CHAPTER 1
A BRIEF HISTORY OF THE CONCEPT “ATOM”
The idea that matter is made of tiny particles is at least 2,500 years old. Through much of history, it was relegated to the fringes of scientific thought for philosophical and religious reasons. But it regained popularity with the rise of science in Europe in the seventeenth and eighteenth centuries. The notion that atoms really do exist only became widely accepted with the rapid rise of atomic physics in the early twentieth century.
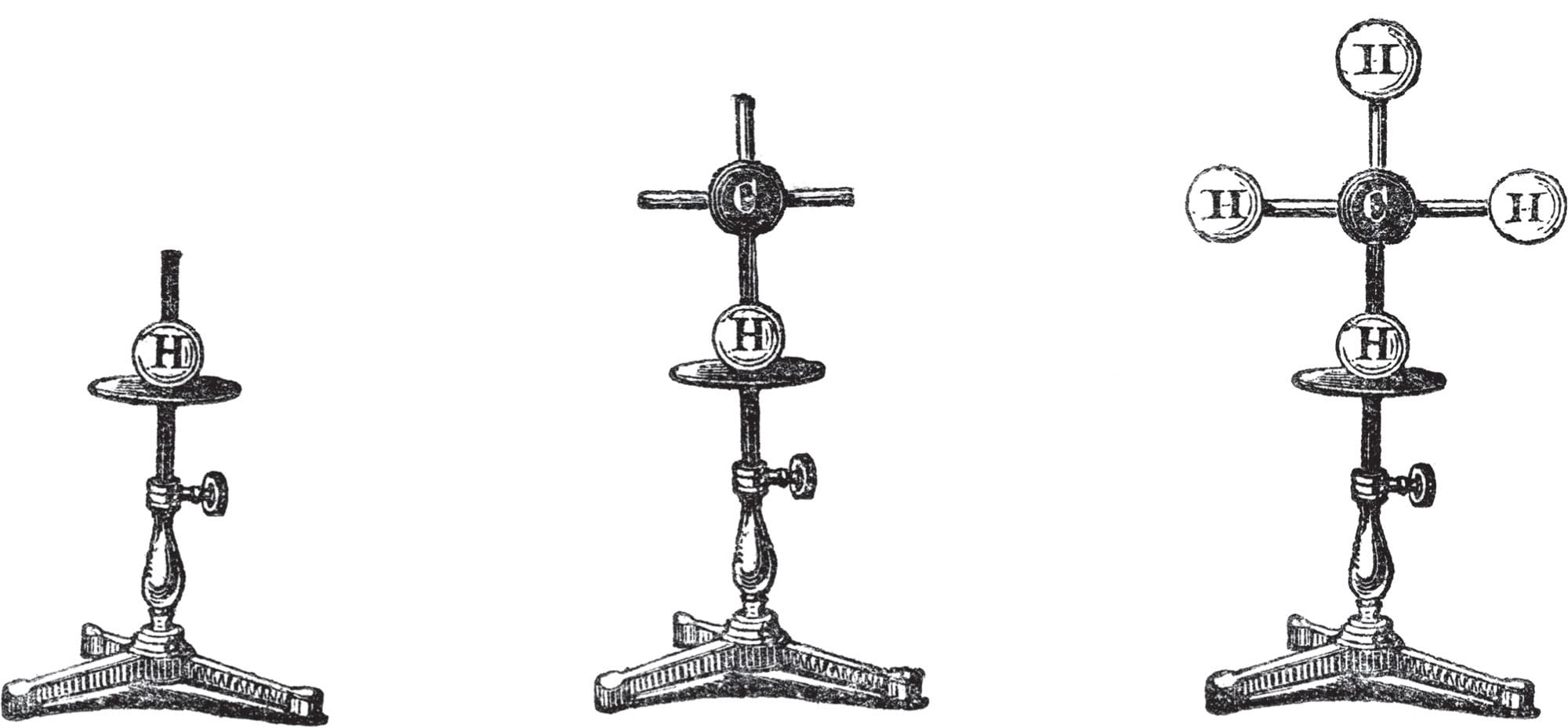
Molecular models presented to the Royal Institution, London, UK, by German chemist August Wilhelm von Hofmann, in 1865. Hoffman used the models in a lecture entitled “The Combining Power of Atoms,” at a time when the existence of atoms was still in doubt.
EVERYTHING AND NOTHING
The roots of modern atomic theory lie in ancient Greece. Oddly, perhaps, the story begins with philosophical wrangling about whether change is real or an illusion—and whether empty space can exist. Despite the development of well-thought-out and convincing atomic theories in both Greece and India, other ideas would come to dominate.
EVERYTHING CHANGES—OR DOES IT?
It was common for ancient Greek philosophers—as it is for scientists these days—to seek order in the world, and in particular to find a unified cause for the huge variety of phenomena we observe. When it comes to the physical world, or matter, the earliest Greek philosophers were “monists.” They proposed that either all matter begins as one kind of substance and then differentiates, or that there actually is only one kind of matter, which manifests in various forms.
One of the earliest Greek philosophers, Thales of Miletus (ca. 625–ca. 545 BCE), suggested that water might be the primary substance from which all other substances derive. Anaximenes, also of Miletus, thought that the primary substance might be air.
Several decades later, another Greek philosopher, Heraclitus of Ephesus (ca. 535–ca. 475 BCE), suggested that the primary material might be fire. His reasoning was that fire is an agent of change—and, our senses report, change is a vital and constant feature of the world. A contemporary of Heraclitus, Parmenides of Elea (born ca. 515 BCE), believed quite the opposite. Parmenides and his followers rejected the empirical experiences of the senses, relying instead on pure reason. They believed that all change is an illusion, that it simply does not exist.
Parmenides’s notion that change is an illusion followed from his belief that “nothingness” could not exist. He argued that the supposedly “changed” state of a thing is different from the original thing and so did not previously exist; it would therefore have to have come from nothing. Parmenides even rejected the idea that things could move. Motion was impossible, he said, because it would require the existence of “void,” or empty space, into which an object could move—and void was the same as “nothingness.” For Parmenides, reality was one perfect, full, unchanging sphere that had always existed and in which nothing ever changed. He called it the plenum. We will return to this notion in the context of modern theoretical physics in chapter seven.
ATOMS—MAKING SENSE OF CHANGE
The views of Parmenides were influential in ancient Greece, and the philosophers who succeeded him felt compelled to take his views into account. One of them was Democritus (ca. 460–370 BCE), widely credited with the first comprehensive atomic theory (although there were similar ideas in India at about the same time, see the box shown here). Democritus attempted to reconcile Parmenides’s notion of reality as an unchanging whole with the fact that change does seem to happen. He did so by making two adjustments to Parmenides’s ideas. First, he suggested that “void,” or empty space, can exist. Second, he proposed that all matter is made of tiny, indivisible particles. The individual particles retain their identity and their total number remain the same, so overall there is no change. But change can occur locally, because particles can move around, collide with one another, join and break apart, and rearrange themselves.
Democritus described his particles as ἄτομος (atomos, meaning “indivisible”). The word comes from ἀ- (a-, “not”) and τέμνω (témnō, “I cut”). He also supposed that the particles are always in motion and that they are identical apart from their size and shape.

The doctrine of monism proposes that everything is made of, or arises from, just one kind of matter—but how can an ocean, rocks, soaring birds, and clouds be made of the same thing?
Democritus’s atomistic philosophy attempted to make sense of the physical properties of matter. He suggested that a dense solid material is made of heavy and more closely-packed atoms, while a gas is made of extremely small, light atoms with a good deal of void between them. He also proposed that atoms link together, with physical connections, such as hooks and eyes, and that those connections can break and reform when chemical reactions take place, or when liquids evaporate and vapors condense—themes we explore in chapter four. Furthermore, the shapes of atoms confer certain properties; atoms of a liquid, for example, are round, so they easily flow over one another, while the atoms of salt are sharp.
Democritus’s theory was not widely accepted, for two main reasons. First, it is a purely materialistic vision of the world; there is no room for metaphysical or spiritual influences. Democritus had envisaged a special type of atom for the soul; these atoms are smaller than the others, able to pass easily between the atoms of the body. The materialistic character of his theory made it unpopular with many people, especially religious thinkers—for how can one reduce the human spirit and imagination to the movement of atoms?
NOTHING REALLY MATTERS
The other main sticking point for Democritus’s theory was its reliance on the notion of void—empty space. This would become increasingly important because of the ideas of one man: Aristotle (384–322 BCE). Aristotle’s ideas about matter were pragmatic, based very much on experience of the world, which is one reason why his works were so influential. Aristotle believed that matter is continuous and, in principle, infinitely divisible. The character, or “form,” of a substance is a separate quality from the matter itself. He stated his ideas as if they were truths, and for centuries that is how most scholars accepted them.
Aristotle firmly believed that empty space cannot exist. Any empty space would immediately be filled by matter around it, he claimed. Perhaps his most famous phrase is horror vacui, normally translated as “nature abhors a vacuum.” Because the existence of void is such a crucial element of Democritus’s theory, a belief that void cannot exist amounted to a strong denial of atomism.
Democritus was born in Abdera, in Thrace, in the fifth century BCE. He wrote on a huge range of subjects, including mathematics, ethics, aesthetics, and epistemology (theory of knowledge), although almost none of his works survives.

Aristotle, a scholar of the fourth century BCE, was one of the most prolific and influential of the Greek philosophers. He wrote on a wealth of topics, including physics, biology, astronomy, weather, ethics, politics, poetry, theater, and language.
A SCHOLASTIC VIEW
Philosophers and other scholars across the early Islamic world and in medieval Europe largely accepted Aristotle’s views on matter without question. Because Aristotle did not believe that matter is made of tiny particles, atomism remained largely in the shadows. But a new spirit of scientific investigation in Europe in the seventeenth century gave a new lease of life to the idea.
