
Figure 56.1 Spectrum of acute brain dysfunction. Courtesy of Vanderbilt University, Nashville, TN. Copyright © 2012. Used with Permission.
BACKGROUND
Delirium is a form of acute brain dysfunction that occurs in 8% to 10% of emergency department (ED) patients.1,2 By contrast, delirium affects 20% to 70% of intensive care unit (ICU) patients, especially those requiring mechanical ventilation.3–5 Historically, delirium was considered a normal and transient part of critical illness that posed little consequence to the patient. Evidence collected in the past decade, however, suggests that delirium may affect patient outcomes profoundly. In the critically ill, delirium is an independent risk factor for death, as well as for long-term cognitive impairment, increased ventilator time, prolonged hospitalizations, and increased hospital costs.6–9
When present, delirium should be considered a medical emergency, as it can be the sole manifestation of an underlying critical illness. ED management of delirium influences clinical outcomes, and the emergency physician must be adept at detecting delirium, identifying its etiology, and initiating potentially lifesaving therapies. This chapter reviews the definition and risk factors for delirium, validated instruments for its detection, and appropriate diagnostic workup and management in confirmed cases.
DEFINITION
Delirium is an acute reversible disturbance in attention and cognition precipitated by an underlying medical illness not attributable to a preexisting or evolving dementia.10 Cognitive change in delirium is rapid, occurring over several hours or days and often fluctuating. The core feature of delirium is inattention; other features may include altered consciousness level, disorganized thinking, and sleep–wake cycle disturbances. It is important to note that delirium lies on a continuum of acute brain dysfunction, the most severe form of which is coma (Fig. 56.1).

Figure 56.1 Spectrum of acute brain dysfunction. Courtesy of Vanderbilt University, Nashville, TN. Copyright © 2012. Used with Permission.
Delirium is classified into three psychomotor subtypes: hypoactive, hyperactive, and mixed type.11 Hypoactive or “quiet” delirium is characterized by decreased psychomotor activity and may manifest in a depressed, sedated, somnolent, or lethargic appearance. Its clinical presentation may be subtle, and it is frequently missed or misdiagnosed as depression or fatigue.12,13 Hyperactive delirium is the most recognizable subtype and is characterized by increased psychomotor activity; patients appear restless, anxious, agitated, and even combative. In mixed-type delirium, patients fluctuate between hypoactive and hyperactive psychomotor activity over a period of minutes to hours. In critically ill patients, hypoactive and mixed type are the commonly observed delirium subtypes; purely hyperactive delirium occurs in <2% of cases.14,15
Excited delirium syndrome (ExDS) is an extreme manifestation hyperactive delirium. Patients with ExDS exhibit extreme agitation, aggressiveness, and violent behavior; they also can appear to possess superhuman strength and to be insensitive to pain.16 Patients with ExDS are an immediate danger to themselves and to the people around them; this unique set of challenges is discussed separately in Chapter 57.
RISK FACTORS FOR DELIRIUM
The onset of delirium involves a complex interaction between patient vulnerability factors and precipitating factors.17 To establish delirium risk, both sets of factors must be considered. Patients who are vulnerable to developing delirium (e.g., an 89-year-old with severe dementia) require a relatively benign insult (e.g., urinary tract infection without signs of sepsis) to develop delirium. Conversely, patients who are not vulnerable to developing delirium (e.g., a healthy 45-year-old) require higher doses of noxious stimuli (e.g., multifocal pneumonia with septic shock) to develop delirium. Therefore, when a patient with low vulnerability presents to the ED with delirium, the clinician should be vigilant in looking for an underlying life-threatening illness.
