vaccine has changed the epidemiology of measles dramatically. Once worldwide in distribution, endemic transmission of measles has been interrupted in many countries where there is widespread vaccine coverage. Historically, measles caused universal infection in childhood in the United States, with 90% of children acquiring the infection before 15 yr of age. Morbidity and mortality associated with measles decreased prior to the introduction of the vaccine as a result of improvements in healthcare and nutrition. However, the incidence declined dramatically following the introduction of the measles vaccine in 1963. The attack rate fell from 313 cases per 100,000 population in 1956–1960 to 1.3 cases per 100,000 in 1982–1988.
A nationwide indigenous measles outbreak occurred in the United States in 1989–1991, resulting in more than 55,000 cases, 11,000 hospitalizations, and 123 deaths, demonstrating that the infection had not yet been controlled. This resurgence was attributed to vaccine failure in a small number of school-age children, low coverage of preschool-age children, and more rapid waning of maternal antibodies in infants born to mothers who had never experienced wild-type measles infection. Implementation of the 2-dose vaccine policy and more intensive immunization strategies resulted in interruption of endemic transmission and in 2,000 measles was declared eliminated from the United States. The current rate is <1 case per 1,000,000 population.
Measles continues to be imported into the United States from abroad; therefore continued maintenance of >90% immunity through vaccination is necessary to prevent widespread outbreaks from occurring (see Fig. 273.1 ).
In 2014 the United States encountered a record number of cases since elimination in 2000, with 667 cases of measles reported to the U.S. Centers for Disease Control and Prevention (CDC). There were 23 outbreaks reported compared with a median of 4 outbreaks reported annually during 2001–2010. The majority of cases were associated with importations from other countries (returning tourists, adoptees, refugees), particularly from the Philippines, with prior year epidemics associated with epidemics in the World Health Organization European Region. Measles cases are largely restricted to unvaccinated individuals. Since 2014, cases continue to result from importations causing multistate outbreaks, but due to increased awareness and vaccination efforts, cases remain <200/annually, with 86 reported in 2016 and 120 to date in 2017.
High levels of measles immunity in a population of ~95% are required to interrupt the endemic spread of measles. In the United States this can be achieved through the current 2-dose immunization strategies when coverage rates are high (>90% 1-dose coverage at 12-15 mo and >95% 2-dose coverage in school-age children). Although measles-mumps-rubella coverage remains high (90–91.5% in children 19-35 mo for 2000–2015), pockets of lower coverage rates exist because of reluctance of parents to vaccinate their children. This variability in vaccination has contributed to outbreaks among school-age children in recent years.
Transmission
The portal of entry of measles virus is through the respiratory tract or conjunctivae following contact with large droplets or small-droplet aerosols in which the virus is suspended. Patients are infectious from 3 days before to up to 4-6 days after the onset of rash. Approximately 90% of exposed susceptible individuals experience measles. Face-to-face contact is not necessary, because viable virus may be suspended in air for as long as 1 hr after the patient with the source case leaves a room. Secondary cases from spread of aerosolized virus have been reported in airplanes, physicians’ offices, and hospitals.
Pathology
Measles infection causes necrosis of the respiratory tract epithelium and an accompanying lymphocytic infiltrate. Measles produces a small-vessel vasculitis on the skin and on the oral mucous membranes. Histology of the rash and exanthem reveals intracellular edema and dyskeratosis associated with formation of epidermal syncytial giant cells with up to 26 nuclei. Viral particles have been identified within these giant cells. In lymphoreticular tissue, lymphoid hyperplasia is prominent. Fusion of infected cells results in multinucleated giant cells, the Warthin-Finkeldey giant cells that are pathognomonic for measles, with up to 100 nuclei and intracytoplasmic and intranuclear inclusions.
Pathogenesis
Measles infection consists of 4 phases: incubation period, prodromal illness, exanthematous phase, and recovery. During incubation, measles virus migrates to regional lymph nodes. A primary viremia ensues that disseminates the virus to the reticuloendothelial system. A secondary viremia spreads virus to body surfaces. The prodromal illness begins after the secondary viremia and is associated with epithelial necrosis and giant cell formation in body tissues. Cells are killed by cell-to-cell plasma membrane fusion associated with viral replication that occurs in many body tissues, including cells of the central nervous system. Virus shedding begins in the prodromal phase. With onset of the rash, antibody production begins, and viral replication and symptoms begin to subside. Measles virus also infects CD4+ T cells, resulting in suppression of the Th1 immune response and a multitude of other immunosuppressive effects.
