11
SEASONINGS: SALT, SPICES, HERBS, AND HOT PEPPERS
Guided Inquiry Activities (Web): 13, Flavor; 14, Cells and Metabolism; 19, Plants; 20, Plants and Color
11.1 INTRODUCTION
We will learn about the molecular structure and behavior of simple inorganic salts, organic compounds that make up spices and herbs, and the more complex biomolecules that are found in hot peppers. We will discuss and investigate how these compounds impact food and how they are best prepared and used and look closely at the history and use of hot peppers.
As you sprinkle salt on your popcorn, have you ever thought about the historical impact of those tiny grains on humans and our livelihood? Humankind’s historic search for salt, spices, and herbs has caused wars, sent peoples to discover new lands, and encouraged the development of new methods to access and acquire particular spices. Seasonings not only enhance the flavor of food, but they have helped to define some cultures and significantly changed the ways in which food can be prepared and stored safely. We all know that the addition of a small amount of a compound or mixture can enhance the flavor of food.
Humankind’s historic search for salt, spices, and herbs has caused wars, sent peoples to discover new routes to access the seasonings, and changed health and food. There are many historical tales of seasonings being used to mask the flavor and odor of tainted foods. In 1939, biochemist J.C. Drummond in The Englishman’s Food: Five Centuries of English Diet tells of the medieval recipes used to mask the flavor and odor of rotten meat [1]. In Food and Cooking in Roman Britain, Marian Woodman describes the difficulty in storing food and use of seasoning to mask the lack of freshness of food [2]. However, food historians will argue these tales as myth and point out that those who could afford spices and herbs were those who were least likely to eat spoiled meat and food. In fact, some seasonings are now known to reduce the incidence of spoilage and contamination, rather being used to “cover” it up. Salts are dry and inhibit bacterial growth in cured meat and some spices and herbs even behave as antibiotics. Thus, it is entirely plausible that people who found certain flavors attractive were more likely to use them in cooking and were less likely to become sick from microbial pathogens. These people would teach the use of spice and pass on the genes for the seasoning taste receptors along with a heightened desire for spiced food. Hot peppers have grown in popularity to rival most herbs and spices, have a very interesting science, and have earned their own place in books on cooking. This may have been particularly important in hot and humid climates where refrigeration was at a premium. Salt, spices, and herbs all play an important role in health and the taste of our food. Let’s start our discussion with the most simple, most utilized, and perhaps most important seasoning of all, salt.
11.2 SALT: FLAVOR ENHANCER AND A DRIVING FORCE OF HISTORY
Salt, whether mined from the ground or dried from the sea, is a critical component of human health and has been integral in shaping much of the world’s history. Many modern roads were initially paths created by animals to salt licks. The discovery and harvest of salt (and spices) created trade routes, resulting in global power shifts and colonization around the world. Romans used salt as part of a soldier’s pay; salt is the root of the term “salary” [3]. In order to understand how salt is indispensable in cooking, baking, and human health, you have to understand its chemistry and molecular structure.
11.2.1 Chemistry of Water and Salt
The chemical definition of a salt is an ionic compound formed by a reaction of an acid and a base. However, commonly, salts are compounds that are composed of cations (positively charged ions) and anions (negatively charged ions) whose charges balance one another out. Common examples of monovalent (singly charged) salts include table salt, sodium chloride (consisting of an equal number of Na+ and Cl− ions), and potassium chloride (K+ and Cl− ions). An example of a divalent salt is magnesium chloride, which consists of one Mg2+ and two Cl− ions. In Chapter 1, we discussed polyatomic ions that can make up a salt, such as that found in sodium sulfate, which consists of two Na+ and one SO42− ion). In the solid state, a salt forms into a well-organized, three-dimensional network called an ionic or crystal lattice, where each ion is surrounded by ions of opposite charge (Fig. 11.1). This ionic attraction holds the cations and anions in place, allowing for the formation of large crystals due to the presence of a repeating geometric pattern.
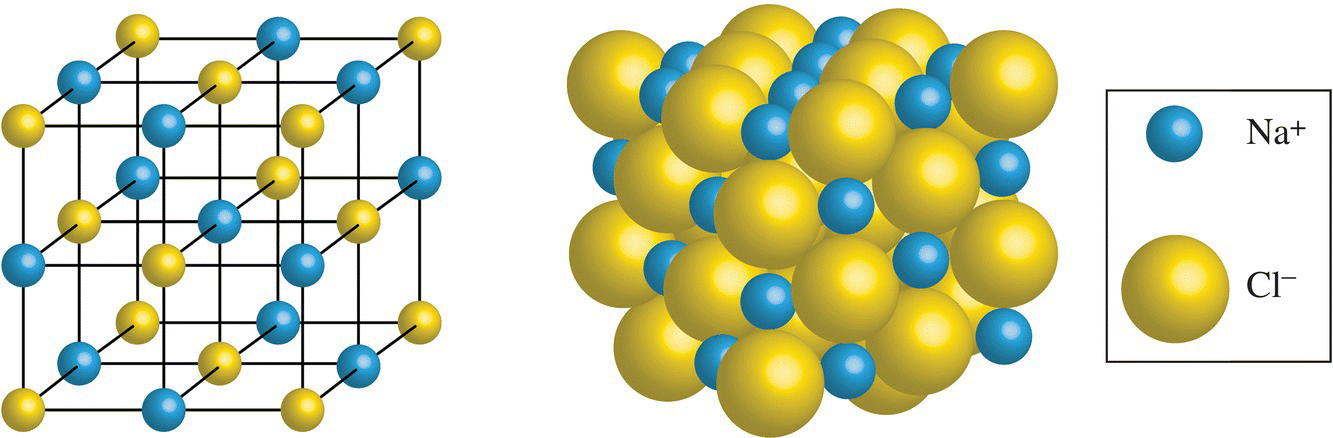
Figure 11.1 Ionic lattice of sodium chloride.
When you dissolve table salt or some other salts in water, the ions dissociate from one another (Fig. 11.2). This means that the cationic and anionic components separate from one another. Why? Remember that water molecules are polar, where the oxygen atom has a partial negative charge due to its attraction or affinity for electrons, while the hydrogen atoms have a partial positive charge. In solution, the water molecule surrounds the salt ions due to its large dielectric constant.
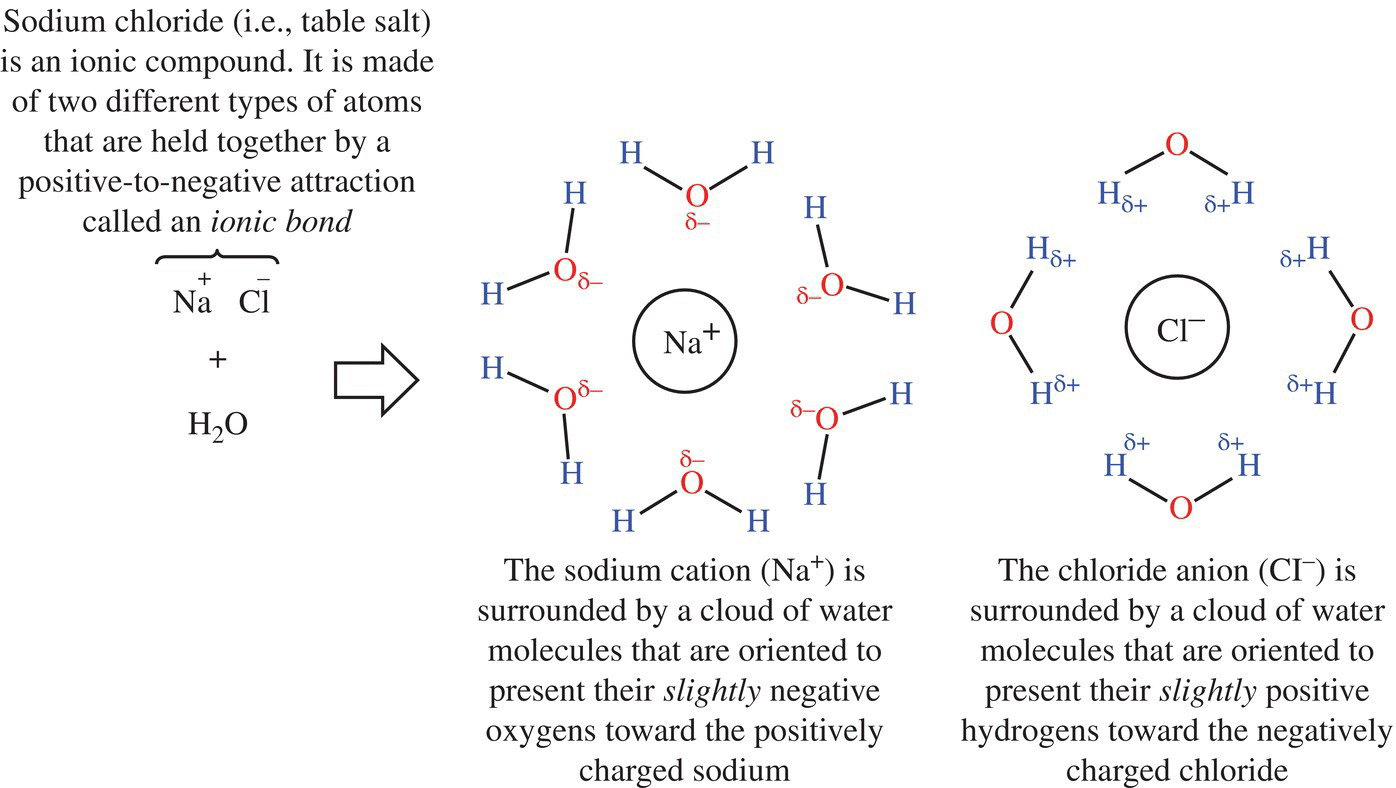
Figure 11.2 Salt dissolves in water. The polar nature of water helps to disrupt the attractive force between ions in a salt crystal.
What in the world is a dielectric constant? A dielectric constant tells you how likely two ions will come apart in a particular solvent. Water has a large dielectric constant because water counters the attraction between the cation and anion of the salt, since water is polar. Basically, water reduces the force (defined by Coulomb’s law; Fig. 11.3) that holds the ions together because it is attracted to and surrounds the cationic and anionic components of the salt. In a salt solution, shells or cages of water surround salts, with the negative or positive pole of water combining to neutralize the ionic compound. This arrangement shields the attraction of positive and negative ions (e.g., Na+ and Cl−) from each other, keeping the ions in solution and the salt dissolved.
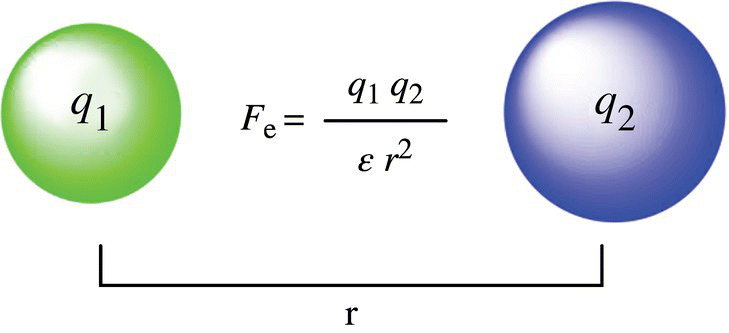
Figure 11.3 Coulomb’s law. The magnitude of force between charged particles is described by Coulomb’s law. The electrostatic force, Fe, is equal to the constant times the two charges all divided by the square of the distance times ε. ε is the dielectric constant and for water it is 80.4 and for benzene is 2.2.
When water is lost due to evaporation, the ions lose some interactions with water molecules, become concentrated, and begin to form into ion clusters (consisting of interacting cations and anions). With increased loss of water, these ion clusters get large enough to form into crystals and precipitate out of the aqueous solution. You may be thinking, do different salts have different precipitation properties? Yes, every salt has a different solubility in water and thus precipitates more or less readily at different water concentrations. In water above 104°F/40°C, KCl is more soluble than NaCl. Thus, by carefully heating a solution (like seawater) that contains both KCl and NaCl, you can remove NaCl from the mixture by precipitation while leaving the bulk of the KCl still dissolved in water. Solubility properties of salts are quite important in the kitchen, so we will come back to this later in the chapter.
11.2.2 Sodium and Health
You have probably seen a “reduced sodium” can of soup in the grocery store or been told to watch your salt intake by your doctor. What do sodium and salt have to do with human health? Everything! Salt is essential for human life. Sodium is found in the blood, in the lymphatic system, and in and around cells throughout the body. Nerve cells use the concentration/charge gradient of sodium and potassium ions to fire signals throughout the nervous system. Sodium and potassium ions are essential in the formation of the high-energy molecule ATP from ADP. A 180 lb adult has nearly 0.2 pounds of sodium; on average, we have 0.1–0.2% sodium in our body. The recommended daily allowance of sodium for adults is 2000–2300 mg, about one half teaspoon of table salt each day, most of which comes from our food and drink. Although this seems like a lot of salt, most of the salt that we ingest does not come from the salt shaker, but from highly processed, canned, and frozen foods or salty snack foods. Diets high in sodium significantly contribute to high blood pressure, heart disease, and stroke. Combined, these diseases kill more people in the United States than all cancers combined. A recent study of over 3000 participants [4] found that a moderate four weeklong decrease in sodium intake from 9–12 to 5–6 g/day decreased blood pressure in those with and without high blood pressure problems. The decrease in sodium intake and blood pressure was accompanied by an increase in the kidney enzyme, renin. While long-term increased levels of renin have negative potential impact on several health issues including diabetes and vascular and renal disease, the benefit of sodium reduction is thought to outweigh such negative impact. A recent study of over 3000 people found that moderate levels of sodium intake did not translate into a greater risk of hypertension or coronary disease, while in this same 2011 study, lower sodium intake was associated with higher heart disease mortality [5].