Knowledge and ideas from ancient Greece and ancient India spread widely from the third century BCE onward, as empires rose and fell—including during the Hellenistic period formed in the wake of conquests by Alexander the Great (356–323 BCE), and, of course, the Roman Empire. Aristotle’s philosophy was championed by early Christian scholars, and also by Arabic scholars from the eighth century onward, in a flourishing empire that was united by a common language (Arabic) and a common religion (Islam). The Islamic empire, characterized by a succession of powerful caliphates and dynasties, extended across a vast region, including the Arabian Peninsula, parts of India, the Middle East, North Africa, southern Italy, and Spain.

Scholasticism was an approach to learning philosophy and theology in European universities, based on the works of Aristotle and the teachings of early Christian thinkers. It was purely didactic, a way of passing on learning, without much room to assess critically what was being learned.
THE ISLAMIC GOLDEN AGE
The period from the ninth to the twelfth centuries is often called the Islamic Golden Age, because of the high culture and sophisticated state of science, mathematics, and engineering across the Islamic empire at that time. Many Arabic scholars absorbed, translated, and reinterpreted the classical works from ancient Greece, as well as made their own contributions in a wide range of subjects. Several scholars developed their own atomic theories, most notably Abū al-Ghazālī (ca. 1058–1111). He believed that all matter was made of indivisible particles that were arranged by Allah.
Two scholars in particular were responsible for saving and promoting the works of Aristotle. They were Ibn Sīnā (known in Europe as Avicenna; ca. 980–1037) and Ibn Rushd (known as Averroes; 1126–98). Both men rejected the kind of atomism proposed by Democritus, and both were highly influential for centuries to come.
INTO EUROPE
Knowledge amassed by Arabic scholars passed to Europe, mainly via Spain, and was adopted into the “scholastic” system—the teaching method used in the new universities of Europe from the eleventh century onward. As far as theories of matter are concerned, it was Aristotle’s ideas that dominated. Aristotle considered matter to have substance and form. The form can change, but the substance remains the same. Most important, matter is continuous and void cannot exist.
Despite the prominence of Aristotelian thinking, the concept of atomism lived on for two main reasons, both of which derived from Aristotle’s own work. First, Aristotle discussed Democritus’s theory extensively in his writing, albeit critically. In fact, Aristotle is one of our most important sources of knowledge about Democritus, because none of his works has survived. Second, Aristotle did discuss the “smallest parts” of matter, which he called minima naturalia. Aristotle’s “minima” were not indivisible particles like the atoms of Democritus; instead, they were the smallest amount of a particular substance. Aristotle wrote, for example, that you can divide flesh into increasingly smaller pieces. Below a certain size, however, the matter would still exist but it would no longer be flesh.
Aristotle did not dwell or particularly elaborate on the concept of minima, and so left room for interpretation. Some medieval scholastics adapted the idea with a leaning toward atomism. The influential Italian scholar Julius Caesar Scaliger (1484–1558) was generally a fierce defender of Aristotle’s ideas at a time when some were beginning to question them, but he considered Aristotle’s minima to be physical objects, the building blocks of matter, not just a limit to the division of a substance. He wrote, for example, about how water can wear away stone, one particle at a time, and how substances are more or less dense because their minima are closer together. There were even some scholars who fully supported atomism in the spirit of Democritus, but they were few. The rise of atomism proper had to wait until scholars began to question Aristotle’s ideas fully to come up with their own ideas and conduct experiments to test them.
QUESTIONING ARISTOTLE
During the Renaissance, which began in the fourteenth century, artists, writers, philosophers, mathematicians, and scientists beyond the scholastic system gained a new interest in the culture of ancient Greece and Rome. These bold thinkers began challenging dogma and creating their own culture in the spirit of the Greeks and the Romans. Johannes Gutenberg’s printing press, invented about 1440, helped spread these new ideas far and wide. Among many important advances, the Renaissance spawned the works of Leonardo da Vinci and Michelangelo, Nicolaus Copernicus’s theory about Earth orbiting the Sun, Andreas Vesalius’s remarkable work on human anatomy, and the Protestant Reformation.
By the early seventeenth century, ideas about how the world works and what it’s made of had shifted from the philosophical to the scientific, with an emphasis on empiricism—a reliance on experience, on an investigation of the real world reported by our senses. In the hands of people such as Galileo Galilei (1564–1642), the newly invented telescope and microscope were highlighting problems with Aristotle’s views, fueling the new spirit of investigation. And in 1620, English politician and scientist Francis Bacon (1561–1626) codified the scientific method, in his book Novum Organum; the title in reference to Aristotle’s Organon, a six-volume work on how to obtain knowledge using logic. With scientists observing, questioning, theorizing, and experimenting across Europe, it was no wonder that views on what matter is made of began to change.
By the mid-seventeenth century, there were several prominent scientists who believed matter is made of particles. One new phenomenon that helped encourage the rise of atomism was the vacuum, the void so vehemently dismissed by Aristotle. In 1643, Italian scientist Evangelista Torricelli (1608–47) observed what seemed to be empty space inside the glass of his mercury barometer. In 1654, German scientist and politician Otto von Guericke (1602–86) invented a crude vacuum pump, able to create partial vacuums inside sealed containers. During the 1660s, von Guericke carried out hundreds of experiments on the partial vacuums he created. His work inspired Anglo-Irish scientist Robert Boyle (1627–91) to develop a much more powerful and effective pump that could produce a much better vacuum. Boyle would also be a pioneer in another important strand in the history of atomism: the science of chemistry.
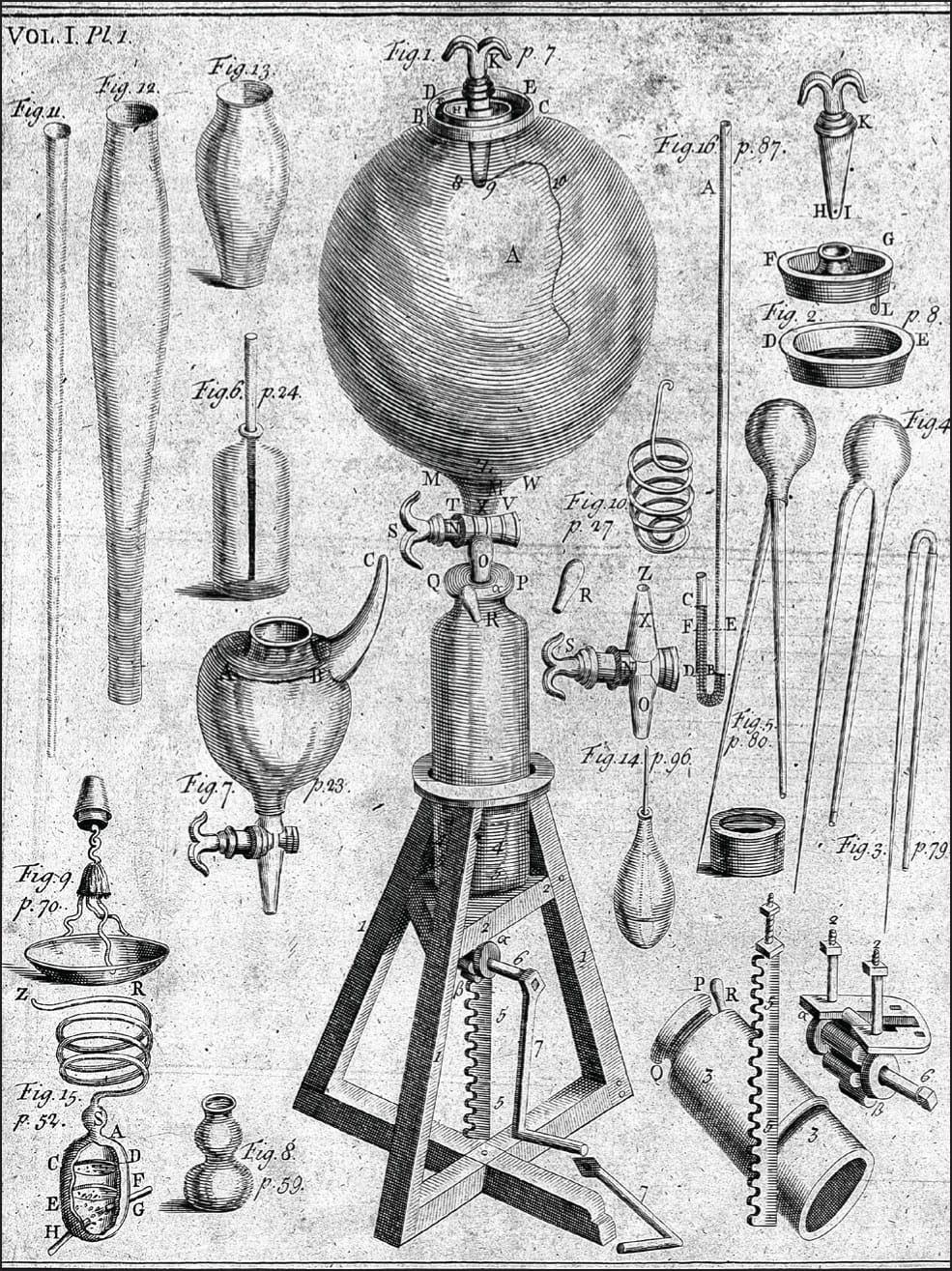
Robert Boyle’s vacuum pump, along with some of the apparatus he used to experiment with low pressure air.

Two hemispheres held together by atmospheric pressure, after von Guericke removed the air from them, could not be pulled apart by teams of horses in a demonstration at Regensberg, Germany, in 1654.
Torricelli’s barometer was a long glass tube sealed at one end and filled with mercury, a dense metal that is liquid at room temperature. When the tube was held upright with the open end submerged in a bowl of mercury, the mercury inside the tube made a column about 30 inches (76 centimeters) tall. Above that was apparently empty space: a vacuum.
A NEW SCIENCE OF MATTER
Founded late in the seventeenth century, the science of chemistry gradually took over its nonscientific predecessor, alchemy. Chemists founded scientific laws about the behavior of matter that would make the rise of atomism inevitable. Inspired by new knowledge about elements and compounds, English chemist John Dalton founded the first modern atomic theory at the beginning of the nineteenth century.
