Steve Moore was once quoted in the New York Times as saying that “our oil supply is infinite. We will never run out,” a statement that provoked outrage. One high school science teacher wrote, “Mr. Moore: Even my fourteen-year-olds know that oil is finite.” That teacher probably became a top science advisor to President Obama.
Oil is the master resource because everything else depends on it. But it is also the most unappreciated resource. Political and opinion leaders are convinced that we have a moral obligation to “conserve” energy, that every barrel of oil we use is one that our children and grandchildren won’t have. Yet the truth is, as the Financial Times has reported, the world “is drowning in oil.”
The Institute for Energy Research recently published a study showing (1) the government’s best estimate of how much oil we had in America fifty years ago, (2) how much of that oil has been drilled out of the ground since then, and (3) what reserves remain (see Figure 4.1 on the following page). Today we have twice the reserves that we had in 1950. And we have already produced almost ten times more oil than the government told us we had back then.
FIGURE 4.1

Technology and innovation have constantly increased the amount of “finite” oil we can produce. Because we discover new sources of oil much faster than we deplete known reserves, oil and natural gas supplies are for all practical purposes nearly inexhaustible. Fracking is the latest game-changer, and the access it gives us to shale oil and gas resources has virtually doubled overnight. And this technology boom in drilling is just getting started.
To become more prosperous over time, we don’t want to conserve energy, we want to use it. Why? Because high energy consumption is an indispensable condition for a modern, prosperous society. Try to imagine your life without the man-made energy flowing through every moment of your day. We are so dependent on energy that its presence, action, and value go unnoticed. And almost all of that energy comes from fossil fuels. Anyone who talks about “decarbonizing” our economy is talking about demodernizing it as well.
Modern Life Uses a Lot of Energy
What do we use our energy resources for? That seems like a question with a patently obvious answer, but it’s shocking how few people know where energy comes from and how it is used. When we speak to high school and college kids, we ask, “Where does your electricity come from?” And most of the kids point to the outlet in the wall. Ah, the millennials. They know everything, don’t they? For those who are wiser and know what they don’t know, here are some energy basics.
The United States consumes around one hundred “quads” of heat energy—that is, one hundred quadrillion British thermal units—every year.1 We use about 40 percent of our energy for electricity, 30 percent for mobility, and 30 percent for “raw heat” to warm ovens and hair dryers and to power countless manufacturing processes. And approximately six of the one hundred quads are converted into “ordered forms of precise power capable of driving radios, microprocessors, lasers and CAT scanners.”2 As Peter Huber and Mark P. Mills remind us in their provocative book The Bottomless Well, since the Industrial Revolution, the conversion of raw heat into focused power has been on a trajectory toward faster, lighter, stronger, cleaner, and more precise power generated within less space.3 The modern wind turbines and solar farms covering millions of acres represent a regression from this improving environmental trend.
Mechanical power was the first pivotal, man-made conversion of thermal energy. Power to move and lift is no longer derived from the backs of men or the muscle of animals. “It is precisely the availability of inanimate sources of power that has enabled man to transcend the limitations of biology and increase his productivity a hundred times over.”4 Electricity was another transformative refinement of thermal energy that vanquished darkness, easing and amplifying human life. Electric power still fuels our work and personal freedoms all day every day. It gives us instant, automatic, compact, silent, clean access to individualized energy services of limitless variety. It provides our food, materials, and mobility and does the work that once required “relentless drudgery,” as the historian Gregory Clark characterizes hard manual labor.
In a more recent development, electricity now delivers knowledge. Electric power is “the sole energizer of electronically transmitted information with unmatchable control, precision, and speed.”5 No energy, no Internet, no Google, Facebook, or Netflix. The vast system supporting the information-communication-technology (ICT) universe now consumes 10 percent of all electric generation, a share that is projected to grow enormously as the use of digital technology increases in developing countries. This estimate accounts for data centers, wired and wireless broadband networks, and devices like personal computers, tablets, and smartphones. This tally does not include the energy powering the manufacture of this hardware. As Mark Mills puts it, “The cloud begins with coal.”6
The ICT system uses approximately 50 percent more electricity than global aviation. Imagine consumers’ response to an EPA rule rationing our access to that system! The essential data centers must have reliable electric power twenty-four hours every day. Wind and solar power, inherently intermittent sources of electric generation, are incapable of providing the digital universe with the reliable generation for which coal remains the mainstay.
Hydrocarbon energy is also embedded in the majority of all the materials used in every home and workplace. Compounds derived from fossil fuels are the raw materials for thousands of synthetic materials. How would a zero-carbon economy replace these goods and the services they provide?
Utterly Dependent on Fossil Fuels
The increase in energy consumption since the Industrial Revolution is staggering. Western Europeans consume on average six to seven times more energy per day than did their forbears of two centuries ago,7 and the average American’s daily consumption of energy is twice that of the Europeans. Some condemn our consumption as profligate, while others simply stand in wonder at how abundant energy has enhanced the lives of people of every income level.
FIGURE 4.2
U.S. Energy Consumption by Source, 2013

Source: U.S. Energy Information Administration (EIA)
Only a trace of those hundred quads that Americans annually consume derives from renewable energy sources. Modern civilization is still utterly dependent on fossil fuels. Non-fossil-fuel energy sources—wood, wind, solar, hydro, geothermal, and nuclear—make up less than 15 percent of the world’s total primary energy supply.8 Nuclear power and hydroelectric power generate far more electricity than all the other renewable sources combined. After several decades of aggressive subsidy, promotion, and even mandates, non-hydroelectric renewable energy sources (wind and solar) in the United States account for only about 3 percent energy consumption.9 See Figure 4.2 above.
