Heart Failure
Joseph W. Rossano
The International Society for Heart and Lung Transplantation (ISHLT) defines heart failure as follows:
A clinical and pathological syndrome that results from ventricular dysfunction, volume, or pressure overload, alone or in combination. It leads to characteristic signs and symptoms, such as poor growth, feeding difficulties, respiratory distress, exercise intolerance, and fatigue, and is associated with circulatory, neurohormonal, and molecular abnormalities. Heart failure has numerous etiologies that are a consequence of cardiac and noncardiac disorders, either congenital or acquired.
Pathophysiology
The heart can be viewed as a pump with an output proportional to its filling volume and inversely proportional to the resistance against which it pumps. As ventricular end-diastolic volume increases, a healthy heart increases cardiac output until a maximum is reached and cardiac output can no longer be augmented (the Frank-Starling principle ; Fig. 469.1 ). The increased stroke volume obtained in this manner is a result of stretching of myocardial fibers, but it also results in increased wall tension, which elevates myocardial oxygen consumption. Hearts working under various types of stress function along different Frank-Starling curves. Cardiac muscle with compromised intrinsic contractility requires a greater degree of dilation to produce increased stroke volume and does not achieve the same maximal cardiac output as normal myocardium does. If a cardiac chamber is already dilated because of a lesion causing increased preload (e.g., a left-to-right shunt or valvular insufficiency), there is little room for further dilation as a means of augmenting cardiac output. The presence of lesions that result in increased afterload to the ventricle (e.g., aortic or pulmonic stenosis, coarctation of the aorta) decreases cardiac performance, thereby resulting in a depressed Frank-Starling relationship.

Systemic oxygen transport is calculated as the product of cardiac output and systemic oxygen content. Cardiac output can be calculated as the product of heart rate and stroke volume. The primary determinants of stroke volume are the afterload (pressure work), preload (volume work), and contractility (intrinsic myocardial function). Abnormalities in heart rate can also compromise cardiac output; for example, tachyarrhythmias shorten the diastolic time interval for ventricular filling. Alterations in the oxygen-carrying capacity of blood (e.g., anemia or hypoxemia) also lead to a decrease in systemic oxygen transport and, if compensatory mechanisms are inadequate, can result in decreased delivery of substrate to tissues.
In some cases of heart failure, cardiac output is normal or increased, yet because of decreased systemic oxygen content (e.g., secondary to anemia) or increased oxygen demands (e.g., secondary to hyperventilation, hyperthyroidism, or hypermetabolism), an inadequate amount of oxygen is delivered to meet the body's needs. This condition, high-output failure , results in the development of signs and symptoms of heart failure when there is no basic abnormality in myocardial function and cardiac output is greater than normal. It is also seen with large systemic arteriovenous fistulas (e.g., vein of Galen malformation). These conditions reduce peripheral vascular resistance and cardiac afterload and increase myocardial contractility. Heart failure results when the demand for cardiac output exceeds the ability of the heart to respond. Chronic severe high-output failure may eventually result in a decrease in myocardial performance as the metabolic requirements of the myocardium are not met.
There are multiple systemic compensatory mechanisms used by the body to adapt to chronic heart failure. Some are mediated at the molecular/cellular level, such as upregulation or downregulation of various metabolic pathway components leading to changes in efficiency of oxygen and other substrate utilizations. Others are mediated by neurohormones such as the renin-angiotensin system and the sympathoadrenal axis. One of the principal mechanisms for increasing cardiac output is an increase in sympathetic tone secondary to increased secretion of circulating epinephrine by the adrenals and increased release of norepinephrine at the neuromuscular junction. The initial beneficial effects of sympathetic stimulation include an increase in heart rate and myocardial contractility, mediated by these hormones’ action on cardiac β-adrenergic receptors, increasing cardiac output. These hormones also cause vasoconstriction, mediated by their action on peripheral arterial α-adrenergic receptors. Some vascular beds may constrict more readily than others, so that blood flow is redistributed from the cutaneous, visceral, and renal beds to the heart and brain. Whereas these acute effects are beneficial, chronically increased sympathetic stimulation can have deleterious effects, including hypermetabolism, increased afterload, arrhythmogenesis, and increased myocardial oxygen requirements. Peripheral vasoconstriction can result in decreased renal, hepatic, and gastrointestinal tract function. Chronic exposure to circulating catecholamines leads to a decrease in the number of cardiac β-adrenergic receptors (downregulation) and also causes direct myocardial cell damage. Therapeutic agents for heart failure are directed at restoring balance to these neuroendocrine systems.
Clinical Manifestations
The clinical manifestations of heart failure depend in part on the degree of the child's cardiac reserve. A critically ill infant or child who has exhausted the compensatory mechanisms to the point that cardiac output is no longer sufficient to meet the basal metabolic needs of the body may present in cardiogenic shock . Other patients may be comfortable when quiet but are incapable of increasing cardiac output in response to even mild activity without experiencing significant symptoms. Conversely, it may take rather vigorous exercise to compromise cardiac function in children who have less severe heart disease.