Measles virus attaches to specific cell receptors to infect host cells. Studies in primates show that the initial targets for measles virus are alveolar macrophages, dendritic cells, and lymphocytes. The cell receptor used appears to be the signaling lymphocyte activating molecule or more properly CD150. Subsequently, respiratory epithelial cells become infected but do not express CD150. The mechanism of infection of respiratory tissues is attachment to the PVRL4 receptor (Nectin4) that is expressed on cells in the trachea, oral mucosa, nasopharynx, and lungs. These 2 receptors, CD150 and PVRL4, account for the lymphotropic and epitheliotropic nature of natural measles virus infection and, along with the prolonged immunosuppressive effects of measles, suggest that it is more characteristic of human immunodeficiency virus infection than a respiratory illness.
Clinical Manifestations
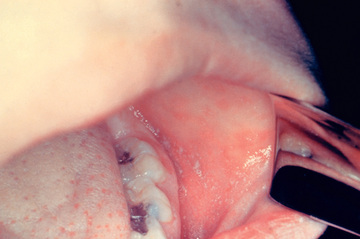
Measles is a serious infection characterized by high fever, an enanthem, cough, coryza, conjunctivitis, and a prominent exanthem (Fig. 273.2 ). After an incubation period of 8-12 days, the prodromal phase begins with a mild fever followed by the onset of conjunctivitis with photophobia, coryza, a prominent cough, and increasing fever. Koplik spots represent the enanthem and are the pathognomonic sign of measles, appearing 1-4 days prior to the onset of the rash (Fig. 273.3 ). They first appear as discrete red lesions with bluish white spots in the center on the inner aspects of the cheeks at the level of the premolars. They may spread to involve the lips, hard palate, and gingiva. They also may occur in conjunctival folds and in the vaginal mucosa. Koplik spots have been reported in 50–70% of measles cases but probably occur in the great majority.
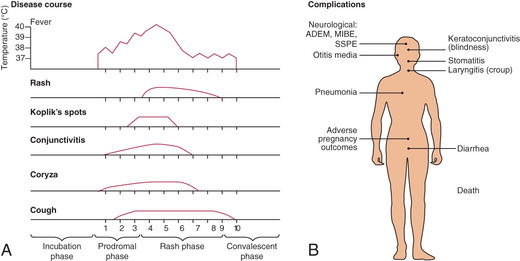
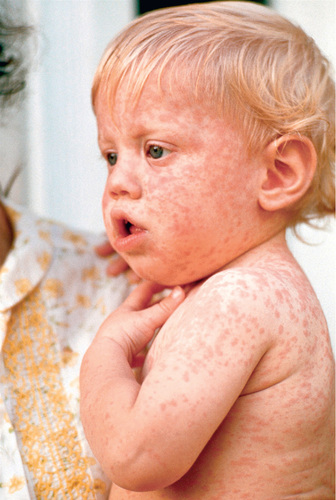
Symptoms increase in intensity for 2-4 days until the 1st day of the rash. The rash begins on the forehead (around the hairline), behind the ears, and on the upper neck as a red maculopapular eruption. It then spreads downward to the torso and extremities, reaching the palms and soles in up to 50% of cases. The exanthem frequently becomes confluent on the face and upper trunk (Fig. 273.4 ).
With the onset of the rash, symptoms begin to subside. The rash fades over about 7 days in the same progression as it evolved, often leaving a fine desquamation of skin in its wake. Of the major symptoms of measles, the cough lasts the longest, often up to 10 days. In more severe cases, generalized lymphadenopathy may be present, with cervical and occipital lymph nodes especially prominent.
Modified Measles Infection
In individuals with passively acquired antibody, such as infants and recipients of blood products, a subclinical form of measles may occur. The rash may be indistinct, brief, or, rarely, entirely absent. Likewise, some individuals who have received a vaccine, when exposed to measles, may have a rash but few other symptoms. Persons with modified measles are not considered highly contagious.
Laboratory Findings
The diagnosis of measles is almost always based on clinical and epidemiologic findings. Laboratory findings in the acute phase include reduction in the total white blood cell count, with lymphocytes decreased more than neutrophils. However, absolute neutropenia has been known to occur. In measles not complicated by bacterial infection, the erythrocyte sedimentation rate and C-reactive protein level are usually normal.
