Chapter 8
Life on the Edge
In the spring of 1977, the geologists Jack Corliss of Oregon State University and John Edmond (1944–2001) of the Massachusetts Institute of Technology boarded the research submarine Alvin for humankind’s first direct look at a mid-ocean ridge. They were following up on observations made two years earlier in the Atlantic that these ridges—a globe-girdling chain of mountains beneath the sea—seemed to consist of freshly solidified basalt, suggesting that an active source of lava was nearby. The researchers were on a hunt for “spreading centers,” where new crust is formed. The existence of such centers was predicted by plate tectonics, a theory that was first proposed by the German geophysicist and meteorologist Alfred Wegener (1880–1930) back in 1912 but, in the mid-1970s, was only recently beginning to achieve widespread acceptance.*
When Alvin reached the slope of the ridge some 2,500 m beneath the surface of the Pacific Ocean, the geologists noticed that the water temperature was five degrees higher than the normal 2°C of the ocean’s depths. At the time, marine geologists had already theorized that the entire volume of the oceans somehow flows through hot volcanic rocks every 8 million years or so—only this could account for the dissolved solids in seawater, which are drastically different from the residue you’d get by evaporating river water to dryness—but no one had yet identified the hydrothermal features that might account for the cycling. This hint of hot springs on the ocean floor was thus a sensational discovery, and the researchers excitedly took samples so that they could later determine the chemical composition of this unexpectedly warm water. Still excited, they piloted Alvin up to the top of the ridge, where a much bigger sensation was waiting for them. Where they had expected to find a stark “desert” of bare, lifeless basalt freshly erupted from the spreading center atop the ridge, they instead found an oasis 100 m across that had warm water seeping through every little crack of the seafloor and was richly populated with clams, crabs, sea anemones, and large pink fish. As Edmond later recalled in Scientific American, they spent the five remaining hours of their dive in frantic excitement. They measured temperature, conductivity, pH, and oxygen content of the seawater, took photographs, and collected specimens of as many of the animal species as they could catch.
Holger Jannasch (1927–1998), a German marine biologist working at the Woods Hole Oceanographic Institution, was one of the first to hear the news. He recalls that he “got a call from the chief scientist, who said he had discovered big clams and tube worms, and I simply didn’t believe it. He was a geologist, after all.”*
The Art of Living Dangerously
Living organisms tend to be sensitive to drastic changes in their environments. Heat and cold, pressure, drought, salinity, and acids and bases all disrupt the crucial interactions that keep biomolecules folded and functional and quickly put an end to the fragile state of chemical disequilibrium we call life. Therefore, scientists have tended to assume that there are strict boundaries to the biosphere that are imposed by Terrestrial life’s requirement for a rather narrow and specific range of physical conditions (see sidebar 8.1).
Discoveries in the past few decades, however, have shown that life isn’t always as sensitive as we might have imagined and that the limits of life on Earth are far from well defined. Historical notions of what is, or is not, a hostile environment have turned out to be erroneous. Methods routinely used for sterilization, including boiling, freezing, and γ-ray treatments, turn out to be deadly for most—but not all—microbes. For every extreme physical condition investigated, extremophilic organisms have shown up that not only tolerate those conditions but often require them for their survival. With these discoveries, the expanse of the known biosphere has grown and the putative boundaries of life have expanded. The decades following that Alvin dive, in particular, have witnessed substantial shifts in what scientists consider the limits of habitable environmental conditions.
While there are certain hard physical limits to the existence of DNA-based cellular life, these limits are far from the normality of common garden-variety organisms such as E. coli and Homo sapiens (a normality admittedly defined by anthropocentric thinking!). Here we take a very brief look at some of the extreme conditions faced by organisms living on our planet, considered under the fundamental astrobiological question of what these findings tell us about the prospect of finding life elsewhere in the Solar System and beyond.
Some Like It Hot
Microbial activity at temperatures above the “normal” range of 20°C to 40°C was reported in the nineteenth century, but the upper limit of life’s known temperature scale has risen rapidly over the past five decades (fig. 8.1). An important foundation was laid in the 1960s when Thomas Brock started culturing heat-resistant bacteria isolated from the hot springs of Yellowstone National Park. His discoveries included Thermus aquaticus, which was to become the first and most common source of heat-stable DNA polymerase for the polymerase chain reaction (PCR) two decades later (see sidebar 8.2). The temperature records set by Brock’s organisms, however, were not destined to last, as even more hostile habitats remained to be discovered.

Figure 8.1 The temperature scale of the best heat-adapted groups of organisms.
Two years after geologists using the research submarine Alvin discovered warm springs on the seafloor that hosted a surprisingly rich ecosystem, another Alvin expedition found the first hydrothermal vents. Here water erupts from chimney-like deposits rising several meters above the seafloor, emerging at temperatures as high as 350°C. (Under the elevated hydrostatic pressure at such depths, the boiling point of water is raised considerably; it reaches 340°C at a depth of 2 km.) The drastically increased solubility of certain minerals in the hydrothermal fluid leads to instant precipitation when the mineral-rich fluid mixes with cold seawater, producing both the characteristic chimney walls and the “smoke plume” that earned these vents the nickname “black smokers” (fig. 8.2). Around these springs, complete ecosystems with complex food webs flourish without daylight or any carbon source more exotic than carbon dioxide.
Detailed investigation of the ecology of hydrothermal vent communities revealed that at the base of their food chain are single-celled chemotrophs, which are organisms that live by taking in abiological nutrients as raw materials to build their cells. These single-celled organisms are, in turn, either bound in a close, mutual symbiosis with, or are eaten by, higher eukaryotes such as clams and the now well-known tube worms that can grow to lengths of more than a meter. These macroscopic, multicellular organisms, however, live in the much cooler water centimeters to meters away from the vents and thus are not themselves particularly thermophilic.
Adaptation to survival at high temperatures is closely linked to the chemical needs of these organisms, specifically to the sulfides they use as their source of reducing potential. These sulfides are provided by the seafloor springs and hydrothermal vents but tend to be insoluble at the 2°C temperature of the deep ocean. The organisms are therefore able to harvest more sulfide the closer they live to the hot spring, and this produces a strong selective pressure for the evolution of extreme thermophilic properties.
