6
Photosynthesis
Every leaf is essentially a solar-powered carbohydrate factory where, fueled by the sun, raw materials such as carbon dioxide and water are transformed by the complex molecular machinery into stable, energy-rich, finished products that help run nature's entire economy. This solar-powered process that makes carbohydrates is known as photosynthesis. It occurs in the leaves of higher plants, as well as in many other plant parts, especially in those that are green. Photosynthesis also occurs in a range of organisms other than higher plants, including algae and some bacteria and protists.
HISTORY
In 1772, Joseph Priestley, a British clergyman and chemist, demonstrated that when a plant or animal was kept alone in an airtight jar, it died. However, when a plant and an animal were put together in an airtight jar, both lived. Seven years later, the Dutch physician Jan Ingen-Housz showed that sunlight was necessary for plants to produce oxygen, although, like Priestley, he knew nothing about oxygen at the time and explained his results in another way. Then in 1782, a Swiss pastor and part-time scientist, Jean Senebier, showed that plants use carbon dioxide (CO2) when they produce oxygen (O2). He suggested that CO2 was converted to O2 during photosynthesis.
Again, it should be stressed that Senebier didn't know which gases were involved. Rather, he reported that the process was dependent upon a particular kind of gas, which he called “fixed air,” and which we now know as carbon dioxide. Then in 1804, the Swiss worker Nicolas-Théodore de Saussure found that water is necessary for the photosynthetic production of organic materials. So by the early nineteenth century, the basic ingredients involved in photosynthesis were already known and could be put in the following equation (see also Figure 6.1).

In 1883, T.W. Engelmann, a German researcher, conducted an experiment that provided circumstantial evidence indicating that chlorophyll, the green pigment of plants, might be important in photosynthesis. He studied the algae Spirogyra species, which has distinctively long, spiral chloroplasts. With a prism, Engelmann directed specific wavelengths of light to different parts of the Spirogyra. He then introduced oxygen-requiring bacteria to the solution, expecting that when the Spirogyra was getting the best light for photosynthesis, the Spirogyra would release the most oxygen, and that would be where the bacteria would move, for the oxygen. The greatest numbers of bacteria clustered where the chloroplasts were absorbing the bands of red and blue light, not by the green wavelengths. From this, Engelmann deduced that oxygen was being produced where the red and blue wavelengths were being absorbed.

Figure 6.1 Photosynthesis, as depicted here, is the process involving chlorophyll molecules that use the energy in sunlight to convert carbon dioxide (CO2) and water (H2O) into carbohydrate.
PHOTOSYNTHETIC PIGMENTS
The reason most leaves look green is because while they absorb the light's red and blue wavelengths, the green passes through, so that's what we see. Since Engelmann's experiments, it has been shown that in addition to the class of green pigments necessary for photosynthesis, which are known as the chlorophylls, there are also yellow, orange, and brown photosynthetic pigments, known as carotenoids and xanthophylls. Some plants have additional photosynthetic pigments, known as anthocyanins, which are stored in large vacuoles. When autumn approaches, certain sugars are converted to anthocyanin pigments that are red under acidic conditions and blue under alkaline conditions, contributing to the fall colors.
PHOTOSYNTHETIC AUTOTROPHS
Unlike organisms known as heterotrophs, which require other plants and animals for their livelihood, autotrophs are organisms that subsist on the inorganic environment. Autotrophs manufacture organic compounds from molecules that are so small, they don't have to be digested. Because the molecules taken in are small enough and sufficiently soluble to pass through the cell membranes, autotrophic organisms do not need to pretreat, break down, or digest their nutrients before taking them into their cells.
Photosynthetic autotrophs are among the most important and widespread organisms alive. They have elaborate systems that enable them, with the use of energy from the sun, to raise electrons to an excited state. When the electron is returned to its more normal state, a portion of its energy is transferred to a form where it may be used by the organism.
So green plants and other photosynthetic organisms create high-energy organic material through photosynthesis. As described previously, this involves converting carbon dioxide and water, in the presence of light, into carbohydrate, oxygen, and water. Photosynthesis is the ultimate source of all the energy-rich carbon compounds used by all organisms; it is responsible for the continual supply of atmospheric oxygen, without which all the aerobic organisms, those that use oxygen for all their oxidative processes, would not exist.
The only organisms that photosynthesize are the green plants and algae, some unicellular green flagellates (see Chapters 2 and 18 for more about one-celled organisms with flagella), and two groups of bacteria. Each year these animals, through photosynthesis, release about one-half of all the oxygen that is currently present in the atmosphere. And at the same time, animals, through their respiratory processes, use that oxygen for their metabolism and replace it with carbon dioxide, which in turn is recycled by the plants.
NUTRIENTS
While animals eat plants, animals, or both, to obtain nutrients, the plants and many microorganisms sometimes obtain their nutrients through inorganic sources, such as the air, water, soil, and the sun. Not really food from a human perspective, the chemicals absorbed by plants and microorganisms are used for metabolic purposes and, therefore, can be categorized as nutrients.
In the same way that the concept of what constitutes food differs, depending on the type of organism being considered, the methods used by organisms to obtain their energy and nutrients also differ. Like all organisms, plants and microorganisms require carbon, oxygen, hydrogen, and nitrogen. In lesser amounts, they also require phosphorus, sulfur, potassium, calcium, magnesium, and iron, as well as trace elements such as molybdenum, boron, copper, and zinc. And certain algae need vanadium and cobalt.
Of the four major elements found in all organisms – carbon, oxygen, hydrogen, and nitrogen – the oxygen and hydrogen are readily obtained from the air or the water. Oxygen often enters plant tissue through the roots and leaves. Inorganic elements, including nitrogen, usually enter higher plants through the roots, and most plants obtain their carbon through the leaves, usually as carbon dioxide. Other essential elements pass through cell membranes by diffusion or active transport (both are defined in Chapter 2), and some cells may ingest particulate matter by pinocytosis and phagocytosis (also defined in Chapter 2).