Diagnosis
In the absence of a recognized measles outbreak, confirmation of the clinical diagnosis is often recommended. Serologic confirmation is most conveniently made by identification of immunoglobulin (Ig) M antibody in serum. IgM antibody appears 1-2 days after the onset of the rash and remains detectable for about 1 mo. If a serum specimen is collected <72 hr after onset of rash and is negative for measles antibody, a 2nd specimen should be obtained. Serologic confirmation may also be made by demonstration of a 4-fold rise in IgG antibodies in acute and convalescent specimens collected 2-4 wk apart. Viral isolation from blood, urine, or respiratory secretions can be accomplished by culture at the CDC or local or state laboratories. Molecular detection by polymerase chain reaction is available through some state and local health departments and through the CDC.
Differential Diagnosis
Typical measles is unlikely to be confused with other illnesses, especially if Koplik spots are observed. Measles in the later stages or modified or atypical infections may be confused with a number of other exanthematous immune-mediated illnesses and infections, including rubella, adenovirus infection, enterovirus infection, and Epstein-Barr virus infection. Exanthem subitum (in infants) and erythema infectiosum (in older children) may also be confused with measles. Mycoplasma pneumoniae and group A streptococcus may also produce rashes similar to that of measles. Kawasaki syndrome can cause many of the same findings as measles but lacks discrete intraoral lesions (Koplik spots) and a severe prodromal cough and typically leads to elevations of neutrophils and acute-phase reactants. In addition, the characteristic thrombocytosis of Kawasaki syndrome is absent in measles (see Chapter 191 ). Drug eruptions may occasionally be mistaken for measles.
Complications
Complications of measles are largely attributable to the pathogenic effects of the virus on the respiratory tract and immune system (Table 273.1 , Fig. 273.2 ). Several factors make complications more likely. Morbidity and mortality from measles are greatest in individuals younger than 5 yr of age (especially <1 yr of age) and older than 20 yr of age. In developing countries, higher case fatality rates have been associated with crowding, possibly attributable to larger inoculum doses after household exposure. Severe malnutrition in children results in a suboptimal immune response and higher morbidity and mortality with measles infection. Low serum retinol levels in children with measles are associated with higher measles morbidity and mortality in developing countries and in the United States. Measles infection lowers serum retinol concentrations, so subclinical cases of hyporetinolemia may be made symptomatic during measles. Measles infection in immunocompromised persons is associated with increased morbidity and mortality. Among patients with malignancy in whom measles develops, pneumonitis occurs in 58% and encephalitis occurs in 20%.
Table 273.1
Complications by Age for Reported Measles Cases, United States, 1987–2000
| COMPLICATION | OVERALL (67,032 CASES WITH AGE INFORMATION) | NO. (%) OF PERSONS WITH COMPLICATION BY AGE GROUP | ||||
|---|---|---|---|---|---|---|
| <5 yr (N = 28,730) | 5-9 yr (N = 6,492) | 10-19 yr (N = 18,580) | 20-29 yr (N = 9,161) | <30 yr (N = 4,069) | ||
| Any | 19,480 (29.1) | 11,883 (41.4) | 1,173 (18.1) | 2,369 (12.8) | 2,656 (29.0) | 1,399 (34.4) |
| Death | 177 (0.3) | 97 (0.3) | 9 (0.1) | 18 (0.1) | 26 (0.3) | 27 (0.7) |
| Diarrhea | 5,482 (8.2) | 3,294 (11.5) | 408 (6.3) | 627 (3.4) | 767 (8.4) | 386 (9.5) |
| Encephalitis | 97 (0.1) | 43 (0.2) | 9 (0.1) | 13 (0.1) | 21 (0.2) | 11 (0.3) |
| Hospitalization | 12,876 (19.2) | 7,470 (26.0) | 612 (9.4) | 1,612 (8.7) | 2,075 (22.7) | 1,107 (27.2) |
| Otitis media | 4,879 (7.3) | 4,009 (14.0) | 305 (4.7) | 338 (1.8) | 157 (1.7) | 70 (1.7) |
| Pneumonia | 3,959 (5.9) | 2,480 (8.6) | 183 (2.8) | 363 (2.0) | 554 (6.1) | 379 (9.3) |
From Perry RT, Halsey NA: The clinical significance of measles: a review, Clin Infect Dis 189(Suppl. 1):S4–S16, 2004.
