

HORMONES. Whenever I mention the word hormones, women have almost instantaneously an intense reaction, either very positive or very negative, not much in-between:
“I don’t want strange chemicals in my body.”
“Being on estrogen has given me my life back.”
“All doctors ever do is prescribe hormone pills.”
“Why didn’t anyone tell me about natural hormones before . . . I feel so much better now!”
“Those are drugs. I don’t take drugs; I want to take natural herbs.”
“I know it’s my hormones out of whack that’s making me feel so bad, but my doctors won’t listen. Why won’t anybody give me hormones?”
“I felt like being on hormones has lifted the fogginess off my brain and I have more energy now.”
This polarization of opinions crops up everywhere these days. Betty Friedan, in a speech at Omega Institute in 1994, called hormones, specifically estrogen therapy, a hoax perpetrated on women by the medical industry. The same year, Dr. Trudy Bush of Johns Hopkins said in several publications that the majority of women after menopause would benefit from estrogen therapy. Both speakers are women, yet 180 degrees apart in their views.
Why such emotional intensity and polarization over hormones? Why is this? What are these things called hormones? What do they do? Why are they important? How do we tell when a woman (or a man, for that matter) needs hormones? This chapter will help clarify these questions. In later chapters, we will look more closely at factors I think contribute to the polarity in views and the intense emotionalism on these issues. I will also provide you with the latest research and clinical information that helps to show often unrecognized hormonal connections affecting many conditions that occur more commonly in women.
A hormone is “a product of a living cell that circulates in body fluids or sap (in plants) and produces a specific effect on the activity of cells remote from its point of origin; or a synthetic substance that acts like a hormone” (Webster’s Collegiate Dictionary 10th edition). I think of hormones as “chemical communicators” or “connectors” that carry messages to and from all organs of the body and serve to connect one organ’s function with another organ’s function to keep the body balanced and functioning optimally. Without our hormones to keep the connections and messages flowing smoothly and our organs functioning in a balanced and integrated manner, we would die. It’s that simple. The secretion and interaction of hormones throughout our bodies every day is a highly complex and ongoing process.
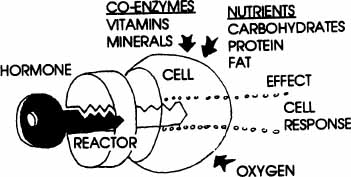
For many years, as I have explained these complex processes to patients and students, I have used the visual analogy of a “key in a lock” to describe the way brain chemicals fit at special receptor sites in the brain and body and the way hormones bind at their specific receptor sites to trigger their action on the target cells. Each hormone, neurotransmitter, neuropeptide, or other chemical messenger is a unique molecular “key,” and each has a “lock” (receptor site) into which it fits (see diagram). Similar keys may fit in a lock, but not be able to turn it. This is a simplistic model, and the hormone-receptor interaction is really more complex than this, but it helps visualize the basic principle to think of it this way.
The receptor sites may be located on cell surfaces (membrane receptors) or inside cells (cytoplasm or nucleus receptors). Each hormone provides a specific set of directions or messages to the target cells to enable the cells to perform certain functions. The cells of the body are like manufacturing plants, making the many chemicals the body needs to function. Hormones have a variety of functions, some similar to those of assembly-line supervisors directing the manufacturing processes, and some similar to those of team facilitators acting as catalysts for chemical processes to occur in the cell. Hormone actions may be extraordinarily rapid if they trigger immediate chemical release via effects on membrane receptors. Hormones act more slowly when they act at the cell nucleus receptors to influence the DNA and direct the manufacture of specific enzymes and proteins. The shape and molecular structure of each hormone fits exactly into the proper receptor site; changes in the molecule shape or makeup produce different notches on the molecule “key.” This means that a different shape may not work, or work as well, at that receptor. This effect of changes in the molecule is evident from the way in which the various types of estrogen molecules produce different results at the estrogen receptors throughout the brain and body. We also see this difference in response with many other hormones and their synthetic “look-alikes.” If the hormone molecules don’t bind just the right way and “click into the lock”, it’s like a key getting stuck in a lock. The door doesn’t open easily (or sometimes at all); the cell doesn’t function properly, and you may experience the body effects of missing that particular hormone.
As a specific example of the importance of molecule shape and makeup, consider the primary human estrogen, 17-beta estradiol, and the primary horse estrogens, called equilins, components of Premarin. All of these types of estrogen have four connecting rings as the basic shape of the molecule. But the equilins have some important differences in the makeup of the rings, which makes them slightly different keys. Consequently, these estrogens don’t fit exactly the same way at the estrogen receptor. The equilin estrogens sometimes keep the human estradiol key from fitting into the lock (receptor), which means the effects can be different from those of the natural human 17-beta estradiol. Horse-derived equilin estrogens attach more strongly, and longer, to our bodies’ estrogen receptors than does our own human estrogen. The process for the body to metabolize equilins also takes much longer because the equilin metabolites are not normal ones for the human body to process, and we don’t have the enzymes needed to break them down. All this because of a few changes in one ring! The body is quite amazing in its ability to recognize its exact molecular keys.
The human female produces three types of estrogen that I will describe more in later chapters so I just want to name them here: estrone (E1) made in the ovary, liver, and fat tissue; 17-beta estradiol (E2) the premenopausal estrogen made in the ovary; and the weaker estriol (E3) produced by the placenta during pregnancy. Premarin contains small amounts of two of these human estrogens (estradiol and estrone), but much higher amounts of the equilin estrogens unique to the horse. All that is needed to activate the human body’s estrogen receptors is the 17-beta estradiol, but the remaining equilin hormones still have to be processed by the liver for excretion in the urine. Equilin isn’t necessarily “bad,” especially for the horse, but it does place an unusual extra demand on the human body that isn’t needed for our bodies to have estrogen’s beneficial effects. For many women this appears to be one of the reasons that they still have symptoms of estrogen loss, even when taking the accepted dose of conjugated equine estrogen (Premarin). The horse estrogen keys are not opening the receptor locks quite as well, or perhaps the horse’s mixed estrogens aren’t providing quite enough of the human form (17-beta estradiol) that woman may need. This may be why the dose is often increased, but that then means even more equilins for the body to have to process, and this contributes to the bloating and breast enlargement women describe. I will talk more about the many effects of some of these differences in the chapters ahead, but I wanted you to begin to realize the crucial importance of the fit of hormone molecules into their receptor sites throughout the body.
Diagram 2.1—Hormones and Receptors:
A Look at How They Work
Each hormone has its own receptor site “lock” at target cells throughout the body. The body’s own hormone molecular “key” fits into its receptor to trigger hormone effects. Molecules with different shapes may also occupy the “lock” for that hormone, but may or may not trigger the hormonal action in the way your body’s molecules do. These different-shaped molecules (examples: equilin, genistein, SERMS) may actually block the body’s own hormones from working properly.