CHLOROPLASTS
As stated in Chapter 2, plastids are the relatively large organelles in plant cells where nutrient storage and/or photosynthesis occurs. Chloroplasts, the plastids containing chlorophyll, are enclosed within an outer envelope composed of two membranes, an outer and inner membrane. Usually quite large and conspicuously green, chloroplasts can be seen under a light microscope. In the chloroplasts are thin, flat, plate-like photosynthetic membranes called lamellae, or thylakoids, located in a protein-rich solution called the stroma. These photosynthetic membranes are arranged in stacks, called grana, throughout the chloroplasts (see Figure 6.2). Within each chloroplast, all the photosynthetic membranes are connected, and they surround an interior space that contains hydrogen ions, which are necessary for the synthesis of ATP (adenosine triphosphate) molecules. Within the thylakoid membranes are chlorophyll and other light-trapping photosynthetic pigments. These pigments are composed of the molecules involved in the electron transport system and the ATP and NADPH2-synthesizing complexes (see Chapter 5).
The photosynthetic thylakoid membranes are arranged in a way that creates considerably more surface area in relation to the total enclosed volume, a key ratio that allows the rapid buildup of hydrogen ions by the activities of all the membraneous surface area. The high relative surface area is also important in allowing the photosynthetic pigments to intercept much of the light energy passing through the leaf, or a specific structure containing the chloroplasts.
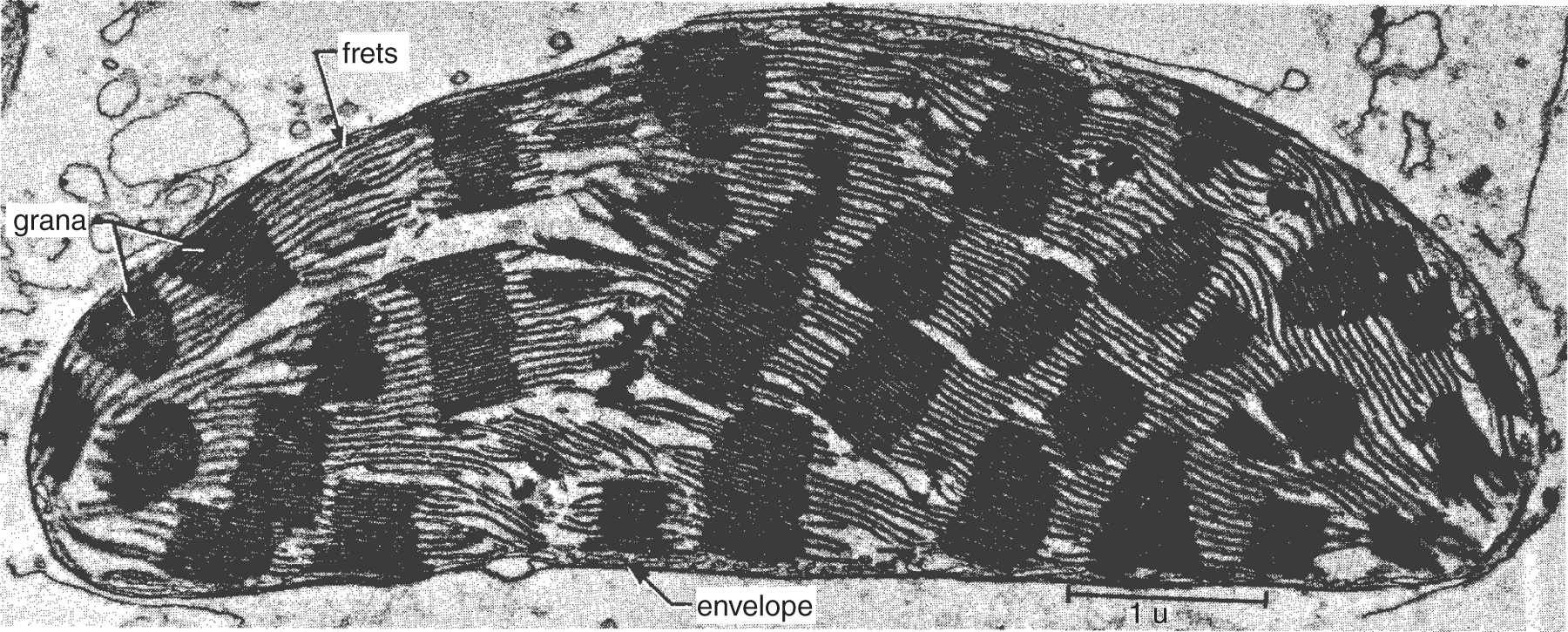
Figure 6.2 Electron micrograph of chloroplast from a corn plant.
The enzymes involved in moving carbon dioxide molecules into carbohydrate molecules are located in the protein-rich stroma surrounding the thylakoids. The ribosomes and deoxyribonucleic acid (DNA) contained within the chloroplasts are also located within the stroma (see Chapter 2). Generally, eukaryotic green algae cells contain between 1 and 40 chloroplasts. Prokaryotic cyanobacteria (also known as blue-green bacteria, and sometimes still called blue-green algae) contain photosynthetic membranes throughout their interior, rather than within distinct chloroplastic organelles.
