

• Inability to conceive a child after six months of unprotected sex at least twice weekly with the same partner in the absence of female causes
• A total sperm count lower than 5 million/ml
• The presence of greater than 50% abnormal sperm
• Inability of sperm to impregnate egg, as determined by the postcoital or hamster-egg penetration tests
Infertility affects about 7.3 million couples in the United States: approximately 12% of the reproductive-age population. It is estimated that one in seven couples in the United States experiences infertility. In about 50% of the cases of infertility the issue is with the female. Current estimates suggest that about 6% of men between the ages of 15 and 50 years are infertile.1
Causes
Most male infertility is due to abnormal sperm count or quality. Although it takes only one sperm to fertilize an egg, there are nearly 200 million sperm in an average ejaculation. However, because of the natural barriers in the female reproductive tract, only about 40 sperm will ever reach the vicinity of an egg. There is a strong correlation between fertility and the number of sperm in an ejaculation.
In about 90% of the cases of low sperm count, the reason is deficient sperm production. Unfortunately, in about 9 out of 10 of those cases, the cause of the decreased sperm formation cannot be identified, and the condition is labeled idiopathic oligospermia (low sperm count) or azoospermia (a complete absence of living sperm).
Causes of Male Infertility
Deficient sperm production
Ductal obstruction
Congenital defects
Postinfectious obstruction
Cystic fibrosis
Vasectomy
Ejaculatory dysfunction
Premature ejaculation
Retrograde ejaculation
Disorders of accessory glands
Infection
Inflammation
Antisperm antibodies
Coital disorders
Defects in technique
Premature withdrawal
Erectile dysfunction
Since the overwhelming majority of men who are infertile suffer from deficient sperm production, that is the major focus of this chapter. Normal sperm are defined as having the following characteristics:
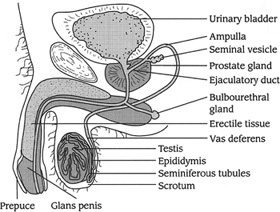
Anatomy of the Male Sexual System
• A smooth, oval-shaped head that is 5 to 6 micrometers long and 2.5 to 3.5 micrometers around (less than the size of a needle point)
• A well-defined cap (acrosome) that covers 40 to 70% of the sperm head
• No visible defect of head, midpiece, or tail
• No fluid droplets in the sperm head that are bigger than one-half the size of the sperm head
Diagnostic Considerations
Semen analysis, which assesses concentration of sperm and sperm quality, is the test most widely used to estimate fertility potential in men. Total sperm count and sperm quality have been deteriorating over the last few decades. In 1940, the average sperm count was 113 million/ml; in 1990, that value had dropped to 66 million/ml; and it is now holding steady at around 60 million/ml. Adding to this problem, the amount of semen in an ejaculation fell almost 20%, from 3.4 ml to 2.75 ml. All together, these changes mean that men are now supplying about 40% of the number of sperm per ejaculation compared with 1940 levels.
The downward trend in sperm count has led to speculation that environmental, dietary, and/or lifestyle changes in recent decades may be interfering with men’s ability to manufacture sperm. Although the theory is controversial, there is substantial supporting evidence.
Possible Causes of Falling Sperm Count
• Increased scrotal temperature
• Tight-fitting clothing and briefs
• Varicoceles (varicose veins that surround the testes)
• Environment
• Increased pollution
• Heavy metals (lead, mercury, arsenic, etc.)
• Organic solvents
• Pesticides (DDT, PCBs, DBCP, etc.)
• Diet
• Increased intake of saturated fats
• Reduced intake of fruits, vegetables, and whole grains
• Reduced intake of dietary fiber
• Increased exposure to synthetic estrogens
As sperm counts in the general population have declined, there has been a parallel reduction in the accepted line between infertile and fertile men, with the minimum sperm count for fertility dropping from 40 million/ml to the current value of 5 million/ml. One of the key reasons these values have dropped so dramatically is that researchers are learning that quality is more important than quantity. A high sperm count means nothing if the percentage of healthy sperm is not also high.
Whenever the majority of sperm are abnormally shaped or are entirely or relatively nonmotile, a man can be infertile despite having a normal sperm concentration. Conversely, a low sperm count does not always mean that a man is infertile. Numerous pregnancies have occurred involving men with very low sperm counts. For example, in studies at fertility clinics, 52% of couples in which the man’s sperm count was below 10 million/ml achieved pregnancy, and 40% of couples in which the man’s sperm count was as low as 5 million/ml were able to achieve pregnancy.1
Semen Terminology |
|
SYNDROME |
DEFINITION |
Aspermia |
An absence of semen despite male orgasm |
Azoospermia |
A complete absence of sperm in the semen |
Oligozoospermia |
Reduced number of normal motile sperm cells in the ejaculate |
Teratozoospermia |
Sperm with abnormal morphology |
Asthenozoospermia |
Reduced sperm motility |
Necrospermia |
Death of sperm |
Oligoasthenoteratozoospermia |
Low sperm count, weak motility, and abnormal morphology |
Because of these confirmed successes in men with low sperm counts, we recommend that conventional semen analysis be interpreted with caution regarding the likelihood of conception. More sophisticated functional tests should also be used, especially in screening couples for in vitro fertilization.
Causes of Temporary Low Sperm Count
• Increased scrotal temperature
• Infections (common cold, flu, etc.)