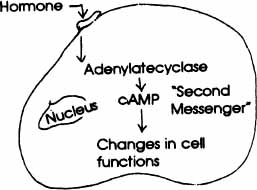
MEMBRANE RECEPTOR MODE OF ACTION The hormone “key” fits into the receptor “lock” located on the membrane surface of the cell. This binding triggers adenylate cyclase, the enzyme in the cell, to increase production of cyclic AMP. cAMP then acts as a “second messenger” to link the hormone at the cell surface with the inside of the cell where the metabolic actions (triggered by thehormone) take place. cAMP is one of a number ofsuch “second messengers” in the body.
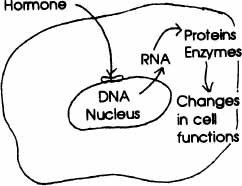
NUCLEAR RECEPTOR MODE OF ACTION This hormone “key” is small and soluble enough (such asestradiol) to pass through the cell membrane and fit into the receptor “lock” in the nucleus of the cell. It triggers its effects on cell metabolism by interacting with DNA in the nucleus. DNA then directs the making of RNA; RNA moves from the nucleus into the cytoplasm and directs the cell’s “metabolic machinery” to make proteins and enzymes for the cell’s many functions throughout the body.
© Elizabeth Lee Vliet, M.D., 1995, revised 2000
Hormones are very potent molecules, and it usually takes miniscule amounts, sometimes as little as a billionth of a gram (nanogram), to exert their effects on cells of the target organs. Estradiol, for example, is measured in picograms, one trillionth of a gram. After initiating actions at the target organs, hormones are broken down and “inactivated” by the target cells themselves or carried by the blood to the liver to be metabolized. The metabolized hormones are used again by the body’s synthesis processes to make new hormone molecules, or the metabolic breakdown products are excreted in the urine (tests can measure hormone breakdown substances in urine or feces).
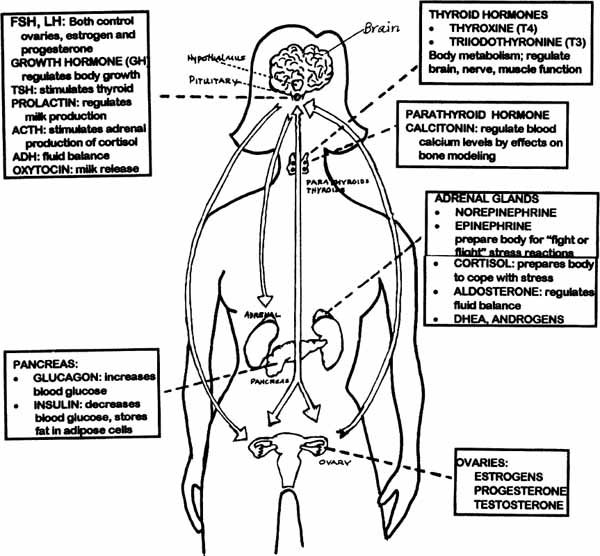
Some of the major hormones, where they are produced, and what they do, are described in the diagrams and charts below. As you read them, think about the brain as the orchestra conductor who provides direction to the various “instruments” (hormones) to produce the “symphony” of our body rhythms and functions. Hormones affect the brain, and the brain affects hormone production and interactions. The brain and the body really are intimately connected.
Diagram 2.2—The Endocrine System: A Look at Key Hormones And What They Do
© Elizabeth Lee Vliet, M.D., 1995, revised 2000
CLASSES OF HORMONES AND WHAT THEY DO
Primary Actions
1. STEROIDS
Cortisol |
many metabolic actions, anti-inflammatory actions, produced in higher amounts during stress and may suppress normal immune function |
Aldosterone |
regulates fluid balance by stimulating kidney to retain sodium and water, excretes potassium |
Androgens (DHEA, others) |
enhance sex drive, produce mild male features in women (e.g., facial hair, male body shape) |
Estrogens (three) |
produces female secondary sex characteristics; key role: menstruation, pregnancy; 400 other functions |
Progesterone |
helps maintain pregnancy, many metabolic effects, high levels give sedative, analgesic effects at brain may produce depressed mood |
Testosterone |
produces male secondary sex patterns; triggers sex drive and arousal in both males and females, many metabolic effects (bone and muscle growth, etc.) |
II. AMINES
Thyroid hormones: |
stimulate body metabolism by increasing cell energy release; increasing heart rate, heat production, and brain activity. Helps normal regulation and growth of nervous, and musculoskeletal systems |
“Adrenaline” hormones: |
“fight or flight” (stress) hormones, prepare body by increasing heart rate; act on brain to lift mood (or in excess, cause anxiety), increase alertness; dilate arteries to key organs to provide more oxygen, glucose, and nutrients |
Insulin |
lowers blood sugar (moves glucose into cells), stimulates fat storage and protein synthesis |
Glucagon |
raises blood glucose (glycogen breakdown and glucose release from liver, gluconeogenesis) |
Somatostatin |
mild effect to raise blood glucose |
Parathyroid (PTH) |
major role: increase blood calcium levels by stimulating bone breakdown, calcium release |
Calcitonin |
involved in regulating blood calcium levels by inhibiting bone breakdown, calcium release |
Thymosin (thymus gland) |
major role in development of immune system |
ACTH |
adrenocorticotropin hormone; stimulates part of the adrenal gland to make Cortisol |
FSH |
stimulates ovaries, activates and promotes follicle growth to produce estrogen |
LH |
triggers ovulation, formation of the corpus luteum, secretion of progesterone, estrogen |
Growth Hormone (GH) |
oversees entire process of normal body growth |
TSH |
stimulates the thyroid gland to release T3, T4 |
Prolactin |
stimulates breast enlargement during pregnancy and milk production after delivery |
Anti-Diuretic Hormone (ADH) |
prevents dehydration by stimulating kidneys to increase reabsorption and retain water |
Oxytocin |
stimulates uterine contractions during labor, helps trigger milk release after delivery |
Melatonin (pineal gland) |
regulation of sleep cycles, body rhythms |
© Elizabeth Lee Vliet, M.D., 1995, revised 2000
The brain and body are interconnected by an incredible array of chemical and electrical circuits, each one interacting with and affecting others. The brain has a multitude of ways to direct the orchestra of the body to respond to what the brain perceives, from inside the body physically, from inside the mind’s thoughts and feelings, or from outside the body. In women’s bodies, the entire process is even more complex, with the menstrual cycle rhythm of changes causing the brain-body systems to continuously adapt to the changing hormonal environment. Unlike the male body, which maintains a fairly steady production (tonic pattern) of testosterone all month, the female body has a cyclic pattern of ovarian hormone rise and fall.
