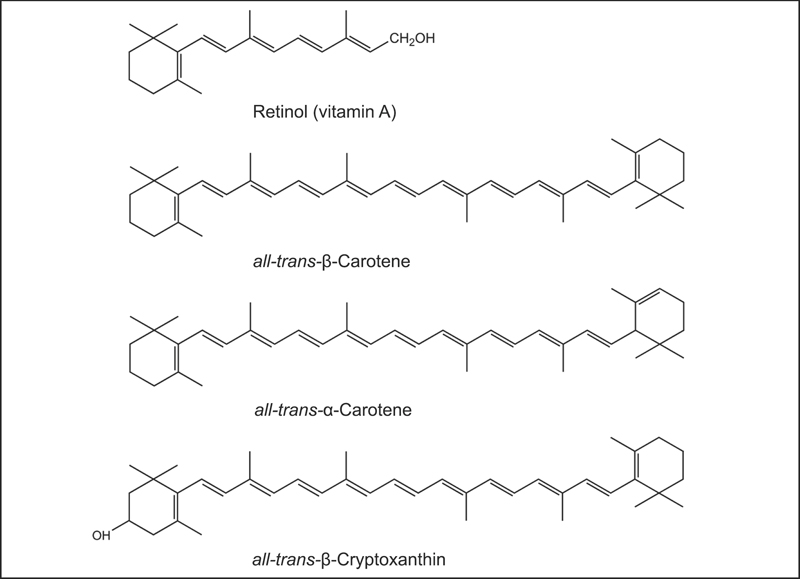
Fig. 8.1 The chemical structures of retinol (vitamin A) and the provitamin A carotenoids, β-carotene, α-carotene, and β-cryptoxanthin.
Carotenoids are a class of more than 600 naturally occurring pigments synthesized by plants, algae, and photosynthetic bacteria. These richly colored molecules are the sources of the yellow, orange, and red colors of many plants.1 Fruits and vegetables provide most of the carotenoids in the human diet. Alpha-carotene, β-carotene, β-cryptoxanthin, lutein, lycopene, and zeaxanthin are the most common dietary carotenoids. Alpha-carotene, β-carotene and β-cryptoxanthin are provitamin A carotenoids, meaning they can be converted by the body to retinol (Fig. 8.1). Lutein, lycopene, and zeaxanthin cannot be converted to retinol, so they have no vitamin A activity (Fig. 8.2). Carotenoids can be broadly classified into two classes, carotenes (α-carotene, β-carotene, and lycopene) and xanthophylls (β-cryptoxanthin, lutein, and zeaxanthin).
For dietary carotenoids to be absorbed intestinally, they must be released from the food matrix and incorporated into mixed micelles (mixtures of bile salts and several types of lipids).2 Therefore, carotenoid absorption requires the presence of fat in a meal. As little as 3–5 g of fat in a meal appears sufficient to ensure carotenoid absorption.3,4 Because they do not need to be released from the plant matrix, carotenoid supplements (in oil) are more efficiently absorbed than carotenoids in foods.4 Within the cells that line the intestine (enterocytes), carotenoids are incorporated into triglyceride-rich lipoproteins called chylomicrons and released into the circulation.2 Triglycerides are depleted from circulating chylomicrons through the activity of an enzyme called lipoprotein lipase, resulting in the formation of chylomicron remnants. Chylomicron remnants are taken up by the liver, where carotenoids are incorporated into lipoproteins and secreted back into the circulation. In the intestine and the liver, provitamin A carotenoids may be cleaved to produce retinal, a form of vitamin A. The conversion of provitamin A carotenoids to vitamin A is influenced by the vitamin A status of the individual.5 Although the regulatory mechanism is not yet clear in humans, cleavage of provitamin A carotenoids appears to be inhibited when vitamin A stores are high.

Fig. 8.1 The chemical structures of retinol (vitamin A) and the provitamin A carotenoids, β-carotene, α-carotene, and β-cryptoxanthin.
Vitamin A is essential for normal growth and development, immune system function, and vision. Currently, the only essential function of carotenoids recognized in humans is that of provitamin A carotenoids (α-carotene, β-carotene and β-cryptoxanthin) to serve as a source of vitamin A.6 The vitamin A activity of β-carotene in foods is 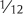 that of retinol (preformed vitamin A), while the vitamin A activities of α-carotene and β-cryptoxanthin are both
that of retinol (preformed vitamin A), while the vitamin A activities of α-carotene and β-cryptoxanthin are both 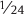 that of retinol.6
that of retinol.6
In plants, carotenoids have the important antioxidant function of quenching (deactivating) singlet oxygen, an oxidant formed during photosynthesis.7 Test tube studies indicate that lycopene is one of the most effective quenchers of singlet oxygen among carotenoids.8 Although important for plants, the relevance of singlet oxygen quenching to human health is less clear. Test tube studies indicate that carotenoids can also inhibit the oxidation of fats (i.e., lipid peroxidation) under certain conditions, but their actions in humans appear to be more complex.9 At present, it is unclear whether the biological effects of carotenoids in humans are a result of their antioxidant activity or other non-antioxidant mechanisms.
The long system of alternating double and single bonds common to all carotenoids allows them to absorb light in the visible range of the spectrum.7 This feature has particular relevance to the eye, where lutein and zeaxanthin efficiently absorb blue light. Reducing the amount of blue light that reaches the critical visual structures of the eye may protect them from light-induced oxidative damage.10
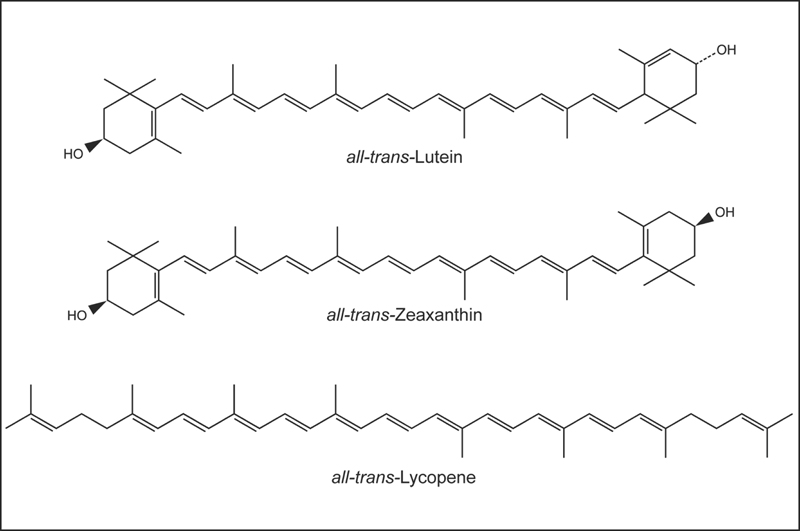
Fig. 8.2 The chemical structures of lutein, zeaxanthin, and lycopene.