A thorough history is extremely important in making the diagnosis of heart failure and in evaluating the possible causes. Parents who observe their child on a daily basis may not recognize subtle changes that have occurred over the course of days or weeks. Gradually worsening perfusion or increasing respiratory effort may not be recognized as an abnormal finding. Edema, which is general absent in infants and young children, may be passed off as normal weight gain, and exercise intolerance as lack of interest in an activity. The history of a young infant should also focus on feeding . An infant with heart failure often takes less volume per feeding, becomes dyspneic while sucking, and may perspire profusely. Eliciting a history of fatigue in an older child requires detailed questions about activity level and its course over several months.
In children, the signs and symptoms of heart failure may be similar to those in adults and include fatigue, effort intolerance, anorexia, dyspnea, edema, and cough. Many children, however, may have primarily abdominal symptoms (abdominal pain, nausea, anorexia) and a surprising lack of respiratory complaints. Attention to the cardiovascular system may come only after an abdominal radiograph unexpectedly catches the lower end of an enlarged heart. The elevation in systemic venous pressure may be gauged by clinical assessment of jugular venous pressure and liver enlargement. Orthopnea and basilar rales are variably present; edema is usually discernible in dependent portions of the body, or anasarca may be present. Cardiomegaly is invariably noted. A gallop rhythm is common; when ventricular dilation is advanced, the holosystolic murmur of mitral or tricuspid valve regurgitation may be heard.
In infants, heart failure may be difficult to distinguish from other causes of respiratory distress. Prominent manifestations of heart failure include tachypnea, feeding difficulties, poor weight gain, excessive perspiration, irritability, weak cry, and noisy, labored respirations with intercostal and subcostal retractions, as well as flaring of the alae nasi. The signs of cardiac-induced pulmonary congestion may be indistinguishable from those of bronchiolitis; wheezing is often a more prominent finding in young infants with heart failure than rales. Pneumonitis with or without atelectasis is common as result of bronchial compression by the enlarged heart. Hepatomegaly usually occurs, and cardiomegaly is invariably present. Despite pronounced tachycardia, a gallop rhythm can frequently be recognized. The other auscultatory signs are those produced by the underlying cardiac lesion. Clinical assessment of jugular venous pressure in infants may be difficult because of the shortness of the neck and the difficulty of observing a relaxed state; palpation of an enlarged liver is a more reliable sign. The etiologies of heart failure are age dependent (Table 469.1 ).
Diagnosis
X-ray films of the chest show cardiac enlargement. Pulmonary vascularity is variable and depends on the cause of the heart failure. Infants and children with large left-to-right shunts have exaggeration of the pulmonary arterial vessels to the periphery of the lung fields, whereas patients with cardiomyopathy may have a relatively normal pulmonary vascular bed early in the course of disease. Fluffy perihilar pulmonary markings suggestive of venous congestion and acute pulmonary edema are seen only with more severe degrees of heart failure. Cardiac enlargement is often noted as an unexpected finding on a chest radiography performed to evaluate for a possible pulmonary infection, bronchiolitis, or asthma.
Chamber hypertrophy noted by electrocardiography may be helpful in assessing the cause of heart failure but does not establish the diagnosis. In cardiomyopathies, left or right ventricular ischemic changes may correlate with other noninvasive parameters of ventricular function. Low-voltage QRS morphologic characteristics with ST-T–wave abnormalities may also suggest myocardial inflammatory disease but can be seen with pericarditis as well. The electrocardiogram (ECG) is the best tool for evaluating rhythm disorders as a potential cause of heart failure, especially tachyarrhythmias.
Echocardiography is the standard technique for assessing ventricular function. Ventricular function as be quantitated simply and reliably with commonly used parameters such as fractional shortening (a single-dimensional variable) and an ejection fraction. The fractional shortening is determined as the difference between end-systolic and end-diastolic diameter divided by end-diastolic diameter. Normal fractional shortening is between approximately 28% and 42%. The ejection fraction uses 2-dimensional data to calculate a 3-dimensional volume; the normal range is 55–65%. In children with right ventricular enlargement or other cardiac pathology resulting in flattening of the interventricular septum, ejection fraction is used because fractional shortening measured in the standard echocardiographic short axis view will not be accurate. Doppler studies can also be used to estimate cardiac output. Doppler assessment of transmitral flow can also be used as a noninvasive assessment of diastolic function. Magnetic resonance angiography (MRA) is also very useful in quantifying left and right ventricular function, volume, and mass as well as coronary artery anatomy. If valvular regurgitation is present, MRA can quantify the regurgitant fraction.