• Increased stress
• Lack of sleep
• Overuse of alcohol, tobacco, or marijuana
• Many prescription drugs
• Exposure to radiation
• Exposure to solvents, pesticides, and other toxins
Until recently, pregnancy was the only proof of the ability of sperm to achieve fertilization. Now there are several functional tests in use. The postcoital test measures the ability of the sperm to penetrate the cervical mucus after intercourse. In vitro variants of this test are also available. One of the most encouraging tests is based on the discovery that human sperm, under appropriate conditions, can penetrate hamster eggs. It has been established that fertile men exhibit a range of penetration between 10 and 100%, and that a penetration rate of less than 10% is indicative of infertility. The hamster-egg penetration test is considered to predict fertility in 66% of cases, compared with about 30% for conventional semen analysis.
Normal Sperm Count |
|
CRITERION |
VALUE |
Volume |
1.5–5.0 ml |
Density |
>20 million sperm/ml |
Motility |
>30% motile |
Normal forms |
>60% |
Another important test in the diagnosis of infertility is the detection of antisperm antibodies. When produced by the man, these antibodies usually attack the tail of the sperm, thereby impeding the sperm’s ability to move and penetrate the cervical mucus. In contrast, the antisperm antibodies produced by women are typically directed against the head of the sperm. The presence of antisperm antibodies in semen analysis is usually a sign of past or current infection in the male reproductive tract.
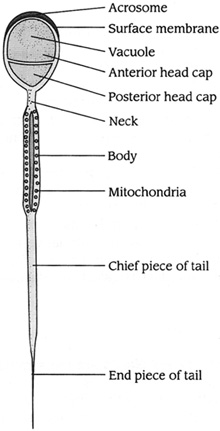
Anatomy of Sperm
Therapeutic Considerations
The fist step in improving sperm counts, morphology, and function is controlling factors that can damage or impair their formation.
The scrotal sac normally keeps the testes at a temperature of between 94 and 96°F.2 At temperatures above 96 degrees, sperm production is greatly inhibited or stopped completely. Typically, the average scrotal temperature of infertile men is significantly higher than that of fertile men. Reducing scrotal temperature in infertile men will often make them fertile. This temperature reduction is best accomplished by not wearing tight-fitting underwear or tight jeans and avoiding hot tubs.
Scrotal temperature can be raised by jogging or the use of rowing machines, simulated cross-country ski machines, or treadmills, especially if a man is wearing synthetic fabrics, tight shorts, or tight underwear. After exercising, a man should allow his testicles to hang free to allow them to recover from heat buildup.
Infertile men should wear boxer-type underwear and periodically take a cold shower or apply ice to the scrotum. They can also choose to use a testicular hypothermia device (also called a testicle cooler) to reduce scrotal temperatures. Still in a primitive stage, the testicle cooler looks like a jock strap from which long, thin tubes extend. The tubes are attached to a small fluid reservoir filled with cold water that attaches to a belt around the waist. The fluid reservoir is also a pump that circulates the water. When the water reaches the surface of the scrotum, it evaporates and keeps the scrotum cool. Because of the evaporation, the reservoir must be filled every six hours or so.
Increased scrotal temperature can be due to the presence of a varicocele. (Varicoceles are varicose veins that surround the testes.) A large varicocele can cause scrotum temperatures high enough to inhibit sperm production and motility. Surgical repair may be necessary, but scrotal cooling should be tried first.
Infections of the male genitourinary tract, including infections of the epididymis, seminal vesicles, prostate, bladder, and urethra, are thought to play a major role in many cases of infertility.3 The exact extent of the role they play is largely unknown because of the lack of suitable diagnostic criteria coupled with the asymptomatic nature of many infections. If there are no other clinical findings, antisperm antibodies or high levels of debris in a semen sample are considered good indicators of a chronic infection.
There are a large number of bacteria, viruses, and other organisms that can infect the male genitourinary system. It is beyond the scope of this chapter to discuss every type of infection, so the discussion will be limited to Chlamydia trachomatis. Chlamydia is now recognized as the most common and the most serious of the infections in the male genitourinary tract.
Chlamydia is considered a sexually transmitted disease. In women, chlamydia infection can lead to pelvic inflammatory disease and scarring of the fallopian tubes. Previous chlamydia infection accounts for a large number of cases of female-factor infertility. In men, chlamydia infection can lead to equally disabling effects. Chlamydia is the major cause of acute nonbacterial prostatitis and urethritis. Typically, the symptoms will be pain or burning sensations upon urination or ejaculation.
More serious is chlamydia infection of the epididymis and vas deferens. The resultant damage to these organs parallels tubal damage in women: serious scarring and blockage can occur. During an acute chlamydia infection, the use of antibiotics is essential. Chlamydia is sensitive to tetracyclines and erythromycin. Unfortunately, because chlamydia lives within human cells, it may be difficult to totally eradicate the organism with antibiotics alone.
While acute chlamydial infections are usually associated with severe pain, chronic infections of the urethra, seminal vesicles, or prostate can occur with few or no symptoms. It is estimated that 28 to 71% of infertile men show evidence of a chlamydial infection. Because of the possible link between chlamydia and low sperm counts, there have been several double-blind studies of the effects of antibiotics on sperm counts. These studies have shown only limited improvements in sperm count and sperm quality. However, there have been isolated cases of tremendous increases in sperm counts and sperm quality after antibiotic treatment. Antibiotics should be used only if there is reason to believe that a chronic infection is present, and both partners should take the antibiotic.
