

• Fasting (overnight) blood glucose concentration greater than or equal to 126 mg/dl on at least two separate occasions
• Following ingestion of 75 g glucose, blood glucose concentration greater than or equal to 200 mg/dl two hours after ingestion and at least one other sample during the four-hour test
• A random blood glucose level of 200 mg/dl or more, plus the presence of suggestive symptoms
• Classic symptoms: increased urination, thirst, and hunger
• Fatigue, blurred vision, poor wound healing, periodontal disease, and frequent infections (often presenting symptoms with type 2 diabetes as well)
Diabetes is a chronic disorder of carbohydrate, fat, and protein metabolism characterized by fasting elevations of blood sugar (glucose) levels and a greatly increased risk of heart disease, stroke, kidney disease, and loss of nerve function. Diabetes can occur when the pancreas does not secrete enough insulin or if the cells of the body become resistant to insulin. Hence, the blood sugar cannot get into the cells, and this condition then leads to serious complications.
Major Complications of Diabetes
• Cardiovascular disease. Adults with diabetes have death rates from cardiovascular disease about two to four times higher than adults without diabetes.
• Hypertension. About 75% of adults with diabetes have high blood pressure.
• Retinopathy. Diabetes is the leading cause of blindness among adults.
• Kidney disease. Diabetes is the leading reason for dialysis treatment, accounting for 43% of new cases.
• Neuropathy. About 60 to 70% of people with diabetes have mild to severe forms of nervous system damage. Severe forms of diabetic nerve disease are a major contributing cause of lower-extremity amputations.
• Amputations. More than 60% of lower-limb amputations in the United States occur among people with diabetes.
• Periodontal disease. Almost one-third of people with diabetes have severe periodontal (gum) disease.
• Pain. Many diabetics fall victim to chronic pain due to conditions such as arthritis, neuropathy, circulatory insufficiency, or muscle pain (fibromyalgia).
• Depression. This is a common accompaniment of diabetes. Clinical depression may begin years before diabetes is fully evident. It is difficult to treat in poorly controlled diabetics.
• Autoimmune disorders. Thyroid disease, inflammatory arthritis, and other diseases of the immune system commonly add to the suffering of diabetes.
Diabetes is divided into two major categories: type 1 and type 2. About 10% of all diabetics are type 1 and about 90% are type 2. Type 1 is associated with complete destruction of the beta cells of the pancreas, which manufacture the hormone insulin. Type 1 patients require lifelong insulin for the control of blood sugar levels. Type 1 results from injury to the insulin-producing beta cells, coupled with some defect in tissue regeneration capacity. In Type 1, the body’s immune system begins to attack the pancreas. Antibodies for beta cells are present in 75% of all individuals with type 1 diabetes, compared with 0.5 to 2% of nondiabetics. It is probable that the antibodies to the beta cells develop in response to cell damage due to other mechanisms (chemical, free radical, viral, food allergy, etc.). It appears that normal individuals either do not develop as severe an antibody reaction or are better able to repair the damage once it occurs.
Type 2 diabetes historically has had an onset after 40 years of age in overweight individuals but today is seen even in children, owing to the obesity epidemic present in all age groups in America as well as those exposed to high levels of persistent organic pollutants (POPs). Initially, insulin levels are typically elevated in type 2, indicating a loss of sensitivity to insulin by the cells of the body. Obesity is a major factor contributing to this loss of insulin sensitivity. Approximately 90% of individuals categorized as having type 2 are obese. Achieving ideal body weight in these patients is associated with restoration of normal blood glucose levels in many cases. Even if type 2 has progressed to the point where insulin deficiency is present, weight loss nearly always results in significant improvements in blood glucose control and dramatic reductions in other health risks such as cardiovascular disease.
Type 2 is a disease characterized by progressive worsening of blood sugar control. It starts with mild alterations in after-meal (postprandial) glucose elevations, followed by an increase in fasting plasma glucose and often ultimately a lack of production of insulin and the need for insulin therapy.
Differences Between Type 1 and Type 2 Diabets |
||
FEATURES |
TYPE 1 |
TYPE 2 |
Age at onset |
Usually younger than 40 |
Usually older than 40 |
Proportion of all diabetics |
<10% |
>90% |
Family history |
Uncommon |
Common |
Appearance of symptoms |
Rapid |
Slow |
Obesity at onset |
Uncommon |
Common |
Insulin levels |
Decreased |
Normal-high initially, decreased after several years |
Insulin resistance |
Occasional |
Often |
Treatment with insulin |
Always |
Usually not required until later in the disease |
There are other types of diabetes, such as gestational diabetes—a type of diabetes that affects about 4% of all pregnant women. About 135,000 cases of gestational diabetes occur each year in the United States. Gestational diabetes occurs more frequently among African-Americans, Hispanic/Latino-Americans, and American Indians. It is also more common among obese women and women with a family history of diabetes. After pregnancy, 5 to 10% of women with gestational diabetes develop type 2; that increases to a 20 to 50% chance of developing diabetes in the 5 to 10 years after pregnancy.
Prediabetes and Metabolic Syndrome
Prediabetes (also called impaired glucose tolerance) is categorized by a fasting glucose of 100–125 mg/dl and/or postprandial glucose of 140–199 mg/dl. It is the first step in insulin resistance and is estimated to affect 57 million Americans. Many people with prediabetes will go on to develop full-blown type 2 despite the fact that prediabetes is usually reversible and, in most cases, diabetes can be completely avoided through dietary and lifestyle changes. Factors implicated in prediabetes, insulin resistance, and the progression to type 2 include a diet high in refined carbohydrates, particularly high-fructose corn syrup; elevated saturated fat intake; overeating due to increased portion sizes of food; increase in inflammatory markers; lack of exercise; industrial pollution; abdominal weight gain; hormonal imbalances; inadequate sleep; and nutrient deficiencies.
Research increasingly indicates that prediabetes is accompanied by serious health risks, especially an increased risk for cardiovascular disease (CVD). Prediabetics often meet the criteria of metabolic syndrome, a cluster of factors that together carry a significantly greater risk for CVD and developing type 2. These factors include:
• Greater waist-to-hip ratio
• Two of the following:
![]() Triglycerides higher than 150 mg/dl
Triglycerides higher than 150 mg/dl
![]() HDL less than 40 mg/dl for men, less than 50 mg/dl for women
HDL less than 40 mg/dl for men, less than 50 mg/dl for women
![]() Blood pressure above 130/85 mm Hg
Blood pressure above 130/85 mm Hg
![]() Fasting blood glucose above 100 mg/dl
Fasting blood glucose above 100 mg/dl
By this definition, and on the basis of data from the Third National Health and Nutrition Examination Survey (NHANES III), the prevalence of metabolic syndrome in the United States is 39% among men and women older than 20.1 Among adolescents, and according to a similar definition, approximately 5.8% meet the established criteria.2 In addition to an elevated risk for cardiovascular disease and diabetes, individuals with metabolic syndrome report poorer health-related quality of life, both physically and mentally.3
Diagnostic Considerations
The classic symptoms of type 1 are frequent urination, weight loss, impaired wound healing, infections, and excessive thirst and appetite. In type 2, because the symptoms are generally milder, they may go unnoticed. For that reason and others, many people with type 2 do not even know they have the disease. Excess abdominal weight, fatigue, blurred vision, poor wound healing, periodontal disease, and frequent infections are often presenting symptoms of type 2.
The standard method for diagnosing diabetes involves the measurement of blood glucose levels. The initial measurement is generally a fasting blood glucose level taken after avoiding food for at least 10 hours but not more than 16. The normal reading is between 70 and 99 mg/dl. If a person has a fasting blood glucose measurement greater than 126 mg/dl (7 mmol/L) on two separate occasions, the diagnosis is diabetes. As mentioned above, a fasting glucose greater than 100 but less than 126 mg/dl is classified as prediabetes.
Glucose Tolerance Test Response Criteria |
|
TYPE |
CRITERIA |
Normal |
No elevation >160 mg/dl (9 mmol/l); <150 mg/dl (8.3 mmol/l) at the end of the first hour, below 120 mg/dl (6.6 mmol/l) at the end of the second hour |
Flat |
No variation more than ± 20 mg/dl (1.1 mmol/l) from fasting value |
Prediabetic |
Blood glucose level of 140 mg/dl (7.8 mmol/l) to 180 mg/dl (10 mmol/l) at the end of the second hour |
Diabetic |
>180 mg/dl (10 mmol/l) during the first hour; 200 mg/dl (11.1 mmol/l) or higher at the end of the first hour; and 150 mg/dl (8.3 mmol/l) or higher at the end of the second hour |
Postprandial and random glucose determinations are also quite helpful in diagnosing diabetes. A postprandial measurement is usually made one to two hours after a meal, while a random measurement is one that is made anytime during the day without regard for the time of the last meal. Any reading greater than 200 mg/dl (11 mmol/l) is considered indicative of diabetes.
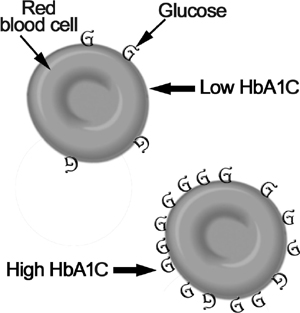
Glycosylation of Red Blood Cells
A valuable laboratory test for evaluating long-term blood glucose levels is measuring glycosylated HbA1C. Proteins that have glucose molecules attached to them (glycosylated peptides) are elevated severalfold in diabetics. Normally, about 4.6 to 5.7% of hemoglobin is combined with glucose. An A1C from 5.7 to 6.4% indicates prediabetes; an A1C of 6.5% or higher can be used to diagnose diabetes. A1C measurements are particularly helpful in patients with unclear results from fasting blood sugar levels. They can be coupled with a fasting blood glucose level and a two-hour postprandial glucose level for a more accurate diagnosis.4 Because the average life span of a red blood cell (RBC) is 120 days, the A1C assay represents time-averaged values for blood glucose over the preceding two to four months. An A1C of 5% indicates that the median blood glucose level for the last three months has been around 100 mg/dl; each point of elevation in the percentage means roughly a 35 mg/dl higher average blood sugar level. Thus, an A1C of 7% means that on average over the last three months the patient’s blood glucose was 170 mg/dl. The A1C test is extremely valuable in providing a simple, useful method for assessing treatment effectiveness and should be checked every three to six months.
Type 1 Diabetes
We know that in type 1 diabetes ultimately the insulin-producing cells of the pancreas are destroyed, in most cases by the body’s own immune system, but what triggers this destruction can vary from one person to another. Genetic factors may predispose the insulin-producing cells to damage through either impaired defense mechanisms, immune system oversensitivity, or some defect in tissue regeneration capacity. The entire set of genetic factors linked to type 1 has been termed “susceptibility genes,” as they modify the risk of diabetes but are neither necessary nor sufficient for disease to develop.5 Rather than acting as the primary cause, the genetic predisposition simply sets the stage for the environmental or dietary factor to initiate the destructive process.6 The very term predisposition clearly indicates that something else needs to occur: less than 10% of those with increased genetic susceptibility for type 1 actually develop the disease.7
In detailed studies, the concordance rate for developing type 1 in identical twins was only 23% in one study8 and 38% in another.9 If one twin develops type 1 after age 24 years, then the rate in the second twin drops all the way down to 6%. These results and others indicate that environmental and dietary factors are more important than a true genetic predisposition in most cases.10
There is additional evidence supporting the need to focus on dietary and environmental triggers:
• There has been a three- to tenfold increase in the number of people with type 1 throughout the world over the past 40 years. Such a rise simply cannot be explained by an increased number of people genetically predisposed to type 1. Changes to the human genetic code across large populations take much more than one generation to occur.11
• The rate of type 1 can increase dramatically when children in areas where type 1 is relatively rare move to developed countries.12 For example, the rate of type 1 increased by nearly fourfold in one 10-year period in children of Asian origin moving to Great Britain, and the rate increased more than sevenfold in Polynesians migrating to New Zealand.13,14 Genetic factors cannot explain such a rapid change.
• There is a strong inverse correlation between maternal vitamin D levels and a child’s risk of developing type 1 diabetes.
Environmental and Dietary Risk Factors
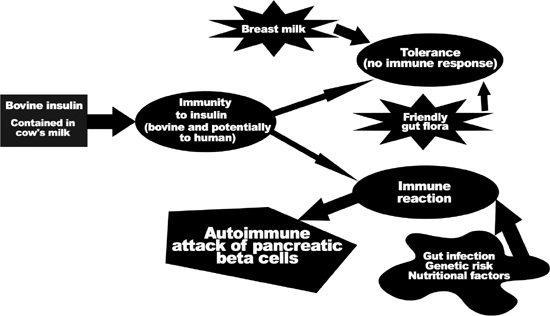
Accumulating data indicate that abnormalities of the gut’s immune system may play a fundamental role in the immune attack on beta cells and the subsequent development of type 1.15 The intestinal immune system serves a vital role in processing the many food and microbial antigens, to protect the body from infection or allergy. What appears to happen in the development of some cases of type 1 is the development by the gastrointestinal immune system of antibodies that ultimately attack the beta cells.
It is interesting to consider that poor protein digestion may contribute to type 1. Poorly digested dietary proteins can cross-react with proteins on or within the beta cells of the pancreas. In humans, two dietary proteins that may be incriminated are those found in milk (which contains bovine serum albumin and bovine insulin) and wheat (which contains gluten). For example, dietary bovine insulin differs from human insulin by only three amino acids. If a person develops antibodies to bovine insulin, there is a good chance that these antibodies will also attack the person’s own insulin. In addition to causing antibody-mediated destruction of the beta cells, bovine insulin is able to activate T cells in those predisposed to diabetes in a manner that can lead to beta cell destruction by direct attack by T killer cells.
Strong evidence implicates dietary factors such as cow’s milk and gluten as important triggers of the autoimmune process that lead to type 1. In contrast, breastfeeding has been identified as an important factor in establishing proper intestinal immune function and reducing the risk of type 1. It is well known that breastfeeding confers a reduction in the risk of food allergies, as well as better protection against both bacterial and viral intestinal infections. In case-controlled studies, patients with type 1 were more likely to have been breastfed for less than three months and to have been exposed to cow’s milk or solid foods before four months of age. A critical review and analysis of all relevant citations in the medical literature indicated that early cow’s milk exposure may increase the risk about 1.5-fold.16 In addition, although the risk of diabetes associated with exposure to cow’s milk was first thought to relate only to intake during infancy, further studies showed that ingestion at any age may increase the risk of type 1.
