TABLE 58.1 Induction Agents for Intubation in the Emergency Department
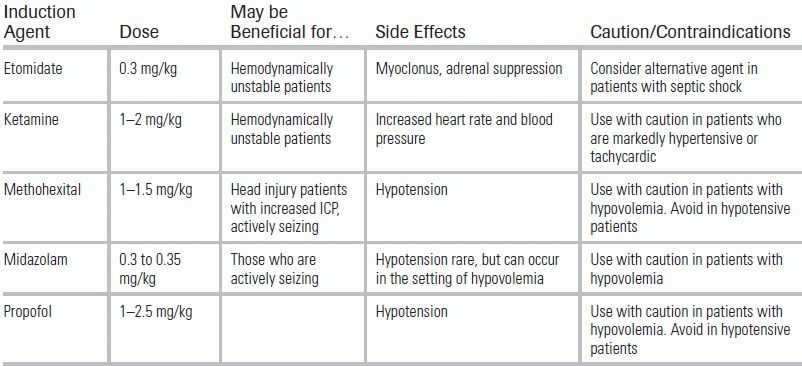
ICP, intracranial pressure. Consider using the lowest dose of induction agent possible to minimize precipitating or exacerbating hemodynamic instability.
BACKGROUND
Sedation is the pharmacologic reduction of agitation and anxiety, and it is an indispensable tool for the clinician treating critically ill emergency department (ED) patients. Sedation is used in the induction of intubation, as well as for maximizing comfort and reducing anxiety in the already intubated patient. Choice of sedative agents in the ED may have ramifications in the intensive care unit (ICU) and hospital course and may affect patient outcomes. This chapter reviews the pharmacologic agents used for the induction for intubation and for the sedation of mechanically ventilated patients.
INDUCTION AGENTS
The ED is frequently tasked with the initial management of a critically ill patient's airway. Induction for endotracheal intubation employs sedatives—called, in this context, induction agents—at doses that typically suppress ventilation. Etomidate, ketamine, barbiturates (methohexital), benzodiazepines (midazolam), and propofol have all been used in this capacity (Table 58.1).
TABLE 58.1 Induction Agents for Intubation in the Emergency Department

ICP, intracranial pressure. Consider using the lowest dose of induction agent possible to minimize precipitating or exacerbating hemodynamic instability.
Etomidate is a carboxylated imidazole derivative that is a potent hypnotic and activates the γ-aminobutyric acid type A (GABA) receptors in the brain; it has no analgesic effects.1 For induction of intubation, the etomidate dose is 0.3 mg/kg given intravenously (IV).2 Etomidate is an ideal induction agent in the ED because it has rapid and predictable onset of action (5 to 15 seconds), a short duration of action (5 to 14 minutes), negligible effect on spontaneous respiration at lower doses, and no direct effects on cardiac output or vascular resistance.1,3,4 Etomidate may be particularly useful in patients with suspected traumatic brain injury or intraocular injuries; by reducing cerebral blood flow and oxygen consumption, it can decrease intracranial and intraocular pressure.3
It should be noted that etomidate use can lead to adrenal suppression, and as such its safety has come into question. Etomidate inhibits the 11β-hydroxylase enzyme, which is involved in the production of cortisol. A single dose of etomidate can cause adrenal suppression for up to 72 hours, but whether this has a clinically relevant effect on outcomes has been a source of significant controversy.5
A recent meta-analysis that included five studies reported that critically ill patients who were septic and received etomidate were more likely to die (relative risk = 1.20).6 However, only two of the five studies included in this meta-analysis were primary analyses of randomized controlled trials.7,8 A recent retrospective cohort study enrolled 2,014 septic patients and reported that one-time etomidate use was not associated with ICU mortality, hospital mortality, vasopressor use, duration of mechanical ventilation, or ICU length of stay (LOS) in the unadjusted and adjusted models.9 However, the limitations of retrospective studies are well documented, and larger randomized controlled trials comparing etomidate with other induction agents are needed.