CHLOROPHYLL AND OTHER PHOTOSYNTHETIC PIGMENTS
There are several different types of chlorophyll molecules, all of which are evolutionarily related. Chlorophyll is the green pigment in plants, algae, and lichens. It is composed of molecules that use light energy to manufacture carbohydrates (sugar). They are referred to as chlorophyll a, chlorophyll b, and so on. Chlorophyll a absorbs the energy from light that is blue and violet, and chlorophyll b absorbs energy from light that is red and blue. Neither of these chlorophylls absorb green light, so the light that plants with chlorophyll pigments transmit and reflect is what's left out of the spectrum, which is why plants look green. Each is quite similar in structure, having two distinct parts (Figure 6.3). The chlorophyll molecule has a complex ring on top, and attached below is a long tail. The complex ring structure contains a magnesium ion at the center, which is the active site where the light energy is trapped.
The long nonpolar end (the tail) is fat soluble and is anchored within the lipids composing the photosynthetic membranes. Lipids constitute important molecular components of cell membranes. The endoplasmic reticulum is a series of cell membranes, membraneous envelopes, and membranous tubules surrounding the cell's organelles, and the membranes within the chloroplasts are all largely composed of lipids, and it is within this membrane in the chloroplasts that the long nonpolar tail of the chlorophyll molecules is attached.
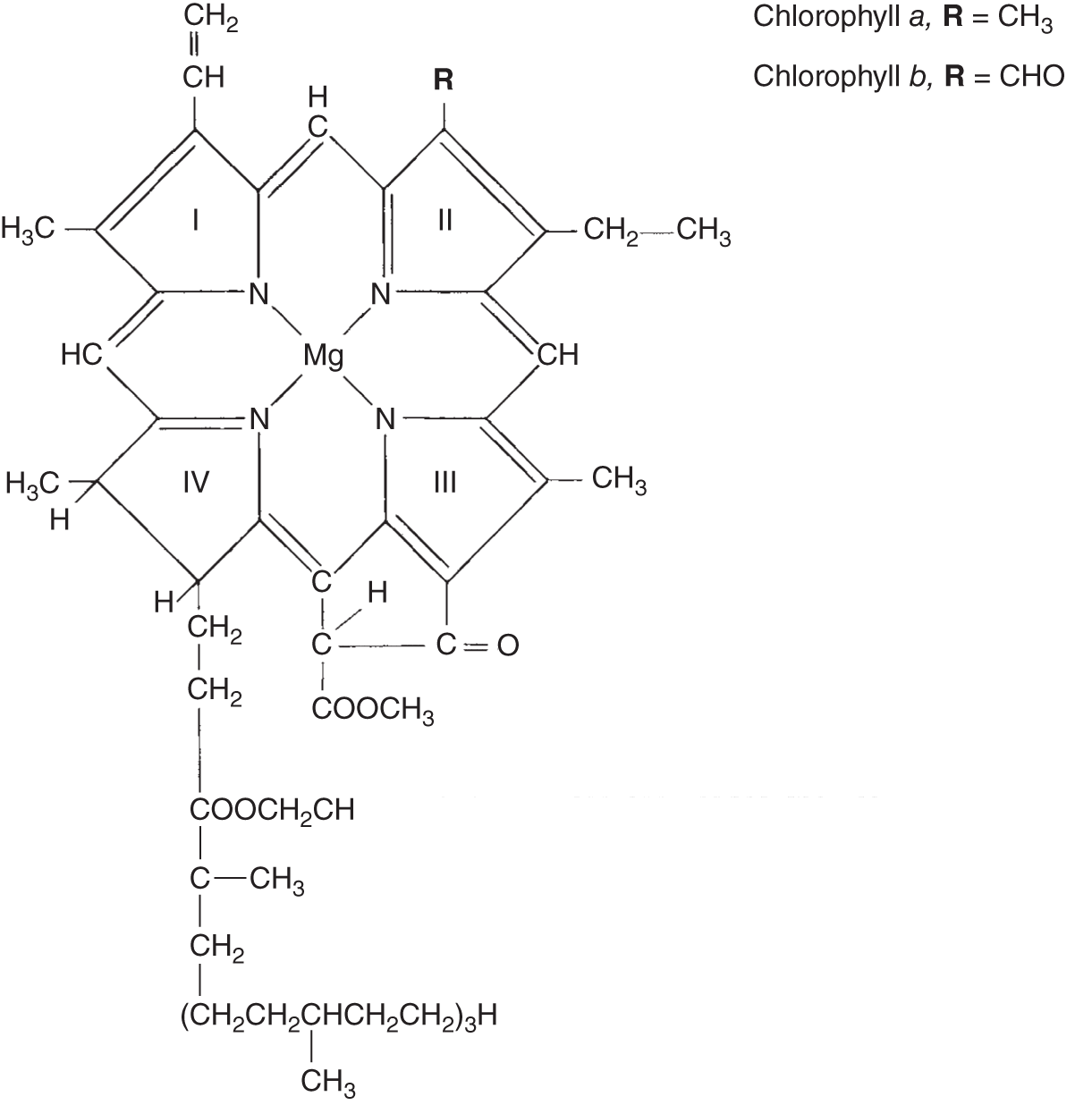
Figure 6.3 Chemical structures of chlorophyll a and chlorophyll b, molecules showing their long hydrocarbon tails. At the upper right of the structure where the chemical structure referred to as “R” is attached, this structure is the only difference between chlorophyll a and chlorophyll b. R = CH3 in the chlorophyll a molecule. R = CHO in the chlorophyll b molecule.
In addition to the structure shown in Figure 6.3, there are actually three or four variations with slightly different absorption spectra. The absorption spectrum of a photosynthetic pigment refers to the different wavelengths of electromagnetic radiation – in this case, light, from the sun – that are absorbed by the specific pigment in question. Figure 6.4 illustrates the absorption spectra of chlorophyll b.
