Septic Arthritis
Eric Robinette, Samir S. Shah
Without early recognition and prompt institution of appropriate medical and surgical therapy, septic arthritis in infants and children has the potential to damage to the synovium, adjacent cartilage, and bone, and cause permanent disability.
Etiology
Staphylococcus aureus (see Chapter 208.1 ) is the most common cause of bacterial arthritis in all age groups. Methicillin-resistant S. aureus (MRSA) accounts for a high proportion (>25%) of community S. aureus isolates in many areas of the United States and throughout the world. Group A streptococcus (see Chapter 210 ) and Streptococcus pneumoniae (pneumococcus; see Chapter 209 ) historically cause 10–20%; S. pneumoniae is most likely in the 1st 2 yr of life, but its frequency has declined since the introduction of the pneumococcal conjugate vaccines. Kingella kingae is recognized as a relatively common etiology with improved culture and polymerase chain reaction (PCR) methods in children younger than 4 yr (see Chapters 220 and 704 ). In sexually active adolescents, gonococcus (see Chapter 219 ) is a common cause of septic arthritis and tenosynovitis, usually of small joints or as a monoarticular infection of a large joint (knee). Neisseria meningitidis (see Chapter 218 ) can cause either a septic arthritis that occurs in the first few days of illness or a reactive arthritis that is typically seen several days after antibiotics have been initiated. Group B streptococcus (see Chapter 211 ) is an important cause of septic arthritis in neonates. Q fever and brucellosis should be considered in endemic areas and with an exposure risk.
Fungal infections usually occur as part of multisystem disseminated disease; Candida arthritis can complicate systemic infection in neonates with or without indwelling vascular catheters. Primary viral infections of joints are rare, but arthritis accompanies many viral (parvovirus, mumps, rubella live vaccines) syndromes, suggesting an immune-mediated pathogenesis.
A microbial etiology is confirmed in approximately 65% of cases of septic arthritis. In addition, some cases treated as bacterial arthritis are actually postinfectious (gastrointestinal or genitourinary) reactive arthritis (see Chapter 182 ) rather than primary infection. Lyme disease produces an arthritis more like a rheumatologic disorder and not typically suppurative.
Epidemiology
Septic arthritis is more common in young children. Half of all cases occur by 2 yr of age and three fourths of all cases occur by 5 yr of age. Adolescents and neonates are at risk of gonococcal septic arthritis.
Most infections in otherwise healthy children arise hematogenously. Less commonly, infection of joints can follow penetrating injuries or procedures such as trauma, arthroscopy, prosthetic joint surgery, intraarticular steroid injection, and orthopedic surgery. Immunocompromised patients and those with rheumatologic joint disease are also at increased risk of joint infection.
Pathogenesis
Septic arthritis primarily occurs as a result of hematogenous seeding of the synovial space. Less often, organisms enter the joint space by direct inoculation or extension from a contiguous focus. The synovial membrane has a rich vascular supply and lacks a basement membrane, providing an ideal environment for hematogenous seeding. The presence of bacterial products (endotoxin or other toxins) within the joint space stimulates cytokine production (tumor necrosis factor-α, interleukin-1) within the joint, triggering an inflammatory cascade. The cytokines stimulate chemotaxis of neutrophils into the joint space, where proteolytic enzymes and elastases are released by neutrophils, damaging the cartilage. Proteolytic enzymes released from the synovial cells and chondrocytes also contribute to destruction of cartilage and synovium. Bacterial hyaluronidase breaks down the hyaluronic acid in the synovial fluid, making the fluid less viscous and diminishing its ability to lubricate and protect the joint cartilage. Damage to the cartilage can occur through increased friction, especially for weight-bearing joints. The increased pressure within the joint space from accumulation of purulent material can compromise the vascular supply and induce pressure necrosis of the cartilage. Synovial and cartilage destruction results from a combination of proteolytic enzymes and mechanical factors.
Clinical Manifestations
Most septic arthritides are monoarticular. The signs and symptoms of septic arthritis depend on the age of the patient. Early signs and symptoms may be subtle, particularly in neonates. As with osteomyelitis, neonates might exhibit pseudoparalysis or pain which limits voluntary movement of the affected extremity (e.g., diaper changes). Septic arthritis in neonates and young infants is often associated with adjacent osteomyelitis caused by transphyseal spread of infection, although osteomyelitis contiguous with an infected joint can be seen at any age (see Chapter 704 ).
Older infants and children might have fever and pain, with localizing signs such as swelling, erythema, and warmth of the affected joint. With involvement of joints of the pelvis and lower extremities, limp or refusal to walk often occurs.