Data regarding the safety of etomidate in nonseptic patients are even more uncertain, as there are few rigorously performed randomized controlled trials comparing etomidate to other induction agents. An association between single-dose etomidate and adverse outcomes (mortality, hospital LOS, ventilator days) has been noted in several retrospective cohort studies of critically ill patients.10,11 One randomized controlled trial enrolled 469 critically patients with and without sepsis and compared etomidate with ketamine.8 Though the etomidate group was more likely to have adrenal sufficiency, no significant difference in 28-day mortality was observed in the septic and nonseptic groups. There was, however, a trend toward increased vasopressor use in the etomidate group compared with the ketamine group (59% vs. 51%).8
Some clinicians have advocated the use of supplemental hydrocortisone and/or fludrocortisone when etomidate is administered for intubation.12 In a secondary analysis of a major randomized controlled trial comparing the role of corticosteroids in septic shock, it was found that patients who received hydrocortisone and fludrocortisone for 7 days had lower 28-day mortality rates compared with patients who received placebo (55% vs. 76%).13,14 However, two additional studies compared hydrocortisone with placebo in patients who received etomidate and observed no improvement in mortality in patients with and without septic shock.15,16
Based upon these limited data, etomidate should be used judiciously in patients with sepsis; however, given its favorable hemodynamic profile, etomidate is still preferable to propofol or barbiturates in unstable patients. In nonseptic patients, despite the reports of adrenal insufficiency, the effect of etomidate on patient outcomes remains uncertain.
Myoclonus is another, albeit less serious, side effect of etomidate and has been reported to occur in 10% to 80% of patients when a paralytic is not used.3 For intubations without neuromuscular blockade, premedication with fentanyl or diazepam prior to etomidate administration may help reduce the incidence of myoclonus.3
Ketamine is a promising alternative to etomidate since it does not, in most cases, affect blood pressure or cardiac output and can be used safely in patients who are hemodynamically unstable. Ketamine is a dissociative agent that has anesthetic, amnestic, and anxiolytic properties. Unlike most other induction agents, it also provides analgesia. Ketamine noncompetitively inhibits glutamate at the N-methyl-d-aspartate receptors and causes dissociation between the thalamoneocortical and limbic regions of the central nervous system (CNS).2 Ketamine may also have theoretical benefit in patients with asthma exacerbations; it causes an increase in serum catecholamine levels and may cause bronchodilation.2 Lastly, patients who receive ketamine are typically able to maintain their respiratory effort and have preserved airway reflexes. For intubation, the dose is 1 to 2 mg/kg IV with an onset of action of approximately 30 seconds.2
Ketamine stimulates catecholamine release, but it can also cause slight myocardial depression.17 Typically, the sympathomimetic stimulation overcomes the myocardial depression and causes an increase in heart rate, blood pressure, and cardiac output.18 Theoretically, patients who have been physiologically stressed for a prolonged period of time may be depleted of endogenous catecholamines, allowing for the myocardial depression to dominate and cause hypotension. Because of this theoretical risk, ketamine should be used cautiously in patients in whom catecholamine depletion is suspected. Because ketamine increases myocardial oxygen demand, it should also be used cautiously in patients with coronary artery disease and avoided in patients who have evidence of myocardial ischemia.18 Ketamine can also cause an increase in heart rate and blood pressure and should be used cautiously in patients who are hypertensive or tachycardic.
Traditionally, ketamine has also been used with caution in patients with traumatic brain injury because early small observational trials observed an increase in intracranial pressure (ICP).19 More recent studies have failed to record a statistically significant increase in ICP, but these studies were similarly limited by their small sample sizes.19 Until more definitive evidence is available, caution should be exercised with ketamine in this population.
Barbiturates, such as methohexital, are CNS depressants that exert effects on the GABA receptors and have anxiolytic and sedative properties. Because barbiturates decreases cerebral blood flow and the brains' metabolic demands, they may have a protective effect in head injury patients. Barbiturates also have anticonvulsant properties and may be advantageous to patients who are actively seizing or who have a history of seizure disorder. However, since barbiturates can cause myocardial depression and peripheral vasodilatation, they are seldom used for intubation in the ED, where patients needing intubation are frequently hemodynamically unstable.2 Barbiturates can also induce aminolevulinic acid synthetase and can precipitate acute porphyric crisis and should be avoided in patients with a history of porphyric disorders.20 Standard induction dose for methohexital is 1 to 1.5mg/kg/IV.2
Benzodiazepines also act on the GABA receptor and have sedative, hypnotic, amnestic, anxiolytic, and anticonvulsant properties, but provide no analgesia.2 Midazolam (0.3 to 0.35 mg/kg) is the most commonly used benzodiazepine for intubation because it has a rapid onset and short duration of action. Although benzodiazepines have minimal cardiovascular effects, they can cause hypotension in patients who are hypovolemic.2 Benzodiazepines have anticonvulsant activities and may be useful in patients who are actively seizing.