Because each photosynthetic pigment absorbs light most efficiently within a specific spectrum, plants have several different photosynthetic pigments, increasing the overall efficiency by capturing light energy from a wider range of wavelengths. For example, the carotenoids, another important group of accessory pigments found in all green plants, absorb energy that is then passed to the chlorophyll molecules, where it is used in photosynthesis. The carotenoids are long molecules consisting of chains of carbon and hydrogen (hydrocarbon chains) with many double bonds throughout and specific attached side groups, such as methyls, and usually there are ring structures at both ends (see Figure 6.5).
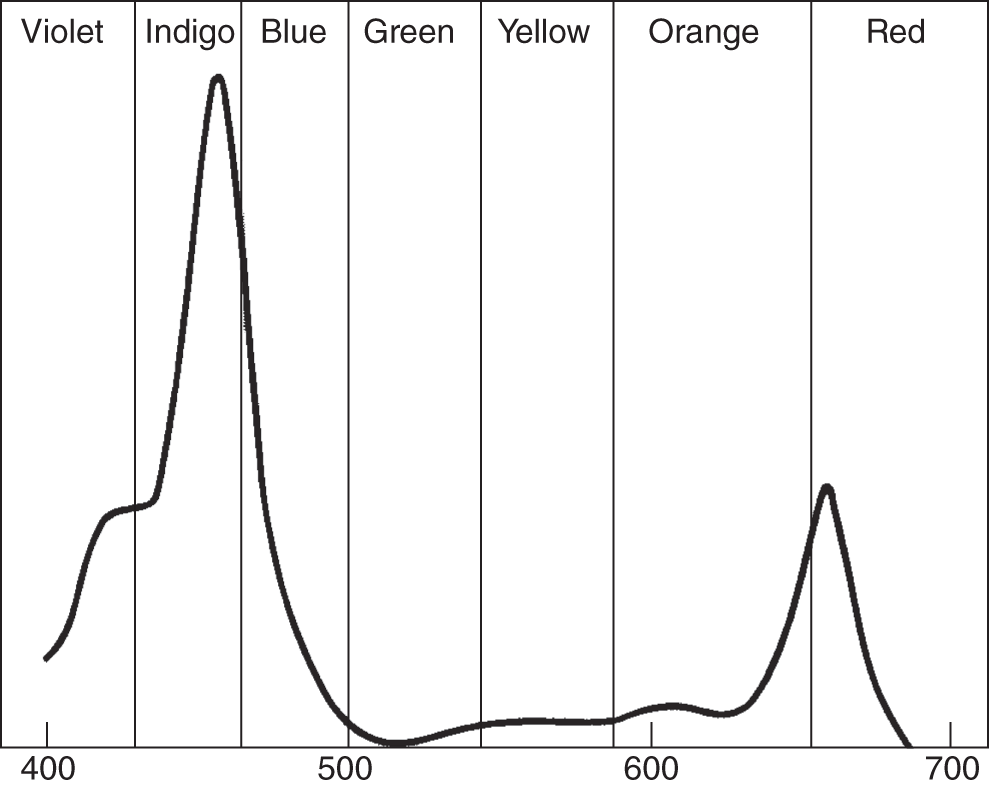
Figure 6.4 Absorption spectrum of chlorophyll b in nanometers (nm) from 400 to 700 nm.
Oxygen molecules are reduced in intense light; this means electrons are usually added to the resulting free radicals, which in this case are oxygen molecules with an odd, or unpaired, electron. These radicals are extremely reactive because of their tendency to gain or lose electrons, and, therefore, they can react with and destroy other molecules. However, because the free radicals rapidly bind with the double bonds in the carotenoid molecules, they are prevented from destroying the chlorophyll.
If the light intensity is too strong, or sustained over too long a period, the backup system may be inadequate and the chlorophyll molecules will be destroyed by the free radicals. In many plants, chlorophyll is broken down in autumn before the onset of cold winter weather. At this time, before certain plants lose their leaves, the chlorophyll is digested and the magnesium and nitrogen are transported to the roots, where they will be stored until the following spring, when they are sent back up the plant and are used. It is the decomposition of chlorophylls that allows the yellows, oranges, and browns of previously masked carotenoids to become visible. And, as mentioned earlier, the colors from the xanthophylls and anthocyanins may also become obvious. Such color changes sometimes signal certain animals, such as birds, luring them in to eat the ripened fruit and distribute the seeds via their feces elsewhere.

Figure 6.5 Chemical structure of three closely related carotenoids: α-, β-, and γ-carotene.
In addition to the photosynthetic pigments discussed above, red algae and blue-green bacteria contain phycobilins, which absorb light energy from wavelengths outside the absorption spectra of chlorophyll a, and then they transfer the energy to chlorophyll a for use in photosynthesis.
LIGHT ABSORPTION
When light energy (photons) is absorbed by the photosynthetic pigments, which are located within the thylakoid membranes, the excess energy in these excited molecules is passed on to chlorophyll a molecules. For instance, when light strikes a pair of chlorophyll molecules, the electron held between them absorbs this energy, raising it from its normal stable energy level to a higher-energy state. This electron then jumps to another molecule. The chlorophyll molecules are left with a net positive charge, which is then neutralized with an electron that comes from either a nearby water-soluble molecule or an electron jumping from another photosynthetic pigment molecule, and the process occurs again.
The energized electrons coming off the chlorophyll molecules get passed from one pigment molecule to the next until they reach either of two specialized forms of chlorophyll a, called P680 or P700. The P is an abbreviation for pigment; P680 has a maximum absorption peak in the red light with a wavelength of 680 nm. P700 has a slightly longer absorption peak of about 700 nm (1 nanometer [nm] = 1 millionth of a millimeter [mm]). Both of these specialized chlorophyll molecules have light absorption peaks in the “long” wavelength end of the spectrum, where the energy is considerably less than at the shorter wavelength end of the spectrum. Higher-energy light with shorter waves is absorbed by other photosystem pigments and then passed down the energy gradient, eventually being trapped at the low-energy end by either P680 or P700. There are discrete photosystems, each of which contains about 200 molecules of chlorophyll a, about 50 molecules of carotenoid pigment, and 1 molecule of either P680 or P700. The former is called photosystem I and the latter is photosystem II.