The major underlying influence of these crucial female hormonal rhythms has never been fully appreciated for the diverse effects on all parts of the female body, not just reproduction. The diagram that follows shows some of the ways that these stimuli (stressors) of all kinds require the body processes to change and adapt. Hormonal change is another one of those stimuli that trigger the body systems to constantly change and adapt. When the body systems are overstressed, a variety of things may occur: we call these changes symptoms if they feel unpleasant to us, or we call them phenomena if they are just normal and don’t bother us. These changes themselves may then become additional “stressors” on the body and contribute to more overload and possible illness. The interconnections and the ways in which hormonal production may in turn be altered by stress on the body are often overlooked when women seek medical care. Keep in mind that Mother Nature’s protective plan is to prevent pregnancy when animals or humans are stressed or sick. It shouldn’t be a surprise to learn that prolonged stress can suppress the ovaries and cause decreases in hormone production. The two-way nature of these pathways is a critical connection throughout all facets of women’s health that is so often overlooked.
Most women learn about menstruation initially from mothers or girlfriends. The first scientific explanation we receive about what happens is often in health class, about the sixth or seventh grade. We then live it each month and don’t really think about which hormone is doing what at any given time. In case you’ve forgotten your health education material, I’d like to give you a quick rundown on what happens in the normal menstrual cycle. My description is based on the average cycle length of twenty-eight days, but keep in mind that cycle variations in length from twenty-five to thirty-five days may be perfectly normal.
We arbitrarily mark the cycle by labeling the first day of bleeding Day 1 (M). We could define any day as Day 1, but it helps to have such a clear-cut marker as bleeding to use as a starting point. Bleeding is really the finishing of the previous cycle with the shedding of the lining of the uterus (womb) that is not needed if there is no fertilized egg to nourish. As the uterine lining is being shed, the body is preparing for the next cycle. The follicle (“little sac”) in the ovary that will become the egg is already being “recruited” by the hormonal changes directed by the brain. Progesterone and estrogen both dropped sharply in the waning days of the previous cycle, and these two hormones are now at their lowest point of the cycle as bleeding begins (Day 1 of next cycle).
From Day 1 onward until ovulation, estradiol (E2), and to a lesser extent estrone (El), levels are rising as the growth of several follicles is being stimulated by FSH (follicle stimulating hormone). Once estrogen reaches a certain level in the bloodstream, all but one of the follicles shrink and die. That dominant follicle remains as the one destined to be the egg released at ovulation. For the entire first half of the cycle, progesterone levels remain extremely low, usually less than 1 nanogram per milliliter (ng/ml). The first half of the cycle is dominated by estrogen and is called either the “Follicular phase” (refers to the egg development in the ovary) or “Proliferative phase” (refers to the process of growth of the endometrial lining of the uterus). Estradiol levels average about 200 pg/ml (picograms per milliliter) across the first two weeks of the cycle, with the low point being Day 1 and the high point being OVULATION, usually about Day 14. Peak estradiol levels at the ovulatory phase are in the range of 350 to 500 pg/ml. The first few days of the cycle, (Days 1–3) estradiol levels are optimally about 80–90 pg/ml, until the decline in estrogen with premenopausal years when estradiol typically drops to less than about 40 pg/ml during Days 1–3 of the cycle.
Thus, keep in mind that in the first two weeks each cycle until ovulation occurs, estrogen levels are high, and progesterone levels are low (in fact, there’s very little progesterone at all present for these first two weeks of each month). Days 1–14 are typically the phase of the cycle during which women experience a sense of well-being, optimal energy level, normal sleep, and an “up” mood; that is, they feel good. Estrogen has an activating effect on brain centers and contributes to enhanced energy and mood as well as clarity of thinking, sharper memory, and ability to concentrate. In chapter 3, I will show you some of the many ways estrogen acts on the brain that are similar to the actions of present antidepressant medications.
FSH is the brain hormone that stimulates the growth of the follicle that contains the egg. FSH begins to rise in the cycle when the brain senses the drop in estrogen at menses, and then FSH stimulates the ovary to produce the next follicle. FSH and LH (luteinizing hormone) are the two primary brain hormones that govern the egg maturation and release for the menstrual cycle. I won’t focus as much on FSH and LH because I want you to have more of an overview about the estrogen and progesterone patterns and effects in the normal cycle. In the perimenopausal years, as estradiol production from the ovary declines, the brain produces more FSH and LH, trying to stimulate the ovary to produce more hormones. So you will see high levels of FSH and LH prior to and after menopause because estradiol levels are then low (usually less than 30 pg/ml).
In the first ten days of each new cycle, rising estradiol levels stimulate the release of LH from the brain. When estradiol reaches its peak level (about 400–500 pg/ml) at mid-cycle, LH triggers the follicle to be released as an egg, called ovulation. Some women can feel ovulation as a brief, sharp pain in the area of the ovary (called “mittelschmerz”); other women do not feel any physical sensations at ovulation. The rapid drop in estradiol as the egg is released may trigger onset of a migraine in some women. It may also contribute to brief disruption in sleep; and for some women, it may trigger brief mood swings as the brain chemicals are changing in response to the drop in circulating estrogen. I will talk more about these important connections in upcoming chapters.
The egg develops into the corpus luteum (from Latin for “yellow body,” due to its yellowish appearance). This half of the cycle is called the luteal phase (if referring to the ovary changes) or secretory phase (if referring to the further thickening of the uterine lining to prepare for fertilization and implantation of the egg). This phase of the cycle is dominated by the hormone progesterone, produced by the corpus luteum. Progesterone is the “pro-gestation” hormone and its primary role is to prepare a woman’s body to sustain and nourish a pregnancy. It is the dominant hormone of the second half of the cycle, with blood levels higher than the level of estrogen. Circulating levels of progesterone reach a peak between 10–25 nanograms per milliliter by about Days 20 to 22, the midpoint of the second half of the cycle (or about the third week from last Day 1 of bleeding). If the egg is fertilized, progesterone levels stay high until the placenta takes over producing the progesterone for pregnancy. If there is no fertilization of the egg, the corpus luteum begins to disintegrate and no longer makes progesterone so the progesterone level drops sharply, triggering the onset of bleeding. Estrogen also drops at this time of the cycle, and the falling estradiol level can be a trigger for migraines, restless sleep, anxiety symptoms, palpitations and pain flares. It is the decrease in progesterone that causes bleeding to occur. Shedding the uterine lining is part of the process of preparing the body to begin a new cycle. Falling progesterone levels may further add to insomnia and anxiety triggered by the falling estradiol.
