Gonadal and Germ Cell Neoplasms
Cynthia E. Herzog, Winston W. Huh
Epidemiology
Malignant germ cell tumors (GCTs) and gonadal tumors are rare, with an incidence of 12 cases per 1 million persons younger than 20 yr. Most malignant tumors of the gonads in children are GCTs. The incidence varies according to age and sex, although the incidence of GCTs in adolescent males has increased over time. Sacrococcygeal tumors occur predominantly in infant females. Testicular GCTs occur predominantly before age 4 yr and after puberty. Klinefelter syndrome is associated with an increased risk of mediastinal GCTs. Down syndrome, undescended testes, infertility, testicular atrophy, testicular microlithiasis, testicular dysgenesis syndrome, and inguinal hernias are associated with an increased risk of testicular cancer . The risk of testicular cancer in patients with cryptorchidism is reduced but not eliminated if orchiopexy is performed before 13 yr of age. The risk of testicular GCT is increased in first-degree relatives and is highest among monozygotic twins.
Pathogenesis
The GCTs and non-GCTs arise from primordial germ cells and coelomic epithelium, respectively. Testicular and sacrococcygeal GCTs arising during early childhood characteristically have deletions at chromosome arms 1p and 6q and gains at 1q, and they lack the isochromosome 12p that is highly characteristic of malignant GCTs of adults. Testicular GCT also may demonstrate loss of imprinting. Ovarian GCTs from older females characteristically have deletions at 1p and gains at 1q and 21. Because GCTs may contain benign and mixed malignant elements in different areas of the tumor, extensive sectioning is essential to confirm the correct diagnosis. The many histologically distinct subtypes of GCTs include teratoma (mature and immature), endodermal sinus tumor, and embryonal carcinoma (Fig. 530.1 ). Non-GCTs of the ovary include epithelial (serous and mucinous) and sex cord–stromal tumors; non-GCTs of the testicle include sex cord–stromal (e.g., Leydig cell, Sertoli cell) tumors. DICER1 mutations have been observed in nonepithelial ovarian cancers, especially in Sertoli-Leydig tumors. Table 530.1 provides a histologic classification of testicular GCTs.

Table 530.1
Main Histologic Types of Testicular Germ Cell Tumors*
| NONINVASIVE GERM CELL NEOPLASIA |
| GERM CELL TUMORS DERIVED FROM GCNIS |
| GERM CELL TUMORS UNRELATED TO GCNIS |
* Based on updated WHO classification of tumors of the testis and paratesticular tissue.
Adapted from Raypert-De Meyts E, McGlynn KA, Okamoto, et al: Testicular germ cell tumours, Lancet 387:1762–1770, 2016 (panel, p 1763).
Clinical Manifestations and Diagnosis
The clinical presentation of germ cell neoplasms depends on location. Ovarian tumors often are quite large by the time they are diagnosed (Fig. 530.2 ). Extragonadal GCTs occur in the midline, including the suprasellar region, pineal region, neck, mediastinum, and retroperitoneal and sacrococcygeal areas (Fig. 530.3 ). Symptoms relate to mass effect, but the intracranial GCTs often present with anterior and posterior pituitary deficits (see Chapter 524 ).

The serum α-fetoprotein (AFP ) level is elevated with endodermal sinus tumors and may be minimally elevated with teratomas. Infants normally have higher levels of AFP, which usually falls to normal adult levels by about age 8 mo; consequently, high AFP levels must be interpreted with caution in this age-group. Elevation of the β subunit of human chorionic gonadotropin (β-hCG ), which is secreted by syncytiotrophoblasts, is seen with choriocarcinoma and germinomas. Lactate dehydrogenase, although nonspecific, may be a useful marker. If elevated, these markers provide important confirmation of the diagnosis and provide a means to monitor the patient for tumor response and recurrence. Both serum and cerebrospinal fluid (CSF) should be assayed for these markers in patients with intracranial lesions.