PASSING THE ELECTRONS
As the light energy is converted into excited electrons that are passed down the chain of pigment molecules, a series of chemical reactions, known as redox reactions, is triggered. Redox is short for reduction and oxidation reactions; reduction means the addition of an electron (storing energy) and is explained more fully at the beginning of Chapter 5; oxidation means the removal of an electron (releasing energy). Since an electron moving from one molecule to another continually takes energy from one molecule and adds it to another, it follows that as one molecule is reduced, another is oxidized.
This electron transport system ultimately stores light energy two different ways. Electrons are taken from P680 or P700 molecules by strong electron acceptors (referred to either as Z or as FRS – ferredoxin-reducing substance). Z passes electrons to ferredoxin, another iron-containing electron acceptor. And when the electrons reach the outer surface of the thylakoid membrane, the stroma, they are passed to the electron acceptor, a hydrogen-carrying coenzyme NADP (nicotinamide adenine dinucleotide phosphate). (NADP is also known as TPN, triphosphopyridine nucleotide.) The NADP retains a pair of energized electrons, and, in this state, an NADP pulls two hydrogen protons, H+, from water to form NADPH2.
Instead of using the notation NADPH2, texts often use NADPH + H+, which indicates that in addition to the NADPH, a hydrogen ion was also added. Either form designates reduced NADP (when the hydrogens are added on, so too are electrons, maintaining a neutral charge).
The source of electrons constantly moving through the system is the accumulation of hydrogen ions in the interior space of the thylakoid membranes, known as the hydrogen ion reservoir. Some of these hydrogen ions are produced by splitting water molecules, and this is where the waste product, oxygen, is produced.
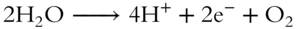
The NADPH2 is valuable because of its great reducing potential. When it reduces another molecule by donating its electrons (at the same time it gives up its hydrogens), the amount of energy it releases is about 50 kcal of energy per mole. (A kcal is a kilocalorie, which is 1,000 calories; a mole is a unit of measurement that always contains the same number of elementary units. That number, by definition, is 6.02 × 1023, which is called Avogadro's Number.)
In photosystem II, the photosynthetic pigments trap the energized electrons that get passed to the electron acceptor Q, which passes them through a chain of acceptor molecules. While the electrons move along this chain, some energy is released that is used to synthesize ATP from adenosine diphosphate (ADP). ATP is a high-energy compound that provides energy for most of the work done by the cell. ATP is a nucleotide, a 5-carbon sugar molecule with a phosphate group and a purine attached; the purine can be either adenosine or guanylate. Nucleotides are the building blocks that make up nucleic acids. Two prominent nucleic acids are DNA and RNA (ribonucleic acid).
The energy supply required for making the ATP molecules that do the cell's work ultimately comes from the sun, via photosynthesis. Respiration releases the energy from the food molecules manufactured during photosynthesis that store the energy from the sun.
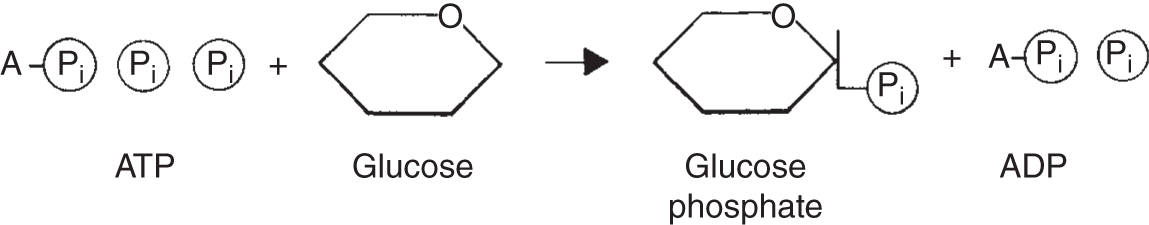
Figure 6.6 The transfer of the ATP molecule's terminal high-energy phosphate to a glucose molecule. This energizes the glucose and converts it to glucose phosphate, which is important in additional chemical reactions.
To synthesize ATP, ADP must be joined to an inorganic phosphate group P. In reverse, ATP is converted to ADP plus an inorganic phosphate group and energy. This process is illustrated in Figure 6.6.
The adenine and the ribose sugar complex compose the adenosine unit. Attached are three phosphate groups; the last two are connected by high-energy bonds. When broken, the resulting products are ADP and a phosphate group, as well as the released energy. Each may be summarized by the following shorthand.
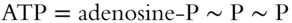
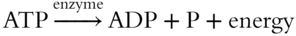
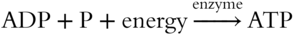
The sequence of events following the movement of electrons through the photosynthetic system may be summarized as follows.
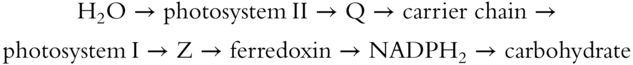
NONCYCLIC PHOTOPHOSPHORYLATION
Noncyclic photophosphorylation is the process of synthesizing NADPH2, in which the high-energy chlorophyll molecule initially donates electrons and then accepts them when in a low-energy state. This photophosphorylation is called noncyclic because the same electrons are not continually passed around the system; rather, an outside source is required. The following equation summarizes this chemical reaction:
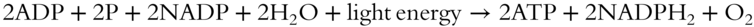
CYCLIC PHOTOPHOSPHORYLATION
In the photosynthetic phosphorylation process, the light energy-driven addition of phosphate groups described above, part of the overall set of reactions, is known as cyclic photophosphorylation. Chlorophyll acts as both an electron donor and acceptor. The electrons are passed from molecule to molecule in a chain of reactions. Each step of the way, the electron loses some of its energy. Finally, when the electrons return to the chlorophyll molecules, they have lost their extra energy.