CHEMICAL REACTIONS
Combustion produces fire and reduces wood to a pile of ashes; fermentation changes plant matter into wine or beer; and smelting produces metals from rocks. Without knowledge of atoms and an understanding of elements and compounds, chemical reactions such as these are mysterious—even magical. Before the science of chemistry began to unlock what is really going on during chemical reactions, philosophers and other scholars based their understanding on the ancient art of alchemy.
Several distinct types of alchemy evolved in several different parts of the world. European alchemists adopted and developed their practices and beliefs from Arabic alchemists of the Islamic Golden Age. The Arabic alchemists developed many of the fundamental laboratory techniques and much of the basic equipment found in chemical laboratories today.
Arabic and European alchemists saw chemical changes as transmutations, substances “changing form.” Their thinking was based on Aristotle’s idea that a substance is made of matter plus form (see here). They also based their thinking on the ancient theory of the four “elements”—earth, air, fire, and water—a theory that Aristotle had also championed and developed. These four substances are not chemical elements as defined by modern science. Some alchemists added mercury and sulfur to the list of elements. Ironically, perhaps, these really are chemical elements. Alchemists saw transmutation as a shift in the proportions of elements within a substance.

According to the theory of the four elements followed by the alchemists, wood is made mostly of fire, water, and earth. When wood burns, the “fire” and “air” components are released, leaving behind earth, which has the appearance of ashes. The modern understanding of the chemical reactions taking place is based on the breaking and making of bonds between atoms in the wood and the air.
ELEMENTS OF CHANGE
Partly as a result of his experiments with vacuums, but more as a result of his firm belief in empiricism, Robert Boyle came to the conclusion that matter must be made of particles. He made his views clear in The Sceptical Chymist (1661), an influential book that helped to found the new science of chemistry.
Boyle adhered to a form of atomism that was gaining popularity in the seventeenth century: corpuscularianism (see here). In the context of this belief, Boyle challenged the theory of the four elements and proposed a new definition for an element:
“certain Primitive and Simple, or perfectly unmingled bodies; which not being made of any other bodies, or of one another, are the Ingredients of all those call’d perfectly mixt Bodies are immediately compounded, and into which they are ultimately resolved.”
In other words, an element is a substance made of just one kind of particle, and the particles of elements mix together to form other, compound, substances. Boyle suggests that it is possible, in principle, to obtain pure elements by breaking down everyday compounds into their components. More important than their support for atomism, and also more important than the new definition of an element, Robert Boyle’s books heralded a new, scientific approach to investigating chemical reactions and matter in general.

In The Sceptical Chymist, Robert Boyle (inset) encouraged people to take a more “philosophical” approach to investigating matter. At the time, most people who used chemical reactions were simply following recipes to make pharmaceuticals.
CHANGE IN THE AIR
One area of chemistry that received particular attention from empirical science was the study of gases, or “airs.” In 1640, Flemish scientist Jan van Helmont (1580–1644) coined the term “gas,” based on the Greek word khaos (meaning “empty space”), as the name of an “air” he discovered—a gas we now know as carbon dioxide.
In the second half of the seventeenth century and the first half of the eighteenth, scientists studied mostly the physical rather than chemical properties of gases. In particular, they worked out the relationships between the pressure, temperature, and volume of gases. In 1662, Robert Boyle discovered that if the temperature of a gas is held constant, its pressure and temperature are inversely proportional to each other. In other words, double the pressure on a gas (compress it twice as hard) and you will halve its volume, and vice versa. This relationship became known as Boyle’s Law. Boyle pictured a gas as made of stationary corpuscles with springs between them. Other scientists worked out similar laws that related pressure, temperature, and volume.
Swiss mathematician Daniel Bernoulli (1700–82) pictured gases differently. In one chapter of his 1738 book Hydrodynamica, Bernoulli suggested that the particles of a gas are tiny solid balls, not large, springy corpuscles. The tiny balls traveled at high speed and collided with each other and with the walls of the container that confined them. The many impacts the particles made on the container walls would account for the pressure exerted by the gas. Bernoulli even related the temperature of the gas to the average speeds of the balls. His mathematical approach led him to an equation that was in excellent agreement with Boyle’s Law and the other gas laws.
Despite this brilliant insight, few of Bernoulli’s contemporaries took any notice of what should have been a crucial piece of evidence in favor of atomism—largely because they could not see why the tiny solid balls would not gradually run out of energy and all end up at the bottom of the container. However, chemists continued to work on gases with great fervor. In the 1750s, Scottish chemist Joseph Black (1728–99) found new ways to produce carbon dioxide, which he named “fixed air.” Research into gases led to the discoveries of “flammable air” (hydrogen, 1766), “phlogisticated air” (nitrogen, 1772). and “dephlogisticated air” (oxygen, 1773).
A drawing from Daniel Bernoulli’s Hydrodynamica illustrates his idea that gases are made of tiny particles in motion.
French chemist Antoine Lavoisier (1743–94) took great interest in these gases, and in particular in the chemical reactions that produced them and in which they took part. Lavoisier was an extremely meticulous experimenter who carefully weighed the chemicals before and after a reaction, including the gases. His research led him to several important conclusions that would firmly establish chemistry as a science, and it would also prepare the ground for the modern atomic theory. Lavoisier found that the total mass of the chemicals taking part in a chemical reaction was exactly the same as the total mass of the reaction products—a phenomenon known as the conservation of mass. He also worked out that combustion involved dephlogisticated air (oxygen) combining with other substances. When flammable air (hydrogen) burned, it combined with dephlogisticated air to produce water. Lavoisier himself came up with the name “hydrogène,” meaning “water maker.” He had found empirical evidence to support Boyle’s idea; hydrogen and oxygen are elements that join together to form a compound, water. He even produced the first list of chemical elements, which he published in his book Traité Élémentaire de Chimie (Elementary Treatise on Chemistry, 1789). In addition to hydrogen and oxygen, Lavoisier’s list also included nitrogen, phosphorus, sulfur, and seventeen metallic elements. But he also included some substances that are actually compounds, not elements—and his list even included light and heat.
In the nineteenth century, in the spirit of Boyle and Lavoisier, chemists began to discover many more previously unknown elements, giving more credence to Boyle’s definition, and more weight of argument against Aristotle’s ideas.
A selection of Antoine Lavoisier’s laboratory equipment, as shown in his book Traité Élémentaire de Chimie (1789).
A NEW ATOMIC THEORY
Lavoisier’s insight into the relationship between elements and compounds, and his penchant for accurate chemical analysis, led French chemist Joseph Proust (1754–1826) to investigate an idea that most chemists of the time assumed to be true, but which had never been tested. The idea was that when elements form compounds, they do so in definite proportions. So, for example, if 100 grams of a compound contains 60 grams of one element and 40 of another, then in a 200-gram sample of the same compound there would be 120 grams of the first element and 80 grams of the second. (Note how the total mass of elements equals the mass of the compound they produce, in accordance with Lavoisier’s conservation of mass.) In the 1790s and 1800s, Proust carried out a large number of experiments on many different compounds, establishing the truth of what chemists had suspected, in his “Law of Definite Proportions.”
Meanwhile, English chemist John Dalton (1766–1844) was studying gases and, in particular, how mixtures of gases behave. He was already convinced that matter is made of particles, and, in the first few years of the 1800s, he was struck by a simple yet powerful qualification of that idea. He realized that every atom of a particular element must have exactly the same mass as all the others, and that this “atomic weight” would be different for each element. The ratio of the masses of elements in a compound are simply the ratios of the atomic weights of the elements involved. Dalton envisaged compounds as being made of molecules, each made of two or more atoms joined together. This vision made sense of Proust’s Law of Definite Proportions; if it holds true for a single molecule, it would hold true for any number of molecules, even in a sample large and heavy enough to weigh.
Dalton went farther. He realized that in some cases, there might be two or three distinct ratios, corresponding to distinct compounds made up of different combinations of the same elements. So, for example, mercury combines with sulfur to form two compounds, which we now call mercury (I) sulfide (HgS), and mercury (II) sulfide (Hg2S)—and the ratios of mercury to sulfur by mass are 25:4 and 50:4. This was Dalton’s Law of Multiple Proportions, and there seems no reasonable explanation of it other than the notion that compounds are made of atoms with definite weights grouped together.
Dalton discussed and outlined the first robust, modern atomic theory in A New System of Chemical Philosophy (1808). He proposed that atoms are indivisible, and cannot be created or destroyed; all atoms of an element are of the same size and mass, and they have the same properties; atoms of different elements combine in simple ratios to form chemical compounds; and chemical reactions involve a rearrangement of atoms. In his book, Dalton listed the known elements with their estimated atomic weights, relative to the lightest of the elements, hydrogen—the first time anyone had done this. Dalton’s theory united the physical and chemical aspects of matter and laid a firm foundation for modern atomic theory. But it did not become mainstream science for many decades.