Arterial oxygen levels may be decreased when ventilation-perfusion inequalities occur secondary to pulmonary edema. When heart failure is severe, respiratory acidosis or metabolic acidosis, or both, may be present. Infants with heart failure often display hyponatremia as a result of renal water retention. Chronic diuretic treatment can decrease serum sodium levels further. Serum B-type (brain) natriuretic peptide (BNP) (or N-terminal pro-BNP), a cardiac neurohormone released in response to increased ventricular wall tension, is elevated in patients with heart failure. In children, BNP may be elevated in patients with heart failure as a result of systolic dysfunction (e.g., cardiomyopathy), as well as in children with volume overload (e.g., left-to-right shunts such as ventricular septal defect). Table 469.2 lists other causes of an elevated BNP.
Treatment
The underlying cause of cardiac failure must be removed or alleviated if possible. If the cause is a congenital cardiac anomaly amenable to surgery, medical treatment of the heart failure is indicated to prepare the patient for surgery. With the current excellent outcomes of primary surgical repair of congenital heart defects, even in the neonatal period, few children require aggressive heart failure management to grow big enough for surgery. In contrast, if the cause of heart failure is cardiomyopathy, medical management provides temporary relief from symptoms and may allow the patient to recover if the insult is reversible (e.g., myocarditis). If the lesion is not reversible, heart failure management usually allows the child to return to normal activities for some period and to delay, sometimes for months or years, the need for heart transplantation.
General Measures
Strict bed rest is rarely necessary except in extreme cases, but it is important that the child be allowed to rest during the day as needed and sleep adequately at night. Some older patients feel better sleeping in a semi-upright position, using several pillows (orthopnea ). As patients respond to treatment, restrictions on activities can often be modified within the context of the specific diagnosis and the patient's ability. Formal cardiopulmonary exercise testing can be used to assess the patient's ability to perform exercise in a controlled environment and is useful for recommending rational exercise restrictions. For patients with pulmonary edema, positive pressure ventilation (PPV) may be required along with other drug therapies. For those in low-output heart failure, PPV can significantly reduce total body oxygen consumption by eliminating the work of breathing and help to reverse metabolic acidosis. β-Adrenergic agonists such as dopamine, dobutamine, and epinephrine may be needed in combination with phosphodiesterase inhibitors such as milrinone for patients with markedly advanced heart failure and cardiogenic shock. If the blood pressure will permit, afterload-reducing agents such as nitroprusside, angiotensin-converting enzyme inhibitors (ACEIs ), or angiotensin receptor blockers (ARBs ) may be beneficial. These agents are initiated in an intensive care setting with proper invasive monitoring of central venous and arterial blood pressure.
Diet
Infants with heart failure usually fail to thrive because of a combination of increased metabolic demands and decreased caloric intake. Increasing daily calories is an important aspect of their management. Increasing the number of calories per ounce of infant formula (or supplementing breastfeeding) may be beneficial. Many infants do not tolerate an increase beyond 24 calories/oz because of diarrhea or because these formulas provide too large a solute load for compromised kidneys.
Severely ill infants and children may lack sufficient strength for effective sucking because of extreme fatigue, rapid respirations, and generalized weakness. In these circumstances, nasogastric feedings may be helpful. In many patients with cardiac enlargement, gastroesophageal reflux is a major problem. The use of continuous drip nasogastric feedings at night, administered by pump, may improve caloric intake while decreasing problems with reflux. Occasionally, especially in infants with heart failure caused by complex congenital heart disease, medical or surgical intervention to correct reflux is necessary (Nissen fundoplication). Continued malnutrition may be an important factor in the decision to undertake earlier surgical intervention in patients who have an operable congenital heart lesion or to proceed with mechanical circulatory support and/or listing for transplantation in patients with cardiomyopathy.
The use of low-sodium formulas in the routine management of infants with heart failure is not recommended because these preparations are often poorly tolerated and may exacerbate diuretic-induced hyponatremia. Human breast milk is the ideal low-sodium nutritional source. The use of more potent diuretic agents allows more palatable standard formulas to be used for nutrition while controlling salt and water balance by chronic diuretic administration. Most older children can be managed with “no added salt” diets and abstinence from foods containing large amounts of sodium. A strict, extremely-low-sodium diet is rarely required or followed.
Diuretics
Diuretics interfere with reabsorption of water and sodium by the kidneys, which results in a reduction in circulating blood volume and thereby reduces pulmonary fluid overload and ventricular filling pressure. Diuretics are usually the first mode of therapy initiated in patients with congestive heart failure.
