Scientific knowledge can be advanced in many ways, and a major development in the search for the origin of pandemic influenza viruses came from a walk on the beach I took in 1967 with the late Graeme Laver. In 2004 he wrote:
The story started in the late 1960s on the south coast of New South Wales in Australia. We noticed that every 10 to 15 m or so there were some dead muttonbirds (shearwaters) washed up on the beach. Knowing that terns in South Africa had been killed by an influenza virus in 1961,26 we wondered if these birds too had died from a flu infection.27
The name ‘muttonbirds’ here refers to the migratory seabirds Puffinus pacificus, which fly on a figure-8 route around the Pacific Ocean and return yearly to nest and raise their young on small islands south of New Zealand and on the Great Barrier Reef islands of Australia. Their common name derives from the fact that in New Zealand these birds served as a ready source of meat for Māori and early European settlers because plump, juicy young birds could be easily pulled from their shallow burrows. Muttonbirds have a dense layer of rich, fishy-tasting fat just under the skin, and they have traditionally been salted and smoked. In recent years they have become a New Zealand delicacy at gourmet restaurants. Laver and I decided that it would be a bit of lark to head to the muttonbird nesting sites on the Great Barrier Reef, on a hunch that they might indeed be infected with influenza.
At that time, I was a graduate student at the Australian National University (ANU) in Canberra, and Laver was a newly recruited junior scientist there. We had no resources to finance this proposed expedition. The islands on the Great Barrier Reef are protected, so we needed to obtain permission for an expedition, and we would also have to lug in all the water, food and equipment we needed by boat. We approached the head of the Department of Microbiology in ANU’s John Curtin School for Medical Research, whose response was ‘You have to be joking! Scientific expedition, my foot. More like a junket to take your friends and families on another of your outback adventures.’
He was largely right, but we did not give up. We knew that Martin Kaplan, head of the Veterinary Virology section of the WHO, was a strong proponent of the theory that swine were a possible source of pandemic influenza in humans, so we took our idea to him. To our delight, Kaplan approved US$500 for the expedition. Back in the late 1960s, $500 was a substantial award that would indeed cover most of the costs. The ANU then reconsidered and decided that there was a scientific aspect to the expedition after all. They agreed to provide one vehicle (a station wagon) and fuel for transportation to and from our chosen port. Participants from ANU came in their own vehicles. The port offering the easiest access to the uninhabited islands where the muttonbirds bred was Gladstone, north of Brisbane in Queensland, some 1500 kilometres by road from Canberra.
Our final destinations were Tryon, North West and Lady Elliot Islands. These are sandy cays with scrubby vegetation but no fresh water. In the days before easy radio or any kind of digital communication, we had to be prepared to be completely self-sufficient for up to two weeks on the islands. That meant bringing 7.5 litres of fresh water per person per day, along with a lot of food, and bird sampling supplies, including Dacron swabs and a large insulated thermos flask (known as a Dewar flask) of liquid nitrogen for keeping samples cold.
Figure 3.1 (opposite) Our search for influenza viruses in birds took us to the islands of the Great Barrier Reef in Australia. This map shows our route from Canberra to Gladstone, the nearest port giving access to reef. Inset photos show (top to bottom):
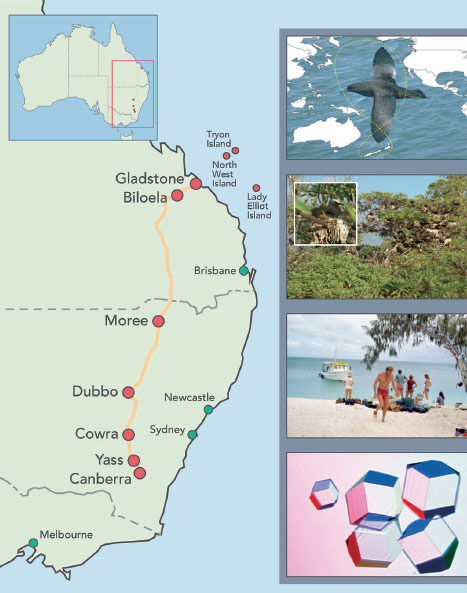
a)The ‘figure 8’ migration route of shearwaters (muttonbirds, Puffinus pacificus) around the Pacific Ocean. The birds breed and raise their young in shallow burrows on the islands south of New Zealand and the islands of the Great Barrier Reef of Australia.