There is also considerable evidence that sensitivity to gluten—the major protein component of wheat, rye, and barley—may also play a role. Gluten sensitivity produces celiac disease, another autoimmune disorder. Celiac disease, like type 1 diabetes, is associated with intestinal immune function abnormalities. And, as with diabetes, breastfeeding appears to have a preventive effect, while the early introduction of cow’s milk is believed to be a major causative factor. The risk of developing type 1 diabetes is higher in children with celiac disease. Not surprisingly, the highest levels of antibodies to cow’s milk proteins are found in people with celiac disease.17
Enteroviruses and Type 1 Diabetes
Recent studies have strengthened the hypothesis that type 1 diabetes can be the result of viral infection.18,19 A working theory is that the immune system has become slightly confused as to which proteins to attack—food-based ones such as those from dairy products or gluten, or similar proteins on the pancreatic beta cells. (Part of this confusion may be due to vitamin D deficiency, as discussed below.) When the person then has a viral infection, the increase in immune system activation sets off the production of more antibodies and sensitized white cells, and those confused immune cells then begin to damage the pancreas. Gastrointestinal infections due to enteroviruses (e.g., coxsackieviruses, echoviruses) and rotavirus are quite common, especially in children. All of these viruses replicate in the gut and cause stimulation of the intestinal immune system; this may activate the insulin-specific immune cells to seek out and destroy beta cells. These viruses and others are also capable of infecting pancreatic beta cells, causing the leukocytes to attack and destroy the beta cells in an attempt to kill the virus. Another possibility is that gastrointestinal virus infections may increase intestinal permeability, leading to absorption of the intact protein; this then enhances the antibody response to dietary bovine insulin. The severe “leaky gut” or increased small-intestine permeability that occurs during and for some time following rotavirus infections (one of the most common causes of acute diarrheal illness in children) exposes the gut-associated immune cells to large quantities of intact proteins.
Vitamin D Deficiency
Emerging evidence indicates that vitamin D supplementation from cod liver oil and other sources during early childhood can prevent type 1 diabetes.20 In the most extensive studies of vitamin D and type 1, all pregnant women in northern Finland who were due to give birth in 1966 were enrolled (more than 12,000 women) and their children were monitored until December 1997.21 Final analysis of 10,366 enrollees demonstrated that children who regularly took vitamin D, primarily from cod liver oil, had an 80% reduced risk of developing type 1, while those with a vitamin D deficiency had a 300% increased risk of developing the disease. One study found that the use of vitamin D from cod liver oil during pregnancy significantly reduced the frequency of type 1 in their children.22 Furthermore, vitamin D levels are much lower in the blood of people with newly diagnosed type 1 than in healthy controls. Because vitamin D can be produced in the body by the action of sunlight on the skin, lack of sun exposure during childhood may also play a role and partially explain the higher type 1 rates in northern countries. In recent observational studies, vitamin D has been shown to prevent the development of autoimmune conditions, including attacks on beta cells; the degree of protection is dose dependent.23
Proposed Triggers of Type I Diabetes
Omega-3 Fatty Acid Deficiency
A strong case can be made for the benefits of omega-3 fatty acids in protecting against the development of type 1 diabetes. In human studies, giving essential fatty acids (EFAs) significantly reduced the onset of type 1; higher red blood cell omega-3 levels are also associated with reduced risk.24 Cod liver oil provides both EPA and DHA, two vital EFAs. Other studies support the benefit of supplementing EFA in mothers and children. The mechanisms responsible for this effect may be related to improved cell membrane function, leading to enhanced antioxidant status and suppression of the formation of inflammatory compounds known as cytokines.25
Nitrates
Clear links have been established between increased levels of nitrate from dietary sources and water and an increased rate for type 1. Nitrates are produced by agricultural runoff from fertilizers; they are also used in cured or smoked meats such as ham, hot dogs, bacon, and jerky to keep the food from spoiling. Nitrates react within the body to form compounds known as nitrosamines. Nitrates and nitrosamines are known to cause diabetes in animals. Infants and young children are believed to be particularly vulnerable to the harmful effects of nitrate exposure.
One of the most alarming features of type 1 is that it is becoming much more prevalent, with a current growth rate of 3% per year worldwide.11 Some areas have been hit particularly hard, such as Finland, Great Britain, Canada, and the United States. Increased nitrate exposure may be a key factor; nitrate levels in ground and surface waters of agricultural regions have increased over the past 40 years. Nitrate contamination occurs in geographic patterns related to the amount of nitrogen contributed by fertilizers, manure, and airborne sources such as automobile and industrial emissions. Nitrate exposure may explain why some geographic pockets have a substantially higher rate of type 1.26,27
Circumstantial evidence from population-based studies also suggests that a higher dietary intake of nitrate from smoked or cured meats is associated with a significantly higher risk for type 1. These foods severely stress body defense mechanisms and should be avoided. Parents would do well to break the habit of feeding children hot dogs, cold cuts, and ham. Health food stores now carry nitrate-free alternatives to these rather toxic food choices. Also, investing in a high-quality water purifier is good insurance against ingesting nitrate-contaminated drinking water.
Early intervention in type 1 designed to affect the autoimmune or oxidative process theoretically may be capable of lengthening the “honeymoon” phase (the time before insulin becomes absolutely necessary) or even completely reversing the damage. Two substances that may have some benefit in this regard are niacinamide and epicatechin.
Niacinamide
The niacinamide form of vitamin B3 has been shown to prevent some of the immune-mediated destruction of pancreatic beta cells and may actually help to reverse the damage.28,29 Observations that niacinamide can prevent the development of type 1 in experimental animals led to several pilot clinical trials that initially confirmed these observations and suggested that if given soon enough after the onset of diabetes, niacinamide could help restore beta cells or at least slow down their destruction. In a study of newly diagnosed type 1 diabetics, seven patients were given 3 g niacinamide per day and nine were given a placebo. After six months, five patients in the niacinamide group and two in the placebo group were still not taking insulin and had normal blood glucose and hemoglobin A1C. At 12 months, three patients in the niacinamide group but none in the placebo group were in clinical remission.30
The results of this pilot study and others suggested that niacinamide can help restore beta cells and prevent type 1 from progressing in some patients if given soon enough at the onset of diabetes. As of 2004, there had been 12 studies of niacinamide treatment in patients with recent-onset type 1, or type 1 of less than five years’ duration, and who still had some functional beta cells. Of 10 double-blind, placebo-controlled studies, 5 showed a positive effect compared with a placebo in terms of prolonging the period in which insulin was not yet required, lower insulin requirements when the hormone was required, improved metabolic control, and increased beta cell function as determined by secretion of a substance known as C-peptide. In the 5 studies that showed a positive result, patients had a higher baseline fasting C-peptide level, and patients were generally older than in the negative studies.31–34
Despite these positive results, it is important to point out that two large studies designed to evaluate the effectiveness of niacinamide in preventing the development of type 1 in high-risk individuals—such as siblings of children who developed type 1, or individuals who already show elevations in antibodies directed against the beta cells—did not show niacinamide to be effective. The first of these studies, the Deutsche Nicotinamide Intervention Study, did not show much of an effect with 1.2 g niacinamide per day; and results from the larger study, the European Nicotinamide Diabetes Intervention Trial (ENDIT), did not show benefit with dosages as high as 3 g per day.35,36 A possible shortcoming of these studies was the choice of a timed-released niacinamide. It is possible that such a formulation did not allow for sufficient peak levels of niacinamide to block autoimmune mechanisms.37
In the best-case scenario, niacinamide will be likely to work for only a few recent-onset type 1 patients. Nonetheless, the fact that some patients have had a complete reversal of their disease makes its use certainly worth the effort, especially since there is currently no other reasonable alternative.
The dosage recommendation is based on body weight: 25 to 50 mg niacinamide per kg of body weight, up to a maximum dosage of 3 g per day, in divided doses. Niacinamide is generally well tolerated and without side effects. In fact, no side effects have been reported in clinical trials involving type 1. It does not cause the skin flushing typical with high doses of niacin. However, because large doses of niacinamide could possibly harm the liver, a blood test for liver enzymes should be performed every three months to rule out liver damage.
Epicatechin
The second natural compound that may offer benefit is epicatechin. The line of research on its potential role in recent-onset type 1 diabetes began with examining the bark from the Malabar kino tree (Pterocarpus marsupium). This botanical medicine has a long history of use in India as a treatment for diabetes. Initially, epicatechin extracted from the bark was shown to prevent beta cell damage in rats. Further research indicated that both epicatechin and a crude alcohol extract of P. marsupium were actually able to assist in the regeneration of functional pancreatic beta cells in diabetic animals.38 Green tea (Camellia sinensis) extract appears to be a better choice than extracts of P. marsupium, as the epicatechin content is higher in a high-quality green tea extract than in extracts of P. marsupium. Second, green tea extract exerts a broader range of beneficial effects. Another reason is that green tea polyphenols exhibit significant antiviral activity against rotaviruses and enteroviruses, two types of virus suspected of being involved in the development of type 1.39 Last, green tea is considerably easier to find commercially than P. marsupium. Recommended dosage for green tea extract in children younger than age 6 is 50 to 150 mg; for children 6 to 12 years old, it is 100 to 200 mg; for children over 12 and adults, it is 150 to 300 mg. The green tea extract should have a polyphenol content of at least 80% and be decaffeinated.
Type 2 Diabetes
The major risk factor for type 2 diabetes is obesity or, more precisely, excess body fat. Approximately 80 to 90% of individuals with type 2 are obese (body mass index greater than 30). When fat cells (adipocytes), particularly those around the abdomen, become full of fat, they secrete a number of biological molecules (e.g., resistin, leptin, tumor necrosis factor, free fatty acids, cortisol) that dampen the effect of insulin, impair glucose utilization in skeletal muscle, promote glucose production by the liver, and impair insulin release by pancreatic beta cells. Also important is that as the number and size of adipocytes (fat cells) increase, this leads to a reduction in the secretion of compounds that promote insulin action, including adiponectin, a protein produced by fat cells. Not only is adiponectin associated with improved insulin sensitivity, but it also has anti-inflammatory activity, lowers triglycerides, and blocks the development of atherosclerosis (hardening of the arteries). The net effect of all of these actions is that fat cells severely stress blood glucose control mechanisms, as well as lead to the development of the major complication of diabetes, atherosclerosis. Because of all these newly discovered hormones secreted by adipocytes, many experts now consider adipose tissue to be part of the endocrine system, joining glands such as the pituitary, the adrenals, and the thyroid.40,41 Measuring blood levels of adiponectin or other hormones secreted by fat cells may turn out to be the most meaningful predictor of the likelihood of developing type 2.42,43
In the early stages of the increased metabolic stress produced by the various secretions of adipocytes and the lack of adiponectin, blood glucose levels remain normal despite the insulin resistance because pancreatic beta cells compensate by increasing insulin output. As metabolic stress increases and insulin resistance becomes more significant, eventually the pancreas cannot compensate and elevations in blood glucose levels develop. As the disease progresses from insulin resistance to full-blown diabetes, the pancreas starts to “burn out” and produces less insulin. Fortunately, the pancreas can recover and continue to secrete insulin for the rest of a person’s lifetime if ideal body weight is achieved and steps to improve insulin sensitivity are taken.
Risk Factors for Type 2 Diabetes
Family history of diabetes (i.e., parent or sibling with type 2 diabetes)
Obesity
Increased waist-to-hip ratio
Age (increasing age is associated with increased risk beginning at 45)
Race/ethnicity (e.g., African-American, Hispanic-American, Native American/Canadian, Native Australian or New Zealander, Asian-American, Pacific Islander)
Previously identified impaired fasting glucose or impaired glucose tolerance
History of gestational diabetes or delivery of baby weighing more than 9 lb
Hypertension (blood pressure greater than 140/90 mm Hg)
Triglyceride level higher than 250 mg/dl
Low adiponectin levels; elevated fasting insulin levels
Polycystic ovary syndrome (consider in any adult woman who is overweight, has acne, and has fertility problems)
Genetics of Type 2 Diabetes and Obesity
In studies of identical twins, the proportion of both twins having the disease (the concordance rate) was between 70 and 90% for type 2. This high concordance points to a strong genetic relationship. Data from family studies also provide additional support: children who have one parent with type 2 have an increased risk of diabetes in their lifetime, and if both parents have the disease, the risk in offspring is nearly 40%.44 However, even with the strongest predisposition, diabetes can be avoided in most cases.
The Case of the Pima Indians
The Pima Indians of Arizona have the highest rate of type 2 and obesity anywhere in the world. Research has demonstrated a strong genetic predisposition, but even with this strong tendency it is extremely clear that the high rate of type 2 in this group is almost totally due to diet and lifestyle. The Pima Indians living traditionally in Mexico still cultivate corn, beans, and potatoes as their main staples, plus a limited amount of seasonal vegetables and fruits such as zucchini, tomatoes, garlic, green peppers, peaches, and apples. The Pimas of Mexico also make heavy use of wild and medicinal plants in their diet. They work hard, have no electricity or running water in their homes, and walk long distances to bring in drinking water or to wash their clothes. They use no modern household devices; consequently, food preparation and household chores require extra effort by the women. In contrast, the Pima Indians of Arizona are largely sedentary and follow the dietary practices of typical Americans. The results are astounding. Although roughly 16% of Native Americans in general in the United States have type 2, 50% of Arizona Pimas have type 2, and 95% of those diabetics are overweight or obese. By contrast, type 2 is a rarity among Mexican Pimas and only about 10% could be classified as obese. The average difference in body weight between the Arizona and Mexican Pima men and women is more than 60 lb.45
Further evidence that diet and lifestyle appear to be able to overcome even the strongest genetic predisposition is shown by some of the intervention studies with Pima Indians. When patients are placed on a more traditional diet along with physical exercise, blood glucose levels improve dramatically and weight loss occurs. The focus right now by various medical organizations such as the National Institutes of Health is to educate children on the importance of exercise and dietary choices to reduce diabetes risk.
Other Genetic and Racial Factors
Racial and ethnic groups besides Pima Indians that have a higher tendency for type 2 include other Native Americans, African-Americans, Hispanic-Americans, Asian-Americans, Australian Aborigines, and Pacific Islanders. In all of these higher-risk groups, again, it is important to point out that when they follow traditional dietary and lifestyle practices, the rate of diabetes is extremely low. It appears that these groups are simply sensitive to the Western diet and lifestyle.
Diet, Exercise, Lifestyle, and Diabetes Risk
Findings from the U.S. government’s Third National Health and Nutrition Examination Survey (NHANES III) make it quite clear that diabetes is a disease of diet and lifestyle. Of individuals with type 2, 69% did not exercise at all or did not engage in regular exercise; 62% ate fewer than five servings of fruits and vegetables per day; 65% obtained more than 30% of their daily calories from fat, with more than 10% of total calories from saturated fat; and 82% were either overweight or obese.46
Insights into the role of modern lifestyle in the development of type 2 can be gleaned from the Old Order Amish. These 30,000 or so individuals, whose ancestors arrived on U.S. shores in the 18th century, maintain religious and cultural beliefs that preclude regular use of modern conveniences such as electrical appliances, telephones, and cars, and they have a physically active lifestyle. By comparison, the 300 million typical Americans living alongside them have, over the past 250 years, willingly adopted advances of modern technology, making life less physically demanding.
