Parainfluenza Viruses*
Holly M. Biggs, Angela J.P. Campbell
Human parainfluenza viruses (HPIVs) are common causes of acute respiratory illness in infants and children and are important causes of lower respiratory tract disease in young children and immunocompromised persons. These viruses cause a spectrum of upper and lower respiratory tract illnesses but are particularly associated with croup (laryngotracheitis or laryngotracheobronchitis), bronchiolitis , and pneumonia .
Etiology
HPIVs are members of the Paramyxoviridae family. Four HPIVs cause illness in humans, classified as types 1-4, with diverse manifestations of infection. Type 4 is divided into two antigenic subtypes, 4a and 4b. HPIVs have a nonsegmented, single-stranded RNA genome with a lipid-containing envelope derived from budding through the host cell membrane. The major antigenic moieties are the hemagglutinin neuraminidase (HN) and fusion (F) surface glycoproteins.
Epidemiology
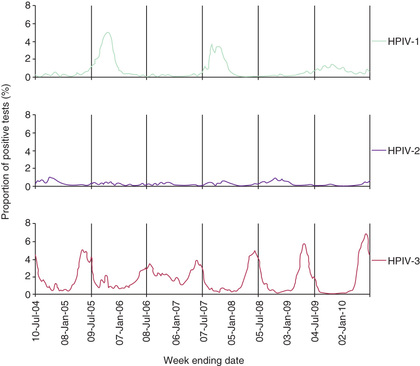
By 5 yr of age, most children have experienced primary infection with HPIV types 1, 2, and 3. HPIV-3 infections generally occur earliest, with half of infants infected by age 1 yr, and over 90% by age 5 yr. HPIV-1 and HPIV-2 are more common after infancy, with approximately 75% infected by age 5 yr. Although HPIV-4 is not recognized as often, about half of children have antibody by the age of 5 yr. In the United States and temperate climates, HPIV-1 has typically been reported to have biennial epidemics in the fall in odd-numbered years (Fig. 286.1 ). HPIV-2 has been reported to cause yearly outbreaks in the fall, but is less common than HPIV-1 or HPIV-3. HPIV-3 can be endemic throughout the year but typically peaks in late spring. In years with less HPIV-1 activity, the HPIV-3 season has been observed to extend longer or to have a second peak in the fall (see Fig. 286.1 ). The epidemiology of HPIV-4 is less well defined, because it is difficult to grow in tissue culture and was often excluded from previous studies, but a recent study suggests it may circulate throughout the year and peak in fall of odd-numbered years. National HPIV trends are created from weekly laboratory test result data that are reported on a voluntary basis, and are available at the Centers for Disease Control and Prevention (CDC) National Respiratory and Enteric Virus Surveillance System (NREVSS) website (https://www.cdc.gov/surveillance/nrevss ).
HPIVs are spread primarily from the respiratory tract by inhalation of large respiratory droplets or contact with infected nasopharyngeal secretions. HPIVs are notable for causing outbreaks of respiratory illness in hospital wards, clinics, neonatal nurseries, and other institutional settings. The incubation period from exposure to symptom onset may range from 2 to 6 days. Children are likely to excrete virus from the oropharynx for 2-3 wk, but shedding can be more prolonged, especially in immunocompromised children, and may persist for months. Primary infection does not confer permanent immunity, and reinfections are common throughout life. Reinfections are usually mild and self-limited, but can cause serious lower respiratory tract illness, particularly in children with compromised immune systems.
Pathogenesis
HPIVs replicate in the respiratory epithelium. The propensity to cause illness in the upper large airways is presumably related to preferential replication in the larynx, trachea, and bronchi in comparison with other viruses. Some HPIVs induce cell-to-cell fusion. During the budding process, cell membrane integrity is lost, and viruses can induce cell death through the process of apoptosis. In children, the most severe illness generally coincides with the time of maximal viral shedding. However, disease severity is likely related to the host immune response to infection as much as to direct cytopathic effects of the virus. Virus-specific immunoglobulin A antibody levels and serum antibodies to the surface HN and F glycoproteins are able to neutralize HPIV, and both likely contribute to host immunity. Cell-mediated cytotoxicity is also important for controlling and terminating HPIV infection.