Among patient vulnerability factors for delirium, dementia is the most consistent across a variety of clinical settings, including the ED and ICU.1,18,19 Age, alcohol use, and depression are additional vulnerability factors in the critically ill.20 Numerous precipitating factors identified in general medical patients are likely equally applicable to the ICU population (Table 56.1). In general, patients with higher illness severity are more likely to develop delirium.21,22 Drug exposures—notably benzodiazepines, opioids, and medications with anticholinergic properties—may also trigger delirium, as can withdrawal from ethanol and benzodiazepines. Other precipitants, especially in the elderly, include cardiovascular illnesses like congestive heart failure and acute myocardial infarction.23,24
TABLE 56.1 Precipitating Factors for Delirium
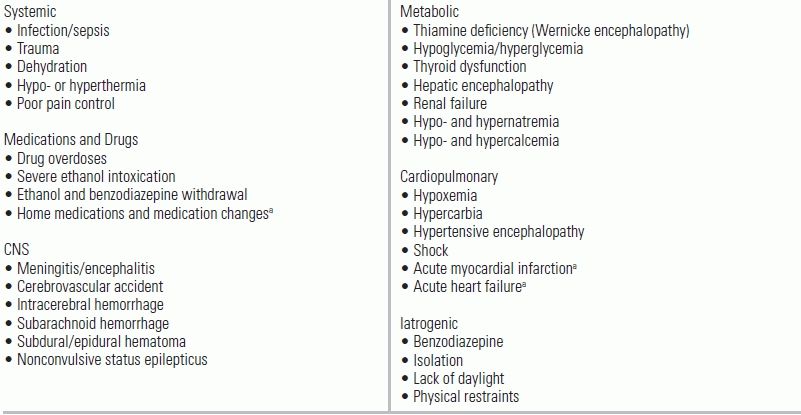
aMore likely to occur in the geriatric patient population, but can occur if the severity of illness is high.
When critically ill patients are boarded for extended periods of time in the ED, emergency physicians become important monitors of potentially preventable iatrogenic risk factors for delirium, specifically the use of deliriogenic medications. Several studies have shown a strong dose–response relationship between benzodiazepine use and the development of delirium in the ICU.25–27 Opioids may also precipitate delirium, but this relationship is less clear.5 Since poorly controlled pain can trigger delirium, opioids in some instances may have protective effect; in burn ICU patients, for instance, opioid pain control reduced delirium incidence by 50%.28,29 Delirium can also be stimulated by negative environmental conditions, such as isolation, lack of daylight, and immobility due to the use of physical restraints.18
ASSESSING FOR DELIRIUM
The diagnosis of delirium is commonly missed.1,12 In the ED, 75% of cases of delirium will go unrecognized; of these, 90% will continue to be overlooked in the inpatient setting.1 When health care providers fail to identify delirium, it is usually because they are unfamiliar with established diagnostic criteria and rely instead on clinical gestalt and the absence of disorientation, hallucinations, delusions, and agitation—features often absent in patients with delirium.30
The American College of Critical Care Medicine, the Society of Critical Care Medicine, and the American Society of Health-System Pharmacists collectively published clinical practice guidelines for pain, agitation, and delirium (2013 PAD Guidelines). The guidelines recommend routine delirium monitoring in critically ill patients, using one of two methods validated in this population: the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) or the Intensive Care Delirium Screening Checklist (ICDSC).31
The CAM-ICU enables assessment of delirium in under a minute—or less if it is performed algorithmically, which allows for early stoppage (Fig. 56.2).32 The CAM-ICU evaluates four cognitive features: (1) altered mental status or fluctuating course, (2) inattention, (3) altered level of consciousness, and (4) disorganized thinking. Since it does not require the patient to speak, it can be performed in both mechanically ventilated and non–mechanically ventilated patients.4,33 For a patient to meet criteria for delirium, features 1 and 2, and either feature 3 or feature 4, must be present.
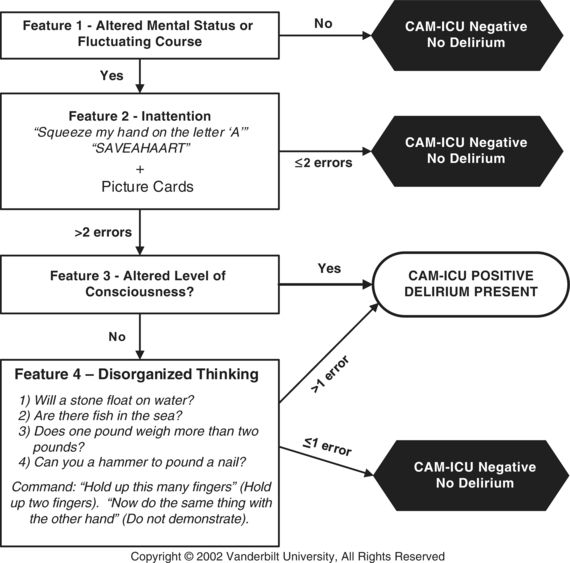
Figure 56.2 Confusion Assessment Method for the Intensive Care Unit. Adapted from www.icudelirium.org. Courtesy of Dr. Wes Ely and Vanderbilt University, Nashville, TN. Copyright © 2002. Used with permission.