Although the typical Amish person’s diet is not very different from the average American’s and the rates of obesity are very similar as well, the rate of diabetes is about 50% lower. Although the percentage of Amish with impaired glucose tolerance (prediabetes) is about the same as the rate among other white populations in America, apparently not as many Amish go on to develop diabetes. This trend suggests that physical activity has a protective effect against type 2, independent of obesity.47,48
Results from other studies corroborate this hypothesis. Lifestyle changes alone are associated with a 58% reduced risk of developing diabetes in people at high risk (those with impaired glucose tolerance), according to results from the Diabetes Prevention Program, a large intervention trial of more than 1,000 subjects. The two major goals of the program were achieving and maintaining a minimum of 7% weight loss and a minimum of 150 minutes per week of physical activity similar in intensity to brisk walking.49
A Diet High in Refined Carbohydrates
Dietary carbohydrates play a central role in the cause, prevention, and treatment of type 2. In an effort to qualify carbohydrate sources as acceptable or not, two tools have been developed: the glycemic index and glycemic load. The glycemic index is a numerical value that expresses the rise of blood glucose after a particular food is eaten. The standard value of 100 is based on the rise seen with the ingestion of glucose. The glycemic index of foods ranges from about 20 for fructose and whole barley to about 98 for a baked potato. The insulin response to carbohydrate-containing foods is similar to the rise in blood sugar. The glycemic index is often used as a guideline for dietary recommendations for people with either diabetes or hypoglycemia. In addition, eating foods with a lower glycemic index is associated with a reduced risk for obesity and diabetes.50–52
One of the shortcomings of the glycemic index is that it tells us only about the quality of the carbohydrates, not the quantity. Obviously, quantity matters too, but the measurement of a food’s glycemic index is not related to portion size. That is where the glycemic load comes into play. The glycemic load takes the glycemic index into account but provides much more accurate information than the glycemic index alone. The glycemic load is calculated by multiplying the amount of carbohydrate in a serving of food by that food’s glycemic index, then dividing it by 100. The higher the glycemic load, the greater the stress on insulin. In Appendix B, we provide the glycemic index and glycemic load for many common foods.
Research studies are just starting to use glycemic load as a more sensitive marker for the role of diet in chronic diseases such as diabetes and heart disease. Preliminary results are showing that the glycemic load of a person’s food intake is a stronger predictor of diabetes than glycemic index.50,52 Researchers are also showing that a high-glycemic-load diet is associated with an increased risk for heart disease. For example, when researchers from the Nurses Health Study used glycemic load measures to assess the impact of carbohydrate consumption on women, they found that high-glycemic-load diets correlated with a significantly greater risk for heart disease because of their association with lower levels of protective HDL cholesterol and higher triglyceride levels.53 Increased risk for diabetes and heart disease started, on average, at a daily glycemic load of 45. Therefore we recommend using the information in Appendix B to help determine how to prevent the total daily GL from exceeding 150. Keep in mind that the GL is directly related to serving size: the larger the serving size, the greater the GL.
The Importance of Dietary Fiber in Reducing the Risk of Diabetes
Population studies, as well as clinical and experimental data, show diabetes to be one of the diseases most clearly related to inadequate dietary fiber intake. Different types of dietary fiber act differently in the body. The type of fiber that exerts the most beneficial effects on blood sugar control is the soluble form. Included in this class are hemicelluloses, mucilages, gums, and pectin substances. These are capable of slowing down the digestion and absorption of carbohydrates, thereby preventing rapid rises in blood sugar. They are also associated with increasing the sensitivity of tissues to insulin and improving the uptake of glucose by the muscles, liver, and other tissues, thereby preventing a sustained elevation of blood sugar.54,55
Particularly good sources of soluble fiber are legumes, oat bran, nuts, seeds, psyllium seed husks, pears, apples, and most vegetables. Although even the simple change from white-flour products to whole-grain versions is associated with a reduced risk for type 2,56,57 our recommendation is to consume at least 35 g fiber a day from various food sources, especially vegetables. Fiber supplements can also be taken to help lower the glycemic load of a food or meal.
The Wrong Types of Fats
Dietary fat plays a central role in the likelihood of developing type 2. Large controlled trials have shown that a reduction of fat intake as part of a healthful lifestyle, combined with weight reduction and exercise, reduces the risk of type 2. However, more important than the amount of fat in the diet is the type of fat.58 The dietary fat profile linked to type 2 is an abundance of saturated fat (mostly found in animal sources) and trans-fatty acids (mostly found in hydrogenated vegetable oils) along with a relative insufficiency of monounsaturated and omega-3 fatty acids.
One of the key factors behind this linkage is the fact that dietary fat determines cell membrane composition. High consumption of saturated and trans fats leads to reduced membrane fluidity, which in turn decreases the binding of insulin to receptors on cellular membranes, decreases insulin action, or both. Trans-fatty acids, found in margarine, shortening, and other foods that are made with partially hydrogenated vegetable oils, are particularly problematic, as they interfere with the body’s ability to use important essential fatty acids. One study estimated that substituting polyunsaturated vegetable oils for margarine would reduce the likelihood of developing type 2 by 40%.59
In contrast to the dampening of insulin sensitivity caused by trans and saturated fats, clinical studies have shown that monounsaturated fats and omega-3 oils improve insulin action.60 Adding further support are population studies showing that frequent consumption of monounsaturated fats (found in, for example, olive oil, raw or lightly roasted nuts and seeds, and nut oils) and omega-3 fatty acids (found in cold-water fish such as wild salmon, trout, anchovies, sardines, halibut, and herring, for example) protect against the development of type 2.
Nuts are particularly helpful in reducing the risk of type 2. Studies have shown that consumption of nuts is inversely associated with risk of type 2, independent of known risk factors for type 2 such as age, obesity, family history of diabetes, physical activity, smoking, and other dietary factors.61 In addition to providing beneficial monounsaturated and polyunsaturated fats that improve insulin sensitivity, nuts are also rich in fiber and magnesium and have a low glycemic index. Higher intakes of fiber, magnesium, and foods with a low glycemic index have been associated with reduced risk of type 2 in several population-based studies.
Low Intake of Antioxidant Nutrients
Cumulative free radical damage leads to cellular aging and is a major factor contributing to type 2, as well as many other chronic degenerative diseases. Several large population-based studies have shown that the higher the intake of fruit and vegetables, the better blood glucose levels are controlled and the lower the risk for type 2.62 Many factors could explain this inverse correlation. Fruits and vegetables are good sources of fiber, have a high nutrient content, and contain high levels of antioxidants. Even something as simple as regular salad consumption is associated with a reduced risk for type 2.63 Studies looking at individual antioxidants have also shown similar inverse correlations—the higher the level of vitamin C, vitamin E, or carotenes, for example, the lower the risk for type 2.64–66
Likewise, the lower the levels of antioxidants and the higher the levels of fats that have been damaged by free radicals (lipid peroxides), the greater the risk for developing type 2.67 In one study 944 men, ages 42 to 60, were followed closely for four years. None of these men had diabetes at the beginning of the study. At the end of this time, 45 men had developed diabetes. What researchers found was that a low vitamin E concentration was associated with a 390% increase in risk of type 2.68
Free Radicals and Diabetes
One of the hallmarks of type 2 is the presence of higher levels of free radicals and pro-oxidants,69 and in particular an increased production of reactive oxygen species and reactive nitrogen species.70 These are associated with high blood glucose and elevated saturated fat levels, and, as already mentioned, they are produced in abdominal fat cells. These compounds oxidize cellular components such as DNA, proteins, and cell membrane fatty acids. In addition to their ability to directly inflict damage on these structures, reactive oxygen and nitrogen species indirectly induce damage to tissues by activating a number of inflammatory compounds that ultimately lead to both insulin resistance and impaired insulin secretion.
Persistent Organic Pollutants (POPs)
These compounds include such chemicals as polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), hexachlorobenzene (HCB), organophosphates, DDE, and bisphenyl A. These compounds have been linked to development of type 2. In addition, research indicates that the body load of POPs not only is a significant predictor of type 2 but may be a more significant risk factor than obesity.71 People with the highest levels of organochlorine pesticides have a five times greater risk for metabolic syndrome.72
Unfortunately, direct measurement of POP levels is difficult and very expensive. However, a good indirect measure is blood levels of gamma-glutamyltransferase (GGTP), a common test to gauge liver function. Individuals with levels above 40 mcg/l have a 20-fold increased risk.73 Interestingly, the level of POPs is a better predictor of diabetes risk than weight.
Environmental Toxins
Environmental pollutants can increase the risk of developing type 2. Reducing chemical exposure by choosing organic food when possible, by using natural cleaners at home, and by not using chemical pesticides is a valid step to help prevent environmental toxins from negatively affecting insulin regulation in the body.
Clinical Monitoring
Knowledge and awareness are the greatest allies for people with diabetes. An individual with diabetes who makes a strong commitment to learning about his or her condition and who accepts the lead role in a carefully supervised monitoring program greatly improves the likelihood of living a long and healthy life. On the other hand, individuals who remain blissfully ignorant about their disease and who refuse to undergo regular testing or self-monitoring are far more likely to face years of unnecessary suffering and, more often than not, catastrophic health problems.
Diabetes can be viewed as a state of biochemical and hormonal anarchy that, unless properly managed and supervised, will lead to organ injury and accelerated aging. Many of the complex control systems that faithfully govern and protect the body are damaged in the diabetic. In order to regain control, a diabetic must learn how to maintain intimate awareness of blood sugar levels, risk factors for atherosclerosis (hardening of the arteries), blood pressure, body mass index, level of fitness, and other factors that determine the risk of developing diabetic complications and eroding quality of life.
Fortunately, diabetics who do pay attention to these risk factors through regular testing and a properly supervised self-monitoring program are also those who are much more likely to benefit from changes in lifestyle and diet, supplements, and, when necessary, medications.
Since its introduction, self-monitoring of blood glucose has revolutionized the management of diabetes.75,76 The publication of the landmark Diabetes Control and Complications Trial,77 which examined intensive glucose control in type 1 diabetics, and the United Kingdom Prospective Diabetes Study,78 which examined intensive glucose control in type 2 diabetics, scientifically proved that the most important factor in determining the long-term risk of serious diabetic complications in both type 1 and type 2 diabetics is blood glucose control. Diabetics who do not remain aware of their blood glucose and who do not make every effort to keep their blood sugar under tight control can expect a significant increase in their risk of serious health problems such as eye, kidney, and heart disease, as well as a number of other problems such as depression, fatigue, impotence, and chronic infections. Self-monitoring of blood glucose is important for various reasons:79
• Modifications of treatment to achieve appropriate blood glucose control
• Detection and diagnosis of hypoglycemia
• The ability to adjust care in response to shifts in daily life circumstances (e.g., food intake, exercise, stress, illness)
• Detection and treatment of severe hyperglycemia
• Increased compliance with therapy (self-monitoring helps to combat apathy and denial, which are factors in noncompliance)
• Improvement in motivation because of immediate positive and negative feedback
Type 1 Diabetes and Self-Monitoring of Blood Glucose Levels
Without a doubt, all type 1 diabetics must monitor their blood glucose frequently if they want to achieve and maintain good health. In the absence of diabetes, the pancreas monitors blood glucose continuously and adjusts its insulin output moment by moment in response to changes in blood glucose. In order to achieve blood glucose levels that are consistently as close to normal as possible, type 1 diabetics must replicate this natural function as closely as possible. This means that they need to monitor their blood glucose frequently, and they must learn to use this information to make ongoing adjustments to their insulin injections, diet, and exercise.
Intensive insulin therapy allows a diabetic to achieve near-normal levels of blood glucose while enjoying improved lifestyle flexibility. With conventional, infrequent insulin injections, the diabetic must structure meals and other aspects of lifestyle around these injections or face serious abnormalities of blood glucose. On the other hand, with intensive insulin therapy that relies on rapid-acting, short-duration insulin or the use of an insulin pump (an electronic device that provides a continuous injection of short-acting insulin with extra boosts before meals), the timing and size of doses can be adjusted to suit the events of the day.80 Even though it may involve multiple injections (usually before each meal and often at bedtime) and blood glucose measurements six times or more each day, intensive insulin therapy results in a higher quality of life and near nondiabetic blood glucose control, which is vital for long-term health.
Type 2 Diabetes and Self-Monitoring of Blood Glucose Levels
Self-monitoring of blood glucose has an important place in the management of type 2 diabetes as well. Each type 2 diabetic lies somewhere on a spectrum, with one end of the spectrum being mild glucose intolerance (accompanied by insulin resistance and higher-than-normal levels of insulin) and the other end of the spectrum being more advanced forms (with more severe insulin resistance, the potential for high blood glucose and ketoacidosis, and partial or nearly complete pancreatic failure with an accompanying lack of insulin). Self-monitoring of blood glucose plays a varying role depending on the severity of the disease. Every type 2 diabetic should own a blood glucose monitor and become familiar with its use. Even those diabetics whose blood glucose is well controlled through diet, lifestyle, and supplements should measure their blood glucose regularly.
Numerous dietary factors, supplements, exercise, stress, and illness can all have a significant impact on blood glucose control. Becoming aware of how all these factors influence diabetes will help motivate type 2 diabetics to make positive changes, and monitoring will provide immediate feedback about the results of any changes.
Diabetics who have a more serious case of disease, with diminished pancreatic insulin production, may benefit from efforts to establish consistently near-normal blood glucose control using intensive insulin therapy similar to that of type 1 diabetics.81 A C-peptide blood test can provide an estimate of how much insulin type 2 diabetics are producing and is one way to help determine the appropriateness of using insulin (discussed below). If diabetics are placed on an intensive insulin therapy program, they must self-monitor their blood glucose as frequently as type 1 diabetics on intensive insulin therapy (usually before and two hours after each meal).
One way to achieve optimal blood glucose in these individuals is to give a daily injection of long-acting insulin (Lantus), which provides a smooth, continual release of insulin for 24 hours, in addition to diet and medication. Diabetics on this type of program definitely need to measure blood glucose frequently.
Guidelines for Self-Monitored Blood Glucose
• Test on awakening and just before each meal. Ideal blood sugar before meals is <120 mg/dl (6.7 mmol/l).
• Test two hours after each meal. Ideal blood sugar two hours after meals is <140 mg/dl (7.7 mmol/l).
• Test at bedtime. Ideal blood sugar level at bedtime is <140 mg/dl (7.7 mmol/l).
Often it is important to know if the pancreas of a diabetic is making insulin, and if so, how much. This assessment can greatly influence treatment, especially in a diabetic hoping to avoid or cease using insulin. The level of pancreatic insulin production can also partially determine the types of medication or natural health products that are more likely to be effective. Once it is known how well the pancreas is producing insulin, the focus may be shifted toward replacing deficiencies in insulin production, stimulating insulin production, preserving pancreatic function, reducing insulin resistance, or a combination of these therapeutic efforts.