b)Noddy terns are resident on North West Island. We caught adult birds for sampling directly from their nests; having never seen humans they were unafraid of us.
c)Dr Graeme Laver (in red trunks) and the research party arriving on Tryon Island.
d)Crystals of the neuraminidase (N) prepared by Graeme Laver from the H11N9 influenza virus isolated from a noddy tern and used in the design of the anti-influenza drug Tamiflu.
There was no shortage of volunteers for the expedition: colleagues from Germany, England and the US raised their hands. We usually had 10–12 participants. Preference was given to families with teenage children, who had the advantage of being lighter than adults and so would be less likely to break through the shallow sand burrows of the muttonbirds and squash them (Figure 3.1).
The organisation of the first expedition served as the model for the subsequent seven field trips that followed at one- or two-year intervals. After assembling all of the scientific supplies and loading them into the vehicles, the team set out in convoy for the two-day trip from Canberra to Gladstone. We followed the inland rather than coastal route to bypass the major cities of Sydney, Newcastle and Brisbane. Another advantage of the outback road was that we could stop for the night wherever we chose and set up camp right by the road. On one trip there was a plague of mice in that part of Australia, and we had their unwelcome company in our tents at night. It was not much of a problem in a tent with a floor, but very early one morning our German colleagues erupted from their floorless tent, flinging mice out of their sleeping bags and clothing. That mouse plague was quite amazing – at night the surface of the road seemed to shimmer as the rodents moved over it.
The boat trip from Gladstone to Tryon Island was always a challenge: the sea could vary from glassy smooth to wild and barely crossable. I was seriously concerned on one expedition when the captain elected to stay behind and let the first mate take the helm. It was so rough that even scopolamine patches failed to suppress the motion sickness. Water washed over the boat with its tied-down hatches. Once we entered the coral reefs surrounding the coral cay, however, the sea was calm and the pristine beaches inviting, and our unpleasant sea crossing was soon forgotten. On the initial trip, the supplies were ferried ashore by rowboat, and the launch returned to Gladstone; on later trips the launch stayed with us in the coral lagoon, enabling us to sample birds on many islands in the area.
A large tent for cooking and eating was the centre of activities, and this base tent was set up in the shade of the abundant trees (Pizonia grandis). Individual pup tents were pitched nearby for sleeping and housing personal gear. We generally spent the days swimming and snorkelling on the most fabulous coral reef in the world, harvesting fish and lobster for our meals. The evenings were spent doing the science. This schedule, I hasten to point out, was dictated by the birds’ behaviour, not just our preferences, since dusk was when the parent birds returned to their burrows with bellies full of fish for their large and rapidly growing juveniles. The rookeries at dusk were a cacophony, with incoming parent birds calling to their impatiently waiting offspring.
After dusk we lit lanterns and walked very carefully into the rookeries. It was the children who usually caught the birds. They would lie on the ground at a burrow that was emitting plenty of ‘bird talk’ and reach in and pull out the bird, holding its feet and wings. When a new recruit had an arm deep in the burrow, Graeme Laver would invariably say, ‘Be careful of the snakes!’ In fact there are no snakes in the coral cays, but the warning always brought the same panicked reaction – a rapid withdrawal with no bird. This initiation rite brought laughter each time.
In the early years we took a throat swab and a blood sample (from the wing vein) from each bird and quickly returned it to its burrow. Later, once we knew that influenza in birds is found in the intestine, we also took a swab from the bird’s cloaca (birds have a cloaca, not an anus). The blood was allowed to clot and separate in the sample tubes overnight, and the clear serum was removed and stored in the aforementioned thermos flask, where it was kept frozen (along with the individual swabs). After sampling some 50 to 60 birds, the group would return to the base tent, the adults enjoyed a glass of sherry and we all partook of a sumptuous seafood meal prepared by the designated cook for that day.