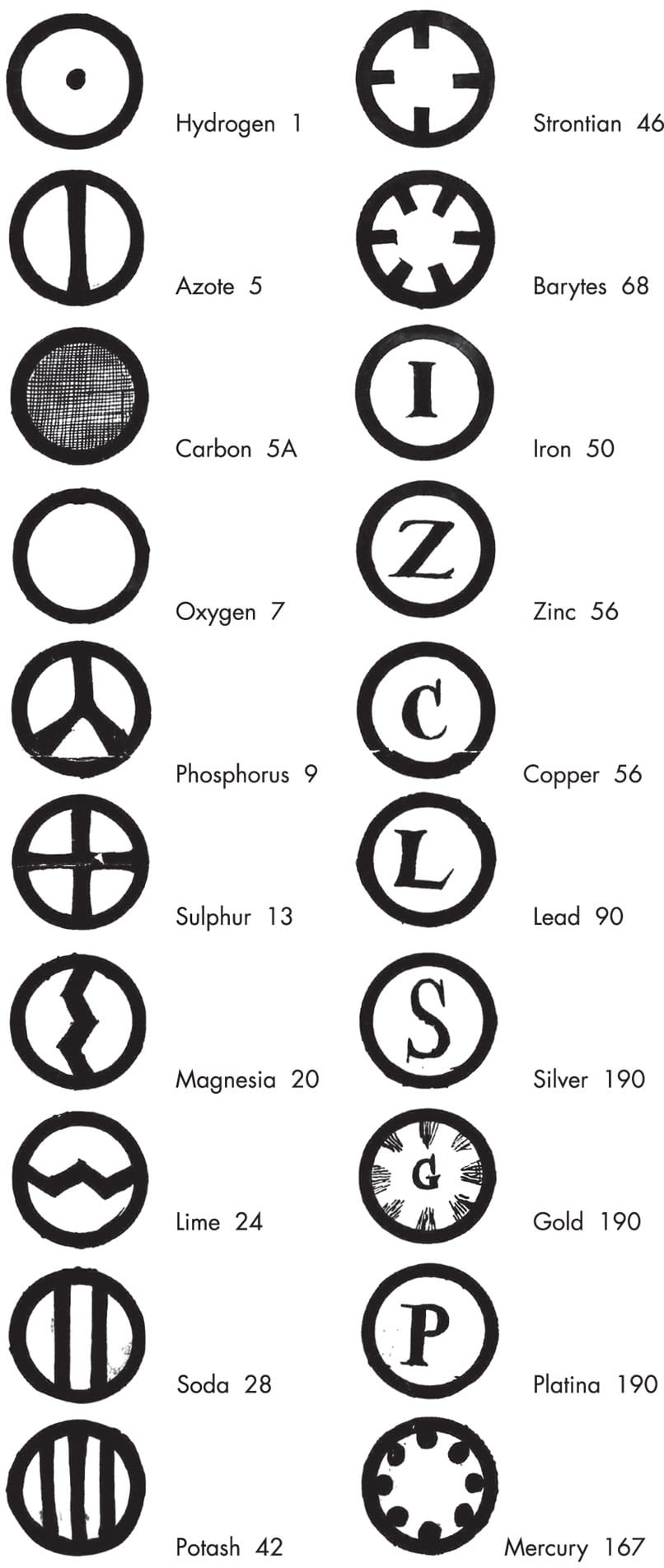
John Dalton used circular symbols for the elements, emphasizing his atomic theory. For Dalton, all the atoms of a particular element had the same mass (the numbers alongside the names, relative to hydrogen, 1). Note that some of the “elements” he lists are actually compounds.
A CONVINCING THEORY
Despite John Dalton’s compelling theory, many nineteenth-century scientists did not believe that atoms could actually be real. But mounting evidence in favor of atomism, both empirical and theoretical, convinced more and more chemists and physicists. By the beginning of the twentieth century, nearly all scientists had accepted that atoms really do exist.
CHEMISTRY—BUSINESS AS USUAL
John Dalton’s atomic theory provided a robust framework for understanding elements, compounds, and chemical reactions in terms of tiny particles. But chemists had little need of a physical theory of matter, so the atom remained a hypothetical object in the eyes of many of them. Despite this, they did measure the “atomic weights” of elements they discovered, and atoms were routinely discussed.
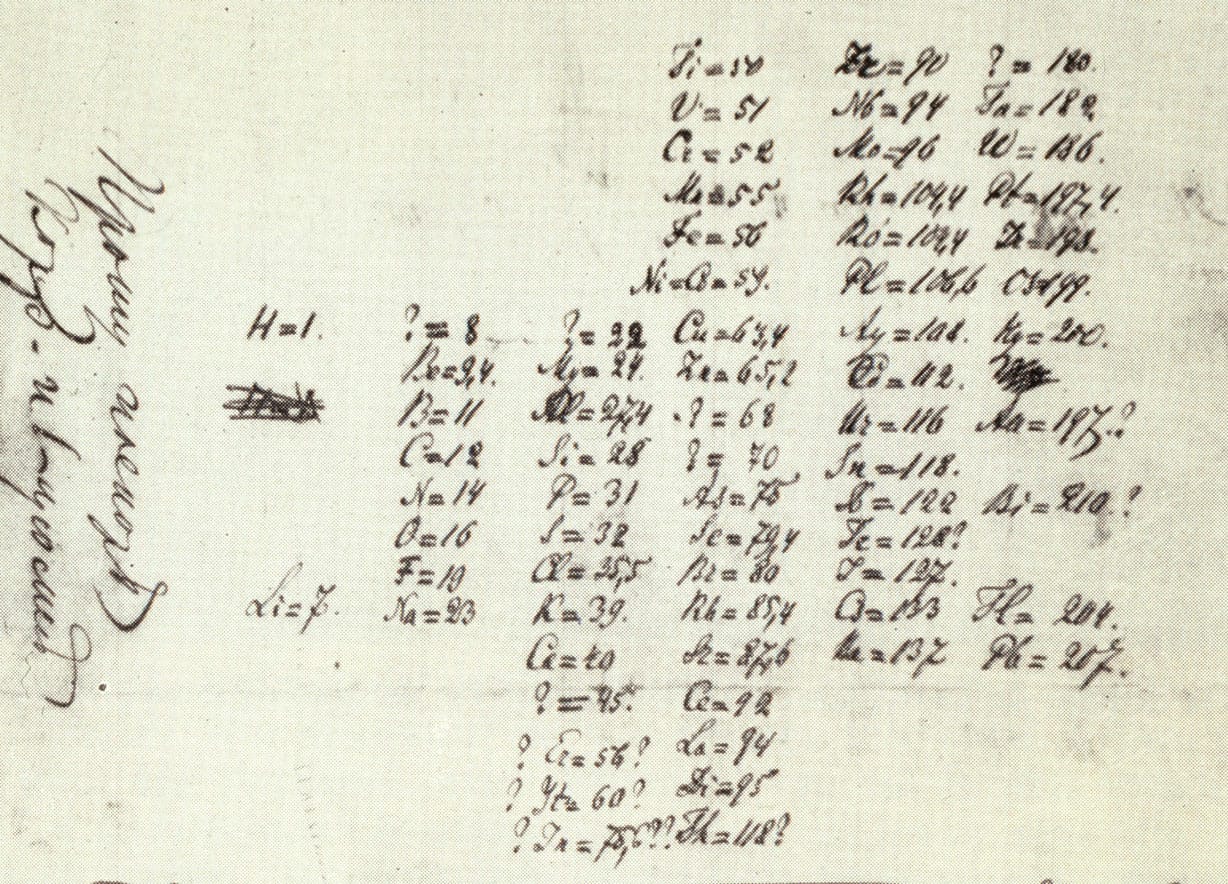
Mendeleev’s handwritten periodic system of the elements (below) is dated February 17, 1869. In honor of Mendeleev (pictured above right), in 1955 a newly discovered element was named mendelevium (Md).

Meanwhile, chemists continued to discover new elements and to investigate a huge range of chemical reactions. One important new tool available to chemists was the electric battery. Investigations of matter using electricity hinted that matter itself has electrical properties. Several scientists used batteries to separate water into hydrogen and oxygen in 1800, just a few months after the invention of the device. English chemist Humphry Davy (1778–1829) used a powerful battery to decompose many compounds into their elements, discovering several elements in the process, among them sodium and potassium (both in 1807). Davy’s colleague Michael Faraday (1791–1867) carried out extensive research into the effect of electricity on chemical compounds dissolved in water or acids. In 1834, he coined the term “ion” for an electrically charged particle that would migrate toward one or other electrodes placed in a solution. Decades later, Swedish chemist Svante Arrhenius (1859–1927) used the term in his theory of how compounds dissolve in water: by dissociating (breaking down) into ions. Arrhenius suggested that atoms contain several charged objects, and that these could be lost or gained, giving the atom a net positive or negative charge. He had predicted the existence of the electron.
Another important tool for chemists was the spectroscope: a device that allowed for careful analysis of the spectrum of light emitted by objects. In the 1820s and 30s, several scientists noticed bright lines in the spectrum of the light given off by certain chemical compounds heated in flames. The particular pattern of lines was characteristic of the elements that were present in the compounds. German chemist Robert Bunsen (1811–99) and physicist Gustav Kirchoff (1824–87) systematically recorded the spectrum of each of the known elements. They discovered the elements cesium (1860) and rubidium (1861) when they observed patterns of bright lines that had not been seen previously. In the hands of other scientists, Bunsen and Kirchoff’s approach was to lead to the discovery of several other elements. Among them was helium, discovered by studying the spectrum of the Sun in 1868, decades before it was found on Earth.
By the 1860s, more than fifty chemical elements were known, and scientists began to notice patterns in their behavior. The elements seemed to fall into groups according to their physical properties, as well as their chemical properties, such as the type of compounds they formed. English chemist John Newlands (1837–98) and German chemist Julius Meyer (1826–1909) noticed that the members of a particular group seemed to be at regular intervals, or periods, in the list of elements when arranged by increasing atomic weight. Meyer constructed a table of some of the elements, with similar elements grouped together. But the periodicity was not perfect, not quite regular enough. Russian chemist Dmitri Mendeleev (1834–1907) was so confident in the periodic nature of the list of elements that he simply left gaps in his “periodic table” to represent elements as yet undiscovered, so that these elements could fall into the right groups. Over the next few decades, Mendeleev was proved right; all the elements he had predicted were discovered. We will examine the periodic table, and how it relates to the inner workings of the atom, in chapter three.