Estrogen (estradiol) levels also rise again from ovulation until about Day 20, reaching the peak for the second half of the cycle at the same time progesterone is peaking. The optimal level of estradiol in the luteal phase of a healthy cycle is somewhat less than in the follicular phase of the cycle. Average peak luteal phase estradiol levels are about 200–300 pg/ml. I find that women we see have worsening premenstrual symptoms when luteal phase estradiol levels fall below about 160–170 pg/ml.
Since progesterone prepares the body for pregnancy, it makes sense that some of the changes you notice—water retention, increased appetite, breast enlargement—are triggered by the metabolic effects of progesterone, since these are necessary changes to help the body provide for a growing fetus. Hormones, such as insulin, that control blood glucose levels are affected by progesterone, and this contributes to some of the “sweet cravings” women describe in the premenstrual week. Tufts University researchers show a 12 percent increase in appetite and metabolic rate under the influence of progesterone. You’re not imagining it, you do notice feelings of hunger more in this half of the cycle for very real, physiological reasons based on the higher levels of progesterone at this phase of the cycle. Progesterone makes you want to eat more so your body could sustain a pregnancy.
Another effect of progesterone is to slow down the movement of food through the gastrointestinal (GI) tract by decreasing the muscular waves (peristalsis) that move material through the entire digestive system. As a result, you hold more contents in the intestine when progesterone is high, so this is one reason you feel full or bloated at this phase of the cycle. Our low-fiber diet is another lifestyle factor that intensifies the natural constipating effects of progesterone. In Stone Age cultures, humans may have eaten 100 grams of fiber a day or more. Thus there was an evolutionary advantage for survival of the species to have progesterone effects in women slow down the gastrointestinal tract, enabling more nutrients to be absorbed from the food into the blood stream. With a high-fiber diet (which causes “rapid transit” through the GI tract) and in times when food was scarce, the hormonal effect of progesterone further helped a woman’s body retain nutrients to sustain a pregnancy. In the typical American high-fat diet, women may get only 10 grams of fiber a day. Slowing down the gastrointestinal tract when you’re eating a low-fiber diet has some very unpleasant side effects, among them constipation. Women in the progesterone-dominant luteal phase (or when taking progesterone or progestins for HRT) frequently describe feeling constipated, bloated, headachy, irritable, and lethargic from fluid retention.
Progesterone and its metabolites have a tranquilizing effect on the brain, much like our current antianxiety medications. For some women, progesterone has such a calming effect that it acts more like a sedative. For some women, the “slowing down” effect of progesterone feels “wonderful,” “soothing,” “relaxed,” “more centered.” For other women these progesterone effects feel like “depression,” fatigue, tiredness, or lethargy. These women say, “I don’t have any get up and go.” “I withdraw.” “I can’t get out of bed.” “I don’t have any energy.” You can see how a hormone having a calming, tranquilizing effect is an evolutionary advantage in the early stages of pregnancy: being calmer or “slowed down” would facilitate diminished activity at a time when the egg is being implanted, thereby helping prevent expulsion from the uterus. This facilitates early development of the embryo. In pregnancy, progesterone levels decrease sharply at the sixth to eighth week and do not rise again until about the thirty-fourth week of gestation to prepare the body for delivery. If you have been pregnant, think about how you felt the last six weeks of pregnancy (when progesterone rises very high) compared with the middle months of pregnancy, when estrogen levels are quite high. You can then see some of the physical and psychological differences between estrogen and progesterone.
The hormonal ebb and flow, which occurs in this cyclical manner, has widespread effects on the brain-body processes, as well as on our psyche. When you understand the specific effects and roles of each of the primary female hormones, you can see how beautifully orchestrated the female endocrine system is for its role in bringing new life into being and keeping the species alive. These hormonal actions make sense from an evolutionary standpoint for the tasks they govern in the body. The problem today is that in our culture, food is no longer scarce; for most people, it is overly available. We don’t eat as much fiber in fruits, whole grains, and vegetables; we have too much sugar, caffeine, and alcohol easily at hand. We’re not getting pregnant as often, and we are trying to do multiple tasks, with a marked increase in overall stress levels. Consequently, some of the natural hormonal metabolic effects are now experienced as negative, unwanted physical-psychological “symptoms” because of our unnatural lifestyles and diet. I think this is one of a number of reasons that we see so many more women experiencing PMS in today’s culture compared to women at the turn of the century when diets had lower overall fat, sugar, salt, alcohol, and caffeine intake and a far higher intake of fruits, whole grains, and vegetables.
All these underlying physiological changes then have a bearing on how we respond to the external world and the impact of external stresses on our brain-body pathways. Please don’t think that I’m saying we are at the mercy of our hormones. If we understand what is happening, we can learn to “go with the flow” in positive ways, rather than making things worse with poor lifestyle choices. What I am concerned about is that we need to better understand the differences and the physiological changes women experience. We desperately need more gender-specific research on these issues so that we can learn to constructively manage our very complicated lives when living in a body that is much more complex than the male body. I don’t think that the hormonal cycles mean that we should do anything less than whatever it is we would like to do with our lives. However, in order to really understand what is happening, we need to know about the hormonal cycles, and we need better medical and health research that will help our therapeutic approaches take into account the unique and natural needs of the female body.
It is important to understand how these hormone messengers are carried in the bloodstream throughout the body. Sex steroid hormones are carried in the bloodstream three ways: bound to two different carrier proteins (sex-hormone–binding globulin (SHBG) and albumin) and in the free form, not bound to carrier protein. Thyroid hormones are carried by thyroid-binding globulin (TBG). Cortisol is carried by corticosteroid binding globulin (CBG). The free amount of all of these hormones is a much smaller percentage than the total circulating hormone.
It was previously thought that only the free form was biologically active, but newer research has shown that both the free portion and the portions weakly bound to albumin are biologically active, at least for the sex steroid hormones. It is less clear whether this is also true for thyroid hormones and cortisol. Serum (blood) assays for ovarian hormones, thyroid, and cortisol include all of these circulating forms: total, weakly bound, and free. The serum assay provides a complete picture of the steroid hormones available to act at tissues of the body. When needed, the “free” fraction of a given hormone can also be measured in the serum.
There has been a great deal of current consumer marketing effort aimed at use of saliva measures of ovarian and other hormones. The makers of the saliva test kits claim that a measurement of the free hormone in saliva gives a more reliable result than the combined fractions measured in serum. These claims have not been borne out in menopause research settings showing saliva hormone testing is a reliable tool for use in assessing adequacy of clinical response and therapeutic effect. In fact, as further research has clarified the complexity of estrogen action at receptor sites and the ability of some tissues to actively utilize both protein bound and free forms of these hormones, it appears to be even more important to use the serum assays that reflect bound and free fractions. Saliva hormone tests only show the small part in the free fraction, and the amount of the free fraction that is excreted into the saliva from serum.