According to experts on the impact of the environment and diet on fetal development, we now live in an environment that can be viewed as “a virtual sea of estrogens.”4,5 Increased exposure to environmental estrogens and other environmental pollutants during fetal development, as well as during the reproductive years, is suggested as a major reason for the tremendous rise in disorders of development and function of the male sexual system.6,7
One can best view the relationship between estrogens and male sexual development by examining the effects of the synthetic estrogen diethylstilbestrol (DES). Between 1945 and 1971 several million women were treated with DES, which is now recognized to have led to problems in their male offspring. As well as being used in humans, DES and other synthetic estrogens were used for 20 to 30 years in the livestock industry to fatten the animals and help them grow faster. DES exposure has been associated with substantial increases in the number of men suffering from developmental problems of the reproductive tract as well as decreased semen volume and sperm counts.4 Although DES is now no longer used, poultry and livestock, especially dairy cows, are still hormonally manipulated. Cow’s milk contains substantial amounts of estrogen because of modern farming techniques. The rise in consumption of dairy products since the 1940s inversely parallels the drop in sperm counts. Avoidance of meat and milk from animals treated with hormones is important for male sexual vitality, especially in men with low sperm counts or low testosterone levels.
There are reports that estrogens have been detected in drinking water.4 The source may be estrogens from excreted synthetic estrogens (birth control pills), which are not removed by water treatment plants. These estrogens may be harmful to male sexual vitality. Purified or spring water may be a suitable option to prevent exposure (but ensure that plastic bottles do not contain BPA, which also can have estrogenic effects).
Many of the chemicals that we have contaminated our environment with during the past 50 years are weakly estrogenic. Most of these chemicals, like polychlorinated biphenyls (PCBs), dioxin, and dichlorodiphenyltrichloroethane (DDT), do not easily biodegrade and are recycled in our environment until they accumulate in our bodies. For example, even though DDT has been banned for nearly 30 years, it is still often found in the soil and root vegetables such as carrots and potatoes. These toxic chemicals are known to interfere with spermatogenesis, but their effects during sexual development may be more important.
All of the estrogenic factors previously discussed are thought to have their greatest impact during fetal development. On the basis of animal studies, these estrogens inhibit the multiplication of Sertoli cells. The number of Sertoli cells is directly proportional to the amount of sperm that can be produced, because each Sertoli cell can support only a fixed number of germ cells that will develop into sperm. Sertoli cell multiplication occurs primarily during fetal life and before puberty and is controlled by follicle-stimulating hormone (FSH). In animal studies, estrogens administered early in life inhibit FSH secretion, resulting in a reduced number of Sertoli cells and, in adult life, diminished sperm counts. The impact of environmental estrogens might persist for multiple generations. For example, vinclozolin is a fungicide used in the wine industry. Alarmingly, exposing a pregnant female rat to this fungicide just once was found to disrupt spermatogenesis in more than 90% of male offspring for at least four generations.8
Heavy Metals
Sperm are also particularly susceptible to the damaging effects of heavy metals such as lead, cadmium, arsenic, and mercury.9 A hair mineral analysis for heavy metals should be performed on all men with reduced sperm counts to rule out heavy metals as a cause. However, a more accurate and sensitive assessment of body load of metals requires challenge testing with a chelating agent. See the chapter “Detoxification and Internal Cleansing.”
Radiation
Cell phones operate at between 400 MHz and 2,000 MHz and emit electromagnetic waves that have been linked to DNA damage.10,11 While the relationship between cell phone use and male infertility remains unclear, there is some evidence that harmful electromagnetic waves emitted from cell phones may interfere with normal spermatogenesis and result in a significant decrease in sperm quality. Specific findings pertaining to sperm motility in humans have also been noted.12,13
In one study the use of cell phones decreased sperm count, motility, viability, and normal morphology.14 The decrease in sperm variables was dependent on the duration of exposure to cell phones and independent of initial semen quality. Of greatest importance was that sperm count, viability, and morphology were reduced as cell phone use increased. Specifically, using the cell phone for more than four hours a day caused a 25% drop in the number of sperm produced, and only 20% of the sperm looked normal.
This preliminary evidence is significant enough for us to discourage cell phone usage, and we definitely recommend that infertile men refrain from storing cell phones in their pockets.
Cigarette Smoking
A common source of oxidants is cigarette smoking, which accelerates DNA damage of the sperm and is associated with decreased sperm counts and sperm motility as well as a higher frequency of abnormal sperm.15–17 Cigarette smoking, as well as the increase in environmental pollution, is thought to be a major contributor to the diminution in sperm counts seen in many industrialized nations during the past few decades.
Alcohol
Excessive alcohol consumption in men is strongly associated with diminished sperm function; however, comprehensive research in this area is limited. Nonetheless, it is prudent to avoid alcohol in the preconception period.
Marijuana and Other Recreational Drugs
The effects of marijuana and other recreational drugs are difficult to determine because their use is illegal. Nevertheless, such drug use generally should be discouraged, particularly because these drugs have well-documented harmful effects on the developing fetus. A known fertility toxicant, marijuana contains cannabinoids, which have been shown to impair signaling pathways, alter hormonal regulation, and cause problems with embryo implantation. In men, cannabinoids have been found to inhibit testosterone production, reduce energy production in human sperm, decrease sperm motility, cause problems with sperm morphology, and decrease sperm function.18–20 Another factor to consider is that some marijuana is contaminated with herbicides and pesticides. These toxins are very efficiently absorbed into the body when smoked.