11.2.3 Use of Salt in Cooking
The culinary use of salt is vital. Salt improves the way we see, taste, and smell food. Salt in food helps to keep proteins from aggregating into a clotted mess. Sodium chloride can stabilize oil-in-water emulsions, reducing the separation of oil and water. We have already discussed the preservative role that salt plays in food. Although excessive salt intake is detrimental to human health, cooks cannot ignore the benefit and role of salt in cooking.
Over 5000 years ago, humans began to use salt to enhance the flavor of and preserve food. How do we taste salt, and how, biologically, does it enhance flavor? Proteins on the surface of taste bud cells transport Na+ into the taste bud, which initiates a signal to the brain that you taste a salty food. However, if you have ever added salt (or forgotten to add the salt) to a cookie recipe, you know that salt does more than just cause a food to taste salty. Low concentrations of salt suppress bitter flavors, thereby allowing other flavors to come through the palate. At high concentrations, salt can increase umami or savory flavors by decreasing sour and sweet flavors. For example, a mixture of table sugar (sweet) and urea (bitter) was found to be equally bitter and sweet by most tasters. However, addition of sodium to the mixture made it seem overwhelmingly sweet. A salad made with a bitter green like spinach or arugula can be made sweeter through the addition of salt. This property of salt may help you to recover and fix a seemingly bitter and ruined meal!
In addition to its contributions to taste, salt impacts the behavior of proteins during cooking and baking. As you know, during cooking, proteins often denature in the presence of acid or heat. The denatured, unraveled protein molecules get tangled up with other protein molecules, forming large protein aggregates that then precipitate. You can recognize this as clots or curds of protein (like you see in curdled or sour milk). How does salt impact protein denaturation? In low salt conditions, salt ions will interact with the protein molecules, preventing their aggregation and forming an insoluble (precipitated) complex. This property comes in handy when you are making a meatloaf or meatballs. Have you ever wondered why you add an egg? The egg works as a binding agent, by helping the meatloaf to hold its shape. This binding property happens to be due to the egg white proteins. What does this have to do with salt? Well, you have to heat up a meatloaf when it cooks to temperatures that are high enough to denature the egg white proteins. However, a meatloaf doesn’t have an eggy-looking exterior or interior like a fried egg. The addition of salt to the egg white proteins helps the proteins to remain in the solution (even in acidic or heated conditions) and not aggregate, thus enhancing their ability to bind the food together.
What about baking? Why does homemade bread that doesn’t contain salt have a horrible taste? In bread, salt plays a different role with proteins. If you recall from Chapter 10, gluten is a complex network of proteins that is responsible for creating a stretchy bread dough due to the formation of cross-links with other wheat proteins. These cross-links govern the texture of the final bread product and cannot form until water hydrates wheat flour. The proteins that make up gluten have many positively charged amino acids; in solution these charges cause the protein molecules to repel each other. The negatively charged chloride ion, provided by table salt, binds to the positive charges in the proteins, allowing them to come close together and to form the cross-links and connections that strengthen the dough. However, the same interaction (between protein molecules and sodium chloride ions) slows down the hydration of gluten proteins because the interaction of proteins with ions reduces interactions between protein molecules and water (since the protein charges are now neutralized by the salt). Bakers will sometimes begin to mix their dough without salt to reduce this effect and then add the salt following hydration of the flour.
Emulsifiers help two solutions that would normally remain separated (like oil and water) and will help maintain two immiscible solutions as one homogeneous mixture. Table salt can be used as an emulsifier; however, other more complex salt compounds such as sodium citrate, phosphates, and tartrate are more commonly used to emulsify foods such as cheese and dairy products. In some foods, table salt will enhance the water binding to proteins, forming the gel and keeping phases from separating.
Do you add salt to your pasta water? The old tale of adding salt to increase the temperature of or make the water boil faster actually has a bit of scientific truth to it; however, in reality, it is not very accurate. It is true that adding salt or other compounds to water will elevate the boiling point of the solution. The boiling or melting point of a solution is called a colligative property. Any substance, whether salt, sugar, or cinnamon, dissolved in water will alter both the boiling and melting point of the solution. The more substance dissolved, the more the melting or boiling point will change. How much is the change and does it matter?
The equation for boiling point elevation is shown below, where ΔTb is the change in boiling point, i is the van’t Hoff Factor, Kb is a constant for the solvent, and m is the molarity or concentration of the solution
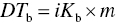
The van’t Hoff Factor, i, is equivalent to “2” for table salt since it dissociates into two ions in water (Na+ and Cl−). Thus, for a water and salt solution, the only factor that will impact the change in boiling point is the mass of table salt dissolved in the water (m). The more the salt, the greater the change in boiling point. Because m is a positive value, ΔTb will be positive and the boiling point will increase. What does this mean for the cook? When you add salt, more heat is required to bring the mixture to a boil than pure water. It will also take longer to boil the same volume of pure water as salt water. However, the boiling saltwater solution will have a higher temperature; therefore, food cooked in salted, boiling water could cook faster than food cooked in unsalted water.
Unfortunately, validation of the old tale ends here. Let’s imagine that you add 533 g (about 21/4 cup) of salt to one gallon of water. The boiling point will increase by a whopping 2.5°C. In other words, the teaspoon of salt that you may add to your water does not change the boiling point by a significant amount. At this point the salt will have a greater impact on the taste of the food than the few degrees of water temperature.
However, the addition of a pinch or two of salt to water is important in cooking! Salt increases the volatility of some compounds, making it easier for these compounds to escape the boiling water and enhance the flavor and aroma of the cooked food. Salt water saturates the starches in boiling pasta, thus enhancing the flavor of the pasta. So, keep adding salt to your boiling water, and it does make a difference in the flavor of the final product.
11.2.4 Too Many Kinds of Salts
A trip to the gourmet grocery store or a close inspection of various recipes may lead you to think there are different kinds of salt: smoked salt, sea salt, table salt, flake salt, kosher salt, etc.—the list is long and confusing. However, in chemistry, salt is any salt, consisting of anions and cations. KCl, NaCl, and magnesium sulfate are all salts. However, in cooking, salt takes on a slightly more focused definition. The culinary definition of salt is sodium chloride, table salt. The various salts that are described in recipes or are present on the grocery store shelf are all sodium chloride; the difference is in the preparation and presence of additional components (contaminants).
How is salt made? Around the world, salt is primarily produced by two techniques: mining or evaporation of seawater. Most table salt comes from rock salt that is mined from the earth. Large, ancient, underground salt formations can be mechanically removed with mining equipment and explosives, ground into small pieces, and dissolved in water. Alternatively, water can be pumped through mines, which dissolves the salt, making a saturated brine solution that is pumped from the caverns. The salt solution is evaporated to concentrate the salt, and the salt crystallizes and precipitates out of the solution. In windy and warm conditions, the brine is left to concentrate in open pans. Modern evaporation techniques use vacuum chambers to reduce the water content. The brines contain a number of contaminating minerals and other compounds; these contaminants are removed by selective precipitation of the contaminants using a variety of reagents.
Seawater, which contains approximately 3.5% NaCl, can provide a nearly inexhaustible source of salt. The simplest method to harvest salt from seawater is to pump the seawater into smaller drying ponds where the water evaporates in the wind and sun. The evaporated seawater is rinsed with a pure, saturated saltwater solution to rinse off impurities, without dissolving the salt crystals. This is a simple process that can easily be re-created if you live near a saltwater body. In short, filter a clean, nonpolluted gallon of seawater (to remove rocks and debris), place it in a shallow pan, and evaporate the water using the heat of an oven or the sun. A gallon of seawater will produce a little less than a cup of salt. If you heat the final product in an oven at a high temperature, you can evaporate the last drops of water and kill any remaining microorganisms. Is there any difference in the sun evaporation versus the oven evaporation methods? Maybe. Rapid evaporation and the addition of small salt crystals (to initiate the crystallization process) will yield the small granular crystals that we associate with table salt. Slow evaporation allows the salt crystals to grow larger and become irregular in size. These salt flakes are sought after due to their sharp edges, which allows the salt to stick to cooking surfaces, producing a crunchy, salty finish to foods. Crystal size and shape are two ways of distinguishing different types of salts. Let’s look at some other differences that lead to the different types of salts that you may see on the grocery store shelf.
11.2.4.1 Table Salt
Table salt, consisting of small, regularly shaped grains, is slow to dissolve because of the shape and purity of each grain or crystal. Iodine is often added to table salt (you may have seen “iodized” and “uniodized” versions) to reduce the incidence of goiter, a problematic thyroid disease associated with problems in mental development, which, prior to iodized salt, plagued the United States and other countries. Glucose is used in very small amounts to stabilize the iodine for long-term storage. To keep the salt free flowing, additional additives are used at low concentrations, such as other sodium, calcium, aluminum, phosphate, and silicon salts. These salts help to absorb any water that may be present in the salt or in the air, thus preventing clumping and caking.
11.2.4.2 Kosher Salt
According to Jewish dietary law, meat must be treated with salt to draw out the blood. Given that salt already adheres to the Jewish dietary law, there are no additional dietary or production requirements for Kosher salt. However, most producers either do not include any additives (iodine or other) or just add anticaking agents. Kosher salt is typically produced by slow evaporation techniques, which produce large, thin sharp-edged salt flakes. The thinner and jagged-edged flakes quickly dissolve and more readily stick to foods than the table salt granules; thus cooks sometimes prefer this form of salt.
11.2.4.3 Canning or Pickling Salt
Canning or pickling salt is produced in the same manner as table salt; however it doesn’t contain any iodine or anticaking or anticlumping agents; these additives are often insoluble at the high salt concentrations required for the canning processes. Both canning and pickling salts are produced and milled to form a small fine-grain crystal that more quickly dissolves than the larger cubed table or rock salt.
11.2.4.4 Rock Salt
Rock salt is the product of raw, undissolved, crushed, or large crystal sodium chloride formation. Because it doesn’t go through a purification process, it contains contaminants of minerals and other compounds. Consequently (as you might predict), rock salt is a less expensive salt that is usually not used for food and cooking, except when making homemade ice cream using a maker that requires use of a salt/ice mixture to freeze the delightfully creamy and rich dessert. Rock salt is not used in the salt grinder that you might see on your dinner table; salt grinders contain large crystals of purified salt.
11.2.4.5 Gourmet Salt
The diversity and custom flavors found in gourmet salts have caught on with chefs and at-home cooks alike (Fig. 11.4). Gourmet salts are flake salts formed from local water sources, which often contain regional or added contaminants (organisms, other salts and minerals) that give the salt a unique color or taste (organisms, other salts and minerals trapped in the salt flakes). Some gourmet salts are made with added flavorings. The most famous and perhaps interesting artisan salt is made by an ancient French technique. Traditional fleur de sel (flower of salt) is made from salt beds in west-central France. Minimally disturbed, the salt flakes grow by dehydration on the surface of the pond and are collected by workers who scrape this layer of salt before the crystals sink to the bottom of the container or ponds. Unrefined and often not washed, these salt flakes contain a range of minerals and even small amounts of algae, all of which contribute to the flavor. Is fleur de sel safe to eat? Yes, this salt is safe for consumption, but given the high cost (approximately $30/lb), it is most frequently used as a garnish or is sprinkled to finish a dish.

Figure 11.4 Gourmet salt. Three types of salt with sea salt on the right.
Himalayan pink salt is crystallized rock salt that comes from the Pakistan Himalayan mountains, where a high mineral content leads to its characteristic color. The lava or coral particles added to make Alaea Hawaiian sea salt gives the salt its red or pink-brown color. You can buy or make numerous other flavored salts, which provide unique and interesting flavors. Common flavored salts include garlic, celery seed, and lemon, but if you check out the spice section at your local grocery store, you will find numerous others that will add character to a dish.
11.2.4.6 Sea Salt
Like gourmet salt, sea salt is produced from solar heating or thermal evaporation of seawater. Since sea salt is less purified and refined than table salt, it is off-colored (sometimes gray) with large pyramid-shaped crystals with sharp edges. Because of the size and shape of the flakes and its expense, sea salt is best used to stick to the surface of a prepared food; its delicate structure will dissolve quickly in the mouth, providing a crunchy, salty sensation. However, there is little evidence to support that sea salt tastes differently or is healthier than other salts. Why might there be a health benefit? The idea is that there is less sodium per tablespoon in sea salt relative to table salt, due to the presence of potassium and calcium salts. However, both table and sea salts contain about 40% sodium by weight. Moreover, the additional minerals found in sea salt are often included in our diet from other sources. The true benefit of sea salt is the quick dissolving, crunchy mouthfeel that is present when the salt is used as a finishing salt for a dish, added immediately prior to serving.
11.3 HERBS AND SPICES
While some argue that salt is a spice, it is not. Salt, herbs, and spices are all seasonings. However, herbs and spices are the products of plants (and you know from our discussion about salt that salt definitely does not come from plants). Table 11.1 shows some common herb–food pairings. Simply defined, herbs are the leaves of a plant, while spices are harvested from the rest of the plant (i.e., the root, stem, bark, seed, or plant fruits). Some plants, like cilantro and dill, produce both spices and herbs, while others like basil produce herbs or spices, respectively. In general, herbs are grown in more temperate climates, while spices grow in warmer or tropical zones. Therefore, NaCl is an inorganic mineral and does not fit into the description of herbs and spices.
