Bronchiectasis
Oren J. Lakser
Bronchiectasis is characterized by irreversible abnormal dilation and anatomic distortion of the bronchial tree and represents the common end stage of many nonspecific and unrelated antecedent events. Its incidence has been decreasing overall in industrialized countries, but it persists as a problem in lower- and middle-income countries and among some ethnic groups in industrialized nations (particularly in aboriginal children). Females are afflicted more frequently than males.
Pathophysiology and Pathogenesis
In industrialized nations, cystic fibrosis (see Chapter 432 ) is the most common cause of clinically significant bronchiectasis. Other conditions associated with bronchiectasis include primary ciliary dyskinesia (see Chapter 433 ), foreign body aspiration (see Chapter 405 ), aspiration of gastric contents, immune deficiency syndromes (especially humoral immunity), and infection, especially pertussis, measles, and tuberculosis (Table 430.1 ). Bronchiectasis can also be congenital, as in Williams-Campbell syndrome , in which there is an absence of annular bronchial cartilage, and Marnier-Kuhn syndrome (congenital tracheobronchomegaly), in which there is a connective tissue disorder. Other disease entities associated with bronchiectasis are yellow nail syndrome (pleural effusion, lymphedema, discolored nails) and right middle lobe syndrome . The right middle lobe syndrome is mostly associated with other generalized causes of bronchiectasis including asthma, cystic fibrosis, primary ciliary dyskinesia, severe pneumonia, aspiration pneumonia, foreign bodies, and immune deficient states. Early phases of the right middle lobe syndrome manifest as persistent or recurrent right middle lobe infiltrates (pneumonia). The right middle lobe syndrome may be classified as intrinsic or extrinsic obstructive (tumors, granulomas, lymphadenopathy) and nonobstructive (aspiration, asthma, cystic fibrosis).
Table 430.1
From Redding GJ: Bronchiectasis in children, Pediatr Clin North Am 56:157–171, 2009, Box 1, p. 158.
Three basic mechanisms are involved in the pathogenesis of bronchiectasis. Obstruction can occur because of tumor, foreign body, impacted mucus because of poor mucociliary clearance, external compression, bronchial webs, and atresia. Infections caused by Bordetella pertussis , measles, rubella, togavirus, respiratory syncytial virus, adenovirus, and Mycobacterium tuberculosis induce chronic inflammation, progressive bronchial wall damage, and dilation. More recently, nontypeable Haemophilus influenzae seems to be a common cause of infection in adults and children with bronchiectasis. Streptococcus pneumoniae and Moraxella catarrhalis are more common in children with bronchiectasis than in adult patients. Chronic inflammation similarly contributes to the mechanism by which obstruction leads to bronchiectasis. Both inadequate and exaggerated/dysregulated immune responses may play a role in the development of bronchiectasis. Activation of Toll-like receptors results in the activation of nuclear factor κB and the release of proinflammatory cytokines interleukin (IL)-1β, IL-8, and tumor necrosis factor-α. IL-8 is a chemoattractant for neutrophils, which are the main inflammatory cell involved in the pathogenesis of bronchiectasis. Once activated, neutrophils produce neutrophil elastase and matrix metalloproteinases, MMP-8 and MMP-9. IL-6, IL-8, and tumor necrosis factor-α are elevated in the airways of patients with bronchiectasis. Eosinophils are also elevated in airways of indigenous children with bronchiectasis which promote neutrophil recruitment, goblet cell hyperplasia, and airway destruction. There is an increase in proinflammatory cytotoxic T lymphocytes in peripheral blood of children with bronchiectasis. The mechanism by which bronchiectasis occurs in congenital forms is likely related to abnormal cartilage formation. The common thread in the pathogenesis of bronchiectasis consists of difficulty clearing secretions and recurrent infections with a “vicious cycle” of infection and inflammation resulting in airway injury and remodeling (Fig. 430.1 ). In early stages, bronchiectasis consists primarily of bronchiolar wall thickening and destruction of elastin resulting in bronchial dilatation. In later stages, the bronchial walls develop cartilage destruction with associated pulmonary artery/arteriole vascular remodeling, resulting in pulmonary hypertension.
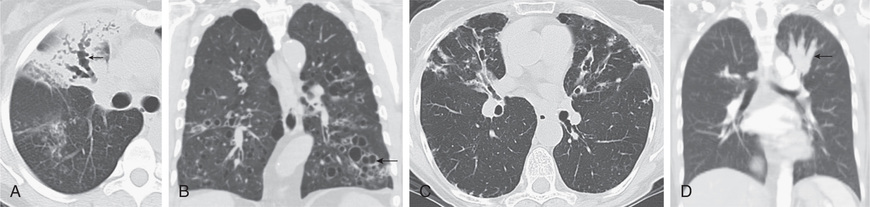
Bronchiectasis can manifest in any combination of 3 pathologic forms, best defined by high-resolution CT (HRCT) scan (Fig. 430.2 ). In cylindrical bronchiectasis, the bronchial outlines are regular, but there is diffuse dilation of the bronchial unit. The bronchial lumen ends abruptly because of mucous plugging. In varicose bronchiectasis, the degree of dilation is greater, and local constrictions cause an irregularity of outline resembling that of varicose veins. There may also be small sacculations. In saccular (cystic) bronchiectasis, bronchial dilation progresses and results in ballooning of bronchi that end in fluid- or mucus-filled sacs. This is the most severe form of bronchiectasis. Bronchiectasis lies within a disease spectrum of chronic pediatric suppurative lung disease. The following definitions have been proposed: prebronchiectasis (chronic or recurrent endobronchial infection with nonspecific HRCT changes; may be reversible); HRCT bronchiectasis (clinical symptoms with HRCT evidence of bronchial dilation; may persist, progress, or improve and resolve); established bronchiectasis (like the previous but with no resolution within 2 yr). Early diagnosis and aggressive therapy are important to prevent the development of established bronchiectasis.