Erythema and edema of the skin and soft tissue overlying the site of infection are seen earlier in septic arthritis than in osteomyelitis because the bulging infected synovium is usually more superficial, whereas the metaphysis is located more deeply. Septic arthritis of the hip is an exception because of the deep location of the hip joint. With Lyme arthritis, joint swelling is typically quite prominent and may be disproportionate to the relatively lesser degree of pain and limited range of motion when compared with suppurative arthritis. Lyme arthritis has a predilection for large joints, particularly the knees and hips, and may be either monoarticular or pauciarticular at presentation.
Joints of the lower extremity constitute 75% of all cases of septic arthritis (Table 705.1 ). The elbow, wrist, and shoulder joints are involved in approximately 25% of cases, and small joints are uncommonly infected, except in gonococcal arthritis. Suppurative infections of the hip, shoulder, elbow, and ankle in infants and children may be associated with an adjacent osteomyelitis of the proximal femur, proximal humerus, proximal radius, and distal tibia because the metaphysis extends intraarticularly. Concomitant osteomyelitis is less common in older children and adolescents as their anatomy and physiology become more adult-like.
Table 705.1
| BONE | PERCENT (%) |
|---|---|
| Knee | ~35 |
| Hip | ~25 |
| Ankle | ~10 |
| Elbow | ~10 |
| Wrist | ~4 |
| Shoulder | ~5 |
| Small joints | ~1-2 |
* Excludes Lyme disease and immune-complex postinfectious arthritis. Viral (rubella, mumps, chikungunya) infectious arthritis is often small and multiple joints. Septic bursitis (shoulder, prepatellar) may be confused with bacterial joint infections.
Diagnosis
The white blood cell count and differential, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) are generally elevated in children with joint infections but elevations are nonspecific and might not be helpful in distinguishing between infection and other inflammatory processes. Most children with septic arthritis will have normal leukocyte counts and ESR at presentation, and normal test results do not preclude the diagnosis of septic arthritis.
Blood cultures should be performed in all cases of suspected septic arthritis but are positive in 20% or fewer cases of proven or probable septic arthritis. Cervical, anal, and throat cultures should be obtained when gonococcus is suspected. Aspiration of the joint fluid provides the optimal specimen to confirm the diagnosis. Most large joint spaces are easy to aspirate, but the hip can pose technical problems; ultrasound guidance facilitates aspiration. Although yield for joint aspirate cultures is higher than from blood cultures, the overall culture yield when combining both methods remains less than 50%. Multiplex bacterial PCR panels appear to have a yield around 50% from joint fluid specimens, but this increase over culture is almost entirely due to their enhanced ability to detect K. kingae. Other strategies to increase detection of K. kingae include prompt inoculation onto solid media and inoculation of the joint fluid in blood culture bottles. A diagnosis of Lyme arthritis is made through via a two-step test of an ELISA or IFA followed by a reflex Western blot for samples that are positive or equivocal by the first methodology. Patients with Lyme arthritis are seropositive because arthritis is a late manifestation of infection. PCR is rarely necessary but can detect Borrelia burgdorferi in joint aspirate specimens in cases of Lyme arthritis.
Synovial fluid analysis for cell count, differential, protein, and glucose has limited utility in diagnosing infectious arthritis. Joint fluid white blood cell counts >50,000 cells/mm3 suggest bacterial infection as the most likely etiology, but this finding is neither sensitive nor specific enough to exclude or confirm a bacterial infection in isolation. When the results of joint aspirate cell counts and culture are not strongly suggestive of a joint infection but the clinical presentation is worrisome for a bacterial etiology, infectious causes of sympathetic joint effusions such as adjacent pyomyositis and osteomyelitis should be investigated by MRI (see Chapter 704 ).
Monitoring elevated CRP may be of value in assessing response to therapy or identifying complications. In addition, patients with adjacent infections complicating septic arthritis more frequently have a CRP >10-13 mg/dL compared with patients with septic arthritis alone. Other findings such as older age, prolonged symptoms, bacteremia, alterations in other lab values (such as elevated ANC and thrombocytopenia), and failure to rapidly improve with therapy have been less consistently associated with adjacent infection. Nonetheless, adjacent infection should be considered in patients demonstrating such multiple risk factors.