Propofol binds to multiple receptors in the CNS including GABA, glycine, nicotinic, and muscarinic receptors. Propofol has sedative, hypnotic, anxiolytic, amnestic, and anticonvulsant properties, but provides no analgesia.21 The dose for induction is 1 to 2.5 mg/kg IV. Propofol has several appealing characteristics for an induction agent. First, it is highly lipophilic and easily crosses the blood–brain barrier, resulting in rapid onset of sedation (1 to 2 minutes). Second, it is rapidly redistributed into the peripheral tissues, resulting in a short duration of action (2 to 8 minutes) even in the setting of renal or hepatic dysfunction. The primary disadvantage of propofol is its negative inotropic effect, which can lead to decreased systemic vascular resistance and cause pronounced hemodynamic depression.22 For this reason, propofol should be used with caution in patients who are volume depleted and should be avoided in patients who are hypotensive.2 Because propofol is dissolved in a 10% lipid emulsion containing egg, soybean oil, and egg lecithin, allergic reactions can be seen in patients with soybean and egg allergies.21
CHOICE OF INDUCTION AGENT
The choice of induction should be guided by the patient's underlying illness and comorbid conditions. Etomidate and ketamine are ideal for use in the ED because of their favorable hemodynamic profiles. Etomidate should probably be avoided in septic patients, although the medical community has not uniformly embraced this recommendation; additional trials are needed to clarify etomidate's safety. Ketamine may be a safer alternative, including in head injury patients. Propofol, barbiturates, and to a lesser extent, benzodiazepines can cause potentially fatal decreases in blood pressure, especially in patients who are volume depleted.
Surprisingly little data exist regarding the effect of induction agent on ease of intubation. One trial randomized 469 septic and nonseptic patients to receive either etomidate or ketamine for the induction of intubation and did not observe any difference in intubation conditions (number of attempts, number of operators, number of alternative techniques, glottis visualization, lifting force, use of external laryngeal pressure, and vocal cord position).8 In a registry study (NEAR II) of 2,380 ED patients who underwent rapid sequence intubation, etomidate, ketamine, and benzodiazepine were associated with a lower likelihood of successful first-attempt intubation compared with barbiturates.23 The authors concluded that using methohexital and propofol facilitated rapid sequence intubation, but that the benefits of these medications should be weighed against their capacity to produce hemodynamic instability.
ANALGESIA AND SEDATION IN THE MECHANICALLY VENTILATED PATIENT
Once a patient is intubated in the ED, a primary goal is to ensure comfort in as safe a manner possible. Endotracheal intubation (as well as other critical care procedures) can result in significant anxiety and agitation, which can lead a patient to self-remove lifesaving medical devices. Unrelieved pain and anxiety may also have long-term psychological consequences, including posttraumatic stress disorder.21
Analgesia and sedation are an integral part to providing comfort to the mechanically ventilated patient (Fig. 58.1). However, special care must be taken to avoid oversedation, which is associated with increased duration of mechanical ventilation, prolonged ICU stays, and delirium.24 Delirium has gained increased attention in the critical care literature over the past decade; it has been shown to be a predictor of death and leads to increased duration of mechanical ventilation, longer ICU stays, and long-term cognitive impairment.25,26
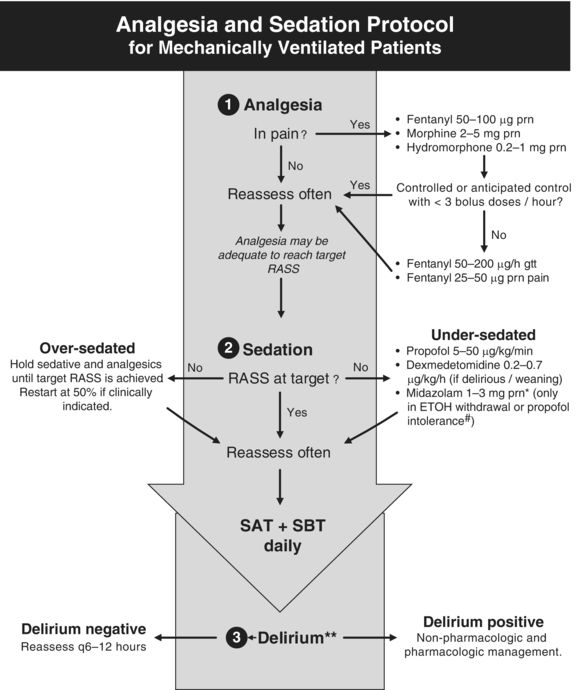
Figure 58.1 Empiric Sedation Protocol. *Midazolam 1 to 3 mg/hour gtt may be used if more than three midazolam boluses are given per hour, for propofol intolerance, or if the patient has been on propofol for >96 hours. #Propofol intolerance may be secondary to propofol infusion syndrome. **Delirium monitoring in critically ill patients is reviewed in Chapter 56. RASS, Richmond agitation and sedation scale; gtt, infusion; prn, as needed; ETOH, ethanol; SAT, Spontaneous awakening trial; SBT, Spontaneous breathing trial. Courtesy of icudelirium.org. Used with permission.