Carotenoids can facilitate communication between neighboring cells grown in culture by stimulating the synthesis of connexin proteins.11 Connexins form pores (gap junctions) in cell membranes, allowing cells to communicate through the exchange of small molecules. This type of intercellular communication is important for maintaining cells in a differentiated state and is often lost in cancer cells. Carotenoids facilitate intercellular communication by increasing the expression of the gene encoding a connexin protein, an effect that appears unrelated to the vitamin A or antioxidant activities of various carotenoids.12
Because vitamin A is essential for normal immune system function, it is difficult to determine whether the effects of provitamin A carotenoids are related to their vitamin A activity or other activities of carotenoids. Although some clinical trials have found that β-carotene supplementation improves several biomarkers of immune function,13–15 increasing the intakes of lycopene and lutein—carotenoids without vitamin A activity—has not resulted in similar improvements in biomarkers of immune function.16–18
Although consumption of provitamin A carotenoids (α-carotene, β-carotene, and β-cryptoxanthin) can prevent vitamin A deficiency, no overt deficiency symptoms have been identified in people consuming low-carotenoid diets if they consume adequate vitamin A.6 After reviewing the published scientific research in 2000, the Food and Nutrition Board of the Institute of Medicine concluded that the existing evidence was insufficient to establish a recommended dietary allowance (RDA) or adequate intake (AI) for carotenoids. The Board has set an RDA for vitamin A. Recommendations by the National Cancer Institute, American Cancer Society, and American Heart Association to consume a variety of fruits and vegetables daily are aimed, in part, at increasing intakes of carotenoids.
Beta-carotene was the first carotenoid to be measured in foods and human blood. The results of early observational studies suggested an inverse relationship between lung cancer risk and β-carotene intake, often assessed by measuring blood levels of β-carotene.19,20 More recently, the development of databases for other carotenoids in foods has allowed scientists to estimate dietary intakes of total and individual dietary carotenoids more accurately. In contrast to early retrospective studies, more recent prospective cohort studies have not consistently found inverse associations between β-carotene intake and lung cancer risk. Analysis of dietary carotenoid intake and lung cancer risk in two large prospective cohort studies in the United States that followed more than 120 000 men and women for at least 10 years revealed no significant association between dietary β-carotene intake and lung cancer risk.21 However, men and women with the highest intakes of total carotenoids, α-carotene, and lycopene were at significantly lower risk of developing lung cancer than those with the lowest intakes. Dietary intakes of total carotenoids, lycopene, β-cryptoxanthin, lutein, and zeaxanthin, but not β-carotene, were associated with significant reductions in risk of lung cancer in a 14-year study of more than 27 000 Finnish male smokers,22 while only dietary in-takes of β-cryptoxanthin, lutein, and zeaxanthin were inversely associated with lung cancer risk in a 6-year study of more than 58 000 Dutch men.23 An analysis of the pooled results of six prospective cohort studies in North America and Europe also found no relationship between dietary β-carotene intake and lung cancer risk, although those with the highest β-cryptoxanthin intakes had a risk of lung cancer that was 24% lower than in those with the lowest intakes.24 While smoking remains the strongest risk factor for lung cancer, results of recent prospective studies using accurate estimates of dietary carotenoid intake suggest that diets rich in several carotenoids—not only β-carotene—may be associated with reduced lung cancer risk. However, a recent systematic review of prospective cohort studies concluded that any protective effect of dietary carotenoids against the development of lung cancer is likely small and not statistically significant.25
The effect of β-carotene supplementation on the risk of developing lung cancer has been examined in three large randomized, placebo-controlled trials. In Finland, the Alpha-Tocopherol Beta-Carotene (ATBC) cancer-prevention trial evaluated the effects of 20 mg/day of β-carotene and/or 50 mg/day of α-tocopherol on more than 29 000 male smokers,26 and in the United States, the β-Carotene and Retinol Efficacy Trial (CARET) evaluated the effects of a combination of 30 mg/day of β-carotene and 25 000 international units (IU)/day of retinol (vitamin A) in more than 18 000 men and women who were smokers or former smokers, or had a history of occupational asbestos exposure.27 Unexpectedly, the risk of lung cancer in the groups taking β-carotene supplements was increased by 16% after 6 years in the ATBC participants and increased by 28% after 4 years in the CARET participants. The Physicians’ Health Study examined the effect of β-carotene supplementation (50 mg every other day) on cancer risk in more than 22 000 male physicians in the United States, of whom only 11% were current smokers.28 In that lower-risk population, β-carotene supplementation for more than 12 years was not associated with an increased risk of lung cancer. Although the reasons for the increase in lung cancer risk are not yet clear, several mechanisms have been proposed29; many experts feel that the risks of high-dose β-carotene supplementation outweigh any potential benefits for cancer prevention, especially in smokers or other high-risk populations.30,31 Beta-carotene is sold as individual supplements and also found in supplements marketed to promote visual health.32
The results of several prospective cohort studies suggest that lycopene-rich diets are associated with significant reductions in the risk of prostate cancer, particularly more aggressive forms.33 In a prospective study of more than 47 000 health professionals followed for 8 years, those with the highest lycopene intake had a risk of prostate cancer that was 21% lower than in those with the lowest lycopene intake.34 Those with the highest intakes of tomatoes and tomato products (accounting for 82% of total lycopene intake) had a risk of prostate cancer that was 35% lower and a risk of aggressive prostate cancer that was 53% lower than in those with the lowest intakes. Similarly, a prospective study of Seventh Day Adventist men found those who reported the highest tomato intakes were at significantly lower risk of prostate cancer,35 and a prospective study of US physicians found those with the highest plasma lycopene levels were at significantly lower risk of developing aggressive prostate cancer.36 However, dietary lycopene intake was not related to prostate cancer risk in a prospective study of more than 58 000 Dutch men.37 A meta-analysis that combined the results of 11 case–control and 10 prospective studies found men with the highest intakes of dietary lycopene or tomatoes had modest, 11%–19% reductions in prostate cancer risk.38 Most recently, a prospective study in a cohort of 29 361 men followed for 4.2 years found no association between dietary lycopene intake and prostate cancer risk.39 Additionally, a recent large prospective study found no association between plasma concentrations of lycopene, or plasma concentrations of total carotenoids, and overall risk of prostate cancer.40 While there is considerable scientific interest in the potential for lycopene to help prevent prostate cancer, it is not yet clear whether the prostate cancer risk reduction observed in some epidemiological studies is related to lycopene itself, other compounds in tomatoes, or other factors associated with lycopene-rich diets. To date, results of short-term, dietary intervention studies using lycopene in patients with prostate cancer have been promising.41 Yet, the safety and efficacy of long-term use of lycopene supplements for prevention or treatment of prostate cancer is not known.41 Largescale, controlled clinical trials would be needed to address these issues.