Obese men are known to have lower sperm counts (up to 50% less), reduced motility, reduced sperm production, increased DNA fragmentation of sperm, and increased levels of erectile dysfunction. Additionally, extra abdominal weight can increase scrotal temperature. Hormonal changes are primarily responsible for the changes in obese men. The level of total and free testosterone is reduced in obese men in proportion to the level of obesity. Estrogen is increased owing to the peripheral aromatization of androgens in adipose tissue. The estrogens produced have a negative feedback effect on gonadotropin production, thereby reducing FSH. This reduction in FSH further reduces testosterone production and sperm production. Additionally, increased body fat and a sedentary lifestyle are associated with raised testicular temperature, which further adversely affects the production of sperm.21
The chapter “A Health-Promoting Diet” provides sound guidance for improving fertility. In particular, it is important to eat the right type of fats. Surrounding the entire sperm is a “shield” of essential fatty acids that protects the sperm, enables movement, and encourages fertilization.22 Avoid trans fats (found in hydrogenated oils), rancid or oxidized fats, and excessive saturated fat intake.
Studies show that sperm motility strongly correlates with levels of sperm membrane omega-3 fatty acids, in particular DHA.23 One study noted that excessive omega-6 compared with omega-3 in seminal fluid produced decreased sperm concentration, sperm motility, and sperm morphology.24 It would be a good idea to restrict the use of popular omega-6 cooking oils such as soy, corn, and safflower. Take omega-3 supplements (optimal dosage is 1,000 to 2,000 mg EPA + DHA); increase consumption of raw nuts and seeds, cold-pressed monounsaturated oils such as olive, canola, and macadamia nut; eat more avocados; and eat more wild and sustainably farmed fish with high levels of essential fatty acids.
In particular, avoid cottonseed oil, as it may contain toxic pesticide residues. Cottonseed also has high levels of gossypol, a substance known to inhibit sperm function. In fact, gossypol is being investigated as the “male birth control pill.” Its use as an antifertility agent began after studies demonstrated that men who had used crude cottonseed oil for cooking were shown to have low sperm counts followed by total testicular failure.25
Antioxidants
A recent Cochrane review assessed the impact of antioxidants on male subfertility by considering 34 trials that involved 2,876 couples.26 The authors concluded that for subfertile men antioxidant supplementation improves the outcome: live births and pregnancy rates. Important points included these:
• Antioxidant use was associated with a statistically significant increase in pregnancy rate compared with controls.
• No studies reported evidence of harmful side effects of the antioxidant therapy used.
• Up to 80% of male factor subfertility may be due to oxidative stress.
• Subfertile men are confirmed as having lower levels of antioxidants in their semen compared with fertile men.
• Free radical levels are significantly higher in sperm samples from infertile men when compared with healthy controls
Free radicals cause fertility problems by damaging the sperm membrane, thus affecting sperm motility and the ability of sperm to penetrate the egg. They can also alter sperm DNA, which affects fertilization and embryo growth.
In healthy men the seminal plasma is naturally rich in antioxidants that protect it from this damage. Antioxidants are also found in the head of the sperm, where they are responsible for protecting the DNA, promoting the survival and longevity of the sperm, and enabling the sperm to detect chemical signals from the egg.
Free radical or oxidative damage to sperm is thought to be responsible for many cases of male infertility. High levels of free radicals are found in the semen of approximately 40% of infertile men.27–29
Although most free radicals are produced during normal metabolic processes, the environment contributes greatly to the free radical load. Men exposed to higher levels of sources of free radicals are much more likely to have abnormal sperm and sperm counts.27–29
Vitamin C. Vitamin C improves all semen variables. A marginal deficiency causes oxidative damage to sperm, resulting in reduced sperm motility and viability. Supplementation leads to improvement in both viability and motility, reduced numbers of abnormal sperm, and reduced sperm agglutination (sperm become agglutinated when antibodies produced by the immune system bind to them; when more than 25% of the sperm are agglutinated, fertility is very unlikely).30–32
In infertile men, vitamin C has been found in reduced quantity in the seminal plasma.33–34 Men with inadequate seminal vitamin C have also been observed to suffer from sperm DNA damage.
When dietary vitamin C was reduced from 250 to 5 mg per day in healthy human subjects, the ascorbic acid content of seminal fluid decreased by 50% and the number of sperm with damage to their DNA rose by 91%.35
It is now well documented that cigarette smoking greatly reduces vitamin C levels throughout the body and that smokers require at least twice as much vitamin C as nonsmokers. In one study, men who smoked one pack of cigarettes a day received either 0, 200, or 1,000 mg vitamin C. After one month, sperm quality improved in proportion to the level of vitamin C supplementation.36
Nonsmokers appear to benefit from vitamin C as much as smokers. In one study, 30 infertile but otherwise healthy men received either 200 or 1,000 mg vitamin C or a placebo per day.37 Sperm count, viability, motility, agglutination, abnormalities, and immaturity were measured weekly. After one week, the 1,000-mg group demonstrated a 140% increase in sperm count, the 200-mg group a 112% increase, and the placebo group no change. After three weeks both vitamin C groups continued to improve, with the 200-mg group catching up to the improvement of the 1,000-mg group. At the beginning of the study all three groups had more than 25% agglutinated sperm. After three weeks, the proportion of agglutinated sperm in the vitamin C groups dropped to 11%. Although this result is significant, the most impressive result of the study was that at the end of 60 days, all of the men in the vitamin C groups had impregnated their wives, compared with none in the placebo group.
Vitamin E. Supplementation with vitamin E appears to be especially warranted because it is the main antioxidant in various cell membranes, including those of sperm. Vitamin E has been shown to play an essential role in inhibiting free radical damage to the unsaturated fatty acids of the sperm membrane.38
In one study, supplementation with vitamin E was found to decrease malondialdehyde (an indicator of lipid peroxidation) and improve sperm motility.39 Even more important, however, 11 of 52 treated infertile men (21%) impregnated their spouses, while none did in the placebo group. Following the completion of the study, 26 of the placebo patients were switched to vitamin E, and four were then able to successfully impregnate their spouses.