In 2013, the American College of Critical Care Medicine, Society of Critical Care Medicine, and American Society of Health-System Pharmacists released a clinical practice guideline for the management of pain, agitation, and delirium in critically ill patients (PAD guidelines).21 These guidelines were developed by a 20-person multidisciplinary task force that reviewed the latest critical care literature and provided consensus recommendations for sedation and analgesia. The subsequent paragraphs provide a summary of these guidelines.
Adequate analgesia is essential to minimizing discomfort, agitation, and delirium in the mechanically ventilated patient (Fig. 58.1).27 Because vital sign abnormalities alone are inaccurate markers for pain, a validated pain assessment should be used for all intubated patients.21 The Behavioral Pain Scale and Critical-Care Pain Observation Tool are two examples of pain scales validated for this patient population.28,29 These scales are based upon the health care providers' observations of the patient's facial expression, upper body movements, and compliance with ventilator.
While it is beyond the scope of this chapter to provide a comprehensive review of analgesia for the mechanically ventilated patient, it is important to note that the PAD guidelines recommend IV administration of opioid medications as first-line treatment of pain related to intubation.21 Longer-acting opioids (such as morphine and hydromorphone) and shorter-acting opioids (such as fentanyl and remifentanil) can be used.24 Of the opioid medications listed above, fentanyl is the most commonly used because of its rapid onset of action, short duration of action, and minimal histaminic release.24 Meperidine is generally avoided because it may be deliriogenic and because it is metabolized into normeperidine, which is neurotoxic and can cause tremors, myoclonus, and generalized tonic–clonic seizures.30,31 Morphine has a less clear role in the development of delirium, with studies producing conflicting results. It is possible that opioid medications may be delirium protective when used for pain control, but deliriogenic in higher doses.32,33 Nonopioid analgesia—such as regional anesthesia, IV acetaminophen, oral, IV or rectal cyclooxygenase inhibitors, or IV ketorolac—can be also used as adjunctive therapy for pain control.21
After adequate pain control has been achieved, the next step (Fig. 58.1) is to provide sedation, if needed, to further minimize anxiety and agitation. Dosing must be guided by ongoing, accurate assessment of a patient's agitation and depth of sedation. Traditionally, descriptors such as lethargic, drowsy, somnolent, restless, agitated, or combative have been used, but these terms may have different meanings for different health providers; instead, arousal scales with standardized definitions should be utilized. The commonly used Richmond Agitation Sedation Scale (RASS, Table 58.2) ranges from −5 (unresponsive to pain and voice) to +4 (extreme combativeness).34 Alternatively, the Riker Sedation–Agitation Scale can be used and ranges from 1 (unarousable) to 4 (calm) to 7 (dangerous agitation).35
TABLE 58.2 Richmond Agitation Sedation Scale
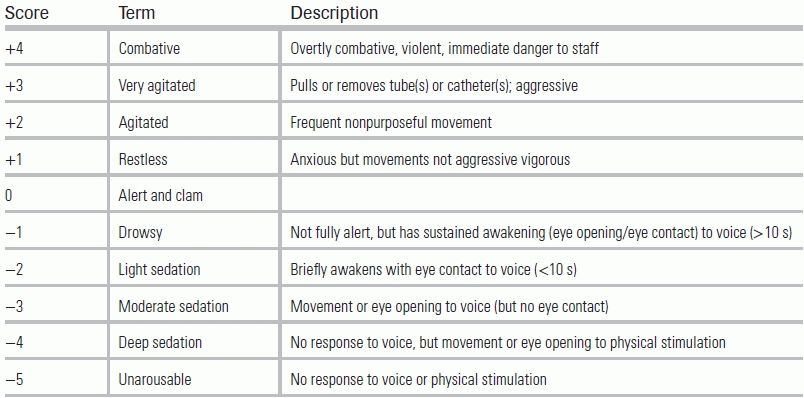
In the time immediately following intubation, it is not uncommon for an ED patient to be overly sedated and minimally responsive to painful stimuli. Prolonged and deep sedation (RASS −3 to −5) within the first 48 hours of mechanical ventilation can lead to delayed extubation times and increased in-hospital and 6-month mortality.36 Ideally, a lighter degree of sedation (RASS −1 or −2) should be targeted, using the least amount of sedation necessary to control agitation and anxiety while maintaining patient comfort.21 Traditionally, benzodiazepines have been the sedative of choice for mechanically ventilated patients.21 Recent evidence, however, suggests that alternative sedative agents such propofol and dexmedetomidine, when available, may improve patient outcomes.