Table 11.1 Herb–Food Suggested Parings.
| Food | Suggested Herb or Spice Combination |
| Beef | Bay leaf, marjoram, nutmeg, onion, pepper, sage, thyme |
| Lamb | Curry powder, garlic, rosemary, mint |
| Pork | Garlic, onion, sage, pepper, oregano |
| Chicken | Ginger, marjoram, oregano, paprika, poultry seasoning, rosemary, sage, tarragon, thyme |
| Fish | Curry powder, dill, dry mustard, marjoram, paprika, pepper |
| Carrots | Cinnamon, cloves, dill, ginger, marjoram, nutmeg, rosemary, sage |
| Corn | Cumin, curry powder, onion, paprika, parsley |
| Green beans | Dill, curry powder, marjoram, oregano, tarragon, thyme |
| Potatoes | Dill, garlic, onion, paprika, parsley, sage |
| Squash | Cloves, curry powder, marjoram, nutmeg, rosemary, sage, cinnamon, ginger |
| Rice | Chives, green pepper, onion, paprika, parsley |
Like salt, herbs and spices have influenced mankind in many ways. Historians find links to trade routes, changes in political power, and geopolitical conflict based on access to herbs and spices. The earliest evidence for the use of herbs or spices comes from ancient humans who wrapped their food in leaves; presumably, they found the food to be more flavorful. However, there is also historical evidence for the use of spice and herbs to preserve food, as perfumes, in religious ceremonies, and for medicinal purpose. Because of the myriad of uses, human desire for access and control of herb and spices drove colonization and expanded exploration routes.
The aroma and flavor associated with most herbs and spices are due to what chemists call volatile organic compounds. Volatile organic compounds are primarily made of carbon atoms (organic); they are not very polar or ionic, and they have a low vapor pressure and low water solubility. Simply put, this means that such molecules have few interactive forces with water and have enough energy at relatively low temperatures to escape from a liquid into a gas phase, where the aroma can reach our nose.
Let’s look at two herbs as an example: cilantro (coriander) and parsley. If you look at the two leafy herbs sitting on the refrigerated shelf in the grocery store, they can be easily confused (although a mnemonic might help you remember that the “c”ilantro has curved leaves, while “p”arsley has pointy leaves). However, crushing a leaf of either plant, which allows the volatile chemicals to escape into the air, will immediately tell you which herb you have. Cilantro’s aroma and flavor mostly come from a family of carbon compounds called decanals (Fig. 11.5). Decanals are 10 carbon-containing molecules with an aldehyde functional group on the first carbon. Some of the decanals also have a double bond within the carbon chain. The compounds associated with the aroma of parsley also lead to a complex scent, but the principal compounds found are 1,3,8-p-menthatriene and limonene (Fig. 11.5). 1,3,8-p-Menthatreine provides parsley with its floral scent, while limonene is the same compound found in oranges and lemon adding to the complex aroma bouquet of parsley.
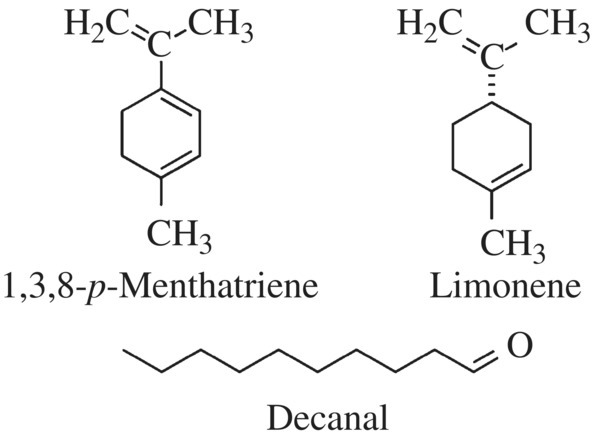
Figure 11.5 Aroma compounds of cilantro and parsley. The two ring compounds 1,3,8-p-menthatriene and limonene are responsible for the smell of parsley, while the long carbon chain decanal gives cilantro its odor.
If you compare all three compounds, you will notice that none of them are charged and all of them are hydrophobic and nonpolar. The structures of the two parsley compounds are very similar, the main difference being the placement of the double bonds in the ring structure, while the shape of decanal is quite distinct. Nevertheless, as you might predict, all of these compounds are poorly soluble in water and have the high vapor pressure that is characteristic of volatile aromatic compounds. The compounds smell differently because you have receptors for taste and smell in your nose that detect the subtle differences with great discrimination and at very low concentration.
The flavorful and aromatic compounds found in herbs and spices are often called essential oils. This is an appropriate term because these numerous organic compounds that are found in the herb or spice more easily dissolve in oil than water. Some of these organic compounds are less volatile than others, providing a longer-lasting flavor and aroma due to the fact that they will remain mostly in the liquid form. What makes a compound more or less volatile? The chemical shape and functional groups contained within the molecule (see Chapter 1). Let’s talk more about chemical shapes and functional groups that are important in herbs and spices here: aldehydes, ketones, alcohols, amines, esters, ethers, terpenes, and thiols.
11.3.1 Terpenes
The most volatile and aromatic molecules found in herbs and spices fall within a family of compounds called the terpenes. Terpenes, found both in plant and animal cells, are comprised of a diverse set of carbon structures that are built from smaller five carbon units called isoprenes or isoprene units (Fig. 11.6).

Figure 11.6 Terpenes of spices and herbs. The terpene is a base unit used by the enzymes in plants to produce an amazingly diverse set of compounds including those shown here.
Terpenes are organized by the number of isoprene units combined to make the compound (Table 11.2). What is the function of terpenes in animal and plant cells? In animal cells, an isoprene unit is the chemical building block for important steroid molecules, including cholesterol, testosterone, estrogen, and steroid hormones (e.g., corticosteroids). In plants, terpenes play a more secondary role but are very common. The blue smoky haze of the Appalachian Mountains forms due to terpene secretion by the pine trees.
Table 11.2 Isoprenes in Herbs and Spices.
| Isoprene Unit | Name | Formula | Use/Example |
| 2 | Monoterpene | C10H16 | Citral, thymol (mandarin orange), menthol, pine, geraniol |
| 3 | Sesquiterpene | C15H24 | Chamomile, cinnamon, clove, ginger |
| 4 | Diterpene | C20H32 | Vitamin A, rosemary |
| 6 | Triterpene | C30H48 | Lanosterol, cholesterol |
Terpenes are common and found in an interesting number of examples beyond cooking. In animal cells, isoprenes are the building blocks for cholesterol, testosterone, estrogen, and sterol hormones including corticosteroids. In plants, terpenes play a secondary role for the plant but are very common. The blue smoky haze of the Appalachian Mountains are formed by terpenes secreted by the pine trees. Monoterpenes also serve as seeds of cloud formation. Terpenes found in herbs and spices are fairly volatile and are the primary component of essential oils. The volatile nature of these compounds is why you immediately smell a strong odor quickly associated with the herb or spice upon heating. Terpenes are also fairly reactive, especially with oxygen; when these reactions occur, the new compound is generated. The combination of terpenes with oxygen is a chemical change that creates a new compound that may not be detected by the receptors in your nose. Thus, the aroma may and is the reason why such scents seem to “disappear” after a while in the air or after aging in oxygen-rich environments. Generally speaking, the larger the terpene compounds, the less volatile the compound is. This is beneficial in cooking because larger, less volatile terpenes will remain in the food during cooking, providing a more enduring taste to your food. Rosemary and ginger both contain less volatile, larger terpene molecules, allowing for their lingering taste and smell in a roasted turkey or batch of gingerbread cookies. Examples of these less volatile, larger compounds include the taste and smell of cooked rosemary and ginger.
11.3.2 Phenols
Thousands of compounds in herbs and spices contain or are derived from a group of molecules called phenols. Phenols are compounds that have a benzene ring (a six-carbon ring system with alternating double bonds) attached to a hydroxyl group (–OH; Fig. 11.7).
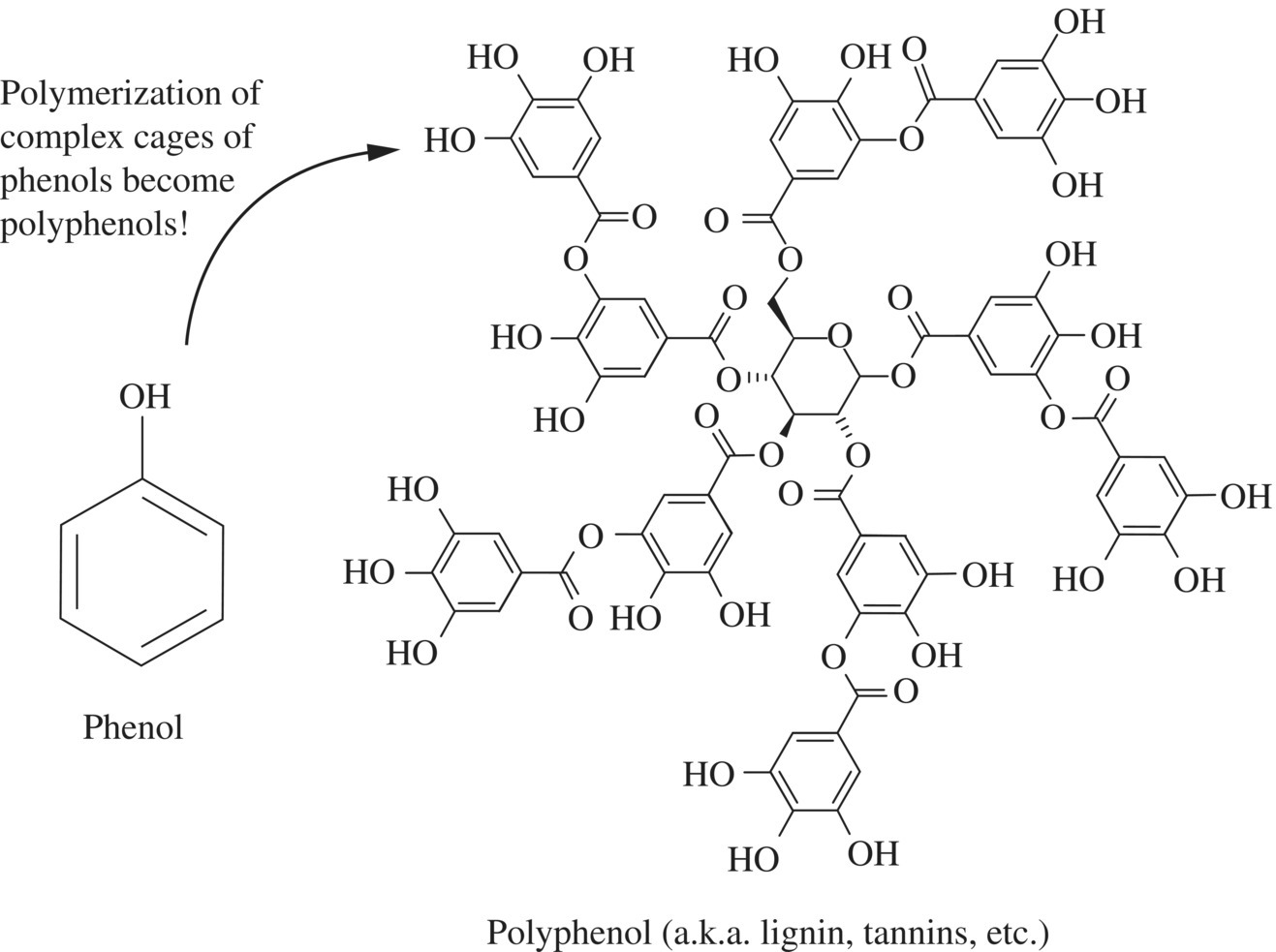
Figure 11.7 The creation of polyphenols. Lignin is shown on the right as an example of a polyphenol.
When several phenol groups are bonded together, a polyphenol is created. Polyphenols have diverse biological and chemical uses; they are used as dyes and in the generation of plastics. In herbs and spices, some polyphenols come from a component of the plant cell wall called lignin, while others are used in defense against herbivores or disease.
One type of polyphenol that is particularly important in food and drink are the tannins (Fig. 11.7). Another complex family of flavor compounds, tannins are polyphenols derived from bark, stems, and woody plant material, which provide a pucker-like feeling called astringency. Several spices contain tannins including tarragon, cumin, vanilla, cinnamon, and cloves.
Oregano, cumin, thyme, bay, and cinnamon are all examples of spices or herbs that contain phenol groups. An extraction and analysis of these five herbs and spices using a sensitive mass spectroscopy analysis found 52 different phenolic compounds [6]. Rosmarinic acid, first found in rosemary plants, is common to all five of these herbs or spices and is found at very high levels in oregano, rosemary, and thyme (Fig. 11.8). Caffeic acid, found in coffee, is another polyphenol compound identified in all five herbs or spices, although it is found in lesser amounts in cinnamon, cumin, and bay (Fig. 11.8). Caffeic acid is a key intermediate in the production of lignin and is found in nearly all plants. A third phenolic compound, chlorogenic acid, is found at relatively similar levels in each of the five herbs or spices (Fig. 11.8). Chlorogenic acid is produced by the modification of caffeic acid and is important in lignin biosynthesis. In addition to its presence as a flavorant and odorant in herbs or spices, it is also found in coffee beans and some fruit.