Because they are very soluble in fat and very insoluble in water, carotenoids circulate in lipoproteins along with cholesterol and other fats. Evidence that low-density lipoprotein (LDL) oxidation plays a role in the development of atherosclerosis led scientists to investigate the role of antioxidant compounds like carotenoids in the prevention of cardiovascular disease.42 The thickness of the inner layers of the carotid arteries can be measured noninvasively using ultrasound technology. This measurement of carotid intima–media thickness is considered a reliable marker of atherosclerosis.43 Several case–control and cross-sectional studies have found higher blood levels of carotenoids to be associated with significantly lower measures of carotid artery intima–media thickness.44–49 Higher plasma carotenoids at baseline have been associated with significant reductions in risk of cardiovascular disease in some prospective studies50–54 but not in others.55–58 While the results of several prospective studies indicate that people with higher intakes of carotenoid-rich fruits and vegetables are at lower risk of cardiovascular disease,58–61 it is not yet clear whether this effect is a result of carotenoids or of other factors associated with diets high in carotenoid-rich fruits and vegetables.
In contrast to the results of epidemiological studies suggesting that high dietary intakes of carotenoid-rich fruits and vegetables may decrease cardiovascular disease risk, four randomized controlled trials found no evidence that β-carotene supplements in doses ranging from 20 to 50 mg/day were effective in preventing cardiovascular diseases.26,28–62,63 Based on the results of these randomized controlled trials, the US Preventive Health Services Task Force concluded that there was good evidence that β-carotene supplements provided no benefit in the prevention of cardiovascular disease in middle-aged and older adults.31,64 Thus, although diets rich in β-carotene have generally been associated with reduced cardiovascular disease risk in observational studies, there is no evidence that β-carotene supplementation reduces cardiovascular disease risk.65
In Western countries, degeneration of the macula, the center of the eye's retina, is the leading cause of blindness in older adults. Unlike cataracts, in which the diseased lens can be replaced, there is no cure for age-related macular degeneration (AMD). Therefore, efforts are aimed at disease prevention or delaying the progression of AMD.
The only carotenoids found in the retina are lutein and zeaxanthin. Lutein and zeaxanthin are present at high concentrations in the macula, where they are efficient absorbers of blue light. By preventing a substantial amount of the blue light entering the eye from reaching the underlying structures involved in vision, lutein and zeaxanthin may protect against light-induced oxidative damage, which is thought to play a role in the pathology of AMD.10 It is also possible, though not proven, that lutein and zeaxanthin act directly to neutralize oxidants formed in the retina. Epidemiological studies provide some evidence that higher intakes of lutein and zeaxanthin are associated with lower risk of AMD.66 However, the relationship is by no means clear-cut. While cross-sectional and retrospective case–control studies found that higher levels of lutein and zeaxanthin in the diet,67–69 blood,70,71 and retina72,73 were associated with a lower incidence of AMD, several prospective cohort studies found no relationship between baseline dietary intakes or serum levels of lutein and zeaxanthin and the risk of developing AMD over time.74–77 Although scientists are very interested in the potential for increased lutein and zeaxanthin intakes to reduce the risk of macular degeneration, it is premature to recommend supplements without more data from randomized controlled trials.78 A clinical trial, the Age-Related Eye Disease Study 2 (AREDS2), is currently under way to evaluate the effect of supplemental lutein and zeaxanthin on the progression of advanced AMD.79 To date, the available scientific evidence suggests that consuming at least 6 mg/day of dietary lutein and zeaxanthin from fruits and vegetables may decrease the risk of AMD.67–69
A randomized controlled trial in patients with atrophic AMD found that supplementation with 10 mg/day of lutein slightly improved visual acuity after 1 year compared with a placebo.80 However, the investigators concluded that further research was needed to assess the effects of long-term lutein supplementation on atrophic AMD.
The first randomized controlled trial (AREDS1) designed to examine the effect of a carotenoid supplement on AMD used β-carotene in combination with vitamin C, vitamin E, and zinc because lutein and zeaxanthin were not commercially available as supplements at the time the trial began.81 Although the combination of antioxidants and zinc lowered the risk of developing advanced macular degeneration in individuals with signs of moderate to severe macular degeneration in at least one eye, it is unlikely that the benefit was related to β-carotene, since it is not present in the retina. Supplementation of male smokers in Finland with 20 mg/day of β-carotene for 6 years did not decrease the risk of AMD compared with placebo.82 A placebo-controlled trial in a cohort of 22 071 healthy US men found that β-carotene supplementation (50 mg every other day) had no effect on the incidence of age-related maculopathy—an early stage of AMD.83 Recent systematic reviews of randomized controlled trials have concluded that there is no evidence that β-carotene supplementation prevents or delays the onset of AMD.84,85
Ultraviolet light and oxidants can damage proteins in the eye's lens, causing structural changes that result in the formation of opacities known as cataracts. As people age, cumulative damage to lens proteins often results in cataracts that are large enough to interfere with vision.7
The observation that lutein and zeaxanthin are the only carotenoids in the human lens has stimulated interest in the potential for increased in-takes of lutein and zeaxanthin to prevent or slow the progression of cataracts.10 Four large prospective studies found that men and women with the highest intakes of foods rich in lutein and zeaxanthin, particularly spinach, kale, and broccoli, were 18%–50% less likely to require cataract extraction86,87 or develop cataracts.88–90 Additional research is required to determine whether these findings are related specifically to lutein and zeaxanthin intake or to other factors associated with diets high in carotenoid-rich foods.66
Evidence from epidemiological studies that cataracts were less prevalent in people with high dietary intakes and blood levels of carotenoids led to the inclusion of β-carotene supplements in several large randomized controlled trials of antioxidants. The results of those trials have been somewhat conflicting. Beta-carotene supplementation (20 mg/day) for more than 6 years did not affect the prevalence of cataracts or the frequency of cataract surgery in male smokers living in Finland.82 In contrast, a 12-year study of male physicians in the United States found that β-carotene supplementation (50 mg every other day) decreased the risk of cataracts in smokers but not in nonsmokers.91 Three randomized controlled trials examined the effect of an antioxidant combination that included β-carotene, vitamin C, and vitamin E on the progression of cataracts. Two trials found no benefit after supplementation for 5 years92 or more than 6 years,93 but one trial found a small decrease in the progression of cataracts after 3 years of supplementation.94 Overall, the results of randomized controlled trials suggest that the benefit of β-carotene supplementation in slowing the progression of age-related cataracts does not outweigh the potential risks.