Pneumonia is the most common cause of death in measles. It may manifest as giant cell pneumonia caused directly by the viral infection or as superimposed bacterial infection. The most common bacterial pathogens are Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus . Following severe measles pneumonia, the final common pathway to a fatal outcome is often the development of bronchiolitis obliterans.
Croup, tracheitis, and bronchiolitis are common complications in infants and toddlers with measles. The clinical severity of these complications frequently requires intubation and ventilatory support until the infection resolves.
Acute otitis media is the most common complication of measles and was of particularly high incidence during the epidemic of the late 1980s and early 1990s because of the relatively young age of affected children. Sinusitis and mastoiditis also occur as complications. Viral and/or bacterial tracheitis is seen and can be life-threatening. Retropharyngeal abscess has also been reported.
Measles infection is known to suppress skin test responsiveness to purified tuberculin antigen. There may be a higher rate of activation of pulmonary tuberculosis in populations of individuals infected with Mycobacterium tuberculosis who are then exposed to measles.
Diarrhea and vomiting are common symptoms associated with acute measles, and diffuse giant cell formation is found in the epithelium in the gastrointestinal tract. Dehydration is a common consequence, especially in young infants and children. Appendicitis or abdominal pain may occur from obstruction of the appendiceal lumen by lymphoid hyperplasia.
Febrile seizures occur in <3% of children with measles. Encephalitis following measles is a long-associated complication, often with an unfavorable outcome. Rates of 1-3 per 1,000 cases of measles have been reported, with greater numbers occurring in adolescents and adults than in preschool- or school-age children. Encephalitis is a postinfectious, immunologically mediated process and is not the result of a direct effect by the virus. Clinical onset begins during the exanthem and manifests as seizures (56%), lethargy (46%), coma (28%), and irritability (26%). Findings in cerebrospinal fluid include lymphocytic pleocytosis in 85% of cases and elevated protein concentrations. Approximately 15% of patients with measles encephalitis die. Another 20–40% of patients suffer long-term sequelae, including cognitive impairment, motor disabilities, and deafness.
Measles encephalitis in immunocompromised patients results from direct damage to the brain by the virus. Subacute measles encephalitis manifests 1-10 mo after measles in immunocompromised patients, particularly those with AIDS, lymphoreticular malignancies, and immunosuppression. Signs and symptoms include seizures, myoclonus, stupor, and coma. In addition to intracellular inclusions, abundant viral nucleocapsids and viral antigen are seen in brain tissue. Progressive disease and death almost always occur.
A severe form of measles rarely seen nowadays is hemorrhagic measles or black measles. It manifested as a hemorrhagic skin eruption and was often fatal. Keratitis, appearing as multiple punctate epithelial foci, resolved with recovery from the infection. Thrombocytopenia sometimes occurred following measles.
Myocarditis is a rare complication of measles. Miscellaneous bacterial infections have been reported, including bacteremia, cellulitis, and toxic shock syndrome. Measles during pregnancy is associated with high rates of maternal morbidity, fetal wastage, and stillbirths, with congenital malformations in 3% of liveborn infants.
Subacute Sclerosing Panencephalitis
Subacute sclerosing panencephalitis (SSPE) is a chronic complication of measles with a delayed onset and an outcome that is nearly always fatal. It appears to result from a persistent infection with an altered measles virus that is harbored intracellularly in the central nervous system for several yr. After 7-10 yr the virus apparently regains virulence and attacks the cells in the central nervous system that offered the virus protection. This “slow virus infection” results in inflammation and cell death, leading to an inexorable neurodegenerative process.
SSPE is a rare disease and generally follows the prevalence of measles in a population. The incidence in the United States in 1960 was 0.61 cases per million persons younger than age 20 yr. By 1980 the rate had fallen to 0.06 cases per million. Between 1956 and 1982 a total of 634 cases of SSPE had been reported to the national SSPE registry. After 1982 approximately 5 cases/yr were reported annually in the United States, and only 2-3 cases/yr were reported in the early 1990s. However, between 1995 and 2000, reported cases in the United States increased and 13 cases were reported in 2000. Nine of the 13 cases occurred in foreign-born individuals. This “resurgence” may be the result of an increased incidence of measles between 1989 and 1991. Although the age of onset ranges from <1 yr to <30 yr, the illness is primarily one of children and adolescents. Measles at an early age favors the development of SSPE: 50% of patients with SSPE had primary measles before 2 yr of age, and 75% had measles before 4 yr of age. Males are affected twice as often as females, and there appear to be more cases reported from rural than urban populations. Recent observations from the registry indicate a higher prevalence among children of Hispanic origin.