Details on how to perform the CAM-ICU and its training manual are available at www.icudelirium.org. Briefly, feature 1 (altered mental status or fluctuating course) is usually obtained from the family member, friend, or caretaker in the ED. Changes and fluctuations in mental status can also be observed by the health care provider during the ED course. Feature 2 (inattention) uses objective assessments and is comprised of an auditory and visual component. For the auditory component, the patient is given a series of 10 letters (“SAVEAHAART”) and is asked to squeeze the rater's hand whenever the letter “A” is heard. For the visual component, the patient is asked to remember 5 objects shown on picture cards and then asked to identify the 5 objects from a series of 10 pictures. Patients who are minimally arousable to verbal stimuli and unable to perform the CAM-ICU's inattention tasks are often erroneously classified as “Negative” or “Unable to Assess” for inattention.34 These patients, however, are actually at the most severe end of the inattention spectrum and should be considered inattentive (feature 2 positive). For feature 3 (altered level of consciousness), a validated arousal scale such as the Richmond Agitation–Sedation Scale is used. For feature 4 (disorganized thinking), the rater asks the patient to answer 4 simple yes/no questions and to perform a simple command.
Initial studies showed the CAM-ICU to have excellent sensitivity (95% to 100%) and specificity (89% to 100%) in detecting delirium in both mechanically ventilated and non–mechanically ventilated patients.4,33 Because of its ease of use, both nurses and physicians can use it reliably.4,33 Subsequent validation studies, however, demonstrated variable diagnostic accuracy. A meta-analysis of nine studies evaluated the performance of the CAM-ICU in critically ill patients and reported a pooled sensitivity of 80% and pooled specificity of 96%.35 While the CAM-ICU's sensitivity for delirium appeared to vary widely between studies (range, 45% to 100%), its specificity remained consistently high, indicating that a positive CAM-ICU is diagnostic of delirium.35 The reasons for such variability in the CAM-ICU's sensitivity remain unclear and deserve further study.
The ICDSC is another assessment tool that uses an eight-item checklist of delirium symptoms, designed for use by ICU nurses over an 8- to 24-hour period. The checklist comprises (1) altered level consciousness; (2) inattention; (3) disorientation; (4) hallucinations, delusions, or psychosis; (5) psychomotor agitation or retardation; (6) inappropriate speech or mood; (7) sleep/wake cycle disturbance; and (8) symptom fluctuation. If a delirium symptom is present, 1 point is assigned; if the symptom is absent, 0 points are assigned. A score of 4 or more is considered positive for delirium. An advantage of the ICDSC is that it does not require additional interaction with the patient, since observations are made during routine clinical care. The ICDSC is, however, more subjective than the CAM-ICU, and its diagnostic performance is dependent on the observer's clinical experience and level of training.
The original validation study of ICDSC found it to be 99% sensitive and 64% specific for delirium, compared to a psychiatrist's evaluation using DSM-IV criteria.36 Subsequent studies have shown variability in the ICDSC's sensitivity and specificity. A meta-analysis of four studies evaluating the diagnostic performance of the ICSDC in the ICU setting reported a pooled sensitivity and specificity of 74% and 82%, respectively.35
A delirium assessment tool that may have promise in the critically ill ED patient is the Brief Confusion Assessment Method (bCAM). The bCAM is a modified CAM-ICU in which the inattention (feature 2) tasks are replaced by having the patient recite the months backward from December to July. The bCAM also decreases the cutoff for disorganized thinking. With these changes, the CAM-ICU's sensitivity improved from 72% to 84%, without a significant impact on specificity.37 This study was performed in older ED patients, and its validity in a broader population of critically ill patients may be limited. The bCAM also requires the patient to speak, so this assessment is not useful in mechanically ventilated patients. Future studies are needed to determine the bCAM's diagnostic accuracy in non–mechanically ventilated patients who are critically ill.