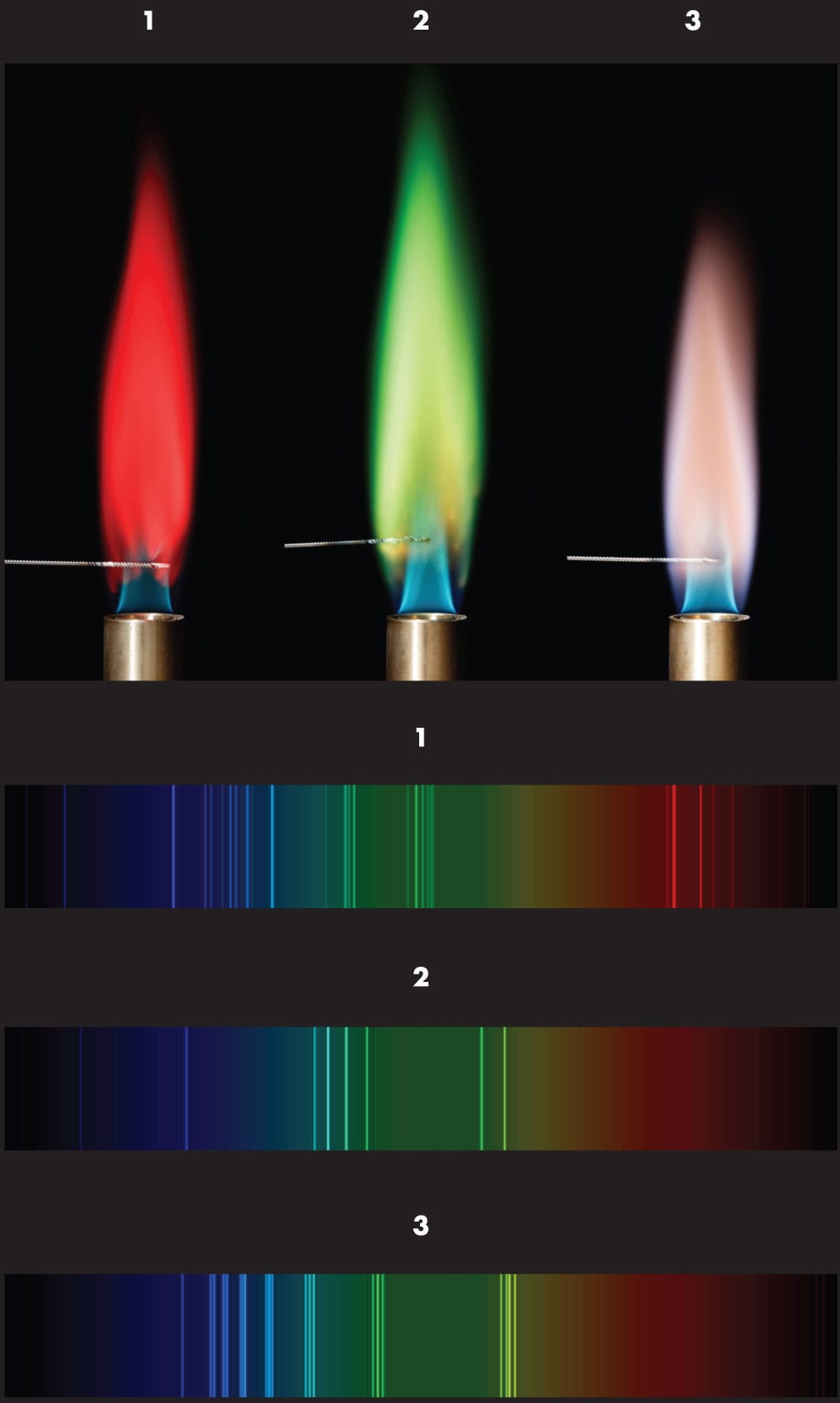
Compounds containing metal held on a wire in a hot flame emit light with colors characteristic of the metallic elements present. Above are: strontium (1), copper (2), and potassium (3). Splitting the light with a spectroscope reveals bright lines at certain parts of the spectrum. This emission spectroscopy was used in the discovery of several previously unknown elements in the nineteenth century.
PHYSICISTS ON ATOMS
While chemists were busy discovering and cataloging new elements, nineteenth-century physicists were trying to make sense of basic phenomena, such as light, heat, electricity, and magnetism. In the previous century, scientists had seen each of these phenomena as a distinct “imponderable fluid” that surrounds or penetrates objects and could be transferred between them. Heat fluid, for example, would flow from a flame to a cooking pot. Increasingly, however, new theories and evidence were undermining the imponderable fluids idea. For example, the discovery of electromagnetism in 1820 showed that electricity and magnetism are intimately linked, so they cannot be separate “fluids.”
Electromagnetism is also intimately related to light. In the 1860s, British physicist James Clerk Maxwell (1831–79) found that light is a wave of undulations in electric and magnetic fields. He had discovered that light is a form of “electromagnetic radiation,” which is produced whenever electric charges are accelerated (their speed or their direction of movement is changed). Light, then, was also not an imponderable fluid.
The idea of heat as a fluid had also been overturned. In its place physicists revisited a notion suggested by several scientists in the past—that heat and temperature are related to the movement of the particles of matter. Heat up a solid substance, and you increase the vibration of the particles of which it is made. If you heat it sufficiently, the particles can break away from each other just enough to move past each other and the solid becomes liquid. Heat a liquid and the particles can break away completely and fly around at high speed, just as Bernoulli had envisaged (see here).
DISTRIBUTION OF PARTICLE SPEEDS AT DIFFERENT TEMPERATURES
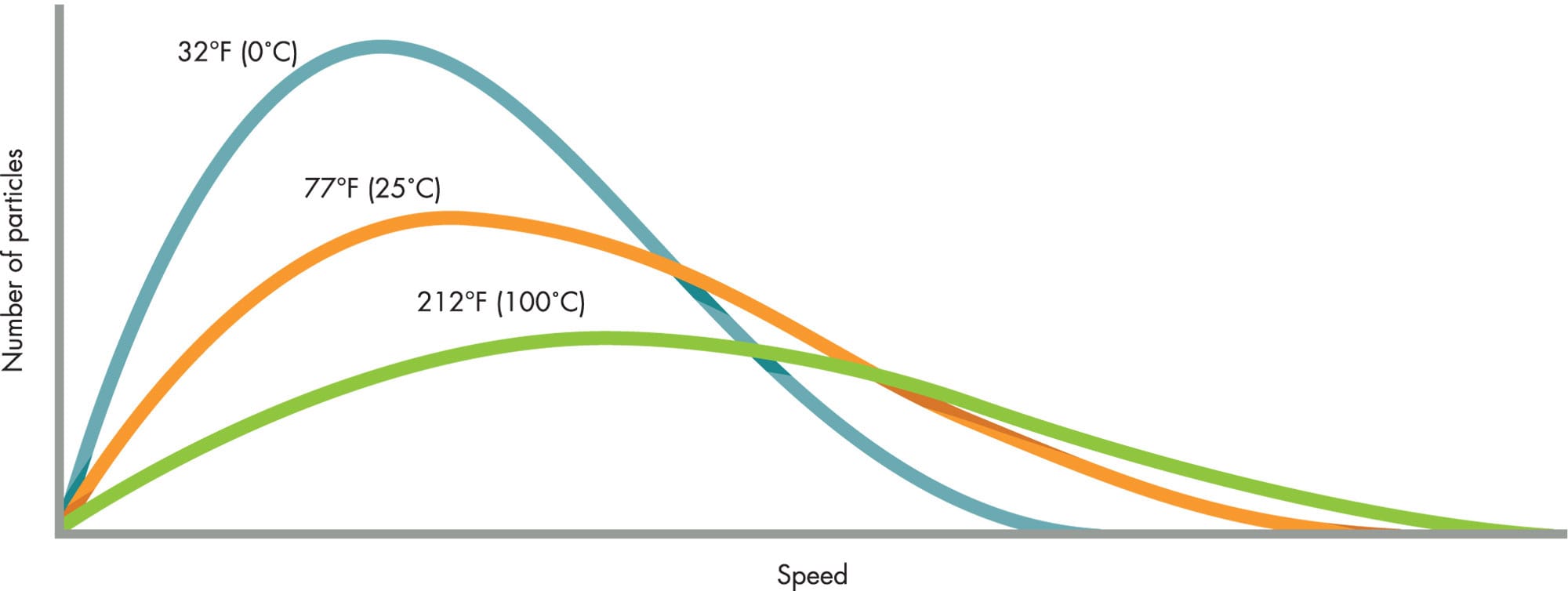
Curves representing the distribution of speeds among the particles of a gas at different temperatures. The total number of particles is the same in each case.
In 1859, Maxwell realized that with so many gas particles colliding randomly, there would be a range of speeds among them. A few would be barely moving, most would have speeds close to the average, and some would be traveling much faster. He used the mathematics of probability to work out the statistical distribution of the particles’ speeds. Austrian physicist Ludwig Boltzmann (1844–1906) improved on Maxwell’s work in the 1870s. The “Maxwell-Boltzmann distribution” helped scientists to make predictions and gain new insight about gases. More importantly, Boltzmann founded “statistical mechanics”—a new branch of science that explains how the properties of countless tiny atoms can determine everyday properties of matter.
CONVINCING THE SKEPTICS
Despite the explanatory power of the Maxwell-Boltzmann distribution and the good sense that atomism made in chemistry, significant numbers of physicists and chemists still rejected atomism, thinking it was just an interesting hypothesis. The last doubters were eventually convinced in 1905, when German physicist Albert Einstein (1879–1955) derived a mathematical explanation for a phenomenon called Brownian motion. In 1827, British botanist Robert Brown (1773–1858) observed through his microscope tiny dust particles moving around in a jittery motion, as if they were being pushed around by even smaller particles that were too small to see. Einstein’s mathematical analysis of Brownian motion proved that the motion of the dust particles was due to the random bombardment of water molecules. Atoms and molecules were real—and matter really was made of tiny particles. However, it was not quite that simple; by now, physicists knew that atoms were not indivisible after all.