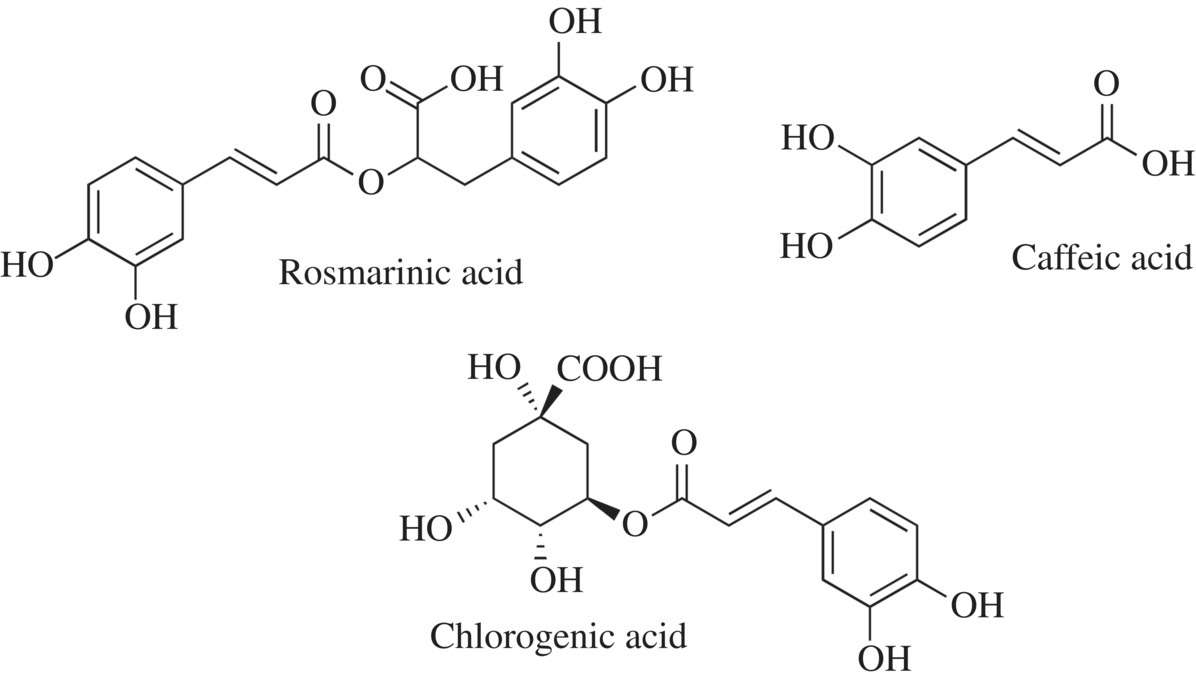
Figure 11.8 Spices as phenols.
11.3.3 Esters
Remember that you have already learned about several chemical functional groups in Chapter 1, including the esters. The presence of functional groups in a compound leads to different chemical characteristics and unique biological activities. Esters are commonly produced from the reaction of carboxylic acids and alcohols (Fig. 11.9). Esters tend to offer a fruity taste and aroma to our food and drink. Pine, cinnamon, and jasmine are a few spices that contain high concentrations of esters.
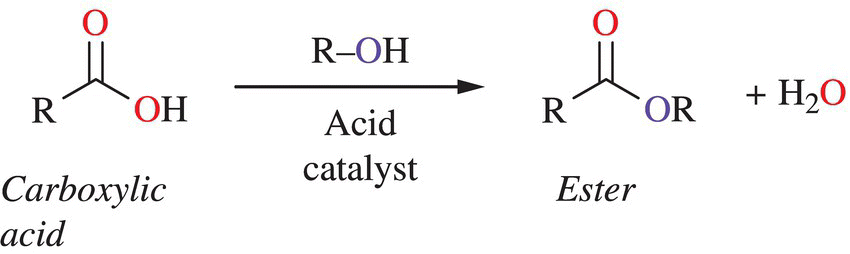
Figure 11.9 Formation of esters from organic acids. Many odorants are generated as esters by the loss (dehydration) of water from a carboxylic acid and combined with another carbon-containing compound.
11.3.4 Pungent
A flavor family that is not defined by the chemical structure of its flavorants and odorants, but by the sensation of heat, “hotness,” or unpleasantness that they bring upon us, is appropriately called pungency. In general, pungent compounds do not bind and activate food and odor receptors, but compounds in this family interact with receptors that signal pain or thermal events. The more formal definition for this type of perception is chemesthesis—the activation of senses in the mouth, nose, or throat for pain, touch, heat, or cold. The cooling sensation of menthol is a chemesthesis event, as well as the heat sensation of wasabi. Horseradish, mustard, wasabi, ginger, pepper, and chilies make up the herbs and spices of this flavor family. Later in this chapter, we will spend some time focusing on chilies and capsaicin. In the meantime, the classification and chemical structure of these compounds can be placed into four categories: thiocyanates, alkylamines or alkaloids, and everything else.
Horseradish, cabbage, wasabi, and mustard all contain a chemical functional group called a thiocyanate (Fig. 11.10). Allyl isothiocyanate is the pungent compound found in horseradish, wasabi, and mustard oil. This compound is produced when the root of each plant is crushed; the crushing process releases enzymes that catalyze the breakdown of the sulfur-containing carbohydrates in the cell wall (called glucosinolates) to isothiocyanates. Although horseradish and wasabi have a similar flavor, the various forms and total amount of the glucosinolates provide the unique flavor profile of wasabi that distinguishes the two. There is almost 10% more of the allyl isothiocyanate in wasabi than in horseradish! However, because of the similarity, the less expensive and easier to obtain horseradish is often tinted green and used as “wasabi.” Don’t let the color fool you though: 10% more allyl isothocyanate makes a world of difference to the receptors in your nose, throat, and mouth!

Figure 11.10 Allyl Isothiocyanate. This sulfur compound is one of the pungent classes of odorants responsible for horseradish and other well-known flavors.
Dried mustard seeds or powders are not very pungent because the drying process halts enzyme activity. However, once hydrated with water, the enzymes are able to produce allyl isothiocyanate, leading to the pungency that you associate with mustard. It can, however, take several minutes to hours for the enzymes to make enough of the isothiocyanate for detection. For example, if mustard is mixed with an acidic solution such as a citric acid or vinegar, the enzymes will function, but at a much slower rate, leading to a less pungent dish. In addition, isothiocyanates are fairly unstable and break down quickly. Although the addition of acid reduces the rate at which the enzymes produce isothiocyanate, the lower pH substantially prevents the allyl isothiocyanate from breaking down in your recipe. Extended exposure of the isothiocyanates to heat also increases breakdown and formation of a nonpungent product. What is to be learned from this discussion? The cook who thrives on the preparation and consumption of pungent dishes should wait until the end of the cooking period to add the mustard or horseradish (Box 11.1).
The second group of chemical structures that define the pungent family of flavors is the alkaloids. Alkaloids are a large diverse family of carbon-based compounds that contain a nitrogen base. In plants, alkaloids are important in the development of the plant, fruit, and seed. However, while they have interesting chemistry and biology, most alkaloids are not flavorants. If, however, you enjoy a blackened grilled salmon or spicy salsa, then two alkaloids are critical to enlivening your taste buds. The alkaloids piperine and capsaicin are responsible for the pungency of black pepper and chili pepper, respectively.
Piperine, produced by black peppers of the fruit of Piper nigrum, acts by binding and exciting the receptors (TRV1) for pain nerve cells (Fig. 11.11). If pepper has ever caused you to feel pain, now you know why! Piperine acts similar to, but has a greater efficacy than allyl isothiocyanate. Three different piperine isomers are found in the pepper fruit: chavicine, isochavicine, and isopiperine. If you remember, isomers are compounds with the same atomic makeup or molecular formula, but they possess a different organization or structure. Chavicine also has a strong bite and aroma, but it, as well as the other isomers, slowly degrades, while piperine remains stable and pungent. The pepper berries include other aromatic volatile compounds, adding to the aroma and pungency of pepper. Terpene, limonene, and linalool all combine with piperine to give fresh black pepper its woody floral taste with a bite of pain.
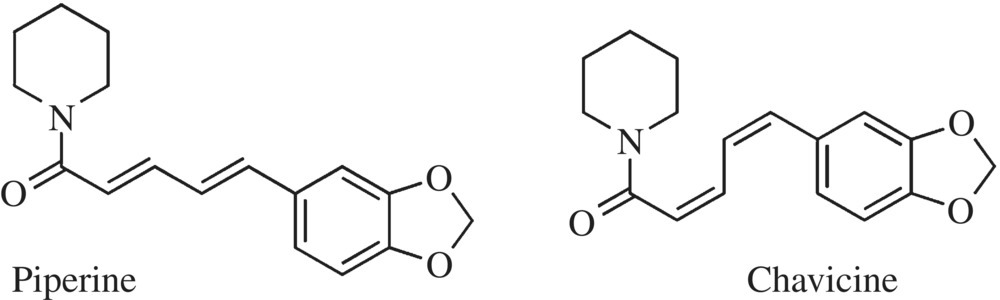
Figure 11.11 Pungent alkaloids. Shown here are isomers of two alkaloids. There are several isomers, which each bind and activate the receptors responsible for sending pungent pepper odor to the brain.
Did you ever wonder why fresh cracked black pepper tastes and smells so different from ground pepper, especially ground pepper that has been left in a shaker for a long time? The difference in taste and aroma is all due to the chemistry and biology of the pepper plant and its compounds. The mature fruit or berries from the pepper vine are dark red and contain a single seed. The blanched and dried berries are left to age in the sun, which ruptures the cell wall of the berries, allowing for enzymatic and Maillard browning reactions to take place. These reactions produce dark-colored polyphenols and other volatile compounds in the intact berry, now called a peppercorn. When you grind peppercorns in your mill, many of the volatile compounds are released into the air, resulting in that “peppery” aroma. Although aged ground pepper still contains many piperine and terpene compounds, a significant portion of the more volatile compounds will have evaporated over time. This is the reason why whole peppercorns are often used for longer forms of cooking or preserving instead of ground pepper.
Have you ever seen white pepper? White pepper is derived from the same P. nigrum vine berries as black pepper; however the outer fruit layer is removed by bacterial decomposition in water. This process results in the loss of most of the terpene aromatic compounds but allows for the retention of much of the pungent piperine molecules. This milder preparation of pepper used for its less aromatic flavor and aroma while still adding pungency to a food. White pepper is often used in foods, salads, and cream sauces in which some of the “pain” of pepper is desired, but not the stronger taste or color of black pepper.
Do chilies and black pepper give you the same “pain” sensation? Yes and no…. This isn’t surprising because the key component in both chilies and capsaicin binds and activates the TRV1 receptor for pain in humans. However, capsaicin does so with an efficacy that is 1000 times greater than black pepper’s piperine. The difference in pungency or hotness in the many varieties of hot chili peppers is primarily due to the level of capsaicin in each pepper. What makes capsaicin so powerful? Notice the nitrogen atom in the middle of the molecular structure of capsaicin. Capsaicin, like piperine, is a relatively large molecule that has some polar functional groups; it is even able to hydrogen-bond with water! This characteristic makes capsaicin much less volatile, which you are likely grateful for if you’ve ever touched a sliced jalapeno and then your eyes! We will talk more about hot peppers later in this chapter.
There are lots of other compounds in the “pungent” family, in which a few compounds are worth noting. Gingerol (found in ginger) closely resembles capsaicin but does not have the nitrogen base of an alkaloid; rather, it is a modified phenol. While pungent, gingerol is rated less pungent than pepper. However, age and heat cause the degradation of gingerol to another compound called shogaol. Shogaol happens to be twice as pungent as its parent, gingerol. Thus, dried ginger has a more pungent flavor than fresh ginger. A lesser known spice compound called paradol is found in the seeds of Guinea pepper. The compound is a phenol, is similar in structure to gingerol, and is rated with the same pungency as piperine. Paradol has an interesting property in that it activates a process called thermogenesis, a biochemical metabolism that burns fat to produce energy. The length of the carbon chain of paradol seems to be critical for its fat-burning ability. In a study in which mice were fed a high-fat diet, the shorter the chain on the compound, the lower the weight gain in the mice that were fed the compound (Box 11.2).
11.4 A CLOSER LOOK AT A FEW HERBS AND SPICES
If you haven’t already gathered this, the biology and chemistry of herbs and spices are pretty interesting. The impact of flavor, the evolution of the originating plants, and the biological impact of herbs and spices are the subjects of many fascinating books. Here, let’s focus on a handful of herbs and spices that have an interesting scientific story and play a significant role in the kitchen.
11.4.1 Vanilla
Vanilla is one of the world’s most popular flavorings, finding its way into food, beverages, perfumes, and even pharmaceuticals! Vanilla, which originated in Mexico and Central America, comes from the vanilla orchid, a vine that produces vanilla beans as a dried seed pod of its fruit. The value of the bean pods was recognized by the Aztecs who used them to flavor their drinks made with powdered cocoa beans, ground corn, and honey. However, due to the hermaphroditic character of the plant (this means that the plant has both male and female reproductive organs), the plant flower requires pollination to set the fruit. This characteristic was problematic during first attempts to cultivate vanilla outside of Mexico and Central America given that the natural pollinator of the flower was not native to other tropical areas where vanilla was first transplanted (Fig. 11.12).