DIAGNOSTIC EVALUATION
If a patient presents with delirium, or develops delirium during the ED course, aggressive efforts are required to uncover the underlying etiology. Early identification and treatment of delirium reduces hospital costs and improves patient outcomes; each additional day of delirium has been shown to increase risk of 1-year mortality by 10%.38 Delirium also prolongs the duration of mechanical ventilation and ICU length of stay and accelerates cognitive decline.7,9
Life-threatening causes of delirium should be considered first, especially in the otherwise healthy patient (Table 56.2).39 Many of these processes can be ruled out at the initial assessment, but others, such as meningitis, demand a more extensive evaluation. Once serious causes have been ruled out, precipitating factors listed in Table 56.1 should be considered.
TABLE 56.2 Life-Threatening Causes of Delirium

Adapted from Caplan GA, et al. Delirium. In: Stern TA, ed. Massachusetts General Hospital comprehensive Clinical Psychiatry. 1st ed. Philadelphia, PA: Mosby/Elsevier; 2008.
The underlying cause of delirium is best diagnosed via a complete history and physical examination. However, as delirious patients have an acute loss in cognition, obtaining an accurate history can be difficult.40 It is best to collect collateral patient history from family members or companions as well as an accurate medication history, including any medication or dosing changes (especially in elderly patients). Medication history can be confirmed with the patient's caregiver or pharmacy.41 If a medication overdose is suspected, every effort should be made to obtain the patient's medication bottles in order to identify the specific medication and amount taken. A careful substance abuse history should also be obtained—preferably from a proxy—as delirium can be precipitated by exposures to, or withdrawal from, benzodiazepines and ethanol.
The physical examination of the delirious patient should be similarly thorough and is summarized in Table 56.3. All patients should be fully exposed to allow for an adequate dermatologic and genitourinary examination looking for signs of infection. Medication patches such as fentanyl and scopolamine should be removed if present.
TABLE 56.3 Physical Examination of the Emergency Department Patient with Delirium
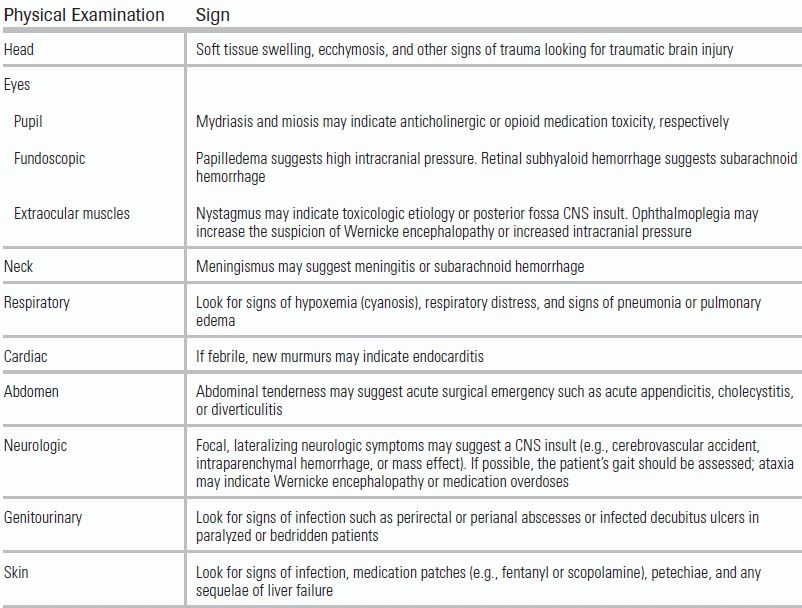
CNS, central nervous system.