The most prevalent carotenoids in North American diets are α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin.6 Carotenoids in foods are mainly in the all-trans form (Figs. 8.1 and 8.2), although cooking may result in the formation of other isomers. The relatively low bioavailability of carotenoids from most foods compared with supplements is partly due to the fact that they are associated with proteins in the plant matrix.2 Chopping, homogenizing, and cooking disrupt the plant matrix, increasing the bioavailability of carotenoids.4 The bioavailability of lycopene from tomatoes is substantially improved by heating tomatoes in oil.95,96
Table 8.1 Alpha-carotene content of selected foods
Food |
Serving |
α-Carotene (mg) |
Pumpkin, canned |
1 cup |
11.7 |
Carrot juice, canned |
1 cup (8 fl oz) |
10.2 |
Carrots, cooked |
1 cup |
5.9 |
Carrots, raw |
1 medium |
2.1 |
Mixed vegetables, frozen, cooked |
1 cup |
1.8 |
Winter squash, baked |
1 cup |
1.4 |
Plantains, raw |
1 medium |
0.8 |
Collards, frozen, cooked |
1 cup |
0.2 |
Tomatoes, raw |
1 medium |
0.1 |
Tangerines, raw |
1 medium |
0.09 |
Peas, edible-podded, frozen, cooked |
1 cup |
0.09 |
Table 8.2 Beta-carotene content of selected foods
Food |
Serving |
β-Carotene (mg) |
Carrot juice, canned |
1 cup (8 fl oz) |
22.0 |
Pumpkin, canned |
1 cup |
17.0 |
Spinach, frozen, cooked |
1 cup |
13.8 |
Sweet potato, baked |
1 medium |
13.1 |
Carrots, cooked |
1 cup |
13.0 |
Collards, frozen, cooked |
1 cup |
11.6 |
Kale, frozen, cooked |
1 cup |
11.5 |
Turnip greens, frozen, cooked |
1 cup |
10.6 |
Pumpkin pie |
1 piece |
7.4 |
Winter squash, cooked |
1 cup |
5.7 |
Carrots, raw |
1 medium |
5.1 |
Dandelion greens, cooked |
1 cup |
4.1 |
Cantaloupe, raw |
1 cup |
3.2 |
Alpha-carotene and β-carotene are provitamin A carotenoids, meaning they can be converted in the body to vitamin A. The vitamin A activity of β-carotene in foods is 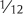 that of retinol (preformed vitamin A). Thus, it would take 12 µg of β-carotene from foods to provide the equivalent of 1 µg (0.001 mg) of retinol. The vitamin A activity of α-carotene from foods is
that of retinol (preformed vitamin A). Thus, it would take 12 µg of β-carotene from foods to provide the equivalent of 1 µg (0.001 mg) of retinol. The vitamin A activity of α-carotene from foods is 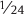 that of retinol, so it would take 24 µg of α-carotene from foods to provide the equivalent of 1 µg of retinol. Orange and yellow vegetables like carrots and winter squash are rich sources of α- and β-carotene. Spinach is also a rich source of β-carotene, although the chlorophyll in spinach leaves hides the yellow–orange pigment. Some foods that are good sources of α-carotene and β-carotene are listed in Tables 8.1 and 8.2, respectively.97
that of retinol, so it would take 24 µg of α-carotene from foods to provide the equivalent of 1 µg of retinol. Orange and yellow vegetables like carrots and winter squash are rich sources of α- and β-carotene. Spinach is also a rich source of β-carotene, although the chlorophyll in spinach leaves hides the yellow–orange pigment. Some foods that are good sources of α-carotene and β-carotene are listed in Tables 8.1 and 8.2, respectively.97
Like α- and β-carotene, β-cryptoxanthin is a provitamin A carotenoid. The vitamin A activity of β-cryptoxanthin from foods is 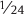 that of retinol, so it would take 24 µg of β-cryptoxanthin from food to provide the equivalent of 1 µg of retinol. Orange and red fruits and vegetables like sweet red peppers and oranges are particularly rich sources of β-cryptoxanthin. Some foods that are good sources of β-cryptoxanthin are listed in Table 8.3.97
that of retinol, so it would take 24 µg of β-cryptoxanthin from food to provide the equivalent of 1 µg of retinol. Orange and red fruits and vegetables like sweet red peppers and oranges are particularly rich sources of β-cryptoxanthin. Some foods that are good sources of β-cryptoxanthin are listed in Table 8.3.97
Lycopene gives tomatoes, pink grapefruit, watermelon, and guava their red color. It has been estimated that 80% of the lycopene in the US diet comes from tomatoes and tomato products like tomato sauce, tomato paste, and catsup (ketchup).98 Lycopene is not a provitamin A carotenoid, meaning the body cannot convert lycopene to vitamin A. Some foods that are good sources of lycopene are listed in Table 8.4.97
Although lutein and zeaxanthin are different compounds, they are both from the class of carotenoids known as xanthophylls. They are not provitamin A carotenoids. Some methods used to quantify lutein and zeaxanthin in foods do not separate the two compounds, so they are typically reported as lutein and zeaxanthin or lutein + zeaxanthin. Lutein and zeaxanthin are present in a variety of fruits and vegetables. Dark green leafy vegetables like spinach and kale are particularly rich sources of lutein and zeaxanthin. One study found that the bioavailability of lutein from lutein-enriched eggs (from chickens fed a lutein-enriched diet) was significantly higher than from spinach or lutein supplements.99 Some foods that are good sources lutein and zeaxanthin are listed in Table 8.5.97
Dietary supplements providing purified carotenoids and combinations of carotenoids are commercially available in the United States without a prescription. Carotenoids are best absorbed when taken with a meal containing fat.
Because it is has vitamin A activity, β-carotene may be used to provide all or part of the vitamin A in multivitamin supplements. The vitamin A activity of β-carotene from supplements is much higher than that of β-carotene from foods. It takes only 2 µg (0.002 mg) of β-carotene from supplements to provide 1 µg of retinol (preformed vitamin A). The β-carotene content of supplements is often listed in IU rather than µg; 3000 µg (3 mg) of β-carotene provides 5000 IU of vitamin A. Most commercial supplements contain 5000–25 000 IU of β-carotene.100
Lycopene has no vitamin A activity. Synthetic lycopene and lycopene from natural sources, mainly tomatoes, are available as nutritional supplements. Many commercial supplements provide 5–20 mg of lycopene.100
Lutein and zeaxanthin have no vitamin A activity. Lutein and zeaxanthin supplements are available as free carotenoids or as esters (esterified to fatty acids). One study found that free lutein and lutein esters had comparable bioavailability,99 while another found that lutein esters were more bioavailable than free lutein.101 Many commercially available lutein and zeaxanthin supplements have much higher amounts of lutein than zeaxanthin.102 Such supplements typically contain 4–20 mg of lutein and 0.2–1 mg of zeaxanthin, although other dosages are available.100 Supplements containing only lutein or only zeaxanthin are also available.