The pathogenesis of SSPE remains enigmatic. Factors that seem to be involved include defective measles virus and interaction with a defective or immature immune system. The virus isolated from brain tissue of patients with SSPE is missing 1 of the 6 structural proteins, the matrix or M protein. This protein is responsible for assembly, orientation, and alignment of the virus in preparation for budding during viral replication. Immature virus may be able to reside, and possibly propagate, within neuronal cells for long periods. The fact that most patients with SSPE were exposed at a young age suggests that immune immaturity is involved in pathogenesis.
Clinical manifestations of SSPE begin insidiously 7-13 yr after primary measles infection. Subtle changes in behavior or school performance appear, including irritability, reduced attention span, and temper outbursts. This initial phase (stage I ) may at times be missed because of brevity or mildness of the symptoms. Fever, headache, and other signs of encephalitis are absent. The hallmark of the 2nd stage is massive myoclonus, which coincides with extension of the inflammatory process site to deeper structures in the brain, including the basal ganglia. Involuntary movements and repetitive myoclonic jerks begin in single muscle groups but give way to massive spasms and jerks involving both axial and appendicular muscles. Consciousness is maintained. In the 3rd stage , involuntary movements disappear and are replaced by choreoathetosis, immobility, dystonia, and lead pipe rigidity that result from destruction of deeper centers in the basal ganglia. The sensorium deteriorates into dementia, stupor, and then coma. The 4th stage is characterized by loss of critical centers that support breathing, heart rate, and blood pressure. Death soon ensues. Progression through the clinical stages may follow courses characterized as acute, subacute, or chronic progressive.
The diagnosis of SSPE can be established through documentation of a compatible clinical course and at least 1 of the following supporting findings: (1) measles antibody detected in cerebrospinal fluid, (2) characteristic electroencephalographic findings, and (3) typical histologic findings in and/or isolation of virus or viral antigen from brain tissue obtained by biopsy or postmortem examination.
Cerebrospinal fluid analysis reveals normal cells but elevated IgG and IgM antibody titers in dilutions >1 : 8. Electroencephalographic patterns are normal in stage I, but in the myoclonic phase, suppression-burst episodes are seen that are characteristic of, but not pathognomonic for, SSPE. Brain biopsy is no longer routinely indicated for diagnosis of SSPE.
Management of SSPE is primarily supportive and similar to care provided to patients with other neurodegenerative diseases. Clinical trials using isoprinosine with or without interferon suggest significant benefit (30–34% remission rate) compared with patients without treatment (5–10% with spontaneous remissions).
It is recognized that carbamazepine is of significant benefit in the control of myoclonic jerks in the early stages of the illness.
Virtually all patients eventually succumb to SSPE. Most die within 1-3 yr of onset from infection or loss of autonomic control mechanisms. Prevention of SSPE depends on prevention of primary measles infection through vaccination. SSPE has been described in patients who have no history of measles infection and exposure only to the vaccine virus. However, wild-type virus, not vaccine virus, has been found in brain tissue of at least some of these patients, suggesting that they had had subclinical measles previously.
Treatment
Management of measles is supportive because there is no specific antiviral therapy approved for treatment of measles. Maintenance of hydration, oxygenation, and comfort are goals of therapy. Antipyretics for comfort and fever control are useful. For patients with respiratory tract involvement, airway humidification and supplemental oxygen may be of benefit. Respiratory failure from croup or pneumonia may require ventilatory support. Oral rehydration is effective in most cases, but severe dehydration may require intravenous therapy. Prophylactic antimicrobial therapy to prevent bacterial infection is not indicated.
Measles infection in immunocompromised patients is highly lethal. Ribavirin is active in vitro against measles virus. Anecdotal reports of ribavirin therapy with or without intravenous gamma globulin suggest some benefit in individual patients. However, no controlled trials have been performed, and ribavirin is not licensed in the United States for treatment of measles.