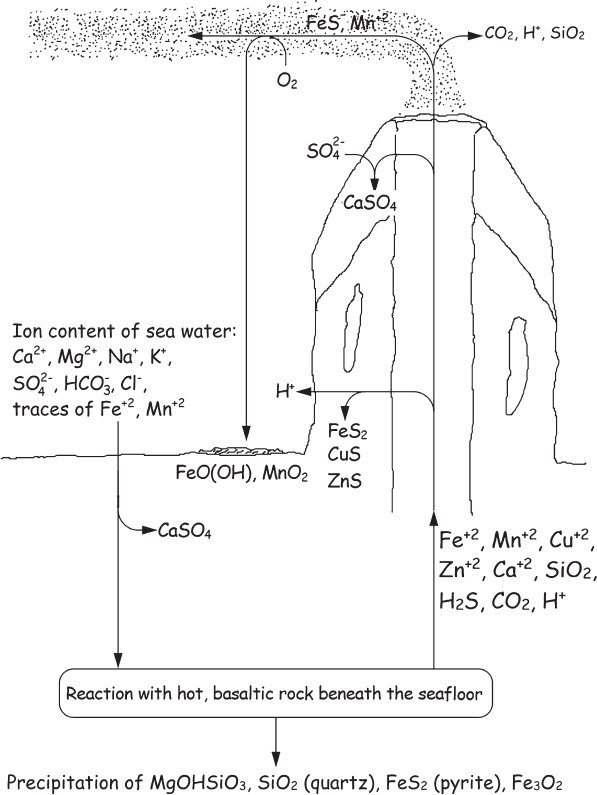
Figure 8.2 A schematic cross section of a hydrothermal vent, or black smoker, showing the reaction paths of the most important minerals. Even before black smokers were discovered, geologists had predicted that such reactions must be occurring at the ocean floor, to account for the unusual salt content of seawater. It is estimated that the entire water volume of our oceans runs through black smokers once every 8 million years.
Other hot environments that have yielded new, extremely thermophilic microbes include solfatare fields, which are volcanic soils permeated by hot vapors that are found in such places as Iceland, Sicily, and America’s Yellowstone National Park. Surface waters in solfatare fields are often near the boiling point and are rather caustic as well, with pH values ranging from an extremely acidic 0.5 to a moderately basic 9.0. Per their name, they also usually contain a diverse mix of sulfur compounds, with deeper layers being anaerobic and thus rich in sulfides. Under such reducing conditions, the sulfides of heavy metals tend to precipitate out of solution, coloring the soils a gooey black. Some of the most extreme thermophilic and acidophilic microbes have been isolated from these environments.
A number of thermophilic organisms have been studied in detail, and several of them are of particular interest to us astrobiologists. These include two of the most thermophilic bacteria, Aquifex pyrophilus and Thermotoga maritima. The 80°C optimal growth temperature and the 89°C and 96°C maximum growth temperatures, respectively, of these microbes, however, have been surpassed by other, more recently characterized species. These include Pyrolobus fumarii, an archaeon isolated from a black smoker, with a growth range of 90°C to 113°C, and “strain 121,” which grows at 121°C (a temperature routinely used in autoclaves to “sterilize” medical or research equipment) and can survive several hours at 130°C but does not grow at all below 85°C. Strain 121 has since been classified as an archaeon of the Crenarchaeota phylum and baptized Geogemma barossii. It is an anaerobe that seems to make its living by extracting electrons from simple reduced compounds in thermal vents and passing them on to Fe+3, reducing it to Fe+2. The current record holder, though, appears to be Methanopyrus kandleri. In 2008, Koki Horikoshi (1932–2016) and his group showed that, as long as the pressure is elevated to the 400 atm of its natural, black-smoker habitat, this carbon-dioxide-and-hydrogen-eating archaeon continues to reproduce at temperatures as high as 122°C.
Life under conditions of extreme heat, especially when coupled with low pH, requires that an organism’s proteins remain folded and active in these seemingly more challenging environments. Most eukaryotic proteins, for example, unfold at modest temperatures or modest deviations from neutral pH, as seen when you boil an egg or when a Peruvian chef makes ceviche by soaking raw seafood in lime juice. The molecular basis for the adaptation to high temperatures has been studied in more detail than any other extreme condition, but no straightforward and universally applicable rules have yet been uncovered that define whether a protein will remain folded and active. One interesting observation has come to light, though: enzymes derived from thermophilic organisms are just as stable at high temperatures as our enzymes are at our body temperature. For example, testing each enzyme at the optimal growth temperature of its source organism yields similar values for key parameters, including thermodynamic stability. This constant stability is achieved through a large number of subtle molecular interactions that combine to produce a rather complex overall picture of thermostabilization.
A final twist regarding the thermophiles is that A. pyrophilus and T. maritima seem to be among the most “ancient” of all prokaryotes. That is, they may be the living organisms most closely related to the putative branch organism from which the domains of Archaea and Bacteria diverged. That these thermophiles might be LUCA’s closest living relative has widely been taken to suggest that life arose under high-temperature conditions, perhaps at the deep-sea vents. Of course, we have to remember that LUCA was quite advanced and that a great many organisms had evolved and disappeared again long before she came on the scene. An alternative scenario is that life on Earth arose someplace far removed from the vents and evolved into organisms that filled many niches, including thermophiles living in the vents, and then the non-vent organisms became extinct. How might this have happened? A meteor or comet impact large enough to boil most of the volume of the Earth’s oceans—an impactor a few dozen kilometers across would do the trick—would kill off everything but the deep-ocean thermophiles.
Cold Adaptation
While hot springs and hydrothermal vents are very important both for biology and geology, most of the water on our planet is rather cold. The bulk of the deep sea is at a constant temperature of 2°C, and in the vicinity of the polar icecaps, liquid seawater may even be cooled to below 0°C as the typical salt content of seawater (3.4%) lowers the freezing point to −1.8°C. When seawater freezes, the crystallization of pure water increases the salinity of the remaining liquid to as high as 15%. Under these conditions, the freezing point may be depressed to as low as −12°C. Numerous species, collectively known as psychrophiles, are now known to thrive under these harsh conditions.