Monitoring for delirium—which affects up to 80% of mechanically ventilated patients and is associated with adverse outcomes—is an essential component of the pain analgesia and sedation protocol (Fig. 58.1).37 Delirium can be the initial manifestation of oversedation or of a change in patient status, such as pain, hypoxemia, hypoglycemia, hypotension, or ethanol withdrawal. If a patient is found to be delirious, every effort should be made to uncover the underlying precipitant. Delirium can be monitored using validated assessments such as the Confusion Assessment Method for the Intensive Care Unit or the Intensive Care Delirium Screening Checklist.37,38 Details of the diagnosis and management of critically ill patients with delirium are described in Chapter 56.
SEDATION AGENTS
Benzodiazepines have been used for sedation for many years in EDs and ICUs. Most benzodiazepines are metabolized by the liver, and their effects can be prolonged in patient with hepatic dysfunction. With the exception of lorazepam, the metabolism of benzodiazepines also produces active metabolites that are renally eliminated. This can result in prolonged sedation in patients with renal dysfunction.24 For all benzodiazepines, elimination is impaired with increased patient age.
Benzodiazepines can also produce respiratory depression and exacerbate hemodynamic instability, especially in patients with preexisting respiratory or cardiac disease.21 Although they are in continued widespread use in the ICU setting, benzodiazepines are known to impair quality of sleep, which can increase the risk for delirium and lead to extended mechanical ventilation time and ICU LOS.24 While there has been a recent push to curtail ICU reliance on benzodiazepines, practice patterns have yet to comply.24,39
For sedation of the mechanically ventilated patient, propofol is initially given as a bolus injection of 5 μg/kg IV over 5 minutes followed by an infusion of 5 to 50 μg/kg/min.23 Propofol crosses the blood–brain barrier with ease, and it is rapidly redistributed into the peripheral tissues, causing it to have a rapid onset and short duration of action. For these reasons, propofol is widely used in the ICU setting, especially for patients that require frequent awakenings for neurologic examinations. In addition, it is useful for performing spontaneous awakening and breathing trials. Note that emergence can be delayed with prolonged propofol infusions once the peripheral tissues have been saturated.
Propofol is a sympatholytic and can lead to hypotension and respiratory depression. Its hemodynamic effects are more pronounced in patients with baseline respiratory insufficiency, cardiovascular instability, or significant hypovolemia. Propofol infusion syndrome (PRIS), although less likely to occur early in a patient's ED or ICU course, is a potentially fatal complication of propofol sedation. The clinical features of PRIS are variable, but can include hypotension and bradycardia, metabolic acidosis, and hypertriglyceridemia.21 Acute kidney injury, hyperkalemia, rhabdomyolysis, and enlarged or fatty liver are also observed.40 PRIS occurs more frequently in patients receiving prolonged (>48 hours) propofol infusions at higher doses >75 μg/kg/min and in patients with acute neurologic or inflammatory illnesses.24,41 When large doses of propofol are used in critically ill patients, it is recommended that serum pH, lactate, creatinine kinase, triglyceride levels, and electrocardiograms (Brugada-type changes) be routinely monitored.24 If PRIS is suspected, treatment consists of discontinuation of the propofol infusion and provision of supportive care.