Clinical Manifestations
The most common complaints in patients with bronchiectasis are cough and production of copious purulent sputum. Younger children may swallow the sputum. Hemoptysis is seen with some frequency. Fever can occur with infectious exacerbations. Anorexia and poor weight gain may occur as time passes. Physical examination typically reveals crackles localized to the affected area but wheezing as well as digital clubbing may also occur. In severe cases, dyspnea and hypoxemia can occur. Pulmonary function studies may demonstrate an obstructive, restrictive, or mixed pattern. Typically, impaired diffusion capacity is a late finding.
Diagnosis
Conditions that can be associated with bronchiectasis should be ruled out by appropriate investigations (e.g., sweat test, immunologic workup). Chest radiographs of patients with bronchiectasis tend to be nonspecific. Typical findings can include increase in size and loss of definition of bronchovascular markings, crowding of bronchi, and loss of lung volume. In more severe forms, cystic spaces, occasionally with air-fluid levels and honeycombing, may occur. Compensatory overinflation of unaffected lung may be seen. Thin-section HRCT scanning is the gold standard because it has excellent sensitivity and specificity. CT provides further information on disease location, presence of mediastinal lesions, and the extent of segmental involvement. The addition of radiolabeled aerosol inhalation to CT scanning can provide even more information. The CT findings in patients with bronchiectasis typically include cylindrical (“tram lines,” “signet ring appearance”), varicose (bronchi with “beaded contour”), cystic (cysts in “strings and clusters”), or mixed forms (see Fig. 430.2 ). The lower lobes are most commonly affected.
Treatment
The initial therapy for patients with bronchiectasis is medical and aims at decreasing airway obstruction and controlling infection. Airway clearance techniques (e.g., gravity-assisted drainage, active cycle of breathing, positive expiratory pressure [PEP], acapella, high-frequency chest wall oscillation), antibiotics, and bronchodilators are essential. Two to 4 wk of parenteral antibiotics is often necessary to manage acute exacerbations adequately. Exacerbations can be defined as the presence of 1 major criteria (wet cough enduring longer than 72 hr, increased cough frequency over 72 hr) plus 1 laboratory criteria (C-reactive protein >3 mg/L, serum IL-6 >2 ng/L, serum amyloid A >5 mg/L, elevated neutrophil percentage), 2 major criteria, or 1 major criteria plus 2 minor criteria (change in sputum color, breathlessness, chest pain, crackles/crepitations, wheeze). Antibiotic choice is dictated by the identification and sensitivity of organisms found on deep throat, sputum (induced or spontaneous), or bronchoalveolar lavage fluid cultures. The most common organisms found in children with bronchiectasis include S. pneumoniae , H. influenzae non–type b, M. catarrhalis , and Mycoplasma pneumoniae . Amoxicillin/clavulanic acid (22.5 mg/kg/dose twice daily) has been particularly successful at treating most pulmonary exacerbations. Viruses (most commonly human rhinovirus) are often found in children with bronchiectasis suffering from an exacerbation. Long-term prophylactic macrolide antibiotics or nebulized antibiotics (e.g., tobramycin, colistin, aztreonam) may be beneficial (reduced exacerbations and hospitalizations, improved lung function) but may also increase antibiotic resistance. Airway hydration (inhaled hypertonic saline or mannitol) also improves quality of life in adults with bronchiectasis. Any underlying disorder (immunodeficiency, aspiration) that may be contributing must be addressed. When localized bronchiectasis becomes more severe or resistant to medical management, segmental or lobar resection may be warranted. Lung transplantation can also be performed in patients with bronchiectasis. A review of randomized trials among children and adult patients with bronchiectasis did not find strong evidence to support the routine use of inhaled corticosteroids, although some studies demonstrate improved quality of life and reduced exacerbations in patients with bronchiectasis treated with inhaled corticosteroids. Although preventative strategies, including immunization against typical respiratory pathogens (influenza, pneumococci), are generally recommended, no studies have been conducted to date to address the efficacy of these recommendations.
Prognosis
Children with bronchiectasis often suffer from recurrent pulmonary illnesses, resulting in missed school days, stunted growth, osteopenia, and osteoporosis. The prognosis for patients with bronchiectasis has improved considerably in the past few decades. Earlier recognition or prevention of predisposing conditions, specialist multidisciplinary management, more powerful and broad-spectrum antibiotics, and improved surgical outcomes are likely reasons.