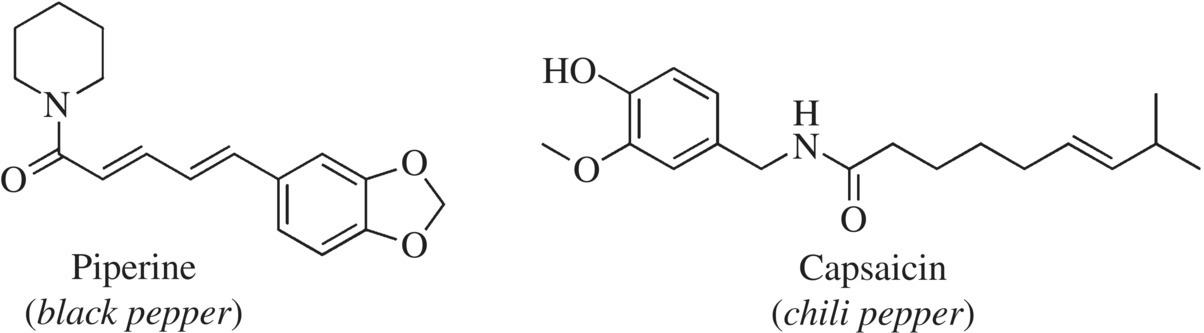
Figure 11.12 Hot or not? Two pungent alkaloids give food a hot flavor but act by very different receptors. Capsaicin but not piperine stimulates our pain receptors, giving a hot feeling.
Due to the great culinary value of the vanilla, cultivars of the plant were brought around the globe in the early to mid-1800s, including the West Indian island of Réunion, where the vine would grow but the pod would not develop. Although this gave the Central American growers a lock on the much-desired vanilla flavor, scientists continued to strive to find a way to cultivate the plant outside of Central America. In 1836 a Belgian botanist discovered the importance of the Melipona bee for pollination; however it wasn’t until 5 years later, in the West Indies, that a 12-year-old slave discovered and developed a hand pollination method for the vanilla flower. This method is still used today. Thanks to 12-year-old Edmond Albius, who won his freedom for the development of this process, vanilla vines can now grow and fruit in many tropical areas around the world.
Madagascar and Indonesia are the world’s largest producers of vanilla. Madagascar and the West Indian island of Réunion (previously called Bourbon) produce Bourbon or Madagascar vanilla. These pods produce the rich flavor that you most often think of as “vanilla.” Tahitian vanilla is derived from a plant hybrid that is grown in the Philippines. While Tahitian vanilla has a desirable flowery and fruity flavor, it is susceptible to breakdown by heat. The sensitivity of Tahitian vanilla to heat is particularly crucial, as part of the curing process of the vanilla bean is to heat the pod, which promotes the browning reactions necessary to form mature vanilla flavors. Mexican and Indonesian beans also have a more subdued vanilla flavor and smoky or wine-like aroma than do the Madagascar/Bourbon pods.
When you cook with a vanilla pod, rather than vanilla extract or flavoring, the food has a much more interesting and complex flavor. Why? Most of the vanilla flavor resides in the sticky material inside the pod, as well as in the small black bean seeds. How do you work with a vanilla bean pod in the kitchen? Slice down the length of a bean pod, scrape out the sticky black material and seeds, and include the combination of scraped seeds and the bean in the recipe (Fig. 11.13). This is particularly delicious when you are making a dish comprised of milk or cream. Because the compounds that provide the flavor and scent of vanilla are more soluble in fat and oil than water, the fats in milk solubilize the vanilla flavor molecules, leading to wonderful concoctions like vanilla milk or vanilla bean ice cream. An interesting additional use of unused sliced pods is to submerge the opened, uncooked pods into a closed container of table sugar; this creates a rich, vanilla-scented sugar that is worthy of baking and candied treats.

Figure 11.13 Vanilla bean pod. An open, close-up image of a vanilla pod. Notice the small black seeds held within the pod. These seeds are used to extract vanillin compound for cooking and baking.
Which molecules give vanilla its characteristic vanilla flavor, and how are these flavoring molecules produced? During the aging and browning process of the bean pods, some of the glycoside components of the seed and plant cell walls are converted to vanillin, the molecule most responsible for vanilla flavor and aroma. Remember that glycosides are sugars that are covalently bonded to other sugars or functional groups via a glycosidic bond. There are many other compounds detected and responsible for part of the flavor of vanilla, but vanillin is responsible for most of the flavor and aroma. The worldwide demand and expense for vanillin far exceed (by about 10-fold) the capacity of the plant to produce the flavor. Therefore, synthetic vanillin accounts for most of the vanilla flavoring market and is produced at one-hundredth the cost of the natural product (Fig. 11.14).
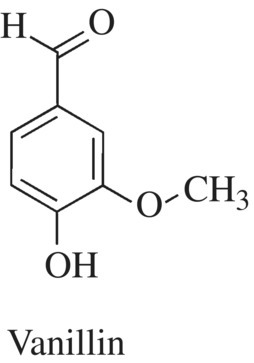
Figure 11.14 Vanilla flavorant. Vanillin is one of the key compounds responsible for the flavor and aroma of vanilla.
What is the difference between a vanilla that is made naturally and synthetically? Natural vanilla extract is a complex mixture that includes vanillin extracted from alcohol-soaked vanilla beans or processed beans that are repeatedly washed over with alcohol. Pure natural vanilla extract is best characterized by its sweet fruity, spicy flavor, and aroma. Most vanilla is a synthetic production of vanillin, which contains added sugar and other compounds. This is still pure vanilla; it is just not naturally produced by the plant. However, regardless of whether vanilla is artificial or a pure vanilla extract, the compound is very volatile, so you should add it later in the cooking process to avoid evaporation and loss.
11.4.2 Coriander and Cilantro
Part of the carrot family and a native Middle Eastern plant, coriander and cilantro are two widely used herbs grown and utilized broadly around the world. Cilantro and coriander come from the same plant; the leaf is used as a herb, while the spice comes from the fruits or seeds of the plant and is typically ground. Coriander and cilantro provide a great example of how herbs and spices are distinguished. Coriander is a spice, as it comes from the fruit of the plant. Cilantro is a herb, since it is the leaf of the plant. If you have ever tasted or smelled cilantro and coriander side by side, you know that there is a distinct difference in flavor and aroma. These differences are due to the distinct molecules that are present in the fruit relative to the leaf of the plant. Cilantro leaves contain 41 different volatile compounds including decanal and similar isomer compounds [7]. Coriander flavor and aroma come from the terpene flavor molecules, linalool and pinene, which give the spice a fruity, pine- or sage-like flavor and odor. Linalool is a branched carbon chain with an alcohol (OH) functional group. Pinene contains a complex carbon ring system and is the molecule also found in pine resin, pine oil, and lemon oil. Mixed with cumin, ground coriander seeds provide the base for many Indian culinary dishes (Fig. 11.15).
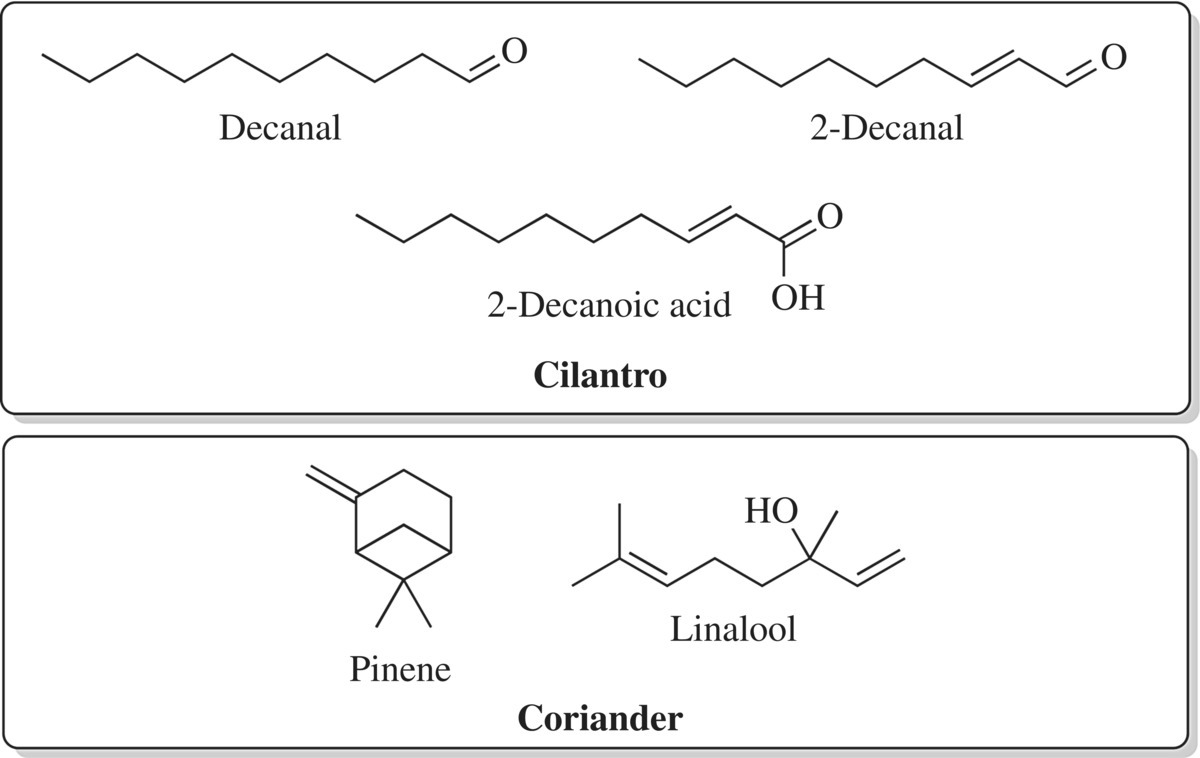
Figure 11.15 Compounds of the coriander plant. Cilantro and coriander, while from the same plant, are a herb and a spice, respectively. The compounds responsible for their unique characteristics are shown here.
Cilantro deserves a bit more discussion because the flavor of this herb is polarizing. Some people love cilantro and mix it with their homemade salsas and Mexican food. Other people hate cilantro, claiming it tastes like soap. Why is there such a love/hate relationship? Let’s take a closer look at the compounds found in the herb and inspect our DNA.
Why the soapy taste? At the molecular level, some of the compounds in soaps are structurally similar to the decanal-based flavor molecules of cilantro. The aldehyde component of the decanals is also structurally similar to the odor molecules that some bugs produce as a defensive weapon. Harold McGee, in a post for The New York Times [8], posits that these similar compounds can remind people of experiences with soap, earth, or even bugs. It is no wonder some people have strong negative feelings about the herb!
What is the connection between cilantro and our DNA? Fourteen to twenty-one percent of people with Asian, European, and African ancestry report detection of a soap-like flavor and a corresponding dislike of cilantro. In contrast, only about 3–7% of people from South Asia, Central America, and the Middle East (where cilantro is heavily used) did not like the taste of cilantro [9]. In a recent study, scientists found a genetic change (a change in a single DNA base) in the chromosomes of about 10% of the population that is linked to a dislike for cilantro. This change in one nucleotide (the chemical building block of DNA) is called a single nucleotide polymorphism (SNP, pronounced “snips”).
SNPs are not uncommon; they are found about every 300 nucleotides on a chromosome. SNPs are part of what brings about genetic diversity in humans, plants, and most organisms. Since the human genome has about three billion nucleotides, there are about 10 million SNPs in our genes. Although 10 million seems like a lot, most of these single mutations do not affect us, as the bulk of the nucleotides that make up our chromosomes do not code for proteins. However, when an SNP happens in a part of the chromosome that does code for a protein, the proteins that these genes code for may have some very unique characteristics.
There are two SNP variants linked to perception of cilantro. How were these variants detected? In one study containing over 14,000 people, individuals were asked whether they detected a soapy cilantro taste and had their genes sequenced. In this study, a connection was made between two SNPs and a group of genes on chromosome 11 that had a single mutation that codes for olfactory receptors [10]. The OR6A2 gene was altered in nearly half of the participating European descendants and codes for a receptor that is highly sensitive to aldehyde-containing compounds. Another study found a link between a dislike for the herb and three different genes. What is the take-home message? Small genetic differences in chromosomes can alter the structure and function of proteins. These genetic differences may be the reason why there is a difference in the perception of flavor and odor between two individuals.
11.4.3 Cinnamon
One of the most popular spices or herbs, cinnamon, is broadly used in the cooking of sweet and savory foods, beverages, and candies or is sprinkled on a piece of toast. Cinnamon is a spice that comes from the inner layer of the bark of tropical evergreen trees and shrubs from the Cinnamomum genus, which consists of 250 trees and shrubs. Given the different species used to make the spice, the term “cinnamon” doesn’t fully capture the different characteristics of molecules present in each preparation. Consistent among all cinnamons is the main flavor ingredient, cinnamaldehyde (Fig. 11.16), while the minor components of the spice will vary from source to source.
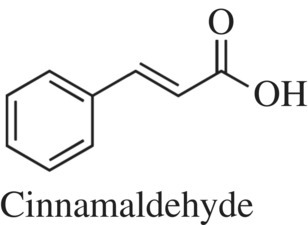
Figure 11.16 Cinnamon flavorant. Cinnamaldehyde is the main compound responsible for the flavor of cinnamon.
What are the different types of cinnamon? “True cinnamon” is derived from the Cinnamomum verum tree that is native to South India and Sri Lanka and has also been transplanted to grow in Madagascar. Because of the historic importance of the spice, true cinnamon is called Sri Lankan (or Ceylon—Sri Lankan’s former name) cinnamon. A second species of tree, Cinnamomum cassia, used to make the spice called “cassia cinnamon” (also called Chinese, Padang, Saigon, or Batavia cinnamon), grows more widely in Vietnam, India, and Indonesia. The Cinnamomum burmannii tree is the source of burmannii cinnamon, which is also called Indonesian or korintje cinnamon. In order to understand what makes these three types of cinnamons different from one another in taste and use, we need to talk about how cinnamon produced.