Urine hormone assays are measuring only the metabolic breakdown products of the various forms of the hormones, not the active forms. The urinary hormone tests are the most indirect (and therefore less useful) measures of circulating levels of the active forms of the ovarian hormones. Compared to the serum assays, the saliva and urinary hormone tests are much less clinically useful in making treatment decisions regarding ovarian hormone therapy because we really need to know more than just the free fraction in order to have a complete picture of what is happening in your body. Serum measures remain the worldwide gold standard in research settings addressing crucial issues in women’s health and certainly are the measure that should be used in making assessments of hormone effects on complex problems.
Many factors affect the amount of hormone that is “free” at any given time. I have shown some of these in the chart that follows. Other factors include medications that are highly protein bound. Such medications compete for the carrier proteins and displace more of a given hormone into the free form, possibly producing symptoms of hormone excess. Or the converse may happen. Some medications will push more of the hormone into the bound and inactive form, leaving less in the free, active fraction. A good example of this problem is the effect of birth control pills on thyroid hormones. BCPs cause more of the thyroid hormones to bind to TBG, moving out of the free and active fraction. This is one way that women on BCPs sometimes develop symptoms of hypothyroidism even thought their TSH and total hormones appear normal on the blood tests.
At birth, a newborn girl’s ovaries contain about 500,000 follicles, and these are the source of her future eggs. By puberty, the number of follicles has decreased to 300,000, and by age thirty-eight to forty, the average woman has only 5,000 to 10,000 follicles remaining. Several follicles are “recruited” each cycle, but only one egg will be released at each ovulation. The follicles and maturing eggs produce estradiol; when the ovaries run out of follicles (and therefore out of eggs), they also run out of the supply of estradiol, our body’s most active form of estrogen. The brain senses the decrease in estradiol, and produces more FSH and LH to try to stimulate the ovaries to produce more hormones. This effort is to no avail because there are no more follicles to develop into hormone-producing eggs. Fundamentally, when our follicle supply is depleted, this is the end of a woman’s reproductive stage, and we no longer menstruate. The end of menses is menopause. The journey from puberty to menopause has many developmental stages along the way. Here are some of the highlights of those stages as they relate to hormone cycles.
Girls in the United States typically enter puberty anytime between about nine and fourteen years of age. Breast development begins to appear first, followed by the appearance of pubic hair. Menstruation usually doesn’t occur until the breasts are well developed and a critical body weight of about 110 pounds is reached. This generally follows soon after the girl’s adolescent growth spurt, at about age eleven or twelve. The average age at the onset of menstruation is about eleven and a half to thirteen, although it may take several years for the menstrual cycle to stabilize into a regular pattern. During these years, a young woman’s ovaries are beginning to develop their individual cycle rhythm. It can be a time of erratic periods, feelings of uncertainty about what’s happening to the body, mood shifts, new physical sensations, and unpredictable menstrual flows. Some young women experience only mild cramps, or no cramps at all, with bleeding; while others have severe and sustained cramping. Some can tell physically when ovulation occurs; others have no awareness of it. Fertility (the ability to conceive and bear children) occurs when ovulation begins. The problem for many adolescent girls is that since ovulation may be erratic in the first year or two of menstruation, it may be difficult to know when they are fertile. This is one reason girls in this age group are surprised when they accidentally become pregnant. It is important for girls who are sexually active at this time to use some form of contraception.
Throughout history many cultures have had ceremonies (rites of passage) for young girls to mark this transition into womanhood. In our culture, this has not been a usual practice, and many young girls are embarrassed by the onset of menstruation. In our culture there is a lot of negative language about menstruation: “on the rag,” “the curse.” Such demeaning terms don’t help young girls feel proud about becoming a woman. Having meaningful ceremonies marking this as a special dimension of being a woman could serve to provide positive images and role models for girls today as they become young women.
During her twenties and early thirties, a woman’s menstrual cycle tends to be fairly regular, unless altered by lifestyle (e.g., extreme dieting, cigarette smoking, strenuous athletic activity, alcohol or drug abuse) or the presence of endocrine or other medical disorders. Polycystic ovary syndrome (PCOS) is an example of an endocrine metabolic disorder that disrupts normal ovary cycles in this age group and often causes infertility. Fertility usually peaks by age twenty-five, and the body is at its physiological optimal point for childbearing during the early and mid-twenties. That does not mean that women cannot become pregnant at a later age; it simply means that in the decade of her twenties, a woman’s circulatory, respiratory, and digestive systems are at their peak to meet the physiological demands of pregnancy with the least stress on the mother’s system.
Hormone production during the twenties and early thirties is more predictable than during either puberty or perimenopause and typically follows the pattern I outlined: estrogen levels high in the first half, and progesterone dominating the second half of each cycle, and testosterone fairly constant throughout the cycle. By the mid-thirties, the menstrual flow may begin changing, at first imperceptibly, and by the late thirties more noticeably, as flow may become lighter and shorter, and cycle length may become either longer or shorter. By the later thirties, women often begin to notice more pronounced premenstrual mood, energy, and appetite changes, which are described as the premenstrual syndrome (PMS) for short. For some women, there is no PMS at all; others experience a minor degree of discomfort; still others have more bothersome symptoms lasting a week or more; and for a small percentage of women, PMS may be severely disruptive of work, family, and social relationships. I describe these changes and some of their causes in chapter 7. As estradiol begins to decline, this is also a time we may see worsening migraines, fibromyalgia, bladder problems, vulvodynia, and other annoying changes.
Between the ages of approximately thirty-five and fifty women enter the phase called the “climacteric” or premenopausal years when the ovaries’ production of estradiol and progesterone begin declining as the woman’s follicles are diminishing. This is a transition phase from our optimal reproductive hormone levels that make us fertile to the lower levels that still produce menstrual periods but don’t necessarily maintain “optimal” metabolic function throughout the body. This is the time that so many women begin having “vague” symptoms as I have been discussing throughout this book. Depending on your lifestyle habits and your genetic makeup, the climacteric may start in the early thirties or may not begin until the mid- or late forties.
During the climacteric or premenopausal years, hormonal levels begin to decline, the amount of estrone rises relative to decreasing estradiol, cycles become more erratic, there are more cycles when you don’t ovulate, and there is more fluctuation in the actual amounts of estrogen and progesterone produced. In cycles when you do ovulate, you will have higher levels of progesterone, and those cycles are usually ones when you have much more PMS: feeling bloated, headachy, irritable, tearful, and just generally awful. In cycles when you do not ovulate, there isn’t much progesterone, so you sail through and don’t notice these PMS-type changes.