The challenges associated with cold adaptation are very different from those associated with a thermophilic lifestyle. While high temperatures speed up reactions, including those that convert the biological macromolecules into smaller and much more stable molecules such as carbon dioxide and water, low temperatures slow reactions down and thus bring biochemical systems to a halt but do not destroy them. The real danger sets in when water is allowed to freeze since its significant expansion during freezing exerts shear forces that can easily cause mechanical damage to cells and their components.*
Two broad solutions to the problem of ice-induced damage have evolved on Earth. The first is the production of antifreeze compounds to prevent the formation of ice. Different species have come up with different ways to avoid freezing. Some organisms simply produce large quantities of osmolytes, such as glycerol, which suppress the freezing point of their tissues by several degrees. Fish of the polar waters have several different kinds of antifreeze proteins (AFPs), ranging from the simple type I AFP, which consists only of a single α helix, through to complex glycoproteins. These proteins typically recognize and bind to structural features of nascent or growing ice crystals, thus blocking their further growth. In contrast, some species of frogs and turtles have found an alternative solution by going in the opposite direction. They have ice nucleation proteins, which facilitate freezing of the animal’s body liquids. By triggering ice formation in many different places at once, these proteins ensure that crystals cannot grow large enough to cause mechanical damage. Essentially, the freezing process is similar to what happens when a small sample of biological material is thrown into liquid nitrogen: the water freezes instantly in all places at the same time, which minimizes the damage to cell structures.
Ice-induced damage, however, is not the only problem that cold-adapted organisms face. Many of the molecular interactions that define our cells are affected by cold, and thus many aspects of biochemistry must be modified for an organism to thrive at low temperatures. These adaptations include the modification of membrane lipids (to avoid stiffening, just as oils often solidify in the refrigerator) and the production of proteins that stop RNA molecules from binding to themselves to form complex structures that could inhibit translation.
A particularly successful strategy of adaptation to the coldest climates on Earth involves the collaboration between two species. Lichens, which colonize much of the rock surfaces in Antarctica, are famously resistant to the dry, cold conditions of their habitat. Although many lichens look like plants, they represent a symbiotic life form made up of a fungus and a photosynthetic organism. The ability to form lichens is spread widely across many families of fungi. In fact, it is estimated that one in five fungal species can perform the trick. Their symbiotic, photosynthetic partners can be algae (e.g., with the fungus Prasiola crispa) or cyanobacteria (e.g., with the fungus Nostoc commune), representing the domains of Eukarya and Bacteria, respectively. The symbionts make a very hardy team. In the wet and relatively mild climates found on the coast of Antarctica, the fungus P. crispa survives on its own by harvesting chemical energy from its environment, but living as a lichen provides an alternative source of energy that allows the fungus (and its algal partner) to thrive even on dry rock faces despite the more limited nutrients and drastic temperature swings typical of this environment. Cold-resistant lichens, forming characteristically colorful, plantlike structures, thrive at very high polar latitudes—even, for example, some 2,200 m up Mount Czegka, which, at a latitude of 86°20′ south, is just 400 km from the pole. This said, the southernmost exposed rock on our planet, some 110 km closer to the pole, on Mount Howe, appears to be completely lacking in multicellular life.
Apart from molecular adaptations and symbiosis, organisms can also exploit their local or even microscopic environment to obtain protection against freezing. Algae, for example, have been shown to survive in tiny liquid pockets encapsulated in the sea ice that surrounds Antarctica during the winter. As the freezing of a closed volume of seawater brings with it an increase in salt concentration, the freezing point is suppressed in these environments. This prevents the organisms from freezing, but as a consequence they have had to adapt to high salinity as well as low temperatures and low light. Other species may “hibernate” most of the year in the sea ice and only spring to life when their habitat is defrosted for a brief period during the southern summer. Similarly, several species of algae have been found to grow a few millimeters under the surface of translucent rocks in Antarctica’s “dry valleys,” the harsh, cold, and extremely dry areas that are widely regarded as the closest thing to Mars on Earth. The overlying rock acts like a greenhouse, significantly extending the growth season of these endolithic (from the Greek endo, meaning inside, and lithos, meaning “rock”) organisms and protecting them from drying out.
A final “ice niche” is located far below the surface of the ice cap in permanently liquid lakes that may have existed for millions of years. Kept warm by geothermal energy (helped by the insulating properties of a kilometers-thick ice cap), these lakes are thought to have been isolated from the rest of the biosphere for the entirety of their existence. Although they were only discovered in the 1990s, during ice-penetrating radar studies, these subglacial lakes are vast. The largest one, Lake Vostok, is located at 77° south beneath the eponymous Russian research station and is thought to contain as much water as North America’s Lake Ontario.
The motivation for exploring Lake Vostok and the other subglacial lakes is clear. The Antarctic lakes, and the methods developed to explore them, are of interest as potential models for the saltwater oceans suspected to be hiding under the ice crusts of several moons in the outer Solar System, which we’ll discuss in detail in chapter 10. Survival in these cold, subglacial lakes may also be relevant for understanding life’s history on our own planet, which is believed to have undergone several “snowball Earth” episodes as recently as 600 million years ago when the carbon dioxide cycle (see chapter 3) that regulates the Earth’s temperature may (or may not—this is still controversial!) have gone haywire.
Motivated by the astrobiological relevance of the lake, researchers gradually advanced drills toward its surface, proceeding with extreme caution so as not to damage the biotope by contaminating it with microbes from the outside world—or by releasing the high pressure, thereby diminishing the freezing point depression and causing the lake to solidify. In 1998, and then again in 2010, drills were halted just 100 m above the water. Ice cores consisting of refrozen lake water were shown to contain evidence of cellular life. Only in February 2012 did research teams punch a hole all the way through the nearly 4 km of ice to the lake water. They allowed the pressurized lake water to rise up by a few meters and then freeze in place, returning a year later to retrieve the frozen lake water for analyses. Severe contamination of the samples with both kerosene and microbes has cast a shadow over the validity of any results obtained, although the researchers have claimed to have identified at least one non-contaminant species. A metagenomic study (see, again, sidebar 7.2) of the ice naturally accreted from Lake Vostok published in 2013 yielded taxonomic classifications for more than 1,600 gene sequences, of which 6% were assigned to eukaryotes and the rest to bacteria.