The light strikes the chlorophyll molecules in photosystem I, causing the electrons to become excited. In turn, the electrons then pass along to the electron acceptor molecule Z, which passes them to other acceptor molecules. Each step follows a downward energy gradient. The released energy fuels the synthesis of ATP from ADP and inorganic phosphate. Eventually, the electrons are returned to the chlorophyll molecules where they started. The term “cyclic” photophosphorylation refers to this cycling of electrons.
CARBON FIXATION AND CARBOHYDRATE SYNTHESIS
That light energy is captured and used to make high-energy molecules of ATP and NADPH2 has been explained above. These energy-rich compounds help synthesize carbohydrates (carbohydrate synthesis), which are the major end products of photosynthesis. The photosynthetic processes in which ATP and NADPH2 are synthesized occur in the presence of light.
Then, with these energy-rich compounds, the synthesis of carbohydrates is carried out in either light or darkness. Cells furnished with the proper biochemical apparatus can synthesize carbohydrates at any time, just as long as they are healthy cells, functioning within the proper temperature range, and are furnished with CO2, ATP, and NADPH2.
Both ATP and NADPH2 fuel the synthesis of carbohydrates. CO2 is pushed up an energy gradient and converted into a series of intermediate compounds until a 3-carbon sugar, PGAL(phosphoglyceraldehyde) or glyceraldehyde 3-phosphate, is formed. See Figure 6.7, which illustrates how these processes work together to synthesize this carbohydrate. Some of the PGAL is used to make ribulose (ribulose biphosphate), the 5-carbon sugar that combines with CO2, making a 6-carbon sugar that is promptly broken into two 3-carbon sugars, or PGA(phosphoglyceric acid; also called 3PG and 3-phosphoglyceric acid), which is converted into energy-rich PGAL.
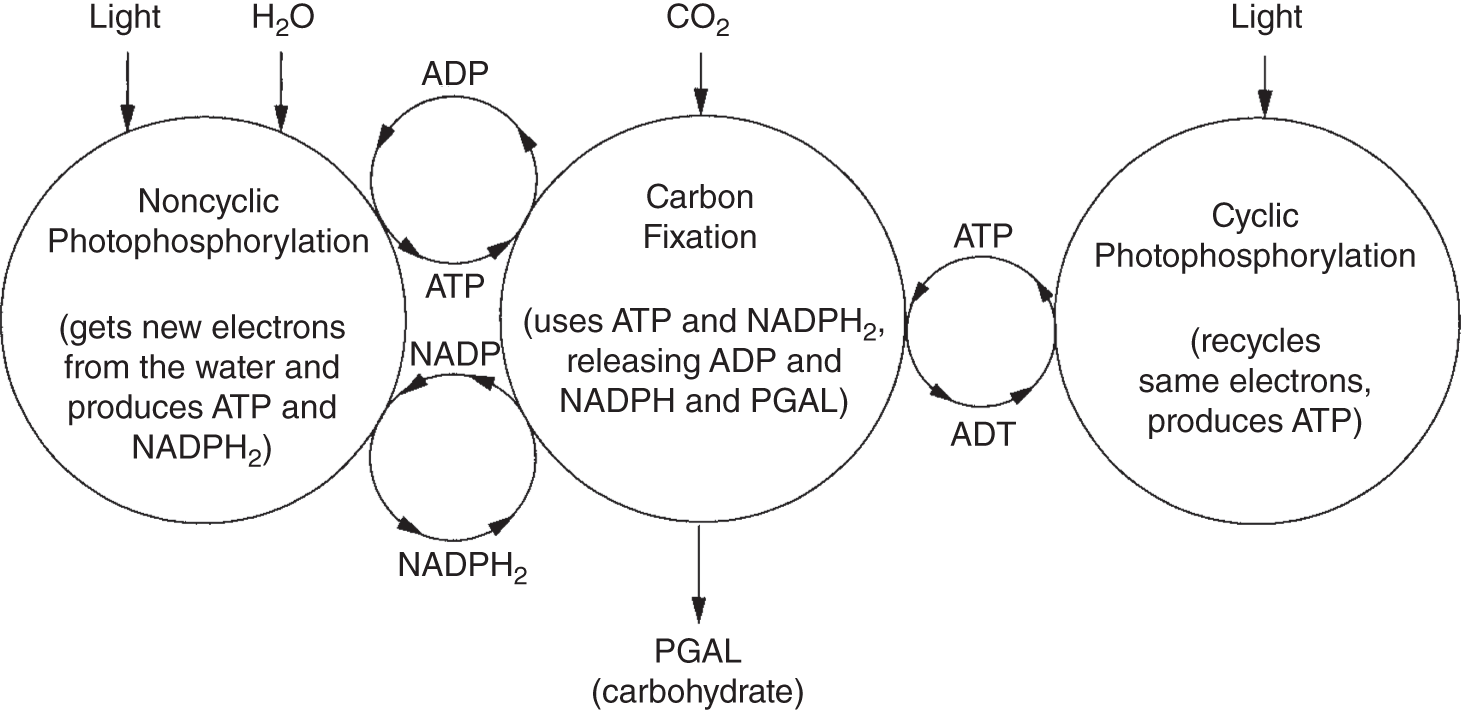
Figure 6.7 Carbohydrate synthesis. Carbohydrates are made from water and CO2 via the carbon-fixation cycle. Through this cycle, three molecules of CO2 produce one molecule of glyceraldehyde 3-phosphate, and this process consumes nine molecules of ATP and six molecules of NADPH.