Cinnamon is obtained by stripping the inner bark from the shoots of 2–3-year-old stems of the tree. The inner bark is dried and curls in a characteristic way that we associate with cinnamon sticks. The cinnamon trees are cut or pruned to allow for the growth of new shoots for the next crop of bark. The work is difficult and requires skilled peelers. Sri Lankan or true cinnamon is paper-thin and forms into a single curl (or quill), while cassia and burmannii cinnamons are thick and curl into a double curl/quill. Moreover, only true cinnamon will have many thin layers rolled into its single quill. Once ground into powder (in the absence of chemical analysis), it is nearly impossible to distinguish between true and cassia cinnamon. Cinnamon powders typically come from low-grade and chipped bark; the leaves and low-grade bark can also be distilled or solvent extracted to harvest cinnamon oil.
Is there a difference in taste between the different types of cinnamon? Sri Lankan cinnamon, which was used in many early European desserts and Mexican recipes, is slightly sweeter and has a mild flavor. Cassia and burmannii cinnamons have a stronger, almost peppery flavor, are the “cinnamon” that you buy in the grocery store, and are the cinnamon spice aromas and flavors that you associate with gum and apple pie. In other countries, cassia cinnamon is distinguished from others by labeling the spice as cassia, not cinnamon.
Since there is a difference in flavor in the different types of cinnamon, you can likely surmise that the different cinnamons must have a different chemical composition. The dried bark of any form of cinnamon or cassia contains 0.5–3% volatile oils that provide most of the flavor and aroma of the spice. The key molecular component, which makes up between 75 and 90% of all the compounds in cinnamon oil, is cinnamaldehyde (Fig. 11.16). Cinnamaldehyde is a modified phenol compound (Fig. 11.17), where a short carbon chain containing an aldehyde has replaced the phenol OH. The compound can be detected at very low concentrations (0.1–0.5 of the total percent of food); upon binding to its receptors, it provides a pungent sensation in addition to the sweet taste.

Figure 11.17 Compounds of cinnamon. While both true and cassia cinnamons have cinnamaldehyde, true cinnamon has more eugenol than cassia, while cassia cinnamon has small amounts of vanillin.
All cinnamons contain cinnamaldehyde. True cinnamon also contains volatile terpene compounds, including the pine-scented pinene and the sweet, floral compounds linalool and eugenol, which are found in many plants that smell of cloves and honey. In contrast, cassia cinnamon also includes a small amount of vanillin, higher concentrations of tannins, and only trace amounts of eugenol. These molecular differences partially explain the difference in flavor (with true being sweet and mild and cassia being more potent and peppery) between the two (Box 11.3).
11.4.4 Saffron
Saffron is a very interesting spice that, perhaps, you have never used (or even heard of). Why is it interesting? For starters, it is the most expensive seasoning, selling for $1500 and $2500 per pound. Why is it so expensive? The spice comes from a flower, the Crocus sativus, which is grown in limited regions of the world; only three threads of the spice are produced by each flower, and harvesting of the spice is performed by hand! Are you intrigued? Let’s learn more about this interesting and expensive spice (Fig. 11.18).

Figure 11.18 The delicate spice saffron.
Saffron is sold as a thin red thread; the threads are the stigmas of the crocus flower, which are picked by hand. Given the small number of saffron threads per flower, it takes about 75,000 plants to produce a pound of the spice; one acre of saffron plants yields only about 10 pounds of the spice annually. Iran, Spain, and Portugal are the main producers of the spice; however the flower is also grown in India. The high cost and low availability of saffron have led to a significant counterfeit market for the spice. True saffron has a unique smell, the threads will turn a cup of water yellow, the resulting water will have a bitter taste, and upon addition of baking soda, the water will remain yellow. Many counterfeit saffron will turn water red or brown with an increase in pH. American or Mexican saffron, which comes from a daisy flower, does not impart the flavor of true saffron. However, turmeric is used in many Indian dishes as a saffron substitute, providing a similar color and flavor to the food.
Like the other spices and herbs we’ve studied, saffron is a complex mixture of volatile substances; over 150 unique compounds have been identified in the spice. Some of the volatile compounds that contribute to the aroma and flavor are produced while drying the stigmas. Analysis of the volatile compounds produced just during the aging process identified 23 different compounds. Although some of these may have been breakdown products produced during the testing process, this is another example of the complex nature of herbs and spices. The two key volatile components are safranal (which makes up ~70% of the volatiles) and beta-isophorone (Fig. 11.19). Both of these small organic compounds have very little water solubility, which you know makes a good volatile fragrance.
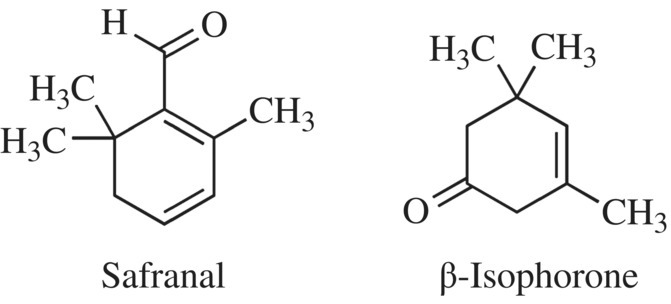
Figure 11.19 Volatile compounds of saffron. The two key components of authentic saffron.
The deep red color of saffron is due to the presence of the fat-soluble carotenoids crocin and crocetin. These strongly colored pigments are formed by the breakdown of a compound called zeaxanthin. A by-product of zeaxanthin degradation is a bitter compound, picrocrocin. Picrocrocin, a glucose derivative and water-soluble flavor molecule, is the telltale compound found in true saffron. Interestingly, the other by-product of this reaction is the aromatic safranal. Why do the stigmas have to be dried to yield these flavor molecules? As we have seen before, the drying process breaks open the cells, releasing the enzymes responsible for these reactions (Fig. 11.20).
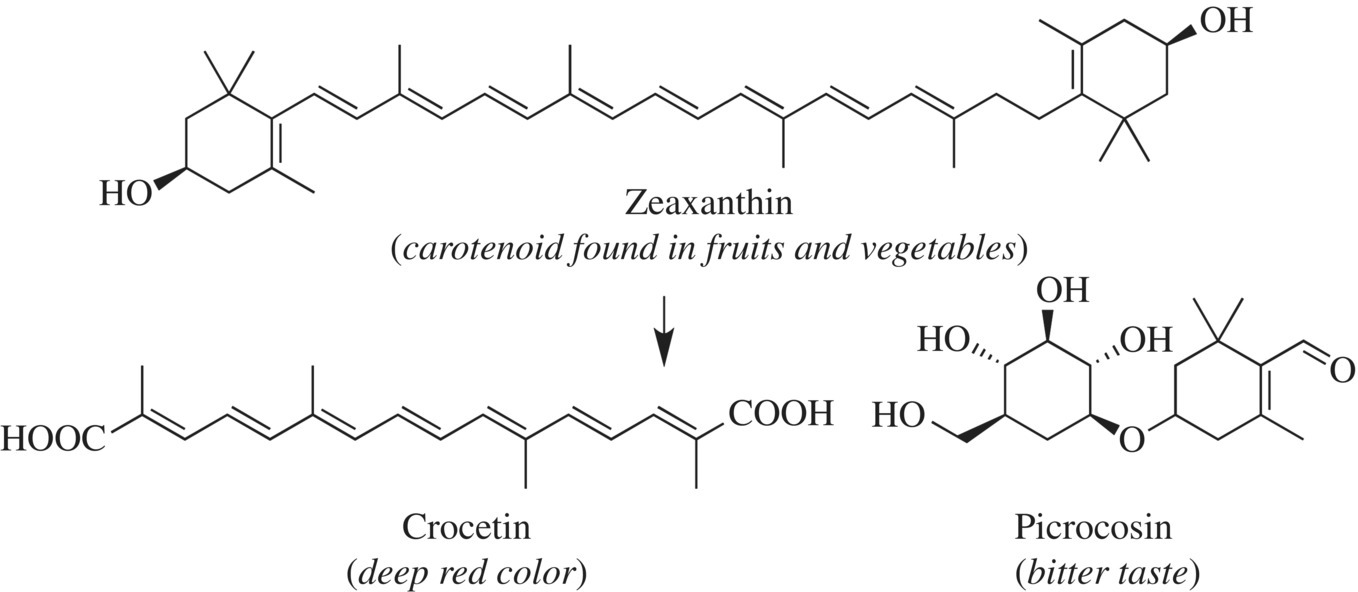
Figure 11.20 Saffron color agents.
Saffron is common in Middle Eastern and Spanish dishes such as rice, risotto, and paella. Fortunately for those cooks who are on a tight budget, a pinch of thread is all that is needed for sufficient flavor and development of the characteristic yellow color. Often, the spice is steeped in warm water or milk for 30 min prior to use; this process draws out the color and assists in generating a homogeneous mixture of flavor in the finished dish.
11.4.5 Nutmeg and Mace
Originally grown in the Indonesian islands, the tropical evergreen Myristica fragrans, commonly known as the nutmeg tree, produces both mace and nutmeg. Nutmeg is made from the seed or pit, while mace comes from the webbing that covers the shell of the pit. Growing nutmeg trees requires great patience. The nutmeg tree is dioecious; this means that there are separate male and female trees. Only the female trees can produce fruit; to complicate matters further, it takes nearly 8 years to identify a tree as male or female. To overcome this complication and reduce the risk of an all-male orchard, cuttings are used to clone the female plants, and 10 female trees are transplanted for each male tree (Fig. 11.21).

Figure 11.21 The nutmeg fruit.
In the United States, nutmeg is used in potato-based dishes, cookies, pastries, sausages, and, during the holidays, eggnog. The savory flavor of nutmeg is used in many Indian, Middle Eastern, and Indonesian dishes. If you have never cooked with mace, you would find it to have a nutmeg-like aroma, but it is more pungent and savory. Although it was once a highly sought-after spice, it is now used as a dominant flavor in spice doughnuts and spice cakes. Both spices have some of the same terpenes as cloves having woody and floral notes. Nutmeg and mace are often included with a mix of other spices including cinnamon, cumin, and vanilla. The compounds contained in both spices are also found in cloves and are terpene based with woody and floral tones.
Off and on in recent years, the potential for nutmeg to produce a drug-like, hallucinogenic high moves through communities. However, consumption of the amount of nutmeg needed to produce the high will result in severe side effects including vomiting, diarrhea, nausea, kidney and central nervous system issues, and irregular cardiac rhythms. The nutmeg molecule that is believed to contribute to the hallucinogenic effect is myristicin. Chemists and biologists interested in this question believe that the drug is either acting directly in the body or must undergo a chemical change to exhibit its activity. Understanding which processes of these is occurring is called the mechanism. There is scientific evidence that myristicin is chemically converted to a psychedelic amphetamine called MMDA in the liver. However, other studies show that myristicin is chemically converted to other nonhallucinogenic compounds in the liver. Other studies show that myristicin itself binds to and stimulates the receptors that are activated by other psychedelic drugs such as amphetamines, serotonin, and dopamine. It is not clear which of these possible mechanisms might be at work in humans, given that these studies were conducted in rodents or petri dishes. However, either way, the dangers and severe unpleasant reaction to myristicin are nasty and can lead to an emergency room visit.
11.4.6 Curry
The term curry is used for both the dish and the powder that is used as a base seasoning for the dish. Distinct from the seasonings that we have discussed thus far, curry powder is actually a mixture of herbs and spices, most of which do not come from either of the two plants with the name “curry.” Curry leaf comes from the Murraya koenigii plant, has a lime–lemony taste with woody overtones. Although most curry powders do not contain curry leaf, Indian curry powder mixtures often include curry leaf, or the leaf is added directly to Southern Indian cuisine on its own. The unrelated curry plant comes from Helichrysum italicum, a plant that is similar to flowering sage bush and smells of curry but is not included in curry dishes or powders. Interestingly (and perhaps confusing), neither is a traditional component of most curry spice mixes. These plants have a large concentration of alkaloids. However, Indian curry powder mixtures often include the leaf, or the leaf is part of Southern Indian cuisine on its own. The unrelated curry plant appears similar in some ways to flowering sage bush and smells of curry but is not involved in curry dishes.
The term curry is more appropriately used to describe a variety of dishes from around the world, including foods from India, Pakistan, Sri Lanka, Singapore, Thailand, and Japan. Like dialect and cultural variation, there are as many kinds of curry spice mixtures as there are villages. In most cases, the recipes for curry powder start by toasting or browning the spice. The traditional spices in a curry powder include turmeric, coriander, cinnamon, allspice, and cumin, to name a few. Northern curry powder, like the Punjabi style of curry known as garam masala, tends to be sweet and contains black pepper, cardamom, and coriander as its main components.
Thai curries, unlike the Southern Indian dishes, do not contain curry leaves, are sold as a powder or paste, and are typically identified by the color of the powder/paste: red, green, and yellow. Thai curry paste tends to be particularly spicy, where the level of spice depends upon on the chili being used. Red Thai curry is made with red chilies, garlic lemongrass, ginger, and shallots. Green Thai curry paste is similar to red curry but has a green chili pepper and includes coriander and cumin. Yellow Thai curry gets its distinctive color from the addition of turmeric and cumin and is a little sweeter and creamier than the green and red versions due to the addition of coconut milk.
A northern Africa/Arabic curry called Ras el hanout is a blend of black pepper, cardamom, sea salt, ginger, cinnamon, mace, turmeric, allspice, nutmeg, and saffron; this blend is common to Moroccan and Arabic cuisines.