Although our colleagues around the world heard only that we were having great fun, day after day of snorkelling and fishing in paradise can actually become boring. Since noddy terns (Anous tenuirostris) were also on the islands, we decided to satisfy our vast scientific curiosity by spending the daylight hours sampling them as well.
The expeditions were not without incident, despite some house rules. One rule stipulated the wearing of a hat, shirt and sneakers at all times during daylight hours, including while swimming. The sunshine was intense, and severe sunburn would have been debilitating. Shoes were required because stonefish with toxic spines and razor clams lurked in the sandy shallows. My two children, Nick and Sally, aged 11 and 13 years respectively, joined the third expedition and were unimpressed, deciding early on that I was being an overprotective parent. This changed on the first evening on Tryon Island. A visiting scientist from the Netherlands had ignored the footwear rule and had been enjoying walking along the beautiful coral beach and clear water in bare feet until he stepped on a sharp object and cut his foot deeply. The visitor was very lucky it was not a stonefish. There was no further static about wearing shoes or the other house rules.
That same night we were woken by severe ground tremors and were all very alarmed. The cause turned out to be a giant sea turtle that was under a corner of our tent digging a hole to lay her eggs! We had pitched our tent on her nesting spot, but she claimed it regardless. Once again we were grateful for our tent floor. The digging went on for nearly an hour before the turtle settled down to lay what appeared to be hundreds of eggs, each about the size of a golf ball, with a flexible, leathery skin. Early the next morning Nick got his own back and rode the massive creature for part of her trip back to the ocean. Although the turtle lumbered along the beach, back in the water she was fast and elegant.
Another rule for uninhabited islands is ‘Don’t swim on an incoming tide’ because this is when sharks may enter the lagoon to feed. Our group did get caught out once when we had to wade in chest-deep water back to the launch at North West Island. We had gone there at low tide to catch noddy terns, a relatively easy task since the terns would stay on the nests, unafraid of predators. Our mother ship was some 275 metres off the beach when the captain blew the recall alarm because a storm had suddenly blown in. The children were put into the rowboat while the adults waded, sharks notwithstanding, to the launch on the incoming tide. The sharks must not have been ready to feed, as we made it all in one piece to the launch. I have never been so happy to be on a storm-tossed boat as when we left that island.
On the first Barrier Reef trip, Laver tested the sera of the muttonbirds right on the island. He put the serum into a small hole in a clear gel beside a hole containing killed influenza virus disrupted with detergent. The next day there was a white line between the virus and the muttonbird sera where a component of the virus combined with antibody in the serum to form a precipitate. This meant the serum from the muttonbird contained antibodies to influenza, which meant in turn that at some time in the past the bird had been infected with an influenza virus (the test did not indicate which). Back in the laboratory at ANU, there was more testing to be done.
Being a biochemist, Laver opted to test the muttonbird sera for their ability to block the activity of the neuraminidase enzyme found on the surface of an influenza virus (see Chapter 2). When this enzyme is active, it releases a chemical that turns the indicator bright red; when the activity is blocked (by an antibody), the indicator remains clear. The question was, which virus should he use in the test? Laver chose the virus responsible for the 1957 Asian pandemic, H2N2. In the first batch of muttonbird sera, one of the 20 samples being tested remained clear. Laver described the excitement: ‘It was one of those rare “eureka” moments that makes scientific research exhilarating.’ The serum from one muttonbird had inhibited the enzyme activity of the human virus, meaning that the bird had previously been infected by an influenza virus related to the human H2N2 influenza virus.
The next step was to isolate the virus itself from the muttonbirds. The initial studies were disappointing: no influenza viruses were isolated from the hundreds of throat swabs taken from the birds. This justified going back to the Barrier Reef and trying again. From the second trip, in 1972, one influenza virus was isolated from the throat swabs of over 200 birds sampled.28 This virus was different from any virus previously reported in that it possessed a novel form of neuraminidase. It was designated A/Shearwater/Australia/1/72 (H6N5). The virus had come from the throat of an apparently healthy bird and did not cause disease when subsequently inoculated into healthy ducks, chickens and turkeys, even though it multiplied to high levels in the birds.