BROWNIAN MOTION
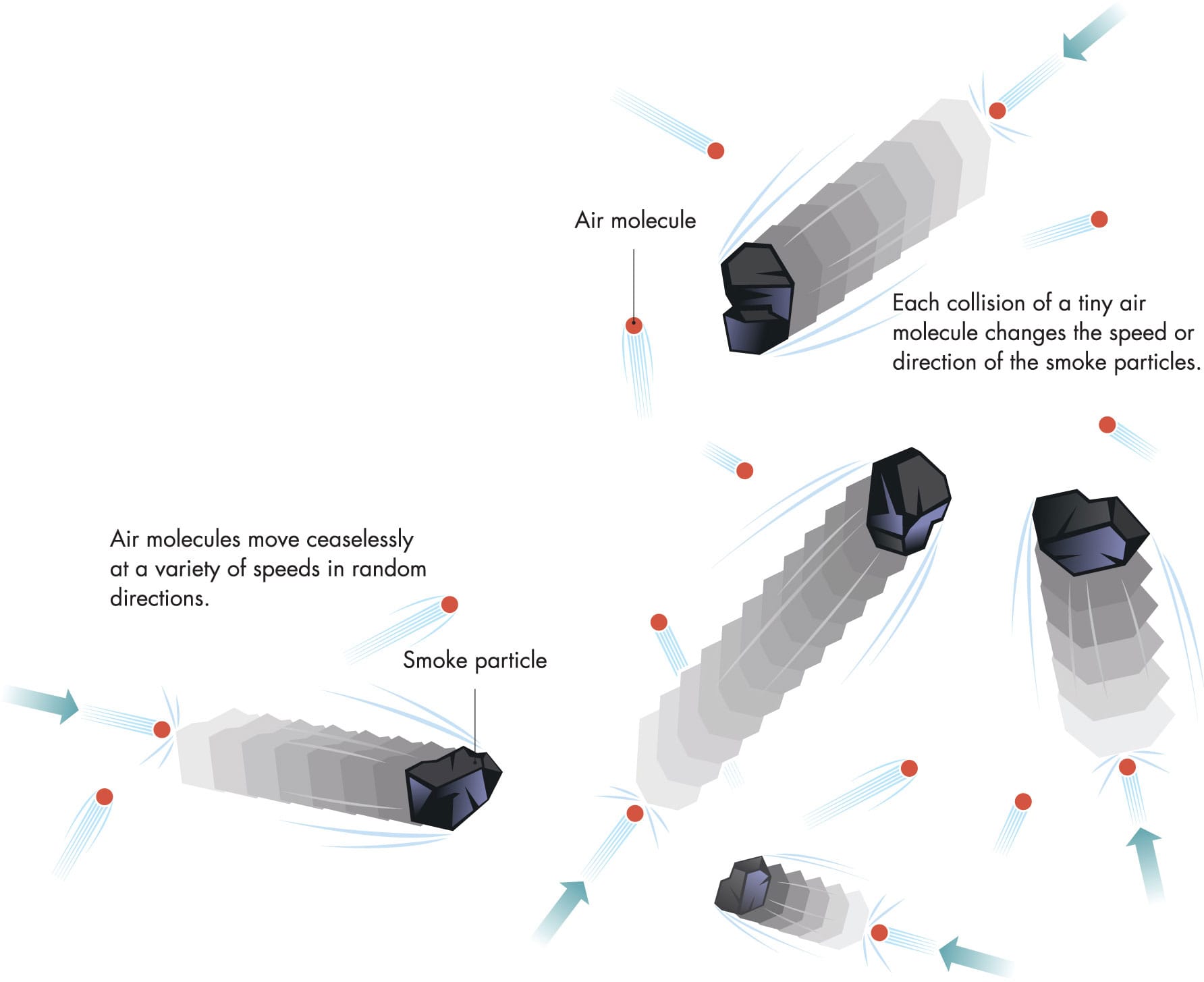
Robert Brown discovered the random buffeting of small particles, known as Brownian motion, when he was looking at pollen dust under a microscope. The phenomenon is best seen with smoke particles in air, because air molecules move faster than water molecules.
INSIDE THE ATOM
With the existence of atoms finally confirmed, the first half of the twentieth century saw an incredibly rapid accumulation of knowledge about the atom’s inner workings. Using a host of new tools and guided by innovative theories, physicists uncovered a bizarre world in which particles are waves and waves are particles—where the laws of nature challenge intuition.
ATOMIC STRUCTURE
In 1897, British physicist Joseph John (J. J.) Thomson (1856–1940) discovered the electron, a particle with negative electric charge. It was much smaller than an atom, and Thomson realized that electrons must in fact be present in every atom: “As corpuscles of this kind can be obtained from all substances, we infer that they form a constituent of the atoms of all bodies,” he wrote.
Physicists knew that each atom must have equal amounts of positive and negative charges, so that it would have no charge overall. In 1904, Thomson proposed that the “negatively charged corpuscles,” as he called electrons, might be arranged in rings inside a fuzzy sphere of positive charge. This idea came to be called the “plum pudding model” of the atom; the electrons were the plums and the positive charge the pudding.
One year before Thomson discovered the electron, French physicist Henri Becquerel (1852–1908) was experimenting with compounds of uranium. Becquerel discovered what Polish-born physicist Marie Curie (1867–1934) would two years later call “radioactivity.” With her husband Pierre (1859–1906), Curie discovered that radiation was coming from the uranium atoms themselves and was not the result of a chemical reaction.
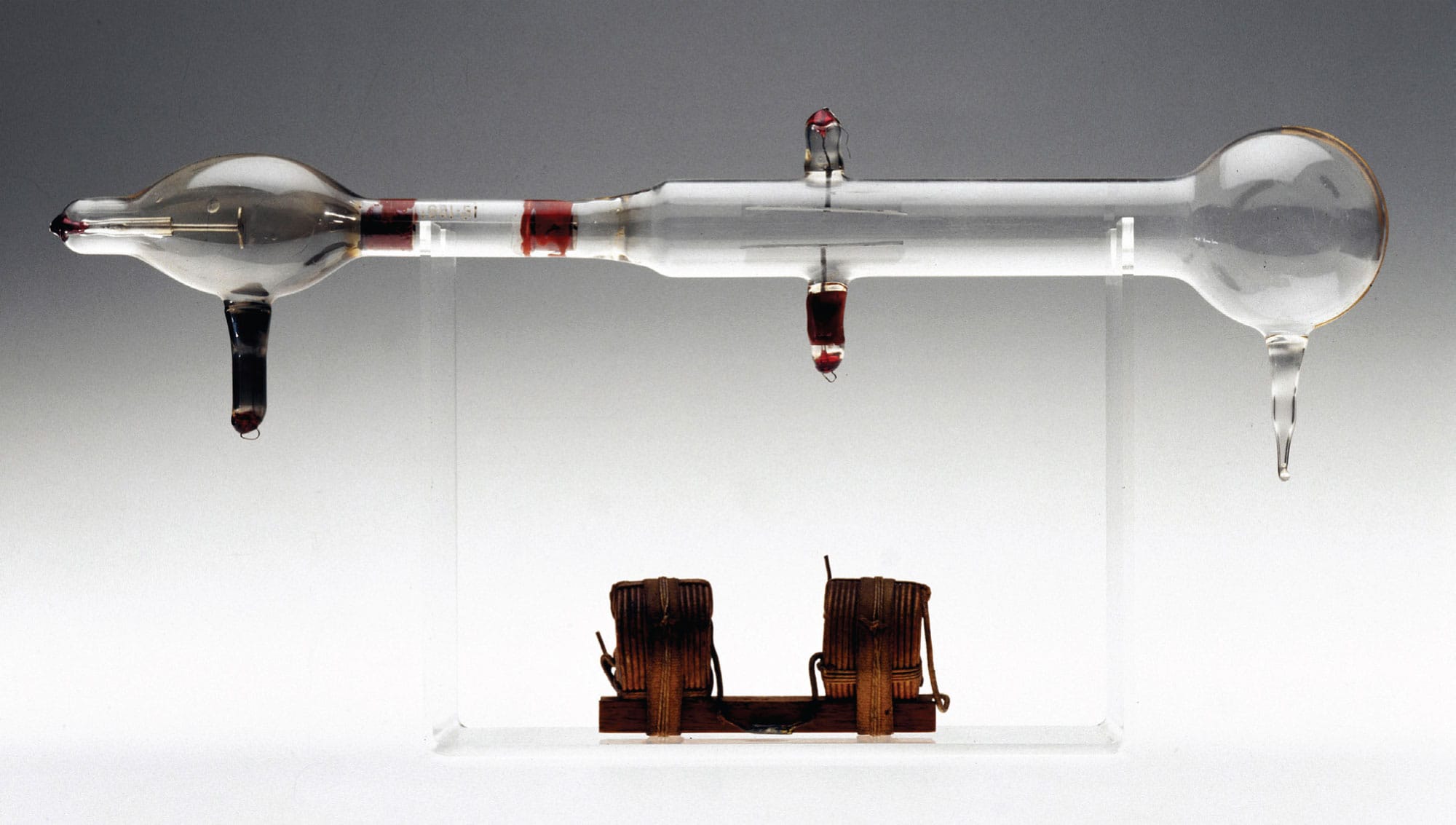
J. J. Thomson discovered the electron using a piece of equipment called a cathode ray tube. Electromagnets and electrified metal plates inside the tube deflected the “cathode rays,” a beam of electrons, which helped Thomson to estimate the mass and charge of the particles.