Furosemide is the most commonly used diuretic in pediatric patients with heart failure. It inhibits the reabsorption of sodium and chloride in the distal tubules and the loop of Henle. Patients requiring acute diuresis should be given intravenous (IV) or intramuscular (IM) furosemide at an initial dose of 1-2 mg/kg, which usually results in rapid diuresis and prompt improvement in clinical status, particularly if symptoms of pulmonary congestion are present. Chronic furosemide therapy is then prescribed at a dose of 1-4 mg/kg/24 hr given between 1 and 4 times a day. Careful monitoring of electrolytes is necessary with long-term furosemide therapy because of the potential for significant loss of potassium. Potassium chloride supplementation is usually required unless the potassium-sparing diuretics are given concomitantly. Chronic administration of furosemide may cause contraction of the extracellular fluid compartment and result in “contraction alkalosis” (see Chapter 68.7 ). Diuretic-induced hyponatremia may become difficult to manage in patients with severe heart failure.
Spironolactone is an inhibitor of aldosterone and enhances potassium retention, often eliminating the need for oral potassium supplementation, which is frequently poorly tolerated. This drug is usually given orally in 2 divided doses of 2 mg/kg/24 hr. Combinations of spironolactone and chlorothiazide are sometimes used for convenience. Adults with heart failure have improved survival when an aldosterone inhibitor is included in the diuretic regimen, likely through multiple effects, including a favorable effect on cardiac fibrosis. Eplerenone is an alternative to spironolactone and does not have the side effect of gynecomastia.
Chlorothiazide is also used for diuresis in children with heart failure. It is less immediate in action and less potent than furosemide, and it affects the reabsorption of electrolytes in the renal tubules only. The usual dose is 10-40 mg/kg/24 hr in 2 divided doses. Potassium supplementation is often required if chlorothiazide is used alone.
Afterload Reducers, Including Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers
The ACEIs and ARBs reduce ventricular afterload by decreasing peripheral vascular resistance and thereby improving myocardial performance. Some of these agents also decrease systemic venous tone, which significantly reduces preload. Afterload reducers may be useful in children with heart failure secondary to cardiomyopathy and in patients with severe mitral or aortic insufficiency. They may also be effective in patients with heart failure caused by left-to-right shunts. ACEIs and ARBs may have additional beneficial effects on cardiac remodeling independent of their influence on afterload by directly influencing cardiac intracellular signaling pathways. In adult patients with dilated cardiomyopathy, the addition of an ACEI to standard medical therapy reduces both morbidity and mortality. Afterload-reducing agents are most often used in conjunction with other anticongestive drugs such as diuretics and, in some patients, digoxin.
Intravenously administered agents such as nitroprusside should be administered only in an intensive care setting and for as short a time as possible. Nitroprusside's short IV half-life makes it ideal for titrating the dose in critically ill patients. Peripheral arterial vasodilation and afterload reduction are the major effects, but venodilation causing a decrease in venous return to the heart may also be beneficial. Blood pressure must be continuously monitored because sudden hypotension can occur. Consequently, nitroprusside is contraindicated in patients with preexisting hypotension. Because the drug is metabolized, small amounts of circulating cyanide are produced and detoxified in the liver to thiocyanate, which is excreted in urine. When high doses of nitroprusside are administered for several days, toxic symptoms related to thiocyanate poisoning may occur (fatigue, nausea, disorientation, acidosis, and muscular spasm). If nitroprusside use is prolonged, blood thiocyanate levels should be monitored. Phosphodiesterase inhibitors (see later) are also excellent, although somewhat less potent afterload reducers but without the toxicity of nitroprusside.
The orally active ACEIs captopril and enalapril produce arterial dilation by blocking the production of angiotensin II, thereby resulting in significant afterload reduction. Venodilation and consequent preload reduction also have been reported. In addition, these agents interfere with aldosterone production and therefore also help control salt and water retention. ACEIs have additional beneficial effects on cardiac structure and function that may be independent of their effect on afterload. Adverse reactions to ACEIs include hypotension and its sequelae (weakness, dizziness, syncope) and hyperkalemia. A maculopapular pruritic rash is encountered in a small number of patients, but the drug may be continued because the rash often disappears spontaneously with time. Neutropenia, renal toxicity, and chronic cough also occur.
While ACEIs/ARBs along with β-adrenergic blocking agents have been shown in multiple prospective, randomized, controlled trials in adults to improve symptoms and mortality in adult heart failure patients, it is unclear if these medications improve the natural history of heart failure in children. Nonetheless, these medications are commonly used for the treatment of heart failure and are recommended by consensus guidelines from the ISHLT and Canadian Cardiovascular Society.
Digitalis Glycosides
Digoxin, once the mainstay of heart failure management in both children and adults, is currently used less frequently, as a result of the introduction of other therapies and the recognition of its potential toxicities. Some cardiologists will use digitalis as an adjunct to ACEIs and diuretics in patients with symptomatic heart failure, whereas others have stopped using it altogether. Despite multiple clinical studies, predominantly in adults, the controversy over digitalis remains. Some data suggest a beneficial effect of digoxin on reducing death among infants with single-ventricle heart disease.