Much of the PGAL goes back into more ribulose to combine with the CO2 molecules. Some PGAL goes straight into the metabolism of the cell, and some, through a series of steps, is combined and rearranged to form the 6-carbon sugar, glucose, which most people recognize as the final product of photosynthesis.
The glucose is then available to be broken down for its energy, which is released and used in the cell's metabolic processes. Other glucose molecules can be used to synthesize additional types of molecules such as fats, or they can be strung together to make more complex carbohydrates, such as sucrose, starch, or cellulose. Sucrose, a water-soluble disaccharide, is the sugar transported in solution through the vascular tissue of plants. Starch is the insoluble carbohydrate that is commonly stored in parts of plants such as the roots.
LEAVES
Most of a plant's surface area contributing to the photosynthetic process occurs in the leaves. Hundreds of millions of years of natural selection have shaped leaves into efficient structures that maximize their exposure to light, control their gas exchange, minimize water loss, and help move water, minerals, and carbohydrates up from the roots and to other parts of the plant.
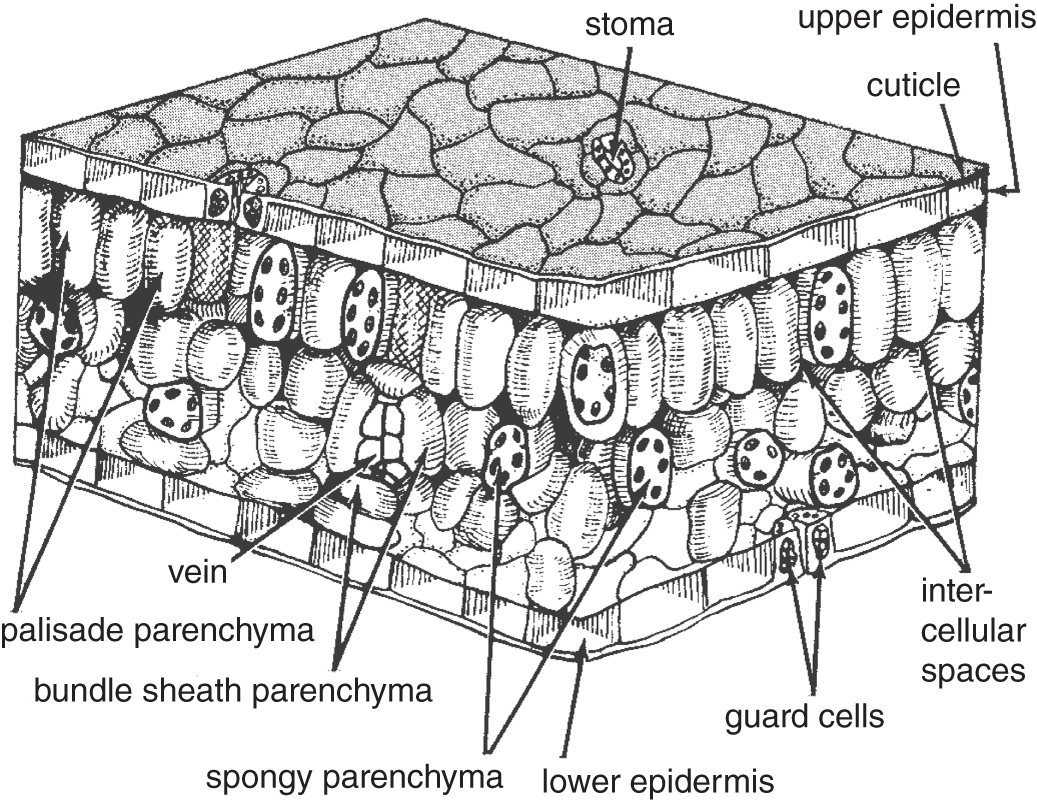
Figure 6.8 Three-dimensional diagram of a leaf section, illustrating external and internal structures.
About 10% of all the photosynthesis that occurs is the product of higher plants, those that we regularly see around us, and the other 90% is the product of algae, most of which occur in the ocean. However, it is the leaves of terrestrial plants that are presented in transverse section in most biology courses and texts, and Figure 6.8 carries on the tradition. Other related structures are described in Chapter 20.
KEY TERMS
| absorption spectrum | cyclic photophosphorylation |
| anthocyanins | electron acceptor Q |
| ATP | Engelmann, T. W. |
| autotrophs | free radicals |
| carbohydrate synthesis | glucose |
| carbon fixation | grana |
| carotenoids | heterotrophs |
| chlorophyll | Ingen-Housz, Jan |
| chloroplasts | lamellae |
| NADP | photosystem II |
| noncyclic photophosphorylation | photosystems |
| oxidation | phycobilins |
| P680 | Priestly, Joseph |
| P700 | redox reactions |
| photosynthetic pigment | reduction |
| phosphoglyceraldehyde (PGAL) | ribulose |
| phosphoglyceric acid (PGA) | Saussure, Nicolas-Théodore de |
| photosynthetic autotrophs | Senebier, Jean |
| photosynthetic phosphorylation | stroma |
| photosynthetic pigments | thylakoids |
| photosynthesis | xanthophylls |
| photosystem I |
SELF-TEST
Multiple-Choice Questions
History, Chloroplasts, Pigments, Autotrophs, and Nutrients
- The process fueled by the sun, where carbon dioxide and water are transformed into carbohydrates, is known as __________.