As estrogen declines, there are many body functions that are affected: sleep, memory, mood, energy level, immune system, body fat distribution, circulatory system, digestive tract, bone metabolism, skin changes (such as increased acne), sexual function, bladder function, and many others that I will be addressing in more detail throughout this book. These changes are gradual and take place over several years; they do not all occur at a single point in time and suddenly you wake up and say “that’s it, it’s menopause.” The final menstrual period happens at a definite (albeit unpredictable) point, but the hormone changes have been leading up to it for a decade or more. These changes in the balance of estradiol, testosterone, DHEA, and progesterone cause a wide variety of physical and psychological experiences. For example, intermittent palpitations around menses and premenstrual migraines may begin to occur for the first time or may suddenly get worse if you have had problems with either one in earlier years.
Another frequent early indicator of declining estradiol, characteristically occurring in the early to mid-forties is changes in sleep patterns. Are you waking up multiple times during the night? I found that for a while I was waking up, looking at the clock, then going back to sleep; an hour later I’d wake up, look at the clock, and go back to sleep. Disrupted sleep is one of the earliest hallmark changes affecting the brain as estradiol levels decline. Mood swings, episodic tearfulness for no reason, irritability, angry outbursts, and brief spells of feeling depressed prior to your period may also occur as a result of estradiol drops that trigger changes in the mood-regulating chemical messengers in the brain.
Another effect of decreased estradiol is the rise in total cholesterol production, decreased HDL, and increased LDL cholesterol that can lead to heart disease. Decreased estradiol also causes loss of bone that can result in osteoporosis. I’ve had a lot of women in their late thirties and forties who were told categorically, without any further evaluation, that they were too young to be having any menopausal symptoms, yet their estradiol levels were already far too low. This is the time that we need to be checking these various health measures so that we can take preventive steps for the future. If you are having premature ovarian decline at age forty, thirty-nine, or even younger, you need to know that, so you can take the necessary steps that will help reduce your risk of heart disease and bone loss in the next five to ten years . . . as well as reduce or eliminate the annoying symptoms that negatively affect your quality of life at this time.
The onset of menopause is defined as one year from the “last menstrual period.” Ovarian function has been declining for several years, and now the levels are so low that menstrual periods cease. The decline in ovarian hormones is sufficient to end the cycling pattern of our reproductive years and produce the steady, low levels of a noncycling hormone pattern characteristic of the postreproductive years.
Menopause is a rather unique situation because it’s the only thing I know of in the medical world that is officially recognized twelve months after it has happened. It is a little hard to plan for it or to take proactive steps to decrease health risks if you don’t define it until twelve months after it has happened. Most of the time women won’t even know when their last menstrual period will be until months have gone by.
At menopause, the ovaries do not cycle any longer, and the overall hormonal production has decreased considerably. After menopause women lose almost all of their estradiol production, and no longer make progesterone. We also lose anywhere from 50–60 percent of our testosterone production. The female ovary produces testosterone and DHEA, so as estradiol is lost after menopause, we begin seeing the “unmasking” of the testosterone that is present. That’s when you notice some changes in skin and facial hair, along with changes in body fat. The “pear” shape female (gynecoid) fat pattern around the hips and buttocks suddenly begins moving up toward the middle of the body to become the “apple” shape (android) more typical of men. Then you wonder, “Gee, what happened to my waistline?” I show this change in the following diagram. These are some of the testosterone effects that begin the body changes that are characteristic of the male body pattern. During these years, the enzyme lipoprotein lipase starts to decrease in the lower body fat tissue and increase in the upper body fat, which further adds to the pattern of increasing body fat around the waist, chest and shoulders, hips and thighs. We see a rise in blood pressure and cholesterol in women that is similar to the pattern in men as the testosterone effects become more pronounced relative to estradiol declines.
As women lose the biologically active estradiol, we begin to see one of the gender differences become a gender similarity: the incidence of cardiovascular disease (CVD) increases dramatically for women. Regardless of age at menopause (whether it’s a natural menopause at age forty-five or a surgical menopause at age forty), the rate of CVD increases. It is based on menopausal hormone levels, not just your chronological age. It is important to know a woman’s endocrine status in order to more completely assess her risk of CVD. You cannot go by age alone, since women experience declining estradiol levels at a variety of numerical ages. This loss of estradiol sends your cholesterol profile into a really negative spin.
Let me share with you a different perspective on menopause and the years that follow. I think we’ve heard many conflicting points about menopause. In our culture it has often been treated with a sense of taboo as well as fear and apprehension. Part of that has to do with the fact that at the turn of the century the average age of death for women in this country was forty-eight; the average age of menopause was fifty-one. Which means that as recently as 1900, most women did not live very long after menopause and ovarian decline. Five hundred years ago women died on average by age thirty-five.
When we read about the wise women of Native American cultures, Asian cultures, and the Celtic and other traditions, the wise, elder women were typically those over thirty, past the age of childbearing in those times. Yet you and I are reading that same material in the context of an unparalleled increase in longevity in the human population. It has only been in the last two to three generations that we have had large numbers of men and women living long enough for us to see some of the brain-body effects of declining ovary hormones for women and declining testicular hormones for men. When I talk about menopause and women’s health needs, I am discussing it from the perspective that we now have an extraordinary length of time—perhaps thirty to fifty years—to live beyond menopause. For many of us, that means we have the potential to live at least half of it after the ovaries have stopped producing the normal quantity of biologically active estradiol and testosterone. Loss of these crucial hormones has an enormous impact on every dimension of our body’s function, literally from head (hair, itchy scalp, and brain function) to foot (joint pain, heel pain), and most everything in-between (skin, teeth, muscles, joints, bones, heart, intestinal tract, immune function, blood pressure, bladder function, and sex drive). You need to be empowered with up-to-date, accurate information to help guide you in your choices for optimal health as you grow older. We now have an incredible wealth of information from outstanding scientific research to show the far-reaching effects on a woman’s body after we lose our optimal estradiol—whether we are twenty or thrity or forty or fifty when that happens. Well-done studies from around the world show that giving estrogen after menopause decreases by 40 to 60 percent the risk of heart attacks, stoke, Alzheimer’s disease, osteoporosis, incontinence, and colon cancer. Loss of estrogen has the potential to rob us of energy, sex drive, sleep, muscle strength, balance, memory, concentration, and general zest, as I will explain further throughout this book.