Vitamin A
Vitamin A deficiency in children in developing countries has long been known to be associated with increased mortality from a variety of infectious diseases, including measles. In the United States, studies in the early 1990s documented that 22–72% of children with measles had low retinol levels. In addition, 1 study demonstrated an inverse correlation between the level of retinol and severity of illness. Several randomized controlled trials of vitamin A therapy in the developing world and the United States have demonstrated reduced morbidity and mortality from measles. Vitamin A therapy is indicated for all patients with measles. Vitamin A should be administered once daily for 2 days at doses of 200,000 IU for children 12 mo of age or older; 100,000 IU for infants 6 mo through 11 mo of age; and 50,000 IU for infants younger than 6 mo of age.
In children with signs and symptoms of vitamin A deficiency, a 3rd age-appropriate dose is recommended 2-4 wk after the 2nd dose.
Prognosis
In the early 20th century, deaths from measles in the United States varied between 2,000 and 10,000 per year, or about 10 deaths per 1,000 cases of measles. With improvements in healthcare and antimicrobial therapy, better nutrition, and decreased crowding, the death:case ratio fell to 1 per 1,000 cases. Between 1982 and 2002, the CDC estimated that there were 259 deaths caused by measles in the United States, with a death:case ratio of 2.5-2.8 per 1,000 cases of measles. Pneumonia and encephalitis were complications in most of the fatal cases, and immunodeficiency conditions were identified in 14–16% of deaths. In 2011, of the 222 cases reported in the United States, 70 (32%) were hospitalized, including 17 (24%) with diarrhea, 15 (21%) with dehydration, and 12 (17%) with pneumonia. No cases of encephalitis or deaths were reported. In the 1st half of 2015, of 159 reported cases 22 (14%) were hospitalized, with 5 pneumonia cases and no deaths.
Prevention
Patients shed measles virus from 7 days after exposure to 4-6 days after the onset of rash. Exposure of susceptible individuals to patients with measles should be avoided during this period. In hospitals, standard and airborne precautions should be observed for this period. Immunocompromised patients with measles will shed virus for the duration of the illness, so isolation should be maintained throughout the disease.
Vaccine
Vaccination against measles is the most effective and safe prevention strategy. Measles vaccine in the United States is available as a combined vaccine with measles-mumps-rubella vaccine, the last of which is the recommended form in most circumstances (Table 273.2 ). Following the measles resurgence of 1989-1991, a 2nd dose of measles vaccine was added to the schedule. The current recommendations include a 1st dose at 12-15 mo of age, followed by a 2nd dose at 4-6 yr of age. However, the 2nd dose can be given any time after 30 days following the 1st dose, and the current schedule is a convenience schedule. Seroconversion is slightly lower in children who receive the 1st dose before or at 12 mo of age (87% at 9 mo, 95% at 12 mo, and 98% at 15 mo) because of persisting maternal antibody; however, this is an evolving situation, with children currently as young as 6 mo unprotected from maternal antibodies and susceptible to measles infection. For children who have not received 2 doses by 11-12 yr of age, a 2nd dose should be provided. Infants who receive a dose before 12 mo of age should be given 2 additional doses at 12-15 mo and 4-6 yr of age. Children who are traveling should be offered either primary measles immunization even as young as 6 mo or a 2nd dose even if <4 yr.
Table 273.2
MMR, measles-mumps-rubella vaccine; MMRV, measles-mumps-rubella-varicella vaccine.
From American Academy of Pediatrics: Measles. In Kimberlin DW, Brady MT, Jackson MA, Long SS, editors: Red book 2018 report of the committee on infectious diseases, ed 31, Itasca, IL, 2018, American Academy of Pediatrics, Table 3.39, p. 543.
Adverse events from the measles-mumps-rubella vaccine include fever (usually 6-12 days following vaccination), rash in approximately 5% of vaccinated persons, and, rarely, transient thrombocytopenia. Children prone to febrile seizures may experience an event following vaccination, so the risks and benefits of vaccination should be discussed with parents. Encephalopathy and autism have not been shown to be causally associated with the measles-mumps-rubella vaccine or vaccine constituents.
A review of the effect of measles vaccination on the epidemiology of SSPE has demonstrated that measles vaccination protects against SSPE and does not accelerate the course of SSPE or trigger the disease in those already infected with wild measles virus.