Supplementation with vitamin E may also be useful for couples undergoing in vitro fertilization. For fertile men with normal sperm who had low fertilization rates, vitamin E (200 mg a day for at least three months) was found to improve the in vitro fertilization rate, possibly by reducing lipid peroxidation.40
Vitamin A, Beta-Carotene, and Lycopene. Vitamin A is an antioxidant required for cellular growth and differentiation, gene expression, regulatory functions, and epithelial tissue integrity. It is necessary for the health of the testes and for sperm production. Low concentrations of vitamin A are associated with abnormal semen variables in men,41 and in animal studies deprivation of vitamin A has been shown to lead to a loss of sperm production.42
Beta-carotene levels are significantly reduced in men whose infertility is due to autoimmune issues—conditions in which antibodies form against sperm components. Beta-carotene intake is associated positively with a higher sperm concentration as well as higher quantities of motile sperm.43 Lycopene may be even more useful than beta-carotene. Lycopene is found in high concentrations in the testes and seminal plasma, and reduced levels have been demonstrated in men with infertility. In one clinical trial, 30 men with fertility problems were administered 2 mg lycopene twice a day for three months. Twenty patients (66%) showed an improvement in sperm concentration, 16 (53%) had improved motility, and 14 (46%) showed improvement in sperm morphology.44
Zinc. Zinc is perhaps the most critical trace mineral for male sexual function and is found in high concentrations within the prostate, testes, and semen (approximately 2.5 mg of zinc is lost per ejaculation). It is involved in virtually every aspect of male reproduction, including hormone metabolism, sperm production, and sperm motility.
Zinc plays an important role in all human living cells, where it is involved in RNA transcription, DNA replication, and protein synthesis, all of which are crucial for reproduction and fertility. Additionally, it protects against free radical damage that may impair sperm. Deficiency of zinc may lead to gonadal dysfunction and has been observed to be associated with male infertility and impotence.45
Zinc levels are typically much lower in infertile men with low sperm counts, indicating that a low zinc status may be the contributing factor in the infertility.45–47 It has also been shown that zinc status directly correlates with an increase in sperm count and improvements in morphology and motility.48 Finally, zinc has been shown to exert an antimicrobial effect in the seminal plasma; this effect can be important if sperm antibodies or underlying genitourinary infection is present.49
Several studies have evaluated the effect of zinc supplementation on sperm counts and motility.50–52 The results of all of the studies support the use of zinc supplementation in the treatment of low sperm count, especially in the presence of low testosterone levels. The effectiveness of zinc is best illustrated by a study of 37 men with infertility of more than 5 years’ duration whose sperm counts were less than 25 million/ml.53 Blood testosterone levels were also measured. The men received a supplement of zinc sulfate (60 mg elemental zinc per day) for 45 to 50 days. In the 22 patients with initially low testosterone levels, mean sperm count rose significantly from 8 milllion to 20 million/ml. Testosterone levels also increased, and 9 of the 22 wives became pregnant during the study. This result is quite impressive given the long-term nature of the infertility and the rapidity of the results. In contrast, in the 15 men who had had normal testosterone levels before the study, sperm count increased slightly, but there was no change in testosterone levels and no pregnancies occurred.
Selenium. Selenium is a potent antioxidant that is essential for male fertility owing to its role in testosterone synthesis, normal sperm maturation, and sperm motility. Clinical trials reveal it has the ability to increase sperm motility and assist in the production of healthy spermatozoa.54,55 Selenium also helps protect the sperm against oxidation.56,57
The effects of selenium on sperm motility are highlighted in a study involving a group of men with poorly motile sperm.58 Over a three-month period men who were given selenium (either on it its own or as a combination of antioxidants that also included vitamins A, C, and E) showed increased sperm motility when compared with the placebo group. Five men in the treatment group (11%) impregnated their wives, in contrast to none in the placebo group. Though this study was small, it suggests that selenium supplementation can increase the chance of successful conception. This outcome is even more significant when one considers the cost and convenience of supplementation in comparison with in vitro fertilization and other methods.
More recently, selenium (200 mcg per day) in combination with the antioxidant n-acetylcysteine (600 mg per day) was found to improve sperm count, sperm motility, and normal sperm morphology.59 However, once supplementation stopped, the sperm reverted back to baseline. This study did not measure pregnancy rate.
Folic Acid and Vitamin B12. Folic acid and vitamin B12 are concentrated within the head of the sperm and are responsible for safeguarding the DNA within.52,60–62 Multiple studies have shown that low levels of folic acid in seminal fluid are associated with increased sperm DNA damage, while a B12 deficiency is strongly associated with reduced sperm motility and count.61,62 As the human body has a high turnover of these nutrients and requires a continual supply, supplementation is advisable for all men experiencing infertility regardless of whether there is a proven deficiency, especially in men who have a sperm count of less than 20 million/ml or a motility rate of less than 50%. In one study, 27% of men with a sperm count less than 20 million/ml who were given 1,000 mg per day of vitamin B12 were able to achieve a total sperm count in excess of 100 million/ml.63 In another study, 57% of men with a low sperm count who took 6,000 mg per day demonstrated improvements.64 As would be expected, men with elevated homocysteine levels—typically due to inadequate intake of B-complex vitamins—have greatly decreased fertility.
Alpha-Lipoic Acid. Alpha-lipoic acid is an antioxidant that is both fat- and water-soluble and assists in the chelation of heavy metals. It is especially useful because of its ability to regenerate other antioxidants, including vitamins C and E, CoQ10, and glutathione.65 In animal studies, alpha-lipoic acid has been shown to protect sperm.66–68 It appears to act as a sort of shield, forming a protective barrier around and inside the midpiece of the sperm. This protection is crucial, as the midpiece has been identified as one of the first places free radicals attack.