Table 8.3 Beta-cryptoxanthin content of selected foods
Food |
Serving |
β-Cryptoxanthin (mg) |
Pumpkin, cooked |
1 cup |
3.6 |
Papayas, raw |
1 medium |
2.3 |
Sweet red peppers, cooked |
1 cup |
0.6 |
Sweet red peppers, raw |
1 medium |
0.6 |
Orange juice, fresh |
1 cup (8 fl oz) |
0.4 |
Tangerines, raw |
1 medium |
0.4 |
Carrots, frozen, cooked |
1 cup |
0.3 |
Yellow corn, frozen, cooked |
1 cup |
0.2 |
Watermelon, raw |
1 wedge (1/16 of a melon 15 in long × 7.5 in diameter [38×19 cm]) |
0.2 |
Paprika, dried |
1 tsp |
0.2 |
Oranges, raw |
1 medium |
0.2 |
Nectarines, raw |
1 medium |
0.1 |
Table 8.4 Lycopene content of selected foods
Food |
Serving |
Lycopene (mg) |
Tomato paste, canned |
1 cup |
75.4 |
Tomato purée, canned |
1 cup |
54.4 |
Tomato soup, canned, condensed |
1 cup |
26.4 |
Vegetable juice cocktail, canned |
1 cup |
23.3 |
Tomato juice, canned |
1 cup |
22.0 |
Watermelon, raw |
1 wedge (1/16 of a melon 15 in long × 7.5 in diameter [38×19 cm]) |
13.0 |
Tomatoes, raw |
1 cup |
4.6 |
Catsup (ketchup) |
1 tablespoon |
2.5 |
Pink grapefruit, raw |
½ grapefruit |
1.7 |
Baked beans, canned |
1 cup |
1.3 |
Table 8.5 Lutein + zeaxanthin content of selected foods
Food |
Serving |
Lutein + Zeaxanthin (mg) |
Spinach, frozen, cooked |
1 cup |
29.8 |
Kale, frozen, cooked |
1 cup |
25.6 |
Turnip greens, frozen, cooked |
1 cup |
19.5 |
Collards, frozen, cooked |
1 cup |
18.5 |
Dandelion greens, cooked |
1 cup |
9.6 |
Mustard greens, cooked |
1 cup |
8.3 |
Summer squash, cooked |
1 cup |
4.0 |
Peas, frozen, cooked |
1 cup |
3.8 |
Winter squash, baked |
1 cup |
2.9 |
Pumpkin, cooked |
1 cup |
2.5 |
Brussels sprouts, frozen, cooked |
1 cup |
2.4 |
Broccoli, frozen, cooked |
1 cup |
2.0 |
Sweet yellow corn, boiled |
1 cup |
1.5 |
Although β-carotene can be converted to vitamin A, the conversion of β-carotene to vitamin A decreases when body stores of vitamin A are high. This may explain why high doses of β-carotene have never been found to cause vitamin A toxicity.103 High doses of β-carotene (up to 180 mg/day) have been used to treat erythropoietic protoporphyria, a photosensitivity disorder, without toxic side effects.6
No toxicities have been reported.104
Increased lung cancer risk. Two randomized controlled trials in smokers and former asbestos workers found that supplementation with 20–30 mg/day of β-carotene for 4–6 years was associated with significant 16%–28% increases in the risk of lung cancer compared with placebo. Although the reasons for these findings are not yet clear, many experts feel that the risks of high-dose β-carotene supplementation outweigh any potential benefits for chronic disease prevention, especially in smokers or other high-risk populations.30,31
Carotenodermia. High doses of β-carotene supplements (30 mg/day or more) and the consumption of large amounts of carotene-rich foods have resulted in a yellow discoloration of the skin known as carotenodermia. Carotenodermia is not associated with any underlying health problems and resolves when β-carotene supplements are discontinued or dietary carotene intake is reduced.
Lycopenodermia. High intakes of lycopene-rich foods or supplements may result in a deep orange discoloration of the skin known as lycopenodermia. Because lycopene is more intensely colored than the carotenes, lycopenodermia may occur at lower doses than carotenodermia.6
Adverse effects of lutein and zeaxanthin have not been reported.102
Unlike vitamin A, high doses of β-carotene taken by pregnant women have not been associated with increased risk of birth defects.6 However, the safety of high-dose β-carotene supplements in pregnancy and lactation has not been well studied. Although there is no reason to limit dietary β-carotene intake, pregnant and breast-feeding women should avoid consuming more than 3 mg/day (5000 IU/day) of β-carotene from supplements, unless they are prescribed under medical supervision.102,103
The safety of carotenoid supplements other than β-carotene in pregnancy and lactation has not been established, so pregnant and breast-feeding women should obtain carotenoids from foods rather than supplements. There is no reason to limit the consumption of carotenoid-rich fruits and vegetables during pregnancy.102,105
The cholesterol-lowering agents, cholestyramine (Questran) and colestipol (Colestid), can reduce absorption of fat-soluble vitamins and carotenoids, as can mineral oil and orlistat (Xenical), a drug used to treat obesity.102 Colchicine, a drug used to treat gout, can cause intestinal malabsorption. However, long-term use of 1–2 mg/day of colchicine did not affect serum β-carotene levels in one study.106 Increasing gastric pH through the use of proton pump inhibitors, such as omeprazole (Prilosec, Losec), lansoprazole (Prevacid), rabeprazole (Aciphex), and pantoprazole (Protonix, Pantoloc), decreased the absorption of a single dose of a β-carotene supplement, but it is not known if the absorption of dietary carotenoids is affected.107
A 3-year randomized controlled trial in 160 patients with documented coronary heart disease and low serum high-density lipoprotein (HDL) concentrations found that a combination of simvastatin (Zocor) and niacin increased HDL2 levels, inhibited the progression of coronary artery stenosis, and decreased the frequency of cardiovascular events, including myocardial infarction (MI) and stroke.108 Surprisingly, when an antioxidant combination (1000 mg of vitamin C, 800 IU of α-tocopherol, 100 µg of selenium, and 25 mg of β-carotene daily) was taken with the simvastatin–niacin combination, these protective effects were diminished. Since the antioxidants were taken together in this trial, the individual contribution of β-carotene cannot be determined. In contrast, a much larger randomized controlled trial of simvastatin and an antioxidant combination (600 mg of vitamin E, 250 mg of vitamin C, and 20 mg of β-carotene daily) in more than 20 000 men and women with coronary artery disease or diabetes found that the antioxidant combination did not diminish the cardioprotective effects of simvastatin therapy over a 5-year period,109 suggesting that the antioxidant combination may have interfered with the HDL-raising effect of niacin in the former trial. Further research is needed to determine potential interactions between antioxidant supplements and cholesterol-lowering agents, such as niacin and HMG-CoA reductase inhibitors (statins).