One way to determine the level of insulin production is by measuring C-peptide. The pancreas manufactures a large protein called proinsulin first. A piece of this protein (C-peptide) is then snipped off by enzymes, and both C-peptide and the remaining insulin are released into the bloodstream. Injected insulin has no C-peptide. Measuring C-peptide can be helpful in both type 1 and type 2, but generally is more so for type 2. In type 1, measuring C-peptide can uncover how much insulin the pancreas is making, which may help indicate how much of the pancreas is still active. In type 2, high C-peptide levels confirm that the patient is very insulin resistant. Low C-peptide levels may indicate that enough damage has occurred to the pancreas that the patient needs to be put on some manner of insulin therapy.
Interpreting C-peptide levels |
|
C-PEPTIDE RESULTS |
INTERPRETATION |
Normal |
Insulin production is at normal levels |
Less than normal |
A. Newly diagnosed type 1 diabetic |
|
B. Long-term type 2 diabetic |
Greater than normal |
A. Newly diagnosed type 2 diabetic |
|
B. Insulinoma (a benign tumor of the pancreas); rare |
Undetectable |
A. Long-term 1 diabetic |
|
B. Post–surgical removal of pancreas; rare |
In any circumstance when the body must derive its primary source of energy from fat, ketones are produced as a by-product. If the level of ketone production is high enough, ketones appear in the urine. In general this is associated only with type 1 diabetic patients, as the vast majority of type 2 patients do not develop ketoacidosis. Ketoacidosis can occur if an insulin-dependent diabetic forgets to take insulin or deliberately avoids taking it. It can also occur when a diabetic becomes ill or injured or is given high doses of cortisone-type drugs. All of these phenomena may result in a severe loss of insulin effectiveness, with the cells unable to take up and use glucose. In such circumstances, blood glucose rises to extraordinarily high levels, large amounts of fat are used by cells that cannot take in glucose, and the blood becomes polluted with toxic levels of acidic ketones. Severe dehydration occurs rapidly because the kidneys are unable to conserve water in the presence of such high levels of blood glucose. This dangerous state is referred to as diabetic ketoacidosis, and it must be treated as a medical emergency, usually necessitating intravenous insulin, high amounts of IV fluids, and careful monitoring, usually in an intensive care unit. Ignoring ketoacidosis can rapidly lead to death.
Because of this, testing the urine for ketones (or, even better, testing the blood for ketones, by use of a special glucometer that has this extra testing capability) remains an important part of monitoring for type 1 patients with no pancreatic function left at all. The presence of urine or blood ketones, accompanied by high blood sugar readings, can help determine how far along the ketoacidosis has developed and what type of medical attention is required. For this reason, all type 1 diabetics should frequently test their urine for ketones during acute illness or severe stress, especially when blood glucose levels are consistently elevated (>300 mg/dl [16.7 mmol/l]), regularly during pregnancy, or when symptoms suggestive of ketoacidosis, such as nausea, vomiting, or abdominal pain, are present.
Although diabetics must take charge of their condition, controlling diet, managing lifestyle, and monitoring blood glucose, they are rarely successful without professional guidance. Numerous studies have determined that physician monitoring of diabetics through laboratory measurements of blood glucose control can have a major impact on a diabetic’s long-term health.
One of the key determinants of blood glucose control is the A1C test, discussed earlier. Unlike direct measurements of blood glucose, which detect the level of blood glucose at the moment of testing, the A1C test reflects the average level of blood glucose over the preceding three months. Studies have shown that the level of A1C closely correlates with the level of risk for diabetic complications. However, an A1C test has a certain potential level of inaccuracy in it. A patient may have steady, well-regulated blood sugars, producing an A1C of 6%, or may have a combination of very high blood sugars and hypoglycemic events, which can also produce the same A1C of 6%.4 Big changes in blood sugar, even if the average is good, are very damaging. Having an A1C of 5.5% or less is ideal, as it reflects that blood glucose levels have averaged in a range that is essentially nondiabetic and no damage is occurring in the body as a result of elevated glucose. All diabetics, type 1 and type 2, should have their A1C level measured every three to four months, depending on the stability of their condition.
Although it is clear that optimal blood glucose control is critical to the health of diabetics, several other risk factors need to be carefully monitored as well. Early detection of problems through a program of regular screening and monitoring will allow for preventive efforts and treatments to be put in place before serious complications or catastrophic problems occur.
Complications of Diabetes
While acute complications of diabetes are relatively rare with proper medical care, long-term complications are extremely common. Elevated blood glucose levels cause inflammatory and oxidative damage that unfortunately leads to chronic disease progression and the development of numerous complications.
The acute complications of diabetes may represent a medical emergency and a possible life-or-death situation. Any diabetic experiencing any symptom even remotely suggestive of an acute complication of diabetes should obtain medical care immediately. The major acute complications of diabetes are hypoglycemia and diabetic ketoacidosis.
Hypoglycemia
Hypoglycemia is usually seen in type 1. Hypoglycemia is the result of injection of too much insulin, decreased or delayed food ingestion, use of alcohol or drugs that interfere with the liver’s production of glucose, or an unusual increase in exercise. Severe hypoglycemia can also occur unpredictably in patients with brittle type 1 or in any diabetic on insulin or sulfonylurea drugs who neglects the need for proper monitoring of blood glucose. Daytime hypoglycemic episodes are usually recognized by their symptoms: sweating, nervousness, tremor, and hunger. Nighttime hypoglycemia may be without symptoms or may be manifested as night sweats, unpleasant dreams, or early-morning headache.
|
QUARTERLY |
ANNUALLY |
Review management plan: |
|
|
Blood glucose self-monitoring results |
X |
|
Medication/insulin regimen |
X |
|
Nutritional plan |
X |
|
Exercise program |
X |
|
Psychosocial support |
x |
|
Physical examination: |
|
|
Weight |
X |
|
Height (for child/adolescent) |
X |
|
Sexual maturation (for child/adolescent) |
X |
|
Skin, including insulin injection sites |
X |
|
Feet: pulses, capillary refill, color, sensation, nails, skin, ulcers |
X |
|
Neurological: reflexes, proprioception, vibratory sensation, touch (distal temperature sensation, distal pinprick or pressure sensation, standardized monofilament) |
|
X |
Regular retinal examination |
X |
|
Dilated retinal examination |
|
X |
Electrocardiogram |
|
X |
Laboratory tests: |
|
|
Fasting or random plasma glucose (target range: 80–120 mg/dl before meals) |
X |
|
Glycosylated hemoglobin (A1C) (target range: <7% in adults, <7.5% in children) |
X |
|
Urinalysis |
X |
|
Glucose, ketones, microalbumin, protein, sediment |
|
|
Complete cardiovascular profile |
|
X |
Cholesterol (target: <200 mg/dl) |
|
|
Triglycerides (target: <200 mg/dl) |
|
|
LDL (target: <130 mg/dl) |
|
|
HDL (target: <35 mg/dl) |
|
|
Lipoprotein (a) (target: <40 mg/dl) |
|
|
C-reactive protein (target: <1.69 mg/l) |
|
|
Fibrinogen (target: <400 mg/l) |
|
|
Homocysteine (target: <16 mmol/l) |
|
|
Ferritin (target: 60–200 mcg/l) |
|
|
Lipid peroxides (target: <normal; note: will vary depending upon the laboratory |
|
|
Serum creatinine (in adults; in children only if protein is present in urine) |
x |
|
Treatment of hypoglycemia follows the “15-15 rule,” whereby patients are told to have 15 g carbohydrates, then recheck their glucose in 15 minutes. If the glucose is still less than 80 mg/dl, ingest another 15 g and check glucose in an hour. When glucose sinks below 55 mg/dl, it is likely that a diabetic will need help from another person; and when glucose is under 20 mg/dl, a seizure is highly likely and is a medical emergency. Any hypoglycemic event should be recorded and reported to a physician.
Diabetic Ketoacidosis
Diabetic ketoacidosis (DKA) is most commonly seen in newly diagnosed type 1 diabetics; in type 1 diabetics with infections (including dental abscesses); in cases of deliberate or accidental omission of insulin; in cases of trauma, heart attack, or stroke; during surgery; and in other miscellaneous situations. The lack of insulin leads to extremely high blood glucose and a buildup of acidic ketone molecules in the body as a result of the burning of fat stores for energy. If progressive, ketoacidosis can result in numerous metabolic problems and even coma or death. Since ketoacidosis is a medical emergency, prompt recognition is imperative. Patients should be taught to check for ketones in their urine or blood when their glucose is above 250 mg/dl for more than a few hours; if they are feverish or have an infection; if they do not feel well; and regularly during pregnancy, as ketoacidosis is usually fatal to the fetus. The symptoms of diabetic ketoacidosis include fruity breath, disorientation, abdominal tenderness, excessive urination and thirst, hyperventilation, and signs of dehydration. Treatment of DKA depends on the severity of the situation and where the glucose level is—it can require insulin injection, insulin injection plus food, or a visit to the emergency room.
Much more common than the acute complications of diabetes are certain long-term complications. The main four areas of the body affected most by diabetic complications are the eyes, the kidneys, the nerves, and the lining of blood vessels and organs. These four areas of the body do not require insulin to absorb glucose into their cells, in contrast to the liver, muscle and fat cells, so when glucose levels are elevated in uncontrolled diabetes, glucose floods those cells and causes significant damage.
Atherosclerosis
Atherosclerosis and other vascular lesions are the underlying factors in the development of many chronic complications of diabetes. Individuals with diabetes have a four- to sixfold higher risk of dying prematurely of heart disease or stroke than a nondiabetic individual, and 55% of deaths in diabetes patients are caused by cardiovascular disease.
Retinopathy
Diabetic retinopathy is the leading cause of blindness in the United States for people between the ages of 20 and 64. In diabetic retinopathy, the retina is damaged by microscopic hemorrhages, scarring, and the attachment of glucose molecules (glycosylation) to structural proteins in the retina. Studies have shown that 20 years after the diagnosis of diabetes, 80% of type 1 and 20% of type 2 diabetics have significant retinopathy. Diabetics are also prone to cataracts.
Neuropathy
Neuropathy usually refers to the loss of peripheral nerve function and is characterized by tingling sensations, numbness, loss of function, and a characteristic burning pain. It commonly occurs noticeably in the feet, but if it progresses it can also spread elsewhere in the body, such as in the autonomic nerves of the gastrointestinal tract, causing diarrhea, constipation, and disturbances in stomach emptying. If it progresses, then impaired heart function, alternating bouts of diarrhea and constipation, and inability to empty the bladder may occur. Approximately 60% of all people with diabetes eventually develop neuropathy. The main problem of peripheral neuropathy is that lack of feeling in the feet can lead to sores and lesions that patients do not notice and that then ulcerate, leading to gangrene and the need for amputation.
Kidney Disease (Nephropathy)
Nephropathy due to diabetes accounts for 40% of the cases of severe kidney disease and is the most common reason for end-stage kidney disease, dialysis, and kidney transplant in patients in America. ACE inhibitors or angiotensin receptor blockers are part of standard care, as they have been shown to protect the kidneys from diabetic damage.
Poor Wound Healing and Foot Ulcers
Poor wound healing is common in diabetes for several reasons, such as functional nutrient deficiencies and microvascular changes that lead to poor circulation. For these reasons and others (peripheral neuropathy, immune system dysfunction leading to chronic infections), foot ulcers are common in individuals with diabetes. Except for trauma, diabetic wounds are the leading cause of limb amputations in the United States. More than 50% of lower limb amputations in the United States (70,000 each year) are due to diabetic foot ulcers.
Immune System Dysfunction
Immune system dysfunction often begins to occur long before a diagnosis of diabetes is made. In fact, in many cases a recurrent vaginal or skin yeast infection is the clue that leads to the detection of diabetes. Immune system problems are made worse by poor glucose control, and this puts the diabetic at risk for serious infections or complications of simple infections. Susceptibility to chronic, hidden infections in the oral cavity, blood, or respiratory tract may be a primary reason for increased risk of cardiovascular disease in diabetics.
Depression and Cognitive Difficulties
Depression and cognitive difficulties are common in diabetics. In fact, depression may begin to occur decades before the onset of type 2 diabetes, when the individual first develops insulin insensitivity. The brain has a greater need for glucose than any other organ, and it appears that the brain cells may suffer from some degree of glucose deprivation when insulin resistance occurs.82 Depression is also much more common in overweight and obese individuals, probably owing to a combined effect from insulin resistance and diminished self-esteem. Cognitive changes begin to occur after the first severe hypoglycemic episode in diabetics. Hypoglycemia is profoundly stressful to the brain, and if severe hypoglycemia occurs many times, significant cognitive impairment is possible. Uncontrolled diabetes is also associated with an increased risk of developing Alzheimer’s disease.
The major factors contributing to the long-term complications of diabetes are listed here, followed by a brief description of each, along with coping measures:
• Poor glucose control
• Glycosylation of proteins (by means of an action similar to glycosylation of hemoglobin)
• Intracellular accumulation of sorbitol
• Increased oxidative damage
• Nutrient deficiency
• Elevated homocysteine levels
• Hypertension
• Changes in blood vessel linings
Poor Glucose Control
A large body of evidence indicates that good blood glucose control significantly reduces the development of complications. Maintaining hemoglobin A1C levels near normal (less than 7%) can dramatically help reduce the risk of eye problems (up to 76%), nerve damage (up to 60%), and kidney disease (up to 56%).
As described previously, glycosylation refers to the binding of glucose to proteins. The poorer the glucose control, the greater the binding of glucose molecules to proteins. This binding leads to changes in the structure and function of the protein. Among the adverse effects of excessive glycosylation are inactivation of enzymes, inhibition of regulatory molecule binding, and formation of abnormal protein structures. For example, when glucose molecules bind to cholesterol-carrying LDL molecules, they block LDL from binding to receptors on the liver that signal the liver to cease manufacturing cholesterol. As a result, the liver “thinks” there is a shortage of cholesterol in the body and continues to produce more and release it into the blood. This is one reason diabetes is almost always associated with high cholesterol levels.
In addition to keeping blood glucose levels as close to ideal as possible, high intakes of antioxidants—especially vitamins C and E, flavonoids, and alpha-lipoic acid (discussed later)—help to reduce glycosylation.
Intracellular Accumulation of Sorbitol
Sorbitol is a sugar molecule that is formed from glucose within cells. In people without diabetes, once sorbitol is formed it is quickly broken down into fructose. This conversion to fructose is critical because the intact sorbitol molecule cannot exit the cell, and if sorbitol levels continue to increase within a cell, the cell leaks small molecules such as amino acids, inositol, glutathione, niacin, vitamin C, magnesium, and potassium to maintain osmotic balance. Because these compounds function to protect cells from damage, their loss results in increased susceptibility to damage.
Intracellular accumulation of sorbitol is a major factor in the development of most complications of diabetes, as evidenced by the fact that elevated sorbitol levels are found in high concentrations in the tissues commonly involved in the major diabetic complications: the lens of the eye, nerve cells, kidney cells, and the cells that line blood vessels.
In addition to controlling blood glucose levels, vitamin C and flavonoids such as quercetin, grape seed extract, and bilberry extract can help lower intracellular sorbitol levels. (Sorbitol accumulation, by the way, has nothing to do with eating foods that contain sorbitol.)