Digoxin is the digitalis glycoside used most often in pediatric patients. It has a half-life of 36 hr and it is absorbed well by the gastrointestinal tract (60–85%), even in infants. An initial effect is seen as early as 30 min after administration, and the peak effect for oral digoxin occurs at 2-6 hr. When the drug is administered intravenously, the initial effect is seen in 15-30 min, and the peak effect occurs at 1-4 hr. The kidney eliminates digoxin, so dosing must be adjusted according to the patient's renal function. The half-life of digoxin may be up to 6 days in patients with anuria because slower hepatic excretion pathways are used in these patients.
Rapid digitalization of infants and children may be carried out intravenously. This should be done with caution in patients with severe heart failure. The dose depends on the patient's age (Table 469.3 ). The recommended digitalization schedule is to give half the total digitalizing dose immediately and the succeeding 2 one-quarter doses at 12-hr intervals later. The ECG must be closely monitored, and rhythm strips obtained before each of the 3 digitalizing doses. Digoxin should be discontinued if a new rhythm disturbance is noted. Prolongation of the P-R interval is not necessarily an indication to withhold digitalis, but a delay in administering the next dose or a reduction in the dosage should be considered, depending on the patient's clinical status. Minor ST segment or T-wave changes are frequently noted with digitalis administration and should not affect the digitalization regimen. Baseline serum electrolyte levels should be measured before and after digitalization. Hypokalemia and hypercalcemia exacerbate digitalis toxicity. Because hypokalemia is relatively common in patients receiving diuretics, potassium levels should be monitored closely in those receiving a potassium-wasting diuretic in combination with digitalis. In patients with active myocarditis, some cardiologists recommend avoiding digitalis altogether and if used, maintenance digitalis should be started at half the normal dose without digitalization because of the increased risk of arrhythmia in these patients.
Table 469.3
Dosage of Drugs Commonly Used for the Treatment of Congestive Heart Failure
| DRUG | DOSAGE* |
|---|---|
| DIGOXIN | |
Digitalization ( initially, followed by
initially, followed by 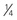 q12h × 2)
q12h × 2) |
Premature: 20 µg/kg Full-term neonate (up to 1 mo): 20-30 µg/kg |
| Infant or child: 25-40 µg/kg | |
| Adolescent or adult: 0.5-1 mg in divided doses | |
| Note: These doses are PO; IV dose is 75% of PO dose | |
| Maintenance digoxin † | 5-10 µg/kg/day, divided q12h |
| Note: These doses are PO; IV dose is 75% of PO dose | |
| DIURETICS | |
| Furosemide (Lasix) | IV: 0.5-2 mg/kg/dose |
| PO: 1-4 mg/kg/day, divided qd-qid | |
| Bumetanide (Bumex) | IV: 0.01-0.1 mg/kg/dose |
| PO: 0.01-0.1 mg/kg/day q24-48h | |
| Chlorothiazide (Diuril) | PO: 20-40 mg/kg/day, divided bid or tid |
| Spironolactone (Aldactone) | PO: 1-3 mg/kg/day, divided bid or tid |
| ADRENERGIC AGONISTS (ALL IV) | |
| Dobutamine | 2-20 µg/kg/min |
| Dopamine | 2-20 µg/kg/min |
| Epinephrine | 0.01-1.0 µg/kg/min |
| PHOSPHODIESTERASE INHIBITORS (ALL IV) | |
| Milrinone | 0.25-1.0 µg/kg/min |
| AFTERLOAD-REDUCING AGENTS | |
| Captopril (Capoten), all PO | Prematures: start at 0.01 mg/kg/dose; 0.1-0.4 mg/kg/day, divided q6-24h |
| Infants: start at 0.15-0.3 mg/kg/dose; 1.5-6 mg/kg/day, divided q6-12h | |
| Children: start at 0.3-0.5 mg/kg/dose; 2.5-6 mg/kg/day, divided q6-12h | |
| Enalapril (Vasotec), all PO | 0.08-0.5 mg/kg/day, divided q12-24h |
| Hydralazine (Apresoline) | IV: 0.1-0.5 mg/kg/dose (maximum: 20 mg) |
| PO: 0.75-5 mg/kg/day, divided q6-12h | |
| Nitroglycerin | IV: 0.25-0.5 µg/kg/min start; increase to 20 µg/kg/min maximum |
| Nitroprusside (Nipride) | IV: 0.5-8 µg/kg/min |
| β-ADRENERGIC BLOCKERS | |
| Carvedilol (Coreg) | PO: initial dose: 0.1 mg/kg/day (maximum: 6.25 mg) divided bid (may use tid in infants), increase gradually (usually 2 wk intervals) to maximum of 0.5-1 mg/kg/day over 8-12 wk as tolerated; adult maximum dose: 50-100 mg/day |
| Metoprolol (Lopressor, Toprol-XL) | PO, non–extended-release form: 0.2 mg/kg/day divided bid, increase gradually (usually 2 wk intervals) to maximum dose of 1-2 mg/kg/day |
| PO, extended-release form (Toprol-XL): given once daily; adult initial dose: 25 mg/day, maximum: 200 mg/day | |
* Pediatric doses based on weight should not exceed adult doses. Because recommendations may change, these doses should always be double-checked. Doses may also need to be modified in any patient with renal or hepatic dysfunction.