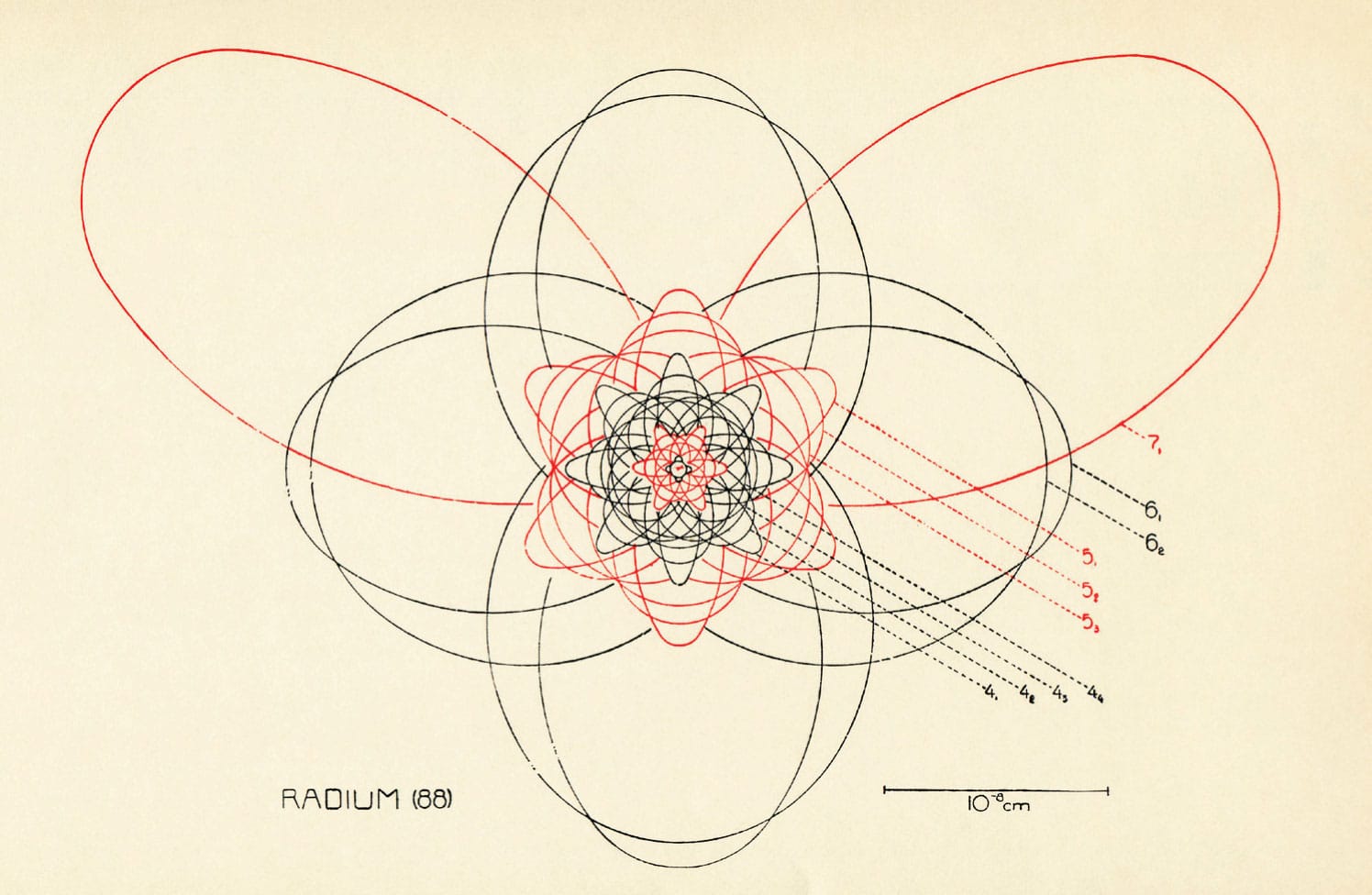
Diagram showing the atomic structure of the chemical element radium (discovered in 1898), based on the model of the atom proposed in 1913 by Danish physicist Niels Bohr and refined by German physicist Arnold Sommerfeld. This diagram was published in The Atom and the Bohr Theory of Its Structure (1926) by Hendrik Anthony Kramers and Helge Holst, originally published in Danish in 1922.
In 1899, New Zealand-born British physicist Ernest Rutherford (1871–1937) found that radioactive substances emit two distinct types of radiation, which he called alpha (α) and beta (β) rays. In 1900, French chemist Paul Villard (1860–1934) discovered a third type, termed gamma rays (γ). Alpha rays are streams of positively-charged particles, and they were to become especially useful tools for probing the atom. In 1911, for example, Rutherford used them to explore the distribution of electric charge in the atom (see box). The results ruled out Thomson’s plum pudding model. Instead, they showed that the atom’s positive charge is concentrated in an extremely tiny object at the center, which Rutherford called the nucleus.
Rutherford proposed a model of the atom in which the electrons orbit the tiny, dense nucleus, held in their orbit by the attraction between their negative charge and the positive charge of the nucleus. This is similar to how the planets of the solar system are held in their orbits by gravitational attraction to the Sun. However, there was a major problem with this model. Any orbiting object constantly changes direction, and, because changing direction constitutes an acceleration, an electron constantly radiates electromagnetic radiation (see here). As it does so, it loses energy, so it orbits lower and lower, spiraling inexorably down toward the nucleus.
WAVES AND PARTICLES
For a solution to Rutherford’s problem, Danish physicist Niels Bohr (1885–1962) looked to a new branch of physics, quantum theory, whose effects are noticeable only at the atomic scale. According to quantum theory, only certain levels of energy are “allowed” in any given system. Because electrons’ energy levels are determined by the distance at which they orbit the nucleus (the farther away, they are the more energy they have), allowed energy levels means “allowed orbits.” In Bohr’s (1913) model of the atom, electrons are not able to spiral into the nucleus or continuously to emit radiation. But they are able to jump between orbits, either when they absorb energy from incoming electromagnetic radiation (to jump up a level) or by emitting electromagnetic radiation (to jump down). The frequency of the radiation depends upon the difference in energy between the two levels.
DE BROGLIE EXPLANATION
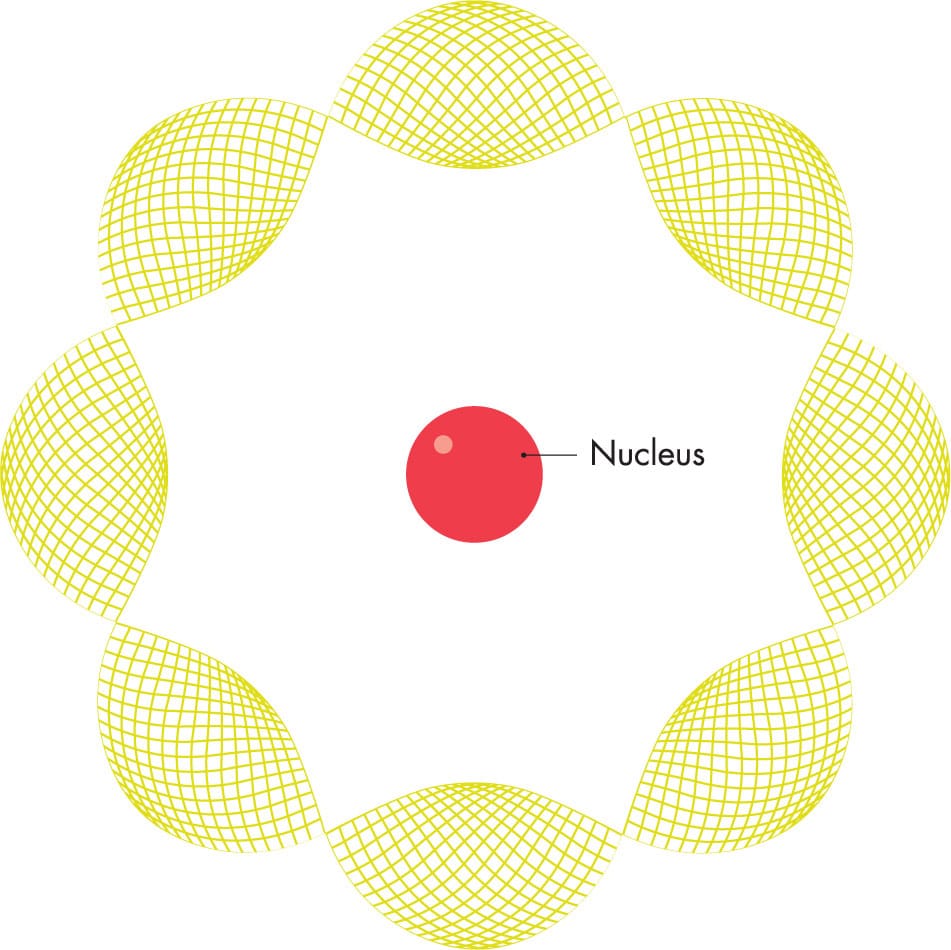
Louis de Broglie suggested that
electrons are wavelike, with a wavelength corresponding to their energies. The wavelengths fitted perfectly into Bohr’s allowed orbits.
BOHR ATOMIC MODEL
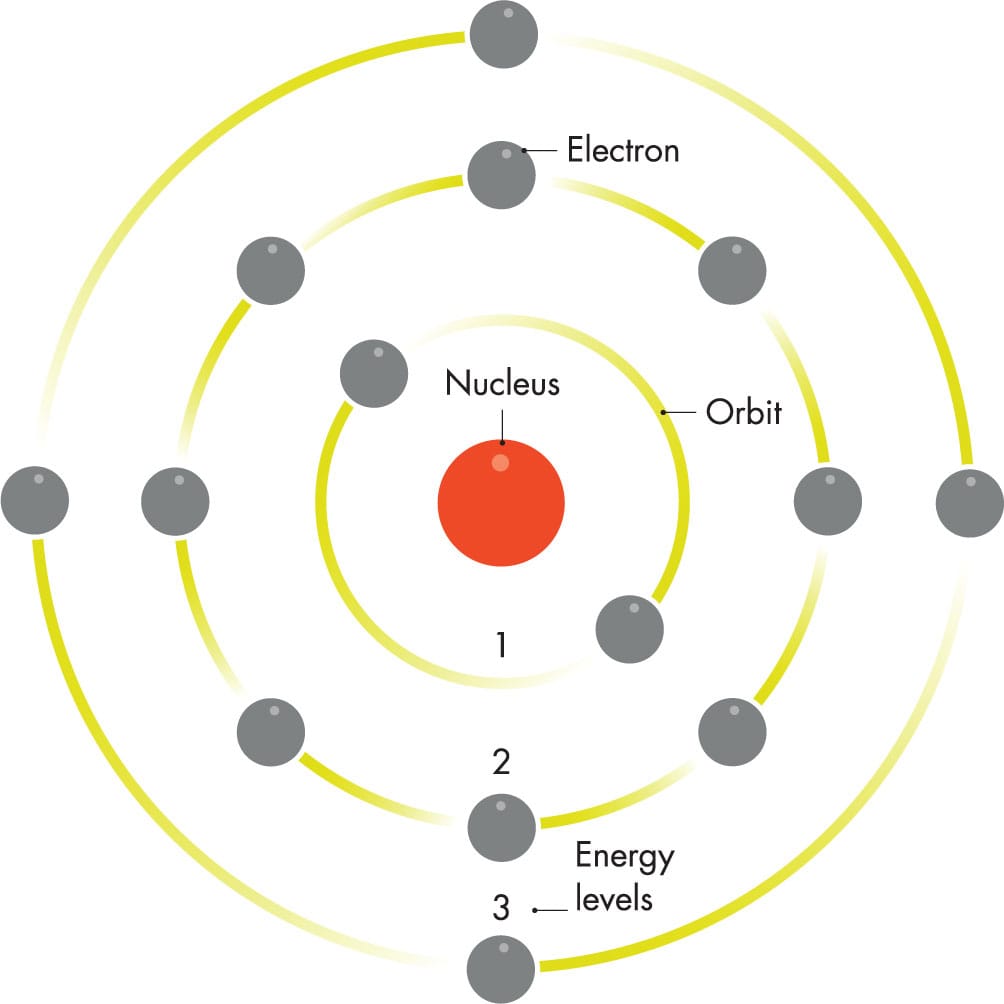
Niels Bohr proposed that an atom’s electrons orbit the nucleus only in specific orbits, and that electrons emit light of specific wavelengths as they “fall” from higher to lower orbits.