In a controlled feeding study, consumption of 18 g/day of the fat substitute Olestra (sucrose polyester) resulted in a 27% decrease in serum carotenoid concentrations after 3 weeks.110 Studies in people before and after the introduction of Olestra-containing snacks to the marketplace found that total serum carotenoid concentrations decreased by 15% in those who reported consuming at least 2 g/day of Olestra.111 One study in adults found that those who consumed more than 4.4 g of Olestra weekly experienced a 9.7% decline in total serum carotenoids compared with those not consuming Olestra.112
Some studies found that the regular use of plant sterol-containing spreads resulted in modest, 10%–20% decreases in the plasma concentrations of some carotenoids, particularly α-carotene, β-carotene, and lycopene113,114 (see Chapter 18 on Phytosterols). However, advising people who use plant sterol- or stanol-containing margarines to consume an extra serving of carotenoid-rich fruits or vegetables daily prevented decreases in plasma carotenoid concentrations.115,116
Alcohol. The relationships between alcohol consumption and carotenoid metabolism are not well understood. There is some evidence that regular alcohol consumption inhibits the conversion of β-carotene to retinol.117 Increases in lung cancer risk associated with high-dose β-carotene supplementation in two randomized controlled trials were enhanced in those with higher alcohol intakes.27,118
The results of metabolic studies suggest that high doses of β-carotene compete for absorption with lutein and lycopene when consumed at the same time.119–121 However, the consumption of high-dose β-carotene supplements did not adversely affect serum carotenoid concentrations in long-term clinical trials.122–125
• Carotenoids are yellow, orange, and red pigments synthesized by plants. The most common carotenoids in North American diets are α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene.
• Alpha-carotene, β-carotene, and β-cryptoxanthin are provitamin A carotenoids, meaning they can be converted by the body to retinol (vitamin A). Lutein, zeaxanthin, and lycopene have no vitamin A activity.
• At present, it is unclear whether the biological effects of carotenoids in humans are related to their antioxidant activity or other non-antioxidant activities.
• Although the results of epidemiological studies suggest that diets high in carotenoid-rich fruits and vegetables are associated with reduced risk of cardiovascular disease and some cancers, high-dose β-carotene supplements did not reduce the risk of cardiovascular diseases or cancers in large randomized controlled trials.
• Two randomized controlled trials found that high-dose β-carotene supplements increased the risk of lung cancer in smokers and former asbestos workers.
• Several epidemiological studies found that men with high intakes of lycopene from tomatoes and tomato products were less likely to develop prostate cancer than men with low in-takes, but it is not known whether lycopene supplements will decrease the incidence or severity of prostate cancer.
• Lutein and zeaxanthin are the only carotenoids found in the retina and lens of the eye. The results of epidemiological studies suggest that diets rich in lutein and zeaxanthin may help slow the development of age-related macular degeneration and cataracts, but it is not known whether lutein and zeaxanthin supplements will slow the development of these age-related eye diseases.
• Carotenoids are best absorbed with fat in a meal. Chopping, puréeing, and cooking carotenoid-containing vegetables in oil generally increases the bioavailability of the carotenoids they contain.
1. International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention: Carotenoids. Lyon, France: International Agency for Research on Cancer; 1998
2. Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr 2002;22:483–504
3. Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP. Serum retinol concentrations in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment. Am J Clin Nutr 1998;68(3): 623–629
4. van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr 2000;130(3):503–506
5. During A, Harrison EH. Intestinal absorption and metabolism of carotenoids: insights from cell culture. Arch Biochem Biophys 2004;430(1):77–88
6. Institute of Medicine, Food and Nutrition Board. Beta-carotene and other Carotenoids. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000:325–400
7. Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd ed. New York, NY: Oxford University Press; 1999
8. Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 1989;274(2):532–538
9. Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys 2001;385(1):20–27
10. Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 2003;23:171–201
11. Bertram JS. Carotenoids and gene regulation. Nutr Rev 1999;57(6):182–191
12. Stahl W, Nicolai S, Briviba K, et al. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis 1997;18(1):89–92
13. van Poppel G, Spanhaak S, Ockhuizen T. Effect of beta-carotene on immunological indexes in healthy male smokers. Am J Clin Nutr 1993;57(3):402–407
14. Hughes DA, Wright AJ, Finglas PM, et al. The effect of beta-carotene supplementation on the immune function of blood monocytes from healthy male non-smokers. J Lab Clin Med 1997;129(3):309–317
15. Santos MS, Gaziano JM, Leka LS, Beharka AA, Hennekens CH, Meydani SN. Beta-carotene-induced enhancement of natural killer cell activity in elderly men: an investigation of the role of cytokines. Am J Clin Nutr 1998;68(1):164–170
16. Hughes DA, Wright AJ, Finglas PM, et al. Effects of lycopene and lutein supplementation on the expression of functionally associated surface molecules on blood monocytes from healthy male nonsmokers. J Infect Dis 2000;182(Suppl 1):S11–S15
17. Watzl B, Bub A, Blockhaus M, et al. Prolonged tomato juice consumption has no effect on cell-mediated immunity of well-nourished elderly men and women. J Nutr 2000;130(7):1719–1723
18. Corridan BM, O'Donoghue M, Hughes DA, Morrissey PA. Low-dose supplementation with lycopene or beta-carotene does not enhance cell-mediated immunity in healthy free-living elderly humans. Eur J Clin Nutr 2001;55(8):627–635
19. Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature 1981;290(5803):201–208
20. Ziegler RG. A review of epidemiologic evidence that carotenoids reduce the risk of cancer. J Nutr 1989;119(1):116–122
21. Michaud DS, Feskanich D, Rimm EB, et al. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am J Clin Nutr 2000;72(4):990–997
22. Holick CN, Michaud DS, Stolzenberg-Solomon R, et al. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol 2002; 156(6):536–547
23. Voorrips LE, Goldbohm RA, Brants HA, et al. A prospective cohort study on antioxidant and folate in-take and male lung cancer risk. Cancer Epidemiol Biomarkers Prev 2000;9(4):357–365
24. Männistö S, Smith-Warner SA, Spiegelman D, et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomarkers Prev 2004;13(1):40–48
25. Gallicchio L, Boyd K, Matanoski G, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr 2008;88(2):372–383
26. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 1994;330(15): 1029–1035
27. Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst 1996;88(21):1550–1559
28. Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 1996;334(18): 1145–1149
29. Palozza P, Simone R, Mele MC. Interplay of carotenoids with cigarette smoking: implications in lung cancer. Curr Med Chem 2008;15(9):844–854
30. Vainio H, Rautalahti M. An international evaluation of the cancer preventive potential of carotenoids. Cancer Epidemiol Biomarkers Prev 1998;7(8):725–728
31. US Preventive Services Task Force. Routine vitamin supplementation to prevent cancer and cardiovascular disease: recommendations and rationale. Ann Intern Med 2003;139(1):51–55
32. Tanvetyanon T, Bepler G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer 2008;113(1): 150–157
33. Giovannucci E. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp Biol Med (Maywood) 2002;227(10):852–859
34. Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 1995;87(23):1767–1776
35. Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 1989;64(3):598–604
36. Gann PH, Ma J, Giovannucci E, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res 1999;59(6):1225–1230
37. Schuurman AG, Goldbohm RA, Brants HA, van den Brandt PA. A prospective cohort study on intake of retinol, vitamins C and E, and carotenoids and prostate cancer risk (Netherlands). Cancer Causes Control 2002;13(6):573–582
38. Etminan M, Takkouche B, Caamaño-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev 2004; 13(3):340–345
39. Kirsh VA, Mayne ST, Peters U, et al. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006;15(1):92–98
40. Key TJ, Appleby PN, Allen NE, et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr 2007;86(3):672–681
41. Dahan K, Fennal M, Kumar NB. Lycopene in the prevention of prostate cancer. J Soc Integr Oncol 2008; 6(1):29–36
42. Kritchevsky SB. beta-Carotene, carotenoids and the prevention of coronary heart disease. J Nutr 1999; 129(1):5–8
43. Bots ML, Grobbee DE. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc Drugs Ther 2002;16(4):341–351
44. Rissanen TH, Voutilainen S, Nyyssönen K, Salonen R, Kaplan GA, Salonen JT. Serum lycopene concentrations and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2003;77(1):133–138
45. Dwyer JH, Paul-Labrador MJ, Fan J, Shircore AM, Merz CN, Dwyer KM. Progression of carotid intima-media thickness and plasma antioxidants: the Los Angeles Atherosclerosis Study. Arterioscler Thromb Vasc Biol 2004;24(2):313–319
46. McQuillan BM, Hung J, Beilby JP, Nidorf M, Thompson PL. Antioxidant vitamins and the risk of carotid atherosclerosis. The Perth Carotid Ultrasound Disease Assessment study (CUDAS). J Am Coll Cardiol 2001;38(7):1788–1794
47. Rissanen T, Voutilainen S, Nyyssönen K, Salonen R, Salonen JT. Low plasma lycopene concentration is associated with increased intima-media thickness of the carotid artery wall. Arterioscler Thromb Vasc Biol 2000;20(12):2677–2681
48. D'Odorico A, Martines D, Kiechl S, et al. High plasma levels of alpha- and beta-carotene are associated with a lower risk of atherosclerosis: results from the Bruneck study. Atherosclerosis 2000;153(1):231–239
49. Iribarren C, Folsom AR, Jacobs DR Jr, Gross MD, Belcher JD, Eckfeldt JH. Association of serum vitamin levels, LDL susceptibility to oxidation, and autoantibodies against MDA-LDL with carotid atherosclerosis. A case-control study. The ARIC Study Investigators. Atherosclerosis Risk in Communities. Arterioscler Thromb Vasc Biol 1997;17(6):1171–1177
50. Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr 2004;79(1):47–53
51. Rissanen TH, Voutilainen S, Nyyssönen K, et al. Low serum lycopene concentration is associated with an excess incidence of acute coronary events and stroke: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr 2001;85(6):749–754
52. Street DA, Comstock GW, Salkeld RM, Schüep W, Klag MJ. Serum antioxidants and myocardial infarction. Are low levels of carotenoids and alpha-tocopherol risk factors for myocardial infarction? Circulation 1994;90(3):1154–1161
53. Ito Y, Kurata M, Suzuki K, Hamajima N, Hishida H, Aoki K. Cardiovascular disease mortality and serum carotenoid levels: a Japanese population-based follow-up study. J Epidemiol 2006;16(4):154–160
54. Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr 2008;138(2):344–350
55. Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in men. Am J Clin Nutr 2005;81(5):990–997
56. Hak AE, Stampfer MJ, Campos H, et al. Plasma carotenoids and tocopherols and risk of myocardial infarction in a low-risk population of US male physicians. Circulation 2003;108(7):802–807
57. Evans RW, Shaten BJ, Day BW, Kuller LH. Prospective association between lipid soluble antioxidants and coronary heart disease in men. The Multiple Risk Factor Intervention Trial. Am J Epidemiol 1998;147(2): 180–186
58. Sahyoun NR, Jacques PF, Russell RM. Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol 1996;144(5):501–511
59. Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 1993;328(20):1450–1456
60. Gaziano JM, Manson JE, Branch LG, Colditz GA, Willett WC, Buring JE. A prospective study of consumption of carotenoids in fruits and vegetables and decreased cardiovascular mortality in the elderly. Ann Epidemiol 1995;5(4):255–260
61. Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Manson JE, Willett WC. Dietary carotenoids and risk of coronary artery disease in women. Am J Clin Nutr 2003;77(6):1390–1399
62. Greenberg ER, Baron JA, Karagas MR, et al. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996;275(9):699–703
63. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334(18):1150–1155
64. Morris CD, Carson S. Routine vitamin supplementation to prevent cardiovascular disease: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139(1):56–70
65. Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr 2006;83(6):1265–1271
66. Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. J Nutr 2002;132(3):518S–524S
67. Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant in-take. Acta Ophthalmol Scand 2002;80(4):368–371
68. Mares-Perlman JA, Fisher AI, Klein R, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol 2001;153(5):424–432
69. Seddon JM, Ajani UA, Sperduto RD, et al; Eye Disease Case-Control Study Group. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994;272(18):1413–1420
70. Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci 2003;44(6): 2461–2465
71. Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol 1993;111(1):104–109
72. Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci 2001;42(1):235–240
73. Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci 2001;42(2):439–446
74. Cho E, Seddon JM, Rosner B, Willett WC, Hankinson SE. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol 2004;122(6):883–892
75. Flood V, Smith W, Wang JJ, Manzi F, Webb K, Mitchell P. Dietary antioxidant intake and incidence of early age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology 2002;109(12):2272–2278
76. Mares-Perlman JA, Klein R, Klein BE, et al. Association of zinc and antioxidant nutrients with age-related maculopathy. Arch Ophthalmol 1996;114(8):991–997
77. Mares-Perlman JA, Brady WE, Klein R, et al. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol 1995;113(12):1518–1523