Nevertheless, senior policymakers in the wealthiest and most educated Western countries remain intent on quickly deriving at least 80 percent of our energy from renewables. Their refusal to take into account fundamental laws of arithmetic and physics threatens the supply of affordable energy and therefore the foundations of modern prosperity. Michael Kelly, a professor of engineering at Cambridge University and a fellow of the Royal Society, explains the scope of what they propose: “Since 90 percent of global improvement in mankind’s estate since 1800 has been enabled by burning fossil fuels, the scale of the decarbonization project is without historical precedent.”10
Exponential growth in energy consumption is a defining characteristic of industrialized countries. Developed countries already consume enormous amounts of energy and will continue to do so. And developing countries, if they wish to become developed countries, will consume more energy as well. The global supply of fossil fuels and primary electricity has increased more than one hundred and sixty times since 1850,11 and the supply must continue to grow unless mankind is going to take a giant step backward.
As long as the sun continues to shower us with light and heat, our energy supply will be unlimited. But “energetic order”—usable power—can be costly. We spend more “on equipment used . . . to concentrate and convert energy—generators, furnaces, car engines, motors, and light bulbs, for example”—than we spend on raw energy itself.12 The supply of energy available for human purposes has not only grown by tremendous volumes, but the extraction, processing, conversions, and end uses of energy have achieved astonishing economic efficiencies as well as environmental sensitivity in prosperous countries. In developed, industrialized economies, we get two to four times more power per unit of energy source than a century ago. Societies are spending less money for far greater energy output. And the physical footprint of our energy infrastructure keeps shrinking. If every computer was as large as the 1960s mainframes, the Internet would not exist. Thanks to tiny microprocessors that operate as the intelligent engines of our computers, we have the Internet, the cloud, and our super smartphones.
And here we encounter the fatal flaw of the renewables so enthusiastically promoted today as a means of averting dangerous climate change. Technologically enhanced from their pre-industrial forms, today’s renewable energy systems are less energy efficient than their hydrocarbon counterparts. In some cases, it takes more electricity to generate electricity from a wind turbine than the turbine produces—hardly a formula for sustained economic growth.
Energy and the Poor
No one has a bigger stake in sound energy policy than the billion human beings in poor countries who still live without electricity. The policies of the U.S. government and of institutions like the World Bank and the International Monetary Fund condition financing for electrification on the use of inferior and expensive renewable energies. As Bjorn Lomborg notes, “In a world in which malnourishment continues to claim at least 1.4 million children’s lives each year, 1.2 billion people live in extreme poverty, and 2.6 billion lack clean drinking water and sanitation, this growing emphasis on climate aid is immoral. . . . Green energy sources may be good to keep on a single light. . . . But they are largely useless for tackling the main power challenges for the world’s poor.”13
The callousness of the doctrinaire warmists is chilling. In an editorial titled “Sacrificing Africa for Climate Change,” Caleb Rossiter, a professor of statistics at American University and a former fellow of the Institute for Policy Studies (IPS), scathingly observed, “Western policies seem more interested in carbon dioxide levels than in life expectancy.”14 For departing from the left-wing orthodoxy on climate policy, Dr. Rossiter’s twenty-year fellowship at IPS was summarily terminated.
Just What Is Energy?
Definitions and distinctions are important. Basic physics distinguishes “energy,” “power,” “force,” and “work.” Aristotle coined the word “energy” by combining the Greek preposition en, meaning “at” or “in,” with the noun ergon, meaning “work.” The dictionary definition of energy—“the capacity to do work”—is useful, but mechanical, or kinetic energy, expended in physical work like typing, pounding a nail, or lifting a book, is only the most visible form of energy; there are many others. “Power,” frequently confused with energy, is defined as energy “at work,” the “rate of energy use,” or more precisely, “the rate at which work is done.”15 “Work” is defined as force multiplied by the distance or resistance through which force acts.
Although it’s everywhere, energy remains an elusive phenomenon even to scientists who have devoted their lives to studying it. Most of the public discussion about global warming reflects an understanding of energy that is shallow at best or totally incorrect at worst. Perhaps the most damaging misconception is the prevalent assumption that wind, solar, or biomass (wood and plants) can accomplish the work now performed by oil, natural gas, and coal. Policies intended to reduce man-made carbon dioxide by 80 to 85 percent represent a huge gamble with humanity’s future.
A grasp of a few hard facts, a little arithmetic, and some basic physics are necessary to avoid calamitous blunders in energy policy. We will therefore take a detour through eighth-grade biology and tenth-grade physics to clarify some fundamental properties about energy. We will examine basic forms, measures, and functions of energy usually missing from current policy debates about fossil fuels.
Forms of Energy
Basic physics distinguishes multiple forms of energy observable in both natural and human processes. The most fundamental forms are heat (thermal), motion (mechanical or kinetic), chemical (food and fuel), and light (electromagnetic). We also live under the earth’s gravitational energy. Damming the flow of rivers in order to generate hydroelectric power is an example of converting gravitational energy.
Thermal
The most fundamental form of energy is heat. The heat energy generated by the sun, converted into chemical energy in food, and then digested by the human body sustains life. Conversion of the high temperatures in fire to useful heat was mankind’s early attempt to control energy, and the overwhelming majority of current systems for generating electricity are still based on heat energy, whether they use fossil fuels, uranium, or concentrated solar power.