I recently saw an eighty-nine-year-old lady who is very active, very sharp mentally, and who enjoys traveling all over the world. She was having trouble with incontinence and wanted to see if estrogen would help. I gave her a script for Estrace 0.5 mg. The pharmacist for her insurance company refused to fill the prescription, saying she was too old for estrogen! When she said she needed it to help incontinence so she could enjoy her activities and travel, he said the insurance company allowed only Detrol for this. Of course Detrol has unpleasant side effects and none of the other benefits of estradiol, but that didn’t seem to matter to the insurance company. I encouraged this woman to go ahead and pay the $25 for Estrace and see how she felt on it. Then she could appeal the insurance company decision later if she thought it had helped. Her daughter reported later that within twenty-four hours of starting Estrace twice a day, the urinary incontinence had significantly improved. After three or four months, the urinary incontinence was very insignificant; she no longer had to wear any kind of protective padding and was feeling great, had more energy, and was so busy traveling she forgot to come in for her follow-up appointment.
Diagram 2.5—WOMEN’S MIDLIFE BODY SHAPE CHANGES
FEMALE (GLYNECOID)
“PEAR” SHAPE
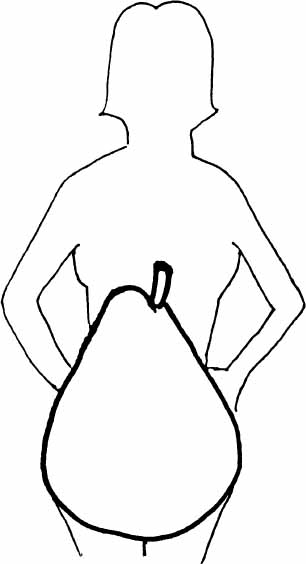
Characteristic of women prior to menopause, when estradiol (E2) greater than estrone (E1), and normal female level androgens.
MALE (ANDROID)
“APPLE” SHAPE
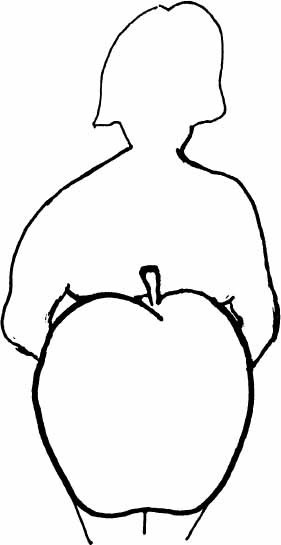
Characteristic of women after menopause (if not on hormone Rx); El greater than E2. Associated with higher risk of heart disease, hypertension, diabetes, insulin resistance. Also seen in women with PCOS due to higher androgens, lower E2, higher than normal El.
© Elizabeth Lee Vliet, M.D., 1995, revised 2000
My message to you is straightforward: Menopause is not a disease, it is a natural transition in our lives if we live long enough. We now have the opportunity to go through this transition and live an extended period beyond. That was not the case for our great grandmothers and their ancestors. Primates such as monkeys, chimpanzees, and gorillas die at the end of their reproductive years, and do not then show postmenopausal changes seen in human females. I think the most critical point for women to understand is that we need to learn what we must know about maintaining our long-term health and reducing our long-term individual disease risks so we can live these additional thirty or forty years with the best possible quality of life and good health. Don’t focus on just treating or “getting through” symptoms now. Look at your big picture and make decisions based on what is needed for your overall vitality and health for many years to come.
Thyroid disease has sometimes been called “the great imitator” because both hyperthyroid and hypothyroid diseases produce myriad symptoms that can be confused with many different illnesses. Hyperthyroid syndromes produce an excess of thyroid hormones, whether you have a disorder such as Graves’ Disease, or are simply taking too much thyroid medication. Hypothyroid disorders involve too little thyroid hormone production, or interference with normal hormone action, such as Hashimoto’s thyroiditis. Thyroid disorders of all types are far more common in women, as much as eight to twenty times the frequency found in men. The incidence of thyroid disorders increases with age in both sexes, but there is a more dramatic increase in women from the mid-forties and on into the seventies. There are a variety of laboratory tests that can identify these problems. What often happens for women is that only the total thyroid hormones themselves are checked without looking also at the brain hormone TSH (thyroid stimulating hormone) that is a much more sensitive indicator of excessive, or declining, thyroid function. Or, if TSH is checked, thyroid antibodies and free hormones are not, and this means early phase thyroiditis (with normal TSH) is missed.
One facet of thyroid disease not generally recognized is its tendency to cause disturbances in mood and in menstrual cycles. Menstrual irregularity, infertility, worsening PMS, atypical depression, new onset depression later in life, postpartum depression, anxiety syndromes, fibromyalgia, excessive fatigue—all of these may be caused or aggravated by thyroid disorders and are “red flags” to me that may indicate an underlying thyroid problem. Yet, all too commonly, women with these syndromes and symptoms are given a psychiatric label, referred to a therapist for “stress management,” and are not evaluated medically any further than a cursory exam and basic blood chemistries. Studies of patients admitted to psychiatric hospitals have repeatedly shown a high incidence of previously unrecognized thyroid disorders that were thought to be the causative factor in the mood disorder. Women at midlife in particular need careful evaluation of thyroid function as part of their medical checkups, and this needs to include more sensitive tests than just the standard profile.
A comment I hear frequently is “you couldn’t have thyroid problems because your TSH (thyroid stimulating hormone) is normal.” When the cluster of symptoms I described above are present in women, I think it is important to go a step further in evaluating the thyroid. I have a series of several hundred patients, all but two are women, who had a normal TSH and turned out to have significantly elevated thyroid antibodies, indicating an autoimmune thyroiditis disorder. This also meant they needed thyroid medication in order to feel normal. This type of oversight is particularly common with a type of thyroid disease called thyroiditis, which is about twenty-five times more common in females than males. There are several different types of thyroiditis, but in general this is an inflammation of the thyroid gland that can result in production of antibodies to the gland tissue (microsomal antibodies) or antibodies to the thyroid hormone itself (thyroglobulin antibodies). These antibodies act like “blocking agents” to keep the gland and its hormones from working properly, and the patient begins to experience the clinical symptoms of declining thyroid gland function. For some period of time (actual length may vary greatly from person to person), there is a gradual or abrupt elevation in the thyroid antibodies before there is a compensating rise in TSH produced by the brain in response to the failing gland. This means a woman may experience the symptoms of disease months to years before TSH goes up. The prevailing “dogma” among endocrinologists and thyroid specialists is that you don’t test for thyroid antibodies if the TSH is normal, even if the patient has classic symptoms of hypothyroidism. That’s where we often run into trouble and fail to diagnose thyroiditis. All too often, and particularly with women, the clinical problems are present and the antibodies are elevated even when TSH is normal. Adding low-dose thyroid medication can be dramatic for these women, and has even helped some of my infertile patients become pregnant!