Whereas benzodiazepine and propofol are GABA receptor agonists, dexmedetomidine is an alpha-2 receptor agonist. It exerts its effects primarily on the presynaptic neurons within the locus ceruleus and spinal cord. Patients sedated with dexmedetomidine are easily arousable to the point of being interactive, and there is minimal associated respiratory depression.21 Unlike propofol and benzodiazepines, dexmedetomidine does not have anticonvulsant properties, but does provide analgesia by an unknown mechanism. The loading dose is 1 μg/kg IV over 10 minutes, and maintenance dose is 0.2 to 0.7 μg/kg/h.21 Studies have shown safety up to 2 g/kg/h but at the expense of increased risk of bradycardia.42 Because dexmedetomidine is metabolized in the liver, lower doses may be required in patients with hepatic dysfunction. There is no need for dose adjustment for patients with renal dysfunction.24
Bradycardia and hypotension are the most common side effects of dexmedetomidine.42 However, the bradycardia observed with dexmedetomidine typically does not require intervention.24 Hypertension may also occur, usually during bolus dosing, via stimulation of the postjunctional alpha-2 receptors located on arterial and venous smooth muscle.24
CHOICE OF SEDATION AGENT
The PAD guidelines currently recommend nonbenzodiazepines (propofol and dexmedetomidine) for sedation of mechanically ventilated patients.21 Based on a recent meta-analysis,43 propofol appears to decrease ICU LOS and slightly decrease the time spent on the ventilator compared with benzodiazepines, but it does not affect mortality. When compared to midazolam, a shorter-acting benzodiazepine, propofol's benefit in reducing ICU LOS disappears.43 It is unclear whether propofol decreases the risk of delirium when compared with benzodiazepines.
Several recent studies have compared dexmedetomidine with a variety of other sedative agents in mechanically ventilated patients.21 The MENDS and SEDCOM studies compared dexmedetomidine with lorazepam and midazolam, respectively, and both studies observed that the dexmedetomidine group was less likely to develop delirium.44,45 Patients receiving dexmedetomidine were also more likely to be close to target sedation compared with patients receiving lorazepam, but no differences were observed when dexmedetomidine was compared with midazolam in this regard. More importantly, the use of dexmedetomidine may facilitate liberation from the ventilator; in the SEDCOM study, patients receiving dexmedetomidine spent a median of two fewer days on the ventilator compared with the midazolam group.45 Dexmedetomidine may also have some mortality benefit in septic patients. In a secondary analysis of the MENDS trial, dexmedetomidine was observed to reduce the risk of mortality by 70% in septic patients compared with patients who received lorazepam.46
More recently, two multicentered randomized controlled trials compared dexmedetomidine with propofol (PRODEX trial) and midazolam (MIDEX trial).47 Time at target arousal was similar between the dexmedetomidine and control (midazolam and propofol) groups.47 Duration of mechanical ventilation was reduced with dexmedetomidine compared with midazolam; no difference was observed with propofol.47 In both trials, patients on dexmedetomidine were better able to communicate pain than those sedated with midazolam or propofol.47
Additional studies are needed to determine if dexmedetomidine should be routinely used for sedation in mechanically ventilated patients and to determine its performance compared with propofol. While there is a push to decrease use of benzodiazepines as the sedation agent of choice, benzodiazepines will continue to play an important role in patients with status epilepticus, or in patients who are withdrawing from ethanol or benzodiazepines.21
INTERRUPTION OF SEDATION
Recently, there has been a paradigm shift in sedation protocols for mechanically ventilated patients, with the goal of reducing duration of mechanical ventilation and patient morbidity. The Awakening and Breathing Controlled (ABC) trial evaluated the efficacy and safety of a “Wake Up and Breathe” protocol that paired management of sedation with ventilation management (Fig. 58.2). This protocol combined spontaneous breathing trials (SBTs), which are standard of care in most intensive care units, with spontaneous awakening trials (SATs), which involve routine interruption of the patient's sedation.

Figure 58.2 “Wake Up and Breathe Protocol.” SAT, Spontaneous awakening trial; SBT, Spontaneous breathing trial. Courtesy of icudelirium.org. Used with permission.
The “Wake Up and Breathe” protocol (SAT + SBT) was compared to the standard of care (SBT alone) in a multicenter randomized control trial that enrolled 336 mechanically ventilated patients.48 Patients who were randomized to the “Wake Up and Breathe” intervention arm spent more days breathing without assistance and had shorter ICU and hospital LOSs. At 1-year follow-up, patients in the intervention arm were less likely to die (44% vs. 58%); for every seven patients treated with the intervention, one life was saved. More patients in the intervention group self-extubated (10% vs. 4%), but there was no difference in patients requiring reintubation. There is natural concern that the SAT may cause undue psychological stress in the patient. However, studies have demonstrated that routine interruption of sedation not only did not result in adverse psychological outcomes but also produced a reduction in symptoms of posttraumatic stress disorder in this population.49
The decision of when to begin SAT and SBT is based on the provider's clinical judgment and the patient's severity of illness. A patient mechanically ventilated because of a drug overdose, for example, will likely begin an SAT + SBT trial earlier than a patient intubated because of a massive traumatic brain injury. As a general rule, ventilator weaning should be initiated within 12 to 24 hours—and in certain circumstances may be initiated in the ED.