Increased Oxidative Damage
Individuals with diabetes typically have elevated levels of free radicals and oxidative compounds.83 These highly reactive compounds bind to and destroy cellular compounds, cause damage all over the body, and increase insulin resistance. They also greatly increase the inflammatory process by increasing the formation of inflammatory mediators such as C-reactive protein.84 One of the critical goals in diabetes prevention and treatment is to flood the body with a high level of antioxidant compounds to counteract the negative effects of free radicals and pro-oxidants. In addition to a basic supplementation program, supplementing the diet with antioxidants such as alpha-lipoic acid and flavonoid-rich extracts is often useful.
Nutrient Deficiency
A deficiency of any one of several nutrients has been shown to contribute to several chronic complications of diabetes. Nutrient supplementation has been found in studies to help diabetic patients with glucose control, to lower blood pressure, and to protect the body from diabetic complications. In general, the risk of long-term complications of diabetes is inversely proportional to micronutrient status. Sometimes the symptoms of nutrient deficiency can mimic closely a chronic complication of diabetes. For example, vitamin B12 deficiency is characterized by numbness, pins-and-needles sensations, or a burning feeling in the hands or feet—symptoms virtually identical to those of diabetic neuropathy. Although vitamin B12 supplementation has been used with some success in treating diabetic neuropathy, it is really not clear if this success is due to correction of a B12 deficiency state or the normalization of the deranged vitamin B12 metabolism seen in diabetics.
High-potency multiple vitamin and mineral supplementation is critical to the management of diabetes. Supplying the diabetic with additional key nutrients improves blood glucose control and reduces the development of the major long-term complications of diabetes.
Elevated Homocysteine Levels
Elevated homocysteine levels are an independent risk factor for dementia, heart attack, stroke, and peripheral vascular disease. In addition, recent research has implicated elevations of homocysteine in the development of long-term complications of diabetes, especially diabetic retinopathy.85
Hypertension
Blood pressure control is essential in preventing the complications of diabetes, especially kidney disease, retinopathy, and stroke. Maintaining blood pressure in the normal range (120–140/80 mm Hg) can reduce the risk of heart disease and stroke by approximately 33 to 50% and reduce microvascular disease (eye, kidney, and nerve disease) by approximately 33% in patients with diabetes.
Changes in Blood Vessel Linings
A single layer of endothelial cells lines all blood vessels and acts as a metabolically active barrier between the components of blood and the blood vessel. These cells regulate many important aspects of blood flow, coagulation and clot formation, and the formation of key regulating compounds, including those that control blood pressure. Endothelial cells are susceptible to damage by oxidized LDL cholesterol and other free radicals—hence the importance of high dietary antioxidant intake, flavonoids, and key supplemental antioxidants such as vitamins C and E and alpha-lipoic acid. All of these factors have been shown to improve endothelial cell function and are critical in the battle against vascular disease in diabetes.86–89
Therapeutic Considerations
The optimal diet for the treatment of diabetes is virtually the same as the program we have presented in the chapter “A Health-Promoting Diet.” The difference is that there often needs to be an even stricter avoidance of foods with a high carbohydrate concentration. What determines how strict the diet needs to be with regard to the intake of carbohydrates is based on the ability to get blood glucose measurements and A1C levels under control and achieve and maintain ideal body weight. Obviously, the poorer the control, the more the carbohydrate intake must be restricted. Initially, some people with diabetes—especially those who have poorly controlled blood glucose levels—may need to avoid meals with a total glycemic load of more than 20 (see Appendix B) and space these meals at least three hours apart. Meals with a higher glycemic load can be consumed if a natural product designed to slow gastric emptying and blunt after-meal blood glucose levels is used (these compounds are discussed later).
Clinical Studies of Diet Therapy in Type 1 Diabetes
Numerous clinical studies have shown impressive results in improving blood glucose control when diets high in fiber and low in glycemic load are followed. This holds for children and pregnant women as well.90–94 We have taken the proven diet to a much higher level by also considering the impact of fats on insulin action.
Clinical Studies of Diet Therapy in Type 2 Diabetes
Diet can often be effective as the sole factor in treating and reversing type 2. Other lifestyle factors and supplements are important, but treatment of type 2 begins with diet. And, just as in type 1, there is considerable evidence from clinical trials that a low-glycemic-load diet is emerging as the most scientifically proved approach, especially when we consider not only its effect on blood glucose levels but also its ability to reduce consequences of diabetes such as high cholesterol levels, cardiovascular disease, hypertension, and other complications.95 One of the key goals is to get the total fiber intake from foods up to at least 25 to 40 g per day. High fiber intake has been shown to lower average daily glucose levels as well as insulin concentrations and total cholesterol levels.96 There is no debate that a low-glycemic-load diet shows significant advantages.97,98
Helping people with diabetes deal with their diagnosis, develop a sense of empowerment, and make important lifestyle changes is an extremely important aspect of proper medical care. Counseling is especially effective in helping adolescents with type 1 cope with their disease, leading to improvements in both mood and blood glucose control.99
Stress adversely affects blood glucose control, as higher stress levels are associated with higher blood glucose levels in both type 1 and type 2.100 There is a simple explanation for this phenomenon. Exposure to stress, whether it be physical, mental, or emotional, leads to activation of the body’s stress response and causes increases in the adrenal gland hormones adrenaline and cortisol. Among other things, these hormones cause blood glucose levels to rise and blunt the response to insulin. They also negatively affect the immune system. Because stress seems to be an inevitable part of modern living, it is critical to develop effective methods to deal with it. Some studies have shown that positive methods for dealing with stress, such as relaxation training, can improve blood glucose control, especially in individuals who are anxious or experiencing significant stress in their lives.101,102
Exercise is absolutely essential in the prevention and management of diabetes. Exercise directly improves insulin sensitivity and blood glucose control because of a combination of increased lean muscle mass and improvement in muscle cell metabolism.103 Exercise also has profound benefits for the cardiovascular system directly, as well as indirectly, through improvements in blood lipids (especially an improvement in HDL or “good” cholesterol). Exercise decreases symptoms of anxiety and depression, improves sexual functioning, and improves confidence and self-esteem. It is important to note that exercise has been shown to help people attain and sustain weight loss.104 Three types of exercise are important for people with diabetes: aerobic, strength training, and stretching.
The treatment of diabetes with natural medicine involves trying to achieve ideal blood glucose control and metabolic targets, as well as reducing the risk of the complications of diabetes by focusing on the following four areas:
1. Providing optimal nutritional status
2. Reducing after-meal elevations in blood glucose levels
3. Improving insulin function and sensitivity
4. Preventing nutritional and oxidative stress
Even though natural products can have significant effects on their own, the proper and effective treatment of diabetes requires the careful integration of diet and lifestyle changes along with any required medication and then natural medicines. Furthermore, all type 1 diabetics and many type 2 diabetics also require conventional medical treatments (oral drugs or insulin), depending on the adequacy of pancreatic insulin production (this can be determined by the C-peptide level) and the response of the diabetic to dietary and lifestyle measures. The most important factor determining whether or not the diabetic needs to be managed by drugs or insulin is the adequacy of blood glucose control.
Providing Optimal Nutritional Status
In addition to a nutrient-dense diet, a high-potency multiple vitamin and mineral formula is an absolute must for people with diabetes. Follow the guidelines given in the chapter “Supplementary Measures.” The individual with diabetes has such an increased need for many nutrients that supplementation is critical. Supplying the diabetic with additional key nutrients has been shown to improve blood glucose control, as well as help prevent or reduce the development of the major complications of diabetes. Taking a multivitamin and mineral supplement has also been shown to boost immune function and reduce infections in diabetics.105 Whenever a diabetic patient adds significant nutrient, fiber, or botanical medicines to his or her protocol, glucose monitoring is recommended, as oral or injectable medicines may need to be reduced.
Chromium. Chromium is vital to proper blood glucose control because it functions in the body as a key constituent of what is known as glucose tolerance factor—a molecule that facilitates the action of insulin. As a result, chromium works closely with insulin in assisting the uptake of glucose into cells. Without chromium, insulin’s action is blocked and glucose levels are elevated. Evidence indicates that marginal chromium status is quite common in the United States. A chromium deficiency may be an underlying contributing factor in the tremendous number of Americans who have diabetes and hypoglycemia and are obese.
More than 20 clinical studies have focused on chromium supplementation in diabetes. In some of these studies involving type 2 diabetics, supplementing the diet with chromium has been shown to decrease fasting glucose levels, improve glucose tolerance, lower insulin levels, and decrease total cholesterol and triglyceride levels while increasing HDL cholesterol levels. Although there are also studies that have not shown chromium to exert much effect in improving glucose tolerance in diabetes, there is no argument that chromium is an important mineral in blood glucose metabolism. At this time, however, it appears, not unexpectedly, that chromium supplementation is likely to produce meaningful improvements in glycemic control only in people who are deficient in this essential trace element.106
Although there is no recommended dietary intake (RDI) for chromium, it appears that at least 200 mg each day in the diet is required. People with diabetes need to supplement this with 400–600 mg per day. Chromium polynicotinate and chromium picolinate may offer the best results, as chromium-rich yeast failed to produce any significant benefit in recent trials.107 In contrast, several recent studies with 600 mcg chromium picolinate in combination with 2 mg biotin showed considerable benefit in helping patients with type 2 improve blood sugar control, as fasting glucose levels dropped 10 mg/dl and A1C levels dropped 0.54%.108 Improvements in blood lipids were also noted in other studies.109
Vitamin C. Because the transport of vitamin C into cells is enhanced by insulin,110 many people with diabetes suffer from a relative deficiency of vitamin C inside their cells even if they consume an adequate amount of vitamin C in their diet. As a result, the individual with diabetes needs to take extra vitamin C.
In addition to its role as an antioxidant, vitamin C is required in immune system function and the manufacture of collagen, the main protein substance of the human body. Because collagen is such an important protein for the structures that hold our body together (connective tissue, cartilage, tendons), vitamin C is vital for wound repair, healthy gums, and prevention of easy bruising. A chronic, latent vitamin C deficiency leads to a number of problems for the diabetic, including an increased capillary permeability, poor wound healing, elevations in cholesterol levels, and a depressed immune system. Vitamin C supplementation has been shown to exert a mild effect in improving glucose control, as evidenced by a slightly lower A1C in the vitamin C group (8.5%) compared with a placebo (9.3%) in one double-blind study.111 Probably more important than any significant effect on improving blood glucose control is the fact that vitamin C supplementation has been shown to reduce the formation of compounds linked to the development of diabetic complications.
In one double-blind study of vitamin C supplementation in type 2, 30 patients who were 45 to 70 years old and had not only type 2 but also hypertension were randomly assigned to take either 500 mg ascorbic acid or a placebo for four weeks. Vitamin C supplementation decreased systolic blood pressure from 142.1 to 132.3 mm Hg and diastolic pressure from 83.9 to 79.5. Additional analytic methods designed to measure vascular resistance also demonstrated significant improvements in arterial flexibility. These results indicate that vitamin C supplementation is effective in improving the elasticity and function of blood vessels in patients with type 2.112
Vitamin C can also prevent sorbitol accumulation (see above). In one study of young adults with type 1, the baseline measurement of sorbitol in RBCs was nearly double in these patients despite adequate dietary intakes of vitamin C. Vitamin C supplementation at a dosage of either 100 mg or 600 mg normalized RBC sorbitol within 30 days. This correction of sorbitol accumulation was independent of changes in diabetic control as monitored by fasting glucose or hemoglobin A1C. In fact, overall diabetic control during the study was moderate to poor, indicating that vitamin C’s effect was not dependent on glucose concentration. Vitamin C inhibits the enzyme aldose reductase, which converts glucose to sorbitol.113
Although vitamin C supplementation is necessary, patients should not rely exclusively on it to meet all of their vitamin C requirements. Foods rich in vitamin C are also good sources of compounds such as flavonoids and carotenes, which work to enhance the effects of vitamin C, as well as exert favorable effects of their own.
Vitamin E. Vitamin E functions primarily as an antioxidant in protecting against damage to the cell membranes. Without vitamin E, the cells of the body would be quite susceptible to damage. Nerve cells are particularly vulnerable. Diabetics appear to have an increased requirement for vitamin E. Vitamin E not only improves insulin action but when taken at dosages ranging from 400 to 800 IU exerts a number of beneficial effects that may aid in preventing the long-term complications of diabetes:
• Prevents free radical damage to LDL cholesterol and the vascular lining114–116
• Improves the functioning of blood vessels and cells that line the blood vessels117,118
• Increases the concentration of magnesium within cells119,120
• Decreases the level of C-reactive protein and other inflammatory compounds121,122
• Increases the level of glutathione—an important intracellular antioxidant—within cells123
• Improves the rate of conduction of the electrical impulse through the nervous system124
• Improves blood flow to the eye and improves diabetic retinopathy
• Improves kidney function and normalizes creatinine clearance—an indicator of kidney function—in diabetics with mild elevations125
Vitamin E supplementation may be particularly helpful for patients with a specific genetic marker—the haptoglobin (Hp) 2-2 genotype—associated with an increased risk for atherosclerosis. In a large study of more than 1,400 diabetics with the Hp 2-2 genotype, those given vitamin E (400 IU per day) for 18 months showed a 50% decrease in the rate of heart attacks, stroke, and death from cardiovascular factors.126
It should be noted that in one study in patients with type 2, treatment with either 500 mg alpha-tocopherol or mixed tocopherols significantly increased systolic blood pressure (approximately 6–7 mm Hg), vs. a placebo, indicating that some patients may have a hypertensive reaction.127 Blood pressure should be monitored in patients taking higher dosages of vitamin E to rule out this negative effect. We suspect this effect was due to the heart’s becoming stronger in response to the vitamin E supplementation.
Niacin and Niacinamide. Enzymes containing niacin (vitamin B3) play an important role in energy production; fat, cholesterol, and carbohydrate metabolism; and the manufacture of many body compounds, including sex and adrenal hormones. Niacin, like chromium, is an essential component of glucose tolerance factor, and therefore is a key nutrient for hypoglycemia and diabetes.
In addition to offering possible benefits in type 1, niacinamide may also help in type 2. Eighteen normal-weight patients with type 2 diabetes who had failed to respond to oral diabetes drugs were randomly assigned to one of three treatments for six months: (1) insulin plus niacinamide (500 mg three times per day); (2) insulin plus a placebo; or (3) an oral diabetes drug plus niacinamide (500 mg three times per day). The indicators assessed included C-peptide, A1C, and fasting and mean daily blood glucose levels. With detailed analysis, niacinamide administration was the only significant factor accountable for the improvement of C-peptide release. The data indicated that niacinamide improved C-peptide release and blood glucose control in type 2 diabetic patients who had previously failed to respond to oral diabetes drugs alone.128
Vitamin B6. Vitamin B6 supplementation appears to offer significant protection against the development of diabetic neuropathy.129 Diabetics with neuropathy have been shown to be deficient in vitamin B6 and to benefit from supplementation.130 The neuropathy of a vitamin B6 deficiency is indistinguishable from diabetic neuropathy. Individuals who have long-standing diabetes or who are developing signs of peripheral nerve abnormalities should definitely supplement their diets with vitamin B6. Vitamin B6 is also important in preventing other diabetic complications.