By contrast, researchers from the Whillans Ice Stream Subglacial Access Research Drilling (WISSARD) project used a specially developed hot-water drill, designed to achieve contamination-free access, to retrieve lake water and sediment samples from Lake Whillans, which forms part of a dynamic drainage system in West Antarctica underneath more than 800 m of ice. In 2014, the team reported the first comprehensive description of a subglacial ecosystem. In the lake water samples they characterized, the researchers found around 130,000 cells per milliliter, which is comparable to cell densities in the deep sea. Analyzing ribosomal RNA genes, they discovered a large number of microbial species in both the water and sediment samples, representing both bacteria and archaea, but not eukaryotes.
Detailed chemical analyses combined with the taxonomic relations of the new species to known microbes suggest that the ecosystem in the lake is able to thrive on a chemoautotrophic basis, that is, deriving energy and nutrients from minerals. Experiments measuring nutrient incorporation rates by the cells retrieved from the lake led the researchers to conclude that the chemoautotrophic production is sufficient to serve as the basis of a complex food web also including heterotrophs, organisms who, like us humans, derive their nutritional needs from consuming complex carbon compounds. Heterotrophs were also found in the lake sediment, presumably feeding on organic material there. A key aspect is the internal nitrogen cycling in the system, which appears to include nitrifying species in the water column that convert abundant ammonium ions into more oxidized nitrogen compounds, along with other species in both the sediment and water column that reduce nitrogen compounds to ammonia.
Drought and Salinity
As we have seen, many extremophiles can survive and even thrive at temperatures above 100°C or below 0°C as long as water remains liquid (because of high pressure or salinity or both). Generally, the availability of liquid water is widely seen as a key requirement for life. Deserts and salt lakes illustrate the struggle for survival in environments where water is either absent or unavailable for chemical reasons.
All known organisms need water as a solvent for their biochemical reactions. But some have evolved ways to survive long periods of drought in a passive state and then carry on living when the water returns. The bacterium Deinococcus radiodurans, for instance, attracted scientists’ attention with its extraordinary resistance to ionizing radiation. First discovered in a can of corned beef that had been “sterilized” with γ-rays, it was also isolated from cooling baths of nuclear reactors. Research showed that the microbe hosts a highly efficient DNA repair mechanism that enables it to survive radiation levels a thousand times higher than those that would kill a human. As it is clear that radioactivity was not the selective factor that produced this mechanism (nuclear reactors being a relatively new habitat), the question as to why a bacterium would have developed this trait was at first a mystery. The consensus now, however, is that the DNA repair mechanism of D. radiodurans evolved as a means of surviving DNA damage induced by severe drought since both irradiated and dried cells suffer similar types of DNA strand breakage due to the presence of active chemical species such as oxygen radicals.
Another impressive defense against the threat of drying is the one employed by the tardigrades (more colloquially known as water bears), a group of microscopically small animals that are found on all continents. Tardigrades are typically found in water droplets suspended in moss and lichens. Remarkably, these organisms have two separate, highly original emergency routines. One is for the case of flooding and associated oxygen shortage and involves inflating to a balloon-like state that allows the tardigrade to float at the surface of the water. The second emergency plan is for the opposite case. When their habitat dries out, tardigrades shrink into a spore-like granule known as the tun state (tun is an archaic word for “barrel,” which is what it looks like). In the tun state, the tardigrade replaces most of its water with the sugar trehalose. When trehalose solutions evaporate, the remaining sugar forms an amorphous glass rather than sharp, damaging crystals, and thus trehalose is an ideal medium for dehydrating proteins and DNA without damaging them.
Once the tardigrade converts into a tun, it is one of the toughest animals on Earth. Tuns found in moss samples from museums have been revived after more than a century of drought. Among other improbable stress treatments, researchers have suspended tuns in perfluorocarbon solvents and subjected them to hydrostatic pressures of up to 6,000 atm, and more than 80% of the animals survived. Tuns can similarly survive temperatures as high as 150°C and as low as 0.2°C above absolute zero. If anybody wanted to send animals to Mars (and who doesn’t?), perhaps tardigrades would be the most likely to survive the trip without much in the way of life support. Indeed, in September 2007, researchers from the University of Kristianstad in Sweden actually sent tardigrades into Earth orbit on the Foton-M3 mission. Twelve days and 189 orbits later, the spacecraft landed safely back on Earth with its cargo safe and sound; the water bears had withstood the vacuum and cold in Earth orbit without breaking a sweat. The hard UV radiation that pervades the Solar System outside the Earth’s ozone layer (which protects us) proved their only real threat: whereas most of the animals in a group protected by UV filters survived, only a few survived in a second group lacking this protection.
In a bittersweet follow-up in April 2019, the Israeli spacecraft Beresheet attempted—but failed—to make a soft landing on the Moon. Along with a “Lunar library,” a 30-million-page archive of human knowledge on a metal disc, it carried more than a thousand tardigrades, sent on the trip by the Arch Mission Foundation, which aims to find a backup habitat for Terrestrial life elsewhere in the Universe. The organization reckons that both the library and the tardigrades may well have survived the crash landing, albeit the latter presumably not for long.
The harshest classical desert on Earth is northern Chile’s Atacama. With the 6,000 m high Andes on the east, which prevent moisture flow from the Amazon, and the Pacific’s cold Humboldt current and the 2,500 m high Chilean Costal Range limiting moisture flow from the west, the heart of the Atacama is considered to be the most Mars-like environment found on our planet. On average, the core of his hyperarid desert receives 1 or 2 mm of rain per decade, and as we can confirm from personal observation, one does not see even a single dead blade of grass over hundreds of kilometers (fig. 8.3). The area is so lifeless, in fact, that NASA uses it as a challenging case study for the development of new life detection technologies, which, by the way, often fail here. (Foreshadowing chapter 10, there are so few bacteria in the soil, and the organics in the soil are so sparse and so refractory, that, had they landed in the Atacama, it is thought that the life detection technologies sent to Mars with the Viking missions would not have found life.) Despite this, in 2006, astrobiologist Chris McKay reported that, even in the driest parts of the Atacama, colonies of cyanobacteria live just a few millimeters below the surface of halite evaporites (rock salt), occupying spaces between the salt crystals. It seems the hygroscopic nature of salt ensures that the crystals absorb some atmospheric water even at the low humidity found in this harsh desert.