† Maintenance digitalis therapy is started approximately 12 hr after full digitalization. The daily dosage, one quarter of the total digitalizing dose, is divided in 2 and given at 12 hr intervals. The oral maintenance dose is usually 20–25% higher than when digoxin is used parenterally. The normal daily dose of digoxin for older children (>5 yr of age) calculated by body weight should not exceed the usual adult dose of 0.125-0.5 mg/24 hr.
IV, Intravenous; PO, oral; bid, twice daily, tid, 3 times daily; qid, 4 times daily; qd, every day.
Patients who are not critically ill may be given digitalis initially by the oral route, and in most instances, digitalization is completed within 24 hr. When slow digitalization is desirable, for example, in the immediate postoperative period, initiation of a maintenance digoxin schedule without a previous loading dose achieves full digitalization in 7-10 days.
Measurement of serum digoxin levels is useful (1) when an unknown amount of digoxin has been administered or ingested accidentally, (2) when renal function is impaired or if drug interactions are possible, (3) when questions regarding compliance are raised, and (4) when a toxic response is suspected. In suspected toxicity, elevated serum digoxin levels are not in themselves diagnostic of toxicity but must be interpreted as an adjunct to other clinical and electrocardiographic findings (rhythm and conduction disturbances). Hypokalemia, hypomagnesemia, hypercalcemia, cardiac inflammation secondary to myocarditis, and prematurity may all potentiate digitalis toxicity. A cardiac arrhythmia that develops in a child who is taking digitalis may also be related to the primary cardiac disease rather than the drug, however, any arrhythmia occurring after the institution of digitalis therapy must be considered to be drug related until proven otherwise. Many drugs interact with digoxin and may increase levels or risk of toxicity, so care should be taken when a patient receiving digoxin is being considered for any additional pharmacologic therapy.
Alpha- and β-Adrenergic Agonists
The α- and β-adrenergic receptor agonists are usually administered in an intensive care setting, where the dose can be carefully titrated to hemodynamic response. Continuous determinations of arterial blood pressure and heart rate are performed; measuring serial mixed venous oxygen saturations or cardiac output directly with a pulmonary thermodilution (Swan-Ganz) catheter may be helpful in assessing drug efficacy, although this technique is used much less in children than adults. Although extremely efficacious in the acute intensive care setting, long-term administration of adrenergic agonists has been shown to increase morbidity and mortality in adults with heart failure and is usually avoided unless the patient is totally dependent on these agents.
Dopamine is a predominantly β-adrenergic receptor agonist, but it has α-adrenergic effects at higher doses. Dopamine has less chronotropic and arrhythmogenic effect than the pure β-agonist isoproterenol. At a dose of 2-10 µg/kg/min, dopamine results in increased contractility with little peripheral vasoconstrictive effect. If the dose is increased beyond 15 µg/kg/min, however, its peripheral α-adrenergic effects may result in vasoconstriction.
Fenoldopam is a dopamine DA1 receptor agonist and is used at a low dose (0.03 µg/kg/min) to increase renal blood flow and urine output. It can cause hypotension, so blood pressure should be carefully monitored.
Dobutamine , a derivative of dopamine, is also useful in treating low cardiac output. It has direct inotropic effects and causes a moderate reduction in peripheral vascular resistance. Dobutamine can be used alone or as an adjunct to dopamine therapy to avoid the vasoconstrictive effects of higher-dose dopamine. Dobutamine is also less likely to cause cardiac rhythm disturbances than isoproterenol.
Isoproterenol is a pure β-adrenergic agonist that has a marked chronotropic effect; it is most effective in patients with slow heart rate. It is often used in the immediate post–heart transplant period.
Epinephrine is a mixed α- and β-adrenergic receptor agonist that is usually reserved for patients with cardiogenic shock and low arterial blood pressure. Although epinephrine can raise blood pressure effectively, it also increases systemic vascular resistance, and therefore increases the afterload against which the heart has to work and is associated with an increased risk of arrhythmia. Additionally, epinephrine is proarrhythmic and can result in direct cardiac toxicity, including myocardial necrosis and apoptosis.