Routine laboratory testing for patients with delirium includes complete blood count, serum electrolytes, blood urea nitrogen and creatinine, blood glucose, liver function studies, and urinalysis. If the patient is on delirium-inducing medications that are amenable to serum measurement (i.e., anticonvulsants, lithium, theophylline, and digoxin), then these levels should be ordered. Thyroid-stimulating hormone and free T4 levels should be considered to rule out thyroid dysfunction. In patients with respiratory complaints or symptoms, an arterial or venous blood gas should be used to identify hypercarbia. Because sepsis is a major precipitant in delirium, blood and urine and cultures should be considered. A lumbar puncture is not routinely performed, but should be obtained in delirious patients in whom a high clinical suspicion for meningitis or encephalitis exists or if the patient has a fever or leukocytosis without an obvious source.42,43 Urine drug screens are typically ordered, but a positive result should be interpreted with caution, as it may mislead the clinician and divert attention from an underlying illness. Urine drug screens can produce false-positive and false-negative results and are qualitative and do not provide drug concentrations.44 In a patient on home opioids or benzodiazepines, it would be difficult to differentiate if positive urine drug screen was the result of an overdose or normal home usage.
If a focal, delirium-inducing process is suspected, imaging is indicated (e.g., chest radiography to evaluate for pneumonia or pulmonary edema in the setting of tachypnea, dyspnea, hypoxemia, or cough). A head CT is not routine, but should be obtained in delirious patients with altered level consciousness, a recent history of a fall or head trauma, or focal neurologic deficits.45,46 A head CT may also be reasonable if no other etiology for delirium is found. Magnetic resonance imaging of the brain (brain MRI) and electroencephalography are not typically performed in the ED, but may be useful in ruling out cerebrovascular accidents and nonconvulsive status epilepticus, both of which can mimic or precipitate delirium.
PHARMACOLOGIC MANAGEMENT OF DELIRIUM
The pharmacologic management of delirium has three guiding principles: pain control, avoidance of deliriogenic medications, and medical therapy to minimize the time of delirium.
Because inadequate pain control can precipitate delirium, intravenous opioid analgesia may be necessary.27 Alternative methods for pain control, such as regional or neuraxial (spinal or epidural) anesthesia may also be considered. Importantly, the delirious patient may not be able communicate his or her needs; every effort should be made to identify factors contributing to or aggravating delirium (e.g., urinary retention) while simultaneously treating the patient's discomfort.
With the notable exception of patients withdrawing from ethanol (delirium tremens) or benzodiazepines, benzodiazepines should be avoided in the delirious patient because they can increase delirium severity.47–49 The same holds true for agitated patients, when possible; initial verbal and nonverbal de-escalation techniques should be attempted, including calming the patient environment by dimming or turning off lights, minimizing auditory stimulation from cardiac monitor or intravenous infusion pump alarms, and having family members and familiar objects from home at the patient's bedside. The PAD guidelines also recommend against the use of benzodiazepines to sedate mechanically ventilated patients, endorsing alternative agents less associated with delirium, such as dexmedetomidine or propofol.31
When nonpharmacologic methods fail, typical (haloperidol) and atypical (olanzapine, ziprasidone, risperidone, quetiapine) antipsychotics may be considered. While some practitioners advocate using antipsychotics for all delirious patients, these medications are typically reserved for delirious patients with agitation or psychotic features (delusions, misperceptions, hallucinations, etc.). Before administering antipsychotic medications, a 12-lead electrocardiogram should be obtained, as these medications can precipitate torsades de pointes in patients with QTc intervals >500 milliseconds.31 This is especially the case for intravenous haloperidol.50
Haloperidol is commonly used in the treatment of delirium and can be given intravenously, intramuscularly, and orally. The 2013 PAD guidelines, however, do not recommend its routine use in the critically ill because of a paucity of supporting data. Only one ICU study—the Modifying the Incidence of Delirium (MIND) Trial—compares haloperidol with placebo for the treatment and prevention of delirium.51 The trial randomized 103 mechanically ventilated ICU patients to haloperidol (5 mg), ziprasidone (40 mg), or placebo every 6 hours for up to 14 days and demonstrated no differences in days alive without delirium or coma, duration of mechanical ventilation, hospital length of stay, or mortality between the three treatment groups.51 The trial was, however, intended for use as a pilot study to assess feasibility and was not adequately powered to determine efficacy.