Kinetic
Also known as mechanical energy, kinetic energy is associated with all motion and moving objects. Mechanical energy operates in wide-ranging natural and human process—weather, weapons, and every motion of the body. The muscles of human beings and animals act as a kind of heat engine to generate mechanical energy for movement and work.
The signal breakthrough of the Industrial Revolution was generation of mechanical energy by means of steam. The steam engine converted into rotary motion the thermal energy released by combustion of the chemical energy stored in coal. A machine could now perform the work that human or animal muscle had performed. With mechanized kinetic energy, the inherent energy limits of pre-industrial societies based on the products of recent photosynthesis were shattered.
The polymath Matt Ridley provides a vivid example of the magnitude of mechanical power gained in the shift from animal muscle to inanimate machine. By 1870, Ridley notes, the mechanical power provided by England’s steam engines was equivalent to the work of six million horses.16
Chemical
Chemical energy is the most pervasive form of energy. The functions of the human body illustrate the potency and versatility of chemical energy. The human body, of course, depends on the daily consumption of what was originally solar energy stored in food to sustain life. A marvelous chemical reactor, the human body transforms the original solar energy stored in plants, a result of photosynthesis, as well as the meat and dairy products derived from animals nourished by plants, into chemical, thermal, mechanical, and electromagnetic energy.
Through the pathways of the body’s metabolism, the human body converts the chemical energy in food and inhaled oxygen to power breathing, maintain temperature, pump blood, digest food, and transmit the brain’s nervous signals. Whether at the intracellular level or in brute bodily motion of the most regular or exceptional form, physical human life is driven by a finely-tuned energy system known as metabolism.
Measuring Energy
Despite its diverse manifestations, energy can be measured in consistent quantitative units. Enabling us to compare different sources, forms, conversions, and uses of energy, these measurements throw considerable light on current debates about fossil fuels and green energy from renewable sources. All energy sources are not created equal, and they are not all suitable for every job.
For example, the energy content of coal is approximately two–four times that of wood, which makes coal a far more efficient and less costly fuel. And the power density of natural gas-fired electric generation—at its maximum efficiency—is almost two thousand times greater than that of wind-generated electricity.17 When all the energy used to produce a gallon of corn-based ethanol is calculated—planting, fertilizing, harvesting, distilling, and transporting—a gallon of ethanol is a net energy loss. In many instances more energy is consumed in producing ethanol than is gained by burning ethanol in the internal combustion system of a vehicle. Not surprisingly, the fuel efficiency of ethanol measured in miles per gallon is about one-third that of gasoline.18
FIGURE 4.3
SI Units for Energy

Throughout this book we will examine such variables as energy density, energy content, energy efficiency, energy intensity, and the energy cost of energy. To understand these concepts, you need a little arithmetic and physics. The numbers, units, and symbols for quantifying energy may be a tedious menu for many readers, but with a little patience, these basic units of measurement are readily intelligible, and they are valuable for understanding the many conundrums of energy policy. These measurements are less intimidating than you might think. In fact, they are similar to the nutritional information printed on food labels. The calorie figure on a soup can is a measure of the energy or heat content of the soup. You’ll be able to compare the flux of energy stored in an egg from a large hen with the energy in a barrel of crude oil. The egg stores almost as much energy as the barrel of oil!19
Energy Measurements
The International System (commonly abbreviated “SI,” from the French version of the name) is the world’s most commonly used units of measurement in science, commerce, and government. English-speaking countries, on the other hand, have been slow to adopt the metric system and the SI units for energy, but they are gradually moving in that direction. Outside of science, the SI units are still not commonly used in the United States. Clinging to miles, foot-pounds, horsepower, calories, and British thermal units (Btu), the customary units remain a dominant system of measurement in this country. There are formulas for converting the measures from one system to another, but this is a cumbersome process for the layperson. For reasons of simplicity and clarity, this book will rely on SI measures with equivalents in customary units wherever necessary.
The most familiar customary measure of energy in the United States is the calorie, which measures the amount of energy—or heat value—in food. For example, one loaf of bread contains roughly 1,400 calories, or 5,714 Btus. As a measure of energy understood as “the capacity to do work,” one person would have to eat twenty-two loaves of bread to complete the same work as a car engine burning one gallon of gasoline, which contains 126,000 Btus.20 One Btu represents the amount of thermal energy (heat) necessary to raise the temperature of one pound of water by one degree Fahrenheit.
Energy Density
Energy density is a straightforward measurement of the amount or content of energy per unit of weight (gravimetric) or per unit of volume (volumetric). Measured in Joules (J) per unit of weight or volume, energy density is a measurement of the heating value of the fuels. Measurement of energy density explains the many energy choices in daily life, historical progress, and economic productivity. Concentrated energy sources confer enormous advantages for extraction, transport, and storage and allow versatile conversions. The relatively higher energy density—and even more so the power densities—of coal, oil, and natural gas is, in large part, why the British Industrial Revolution and its aftermath achieved such unprecedented gains in economic productivity.
By weight, energy density is calculated as Joules per gram (J/g) or megajoules per kilogram (MJ/kg). By volume, energy density is calculated as Joules per cubic centimeter (J/cm3) or megajoules per liter (MJ/L).