When Bohr put numbers into his model that correspond to real values, such as the electric charge on an electron, he calculated exactly the frequencies of the red, blue, and ultraviolet light that real hydrogen atoms emit. Bohr’s hypothetical orbits seemed to match what spectroscopists had found (see here).
A few years before Bohr devised his atomic model, Einstein discovered that light and other electromagnetic radiation are formed of a stream of particles, which he named “photons,” as much as the waves described by James Clerk Maxwell (see here). In 1924, French physicist Louis-Victor de Broglie (1892–1987) suggested that this “wave-particle duality” might also apply to entities that had always been considered as particles—in particular, to electrons. De Broglie reenvisaged Bohr’s orbits, fitting “electrons as waves” around the orbits instead of a solid moving particle. Again, the numbers worked out perfectly; the distances at which the electron waves fitted exactly around the nucleus corresponded perfectly with Bohr’s orbits.
SCHRÖDINGER WAVE FUNCTION
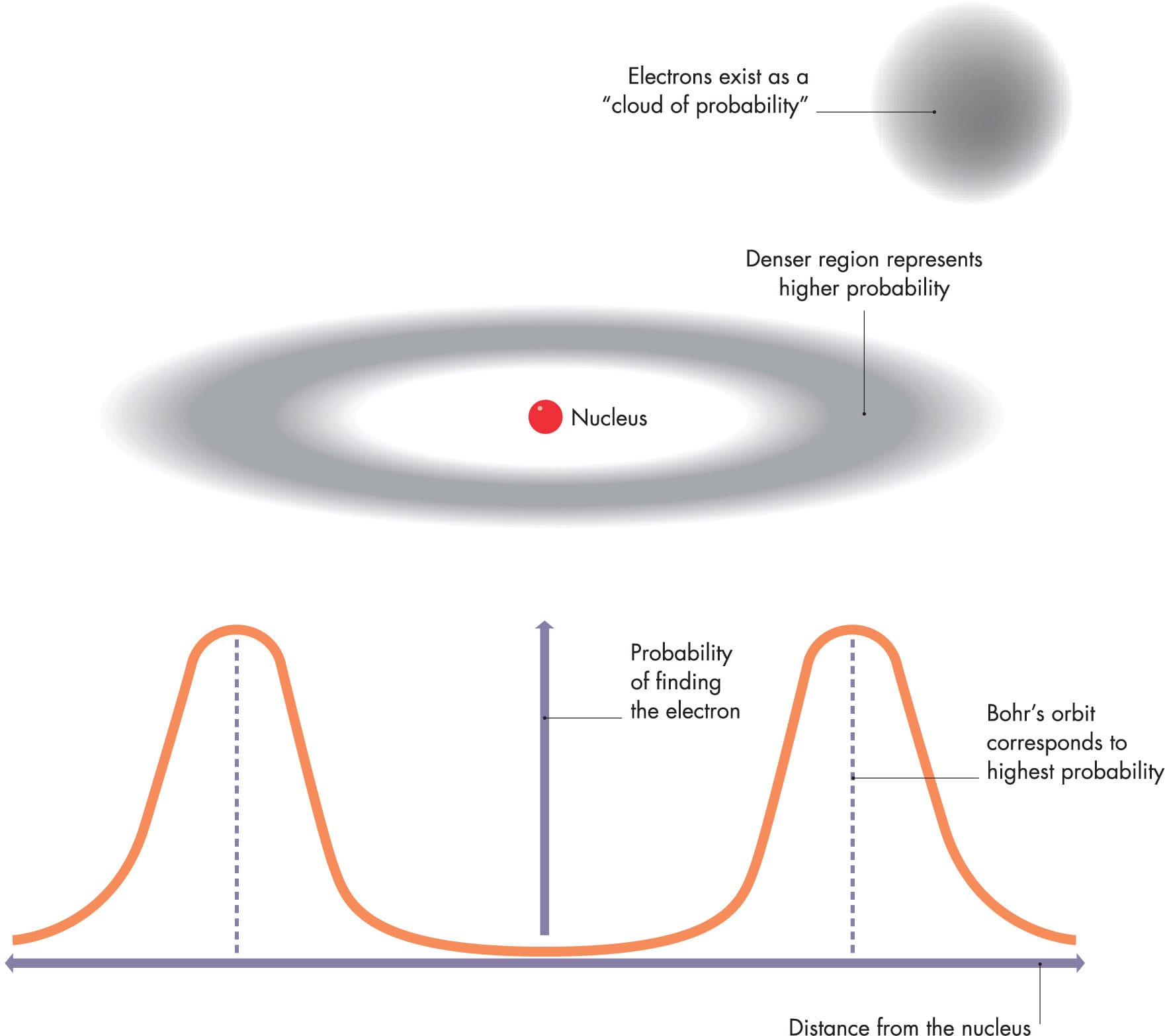
The wave function of a hydrogen atom as a graph of the probability of an electron being at a particular location, plotted against distance from the nucleus—from zero at the nucleus to a maximum, then tailing off to zero again. In three dimensions, the graph describes a hollow “cloud” of varying probability density.
The following year, Austrian physicist Erwin Schrödinger (1887–1961) formulated an equation that allowed physicists to calculate and predict the behavior of wavelike particles (or particle-like waves) in atoms. The Schrödinger equation predicts the behavior of electrons in atoms, and in fact any quantum system, as a “wave function”: a mathematical construct that determines parameters such as the position and speed of a particle. Bizarrely, perhaps, the wave function can actually give only the probability of those parameters having certain values at certain times and places. So not only does an electron behave as both a wave and particle, it exists throughout a particular region of space, as a “cloud of varying probability.” We will explore the strange rules of the quantum world, including some of the later developments in understanding electrons, later in the book.
INTO THE NUCLEUS
In 1911, Dutch physicist Antonius van den Broek (1870–1926) proposed that each element has a unique “atomic number,” which would be equal to the number of positive charges in the nucleus. A theory from a hundred years earlier had suggested something similar. English chemist William Prout (1785–1850) had noticed that atomic weights seemed to be exact multiples of the atomic weight of hydrogen, and suggested that the atoms of other elements might be built up as aggregations of hydrogen atoms. Prout called his hypothetical prototype atom a “protyle.” In 1917, Ernest Rutherford proved that the nucleus of a hydrogen atom, with a charge of +1, is indeed found in other nuclei. Rutherford gave it the name “proton.”
It was clear, however, that the nucleus must contain another type of particle, because doubling the atomic number (the amount of positive charge in the nucleus, equal to the number of protons) would more than double the atomic weight. For example, while hydrogen has atomic number 1 and atomic weight 1, helium, with atomic number 2, has a weight of 4. In 1920, Rutherford proposed that another particle must inhabit the nucleus, and suggested it is a combination of a proton plus an electron. The combination of proton (+) plus electron (-) would give this particle a neutral charge overall, so Rutherford named it “neutron.” British physicist James Chadwick (1891–1974) discovered the neutron in 1932, but it was not a combination of a proton and an electron: it was a particle in its own right.
One of the big mysteries of the 1930s was just how the nucleus could hold together. After all, protons are all positively charged, and objects carrying electric charge of the same type repel. Packed so closely together in the nucleus, the forces pushing the protons apart must be very strong. Several scientists suggested that there must be an attractive force that holds together the protons and neutrons inside the nucleus. Because neutrons contribute to the attractive force but not the repulsive one (because they have no electric charge), they act as a kind of glue to hold the nucleus together.
In 1935, Japanese theoretical physicist Hideki Yukawa (1907–81) suggested a mechanism by which this “strong nuclear force” might work. He proposed that protons and neutrons constantly pass between them mediating particles called “mesons”—and that the exchange of these particles is the source of the force. It was a bold theory but, nevertheless, Yukawa’s mesons were discovered in 1947. We will explore the nucleus, including some of the later experimental and theoretical developments, in later chapters.
A DEEPER ATOMISM
Yukawa’s meson was a member of a burgeoning family of tiny particles, all much smaller than atoms. These particles were being discovered in cosmic rays (streams of particles coming from space) and in particle accelerators. In 1964, American physicist Murray Gell-Mann (born 1929) suggested that some subatomic particles are “composite”—made up of yet smaller particles, called quarks. According to Gell-Mann’s theory, protons and neutrons are composed of three quarks, while other particles, such as mesons, are composed of two. Other particles, such as the electron, are truly fundamental. The current best theory for understanding the plethora of fundamental and not-so-fundamental particles—the bastion of modern atomism—is the Standard Model, which we explore in chapter seven.