The PAD guidelines support the use of atypical antipsychotics in the treatment of delirium, but this is again based on limited evidence. One small double-blinded randomized control trial compared quetiapine (50 mg q12h, titratable up to 200 mg q12h) with placebo in 36 ICU patients, with both groups receiving additional haloperidol as needed.52 The quetiapine group had shorter delirium duration and less agitation.52 A trend toward increased likelihood of hospital discharge to home rather than to rehabilitation was also observed.52 Atypical antipsychotics are increasingly favored in the treatment of delirium because of a lower association with extrapyramidal side effects. As new clinical trial data emerge, the PAD recommendations for typical and atypical antipsychotic medications will likely evolve.
Finally, because the pathogenesis of delirium is thought to be due in part to increased anticholinergic central nervous system activity, rivastigmine, a cholinesterase inhibitor, was evaluated for use in elderly patients with delirium. A recent multicenter trial comparing rivastigmine with placebo in critically ill patients was, however, stopped early when the rivastigmine group was noted to have longer duration of delirium and higher mortality.53
NONPHARMACOLOGIC MANAGEMENT OF DELIRIUM
Data concerning the nonpharmacologic management of delirium are largely obtained from the geriatric literature, but certain components may be applicable to the critically ill patient. Most of these interventions have multiple components and emphasize (1) encouraging early mobility and avoiding physical restraints; (2) providing a calm and quiet environment, especially at night; (3) reestablishing the sleep–wake cycle reversal commonly observed in delirious patients through environmental modifications (e.g., limit light and noise at night and provide the majority of clinical care during the day) and nonpharmacologic sleep aids (e.g., soothing music, massages, earplugs); (4) reorienting the patient using large clocks or dated whiteboards; (5) performing cognitive stimulating activities such as word games; (6) placing familiar persons or objects near the patient; and (7) reducing sensory deprivation during daytime hours by offering eyeglasses or hearing devices.54 The efficacy of such bundled protocols in the critically ill, though intuitive, is still not well defined and requires future study. One randomly controlled trial, however, proved the efficacy of the simple and cost-effective earplug in the noisy ED and ICU environment: In 136 critically ill patients, the use of ear plugs at night reduced the onset of delirium by half (hazard ratio 0.47, 95% CI, 0.27 to 0.82).55
ABCDE Bundle for Mechanically Ventilated Patients
The ABCDE bundle is a recently proposed approach to the management and prevention of delirium in mechanically ventilated patients. The acronym stands for Awakening and Breathing Coordination, Choice of Medication, Delirium monitoring and Exercise/Early mobility bundle (ABCDE).56
The first two steps of the bundle are the Awakening and Breathing Coordination, which comprise a daily spontaneous awaking trial (SAT) and spontaneous breath trials (SBT) implemented by bedside nurses and respiratory therapists. Details of these steps are provided in Chapter 58. The key component of the ABC portion of the bundle is the daily interruption of sedation. To pass the SAT, patients must open their eyes to verbal stimuli or tolerate the interruption of sedation for 4 or more hours, without meeting any of the failure criteria. Following a successful SAT, patients proceed to the SBT. Use of this portion of the bundle alone has been shown to decrease both days spent in coma and 1-year mortality.57
The third step is Choice of sedation for the mechanically ventilated patient. As previously noted, benzodiazepines should be avoided except in the cases of ethanol or benzodiazepine withdrawal. Preferred alternatives include propofol or dexmedetomidine, both of which have a reduced risk of delirium. Use of dexmedetomidine, when compared to benzodiazepines, is also associated with more ventilator-free days.58,59
The fourth step is Delirium monitoring. This is particularly important for patients boarded in the ED for prolonged periods. Using validated delirium assessment tools, such as the CAM-ICU or ICDSC in combination with a validated sedation scale (e.g., Richmond Agitation–Sedation Scale), facilitates early delirium recognition and helps tailor sedation management to specific patients' needs. Standardized assessment instruments also provide a structured framework for communication between providers. While the term “altered” may suggest a range of cognitive capacity, “RASS −3 and CAM-ICU positive” provides a clear and succinct description of a patient's mental status.