Phosphodiesterase Inhibitors
Milrinone is useful in treating patients with low cardiac output who are refractory to standard therapy. It has been shown to be highly effective in managing the low-output state present in children after open heart surgery. It works by inhibition of phosphodiesterase, which prevents the degradation of intracellular cyclic adenosine monophosphate. Milrinone has both positive inotropic effects on the heart and peripheral vasodilatory effects and has generally been used as an adjunct to dopamine or dobutamine therapy in the intensive care unit. It is given by IV infusion at 0.25-1 µg/kg/min, sometimes with an initial loading dose of 50 µg/kg. A major side effect is hypotension secondary to peripheral vasodilation, especially when a loading dose is used. The hypotension can generally be managed by the administration of IV fluids to restore adequate intravascular volume. Long-term milrinone is often used to support patients while listed for heart transplantation, and in select patients can be used in the outpatient setting.
Chronic Treatment With β-Blockers
Studies in adults with dilated cardiomyopathy show that β-adrenergic blocking agents, introduced gradually as part of a comprehensive heart failure treatment program, improve exercise tolerance, decrease hospitalizations, and reduce overall mortality. The agents most often used are carvedilol , with both α- and β-adrenergic receptor–blocking as well as free radical–scavenging effects, and metoprolol , a β1 -adrenergic receptor selective antagonist. β-Blockers are used for the chronic treatment of patients with heart failure and should not be administered when patients are still in the acute phase of heart failure (i.e., receiving IV adrenergic agonist infusions). Although very efficacious in adults, clinical studies in children have shown mixed results, potentially from the significant heterogeneity of the populations being studied and differences in the types of β-blocking agents.
New Therapies
Several newer medications have shown promise in the treatment of adult heart failure patients are now also being studied in pediatric patients. Serelaxin , recombinant human relaxin-2, resulted in fewer deaths when used for the treatment of acute heart failure in hospitalized patients. For chronic heart failure, ivabradine has been studied in patients with elevated heart rates. Ivabradine is a selective inhibitor of the If current in the sinus node and lowers heart rates without decreasing myocardial contractility. The use of ivabradine was associated with improved outcomes in heart failure patients, including decreased hospital admissions and cardiovascular death. A large, prospective randomized trial showed that the combination of an ARB and a neprilysin inhibitor can lead to several beneficial effects, including vasodilation, decreased aldosterone levels, and improved natriuresis, and patients randomized to the medication had a lower risk of death and hospitalization. Further studies are needed to determine what role, if any, these medications will have in the treatment of pediatric heart failure.
Electrophysiologic Approaches to Heart Failure Management
Significant improvements in symptomatology and functional capacity have been achieved in select adult patients with cardiomyopathy using biventricular resynchronization pacing . This technique improves cardiac output by restoring normal synchrony between right and left ventricular contraction, which is often lost in patients with dilated cardiomyopathy (these patients usually manifest a left bundle branch block on ECG). There is growing experience with resynchronization pacing in children, but it remains uncertain which population of patients with heart failure benefit from this therapy.
Arrhythmias are a leading cause of sudden death in patients with severe cardiomyopathy (both dilated and hypertrophic). Although antiarrhythmic medications can sometimes reduce this risk, for patients at particularly high risk (e.g., those with a condition known to be associated with a high risk of ventricular arrhythmia or those who have already experienced a “missed sudden death” episode), use of an implantable cardioverter-defibrillator can be lifesaving (see Chapter 463 ).
Cardiogenic Shock
Joseph W. Rossano
Cardiogenic shock may be caused by (1) severe cardiac dysfunction before or after cardiac surgery, (2) septicemia, (3) severe burns, (4) anaphylaxis, (5) cardiomyopathy, (6) myocarditis, (7) myocardial infarction or stunning, and (8) acute central nervous system (CNS) disorders. It is characterized by low cardiac output and results in inadequate tissue perfusion (see Chapter 88 ).
Treatment is aimed at reinstitution of adequate cardiac output to prevent the untoward effects of prolonged ischemia on vital organs, as well as management of the underlying cause. Under normal physiologic conditions, cardiac output is increased as a result of sympathetic stimulation, which increases both contractility and heart rate. If contractility is depressed, cardiac output may be improved by increasing heart rate, increasing ventricular filling pressure (preload) through the Frank-Starling mechanism, or by decreasing systemic vascular resistance (afterload). Optimal filling pressure is variable and depends on a number of extracardiac factors, including ventilatory support and intraabdominal pressure. The increased pressure necessary to fill a relatively noncompliant ventricle should also be considered, particularly after open heart surgery, or in patients with restrictive or hypertrophic cardiomyopathies. If carefully administered incremental fluid does not result in improved cardiac output, abnormal myocardial contractility or an abnormally high afterload, or both, must be implicated as the cause of the low cardiac output. Although an increase in heart rate may improve cardiac output, an excessive increase in heart rate may reduce cardiac output because of decreased time for diastolic filling. Additionally, high heart rates will increase myocardial oxygen demand, which may be counterproductive in a state of limited tissue oxygen supply.