Figure 8.3 The heart of the Atacama Desert in northern Chile is considered to be the most Mars-like environment found on our planet. And yet, even in this hyperarid desert, where only a few millimeters of rain falls a few times a decade and one does not see even a single dead blade of grass over many hundreds of kilometers, there is life: endolithic colonies of cyanobacteria living millimeters below the surface of halite (rock salt), occupying spaces between the salt crystals and living off atmospheric water vapor drawn in by the salt crystals. (Photo credit: Jade Dutcher)
Living in condensed dew that’s wicked between salt crystals presents another problem: while water may be present, it is not chemically available. Think of the so-called Dead Sea, which despite its name is very much alive. Evaporation lakes like the Dead Sea are saturated with salt, so all the water is essentially taken up with the task of solvating salt ions. Living organisms have to compete with these ions for water, lest the osmotic gradient suck them dry. Several species of algae, along with bacteria and archaea, have adapted to such high-salt environments. While the algae tend to be halotolerant, meaning that they can live with the salt but may be better off without it, many of the archaea found in salt lakes are obligate halophiles—that is, they grow better, or only, when high salt concentrations are present. In response to the salt, all these adapted organisms maintain very high concentrations of other solutes in the cell to keep their insides in osmotic balance with the outside world. While salt-adapted algae and bacteria tend to use small organic molecules for this purpose, highly halophilic archaea fight fire with fire by keeping extremely high concentrations of potassium chloride within their cells. This literally takes away the pressure from the membrane, but it shifts the stress and the requirement for adaptation onto the molecular machinery of the cell. All the proteins in a halophile have to be optimally folded and functional at saturated salt conditions, much the same way that the proteins of hyperthermophiles must function near 100°C. Researchers have studied the amino acid sequences, structures, and functional characteristics of halophilic proteins in comparison with thermophilic and mesophilic (“normal”) proteins in order to gain some insights into the evolutionary strategies used to adapt proteins to stress conditions, but the picture remains far from complete.
Extremes of pH
Another part of the “normality” that we rarely question is that the pH of most liquids in a biological context is fairly neutral, typically ranging from 7.0 to 7.4 (fig. 8.4). Where acids come into play, such as in the saliva and stomach fluids, they are meant to destroy biological material and any surviving foodborne organisms. However, there are habitats (such as the Yellowstone solfatare fields described above) where both fungi and archaea thrive at pH values around zero, which is even more acidic than pure stomach fluids. Although extremely acidophilic organisms have been known for decades, the nature of their specific adaptation is far less studied than that of thermophiles.
In contrast, the base-loving alkaliphiles have been studied in some detail, particularly by researchers interested in bioenergetics. The issue is that most aerobic organisms generate energy in the form of ATP via reactions that actively pump protons out of the cell or out of the mitochondria (rendering the pH inside more basic than the pH outside) and then generate ATP utilizing a millstream-like flow of hydrogen ions back down this gradient and through the enzyme F-ATPase.* This seemingly odd arrangement allows energy-producing reactions whose energy is not quite enough to make one ATP, or not quite enough to make two ATP, to donate their energy to a single energy “reservoir” from which the F-ATPase can then extract exactly one ATP’s worth of energy at a time. This ensures the efficient utilization of the available energy and allows many diverse reactions to all contribute to making ATP.
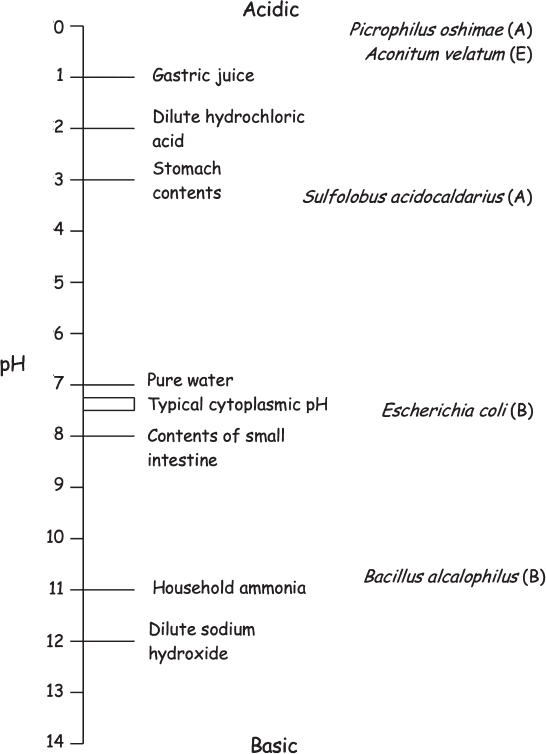
Figure 8.4 The pH scale is shown with examples of acidic and basic fluids and some pH extremophiles (A, archaeon; B, bacterium; E, eukaryote).
The mystery with the alkaliphiles is that, if the “normal” pH gradient is reversed, the F-ATPase reverses, consuming ATP and pumping protons out of the cell. That is, if a normal cell found itself in a high pH environment, it might end up wasting its energy trying to acidify the outside world. Adaptation to high pH thus likely involves fairly drastic reorganization of metabolic electrochemistry and, perhaps for this reason, is found in only a few branches of the tree of life. The standard model organism for alkaliphile studies is Bacillus alcalophilus, an obligate alkaliphile that can only live at pH values between 9 and 12. To do so, it actively pumps sodium ions out of the cell and then uses the resulting charge imbalance to drive protons back inside, thus maintaining the pH of its cytoplasm below 9.5. How it generates its ATP with the pH gradient “backward,” though, remains a mystery.