Vitamin B6 supplementation can be a safe and effective treatment for gestational diabetes (diabetes caused by pregnancy). One study of 14 women with gestational diabetes given 100 mg vitamin B6 per day for two weeks resulted in eliminating the diagnosis in 12 of the 14 women.131
Magnesium. Like chromium, magnesium is involved in glucose metabolism. Considerable evidence indicates that diabetics should take supplemental magnesium, the reasons being that more than half of all people with diabetes show evidence of magnesium deficiency and magnesium may prevent some of the complications of diabetes such as retinopathy and heart disease. Magnesium levels are usually low in diabetics and lowest in those with diabetic complications such as retinopathy and neuropathy. Clinical studies have shown that magnesium supplementation (usually 400 to 500 mg per day) improves insulin response and action, glucose tolerance, and the fluidity of the RBC membrane in patients with diabetes.132,133
The RDI for magnesium is 420 mg per day for adult males and 320 mg per day for adult females. Diabetics may need twice this amount because they tend to lose excessive magnesium through their kidneys.134 Most of the magnesium should be derived from the diet. The average intake of magnesium by healthy U.S. adults ranges from 143 to 266 mg per day. This is obviously far below the RDI. Food choices are the main reason. Although magnesium occurs abundantly in whole foods, food processing refines out a large portion of a food’s magnesium. The best dietary sources of magnesium are tofu, seeds, nuts, and green leafy vegetables. Fish, meat, milk, and the most commonly eaten fruits are low in magnesium. Most Americans consume a low-magnesium diet because their diet is high in refined foods, meat, and dairy products.
In addition to eating a diet rich in magnesium, diabetics should supplement their diet with 300 to 500 mg magnesium daily. For best results, highly absorbable sources of magnesium such as magnesium aspartate or citrate should be taken. Diabetics should also be sure to get at least 25 mg vitamin B6 per day, as the level of vitamin B6 inside the cells of the body appears to be intricately linked to the magnesium content of the cell. In other words, without vitamin B6 (as well as vitamin E), magnesium will not get inside the cell and will therefore be useless.
Zinc. Zinc functions in more enzymatic reactions than any other mineral, as it is a cofactor in more than 200 different enzymes. Although severe zinc deficiency is rare in developed countries, many individuals in the United States have marginal zinc deficiency. This is particularly true of the elderly population, as well as of people with diabetes. Low levels of zinc in the body are associated with increased susceptibility to infection, poor wound healing, a decreased sense of taste or smell, or skin disorders. It has also been suggested that zinc deficiency, like chromium deficiency, plays a role in the development of diabetes.135
Zinc is involved in virtually all aspects of insulin metabolism: synthesis, secretion, and utilization. Zinc also has a protective effect against beta cell destruction and has well-known antiviral effects. Diabetics typically excrete too much zinc in the urine and therefore require supplementation. Diabetics should take at least 30 mg zinc per day. Zinc is also found in good amounts in nuts and seeds.
Manganese. Manganese functions in many enzyme systems, including those involved in blood glucose control, energy metabolism, and thyroid hormone function. Manganese also functions in the antioxidant enzyme superoxide dismutase (SOD). In guinea pigs, a deficiency of manganese results in diabetes and an increase in the number of offspring that develop pancreatic abnormalities or have no pancreas at all. Diabetics have been shown to have only one-half the manganese of normal individuals. A good daily dose of manganese for a diabetic is 3 to 5 mg.
Biotin. Biotin is a member of the B vitamin family and functions in the manufacture and utilization of carbohydrates, fats, and amino acids. Without biotin, sugar metabolism is severely impaired. Biotin supplementation has been shown to enhance insulin sensitivity and increase the activity of glucokinase, the enzyme responsible for the first step in the utilization of glucose by the liver. Glucokinase concentrations in diabetics are low. Evidently, supplementing the diet with high doses of biotin improves glucokinase activity and glucose metabolism in diabetics. In one study, 16 mg biotin per day resulted in significant lowering of fasting blood glucose levels and improvements in blood glucose control in type 1 diabetics. In another study, involving type 2 diabetics, similar effects were noted with 9 mg biotin per day.136 Biotin therapy has also been shown to be quite helpful in the treatment of diabetic neuropathy.137
Omega-3 Fatty Acids from Fish Oil. Omega-3 fatty acids are vital supplements for diabetic patients to take. They offer significant protection against heart disease in diabetes, helping to lower lipids and blood pressure. They are anti-inflammatory and promote insulin sensitivity. Omega-3 oils are usually nearly completely lacking in the basic diet of a diabetic patient. Foods that contain omega-3s include oily fishes such as wild salmon, anchovies, sardines, herring, trout, and mackerel; walnuts; grass-fed beef; wild game meat; omega-3 eggs; and ground flax, hemp, and chia seeds. Initially there were concerns that omega-3 fatty acid supplementation might adversely affect blood glucose control, but two intensive investigations, one conducted at Oxford University and the other at the Mayo Clinic, analyzed data from 18 double-blind clinical trials involving 823 participants followed for an average of 12 weeks.138,139 Both evaluations came to the same conclusions: fish oil supplementation has no adverse effect on blood sugar control, but it does appears to offer the same protection against cardiovascular disease in people with diabetes that it does in people without diabetes.140 Importantly, many studies of patients with diabetes were conducted with lower-quality fish oil products that contained significant amounts of cholesterol and lipid peroxides. As a result, in some of these studies an elevation in LDL cholesterol was noted. It is important for diabetic patients to ingest a high-quality fish oil product. The combined total EPA + DHA level should be approximately 1,000 mg per day.
Reducing After-Meal Elevations in Blood Glucose Levels
Elevations of blood glucose levels after a meal can wreak biochemical havoc in both type 1 and type 2 diabetics. In fact, an elevation in postprandial blood glucose levels is the major contributor to the development of diabetic complications, especially cardiovascular disease and diseases of the microvasculature (retinopathy, neuropathy, and nephropathy). For example, patients who have a normal fasting blood glucose measurement but an average 2-hour postprandial glucose level greater than 200 mg/dl (11 mmol/l) have a threefold increase in the incidence of diabetic retinopathy.141 Therefore, blunting the after-meal increase in blood glucose levels is an important goal.
In addition to low-glycemic-load meals, several natural products can be used to reduce postprandial blood glucose levels. The best supplements to use in this regard are fiber supplements and natural glucosidase inhibitors.
Fiber Supplements. Fiber supplements have been shown to enhance blood glucose control, decrease insulin levels, and reduce the number of calories absorbed by the body. The best fiber sources for these purposes are those that are rich in soluble fiber, such as glucomannan (from konjac root), psyllium, guar gum, defatted fenugreek seed powder or fiber, seaweed fiber (alginate and carrageenan), and pectin.
Clinical studies have repeatedly shown that after-meal blood glucose levels decrease as soluble fiber viscosity increases.142,143 This relationship has also been shown to hold true for the other physiological benefits produced by soluble fiber, including increased insulin sensitivity, diminished appetite, significant weight control, improved bowel movements, and decreased serum cholesterol.144
When taken with water before meals, these fiber sources bind to the water in the stomach and small intestine to form a gelatinous, viscous mass that not only slows down the absorption of glucose but also induces a sense of satiety (fullness) and reduces the absorption of calories.
One of the most viscous naturally occurring dietary fibers is glucomannan, a soluble fiber obtained from the root of konjac, a plant that has been used as a food and remedy for thousands of years in Asia. Highly refined glucomannan possesses the greatest viscosity of any single dietary fiber. It is three times more viscous than guar and approximately seven times more viscous than psyllium. Konjac fiber is now easily available in noodles made with konjac root.
PGX is a novel natural polysaccharide matrix composed of three natural compounds (glucomannan, alginate, and xanthan gum) that are combined in a proprietary process that leads them to coalesce to form the most viscous fiber ever discovered.145,146 When glucomannan is bonded with alginate and xanthan gum, its viscosity can be amplified three to five times. PGX reduces the glycemic index of any food or beverage by 15% to 70% and also reduces postprandial glucose levels when added to or taken with foods.147,148 In a double-blind study with an earlier version of PGX, three weeks of supplementation with meals lowered postprandial blood glucose by approximately 20% and lowered insulin secretion by approximately 40% to produce a whole-body insulin-sensitivity index improvement of nearly 50%.149 It was also shown to reduce total cholesterol (by 12.4%), LDL cholesterol (by 22.3%), the ratio of LDL to HDL (by 15%), and serum fructosamine (by 5%).149 In another study involving similar patients, postprandial blood glucose (27%), postprandial insulin levels (41%), and insulin resistance were estimated to be improved by 56%.150 Typical dosage for PGX is 2.5 to 5 g before meals. PGX is discussed further in the chapter “Obesity and Weight Management.”
Natural Glucosidase Inhibitors. Starches, complex carbohydrates, and even simple sugars (disaccharides) such as sucrose are broken down in the digestive tract into glucose by the action of certain enzymes. Among the most important enzymes are the alpha-glucosidases, found in the intestines. Because these enzymes are essential for the breakdown of starches, complex carbohydrates, maltose, and sucrose into absorbable glucose molecules, their inhibition can diminish the after-meal rise in both glucose and insulin.
Acarbose (Precose) and miglitol (Glyset) are approved drugs for treating diabetes by inhibiting alpha-glucosidase. Although clinical studies have shown them to be quite effective, they are also characterized by a high frequency of mild to moderate gastrointestinal side effects such as flatulence, diarrhea, and abdominal discomfort. Although these side effects generally diminish in frequency and intensity with time, few patients are willing to put in the necessary time to get over them.
Instead of the drug acarbose, we recommend trying extracts of either touchi or mulberry, which are natural and superior to their drug counterparts. Touchi is a fermented soybean product that has been used in China and Japan for more than 3,000 years. Touchi extract is concentrated to possess high levels of naturally occurring alpha-glucosidase inhibitors. Several clinical studies have documented its effectiveness in reducing postprandial elevations in blood glucose levels.151 Longer-term studies have also shown benefit.152,153 For example, when type 2 patients took 300 mg touchi extract before each meal for six months, there were moderate changes in fasting blood glucose and hemoglobin A1C levels. The effects were apparent after only one month of use. After six months, fasting blood glucose dropped more than 10 mg/dl in nearly 80% of the patients, and hemoglobin A1C levels fell by more than 0.5% in 60% of patients. Surprisingly, touchi extract also had a mild effect in lowering triglyceride and cholesterol levels, probably through a decrease in insulin resistance. With touchi extract—unlike the drug alpha-glucosidase inhibitors—no side effects have ever been seen and no one in the clinical trials has ever complained of the gastrointestinal side effects that are so characteristic of acarbose.
The mulberry plant (Morus indica) is probably best known as food for silkworms, but it has also been highly regarded in traditional Chinese and Japanese medicine. It has been shown to have significant hypoglycemic effects in animal studies, and it contains an effective alpha-glucosidase inhibitor, along with other compounds that appear to improve blood glucose control.154,155 Mulberry extract has been studied in type 2, and the results are excellent. In one study, researchers decided to investigate its effect on blood and RBC lipids, as well as compare its blood-glucose-lowering actions with the oral diabetes drug glyburide.156 Patients were given either dried mulberry leaves at a dose of 3 g per day or one tablet of glyburide (5 mg per day) for four weeks. Mulberry therapy significantly improved diabetic control in type 2 diabetic patients (see the table below). The results clearly show that the fasting blood glucose concentrations were significantly lowered with mulberry therapy, suggesting that it is effective in controlling diabetes. Mulberry therapy significantly reduced fasting blood glucose concentration of diabetic patients by 27% compared with glyburide, which reduced it by only 8%. Mulberry extract was also superior to glyburide in its ability to decrease hemoglobin A1C, total cholesterol, LDL, and triglycerides. It also resulted in an increase in HDL, the “good” cholesterol. Although these changes were not statistically significant, there are strong suggestions that this natural product is clearly superior to an established drug treatment for type 2 diabetes.
In addition to the beneficial effects on blood glucose levels and blood lipids, mulberry therapy was also shown to reduce the amount of lipid peroxidation in the cell membranes of RBCs, indicating a significant antioxidant effect. Additionally, mulberry therapy significantly decreased membrane cholesterol of type 2 diabetic patients.
Improving Insulin Function and Sensitivity
The first step in improving insulin function and sensitivity is achieving ideal body weight and following the dietary and lifestyle recommendations given earlier, including taking a high-potency multiple vitamin and mineral formula to ensure the body has all of the necessary essential vitamins and minerals that proper insulin sensitivity requires. If additional support is necessary to bring blood glucose levels under control, we would recommend using in isolation or in scientifically formulated combinations one or more of the following: Gymnema sylvestre extract, bitter melon, Panax quinquefolius (American ginseng) or Panax ginseng (Chinese ginseng), and fenugreek seed extract. We also recommend increasing the intake of onions and garlic.
Influence of Mulberry and Glyburide Treatments on Blood Glucose, Glycosylated Hemoglobin, and Serum Lipids of Patients with Type 2 Diabetes |
||||||
VARIABLE |
GLYBURIDE |
MULBERRY |
||||
BEFORE |
AFTER |
CHANGE (%) |
BEFORE |
AFTER |
CHANGE (%) |
|
Fasting blood glucose (mg/dl) |
154.4 |
141.8 |
–8 |
152.7 |
110.5 |
–27 |
A1C (%) |
12.5 |
12.4 |
0 |
12.5 |
11.2 |
–10 |
Cholesterol (mg/dl) |
190 |
182 |
–4 |
193.7 |
170.3 |
–12 |
LDL (mg/dl) |
102.5 |
95.5 |
–7 |
102.1 |
78.7 |
–23 |
HDL (mg/dl) |
49.8 |
51.3 |
+3 |
50.1 |
59.2 |
+18 |
Triglycerides (mg/dl) |
199.5 |
180 |
–10 |
200.4 |
168 |
–16 |
Free fatty acids (pmol/dl) |
589.8 |
580 |
–2 |
590.1 |
520 |
–12 |
Gymnema sylvestre. Gymnema is a plant from India that has long been used as a treatment for diabetes. Recent scientific investigation has upheld its effectiveness in both type 1 and type 2. Gymnema extracts have been shown to enhance glucose control in diabetic dogs and rabbits. Interestingly, in animals that have their pancreas removed, gymnema has no apparent effects, suggesting that it enhances the production or activity of insulin. There is evidence in animal studies that gymnema promotes the regeneration of insulin-producing beta cells in the pancreas. Studies with humans also seem to support the possibility of pancreas regeneration.157
An extract of the leaves of G. sylvestre given to 27 patients with type 1 on insulin therapy was shown to reduce insulin requirements and fasting blood glucose levels, as well as to improve blood glucose control.158 These results indicate that gymnema enhances the action of insulin, as these diabetics were not recently diagnosed. Clinical experience also shows that gymnema has a significant benefit in decreasing sugar cravings and enabling patients to follow a lower-carbohydrate diet.