I do not advocate using thyroid hormone if all of the laboratory studies are completely normal, including thyroid antibodies. There are potential serious adverse effects of taking thyroid when you don’t actually need it, and these problems are worse for women: heart rhythm disturbances and bone loss are the two most important. The problem I have found is that too often women are told their thyroid is normal without having the complete thyroid tests done. Of course, what most people, and many physicians, don’t realize is that (1) a “normal range” on a laboratory report is just that: a range. A given person may require higher or lower levels to feel well and to function optimally. I think we must look at the lab results along with the clinical picture described by the patient. After all, we are supposed to be treating people, not lab values. (2) It is also possible that one or more lab measures may still fall in the normal range and yet other, more subtle measures, may be abnormal. Hashimoto’s Thyroiditis may often occur with normal total T4 and T3 but markedly elevated antibodies and very low free T4 and/or free T3. We have to listen to the woman and her descriptions with an open mind and with trust in what she says and knows about her body. Several cases from my practice will illustrate this point very clearly.
Arla was thirty-two when I first saw her. Her last child (of two) had been born five years earlier. Over the past three years, she had been experiencing marked loss of energy, decreased concentration at work, premenstrual mood swings, and severe chocolate cravings. She described feeling “so tired I can hardly make it through the day, that just isn’t like me. My memory isn’t as sharp as usual, I get dark circles under my eyes all the time, I can’t lose weight no matter how much I exercise. I just feel so loggy all the time. I thought there might be a thyroid problem, but my family doctor said my thyroid hormones were normal. What’s wrong with me?”
Her routine lab studies were normal, and there was nothing particularly unusual on her physical exam except for the moderate degree of obesity. Her standard thyroid profile was normal for the T3, T4 hormones and her TSH was 2.21 (normal range is 0.3 to 5.0 and the standard teaching is that no thyroid treatment should be given until the TSH reaches about 7 or 8). The striking abnormality was the thyroid antibody result: her antithyroglobulin antibody was 370 when the normal range is 0.00 to 0.30 units/ml. She had a level of 1,660 on a later check of her antithyroglobulin antibody, even though her TSH was still normal at that time also. The antibody to the thyroglobulin was acting like a “blocking agent” keeping the thyroid hormone from being able to attach to the receptor sites and work properly, even though the thyroid gland was making enough of the hormone. She was certainly relieved to know what had been causing her diverse symptoms. I started her on Synthroid, initially at a small dose of only 0.025 mg. She felt better fairly soon but had to have several more dose adjustments before she reached the optimal amount of medication. A year later, she described feeling “back to my old self”!
Another woman, a twenty-year-old college student, illustrates the potential for marked effects on brain phenomena when the thyroid function is diminished. Her father asked if I would do a consult for her because they had not been able to find help for her severe PMS. She had not been doing well in school although previously had been an excellent student. When I saw her, she described having a lot of difficulty keeping her concentration focused to be able to study, her memory was much worse than normal, and she had a deterioration in her grades even though she was studying more than usual. She talked about feeling embarrassed by severe anger outbursts before her period, “I’d be so irritable I thought I would explode, and then I would cry over little things and couldn’t stop.” Her family described her as usually very energetic and vivacious, but over the past year, she said she could barely make it through the day and often cut class to take naps in the afternoon because she was so tired. She had gained about twenty pounds over the past year and had not previously had a weight problem. There was no evidence of a major depression. Her primary doctor had told her that all her tests, including the thyroid hormones, were normal—TSH was not done. The physician suggested she see a counselor to help her cope with the stress of being away from home at college. When I interviewed her with her parents, I did not find any indication that she had problems adjusting to the school setting. I did, however, find out an interesting point in the family history: the patient’s mother had thyroid disease and had been on thyroid medication for many years, as had her maternal grandmother.
Shellie had a complete evaluation that came back normal except for the thyroid antibodies and TSH. Her antithyroglobulin antibody was 6,000 IU/L (the normal range this time was 0 to 100 IU/L), the antimicrosomal antibody was 475 (same normal range of 0–100). Her TSH was 7, a level that many doctors simply “watch” for six months and recheck. But since we had the critical information about the marked abnormalities of the antibodies, I thought it was important to begin thyroid medication right away. Clearly she was significantly hypothyroid, even though the T3 and T4 were normal. A thyroid scan showed a diffusely enlarged gland typical of thyroiditis. The high antibody levels were preventing the thyroid hormones from working properly at critical body pathways, especially the brain. She was started on thyroid medication and gradually increased to the right amount for her. The brain symptoms have resolved, and her PMS is no longer a major problem. She is gradually losing weight with exercise and a healthy meal plan combined with her thyroid medication.
Both of these women show how important it is to pay attention to changes like this in brain function when the person has previously not had such difficulties. The brain is often the first organ to show the effects of subtle changes in either thyroid or ovarian hormone function because the brain is so exquisitely dependent upon normal balance for optimal function. When I talk with physicians about these issues, I emphasize that the overall “pattern” is what helps determine the tests to do. We are treating patients, not lab values. Since space is limited in this book, you may want to read Living Well with Hypothyroidism, by Mary Shomon, or The Thyroid Solution by Dr. Arem, for excellent and much more in-depth discussions on getting help for thyroid problems. I also have explained more about the thyroid connection in PMS and fibromyalgia in upcoming chapters.
COMMON SYMPTOMS OF HYPOTHYROIDISM
• severe fatigue, loss of energy, feeling “sluggish”
• weight gain, difficulty losing weight
• depressed mood, usually not as severe as major depression, but may be mistaken for primary depression
• menstrual irregularities, difficulty becoming pregnant/infertility
• low body temperature*
• PMS symptoms, with premenstrual mood changes common
• dry, scaly, Itchy skin and scalp
• dry, brittle hair and nails
• losing hair (alopecia)
• hoarseness
• chronic constipation
• puffiness of face, lower legs, and feet
• slowed heart rate, unusually low blood pressure
• diminished reflexes
• difficulty tolerating cold environments, climate (“can’t get warm”)
• sleeping much more than normal
• tingling in wrists/hands, mimicking “carpal tunnel syndrome”
• clotting problems
• multiple joint aches (arthralgias)
• achy muscles (myalgias), leg cramps, muscle weakness
• diminished or lost sexual desire (libido)
• decreased memory, concentration (also brain effect of low thyroid may be misdiagnosed as dementia in an older person)
• Worsening allergies
• ABNORMAL LAB TESTS: high TSH, low thyroid hormones, high total cholesterol, lower than normal HDL, possibly elevated liver enzymes
*keep in mind, many of these changes including low basal body temperature may also occur in other conditions, particularly when estradiol and testosterone loss occurs. You can’t go by symptoms or basal body temperature alone—you must have the proper hormone levels fully checked to determine which problem you have.
© Elizabeth Lee Vliet, M.D., 1995, revised 2000