- respiration
- oxidation
- reduction
- carbosynthesis
- photosynthesis
- The following investigator demonstrated in 1772 that a plant alone in an airtight jar will die, and an animal alone in an airtight jar will die. However, when a plant and an animal are both placed together in an airtight jar, both survive.
- Hooke
- Schwann
- Schleiden
- Priestly
- Senebier
- In 1779, the following researcher showed that sunlight was necessary for plants to produce oxygen:
- Hooke
- Schwann
- Priestly
- Senebier
- Ingen-Housz
- In 1782, the part-time scientist __________ showed that plants use carbon dioxide when they produce oxygen.
- Schleiden
- Priestly
- Senebier
- Ingen-Housz
- Schwann
- In 1804, __________ found that water is necessary for the photosynthetic production of organic materials.
- Priestly
- Senebier
- Ingen-Housz
- Engelmann
- De Saussure
- In 1883, the following researcher conducted an experiment that provided circumstantial evidence indicating chlorophyll, the green pigment in plants, might be important to photosynthesis:
- Priestly
- Senebier
- Ingen-Housz
- Engelmann
- De Saussure
- The following are photosynthetic pigments:
- chlorophyll a and b
- xanthophylls
- anthocyanins
- carotenoids
- all of the above
- Based on their mode of nutrition, the following category of organisms subsists on the inorganic environment, taking in small molecules that do not have to be digested, from which they manufacture organic compounds:
- inorganotrophs
- organotrophs
- homotrophs
- heterotrophs
- autotrophs
- The relatively large organelles in plant cells where nutrient storage and/or photosynthesis occur are known as __________.
- thylakoids
- lamellae
- chlorophyll
- plastids
- none of the above
- The plastids containing chlorophyll are called __________.
- thylakoids
- lamellae
- chloroplasts
- all of the above
- none of the above
- The thin, flattened sacs inside the chloroplasts are called __________.
- thylakoids
- lamellae
- stroma
- a and b
- a and c
- The stack-like groupings of the photosynthetic membranes located inside the chloroplasts are known as __________.
- grana
- stroma
- plastids
- chloroplasts
- all of the above
- Surrounding the thylakoids is a protein-rich solution, the __________, which contains enzymes involved in moving carbon dioxide molecules into carbohydrate molecules.
- grana
- stroma
- plastids
- chloroplasts
- none of the above
Chlorophyll, Light Absorption
- The green pigments necessary for photosynthesis are known as __________.
- xanthophylls
- carotenoids
- chlorophylls
- anthocyanins
- all of the above
- There are several different types of chlorophyll molecules; each is quite __________.
- similar
- different
- red
- blue
- orange
- Chlorophyll molecules have two distinct parts. There is a long nonpolar end that is soluble and is anchored within the lipids composing the photosynthetic membranes. The other end of the chlorophyll molecules contains a complex ring structure with a __________ ion at the center, which is the active site where the light energy is trapped.
- iron
- cadmium
- calcium
- manganese
- magnesium
- __________ refer(s) to the different wavelengths of electromagnetic radiation that are absorbed by the specific pigment in question.
- x-rays
- gamma rays
- ionizing radiation
- prismatic spectrum
- absorption spectra
- The __________ are an important group of accessory pigments found in all green plants. There is evidence that they absorb energy, which is then passed to the chlorophyll molecules that are used in photosynthesis.
- free radicals
- phycobilins
- nucleotides
- carotenoids
- adenoids
- Chlorophyll may be broken down in the autumn before the onset of winter. At this time, some plants digest their chlorophyll to save the __________ and __________ atoms, which are then transported and stored in their roots.
- calcium, iron
- hydrogen, oxygen
- nitrogen, magnesium
- chlorine, potassium
- carbon, iodine
- In addition to the photosynthetic pigments found in most advanced plants, red algae and blue-green bacteria contain __________, which absorb light energy from wavelengths outside the absorption spectra of chlorophyll a and then transfer this energy to chlorophyll a to be used in photosynthesis.
- carotenoids
- xanthophylls
- anthocyanins
- phycobilins
- none of the above
Light Absorptions, Passing the Electrons
- When light is absorbed by the photosynthetic pigments that are located within the __________, the energy in these excited molecules is passed on to chlorophyll a molecules.
- nucleus
- endoplasmic reticulum
- thylakoid membranes
- adenoids
- none of the above
- The energy supply required for making the ATP molecules, which do the cell's work, either comes from photosynthesis, which captures and stores the sun's energy in food molecules, or it comes from __________, the process that breaks down food molecules, releasing their energy.
- respiration
- transpiration
- ingestion
- refraction
- inspiration
- Cyclic photophosphorylation and noncyclic photophosphorylation may occur in __________.
- the dark
- the light
- roots
- all of the above
- none of the above
- ATP and NADPH2 are synthesized in the __________.
- dark
- light
- roots
- all of the above
- none of the above
- With ATP and NADPH2, the synthesis of carbohydrates can be carried out. Unlike photophosphorylation, carbohydrate synthesis can occur in the __________.
- dark
- light
- bark
- all of the above
- none of the above
ANSWERS
- e
- d
- e
- c
- e
- d
- e
- e
- d
- c
- d
- a
- b
- c
- a
- e
- e
- d
- c
- d
- c
- a
- b
- b
- a
Questions to Think About
- Give a brief history of how the basic fundamentals of photosynthesis were first discovered.
- What are the different photosynthetic pigments, and why are there different ones?
- Describe the differences between autotrophs and heterotrophs.
- List some of the most important nutrients to a plant, and tell where they are most likely to come from.
- How does chlorophyll work?
- Where is chlorophyll usually located, and why?
- What is noncyclic photophosphorylation?
- What is cyclic photophosphorylation?
- How do plants synthesize carbohydrates?