Going Deep
The effects of pressure on organisms are not so widely appreciated. Changes in atmospheric pressure may be indicative of a change in the weather, but otherwise they don’t normally affect organisms that, like us, live on dry land. When we climb a mountain, the decline in the partial pressure of oxygen affects us quite strongly, but the overall change of atmospheric pressure does not have any notable effects.
The situation changes drastically when we start diving into the ocean’s depths, where the hydrostatic pressure increases by about an atmosphere for every 10 m of descent (fig. 8.5). The increasing pressure and associated changes in the solubility of gases and toxicity of oxygen limit the reaches of recreational scuba divers to around 40 m and divers with optimized gas mixtures, to around 300 m. Beyond that, humans need submarines to explore life under hydrostatic pressure, which is one of the reasons we know a lot less about barophiles (pressure-adapted organisms) than about thermophiles.
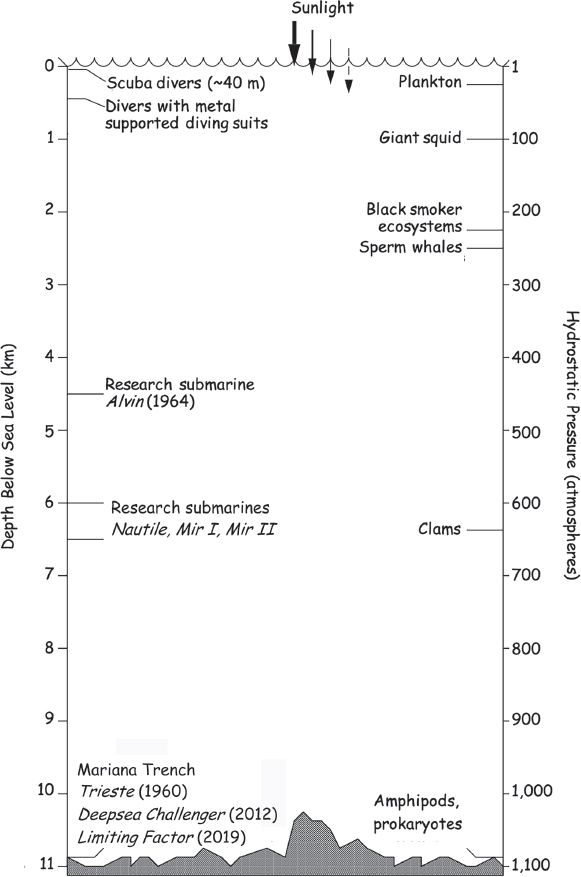
Figure 8.5 The depths below sea level that can be reached by divers, undersea vessels, and some pressure-adapted organisms are shown with the associated hydrostatic pressures.
There is no doubt that barophilic adaptation exists and is widespread in the oceans. Research vessels have consistently found life on the seafloor over the whole depth range of the oceans down to the 11 km maximal depth of the Mariana Trench, where the pressure reaches 1,086 atm. Most non-pressure-adapted microorganisms, however, stop growing at pressures above 500 atm, and mammals like us, with lungs and an entire metabolism depending critically on the equilibrium of gases (oxygen and carbon dioxide) transported through the blood, are more pressure sensitive still. Among mammals, the sperm whale, with its 2,440 m (230 atm) diving range, is a very lonely record holder.
The difficulty in obtaining samples from deep-sea habitats and the challenges of conducting biochemical experiments under high pressure conditions in the laboratory have conspired to make this research field one of the less comprehensively studied. For example, not until 2000 did researchers identify the first genes unequivocally involved in adaptation to high hydrostatic pressure. Most of the mechanisms that allow life to thrive even at the greatest depths of the oceans remain to be discovered.
Intraterrestrial life
Almost all life on Earth depends, ultimately, on the Sun for its energy. Plants utilize solar energy directly, obviously, and we do so indirectly by eating plants (or things that ate plants) and breathing oxygen. And while the “fixed” carbon and oxygen produced by plants are not the only sources of reduction potential (high-energy electrons) and oxidative potential (high-energy electron sinks) that are employed in the biosphere, the vast majority of organisms use at least one of these two (fig. 8.6). We humans, for example, oxidize photosynthetically derived fixed carbon using photosynthetically derived oxygen. But there are other organisms that, instead of using oxygen as their electron sink, use minerals such oxidized iron (Fe+3) or manganese (Mn+4). Not surprisingly, these organisms are found in environments lacking free oxygen, such as the deep mud at the bottom of lakes. But they are still heterotrophs, however, since they feed off the organic material that rains down from the water column, and thus their metabolism is, like ours, ultimately coupled to sunlight. The hydrothermal vent communities described in the opening of this chapter are likewise coupled to sunlight; while they use the reduction potential available from sulfides (such as H2S) extracted by the hot vent water from the crust, the reason this provides energy is that a mixture of sulfides and oxygen is out of equilibrium relative to sulfate (SO4−2) and water. The chemical disequilibrium on which the entire vent ecology is founded thus relies on the availability of photosynthetically produced molecular oxygen, without which the vent water would presumably be too close to chemical equilibrium to provide enough energy for anything but the simplest of living communities.
If life depended on light, either directly through photosynthesis or indirectly via reactions such as those presented in fig. 8.6, it would be limited to the surfaces of planets. Given, as we’ll see in chapter 9, that the surfaces of most planets are too hot or too cold to support liquid water, this would be a major constraint on potential habitats. In the late twentieth century, however, our view of habitability was greatly broadened with the discovery that there are biotopes completely cut off from the energy of the Sun. They reside in the hot, permeable rocks deep beneath our feet.