FIGURE 4.4
Energy Density by Weight and Volume
From Table A.6, Smil, Energy in Nature and Society, at 392
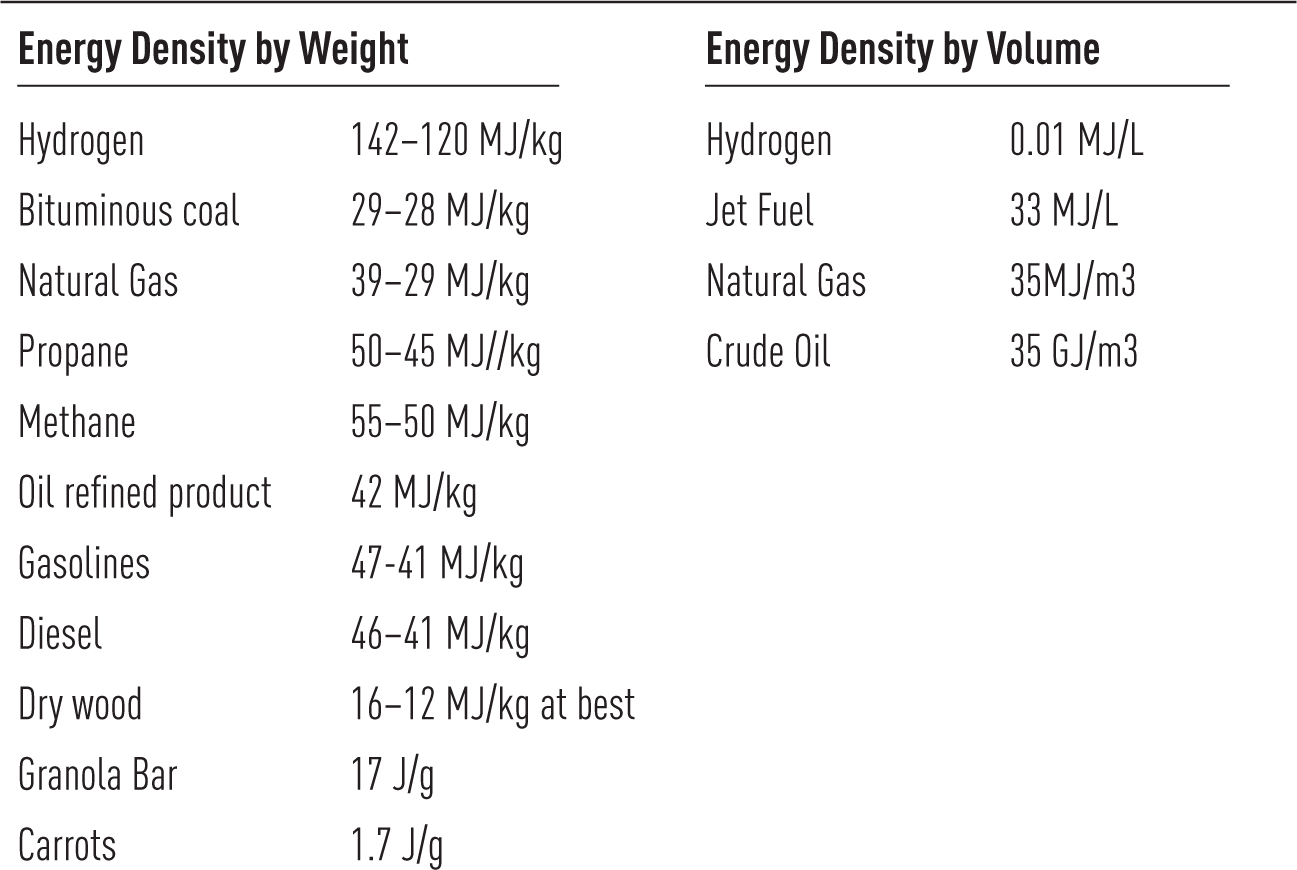
FIGURE 4.5
Power Density21

Source: Vaclav Smil
In Figure 4.4, note that the volumetric density of crude oil, measured in gigajoules (GW), is a thousand times higher than that of natural gas. This is why, without compressing or liquefying natural gas, the transatlantic shipment of natural gas does not make economic sense. And natural gas is not a suitable fuel for aviation, where volume or weight is limited. Hydrogen has an energy density by weight far higher than that of any other fuel at 143 MJ/kg, but its volumetric density is minuscule compared with that of crude oil. Kerosene, the traditionally preferred fuel for aviation, has a volumetric density of 33 MJ/L—more than three thousand times that of hydrogen.
Quantifying the power density of different fuels reveals glaring contrasts between renewable energy sources and fossil fuels. If energy density measures the energy content per unit of weight or volume, power density measures the energy flow that can be harnessed from a given unit of volume, area, or mass. Although there are different formulas for quantifying power density, the broadest measure estimates in watts per square meter (W/m2) and may include all the ancillary area beyond the generating site to include production of materials, transport, and related infrastructure.22 Different methods of measuring the power density yield different numbers. What remains constant across different numbers is the massive differential between the power densities of renewable generation and of conventional fossil-fueled and nuclear generation.