In the interim, I had moved to St Jude Children’s Research Hospital in Memphis, Tennessee. One of the things I was keen to do was examine populations of migratory waterfowl on other continents, such as migratory ducks in Canada.
We know now that the influenza virus in aquatic birds multiplies mainly in the intestinal tract and was spread to other birds by means of faeces excreted into the water.29 We realised that for years we had been looking at the wrong end of the bird for influenza viruses – the throat instead of the cloaca. From samples collected in 1975 we had isolated eight influenza viruses from apparently healthy noddy terns and one from the throat of a healthy shearwater. Full characterisation of the viruses revealed that the one from the shearwater was related to the H5N3 influenza virus that had killed many terns on the coast of South Africa in 1961. However, the shearwater virus caused no apparent disease, either in the host bird or in our experiments with ducks, chickens and turkeys.
This led to the key finding that harmless versions of influenza viruses can be carried by apparently healthy migratory birds but that the same viruses can undergo changes and become killers. Perhaps the most important of all the viruses isolated from birds on the Barrier Reef was from the 70th cloacal swab of a noddy tern caught on North West Island by Adrian Gibbs. This influenza virus was characterised as an H11N9 and contained a neuraminidase that had not been previously described.
The ultimate goal of our studies was to provide treatments and cures for influenza, but first we had to better understand the origin of the viruses. If we could determine the structure of an influenza virus and its components, a drug to prevent or cure the disease could be developed. So the goal was to find the ‘active site’ on the neuraminidase molecule, the part that enables it to separate from a host cell and spread through the body. Laver set out to do this by bombarding the molecule with x-rays at a cyclotron in Switzerland.
Although breakthroughs in medicine are often illustrated by images of scientists hunched over microscopes to discover disease agents, molecules are too small to be observed by ordinary visual magnification. One technique for examining them is to place them in a beam of x-rays, which have a much shorter wavelength than visible light. The molecular structure can be determined from the way the molecule diffracts the x-ray beam. However, to use this technique, samples must be prepared in the form of crystals through which the x-ray beam can pass. This typically involves placing the biological sample in a chemical solution (such as a salt solution) under conditions that encourage the formation or ‘growth’ of crystals, like creating rock candy from a sugar solution.
Growing good crystals is a challenging and delicate task. At the time, Laver was probably the greatest crystal grower in the world. He isolated the N9 neuraminidase from a virus isolated from the noddy tern and set out to grow the best crystals the world had ever seen. One of the techniques at that time, believe it or not, was to send the material into space on a NASA space shuttle, where microgravity allowed large crystals to form. Unfortunately, the Challenger explosion in January 1986 brought that method to a halt. Not to be stopped, Laver approached scientists of the Soviet Union and persuaded them to send the influenza neuraminidase protein to the MIR space station. The possibility that the Soviets might achieve a major breakthrough in crystal making worried American strategists, but the plan went ahead. (Laver loved to stir the pot and revelled in creating problems for American and Soviet strategists.)
The strategists need not have worried, however, for the crystals were, at best, only marginally better than those grown on Earth. Some scientists speculated that the re-entry and bump-down of the supply spacecraft in Kazakhstan may not have been beneficial to the crystals, and that perhaps top-quality crystals had indeed been grown on the MIR and then wrecked. Regardless, subsequent advances in robotics enabled the creation of optimal conditions for crystallisation, making it possible to grow large, high-quality crystals safely on Earth.
At the time, an anti-influenza drug called Relenza had been developed based on the structure of the human H2N2 neuraminidase, but it had to be puffed into the respiratory tract of patients, which is not easy. An easier delivery system was needed. Laver provided crystals of the isolated N9 neuraminidase from the noddy tern, H11N9, to Gilead Sciences of California to assist in the design of a drug that could be taken as a pill. The drug now known as Tamiflu was developed by Gilead scientists and was later acquired by the Roche company. It is currently the most widely used drug for treatment of influenza. As Laver pointed out, Tamiflu could have been developed using N2 neuraminidase crystals, but when Gibbs caught the noddy tern whose sample provided the large and near-perfect N9 crystals, it made the job a whole lot easier. So a walk on the beach provided a vital insight into the origin of pandemic influenza viruses and contributed to the development of an important new drug.