Myocardial contractility usually improves when treatment of the basic cause of shock is instituted, hypoxia is eliminated, and acidosis is corrected. β-Adrenergic agonists such as dopamine, epinephrine, and dobutamine improve cardiac contractility, increase heart rate, and ultimately increase cardiac output. However, some of these agents also have α-adrenergic effects, which cause peripheral vasoconstriction and increase afterload, so careful consideration of the balance of these effects in an individual patient is important. The use of cardiac glycosides to treat acute low cardiac output states should be avoided.
Patients in cardiogenic shock may have a marked increase in systemic vascular resistance (SVR) resulting in high afterload and poor peripheral perfusion. If the increased SVR is persistent and the administration of positive inotropic agents alone does not improve tissue perfusion, the use of afterload-reducing agents may be appropriate, such as nitroprusside or milrinone in combination with a β-adrenergic agonist. Milrinone, a phosphodiesterase inhibitor (see earlier), is also a positive inotropic agent, and combined with a β-adrenergic agonist, it works synergistically to increase levels of myocardial cyclic adenosine monophosphate.
Sequential evaluation and management of cardiovascular shock are mandatory (see Chapter 88 ). Table 469.4 outlines the general treatment principles for acute cardiac circulatory failure under most circumstances. In addition to cardiac-specific medications, other treatments aimed at improving oxygen capacity (e.g., blood transfusion for patients with anemia) and decreasing oxygen demand (e.g., intubation, mechanical ventilation, sedation) can be beneficial. Treatment of infants and children with low cardiac output after cardiac surgery also depends on the nature of the operative procedure, any intraoperative complications, and the physiology of the circulation after repair or palliation (see Chapter 461 ). If cardiogenic shock does not respond rapidly to medical therapy, consideration of mechanical support is warranted.
Table 469.4
Treatment of Cardiogenic Shock*
| DETERMINANTS OF STROKE VOLUME | |||
|---|---|---|---|
| Preload | Contractility | Afterload | |
| Parameters measured | CVP, PCWP, LAP, cardiac chamber size on echocardiography | CO, BP, fractional shortening or ejection fraction on echocardiography, MV O2 saturation | BP, peripheral perfusion, SVR |
| Treatment to improve cardiac output | Volume expansion (crystalloid, colloid, blood) | β-Adrenergic agonists, phosphodiesterase inhibitors | Afterload-reducing agents: milrinone, nitroprusside, ACEIs |
* The goal is to improve peripheral perfusion by increasing cardiac output, where: cardiac output = heart rate × stroke volume.
ACEIs, Angiotensin-converting enzyme inhibitors; BP, blood pressure; CO, cardiac output (measured with a thermodilation catheter); CVP, central venous pressure; LAP, left atrial pressure (measured with an indwelling LA line); MV O2 saturation, mixed venous oxygen saturation (measured with a central venous catheter); PCWP, pulmonary capillary wedge pressure (measured with a thermodilation catheter); SVR, systemic vascular resistance (calculated from CO and mean BP).
Mechanical Circulatory Support
Extracorporeal membrane oxygenation (ECMO) , which can provide total cardiopulmonary support, is the most common short-term modality to support circulatory failure in children. In experienced centers, children can be placed on ECMO rapidly, and therefore the modality can be used in multiple settings, including low cardiac output syndrome (low-output heart failure) after cardiac surgery, rapidly deteriorating hemodynamics in several scenarios (e.g., myocarditis), and as resuscitation from refractory cardiac arrest. The modality is ideal for short-term support when the underlying disease requiring EMCO is expected to resolve within days to weeks. For multiple reasons, including the relatively high complication rate and decreased mobility of many patients on ECMO, it is not an ideal support modality for long-term myocardial support.
Given of limitations of ECMO, there is a need to develop long-term support options for children with refractory heart failure. With advancements in the current era, almost 50% of children with dilated cardiomyopathy will be supported on a ventricular assist device (VAD) prior to heart transplantation.
For infants and small children, the most commonly used VAD is the Berlin Heart EXCOR. This device can be used for left, right, or biventricular support. It is classified as a paracorporeal pneumatic pulsatile device, and the pump sits outside the body. Among adults, these older types of devices have been replaced by newer-generation devices classified as intracorporeal continuous flow devices. These are completely internalized except for a drive line that connects to the power source (Fig. 469.2 ). These VADs have fewer complications and can provide long-term durable support outside the hospital. These devices are often used in older children and adolescents, with many of these patients discharged home on VAD support.

Other types of devices, including temporary VAD for short-term support and the total artificial heart for long-term support, have also been used in children, but less frequently. In children, most of these devices are used with the intention of subsequently performing a heart transplantation, although the devices can be removed if myocardial function recovers. This is in contrast to adult patients, many of whom are placed on these devices with no plan for heart transplantation, the so-called destination therapy. Successfully managing patients on VAD support requires a dedicated multidisciplinary team.