Diagnosis begins with physical examination and imaging studies, including plain radiographs of the chest and ultrasonography of the abdomen. CT or MRI can further delineate the primary tumor. If germ cell malignancy is strongly suggested, preoperative staging with CT of the chest and bone scan is appropriate. Primary surgical resection is indicated for tumors deemed resectable. For older patients with testicular tumors, ipsilateral retroperitoneal lymph node sampling may be required to determine extent of disease and aid in treatment planning. Ovarian tumors also require detailed surgical evaluation, including lymph node removal and pelvic washings for cytologic analysis for peritoneal spread. Diagnosis of intracranial lesions can be established with imaging and AFP or β-hCG determinations of serum and CSF.
Gonadoblastomas often occur in patients with gonadal dysgenesis and all or parts of a Y chromosome. Gonadal dysgenesis is characterized by failure to fully masculinize the external genitalia. If this syndrome is diagnosed, imaging of the gonad with ultrasonography or CT is performed, and surgical resection of the tumor usually is curative. Prophylactic resection of dysgenetic gonads at the time of diagnosis is recommended, because gonadoblastomas, some of which contain malignant GCT elements, often develop. Gonadoblastomas may produce abnormal amounts of estrogen.
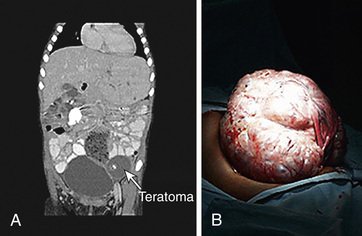
Teratomas occur in many locations, presenting as masses. They are not associated with elevated markers unless malignancy is present. The sacrococcygeal region is the most common site for teratomas. Sacrococcygeal teratomas occur most commonly in infants and may be diagnosed in utero or at birth, with most found in girls. The rate of malignancy in this location varies, ranging from <10% in children younger than 2 mo to >50% in children older than 4 mo.
Germinomas occur intracranially, in the mediastinum, and in the gonads. In the ovary, they are called dysgerminomas, and in the testis, they are called seminomas. They usually are tumor-marker–negative masses despite being malignant. Endodermal sinus or yolk sac tumor and choriocarcinoma appear highly malignant by histologic criteria. Both occur at gonadal and extragonadal sites. Embryonal carcinoma most often occurs in the testes. Choriocarcinoma and embryonal carcinoma rarely occur in the pure form and are usually found as part of a mixed malignant GCT.
Non–germ cell gonadal tumors are very uncommon in pediatrics and occur predominantly in the ovary. Epithelial carcinomas (usually an adult tumor), Sertoli-Leydig cell tumors, and granulosa cell tumors may occur in children. Carcinomas account for about one third of ovarian tumors in females <20 yr old; most of these occur in older teens and are of the serous or mucinous subtype. Sertoli-Leydig cell tumors and granulosa cell tumors produce hormones that can cause virilization, feminization, or precocious puberty, depending on pubertal stage and the balance between Sertoli cells (estrogen production) and Leydig cells (androgen production). Diagnostic evaluation usually focuses on the chief complaint of inappropriate sex steroid effect and includes hormone measurements, which reflect gonadotropin-independent sex steroid production. Appropriate imaging also is performed to rule out a functioning gonadal tumor. Surgery usually is curative. No effective therapy for nonresectable disease has been found.
Treatment
Complete surgical excision of the tumor usually is indicated, except for patients with intracranial tumors, for whom the primary therapy consists of radiation therapy and chemotherapy. For testicular tumors, an inguinal approach is indicated, and complete resection should include the entire spermatic cord. When complete excision cannot be accomplished, preoperative chemotherapy is indicated, with second-look surgery. For teratomas, both mature and immature, and completely resected malignant tumors of the testes and ovary, surgery alone is the treatment. For ovarian tumors, unless the contralateral ovary is obviously also involved by tumor, a fertility-sparing surgery should be performed. Cisplatin-based chemotherapy regimens usually are curative in GCTs that cannot be completely resected, even if metastases are present. However, sex cord–stromal tumors tend to be refractory to chemotherapy. Except for GCTs of the central nervous system, radiation therapy is limited to those tumors that are not amenable to complete excision and are refractory to chemotherapy.
Prognosis
The overall cure rate for children with GCTs is >80%. Age is the most predictive factor of survival for extragonadal GCTs. Children >12 yr old have a 4-fold higher risk of death and a 6-fold higher risk if the tumor is thoracic. Histology has minimal effect on prognosis. Patients with nonresected extragonadal GCTs have a slightly worse prognosis.