The fifth step is Early Exercise. One randomized controlled trial (RCT) compared mechanically ventilated patients given daily interruptions of sedation with exercise to patients given daily interruption of sedation alone. Patients who received protocolized exercise early in their ICU course experienced an average of two fewer days of delirium, two more ventilator-free days, and a 5-day improvement in time to mobilization out of bed.60 Patients in the intervention group were also more likely to return to independent functional status at hospital discharge (59% vs. 35%).
A recent study of the ABCDE bundle in 296 mechanically ventilated patients demonstrated more delirium-free and ventilator-free days than historical controls.61 While these results are encouraging, the study's use of historical controls made it subject to bias from general improvements in care over time; however, obtaining more robust data from randomized controlled trials may not be ethical or feasible.
Conclusion
Delirium is a form of acute brain dysfunction that is commonly observed in critically ill patients in the ED. It is associated with accelerated cognitive decline and higher mortality. Delirium follows from a complex interaction between patient vulnerability and precipitating factors and can be diagnosed using a validated assessment such as the CAM-ICU or ICDSC. Once detected, the primary clinical goal is to identify and treat the underlying precipitant. Beyond this, the optimal management of delirium remains unclear. Environmental modifications to calm patients and restore natural sleep cycles may be helpful to all patients. Pharmacologically, benzodiazepines should be avoided whenever possible, including for sedation of mechanically ventilated patients, where alternative sedatives, including propofol or dexmedetomidine, may be used. Atypical antipsychotics, such as quetiapine, may improve outcomes in all critically ill delirious patients, but larger trials are needed to confirm these findings. The ABCDE bundle, which consists of interruption of sedation in mechanically ventilated patients, appropriate choice of medicine, delirium monitoring, and early mobilization, may be a useful model for the treatment and prevention of delirium.
Dr. Han is supported by the National Institutes of Health (K23AG032355). Dr. Vasilevskis is supported by the National Institutes of Health (K23AG040157). Dr. Ely has received grant support and honoraria from Eli Lilly, Hospira, and Pfizer and is supported by the National Institutes of Health (R01 AG035117-02, R01 AG 027472–05). Drs. Ely and Vasilevskis are also supported by the Veterans Affairs Clinical Research Center of Excellence and the Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC).
LITERATURE TABLE

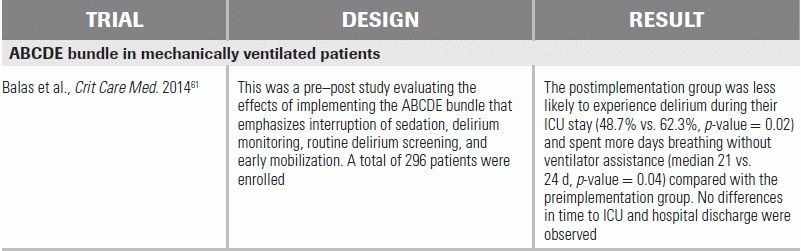
95% CI, 95% confidence interval; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; ICU, intensive care unit; Intensive Care Delirium Screening Checklist, ICDSC; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; LOS, length of stay; RCT, randomized control trial.
1.Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16:193–200.
2.Hustey FM, Meldon SW, Smith MD, et al. The effect of mental status screening on the care of elderly emergency department patients. Ann Emerg Med. 2003;41:678–684.
3.Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304.
4.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29:1370–1379.
5.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41.
6.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900.
7.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
8.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962.
9.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316.
10.American Psychiatric Association. American Psychiatric Association. Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994.
11.Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5:75–85.
12.Inouye SK, Foreman MD, Mion LC, et al. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–2473.
13.Nicholas LM, Lindsey BA. Delirium presenting with symptoms of depression. Psychosomatics. 1995;36:471–479.
14.Pandharipande P, Cotton BA, Shintani A, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33:1726–1731.
15.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484.
16.Vilke GM, Payne-James J, Karch SB. Excited delirium syndrome (ExDS): redefining an old diagnosis. J Forensic Leg Med. 2012;19:7–11.
17.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–857.
18.Van Rompaey B, Elseviers MM, Schuurmans MJ, et al. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13:R77.
19.Pisani MA, Murphy TE, Van Ness PH, et al. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–1634.
20.Brummel NE, Girard TD. Preventing delirium in the intensive care unit. Crit Care Clin. 2013;29:51–65.