Watts per square meter is a measure of how much of the surface area of the earth is required to generate electricity. This measure can also include the amount of land used to extract and process raw materials and transmission infrastructure to calculate the power density of the entire system.23 Coal, natural gas, and nuclear generation have far greater power density than wind, sunlight, or wood (biomass) as a source of generation. That the power densities achievable in fossil-fuel-fired electric generation can be thousands of times greater than in renewable systems was the key insight of Google’s RE<C engineers.24
Generating electricity from renewable sources requires huge amounts of land and material.25 When used as a generating fuel, the power densities of wind and wood range from 0.5 to 1.5 W/m2, whereas natural-gas-fired generation systems can achieve a power density of two thousand W/m2.26
In a brief essay, Vaclav Smil points out meeting 20 percent of the world’s demand for electricity with wind would be a Herculean task.27 This is a modest amount of wind generation compared with what is envisioned in most climate policies. Because the wind blows intermittently, wind has a capacity factor on average of only 20–30 percent. Thermal power plants using the heat from the combustion of fossil fuels can reach a capacity factor of 75 percent or higher. Thus, to meet demand with wind requires far more generating hardware (turbines and land area) on which to erect that hardware than fossil fueled power plants. Even with the larger, improved wind turbines of 3 MW installed capacity, you would need to manufacture, transport, and install four hundred thousand new turbines to meet 20 percent of demand.28 This is the insight of Google’s engineers when they noted, “The scale of the building would be like nothing ever attempted by the human race.”29
Replacing our current fossil-fuel-based electric system with wind, solar, or biomass would require thousands of times more land than is now used for fuel extraction, electric generation, and transmission, encroaching on millions of acres of the surface of the earth. Most advocates of rapidly replacing fossil fuels with renewable fuels seem unaware of this challenge. The implications of trying to use energy fuels with the inherently inferior power densities of wind, solar, and biomass will be addressed throughout this book.
Energy and Climate
The theories of man-made global warming and predictions of catastrophic climate change are based on assumptions about the earth’s climate system that are not confirmed by observational evidence. The empirical sciences have long understood that measurement, observation, and experiment are the essential means of validating a scientific hypothesis. Claims of consensus cannot trump physical evidence. As the renowned paleogeologist Ian Plimer, of the University of Adelaide, argues, “The theory of human induced global warming is not science because research is based on a pre-ordained conclusion, huge bodies of evidence are ignored, and the analytical procedures [climate models] are treated as evidence.”30
To oversimplify, it is said that the relatively small increment of man-made carbon dioxide added to the natural atmospheric concentration of carbon dioxide since the Industrial Revolution disrupts the natural dynamics of climate. Adding more carbon dioxide, a greenhouse gas, to the atmosphere could lead to some warming, but could the relatively small additional increment of man-made carbon dioxide overpower the natural variables of climate, leading to planetary catastrophe?
Since 1988, the “official” scientific account of global warming has been under the aegis of the United Nations’ Intergovernmental Panel on Climate Change (IPCC), composed of scientists appointed by member countries. Every four or five years, the IPCC issues an “assessment report” on the current state of the science. The IPCC is charged with providing the science to support the theory that mankind’s consumption of fossil fuels is causing global warming (the physical science), predicting the symptoms (effects, adaptation, and vulnerability), and prescribing remedies (mitigation) to human-induced emissions of carbon dioxide assumed to cause the alleged disease of global warming. The panel’s multiple roles of scientist, soothsayer, and policy maker corrupted the scientific process from its inception. As Richard Lindzen, a revered professor emeritus of atmospheric sciences at MIT, puts it, “The charge to the IPCC is not simply to summarize [the state of climate science], but rather to provide the science with which to support the [UN’s] negotiating position whose aim is to control greenhouse gas levels. This is a political rather than a scientific charge.”31
FIGURE 4.6
Global Bulk Atmospheric Temperature (Surface–50k ft)

Source: John R. Christy, University Alabama in Huntsville, Testimony before the U.S. House Committee on Science, Space & Technology (2 Feb 2016); model output, KNMI Climate Explorer.
In a 2009 scandal popularly known as “Climategate,” e-mails between key authors of the IPCC’s 2008 assessment report revealed an even darker side of the IPCC: active efforts to suppress, destroy, and manipulate data, to exaggerate temperature records and to prevent the publication of works by dissenting scientists in academic journals.32 Several investigations followed, and some leading scientists called for a dissolution of the IPCC, but the UN-driven process limped on.
To the surprise of many, the 2013 IPCC assessment report acknowledged the gap between the modeled predictions of warming and physically observed temperatures lower than the models. The report also concluded that meteorological records do not indicate a higher incidence of extreme weather events.33 The political alarmists, including President Obama and many European leaders, however, continue to issue urgent warnings about catastrophic climate change in the here and now that are at odds with the IPCC’s latest assessment report. (The IPCC document that makes headlines and is fodder for politicians and activists is not the extensive report written by the scientists but the “Summary for Policy Makers,” written by appointed government officials and only a few of the scientists.)
The politicians, the media, and the activists bombard the public with horror stories of imminent and irreversible climate catastrophe. Yet many of the most scientifically prominent alarmists recognize that the evidence does not support such stories. Gavin Schmidt of NASA’s Goddard Institute of Space Studies grants that “general statements about extremes are almost nowhere to be found in the [scientific] literature but seems to abound in the popular media.”34 And he is not alone.
Energy: From the Cosmos to Your Body
The climate issue now pervades our culture and is institutionalized in law. The phrase “climate change” has been used in so many vague ways that it has become virtually meaningless. Of course the climate changes. The real issue is energy: the natural forces operating in the galaxy in which the earth resides as well as the energy now harnessed for human purposes. A consideration of some basic physical realities about energy in its many forms and pathways is a useful filter for the exaggerated nonsense typical of official discourse about climate change.