Madras curry sauce is a British version of a hot Indian-inspired curry paste, containing chili powder, turmeric, cumin, and cinnamon, which is added for its pungent and savory flavor that contributes to the “heat” of a Madras curry. Given that we are talking about the “heat” associated with a curry, let’s talk more about the chilies and capsaicin that give many other dishes “heat.”
11.4.7 Chilies, Capsaicin, and Heat
The fruits of the flowering plant Capsicum include the mild green pepper and the hottest peppers ghost pepper or bhut jolokia, Carolina Reaper, and the Trinidad moruga scorpion. The primary compound contained within capsicum plants is capsaicin; however the name of the fruit that produces the compound is not universally accepted. At times “chili” is used to describe the pepper, believed to be derived from chili con carne, a tomato-based dish that is made with the pepper. Eventually, the name for the food and the pepper was shortened to chili. Chile, the name of the South American country, and consistent with the Spanish “e” ending, has also been used as a name for hot peppers. However, perhaps the most historically correct way to refer to a pepper is chili. A Spanish physician and botanist, Francisco Hernández de Toledo, in Four Books on the Nature and Virtues of Plants and Animals for Medicinal Purposes in New Spain, used the Aztec native language to describe white habanero peppers as “arbol chili” in 1615. We will use this historical common name, chili or pepper here.
Chilies belong to a larger family of flowering plants including tomato, potato, and petunia plants and over 2700 other species called Solanaceae (nightshade). Plants are organized from this larger family into smaller subsets (or genus). Chili is in the Capsicum genus, which includes 22 wild and more than three domestic species. Most hot peppers lie in the Capsicum annuum, including bell, anaheim, banana, jalapenos, cayenne, and some of other commonly used peppers. The breadth of taste and heat that lie within the C. annuum species is somewhat surprising. A few familiar peppers do belong to a unique species: Tabasco and Thai (Capsicum frutescens), habanero, and Scotch bonnet (Capsicum chinense).
11.4.7.1 Anatomy of a Chili
Have you ever thought about a chili as a hollow container for seeds (Fig. 11.22)? That is essentially what it is! Inside of the hollow pod, the thin-shelled seeds are attached to the glands and placenta of the fruit. Chilies are mostly water (70% or more); the dry mass consists of fibrous, soluble, and insoluble complex carbohydrates with a significant concentration of glucose and free amino acids that provide flavor that is hidden behind the heat of a chili. Although there are volatile oils and other fats that also contribute to the flavor and aroma of the fruit, most of the characteristic colors, flavors, and aromas come from molecules called the carotenoids and capsaicinoids.

Figure 11.22 Anatomy of a pepper. Most of the “hot” compound, capsaicin, is found in the placenta. The seeds are filled with bitter-tasting cell wall material and are coated by the oil glands with capsaicin.
11.4.7.2 Color
Have you ever seen or tasted an orange bell pepper? The varied colors of raw and powdered peppers are highly valued for the aesthetic component that these vivid colors bring to a food. The pigments that give plants and select microorganisms yellow and red colors are the isoprene and phenol-based compounds called the carotenoids. There are over 20 different carotenoids in the fruit of chilies.
Beta-carotene is responsible for much of the yellow-orange color peppers, while the red color of cayenne, red bells, and even some red spices like paprika comes from the less common carotenoids, capsanthin and capsorubin (Fig. 11.23).
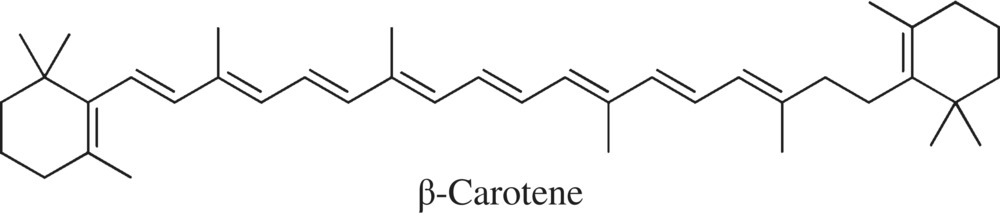
Figure 11.23 Beta-carotene. The compound responsible for many of the colors in plants and vegetables. Metabolism of this compound generates many different colors in vegetables and fruits.
What about green peppers and plants? As you may recall from high school biology, chlorophyll gives plants their green color. While there are several different types of chlorophyll (a, b, c, d) with slight structural differences, all of the forms have the four-ringed structure shown in Figure 11.24. This structure causes chlorophyll to absorb blue and red light. Thus, chlorophyll reflects green light, which is the light and color that we see. However, you know that there are a variety of colors of green in chilies. This variation happens because green chilies not only contain the different types of chlorophyll (in different amounts) but also the other carotenoid pigments that we have already discussed. When the various combinations of beta-carotene, the chlorophylls, and other carotenoids come together, red, blue-green, and blue lights are all absorbed, leaving a spectrum of green light to be reflected from different types of chili fruits.
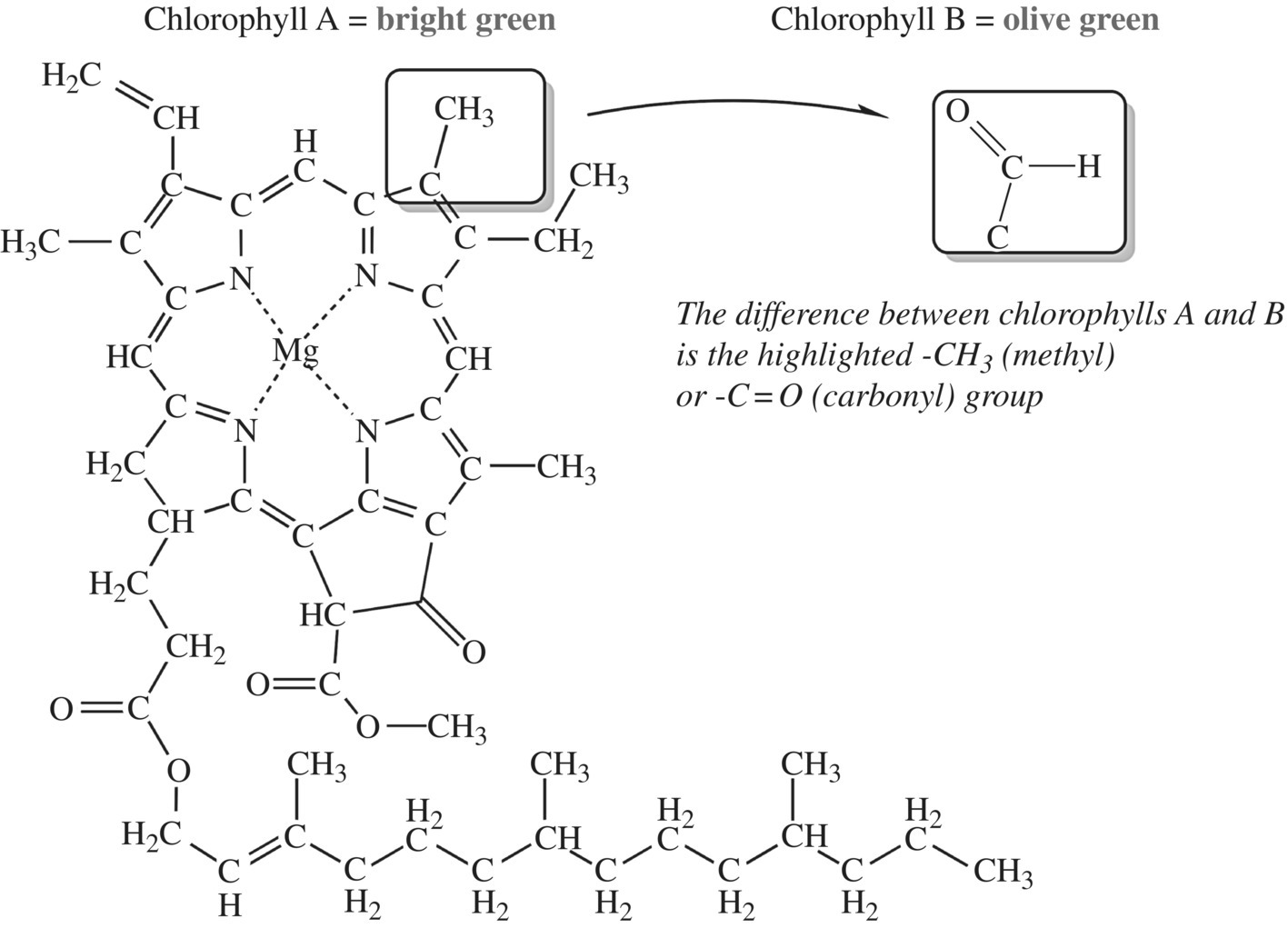
Figure 11.24 Chlorophyll.
Have you ever seen a pepper fruit change color while on the plant in your garden? Chlorophyll is an unstable compound, but when the plant stops producing it or if fruit is harvested, the green pigment decomposes and will no longer absorb light. Carotene is responsible for the color change, as it is more stable and degrades more slowly than chlorophyll. Thus, as your green pepper sits on a dying plant, it will turn yellow or red, or if you place a green pepper on your kitchen counter, it will eventually also turn yellow/orange/red. Moreover, in several fruit, the red carotenoids are not significantly produced until maturity, at which time the plant hormones shut off chlorophyll production in the fruit. Thirty-four different carotenoids were identified in a ripening extract of Hungarian capsicum. Yellow carotenes are less stable than red carotenes, giving aged pepper powder its characteristic red color.
Where do the unusual pepper colors come from, such as purple bell peppers? These less typical pepper colors are due to another group of molecules, called the anthocyanins (the basic anthocyanin molecule is shown in Chapter 7). These molecules can change color in response to the pH of the fruit (red for acidic and purple in more basic conditions).
11.4.7.3 Capsaicinoids
What is the star of hot chicken wings, extra spicy salsa, or an eye-tearing Tabasco sauce? The heat of course! The molecule responsible for the heat is actually a group of alkaloid compounds called capsaicinoids (Fig. 11.25).
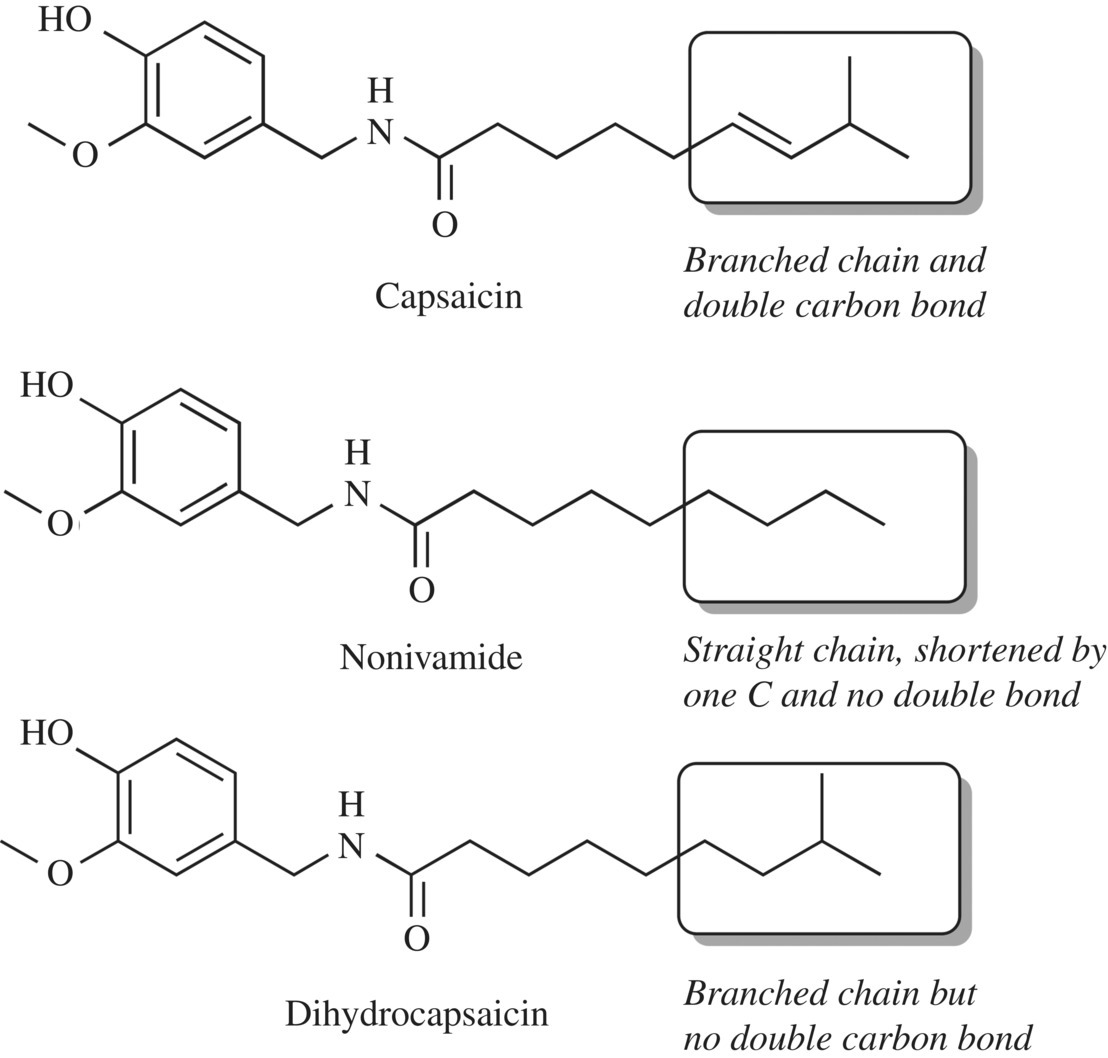
Figure 11.25 Capsaicinoids. Capsaicin is the parent compound of the capsaicinoids. Carefully inspect the differences in the tails; the remaining structures are all identical.