Carnitine. Carnitine is a naturally produced compound in the body. It is derived from the amino acids lysine and/or methionine and plays a vital role in fatty acid metabolism. It works synergistically with coenzyme Q10. Carnitine exerts protective antioxidant effects and provides energy to the testicles and sperm. Several studies comparing fertile men with infertile men found that fertile men had a statistically significant larger amount of carnitine in their seminal sample than the infertile men, and that low levels of L-carnitine in the seminal plasma may be a potent marker for infertility.69
Carnitine concentrations are extremely high in the epididymis and sperm, suggesting a role in male reproductive function. The epididymis derives the majority of its energy requirements from fatty acids, as do the sperm during transport through the epididymis. After ejaculation, the motility of sperm correlates directly with carnitine content—the higher the carnitine content, the more motile the sperm. Conversely, when carnitine levels are low, sperm development, function, and motility are drastically reduced.
Several clinical studies have shown that carnitine supplementation can produce dramatic improvements in sperm counts and sperm motility.69 In the Italian Study Group on Carnitine and Male Infertility, 100 subjects were given 3,000 mg L-carnitine per day for four months.70 Carnitine was able to increase sperm counts and sperm motility:
• The number of ejaculated sperm per ml increased from 142 billion to 163 billion.
• The proportion of motile sperm increased from 26.9 to 37.7%.
• The proportion of sperm that are able to swim in a straight line increased from 10.8 to 18%.
• The mean sperm velocity (how fast the sperm are able to swim) increased from 28.4 to 32.5%.
The results are even more impressive if results for only the patients with the poorest sperm motility are examined. This subgroup saw even more significant gains on all variables. For example, the proportion of motile sperm increased from 19.3 to 40.9%, and the proportion of sperm that are able to swim in a straight line increased from 3.1 to 20.3%. These results have been confirmed in several double-blind studies.71–75
Coenzyme Q10 (CoQ10). CoQ10 is concentrated in the head and midpiece (neck) of the sperm, and is also found in seminal fluid. It is considered to be the most crucial and powerful antioxidant in sperm structure owing to its role in mitochondrial energy release. It is believed to promote motility, foster sperm survival, and provide energy to assist the sperm’s travel on its journey to the egg.
As a fat-soluble antioxidant and free radical scavenger, CoQ10 is required for the maintenance of healthy cell membrane integrity and cell functioning, specifically for new cells such as sperm. Decreased levels have been found in the seminal fluid and sperm of men with idiopathic and varicocele-associated asthenospermia.76–78
Arginine. The amino acid arginine is required for the replication of cells, so it is essential in sperm formation. A number of studies have shown that arginine can improve sperm count and motility. Stress in particular has been found to decrease the levels of arginine in the sperm production pathways. Arginine supplementation is often, but not always, an effective treatment for male infertility. The critical determinant appears to be the sperm count: if it is under 20 million/ml, arginine supplementation is less likely to be of benefit. In order to be effective, the dosage of arginine must be at least 4 g per day for three months. In perhaps the most favorable study, 74% of 178 men with low sperm count had significant improvements in sperm count and motility after arginine therapy.79
In one double-blind, randomized, placebo-controlled, crossover clinical trial, an improvement in semen variables was observed after administration of Prelox, a combination of 80 mg Pycnogenol and 3 g L-arginine.80 Over a treatment period of four weeks, fifty men with idiopathic infertility experienced significant increase in ejaculate volume, concentration and number of sperm, and percentage of vital spermatozoa compared with the placebo group. The percentage of sperm with good motility also increased significantly, while the percentage of immotile sperm decreased. This effect appears to be due to a combination of the antioxidant activity of Pycnogenol and/or the ability of arginine to stimulate production of nitric oxide. Pycnogenol alone (200 mg per day for 90 days) was shown to improve sperm morphology by 38% and viability by 19% in a small pilot study.81
Chinese Ginseng
Current scientific investigation suggests that Chinese ginseng (Panax ginseng) can be useful in supporting male fertility. It has a long history of use as a male tonic. In animal studies, Chinese ginseng has been shown to promote the growth of the testes, raise sperm formation and testosterone levels, and increase sexual activity and mating behavior. The active constituents (ginsenosides) have been shown to enhance nitric oxide production, thus improving fertilization ability and sperm motility.82,83 Additionally, they have been shown to improve the functioning of parts of the endocrine system, which can assist in modulating stress-induced infertility or lowered testosterone from insufficient DHEA synthesis.84 In clinical trials Panax ginseng has been shown to increase testosterone levels in men with low levels, improving erectile function and libido; it also improved sperm count and motility (including in some patients with varicoceles).84–85 Note that ginseng does not increase testosterone in men with normal levels.
Pygeum
Pygeum (Pygeum africanum) has been shown to increase prostatic secretions and improve the composition of seminal fluid.86–88 Specifically, pygeum administration to men with decreased prostatic secretion led to an increased total amount of seminal fluid plus increases in alkaline phosphatase and protein content. Pygeum appears to be most effective in men in whom the level of alkaline phosphatase activity is reduced (i.e., less than 400 IU/cm3) and there is no evidence of inflammation or infection (i.e., absence of white blood cells and immunoglobulin A [IgA]). The absence of IgA in the semen is a good indicator of clinical success. In one study, patients with no IgA in the semen demonstrated an alkaline phosphatase increase from 265 to 485 IU/cm3.88 In contrast, patients with IgA showed only a modest increase from 213 to 281 IU/cm3.