Energy operates incessantly throughout our physical world, within our solar system, atmosphere, plant and animal life, and the human body. Everything and everyone are constantly moving and changing. The sun orbits the gravitational center of the Milky Way galaxy; the earth orbits the sun, and our moon orbits the earth. Energy pulses through the universe on a galactic scale and through your body as it digests this morning’s breakfast.
There are fundamental parallels between the pathways and transformations of energy in the natural world, in the human body, and in fossil-fueled societies. For instance, the way the human body burns the chemical energy contained in food is similar to the way the internal combustion engine of an automobile burns the chemical energy in gasoline.
Both natural and man-made energy systems rely on a constant supply of energy, require water, and emit heat. Both systems oxidize carbon. But while the body’s natural metabolic system is fixed, the man-made energy system is continuously expanding—amplifying, ordering, and accelerating the transmission of energy and power for human use whenever someone flips a switch or taps on a smartphone. The evolution from Edison’s first light bulb to low-emission diode bulbs to state-of-the-art diode lasers is marked by improved efficiency, performance, access, and speed.35
Modern man’s energy system is still interconnected with nature’s energy system. Like the man-made system operating across the world, the earth’s biosphere is “an intricate, interactive assembly of energy stores and flows.” The term “biosphere,” in this regard, refers to the physical home of human beings—the earth and its atmosphere. The earth’s biosphere is unique in the known universe. Indeed, as Paul Davies shows in his provocative book The Cosmic Jackpot, given all the conditions that had to coalesce with astonishing precision to support life, the earth is an extraordinarily lucky planet.36 It is the only planet in our solar system—the only one known at this time—with enough atmospheric oxygen to support human life. And oxygen is a kind of waste product of photosynthesis!
The carbon cycle—the earth’s system that supports animal and plant life—operates through the constant reception and conversion of external energies.37 And among those conversions of energy necessary to maintain animal life is inhalation of oxygen and exhalation of carbon dioxide. Plant life operates in the reverse, inhaling carbon dioxide and exhaling oxygen. Atmospheric carbon dioxide is an essential nutrient for life on this planet and hence called the gas of life. Human beings exhale 4 to 5 percent more carbon dioxide by volume than they inhale.38 This makes the concentration of carbon dioxide in human expiration about forty thousand parts per million (ppm)! The current atmospheric concentration of carbon dioxide is now about four hundred ppm, including man-made contributions from the combustion of fossil fuels.39 Atmospheric levels of carbon dioxide in 1850 are thought to have been about 280 ppm.
The earth’s location in our solar system is also essential to its ability to support life. The position of the earth gives it continuous access to a massive source of energy—the star we call the sun. The earth is a planet orbiting one of two hundred to four hundred million stars within the constellation Sagittarius in the Milky Way. Unlike Venus, which is too hot to support carbon-based life, or Mars, which is too cold, the earth rotates on its axis at an optimal angle and distance from the sun.40
The sun is the original source of almost all the energy accessible to mankind.41 With the exception of the geothermal energy inside the earth and tidal energy driven by gravitational forces, the energy generated by the sun is the source of all energy on the earth, including wind. Geothermal energy is responsible for tectonic shift, volcanoes, and earthquakes. And the heat inside the earth played an important role in transforming dead plant and animal matter into fossil fuels over millions of years of compression. Tidal energy originates from shifting gravitational interaction of the orbits of the sun, earth, and the moon.
“That the sun influences our climate should not be surprising . . . when we consider that 99.98 percent of the total energy of the world’s climate comes from the sun.”42 Oddly, the UN’s IPCC, whose “science” is the authoritative support for climate policy to supplant fossil fuels, largely dismisses the sun’s effect on climate.43 The IPCC assumes that the increased man-made emissions of carbon dioxide over the previous two centuries overpower the influence of the sun—and of all other natural climactic variables, such as water vapor, clouds, and aerosols—on climate.
The question of the climate’s sensitivity to anthropogenic carbon dioxide added to the atmosphere is crucial to the IPCC’s predictions of warming in the future. IPCC models assume anthropogenic carbon dioxide amplifies the greenhouse effect of natural water vapor and thus may double warming caused by carbon dioxide. Since the mid-1990s, the rate of warming predicted by the IPCC’s models has not occurred. The temperatures actually measured by NASA’s remote sensing satellites and balloons are substantially lower than the IPCC’s modeled forecast. As shown in Figure 4.6 on page 86, the average warming of the IPCC’s 102 climate models is one degree Celsius for the period 1979–2013. Observational measurement of global temperature over the same period is only 0.2 degree Celsius. This means that the climate sensitivity (or warming) expected from the doubling of atmospheric carbon dioxide is more likely to be three times lower than the IPCC models assume.44
In its Fifth Assessment Report, issued in 2013, the IPCC concedes that its models may assume too much sensitivity to man-made emissions of carbon dioxide and too little natural variability. Yet the first pages of the report’s “Summary for Policy Makers” conclude with high degree of certainty that human influences prevail. Although climate sensitivity to man-made carbon dioxide is a core assumption of the models, the IPCC’s acknowledgment of its uncertainty is buried under a host of qualifications.45
Human Activity Trumps the Power of the Sun?