Passively administered immune globulin may inhibit the immune response to live measles vaccine, and administration should be delayed for variable amounts of time based on the dose of Ig (Table 273.3 ).
Table 273.3
Suggested Intervals Between Immunoglobulin Administration and Measles Immunization*
| INDICATION FOR IMMUNOGLOBULIN | DOSE | |||
|---|---|---|---|---|
| Route | Units (U) or Milliliters (mL) | mg IgG/kg | Interval (mo) † | |
| Tetanus (as tetanus Ig) | IM | 250 U | 10 | 3 |
| Hepatitis A prophylaxis (as Ig): | ||||
| Contact prophylaxis | IM | 0.02 mL/kg | 3.3 | 3 |
| International travel | IM | 0.06 mL/kg | 10 | 3 |
| Hepatitis B prophylaxis (as hepatitis B Ig) | IM | 0.06 mL/kg | 10 | 3 |
| Rabies prophylaxis (as rabies Ig) | IM | 20 IU/kg | 22 | 4 |
| Varicella prophylaxis (as VariZIG) | IM | 125 U/10 kg (maximum 625 U) | 20-40 | 5 |
| Measles prophylaxis (as Ig): | ||||
| Standard | IM | 0.50 mL/kg | 80 | 6 |
| Immunocompromised host | IV | 400 mg/kg | 8 | |
| Respiratory syncytial virus prophylaxis (palivizumab monoclonal antibody) ‡ | IM | — | 15 mg/kg (monoclonal) | None |
| Cytomegalovirus immune globulin | IV | 3 mL/kg | 150 | 6 |
| Blood transfusion: | ||||
| Washed RBCs | IV | 10 mL/kg | Negligible | 0 |
| RBCs, adenine-saline added | IV | 10 mL/kg | 10 | 3 |
| Packed RBCs | IV | 10 mL/kg | 20-60 | 6 |
| Whole blood | IV | 10 mL/kg | 80-100 | 6 |
| Plasma or platelet products | IV | 10 mL/kg | 160 | 7 |
| Replacement (or therapy) of immune deficiencies (as IVIG) | IV | — | 300-400 | 8 |
| ITP (as IVIG) | IV | — | 400 | 8 |
| ITP | IV | — | 1,000 | 10 |
| ITP or Kawasaki disease | IV | — | 1,600-2,000 | 11 |
* Immunization in the form of measles-mumps-rubella (MMR), measles-mumps-rubella-varicella (MMRV), or monovalent measles vaccine.
† These intervals should provide sufficient time for decreases in passive antibodies in all children to allow for an adequate response to measles vaccine. Physicians should not assume that children are fully protected against measles during these intervals. Additional doses of Ig or measles vaccine may be indicated after exposure to measles.
‡ Monoclonal antibodies, such as palivizumab, do not interfere with the immune response to vaccines.
Ig, immunoglobulin; IgG, immunoglobulin G; ITP, immune (formerly termed “idiopathic”) thrombocytopenic purpura; IVIG, intravenous Ig; RBCs, red blood cells.
From American Academy of Pediatrics: Red book: 2015 report of the Committee on Infectious Diseases , ed 30, Elk Grove Village, IL, 2015, American Academy of Pediatrics, Table 1.10, p. 39.
Live vaccines should not be administered to pregnant women or to immunodeficient or immunosuppressed patients. However, patients with HIV who are not severely immunocompromised should be immunized. Because measles virus may suppress the cutaneous response to tuberculosis antigen, skin testing for tuberculosis should be performed before or at the same time as administration of the vaccine. Individuals infected with M. tuberculosis should be receiving appropriate treatment at the time of administration of measles vaccine.
Postexposure Prophylaxis
Susceptible individuals exposed to measles may be protected from infection by either vaccine administration or with Ig. The vaccine is effective in prevention or modification of measles if given within 72 hr of exposure. Ig may be given up to 6 days after exposure to prevent or modify infection. Immunocompetent children should receive 0.5 mL/kg (maximum dose in both cases is 15 mL/kg) intramuscularly (IM). For severely immunocompromised children and pregnant woman without evidence of measles immunity, Ig intravenously is the recommended IG at 400 mg/kg. Ig is indicated for susceptible household contacts of measles patients, especially infants younger than 6 mo of age, pregnant women, and immunocompromised persons.