CONCLUSION
Sedation is an integral part of ED care of the critically ill patient. For induction of intubation, etomidate has been the medication of choice, but its use is controversial, as a one-time dose causes adrenal suppression and may lead to higher mortality, especially in septic patients. Ketamine is a viable alternative induction agent notable for its minimal impact on a hemodynamic status. Propofol, methohexital, and to a lesser extent midazolam are more likely to cause hypotension, especially in patients who are hypovolemic. Once a patient is intubated and on mechanical ventilation, achieving adequate analgesia and sedation is critical to optimizing outcome. For sedation of most patients, propofol and dexmedetomidine should—when available—be used in place of benzodiazepines. If a patient is anticipated to be the ED for more than 12 hours, SAT and SBT trials should be considered, as these can facilitate early extubation and improve mortality.
Dr. Han is supported by the National Institutes of Health (K23AG032355).
LITERATURE TABLE
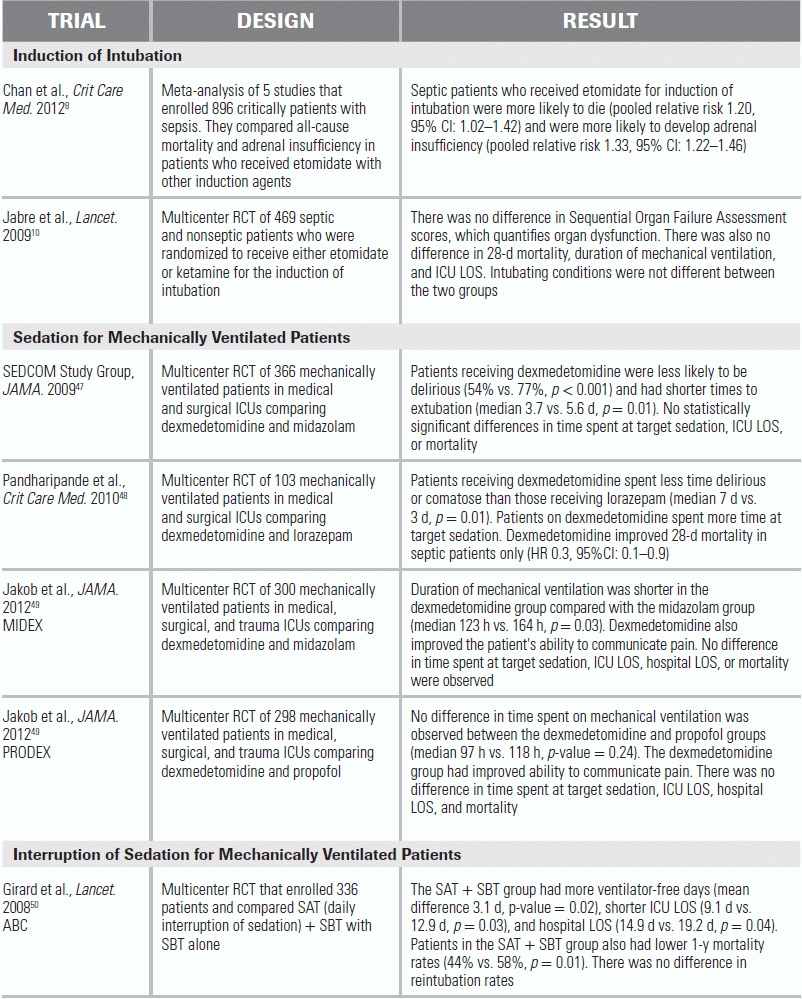
RCT, randomized control trial; CI, confidence interval; HR, hazard ratio; LOS, length of stay.
1.Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology. 2011;114:695–707.
2.Mace SE. Challenges and advances in intubation: rapid sequence intubation. Emerg Med Clin North Am. 2008;26:1043–1068, x.
3.Bergen JM, Smith DC. A review of etomidate for rapid sequence intubation in the emergency department. J Emerg Med. 1997;15:221–230.