Clinical Manifestations
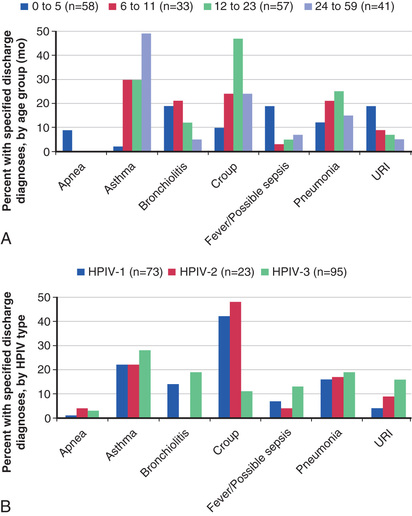
The most common type of illness caused by HPIV infection consists of some combination of low-grade fever, rhinorrhea, cough, pharyngitis, and hoarseness, and may be associated with vomiting or diarrhea. Rarely, HPIV infection is associated with parotitis. HPIVs have also been associated with a variety of skin manifestations, including typical maculopapular viral exanthems, erythema multiforme, and papular acrodermatitis, or Gianotti-Crosti syndrome (see Chapter 687 ). Although often mild, more serious HPIV illness may result in hospitalization, with common discharge diagnoses of bronchiolitis, fever/possible sepsis, and apnea among younger children, and croup, pneumonia, and asthma among older children (Fig. 286.2 ). HPIVs account for 50% of hospitalizations for croup and at least 15% of cases of bronchiolitis and pneumonia. HPIV-1, and to a lesser extent HPIV-2, cause more cases of croup, whereas HPIV-3 is more likely to infect the small air passages and cause pneumonia, bronchiolitis, or bronchitis. HPIV-4 causes a similar range of illness as the other types. Any HPIV can cause lower respiratory tract disease, particularly during primary infection or in patients with compromised immune systems. In children and adult patients with hematologic malignancies and undergoing hematopoietic stem cell transplantation, lymphopenia has repeatedly been shown to be an independent risk factor for progression from upper to lower respiratory tract disease.
Diagnosis and Differential Diagnosis
The diagnosis of HPIV infection in children is often based on only clinical and epidemiologic criteria. Croup is a clinical diagnosis and must be distinguished from other diagnoses, including foreign body aspiration, epiglottitis, retropharyngeal abscess, angioedema, and subglottic stenosis or hemangioma. Although the radiographic steeple sign , consisting of progressive narrowing of the subglottic region of the trachea, is characteristic of croup, differential considerations include acute epiglottitis, thermal injury, angioedema, and bacterial tracheitis. Manifestation of HPIV lower respiratory tract disease may be similar to that of a number of other respiratory viral infections; therefore identification of virus should be sought by the most sensitive diagnostic means available for certain severe illnesses, such as pneumonia in immunocompromised children.
Sensitive, specific, and rapid molecular assays such as multiplex polymerase chain reaction assays, have become more widely available and greatly increase sensitivity of HPIV detection. For immunocompromised patients, these highly sensitive platforms provide the critical ability to make a prompt diagnosis by detecting a wide range of viral pathogens, including HPIVs, thus allowing for early implementation of infection prevention measures and potential treatment. Conventional laboratory diagnosis is accomplished by HPIV isolation in tissue culture, although time to result can take up to a week or longer; this can be shortened to 1-3 days using a rapid shell viral culture system. Direct immunofluorescent staining is available in some laboratories for rapid identification of virus antigen in respiratory secretions.
Treatment
There are no specific antiviral medications approved for the treatment of HPIV infections. For croup, the possibility of rapid respiratory compromise should influence the level of care and treatment given (see Chapter 412 ). Humidified air has not been shown to be effective. Corticosteroids, including dexamethasone orally or by injection and less often budesonide via nebulizer, improve symptoms within 6 hr after treatment, lessen the need for other medications, and shorten hospital stays. In general, because of its safety, efficacy, and cost-effectiveness, a single dose of oral dexamethasone (0.6 mg/kg) is the primary treatment for croup in the office or emergency room setting. A single dose of intramuscular dexamethasone or budesonide (2 mg [2 mL solution] via nebulizer) may provide an alternative to dexamethasone for children with severe respiratory distress or vomiting. The dose may be repeated, but this should not be necessary on a routine basis, and there are no guidelines to compare outcomes of single- and multiple-dose treatment schedules. Moderate to severe symptoms that persist for more than a few days should prompt investigation for other causes of airway obstruction.