Radiographic Evaluation
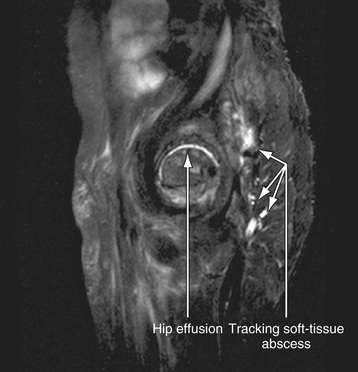
Radiographic studies play a crucial role in evaluating septic arthritis. Conventional radiographs and ultrasonography are performed as part of the routine workup. CT, MRI, and radionuclide studies can all contribute to establishing the diagnosis in selected cases (Fig. 705.1 ).
Plain Radiographs
Plain films can suggest the diagnosis of septic arthritis by showing: widening of the joint capsule, soft tissue edema, and obliteration of normal fat lines. Plain films can also help to exclude other causes of joint pain such as fractures. Plain films of the hip can show medial displacement of the obturator muscle into the pelvis (the obturator sign), lateral displacement or obliteration of the gluteal fat lines, and elevation of Shenton's line with a widened arc.
Ultrasonography
Ultrasonography is included with plain films in routine evaluations because it is particularly helpful in detecting joint effusion and fluid collection in the soft tissue and subperiosteal regions. Ultrasonography is highly sensitive in detecting joint effusion, particularly for the hip joint, where plain radiographs are normal in more than 50% of cases of septic arthritis of the hip. Ultrasonography can serve as an aid in performing hip aspiration.
Magnetic Resonance Imaging and Computed Tomography
MRI and CT can confirm the presence of joint fluid in patients with suspected osteoarthritis infections but are not routinely indicated. MRI is useful in evaluating for adjacent osteomyelitis or pyomyositis but is typically reserved for cases when the index of suspicion for these conditions is high. Considerations include: patient factors such as younger age, the clinical presentation (e.g., protracted pain preceding joint swelling), the results of laboratory investigations such as joint aspiration and CRP, and response to therapy.
Radionuclide Imaging
Radionuclide imaging, although not routinely indicated, is more sensitive than plain radiographs in providing supportive evidence of the diagnosis of septic arthritis; a scan may be positive within 2 days of the onset of symptoms. Three-phase imaging with technetium-99 methylene diphosphonate shows symmetric uptake on both sides of the joint, limited to the bony structures adjacent to the joint. Radionuclide imaging is also useful for evaluating the sacroiliac joint.
Differential Diagnosis
The differential diagnosis of septic arthritis depends on the joint or joints involved and the age of the patient. For the hip, toxic synovitis, pyomyositis, Legg-Calvé-Perthes disease, slipped capital femoral epiphysis, psoas abscess, and proximal femoral, pelvic, or vertebral osteomyelitis, as well as diskitis, should be considered. For the knee, distal femoral or proximal tibial osteomyelitis, pauciarticular rheumatoid arthritis, and referred pain from the hip should be considered. Knee or thigh pain may be referred from the hip. Other conditions such as trauma, cellulitis, pyomyositis, sickle cell disease, hemophilia, Lyme arthritis, and Henoch-Schönlein purpura can mimic purulent arthritis. When several joints are involved, serum sickness, collagen vascular disease, rheumatic fever, and Henoch-Schönlein purpura should be considered. Arthritis is one of the extraintestinal manifestations of inflammatory bowel disease. Reactive arthritis following a variety of bacterial (gastrointestinal or genital) and parasitic infections, streptococcal pharyngitis, or viral hepatitis can resemble acute septic arthritis (see Chapter 182 ).
Treatment
Optimal treatment of septic arthritis requires cooperation of pediatricians, orthopedic surgeons, and radiologists.
Antimicrobial Therapy
The initial empirical antimicrobial therapy is based on likely bacterial pathogens at various ages, the results of the Gram stain of aspirated material, and additional considerations. In neonates, an antistaphylococcal penicillin, such as nafcillin or oxacillin (150-200 mg/kg/24 hr divided q6h IV), and a broad-spectrum cephalosporin, such as cefepime (100 to 150 mg/kg/24 he divided q 12 IV), provide coverage for the S. aureus, group B streptococcus, and Gram-negative bacilli. If MRSA is a concern, vancomycin is selected instead of nafcillin or oxacillin. If the neonate is a small premature infant or has a central vascular catheter, the possibility of nosocomial bacteria (S. aureus , Gram-negative enterics, or Pseudomonas aeruginosa ) or fungi (Candida) should be considered.
In older infants and children with septic arthritis, empirical therapy to cover for S. aureus , streptococci, and K. kingae includes at minimum cefazolin (100-150 mg/kg/24 hr divided q8h) or nafcillin (150-200 mg/kg/24 hr divided q6h).