Pygeum extract has also shown an ability to improve the capacity to achieve an erection in patients with benign prostatic hypertrophy or prostatitis, as determined by nocturnal penile tumescence in a double-blind clinical trial.89
Tribulus
Tribulus (Tribulus terrestris) has been used traditionally in ayurvedic medicine as a tonic and aphrodisiac and in European folk medicine to increase sexual potency. A steroidal saponin, protodioscin, is considered the chief constituent responsible for the herb’s effects on libido and sexual functioning. Of prime importance is the source of the extract. All of the clinical studies showing positive effects have used a leaf extract from Bulgaria, as it has been shown to be highest in protodioscin. A tribulus product made from the root or fruit of the plant or sourced from anywhere besides eastern Europe will probably contain low levels of protodioscin.
In animal studies tribulus has been shown to increase certain sex hormones (including testosterone) as well as nitric oxide synthesis;90 however, these results have not been observed in some human studies.91 One possible reason is differences in the extract used; another is that many of the studies have used healthy men with normal testosterone levels rather than men with testosterone abnormalities.
Tribulus appears to enhance male fertility owing to its ability to increase sperm count, viability, and libido; however, published studies are poorly designed and have produced conflicting results.92
![]()
QUICK REVIEW
• The average sperm count has declined by 40% since 1940.
• A high sperm count means nothing if the percentage of healthy sperm is not also high.
• Reducing scrotal temperature in infertile men will often make them fertile.
• Infertile men should wear boxer-type underwear and periodically take a cold shower or apply ice to the scrotum.
• The presence of antisperm antibodies or of high levels of debris in a semen sample is considered a good indicator of a chronic infection.
• Increased exposure to environmental estrogens and other environmental pollutants during fetal development is suggested as a major cause of the tremendous rise in disorders that affect the development and function of the male sexual system.
• In one study cell phone use decreased sperm count, motility, viability, and normal morphology.
• Obese men are known to have lower sperm counts, reduced motility, reduced production of sperm, increased DNA fragmentation in sperm, and increased levels of erectile dysfunction.
• Free radical damage to sperm is thought to be responsible for many cases of male infertility.
• Antioxidants such as vitamin C, beta-carotene, selenium, and vitamin E have been shown to be very important in protecting the sperm against damage and improving male fertility.
• Zinc supplementation can be very helpful in achieving fertility, especially in men with low testosterone levels.
• Multiple studies have shown low levels of folic acid in seminal fluid to be associated with increased sperm DNA damage, while a vitamin B12 deficiency is strongly associated with reduced sperm motility and count.
• Carnitine supplementation can lead to improvements in sperm counts and sperm motility.
• Pygeum has been shown to increase prostatic secretions and improve the composition of the seminal fluid.
• Ashwagandha inhibited lipid peroxidation, improved sperm count and motility, and had a positive effect on hormone levels.
Velvet bean (Mucuna pruriens) has been used in ayurvedic medicine to improve stress endurance, increase general resistance against infection, retard the aging process, and improve male sexual function. It has been shown to help alleviate disorders including psychogenic impotence and unexplained infertility.93 One paper showed that Mucuna pruriens seed powder produced dramatic improvements in 70% of study participants, helping to fight stress-mediated poor semen quality and acting as a restorative and invigorator in infertile subjects.94 The effect appears to be due to significant improvements in levels of testosterone, luteinizing hormone, dopamine, adrenaline, and noradrenaline, plus reduction in levels of follicle-stimulating hormone and prolactin.95 Sperm count and motility were also significantly improved in infertile men after treatment.
Ashwagandha
Ashwagandha (Withania somnifera) has shown considerable anti-stress and adaptogenic effects. In a three-month clinical trial, 75 normal healthy fertile men (control subjects) were compared with 75 men undergoing infertility screening who received 5 g powdered root per day. Results showed that ashwagandha inhibited lipid peroxidation and improved sperm count and motility. Treatment also significantly increased serum testosterone and luteinizing hormone and reduced levels of follicle-stimulating hormone and prolactin—all beneficial effects in infertile men.96
![]()
TREATMENT SUMMARY
There are many factors involved in male infertility, and a comprehensive treatment plan is essential to success. We recommend consulting a urologist or fertility specialist for a complete evaluation. It is advisable to go on a detoxification program at the start of treatment; see the chapter “Detoxification and Internal Cleansing.”
Because elevated scrotal temperature is a common cause of infertility, we recommend scrotal cooling through the use of loose underwear, avoidance of activities that raise testicular temperature (e.g., hot tubs), and application of cold water or ice to the testes.
Optimize nutritional status and eliminate any unhealthy lifestyle practices; add fertility-enhancing nutritional supplements and botanicals as needed. Avoid pollutants and toxic substances such as cigarette smoke.