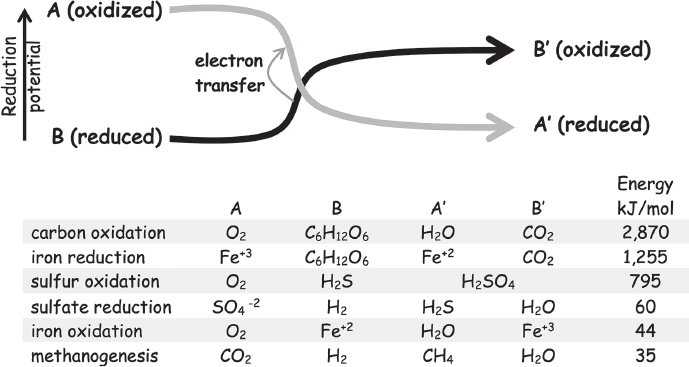
Figure 8.6 The redox interchange of life. Energy metabolism in all life on Earth involves something getting oxidized and something else getting reduced in exchange. Heterotrophs, for example, oxidize the “organics” (fixed carbon) obtained ultimately from plant material using either molecular oxygen (as we do) or oxidized minerals (as many microorganisms do in anoxic lake bed and seafloor muds). Deep-sea vent archaea, in contrast, harvest their reduction potential from abiological sources, such as sulfides or other reduced minerals leaching from the crust. These organisms nevertheless use photosynthetically produced oxygen as their oxidizer, which links their metabolism, like ours, to the energy of Sun. Only a few subsurface life-forms, including the subsurface methanogens and sulfate-reducing microorganisms, use geology rather than photosynthesis to provide their sources of both reductant and oxidizer, and thus live completely uncoupled from sunlight. This independence is achieved, however, at some cost: these reactions provide relatively meager amounts of energy.
The idea of abundant microbial life deep within the Earth’s crust was first promoted by the Cornell University astrophysicist Thomas Gold (1920–2004), who, in 1992, published a paper in the Proceedings of the National Academy of Sciences entitled “The Deep, Hot Biosphere.” Gold’s ideas regarding life below the Earth’s surface were an extension of his fairly heretical theories about the origins of fossil fuels. The traditional view is that coal and oil are the remains of long-dead plant matter and marine algae, respectively. Gold believed, instead, that these hydrocarbons were incorporated into the Earth during accretion and that they provide a ready carbon source for a complex and rich underground ecosystem that could even exceed the mass of the surface biosphere we see around us.
And is there any evidence for Gold’s claims? Over the last few decades, the answer has moved increasingly toward “yes,” at least with regard to a biosphere eking out a living deep within the crust (less so for his ideas regarding the origins of fossil fuels). For example, in the 1990 study that spurred Gold’s paper, a hole drilled some 6.7 km into the granite of Siijan in Sweden turned up a thick, black, strongly odiferous fluid from which two different species of thermophilic, iron-reducing bacteria were later cultured.* About the same time, complex ecosystems were being reported within rocks freshly harvested from 3 km down in South African gold mines. But the idea of microbes living in what appeared to be solid rock far beneath the surface didn’t sit well with the microbiology community, most of whom seemed to believe that the purported subsurface microbes were simply normal surface organisms that had contaminated the relevant samples after they were harvested. In 1993, however, Todd Stevens and James McKinley of the Pacific Northwest Laboratory probed for ecosystems deep below eastern Washington state and turned the tide of scientific consensus.
Stevens and McKinley’s identification of subsurface organisms relied on two approaches. First, they went to exceptional lengths to collect their samples aseptically from a thick bed of basalt several hundred meters down. On retrieving their samples, they isolated methanogens, microbes that use the (quite limited) energy available from the conversion of carbon dioxide and hydrogen to methane and water (see fig. 8.6). In many, perhaps better-known, habitats (such as swamp mud and cow stomachs), methanogens consume hydrogen that is produced biologically, but the water samples from the deep drill contained more hydrogen than could be explained by any biological mechanism. Investigating further, the researchers found that the hydrogen had been produced by the reaction of reduced iron from the basalt with hot, oxygen-free groundwater. Putting these observations together, the researchers theorized that their subsurface ecosystem was fueled by abiologically produced hydrogen and carbon dioxide, thus rendering it completely independent of solar energy in any of its many guises. Second, to clinch their hypothesis, Stevens and McKinley demonstrated that their bacteria could survive for more than a year when sealed in a flask with nothing more than hot water, carbon dioxide, and basalt to munch on. Indeed, the microbes not only survived but actually multiplied under these seemingly extreme culture conditions, suggesting that they were well adapted to their diet of reduced rocks, water, and carbon dioxide. With this second line of evidence in hand, the case for subsurface ecosystems was clinched and today is no longer in doubt.
Following on the work of Stevens and McKinley, in 2008, Dylan Chivian of the Lawrence Berkeley National Laboratory in Berkeley, California, circled back to those South African gold mines, where he discovered a subsurface biotope in fractured rock some 2.8 km deep. Metagenomic sequencing of DNA extracted from water collected from the fracture revealed that at least 99.9% of the microorganisms in the sample belonged to a single bacterial species, which he named Desulforudis audaxviator, from a Latin quote in Jules Verne’s Journey to the Center of the Earth: “Descende, audax viator, et terrestre centrum attinges” (Descend, bold traveler, and you will attain the center of the Earth).
The lack of species other than D. audaxviator in the deep rock of South Africa is odd; the vast majority of living things on Earth today rely on other living things for at least some of their nutrients. Plants, for example, can fix carbon from carbon dioxide, but they generally receive much of their nitrogen as nitrates produced by soil bacteria. The genome of the gold mine microbe, however, suggests that this alkaline-resistant (to pH 9.3) thermophile (to 60°C) harvests its carbon and nitrogen from inorganic materials leached from the rock and uses hydrogen produced by the radiolysis (radiation-induced decomposition) of water as its electron source and mineral-derived sulfate as its electron sink. Chivian and colleagues couldn’t exclude the presence of other species, which might, for instance, cling to the rocks so tightly that they weren’t retrieved during the sampling process. But they concluded that the species they discovered may well constitute an ecosystem all by itself, living off only the inorganic materials that geology provides for it. And it seems to be good at it; in 2019, researchers reported isolating the same species from 2 km down in western Siberia, suggesting that this deep crustal organism has somehow spread around the globe. Indeed, the South African and Siberian strains differed by only 800 nucleotides across their entire genomes, suggesting that the microbe can migrate from environment to environment relatively rapidly or that its extremely slow replication rate (brought on by its exceedingly slow metabolism) resulted in the observed high genetic similarity despite the 200 million years that have passed since the Pangea supercontinent broke up and Eurasia separated from Africa.