4.Zed PJ, Abu-Laban RB, Harrison DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergency department: an observational cohort study. Acad Emerg Med. 2006;13:378–383.
5.Vinclair M, Broux C, Faure P, et al. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34:714–719.
6.Chan CM, Mitchell AL, Shorr AF. Etomidate is associated with mortality and adrenal insufficiency in sepsis: a meta-analysis*. Crit Care Med. 2012;40:2945–2953.
7.Tekwani KL, Watts HF, Sweis RT, et al. A comparison of the effects of etomidate and midazolam on hospital length of stay in patients with suspected sepsis: a prospective, randomized study. Ann Emerg Med. 2010;56:481–489.
8.Jabre P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300.
9.McPhee LC, Badawi O, Fraser GL, et al. Single-dose etomidate is not associated with increased mortality in ICU patients with sepsis: analysis of a large electronic ICU database. Crit Care Med. 2013;41(3):774–783.
10.Warner KJ, Cuschieri J, Jurkovich GJ, et al. Single-dose etomidate for rapid sequence intubation may impact outcome after severe injury. J Trauma. 2009;67:45–50.
11.Hildreth AN, Mejia VA, Maxwell RA, et al. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. J Trauma. 2008;65:573–579.
12.Bloomfield R, Noble D. Etomidate and intensive care physicians. Intensive Care Med. 2005;31:1453; author reply 1454.
13.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871.
14.Annane D. Etomidate and intensive care physicians. Intensive Care Med. 2005;31:1454.
15.Payen JF, Dupuis C, Trouve-Buisson T, et al. Corticosteroid after etomidate in critically ill patients: a randomized controlled trial. Crit Care Med. 2012;40:29–35.
16.Cuthbertson BH, Sprung CL, Annane D, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009;35:1868–1876.
17.Gelissen HP, Epema AH, Henning RH, et al. Inotropic effects of propofol, thiopental, midazolam, etomidate, and ketamine on isolated human atrial muscle. Anesthesiology. 1996;84:397–403.
18.Tweed WA, Minuck M, Mymin D. Circulatory responses to ketamine anesthesia. Anesthesiology. 1972;37:613–619.
19.Filanovsky Y, Miller P, Kao J. Myth: ketamine should not be used as an induction agent for intubation in patients with head injury. CJEM. 2010;12:154–157.
20.James MF, Hift RJ. Porphyrias. Br J Anaesth. 2000;85:143–153.
21.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:278–280.
22.Gauss A, Heinrich H, Wilder-Smith OH. Echocardiographic assessment of the haemodynamic effects of propofol: a comparison with etomidate and thiopentone. Anaesthesia. 1991;46:99–105.
23.Sivilotti ML, Filbin MR, Murray HE, et al. Does the sedative agent facilitate emergency rapid sequence intubation? Acad Emerg Med. 2003;10:612–620.
24.Hughes CG, McGrane S, Pandharipande PP. Sedation in the intensive care setting. Clin Pharmacol. 2012;4:53–63.
25.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520.
26.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
27.Agarwal V, O'Neill PJ, Cotton BA, et al. Prevalence and risk factors for development of delirium in burn intensive care unit patients. J Burn Care Res. 2010;31:706–715.
28.Payen JF, Bru O, Bosson JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29:2258–2263.
29.Gelinas C, Fillion L, Puntillo KA, et al. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15:420–427.
30.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518–1522.
31.Armstrong PJ, Bersten A. Normeperidine toxicity. Anesth Analg. 1986;65:536–538.
32.Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304.
33.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:M76–M81.
34.Sessler CN, Gosnell MS, Grap MJ, et al. The richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
35.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329.
36.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
37.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–2710.
38.Bergeron N, Dubois MJ, Dumont M, et al. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864.
39.Skrobik Y. Counterpoint: should benzodiazepines be avoided in mechanically ventilated patients? No. Chest. 2012;142:284–287; discussion 287–289.
40.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690–701.
41.Vasile B, Rasulo F, Candiani A, et al. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29:1417–1425.
42.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36:926–939.
43.Ho KM, Ng JY. The use of propofol for medium and long-term sedation in critically ill adult patients: a meta-analysis. Intensive Care Med. 2008;34:1969–1979.
44.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653.
45.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499.
46.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38.
47.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151–1160.
48.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134.
49.Kress JP, Gehlbach B, Lacy M, et al. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168:1457–1461.