To get a sense of the power of the sun, consider that it burns over six hundred million metric tons of hydrogen every second. If the resulting heat were converted to electricity, it could meet the energy needs of the earth’s current human population for eons.46 The sun’s energy is produced through nuclear fusion, in which the atomic nuclei of hydrogen and helium are fused. Man has applied nuclear fission, in which atomic nuclei are split, but the practical applications of nuclear fusion remain elusive. If nuclear fusion were commercially viable, we would have at our disposal an almost infinite and pollution-free source of energy. Clearly, the physical dynamic of our planet generates a surfeit of energy. The challenge is how to access and convert the energy into forms affordable, adaptable, and acceptable to human societies.
Just Where Does Energy Come From?
Energy and how it operates are questions that have preoccupied philosophers, scientists, engineers, and artists for thousands of years. Energy is so intimately connected to life itself that it almost seems equivalent to physical life. Major advances in the scientific understanding of energy and its engineering applications have occurred only within the past two centuries. Although we know more about how energy operates in the natural world and how it can be harnessed for human purposes, we still lack a clear understanding of what energy is. Is it a particle or a wave? Is it a capacity or something physical? Albert Einstein’s famous formula—energy equals mass times the speed of light squared (E = mc2)—and that formula’s practical applications in nuclear physics make this much clear: matter itself is a repository of massive energy.
Understanding the operation of energy in our lives, society, and the natural world is important, because if now misunderstood, current government actions to supplant fossil fuels and to decrease energy consumption could reverse the trajectory of progress enjoyed over the last century by an increasing percentage of the world population. The late Nobel laureate physicist and author of many popular books Richard Feynman concluded that energy remains an elusive, subtle concept. “It is very, very difficult to get right.”47 Current energy policies to replace coal plants with subsidized solar panels and wind turbines do not get energy right unless a return to energy scarcity is the goal.
Made of Sunlight
The human body—like all complex plant and animal life on earth—depends on transformations of the sun’s light energy. Solar radiation—also known as visible sunlight—is a form of the electromagnetic energy generated by the sun in between infrared and ultra-violet radiation on the electromagnetic spectrum.
To a large extent, our bodies are constructed by sunlight. And the agent is the most fundamental natural energy conversion on the earth, commonly known as photosynthesis catalyzed by atmospheric carbon dioxide. The transformation of solar radiation into chemical energy sustains the growth of the tissue in living plants. Carbohydrates, the food base on which human life depends, originate through photosynthesis.
Typically taught without inspiration in eighth-grade science classes, photosynthesis is the most important energy conversion on the planet. James Watt’s steam engine, which converted the heat energy in coal to mechanical energy in a machine, is the most important anthropogenic conversion of energy. Before mankind harnessed fossil fuels on a large scale in the Industrial Revolution, the energies available for human use were limited to what recent photosynthesis could provide in the form of fuel for heat, food, and raw material for clothing and shelter.
All human beings depend on the chemical energy available in plants for food, and the world’s poorest societies still depend on it for heating fuel as well. Developed societies, on the other hand, no longer rely on woody plants as a source of thermal and chemical energy. For that purpose, they depend on the products of ancient photosynthesis—the remains of life from long-dead plants and organisms transformed and concentrated through millions of years of compression and heat inside the earth—that is, fossil fuels.
Don’t Forget the Diamonds!
Carbon, indeed, is the stuff of life. It occurs in countless forms. Diamonds represent a relatively pure form of carbon, noted for their incomparable hardness and conductivity. Graphite—good old pencil lead—is another relatively pure form of carbon. The opposite of a diamond, graphite is dark and soft.
In the second decade of the twenty-first century, humanity sits atop two centuries of major advances in physics, biology, and chemistry that have applied hydrocarbon compounds for mankind’s benefit. Yet the doomsayers of our age employ the term “carbon” as if it were a poison threatening the survival of civilization.
The American Heritage Dictionary provides a concise explanation of photosynthesis: “Almost all life on earth depends on food made by organisms that can perform photosynthesis such as green plants, algae, and certain bacteria. These organisms make carbohydrates from carbon dioxide and water using the light energy from the sun. . . . Almost all of the oxygen in the earth’s atmosphere was produced as waste by photosynthetic organisms.”48
The primal energy conversion for life on the earth, photosynthesis is relatively inefficient. The amount of solar radiation that reaches the earth is the energy equivalent of twenty million calories per day per surface acre, and the average percentage of that radiant energy that plants capture and convert into plant tissue is relatively minute—averaging less than 0.3 percent.49
Human beings, however, digest carbohydrates produced by photosynthesis with an energy efficiency (the ratio between the amount of energy input and the actual energy output) of 99 percent.50 Many machines, or “prime movers” as engineers call them, that convert energy from one form to another, like the turbines used by modern thermal power plants, can convert 99 percent of the turbine’s mechanical energy into electricity.52 Given that the sun’s radiant energy is showered on the earth every day, the energy that goes into the photosynthetic conversion is largely irrelevant. In this sense, the earth is not a closed system of energy. The sun will come up again tomorrow, and human innovations will continue to surprise us.
Questions about Climate Science
Climatological research over the past three decades, supported by hundreds of billions of dollars from taxpayers world-wide, nominally excluded research on natural climate variables such as the sun and water vapor. In the U.S., the federal government selects the research projects and provides the majority of research funds. The overwhelming majority of those research grants were devoted to studies that reinforced—but did not prove by empirical evidence—the original theory that mankind’s use of fossil fuels will lead to “dangerous interference with the climate.”51
As shown in Figure 4.6 on page 86 (modeled temperature predictions), the IPCC’s all-important computer models have substantially exaggerated predicted warming for decades. Many senior scientists in the field conclude that it is time to declare the IPCC’s methodology and computer models a failure. A portion of the federal research funds should be allocated to those highly credentialed scientists—now marginalized as skeptics—to assess the IPCC’s work over the last several decades and to offer alternative theories and evidence.