The severity assessment of croup generally incorporates a number of clinical features, which include the presence and degree of chest wall retractions, whether stridor is present at rest, and evaluation of the child's mental status (e.g., for agitation, anxiety, lethargy). For obstructive airway symptoms associated with moderate to severe croup, nebulized epinephrine (either racemic epinephrine 2.25% solution, 0.05 to 0.1 mL/kg/dose, maximum dose 0.5 mL, diluted, in 3 mL of normal saline; or L -epinephrine, 0.5 mL/kg/dose of 1 : 1,000 solution in normal saline, maximum dose 5 mL) is recommended and may also provide temporary symptomatic improvement. Children should be observed for at least 2 hr after receiving epinephrine treatment for return of obstructive symptoms. Repeated treatments may be provided, depending on the duration of symptoms. Oxygen should be administered for hypoxia, and supportive care with analgesics and antipyretics is reasonable for fever and discomfort associated with HPIV infections. The indications for antibiotics are limited to well-documented secondary bacterial infections of the middle ear(s) or lower respiratory tract.
Ribavirin has some antiviral activity against HPIVs in vitro and in animal models. Inhaled ribavirin has been given to severely immunocompromised children with HPIV pneumonia; however, the majority of data have not shown improved outcomes, and randomized, controlled studies are lacking. Some institutions use intravenous immunoglobulin for HPIV pneumonia in children with hematologic malignancies or who have undergone hematopoietic stem cell transplantation; the impact of this treatment strategy on clinical outcomes is also limited by lack of controlled studies. Use of investigational antiviral DAS181, a novel sialidase fusion protein inhibitor, has shown clinical potential when used for treatment of HPIV lower respiratory tract disease among solid-organ and hematopoietic stem cell transplant recipients, but further study is needed. Other potential strategies for drug development include hemagglutinin-neuraminidase inhibitors, transcription inhibitors, and synthetic small interfering RNAs.
Complications
Eustachian tube obstruction can lead to secondary bacterial invasion of the middle ear space and acute otitis media in 30–50% of HPIV infections. Similarly, obstruction of the paranasal sinuses can lead to sinusitis. The destruction of cells in the upper airways can lead to secondary bacterial invasion and resultant bacterial tracheitis, and antecedent HPIV infection of lower airways may predispose to bacterial pneumonia. Nonrespiratory complications of HPIV are rare but include aseptic meningitis, encephalitis, acute disseminated encephalomyelitis, rhabdomyolysis, myocarditis, and pericarditis.
Prognosis
The prognosis for full recovery from HPIV infection in the immunocompetent child is excellent, with no long-term pulmonary sequelae. Deaths may rarely occur, particularly in immunocompromised children with lower respiratory tract infection.
Prevention
Vaccine development has focused largely on live-attenuated intranasal HPIV-3 vaccines. Candidates include a recombinant human HPIV-3 virus (rcp45) derived from complementary DNA, as well as a complementary DNA–derived chimeric bovine/human HPIV-3 virus; these candidates are well tolerated and immunogenic in infants and young children. Constructs using chimeric bovine/human HPIV-3 virus in addition to the F or both F and G proteins of respiratory syncytial virus are also under investigation. Although at a less advanced state in development, live attenuated candidate HPIV-1 and HPIV-2 vaccines have undergone phase 1 clinical studies (www.clinicaltrials.gov ). The measure of protection afforded by vaccines will be difficult to assess, because symptomatic reinfection occurs and the frequency of serious infection in the general population is low. Nonetheless, it is clear that prevention of acute respiratory illness caused by HPIVs, particularly lower respiratory tract infections among infants and young children, is a worthwhile goal.