Building on the work of Stevens, McKinley, Chivian, and others, in 2009, a group of several dozen scientists from three continents banded together to create a virtual institute called the Deep Carbon Observatory, the goal of which was to systematically characterize the distribution of carbon within the Earth’s crust. This interdisciplinary program, which ran until 2019, addressed four key areas: the ecology of subsurface life (the Deep Life directorate), the reservoirs and fluxes of carbon within the planet, their implications for energy, and the extreme chemistry and physics of carbon materials in a geological context.
The Deep Life directorate, chaired by Isabelle Daniel from the Université Claude Bernard in Lyon, France, set out to find, metagenomically identify, and, if possible, culture microbial samples from deep subsurface habitats around the world. Drilling 2.5 km into the seafloor and sampling microbes from continental mines and boreholes more than 5 km deep, for example, the researchers used the samples from hundreds of sites around the globe to construct models of the ecosystems that lie deep within our planet’s crust. From these studies, the directorate estimates that, at about 2 billion cubic kilometers, the volume of habitable rock beneath our feet is almost twice the volume of all the oceans and that the total carbon mass of the biosphere inhabiting this zone weighs in at 15 to 23 billion tons, which is around 300 times the mass of all humans alive. Among the billions of tons of life in the deep biosphere, the researchers identified representatives of all three domains of life, but, not surprisingly, found that bacteria and archaea were predominant.
Another surprise that the effort uncovered is that the microbial biodiversity found below ground is comparable to that found on the surface. This despite the fact that, at first glance, one might assume that the environmental diversity is lower below ground than above. The microbial diversity is likewise a surprise due to the slow reproduction of deep biosphere organisms, which should slow their evolutionary drift and subsequent speciation. Although few of these deep biosphere microorganisms have been cultured in the laboratory, and thus we do not know their replication rates, measurements of the rate with which they increase their mass suggest that their doubling times are often measured in decades if not centuries. Life is not easy when nutrients are so limited.
And how deep does the deep, hot biosphere go? As of yet, we do not know: even at the bottom of the deepest drillings performed by the Deep Carbon Observatory, some 5 km down, researchers still found life, and thus the true depth of the biosphere remains unplumbed.
Conclusions
In some sense, the Terrestrial extremophiles simultaneously tell us both a great deal and very little about the ability of life to arise and thrive on other planets. The ability of life to survive in such unlikely, resource-poor environments as solid rock kilometers below the surface does suggest that the range of niches suitable for life may be much greater than many scientists previously suspected. Similarly, tardigrades’ ability to survive desiccation down to a water content of 3% suggests that oceans and rivers may not be required even for advanced, multicellular organisms. It is quite plausible that the rock-eating archaea that live within the Earth’s crust could survive within the crust of Mars as well, and, although probably less likely, perhaps the subglacial microbes of Lake Vostok could survive in the ocean hidden beneath the ice shield of Jupiter’s moon Europa (which we’ll learn about in the next chapter). But even if such life could survive on Mars or Europa, could it have arisen there in the first place (see sidebar 8.3)? Or was there something special about the early Earth that rendered it much more suitable than elsewhere in the Solar System for the formation of life? Perhaps the answers to these questions will be found as humanity reaches out to explore the cosmos—which is what we explore in the remaining chapters.
Further Reading
Discovery of Seafloor Biotopes
von Damm, Karen, and John M. Edmond. “Hot Springs on the Ocean Floor.” Scientific American 248, no. 4 (1983): 70–85.
Extremophiles
Gross, Michael. Life on the Edge. Cambridge, MA: Perseus, 2001.
Strain 121
Kashefi, Kazem, and Derek R. Lovley. “Extending the Upper Temperature Limit for Life.” Science 301, no. 5635 (2003): 934.
Life under Extreme Aridity
Wierzchos, Jacek, Carmen Ascaso, and Christopher P. McKay. “Endolithic Cyanobacteria in Halite Rocks from the Hyperarid Core of the Atacama Desert.” Astrobiology 6, no. 3 (2006): 415–22.
Subsurface Ecosystems
Chivian, Dylan, Eoin L. Brodie, Eric J. Alm, David E. Culley, Paramvir S. Dehal, Todd Z. DeSantis, Thomas M. Gihring, et al. “Environmental Genomics Reveals a Single-Species Ecosystem Deep within Earth.” Science 322, no. 5899 (2008): 275–78.
Gold, T. “The Deep, Hot Biosphere.” Proceedings of the National Academy of Sciences USA 89, no. 13 (1992): 6045–49.
Magnabosco, C., L.-H. Lin, H. Dong, M. Bomberg, W. Ghiorse, H. Stan-Lotter, K. Pedersen, T. L. Kieft, E. van Heerden, and T. C. Onstott. “The Biomass and Biodiversity of the Continental Subsurface.” Nature Geoscience 11 (2018): 707–17.
Stevens, Todd O., and James P. McKinley. “Lithoautotrophic Microbial Ecosystems in Deep Basalt Aquifers.” Science 270, no. 5235 (1995): 450–55.
The Deep Carbon Observatory
- * More precisely, Wegener had proposed continental drift based on the close fit of eastern South America with the western coast of Africa and coincident mineral formations on either side of the Atlantic, but his theory did not provide details as to why or how the continents might be moving. The idea of plate tectonics as we know it was developed in the 1950s.
- * Quoted in TIME, August 14, 1995.
- * If this kind of problem is excluded, many kinds of microorganisms can be frozen and thawed without harm. The crucial point is that a sufficiently low temperature has to be reached without any formation of ice crystals, which in the laboratory is generally achieved by flash freezing in liquid nitrogen at −200°C.
- * The millstream analogy is better than you might imagine. In 1997, Masasuke Yoshida and his research team filmed the F-ATPase rotating under the influence of this proton flow as a critical step in the enzyme’s synthesis of ATP.
- * An important caveat is that, back then, prior to metagenomics, microbiologists could identify new bacterial species only if the organisms could be cultured (grown) in the laboratory. As many extremophiles do not survive under laboratory conditions, there may well have been more than two species in this mud.