• Maintain scrotal temperatures between 94° and 96.8°F
• Avoid exposure to free radicals
• Identify and eliminate environmental pollutants
• Talk to your doctor about stopping or reducing drugs such as antihypertensives, antineoplastics (e.g., cyclophosphamide), and anti-inflammatories (e.g., sulfasalazine)
• Utilize effective stress reduction techniques; employ psychological counseling if needed
• Avoid cigarette smoking and recreational drugs
• Follow the guidelines in the chapter “A Health-Promoting Diet”
• Avoid dietary sources of free radicals, saturated fats, and trans-fats; also avoid cottonseed oil
• Increase consumption of legumes (especially soy); good dietary sources of antioxidant vitamins, carotenes, and flavonoids (many dark- or bright-colored vegetables and fruits); and essential fatty acids and zinc (from nuts and seeds)
• Eat 8 to 12 servings of vegetables and 1 to 2 servings of fresh fruits per day
• Optimize protein intake from both vegetarian and organic animal sources
• Drink 6 to 8 glasses of water per day
• Eliminate caffeine, alcohol, sugar, and food additives (such as preservatives and colorings)
• A high-potency multiple vitamin and mineral formula as described in the chapter “Supplementary Measures”
• Key individual nutrients:
![]() Vitamin B6: 25 to 50 mg per day
Vitamin B6: 25 to 50 mg per day
![]() Folic acid: 800 mcg to 2 mg per day
Folic acid: 800 mcg to 2 mg per day
![]() Vitamin B12: 800 mcg per day
Vitamin B12: 800 mcg per day
![]() Vitamin C: 500 to 1,000 mg per day
Vitamin C: 500 to 1,000 mg per day
![]() Vitamin E (mixed tocopherols): 200 to 400 IU per day
Vitamin E (mixed tocopherols): 200 to 400 IU per day
![]() Beta-carotene: 15,000 to 30,000 IU per day (preferably as mixed carotenoids)
Beta-carotene: 15,000 to 30,000 IU per day (preferably as mixed carotenoids)
![]() Magnesium (bound to aspartate, citrate, fumarate, malate, or succinate): 200 to 300 mg three times per day
Magnesium (bound to aspartate, citrate, fumarate, malate, or succinate): 200 to 300 mg three times per day
![]() Selenium: 100 to 200 mcg per day
Selenium: 100 to 200 mcg per day
![]() Zinc: 30 to 45 mg per day
Zinc: 30 to 45 mg per day
![]() Vitamin D3: 2,000 to 4,000 IU per day (ideally, measure blood levels and adjust dosage accordingly)
Vitamin D3: 2,000 to 4,000 IU per day (ideally, measure blood levels and adjust dosage accordingly)
![]() Fish oils: 1,000 mg EPA + DHA per day
Fish oils: 1,000 mg EPA + DHA per day
• One of the following:
![]() Grape seed extract (>95% procyanidolic oligomers): 100 to 300 mg per day
Grape seed extract (>95% procyanidolic oligomers): 100 to 300 mg per day
![]() Pine bark extract (>95% procyanidolic oligomers): 100 to 300 mg per day
Pine bark extract (>95% procyanidolic oligomers): 100 to 300 mg per day
![]() Some other flavonoid-rich extract with a similar flavonoid content, super greens formula, or another plant-based antioxidant that can provide an oxygen radical absorption capacity (ORAC) of 3,000 to 6,000 units or more per day
Some other flavonoid-rich extract with a similar flavonoid content, super greens formula, or another plant-based antioxidant that can provide an oxygen radical absorption capacity (ORAC) of 3,000 to 6,000 units or more per day
• Specialty supplements:
![]() Lycopene: 2 to 5 mg per day
Lycopene: 2 to 5 mg per day
![]() CoQ10: 200 to 400 mg per day
CoQ10: 200 to 400 mg per day
![]() L-carnitine: 2,000 to 3,000 mg per day
L-carnitine: 2,000 to 3,000 mg per day
![]() L-arginine: 4,000 mg per day
L-arginine: 4,000 mg per day
One or more of the following:
• Chinese ginseng (Panax ginseng):
![]() High-quality crude ginseng root: 1.5 to 2 g per day
High-quality crude ginseng root: 1.5 to 2 g per day
![]() Fluid extract (containing a minimum of 10.5 mg/ml ginsenosides with Rg1:Rb1 greater than or equal to 0.5 by HPLC): 2 to 6 ml (1/2 to 11/2 tsp) per day
Fluid extract (containing a minimum of 10.5 mg/ml ginsenosides with Rg1:Rb1 greater than or equal to 0.5 by HPLC): 2 to 6 ml (1/2 to 11/2 tsp) per day
![]() Dried powdered extract standardized to contain 5% ginsenosides with an Rb1/Rg1 ratio of 2:1: 250 to 500 mg per day
Dried powdered extract standardized to contain 5% ginsenosides with an Rb1/Rg1 ratio of 2:1: 250 to 500 mg per day
• Pygeum (Pygeum africanum), liposterolic extract standardized to contain 14% triterpenes: 100 to 200 mg per day in divided doses
• Tribulus (Tribulus terrestris):
![]() Dried leaf with a protodioscin content of 12.22 mg/g: 9 to 18 g per day
Dried leaf with a protodioscin content of 12.22 mg/g: 9 to 18 g per day
![]() Dried powdered extract standardized to contain 45% steroidal saponins: 250 to 500 mg per day
Dried powdered extract standardized to contain 45% steroidal saponins: 250 to 500 mg per day
![]() Fluid extract (2:1): 7 to 21 ml per day
Fluid extract (2:1): 7 to 21 ml per day
• Velvet bean (Mucuna pruriens): dosage equivalent to 5 g powdered dried seed per day
• Ashwagandha (Withania somnifera):
![]() Powdered root: 5 g per day
Powdered root: 5 g per day
![]() Dried powdered extract (root and leaves) standardized to contain 8% withanolide glycoside conjugates and 32% oligosaccharides: 125 to 250 mg per day
Dried powdered extract (root and leaves) standardized to contain 8% withanolide glycoside conjugates and 32% oligosaccharides: 125 to 250 mg per day
![]() Fluid extract (2:1), containing a minimum of 4 mg/ml withanosides: 2.5 to 5.0 ml per day
Fluid extract (2:1), containing a minimum of 4 mg/ml withanosides: 2.5 to 5.0 ml per day