21.Inouye SK, Viscoli CM, Horwitz RI, et al. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119:474–481.
22.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097–1101.
23.Kolbeinsson H, Jonsson A. Delirium and dementia in acute medical admissions of elderly patients in Iceland. Acta Psychiatr Scand. 1993;87:123–127.
24.Bayer AJ, Chadha JS, Farag RR, et al. Changing presentation of myocardial infarction with increasing old age. J Am Geriatr Soc. 1986;34:263–266.
25.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26.
26.Pisani MA, Murphy TE, Araujo KL, et al. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37:177–183.
27.Agarwal V, O'Neill PJ, Cotton BA, et al. Prevalence and risk factors for development of delirium in burn intensive care unit patients. J Burn Care Res. 2010;31:706–715.
28.Vaurio LE, Sands LP, Wang Y, et al. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102:1267–1273.
29.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:M76–M81.
30.Meagher DJ, Moran M, Raju B, et al. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br J Psychiatry. 2007;190:135–141.
31.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:278–280.
32.Ely EW, Truman B, Manzi DJ, et al. Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med. 2004;30:1537–1543.
33.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–2710.
34.Woien H, Balsliemke S, Stubhaug A. The incidence of delirium in Norwegian intensive care units; deep sedation makes assessment difficult. Acta Anaesthesiol Scand. 2013;57:294–302.
35.Gusmao-Flores D, Figueira Salluh JI, Chalhub RA, et al. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16:R115.
36.Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864.
37.Han JH, Wilson A, Graves AJ, et al. Validation of the Brief Confusion Assessment Method for Older Emergency Department Patients. Ann Emerg Med. 2011;60(suppl):S28.
38.Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097.
39.Caplan GA, Cassem NH, Murray GB. Delirium. In: Stern TA, ed. Massachusetts General Hospital Comprehensive Clinical Psychiatry. 1st ed. Philadelphia, PA: Mosby/Elsevier; 2008:xvii, 1273.
40.Han JH, Bryce SN, Ely EW, et al. The effect of cognitive impairment on the accuracy of the presenting complaint and discharge instruction comprehension in older emergency department patients. Ann Emerg Med. 2011;57:662–671.
41.Mazer M, Deroos F, Hollander JE, et al. Medication history taking in emergency department triage is inaccurate and incomplete. Acad Emerg Med. 2011;18:102–104.
42.Warshaw G, Tanzer F. The effectiveness of lumbar puncture in the evaluation of delirium and fever in the hospitalized elderly. Arch Fam Med. 1993;2:293–297.
43.Metersky ML, Williams A, Rafanan AL. Retrospective analysis: are fever and altered mental status indications for lumbar puncture in a hospitalized patient who has not undergone neurosurgery? Clin Infect Dis. 1997;25:285–288.
44.Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83:66–76.
45.Naughton BJ, Moran M, Ghaly Y, et al. Computed tomography scanning and delirium in elder patients. Acad Emerg Med. 1997;4:1107–1110.
46.Hardy JE, Brennan N. Computerized tomography of the brain for elderly patients presenting to the emergency department with acute confusion. Emerg Med Australas. 2008;20:420–424.
47.Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153:231–237.
48.Mayo-Smith MF, Beecher LH, Fischer TL. et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164:1405–1412.
49.American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156:1–20.
50.Hassaballa HA, Balk RA. Torsade de pointes associated with the administration of intravenous haloperidol:a review of the literature and practical guidelines for use. Expert Opin Drug Saf. 2003;2:543–547.
51.Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38:428–437.
52.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–427.
53.van Eijk MMJ, Roes KCB, Honing MLH, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–1837.
54.Chong MS, Chan MP, Kang J, et al. A new model of delirium care in the acute geriatric setting: geriatric monitoring unit. BMC Geriatr. 2011;11:41.
55.Van Rompaey B, Elseviers MM, Van Drom W, et al. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16:R73.
56.Vasilevskis EE, Pandharipande PP, Girard TD, et al. A screening, prevention, and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med. 2010;38:S683–S691.
57.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134.
58.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653.
59.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499.
60.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
61.Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle*. Crit Care Med. 2014;42:1024–1036.