78. Mares-Perlman JA. Too soon for lutein supplements. Am J Clin Nutr 1999;70(4):431–432
79. Coleman H, Chew E. Nutritional supplementation in age-related macular degeneration. Curr Opin Ophthalmol 2007;18(3):220–223
80. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75(4):216–230
81. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119(10):1417–1436
82. Teikari JM, Laatikainen L, Virtamo J, et al. Six-year supplementation with alpha-tocopherol and beta-carotene and age-related maculopathy. Acta Ophthalmol Scand 1998;76(2):224–229
83. Christen WG, Manson JE, Glynn RJ, et al. Beta carotene supplementation and age-related maculopathy in a randomized trial of US physicians. Arch Ophthalmol 2007;125(3):333–339
84. Evans JR, Henshaw K. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev 2008; (1):CD000253
85. Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye (Lond) 2008;22(6):751–760
86. Brown L, Rimm EB, Seddon JM, et al. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am J Clin Nutr 1999;70(4):517–524
87. Chasan-Taber L, Willett WC, Seddon JM, et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr 1999;70(4):509–516
88. Lyle BJ, Mares-Perlman JA, Klein BE, Klein R, Greger JL. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol 1999;149(9):801–809
89. Christen WG, Manson JE, Glynn RJ, et al. A randomized trial of beta carotene and age-related cataract in US physicians. Arch Ophthalmol 2003;121(3):372–378
90. Moeller SM, Voland R, Tinker L, et al; CAREDS Study Group; Women's Helath Initiative. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an Ancillary Study of the Women's Health Initiative. Arch Ophthalmol 2008;126(3):354–364
91. Christen WG, Manson JE, Glynn RJ, et al. A randomized trial of beta carotene and age-related cataract in US physicians. Arch Ophthalmol 2003;121(3):372–378
92. Gritz DC, Srinivasan M, Smith SD, et al. The Antioxidants in Prevention of Cataracts Study: effects of antioxidant supplements on cataract progression in South India. Br J Ophthalmol 2006;90(7):847–851
93. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol 2001;119 (10):1439–1452
94. Chylack LT Jr, Brown NP, Bron A, et al. The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol 2002; 9(1):49–80
95. Gärtner C, Stahl W, Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am J Clin Nutr 1997;66(1):116–122
96. Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr 1992;122(11):2161–2166
97. US Department of Agriculture, Agricultural Research Service. USDA Nutrient Database for Standard Reference, Release 21. 2008. Available at: http://www.nal.usda.gov/fnic/foodcomp/search/. Accessed May 2, 2012
98. Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev 1998;56(2 Pt 1):35–51
99. Chung HY, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr 2004;134(8):1887–1893
100. Natural Medicines Comprehensive Database [Web site]. Available at: http://www.naturaldatabase.com. Accessed May 2, 2012
101. Bowen PE, Herbst-Espinosa SM, Hussain EA, Stacewicz-Sapuntzakis M. Esterification does not impair lutein bioavailability in humans. J Nutr 2002; 132(12):3668–3673
102. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. Montvale, NJ: Medical Economics Company, Inc; 2001
103. Beta-Carotene. Natural Medicines Comprehensive Database [Web site]. Available at: http://www.naturaldatabase.com/monograph.asp?mono_id=999&brand_id=. Accessed May 2, 2012
104. Solomons NW. Vitamin A and carotenoids. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition. 8th ed. Washington, DC: ILSI Press; 2001:127–145
105. Lutein. Natural Medicines Comprehensive Database [Web site]. Available at: http://www.naturaldatabase.com/monograph.asp?mono_id=754&brand_id=. Accessed May 2, 2012
106. Ehrenfeld M, Levy M, Sharon P, Rachmilewitz D, Eliakim M. Gastrointestinal effects of long-term colchicine therapy in patients with recurrent polyserositis (familial mediterranean fever). Dig Dis Sci 1982; 27(8):723–727
107. Tang G, Serfaty-Lacrosniere C, Camilo ME, Russell RM. Gastric acidity influences the blood response to a beta-carotene dose in humans. Am J Clin Nutr 1996;64(4):622–626
108. Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001;345(22):1583–1592
109. Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract 2002;56(1):53–56
110. Koonsvitsky BP, Berry DA, Jones MB, et al. Olestra affects serum concentrations of alpha-tocopherol and carotenoids but not vitamin D or vitamin K status in free-living subjects. J Nutr 1997;127(8,Suppl): 1636S–1645S
111. Thornquist MD, Kristal AR, Patterson RE, et al. Olestra consumption does not predict serum concentrations of carotenoids and fat-soluble vitamins in free-living humans: early results from the sentinel site of the olestra post-marketing surveillance study. J Nutr 2000;130(7):1711–1718
112. Neuhouser ML, Rock CL, Kristal AR, et al. Olestra is associated with slight reductions in serum carotenoids but does not markedly influence serum fat-soluble vitamin concentrations. Am J Clin Nutr 2006;83(3):624–631
113. Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R; Stresa Workshop Participants. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 2003;78(8):965–978
114. Weststrate JA, Meijer GW. Plant sterol-enriched margarines and reduction of plasma total- and LDLcholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 1998;52(5):334–343
115. Ntanios FY, Duchateau GS. A healthy diet rich in carotenoids is effective in maintaining normal blood carotenoid levels during the daily use of plant sterol-enriched spreads. Int J Vitam Nutr Res 2002; 72(1):32–39
116. Noakes M, Clifton P, Ntanios F, Shrapnel W, Record I, McInerney J. An increase in dietary carotenoids when consuming plant sterols or stanols is effective in maintaining plasma carotenoid concentrations. Am J Clin Nutr 2002;75(1):79–86
117. Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr 1999;69(6):1071–1085
118. Albanes D, Heinonen OP, Taylor PR, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 1996;88(21):1560–1570
119. van den Berg H. Carotenoid interactions. Nutr Rev 1999;57(1):1–10
120. Micozzi MS, Brown ED, Edwards BK, et al. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr 1992;55(6):1120–1125
121. Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr 1995;62(3):604–610
122. Albanes D, Virtamo J, Taylor PR, Rautalahti M, Pietinen P, Heinonen OP. Effects of supplemental beta-carotene, cigarette smoking, and alcohol consumption on serum carotenoids in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr 1997;66(2):366–372
123. Nierenberg DW, Dain BJ, Mott LA, Baron JA, Greenberg ER. Effects of 4 y of oral supplementation with beta-carotene on serum concentrations of retinol, tocopherol, and five carotenoids. Am J Clin Nutr 1997;66(2):315–319
124. Wahlqvist ML, Wattanapenpaiboon N, Macrae FA, Lambert JR, MacLennan R, Hsu-Hage BH; Australian Polyp Prevention Project Investigators. Changes in serum carotenoids in subjects with colorectal adenomas after 24 mo of beta-carotene supplementation. Am J Clin Nutr 1994;60(6):936–943
125. Mayne ST, Cartmel B, Silva F, et al. Effect of supplemental beta-carotene on plasma concentrations of carotenoids, retinol, and alpha-tocopherol in humans. Am J Clin Nutr 1998;68(3):642–647