In areas where methicillin resistance is noted in ≥10–15% of community-acquired methicillin-resistant S. aureus (CA-MRSA) strains, adding an antimicrobial that is effective against local CA-MRSA isolates is suggested. Vancomycin (15 mg/kg q6h IV) is preferred in patients who are ill-appearing, suspected to be bacteremic, or if local clindamycin resistance is more than 10–15%. Clindamycin (40 mg/kg divided q6h) is a reasonable alternative when treating CA-MRSA infections. For immunocompromised patients, combination therapy is usually initiated, such as with vancomycin and ceftazidime, cefepime, or piperacillin/tazobactam, with or without an aminoglycoside. Adjunct therapy with dexamethasone for 4 days with antibiotic therapy has been shown to decrease the duration of fever and promote a more rapid decline in inflammatory markers. These studies have had some significant limitations and a favorable impact on long-term outcomes has not yet been clearly demonstrated in humans thus this practice has not yet been adopted as part of routine care. Lyme arthritis is treated with doxycycline (4 mg/kg/24 hr divided Q12 PO) for 28 days in children >8 yr old. For children < 8 yr old, amoxicillin (50 mg/kg/24 hr divided Q8 PO) or cefuroxime (30 mg/kg/24 hr divided Q12) is recommended. A second 28-day course may be considered for patients with persistent or recurrent symptoms after completing the initial course of treatment. Intravenous ceftriaxone (50 mg/kg Q24 IV) for 14-28 days may be considered as an initial or second course of therapy for severe or refractory cases.
Empirical antimicrobials are narrowed to targeted therapy when the pathogen is identified. If a pathogen is not identified and a patient's condition is improving, therapy is continued with the antibiotic selected initially. If a pathogen is not identified and a patient's condition is not improving, consideration should be given to the need for reaspiration, the presence of an extraarticular infection requiring surgical debridement or the possibility of a noninfectious etiology. In such cases, MRI may be performed to assist with subsequent management decisions.
Duration of antibiotic therapy is individualized depending on the organism isolated and the clinical course. Ten to 14 days is usually adequate for streptococci, S. pneumoniae , and K. kingae; longer therapy may be needed for S. aureus and Gram-negative infections (3 wk), concomitant osteomyelitis (4 wk), extensive disease, or slow response to treatment. Normalization of CRP in addition to a normal examination supports discontinuing antibiotic therapy. The prognostic significance of an improved but still minimally elevated ESR in the 3rd or 4th week of therapy is not clear if all other clinical and laboratory parameters are favorable. In selected patients, obtaining a plain radiograph of the joint before completing therapy can provide evidence (typically periosteal new bone) of a previously unappreciated contiguous site of osteomyelitis that would likely prolong antibiotic treatment. Oral antibiotics can be used to complete therapy once the patient is afebrile for 48-72 hr and is clearly improving.
Surgical Therapy
Infection of the hip is generally considered a surgical emergency because of the vulnerability of the blood supply to the head of the femur. For joints other than the hip, daily aspirations of synovial fluid may be required. In general, one or two subsequent aspirations suffice. If fluid continues to accumulate after 4-5 days, arthrotomy or video-assisted arthroscopy is needed. At the time of surgery, the joint is flushed with sterile saline solution. Antibiotics are not instilled because they are irritating to synovial tissue, and adequate amounts of antibiotic are achieved in joint fluid with systemic administration.
Prognosis
Improvement in signs and symptoms occurs rapidly following joint drainage and antibiotic administration. Failure to improve or worsening by 48-72 hr requires review of the appropriateness of the antibiotic therapy, the need for surgical intervention, and the correctness of the diagnosis. Acute phase reactants may be useful as monitors. Failure of either of these acute phase reactants to follow the usual course should raise concerns about the adequacy of therapy. Recurrence of disease and development of chronic infection after treatment occur in <10% of patients.
Septic arthritis can lead to numerous long-term sequelae in children, including leg-length discrepancy or angular deformity from growth arrest, limitations in range of motion due to chondral damage, and avascular necrosis of the femoral head from septic arthritis of the hip. The overall rate of these sequelae with current therapies is <5%. However, children are in a dynamic state of growth, so these abnormalities might not become apparent for months or years; therefore long-term follow-up is necessary, with close attention to range of motion of joints and bone length. Involvement of the hip is associated with a higher rate of sequelae. Although firm data about the impact of delayed treatment on outcome are not available, it appears that initiation of medical and surgical therapy within 1 wk of onset of symptoms provides a better prognosis than delayed treatment.