In type 2 diabetes, gymnema extract appears to work by enhancing the action of insulin. In one study, 22 type 2 diabetics were given gymnema extract along with their oral diabetes drugs.159 All patients demonstrated improved blood glucose control; 21 of the 22 were able to reduce their drug dosage considerably, and 5 were able to discontinue their medication and maintain blood glucose control with the gymnema extract alone.
The dosage for gymnema extract (standardized to contain 24% gymnemic acid) can range between 200 mg twice a day and 2,400 mg per day. No side effects have been reported from gymnema extract.
Bitter Melon. In addition to being eaten as a vegetable in Asia, unripe bitter melon (Momordica charantia) has been used extensively in folk medicine as a remedy for diabetes. The blood-glucose-lowering action of the fresh juice or extract of the unripe fruit has been clearly established in modern scientific studies in both type 1 and type 2.
Bitter melon contains several compounds with confirmed blood-glucose-lowering properties. Charantin, extracted by alcohol, is a hypoglycemic agent composed of mixed steroids that is more potent than the oral hypoglycemic drug tolbutamide. Bitter melon also contains an insulin-like polypeptide, polypeptide-P, which lowers blood glucose levels when injected like insulin into type 1 diabetics. Because it appears to have fewer side effects than insulin, it has been suggested as a replacement for insulin in some patients, although the likelihood that this application will ever be developed is extremely remote. Fortunately, taking as little as 2 fl oz of the juice has shown good results in clinical trials.160,161
Unripe bitter melon is available primarily at Asian grocery stores. Health food stores may have bitter melon extracts, but the fresh juice is probably the best to use, as this was what was used in the studies. Bitter melon juice is difficult to make palatable. As its name implies, it is quite bitter, so we recommend that patients hold the nose and take a 2-fl-oz shot of the juice. The dosage of other forms should approximate this dose.
American Ginseng. Research conducted at the University of Toronto’s Risk Factor Modification Center has uncovered important properties of some ancient natural medicines. In a study at the center, 3 g whole powdered American ginseng (Panax quinquefolius) root taken before each meal reduced postprandial blood glucose significantly in type 2 diabetics.162–166 American ginseng is now considered by authorities to be the herbal therapy with the strongest evidence of efficacy in type 2.167
Panax ginseng (Chinese ginseng) can also be helpful. In a double-blind, controlled study, 36 non-insulin-dependent diabetic patients were treated for eight weeks with ginseng extract at 100 or 200 mg or with a placebo. Ginseng elevated mood, improved both physical and mental performance, and reduced fasting blood glucose and body weight. The 200-mg dose improved A1C levels and physical activity.168
Fenugreek. Fenugreek seeds have demonstrated significant antidiabetic effects in experimental and clinical studies. The active principles are the special soluble fiber of fenugreek, along with the alkaloid trigonelline and 4-hydroxyisoleucine. Fenugreek appears to be helpful in both type 1 and type 2 diabetes. Defatted fenugreek seed powder given to type 1 diabetics twice per day at a 50-g dose resulted in a significant reduction in fasting blood glucose and improved glucose tolerance test results.169 A 54% reduction in two-hour urinary glucose excretion and significant reductions in LDL and VLDL cholesterol and triglyceride values also occurred. In type 2 diabetics, the addition of 15 g powdered fenugreek seed soaked in water significantly reduced postprandial glucose levels during the meal tolerance test.170 However, that is a very large dose and impractical for daily supplementation. In another study, however, 25 patients with type 2 were randomly assigned to receive 1 g per day of fenugreek seed extract or placebo capsules for 2 months.171 Complex analysis of the data produced an interesting finding. The group taking the fenugreek seed extract had improved blood glucose measurements (e.g., fasting blood glucose levels dropped from 148.3 to 119.9 mg/dl), but there was a significant decrease in insulin output. This finding indicates that there was a significant improvement in insulin sensitivity. This effect is most likely due to the 4-hydroxyleucine.
Onions and Garlic. Onions (Allium cepa) and garlic (Allium sativum) appear to have significant blood-glucose-lowering action. The active principles are believed to be the sulfur-containing compounds allyl propyl disulfide (APDS) and diallyl disulphide oxide (allicin), respectively, although other constituents such as flavonoids may play a role as well.
Although garlic generally has more potent effects, onions can be given at higher dosages and the active compounds appear to be more stable than allicin. Graded doses of onion extracts (1 ml extract = 1 g whole onion) at levels sometimes found in the diet (i.e., 1 to 7 oz onion) reduced blood glucose levels during an oral glucose tolerance test in a dose-dependent manner. The effects are similar with both raw and boiled onion extracts, indicating that the active components are probably stable.172
Garlic has a wide range of additional well-documented effects useful for the diabetic, including helping to improve blood glucose control, lower cholesterol and blood pressure, and inhibit some of the factors associated with increased risk for vascular complications of diabetes such as increased fibrinogen levels.
Preventing Nutritional and Oxidative Stress
Diabetes is characterized by increased nutritional and oxidative stress. Individuals with diabetes typically have elevated levels of free radicals and oxidative compounds. These highly reactive compounds bind to and destroy cellular compounds. They also greatly increase the inflammatory process by increasing the formation of inflammatory mediators such as C-reactive protein.
One of the critical goals in nutritionally supporting individuals with diabetes is to flood the body with a high level of antioxidant compounds to counteract the negative effects of free radicals and pro-oxidants. The implementation of this goal is achieved by using the recommendations given earlier, along with taking a flavonoid-rich extract and alpha-lipoic acid.
Flavonoids. Recent research suggests that flavonoids may be useful in treating diabetes, as well as in preventing long-term complications. Flavonoids such as quercetin promote insulin secretion and are potent inhibitors of glycosylation and sorbitol accumulation, while flavonoid-rich extracts such as bilberry and hawthorn have been shown to be helpful in diabetic retinopathy and microvascular abnormalities.173
Flavonoids for the Treatment of Diabetes and Diabetic Complications |
||
EXTRACT |
DAILY DOSE |
INDICATION |
Bilberry extract (25% anthocyanidins) |
160–320 mg |
Best choice in diabetic retinopathy or cataracts. |
Ginkgo biloba extract (24% ginkgo flavonglycosides) |
120–240 mg |
Best choice for most people older than 50. Protects brain and vascular lining. Very important in improving blood flow to the extremities (useful for neuropathy and foot ulcers). |
Grape seed extract or pine bark extract (>95% procyanidolic oligomers) |
150–300 mg |
Systemic antioxidant; best choice for most people younger than 50, especially if retinopathy, hypertension, easy bruising, and poor wound healing exist. |
|
|
Also specific for the lungs, varicose veins, and protection against cardiovascular disease. |
Green tea extract (>80% total polyphenols) |
150–300 mg |
Best choice in the early stage of type 1 diabetes or if there is a family history of cancer. |
Hawthorn extract (10% proanthocyanidins) |
450–600 mg |
Best choice in cardiovascular disease or hypertension. |
Milk thistle extract (70% silymarin) |
210–350 mg |
Best choice if there are signs of impaired liver function. |
Mixed citrus flavonoids |
1,000–2,000 mg |
Least expensive choice but may not provide same level of benefit; OK if no complications currently present. |
The beneficial effects of flavonoids in battling the complications of diabetes are numerous and include the fact that flavonoids are generally more potent and effective against a broader range of oxidants than the traditional antioxidant nutrients vitamins C and E, beta-carotene, selenium, and zinc. Other beneficial effects include increasing intracellular vitamin C levels, decreasing the leakiness and breakage of small blood vessels (preventing easy bruising), promoting wound healing, and providing immune system support. Good dietary sources of flavonoids include citrus fruits, berries, onions, parsley, legumes, green tea, and red wine.
For individuals with diabetes who are already showing signs of long-term complications, it is extremely important to take a flavonoid-rich extract. Because certain flavonoids concentrate in specific tissues, it is possible to take flavonoids that target specific body tissues. For example, because the flavonoids of bilberry (Vaccinium myrtillus) have an affinity for the eye, including the retina, bilberry is probably the best choice for a diabetic already exhibiting signs of diabetic retinopathy. Identify which flavonoid or flavonoid-rich extract is most appropriate and take it according to the recommended dosage (see the table opposite). There is tremendous overlap among the mechanisms of action and benefits of flavonoid-rich extracts; the key point here is to take the one that is most specific to your needs.
Alpha-lipoic Acid. Alpha-lipoic acid is a vitamin-like substance that is often described as “nature’s perfect antioxidant.” First of all, alpha-lipoic acid is a small molecule that is efficiently absorbed and easily crosses cell membranes. Unlike vitamin E, which is primarily fat soluble, and vitamin C, which is water soluble, alpha-lipoic acid can quench either water- or fat-soluble free radicals both inside the cell and outside in the intracellular spaces. Furthermore, alpha-lipoic acid extends the biochemical life of vitamin C and E, as well as other antioxidants such as glutathione, the most important intracellular antioxidant.
Alpha-lipoic acid is an approved drug in Germany for the treatment of diabetic neuropathy and has been successfully used there for more than 30 years. The beneficial effects of alpha-lipoic acid in diabetic neuropathy have been confirmed in several double-blind studies at a dosage of 400 to 600 mg per day.174,175 Although the primary effect of alpha-lipoic acid in improving diabetic neuropathy is thought to be the result of its antioxidant effects, it has also been shown to lead to an improvement in blood glucose metabolism, improve blood flow to peripheral nerves, and actually stimulate the regeneration of nerve fibers. Its importance in treating diabetic neuropathy cannot be overstated.
Following are additional recommendations for dealing with specific complications of diabetes. The most important method for reducing the risk of all these complications is achieving optimal blood glucose control.
Elevated Cholesterol Levels
Key natural products to lower cholesterol levels in diabetes are soluble fiber, garlic, and niacin. These agents are discussed fully in the chapter “High Cholesterol and/or Triglycerides.” Because taking niacin at higher dosages (3,000 mg or more) can impair glucose tolerance, many physicians have avoided using niacin therapy for diabetics, but newer studies with slightly lower dosages (1,000 to 2,000 mg) of niacin have not shown it to adversely affect blood glucose regulation.176 For example, during a 16-week, double-blind, placebo-controlled trial, 148 type 2 patients were randomly assigned either to a placebo or to 1,000 or 1,500 mg per day of niacin; in the niacin-treated groups there was no significant loss in glycemic control, and the favorable effects on blood lipids were still apparent.177 Other studies have actually shown hemoglobin A1C to drop, indicating improvement in glycemic control.178
The most common blood lipid abnormalities in type 2 diabetic patients are elevated triglyceride levels, decreased HDL cholesterol levels, and a preponderance of smaller, denser LDL particles—the worst type. Niacin has been shown to address all of these areas much more significantly than the statin or other lipid-lowering drugs. However, one reason that niacin may not be as popular as it should be is the side effect of skin flushing—like a prickly heat rash—that typically occurs 20 to 30 minutes after the niacin is taken and disappears after about the same amount of time. Other occasional side effects of niacin include gastric irritation, nausea, and liver damage. The liver damage risk is very pertinent. Diabetic patients who are overweight frequently have developed a fatty liver (see the chapter “Non-Alcoholic Fatty Liver Disease [NAFLD]/Non-Alcoholic Steatohepatitis [NASH]”). Fatty liver is now considered to be as damaging as the effects of alcohol dependence and hepatitis C, and can also lead to fibrosis and cirrhosis of liver tissue. Taking niacin may put extra stress on that vital organ. With overweight diabetic patients, therefore, high-dose niacin is to be used only under a physician’s recommendation. To reduce the side effect of skin flushing, use intermediate-release niacin, which is identical in dissolution pattern to the prescription niacin product Niaspan. Taking an intermediate-release product just before going to bed is recommended, as most people sleep right through any flushing reaction if one should occur. Another approach to reduce flushing is to use inositol hexaniacinate. This form of niacin has long been used in Europe to lower cholesterol levels and also to improve blood flow in intermittent claudication, a peripheral vascular disease that is quite common in diabetes.179 If inositol hexaniacinate does not work, regular niacin can be tried.
If regular niacin or inositol hexaniacinate is being used, a dose of 500 mg should be given at night, before bed, for 1 week. The dosage should be increased to 1,000 mg the next week and 1,500 mg the following week. The 1,500 mg dosage should be given for two months before checking the response; the dosage can be adjusted up or down depending on the response. Intermediate-release niacin products such Niaspan can be used at the full dosage of 1,000 to 2,000 mg at night from the beginning. Regardless of the form of niacin being used, periodic checking (minimum every three months) of cholesterol, A1C, and liver function is strongly indicated.
Retinopathy and Cataracts
Diabetic retinopathy has two forms: (1) simple retinopathy, with bursting of blood vessels, hemorrhages, and swelling; and (2) proliferative retinopathy, with newly formed vessels, scarring, more serious hemorrhage, and retinal detachment. The development of laser photocoagulation therapy is an important treatment for the more severe proliferative retinopathy but is not indicated in milder forms of retinopathy, because the risk of visual loss usually outweighs the benefits.
Extremely important in the battle against retinopathy are flavonoid-rich extracts, especially bilberry, pine bark, or grape seed extract. Flavonoids increase intracellular vitamin C levels, decrease the leakiness and breakage of capillaries, prevent easy bruising, and exert potent antioxidant effects. These effects are of particular value in dealing with the microvascular abnormalities of diabetes. Because the flavonoids in bilberry, pine bark, and grape seed extract have an affinity for the blood vessels of the eye and improve circulation to the retina, they are particularly helpful in slowing the progression of diabetic retinopathy, as evidenced by positive results in more than a dozen clinical trials.180,181
In addition to alpha-lipoic acid and the basic supplementation program, three natural medicines along with acupuncture deserve mention:
• Gamma-linolenic acid (GLA) has been shown to improve and prevent diabetic neuropathy. Diabetes is associated with a substantial disturbance in essential fatty acid metabolism. One of the key disturbances is the impairment in the process of converting linoleic acid to GLA. As a result, providing GLA in the form of borage, evening primrose, or black currant oil can bypass some of this disturbance. In the GLA Multicenter Trial, 111 patients with mild diabetic neuropathy were given either GLA at a dose of 480 mg per day or a placebo for one year. Sixteen different variables were assessed, including conduction velocities, hot and cold thresholds, sensation, tendon reflexes, and muscle strength. After one year, all 16 of these improved, 13 of them to a statistically significant degree. Treatment was more effective in patients with relatively well-controlled diabetes than in those with poorly controlled disease.