President Obama often speaks of the “dictates” of climate science. Yet the predictions of computer models that are contradicted by measured observation—actual temperatures—hardly offer scientific justification for eliminating the energy sources on which modern society is utterly dependent.
As we shall see later, the chief factor limiting plant productivity—photosynthetic efficiency—is the level of atmospheric carbon dioxide, which is currently at a relatively low level compared with previous eras in the earth’s long history. Yes, you read that correctly. Agricultural productivity would be substantially increased if the atmospheric concentration of carbon dioxide were much higher. This is why nurseries often pump carbon dioxide into greenhouses.
The earth’s atmosphere is now 78 percent nitrogen and 20 percent oxygen; the remaining 2 percent consists of trace gases such as carbon dioxide and argon. Natural carbon dioxide accounts for a minuscule 0.039 percent of the atmospheric gasses we actually breathe at the tropospheric level. Human activity—breathing as well as burning oil, natural gas, and coal—accounts for 3 to 5 percent of the atmospheric level of carbon dioxide, that is, about 0.002 percent of all the gasses in the atmosphere.53 We wonder how this trace of carbon dioxide from human activity could override the power of the sun in matters of climate or weather. Observed temperatures do not reflect the assumed climate sensitivity to carbon dioxide that drives the models.
Carbon: The Chemical Basis of Life or a “Weapon of Mass Destruction”?
The political use of the word “carbon” to denote a pollutant that, if not eliminated, will lead to a planetary meltdown is utterly detached from reality. In an address to a large international gathering, Secretary of State John Kerry stated that carbon is “among the worst weapons of mass destruction.” President Obama and Christina Figueres, head of the UN’s climate program, regularly declare the urgent need to decarbonize human societies. This is a shorthand slogan for eliminating the use of fossil fuels now providing 80 to 90 percent of energy across the world.
Communism, says Figueres, is more likely to save the planet than democracy.54 The long-sought goal of a global agreement on reducing carbon emissions assumes mandates imposed by strong central governments. Surprisingly, the long-awaited climate agreement reached in Paris in 2015 does not appear to bind any country in a legally enforceable manner, but international politics may be a different story. The Paris climate pact incorporates “pledges” to reduce greenhouse gases submitted by each country. The pledges—known as “Intended Nationally Determined Contributions” (INDCs) to reducing greenhouse gases—are voluntary, but the text repeatedly speaks of the agreement’s legal effect and “entry into force.” At the least, a huge international bureaucracy is now empowered to badger national governments about their pledges.
Governments and the private sector have already directed hundreds of billions of dollars to ridding the world of carbon. Betting on the success of this effort would be a highly risky investment. Carbon, after all, is defined as “the chemical basis of all known life.”55 Have people forgotten this fact?
How can carbon be a weapon of mass destruction and the basis of all known life? As Humpty Dumpty said to Alice when she asked if a word can mean anything you like, “The question is, which is to be master—that’s all.”56 Climate policy is ultimately about power—will energy be controlled and allocated by big, centralized governments or by free persons acting in competitive market economies?
Hydrocarbon compounds represent a host of useful natural chemicals and synthesized compounds, providing the chemical building blocks for such ubiquitous materials as plastics, polyester, lubricants, and solvents. With the addition of phosphorous and other elements, carbon also naturally forms DNA and RNA as well as adenosine triphosphate (ATP). ATP is the most important energy-transferring molecule in all living cells. Decarbonizing anyone?
Policy vs. Reality
One of our favorite New Yorker cartoons shows a giant electric fan blowing into a windmill to get the turbines to rotate. It’s a humorous reminder that the aggressive political pursuit of green energy as the dominant source of electric power runs afoul of some basic physical realities. Given the magnitude of the human damage and geopolitical weakness that would follow a collapse of the complex energy systems on which modern societies depend, a more critical assessment of renewable energy is urgently needed. Engineers and physicists typically have an intuitive understanding of why wind and solar power are not promising candidates to provide base load electric power for large cities. Climate scientists and most policy makers do not.
Energy remains an elusive concept, easily misunderstood by policy makers who would impose grand plans on society. The late economist Julian Simon reminded us that energy is the master resource because it allows the extraction, transport, and transformation of all other natural resources. If world leaders continue to impose energy policies that contradict fundamental energy realities, the damage will extend far beyond higher electricity rates and could lead to a world much poorer and less free.
Without abundant, affordable, reliable, and versatile energy, economic growth will be undermined. The productivity achieved through man’s relatively recent energy enrichment, commonly known as the Industrial Revolution, will be unraveled. Poverty will increase and the middle class will shrink, a trend already occurring across the world, even in the United States. The ruling elites will go unscathed, protected as they were before the Industrial Revolution, while the rest of mankind reaps the bitter harvest of false green hopes.
The global warming alarmists and many educated elites have lost their faith in man’s ability to adapt and to tame his natural surroundings. Making carbon the enemy of the planet means that mankind is the enemy of the planet. Our bones, blood, and flesh are made of carbon. As in so many earlier fits of pessimism, the source of these damaging policies is the misanthropic view that the “common enemy of man” is man.57 The prophets of doom have the story backward: the abundant energy that is a product of human ingenuity makes our planet habitable, not inhabitable.