• Benfotiamine is a fat-soluble form of thiamine (vitamin B1) that is more effective in raising blood thiamine levels (up to 120–240% vs. regular thiamine). In studies of diabetics, benfotiamine decreased advanced glycosylated end-product formation, decreased sorbitol accumulation, and reduced oxidative cellular damage.182 However, results in the treatment of diabetic neuropathy and nephropathy in small clinical trials with benfotiamine alone show modest to no benefit.183,184 It is possible that benfotiamine should be combined with alpha-lipoic acid. In a small study of patients with type 1, treatment with 600 mg benfotiamine with 300 mg alpha-lipoic acid produced better results in reducing the effects of hyperglycemia than benfotiamine alone.185
• Capsaicin is the active component of cayenne pepper (Capsicum frutescens), which stimulates and then blocks the small nerve fibers that transmit the pain impulse by depleting these fibers of a transmitting substance known as substance P.186 Topically applied capsaicin has been shown to be of considerable benefit in relieving the pain of diabetic neuropathy in numerous double-blind studies. Roughly 80% of people with diabetic neuropathy experience tremendous pain relief.187 Commercial ointments containing 0.025% or 0.075% capsaicin are available over the counter. Apply the 0.075% cream twice per day to the affected area (cover the hand with plastic wrap or use disposable gloves to avoid the chance that the capsaicin will come into contact with the eyes or mucous membranes). It may take a few days for the cream to start working, and it will continue to work only with regular application.
• Acupuncture can also be helpful in improving neuropathy. The scientific investigation of acupuncture in diabetes includes both experimental and clinical studies. For example, animal experiments have shown that acupuncture can act on the pancreas to enhance insulin synthesis, increase the number of receptors on target cells, and accelerate the utilization of glucose, resulting in lowering of blood glucose.188 However, the best-documented use for acupuncture is in treating chronic painful diabetic neuropathy. In one clinical study, 77% of patients treated with acupuncture noted significant improvement in their symptoms, with 21% noting that their symptoms were completely eliminated.189 That success rate is excellent considering the long-standing nature of the condition in most of the patients and the fact that no side effects were observed.
Nephropathy
Particularly important for kidney protection in diabetics is dietary fiber. Dietary fiber (especially soluble fiber) is fermented in the colon to produce short-chain fatty acids. These by-products are the primary fuel for the cells of the colon, and if present in high amounts, they greatly increase the colon’s waste removal capabilities. It has been shown that in the presence of a high-fermentable-fiber diet, the colon turns into a “second kidney,” collecting nitrogenous wastes from the blood and disposing of them in the feces. This has been shown to greatly reduce stress on the kidneys.190
Highlighting just how important some of the basic supplement recommendations are in halting the progression of diabetic nephropathy are the results of a study of 30 type 2 patients with elevated albumin in their urine. The patients received vitamin C (1,250 mg) and vitamin E (680 IU) per day or a placebo for four weeks, followed by a three-week washout period before being switched to the other treatment.191 The results were that the vitamins were successful in reducing urinary albumin levels by an average of nearly 20%, indicating that antioxidant therapy may slow or halt the progression of kidney disease in diabetics.
If a diabetic has developed serious kidney failure, then following a low-protein, low-potassium diet is necessary; unfortunately, that does not aid good glucose control, which can then promote worse kidney functioning. The main goal is to prevent end-stage renal disease from developing in the first place.
If drugs are necessary, the angiotensin-converting enzyme (ACE) inhibitors and ACE receptor blockers offer the greatest benefits in dealing with diabetic nephropathy.192 They are now often prescribed in low doses to help prevent nephropathy, even in the absence of high blood pressure. Alternatively, a special preparation of bonito peptides has been shown to exert similar anti-ACE activity (see the chapter “High Blood Pressure” for more information) and may prevent the need for actual ACE inhibitors.
Poor Wound Healing
A deficiency of virtually any essential nutrient can lead to impaired wound healing. Key nutrients include vitamin C and zinc, both of which are often deficient in the diabetic. Taking a high-potency multiple vitamin and mineral formula should improve nutritional status and promote proper wound healing. For topical application pure (100%) aloe vera gel can be used. Aloe vera contains a number of compounds necessary for wound healing, including vitamin C, vitamin E, and zinc, and has been shown to stimulate many factors important to wound repair. Apply it to affected areas (not severe open wounds) two or three times per day. Another option is a proprietary product called Amerigel, a topical ointment featuring an oak extract (Quercus rubra) that contains quercitannic acid, catechin, ellagitannin, and proanthocyanidin, readily absorbed into damaged skin.
Foot Ulcers
Lack of blood supply, poor wound healing, and peripheral neuropathy are key factors in the development of diabetic foot ulcers. Key strategies in prevention and treatment are proper foot care (including care of nails and calluses), preferably by a podiatrist; regular examination of the feet by a physician; avoidance of injury; avoidance of tobacco in any form; and methods to improve local circulation. Proper foot care includes keeping the feet clean, dry, and warm and wearing well-fitting shoes. Tobacco use in any form constricts the peripheral blood vessels and can lead to more serious peripheral vascular disease with severe arterial blockages. Circulation can be improved by exercising regularly, avoiding sitting cross-legged or in other positions that compromise circulation, and massaging the feet lightly upward. Ginkgo biloba or grape seed extract can also be used to support optimal circulation.
QUICK REVIEW
• Diabetes is divided into two major categories: type 1 and type 2.
• Type 2 diabetics, who are not dependent upon insulin, account for 90% of all cases of diabetes.
• Although genetic factors appear important in susceptibility to diabetes, environmental factors are required to trigger diabetes.
• Obesity is a major factor in type 2 diabetes, as 90% of type 2 diabetics are obese.
• Exposure to a protein in cow’s milk (bovine albumin peptide) in infancy may trigger the autoimmune process and subsequent type 1 diabetes.
• The trace mineral chromium plays a major role in the sensitivity of cells to insulin.
• To reduce the risk of developing the complications of diabetes, it is important to control against elevations in blood sugar by careful monitoring.
• Dietary modification and dietary treatment are fundamental to the successful treatment of diabetes, whether it be type 1 or 2.
• The treatment of diabetes requires nutritional supplementation, as diabetics have a greatly increased need for many nutrients.
• Since the transport of vitamin C into cells is facilitated by insulin, many diabetics do not have enough intracellular vitamin C.
• Some newly diagnosed type 1 diabetics have experienced complete reversal of their diabetes with niacinamide supplementation.
• Vitamin B6 supplementation appears to offer significant protection against the development of diabetic nerve disease.
• Diabetics appear to have an increased requirement for vitamin E and benefit from high-dose supplementation.
• Flavonoid-rich extracts such as bilberry, grape seed, or pine bark are extremely important in protecting against the long-term complications of diabetes.
• Onions and garlic have demonstrated blood-sugar-lowering action in several studies and help reduce the risk of cardiovascular disease.
• The oral administration of bitter melon preparations has shown good results in clinical trials in patients with both type 1 and type 2 diabetes.
• Recent scientific investigation has upheld the effectiveness of Gymnema sylvestre in treating both type 1 and type 2 diabetes.
TREATMENT SUMMARY
Effective treatment of the diabetic patient requires the careful integration of wide-ranging therapies and a willingness to substantially improve diet and lifestyle. Type 2 is usually the end result of many years of chronic metabolic insult, and although it is treatable with the natural approach presented here, resolving it will take persistence.
The first step in the therapy of either type 1 or type 2 is a thorough diagnostic workup. Of particular importance is identifying any of the complications of diabetes. Diet, environment, and lifestyle need to be carefully studied to rule out any exposure to agents that may be inducing glucose intolerance. Then a diet, exercise, and supplement program that meets your personal needs must be developed. For maximum efficacy, ideal body weight must be achieved (see the chapter “Obesity and Weight Management”).
Monitoring—both by the diabetic and by a physician—is very important in diabetes. Home glucose monitoring and the HbA1C test are essential. It is important to recognize that as the natural therapies described in this chapter take effect, drug dosages must be altered, and so a good working relationship with the prescribing doctor is required. The ultimate goal is to reestablish normal blood sugar control and prevent the development of (or ameliorate) the complications of diabetes.
The optimal diet detailed in the chapter “A Health-Promoting Diet” is clearly the diet of choice. Avoid all simple, processed, and concentrated carbohydrates. A low-glycemic diet rich in high-fiber foods should be stressed, and sources of healthful fats should be ingested. Low-glycemic vegetables, including onions and garlic, are particularly useful.
The recommended supplementation program depends on the existing degree of blood glucose control, as indicated by self-monitored blood glucose and A1C levels.
• High-potency multiple vitamin and mineral formula as described in the chapter “Supplementary Measures”
• Fish oils: 1,000 mg EPA + DHA per day
• Vitamin C: 500 to 1,500 mg per day
• Vitamin E (mixed tocopherols): 100 to 200 IU per day
• Vitamin D: 4,000 to 10,000 IU per day (ideally, determine dosage according to blood levels)
• Niacinamide: 25 to 50 mg/kg body weight
• Green tea extract: Recommended dosage for children under 6 years is 50 to 150 mg; for children age 6 to 12, 100 to 200 mg; for children older than 12 and adults, 150 to 300 mg. The green tea extract should have a polyphenol content of >90% and be decaffeinated.
• A high-potency multiple vitamin and mineral formula as described in the chapter “Supplementary Measures”
• Key individual nutrients:
![]() Vitamin B6: 25 to 50 mg per day
Vitamin B6: 25 to 50 mg per day
![]() Folic acid: 800 mcg per day
Folic acid: 800 mcg per day
![]() Vitamin B12: 800 mcg per day
Vitamin B12: 800 mcg per day
![]() Vitamin C: 500 to 1,000 mg three times per day
Vitamin C: 500 to 1,000 mg three times per day
![]() Vitamin E (mixed tocopherols): 400 to 800 IU per day
Vitamin E (mixed tocopherols): 400 to 800 IU per day
![]() Selenium: 100 to 200 mcg per day
Selenium: 100 to 200 mcg per day
![]() Zinc: 30 mg per day
Zinc: 30 mg per day
![]() Vitamin D3: 4,000 to 10,000 IU per day (ideally, measure blood levels and adjust dosage accordingly)
Vitamin D3: 4,000 to 10,000 IU per day (ideally, measure blood levels and adjust dosage accordingly)
• Fish oils: 1,000 mg EPA + DHA per day
• Alpha-lipoic acid: 400 to 600 mg per day
• One of the following:
![]() Grape seed extract (>95% procyanidolic oligomers): 100 to 300 mg per day
Grape seed extract (>95% procyanidolic oligomers): 100 to 300 mg per day
![]() Pine bark extract (>95% procyanidolic oligomers): 100 to 300 mg per day
Pine bark extract (>95% procyanidolic oligomers): 100 to 300 mg per day
![]() Green tea extract (>80% polyphenol content): 150–300 mg per day
Green tea extract (>80% polyphenol content): 150–300 mg per day
• Level 1 supplements
• Gymnema sylvestre extract (24% gymnemic acid): 200 mg twice per day
• Biotin: 8 mg twice per day
• Bitter melon juice (optional): 2 to 4 fl oz per day
The recommended supplementation program depends on the degree of blood glucose control, as evidenced by self-monitored blood glucose and A1C levels.
• Same recommendations as for Level 1 for type 1 diabetes, with the addition of PGX, glucomannan, or another source of soluble fiber, 2,500 to 5,000 mg before meals
• Level 1 supplements
• One of the following insulin enhancers:
![]() Gymnema sylvestre extract (24% gymnemic acid): 200 mg twice per day
Gymnema sylvestre extract (24% gymnemic acid): 200 mg twice per day
![]() Fenugreek extract: 1 g per day
Fenugreek extract: 1 g per day
![]() Garlic: minimum 4,000 mcg of allicin per day
Garlic: minimum 4,000 mcg of allicin per day
• One of the following glucosidase inhibitors:
![]() Touchi extract: 300 mg three times per day with meals
Touchi extract: 300 mg three times per day with meals
![]() Mulberry extract: equivalent of 1,000 mg dried leaf three times per day
Mulberry extract: equivalent of 1,000 mg dried leaf three times per day
If self-monitored blood glucose levels do not improve after four weeks of following the recommendations for the current level, move to the next highest level. If you are already at Level 2, the next step is to add a prescription medication (either an oral hypoglycemic drug or insulin).
• For high cholesterol levels and other cardiovascular risk factors:
![]() Total cholesterol greater than 200 mg/dl or LDL cholesterol greater than 135 mg (100 mg if history of heart attack); HDL cholesterol below 45 mg/dl; lipoprotein (a) above 40 mg/dl; or triglycerides above 150 mg/dl
Total cholesterol greater than 200 mg/dl or LDL cholesterol greater than 135 mg (100 mg if history of heart attack); HDL cholesterol below 45 mg/dl; lipoprotein (a) above 40 mg/dl; or triglycerides above 150 mg/dl
![]() Niacin (or Niaspan or inositol hexaniacinate): 1,000–2,000 mg at night at bedtime
Niacin (or Niaspan or inositol hexaniacinate): 1,000–2,000 mg at night at bedtime
![]() Garlic: minimum of 4,000 mcg of allicin per day
Garlic: minimum of 4,000 mcg of allicin per day
• For hypertension:
![]() Garlic: minimum of 4,000 mcg of allicin per day
Garlic: minimum of 4,000 mcg of allicin per day
![]() CoQ10: 100 to 200 mg per day
CoQ10: 100 to 200 mg per day
![]() One of the following:
One of the following:
– Hawthorn extract (10% proanthocyanidins or 1.8% vitexin-4¢-rhamnoside): 100 to 250 mg three times per day
– Olive leaf extract (17% to 23% oleuropein content): 500 mg two times per day
– Hibiscus: three 240-ml servings/day or an extract providing 10–20 mg anthocyanidins per day
• For diabetic retinopathy:
![]() Bilberry extract: 160 to 320 mg per day or grape seed extract: 150 to 300 mg per day
Bilberry extract: 160 to 320 mg per day or grape seed extract: 150 to 300 mg per day
• For diabetic neuropathy:
![]() Gamma-linolenic acid from borage, evening primrose, or blackcurrant oil: 480 mg per day
Gamma-linolenic acid from borage, evening primrose, or blackcurrant oil: 480 mg per day
![]() Benfotiamine: 600 mg per day
Benfotiamine: 600 mg per day
![]() Capsaicin (0.075%) cream: apply to affected area twice per day
Capsaicin (0.075%) cream: apply to affected area twice per day
• For diabetic nephropathy:
![]() Follow recommendations for high blood pressure, above, unless kidney function falls below 40% of normal; seek a physician’s advice regarding magnesium and potassium supplements
Follow recommendations for high blood pressure, above, unless kidney function falls below 40% of normal; seek a physician’s advice regarding magnesium and potassium supplements
![]() Benfotiamine: 600 mg per day
Benfotiamine: 600 mg per day
• For poor wound healing:
![]() Aloe vera gel: Apply to affected areas twice per day.
Aloe vera gel: Apply to affected areas twice per day.
• For diabetic foot ulcers, one of the following:
![]() Gingko biloba extract: 120 to 240 mg per day
Gingko biloba extract: 120 to 240 mg per day
![]() Grape seed extract: 150 to 300 mg per day
Grape seed extract: 150 to 300 mg per day