Often, the capsaicinoids are called capsaicin, as this is the single molecule that comprises the majority (64–72%) of the capsaicinoid compounds in a chili. The second highest capsaicinoid, dihydrocapsaicin, accounts for about 22% of the pungent compounds. Five other closely related compounds comprise about 10–20% of the capsaicinoids. A close inspection of the compounds shows that they are similar in molecular structure to other flavorants, such as piperine, gingerol, and vanillin, particularly in the ring component of the molecules. In fact, capsaicinoids are part of a group of molecules called vanilloids (e.g., gingerol, vanillin, and capsaicin) due to the presence of the phenol group. However, the carbon “tails” provide great diversity in the signaling of each compound to our brain; the longer, more hydrophobic tail of capsaicin is chemically very different from the OH and straight chain found in gingerol. Because of these structural differences in particular areas of the molecules, each compound binds to entirely different receptors and sends very different signals to our sensory system.
Perhaps you have noticed a difference in taste when you include (or don’t include) jalapeno seeds in your homemade salsa. Many people associate the heat of a chili with the seeds. However, capsaicin is produced in the placenta of the fruit. Thus, while some of the compound finds its way to the thin delicate seeds, the seeds are not the source of capsaicin, nor do they make positive contributions to taste, as they have membranes with various tannins and polyphenols and are often bitter tasting. Thus, the best way to appreciate the heat, retain the flavor, and reduce the less appealing, bitter overtones of a chili is to remove the seeds and retain the rest of the chili in your dish. Perhaps you have also noticed that the same type of chili (like a jalapeno) may have different levels of heat. The more stress from heat or dry conditions during the growing process increases the capsaicinoid level in the fruit. The fruit also increases the production and secretion of capsaicinoids as the color begins to change and the fruit begins to wither (Fig. 11.26).

Figure 11.26 The vanilloids. Based on the common phenol head group, the vanilloids are a diverse set of molecules responsible for many odors and flavors. Note the diversity in tails or nonphenol components for each class of compound. Each compound will bind to distinct receptors eliciting a very different response.
When you bite into a chili, the sensory impact is obvious: pain. However, there is little to no real biological damage that occurs when you eat chilies. Capsaicin (and related compounds) interacts and binds to a pain-sensitive nerve, not a taste receptor! More precisely, capsaicin compounds bind to a class of protein receptors located on the surface of specialized sensory nerves called nociceptors. Nociceptors, found on the skin and mucous membranes, are typically activated by a noxious stimulus that provides a damage signal to the body. Damaged tissue associated with trauma, heat, or chemicals leads to the activation of nociceptor nerve fibers, which provides a signal to the person experiencing the event to withdraw, stop, or get away from the event. Thus, capsaicin does not cause damage; it just sends a signal that pain or heat is being experienced. Let’s take a closer look at the biology of this process.
Each of the capsaicinoids binds to a protein located in the nociceptor nerve fibers called the transient receptor potential vanilloid type 1 ion channel. That is a mouthful; fortunately, the receptor is also known as the capsaicin receptor or TRPV1. TRPV1 responds to a range of different signals including capsaicin, the pungent compound allyl isothiocyanate, heat above 109°F/43°C, acid conditions below pH 5.3, and even electrical current. At higher concentrations gingerol binds to the TRPV1 receptor, providing the pungency for aged ginger powder. Once stimulated, TRPV1 activates dozens of proteins within the cells and opens the membrane for calcium and other ions to enter into the nerve cell. The result is a slew of biological signals that propagate the activation of pain nerve fibers. As long as capsaicin binds its receptor, the pain signal is sent, and capsaicin binds the TPRV1 receptor very well, with an affinity of less than 700 nM. What does this mean? Humans have the capacity to detect the powerful stimulant in solutions of 10 parts per million, that is, about 3.5 teaspoons of the drug dissolved in an Olympic-sized swimming pool. There is plenty of capsaicin in most peppers to be noticed (Fig. 11.27)!

Figure 11.27 Capsaicin activation of pain nerves. Several signaling cues can activate the membrane receptor TRPV1 including capsaicin. This results in an influx of calcium and sodium ions and sets off pain signals to the CNS.
It is perplexing then why, despite the pain and discomfort, so many people find pleasure in eating chilies and, often, the hotter the chili, the better! As you can surmise, the more capsaicin present in the fruit, the more likely the TPRV1 receptor will bind and send its signal of burning pain to the brain. However, even after eating a hot chili, most individuals will eventually want to stop the pain and limit spreading the pain! The key solution to this problem lies in the structure of capsaicin. The compound is fat soluble and not water soluble, so drinking water or soda will only make things worse. In fact, water will spread the hydrophobic oil-like capsaicin throughout the mouth to mix and coat other membranes, which will set off more nerve fibers! Soda may make things even worse as the acidic drink seems to synergize the pain signal. The solution is to remove the unbound molecule from the system and to tease the capsaicin from its receptor with a good glass of fat-containing milk or oily food that you spit out. Swallowing the now-capsaicin-soaked milk would only help deliver the compound to the rest of your digestive track, providing you with fun for a long time.
Another piece of advice is don’t touch your eyes or any other mucous membrane tissue if you’ve been cutting chili with your bare hands! Because the capsaicinoids are hydrophobic and primarily nonpolar, the molecules will dissolve in fat. On the skin, a rub in butter will help solubilize the compound into the lipid where it can be washed away.
Do you know anyone who doesn’t seem to be bothered by spicy, hot foods? This tolerance for hot food is easily explained at the TRPV1 receptor level. If the receptor is activated for long periods of time or repeatedly stimulated due to the presence of capsaicinoids, enzymes modify TPRV1, shutting down the nerve’s ability to send signals to the brain. This property, called desensitization, has been used therapeutically for a long time. Ancient Mayans used capsaicin (chilies) to treat sore throats, while the Aztecs used them to treat toothache. The desensitization caused by capsaicin continues to be used by the pharmaceutical industry. Capsaicin is topically used to alleviate pain and is a key ingredient in the creams that are used as treatment for shingles, muscle aches, and pain patches; it is even sold to reduce the pain of arthritis. A second mode of action utilized by capsaicin to relieve pain is through a peptide called substance P. Substance P is associated with several chronic pains including arthritis; it is responsible for some of the pain signals of different nociceptor nerve fibers that respond directly to capsaicin. Routine application of capsaicin depletes the amount of substance P available; with less substance P, there is less pain associated with some chronic illnesses. Thus as a drug, capsaicin is used with modest success in treating the pain associated with shingles, fibromyalgia, osteoarthritis, and even postcancer surgery.
11.4.7.4 How Hot Is It?
Inspection of the structures of a few of the more than 20 known capsaicinoids shows the seemingly minor changes in double bonds and orientation of the tail or other functional groups (see Figure 11.25 for a simple example). However, these structural differences significantly alter the ability of each compound to bind and send its signal through the TRPV1 receptor. Capsaicin and dihydrocapsaicin, which represent 80–90% of the compounds in the family, have the highest binding affinity for the TRPV1 receptor. There are more than 20 known capsaicinoids that each bind to TRPV1 with varying efficiency. However, there isn’t a significant detectable difference between these less common capsaicins. Therefore, if you are looking for the hottest chili, you should focus on the overall amount of capsaicin. The more the compounds, the more receptors that are activated and the more nerves that send the painful burning signal to the brain.
The famous Scoville heat unit (SHU) is the unit by which the “heat” of a chili is measured. The SHU is a rather arbitrary and difficult measurement technique. How did a semiquantifiable scale come about? Parke-Davis pharmaceutical chemist Dr. Wilbur Scoville was following up on some initial studies performed by a Hungarian physician, Hőgyes. In 1878, Hőgyes had noted that ingestion of capsaicin created a sharp sensation on his tongue and warmth in his gut, followed by belching and flatulence. While Hőgyes’ work eventually led to the pharmacological use for the drug, Dr. Scoville was searching for a way to measure the “hot” compound in ground peppers. Why was it necessary? Well, chilies vary significantly in heat from plant to plant or season to season. Thus, in 1912, Dr. Scoville published his one-and-a-half-page paper “Note on Capsicums,” in which he described the process to measure “heat” to further the drug discovery potential for capsaicin [11].
In this historical method, Scoville ground an exact amount of dried chili (a grain or about 64 mg of fruit) in 100 ml of ethanol to dissolve the capsaicin. He then diluted the extract with sugar water. The dilution in which the pungency was no longer detected (by the human tongue) was the measurement of the fruit’s capsaicin strength. The heat scale was based on this dilution and later came to be known as the Scoville heat index. However, this taste-based (organoleptic) test method is problematic, as tasters will wear out, desensitizing their receptors. Thus another more modern method, which utilizes high-performance liquid chromatography (HPLC), has been devised. Similar to the procedure described by Scoville, a mass of chili is ground in 95% ethanol with sodium acetate. The fluid is then run through fine, silt-like beads in a steel column that binds and releases the compounds present in the extract differently. Some compounds do not bind with the beads and flow through the column quickly, while other compounds bind tightly and are only released after a long time, or anything in between. This process, called chromatography, separates the compounds present in a complex mixture since each compound flows through the column beads at a different rate. How are these compounds measured or detected? Each compound can be measured by its absorbance of light as the solution flows through a light detector: the larger the absorbance peak, the greater the amount of compound present. Based upon the structure of the compound, the properties of the column beads, and the properties of the fluid (or solvent) that is running through the system, a scientist can predict which peak corresponds to which molecule. Now, scientists can carefully determine the heat of a chili using this standardized technique. SHU (from Scoville’s historical method) are still used to measure the heat of chilies. Using the HPLC method, the parts of capsaicinoid present per million of solvent (ppm) is multiplied by 15 to obtain SHU.
For years, the hottest pepper crown was given to the bhut jolokia from India. This pepper was commonly called the “ghost bhut” because of how the pain sneaks up on the consumer or it makes you feel as you have given up the ghost. However, the pepper lost its standing in the Guinness Book of World Records in 2012 to the Trinidad moruga scorpion pepper. Dr. Bosland from the New Mexico State University Chile Pepper Institute using a very standardized approach to testing a batch of plants found the bhut jolokia to have an SHU rating of 1.02 million, with the hottest plant possessing a rating of 1.58 million SHU. The C. chinense variety Trinidad averaged 1.2 million SHU; the hottest individual pepper was found to have just over 2.0 million SHU. To ensure the uniqueness of this pepper, the scientists measured several genes with unique SNPs for each. This showed the C. frutescens bhut was not a variety of the Trinidad pepper. The top spot in Guinness was taken over by the Carolina Reaper 1 year later. Measured in an undergraduate analytical chemistry laboratory at Winthrop University, the average pepper was 1.57 million SHU, while the top hot pepper produced a mouth screaming 2.2 million SHU. To give you some perspective on this heat, pure capsaicin has a rating of 16 million SHU. For better perspective the Carolina Reaper is 440 times hotter than an average jalapeno pepper. Those plugged into the pepper world have heard rumors of new variants that are even hotter being developed and tested. It will be interesting to see how much capsaicin a single pepper fruit can produce!
11.4.7.5 Why? Chemical Warfare!
The idea of eating a chili with two or more million SHU is beyond most people; however the curious (but not crazy) might ask why a plant would produce such a noxious compound? Every molecule that is produced by a cell requires energy. Thus, if, over time, an organism retains the gene that allows it to produce a molecule, there is usually a biological rationale behind it. There are two biological reasons why chili plants produce capsaicin: chemical defense and seed dispersion.
Plants do not have an immune system, nor can they move if attacked or threatened. To defend themselves against being eaten or killed by pathogens, plants have developed an amazing assortment of defense mechanisms to detect and stop invading organisms and animals. The bitter taste of some plants acts as a deterrent to animals biting and eating the plant. Damage caused by insects opens a wound that allows for microbes to enter, damage, and kill the plant. Thus in response to insect or animal damage, plants release volatile repellant compounds such as terpenes and polyphenols to deter further attacks. In other words, the same volatile oils that provide the flavor and aroma of spices act as insect repellants. In addition to repelling further damage, breaking the cell wall in some plants releases enzymes, such as the polyphenol peroxidases (discussed in Chapter 6) that chemically transform the lignin carbohydrates in the cell wall. The modified lignins act as a scar and patch the wounded area, slowing the growth of microbial pathogens already infecting the plant.
Capsaicins also play a very different role in chili plant survival. Most plants need to disperse seeds away from the parent plant to avoid the new plants competing with the parent for sunlight and nutrients. One method that plants use to achieve this is called zoochory. Zoochory occurs when seeds are dispersed through the gut of animals. In fact, zoochory is why the flesh (fruit) surrounding seeds is attractive in color, flavor, and aroma. The fruit acts as an attractant for an animal to eat and spread the seed through its feces. However, this method of seed dispersal is problematic for chilies because the seeds of most chilies are more fragile than seeds found in other fruits. Yet, the chili fruit has considerable sugar content and an attractive color. Let’s consider this problem with an example. Pear, orange, and apple seeds have a tough coating. A herbivore with flat grinding teeth would not destroy an apple seed, but a chili seed would be seriously damaged. The conundrum is to attract the right animal to eat and disperse the seed and deter those that would damage the seed and block propagation. Fortunately, there was an evolutionary change that generated a molecule (capsaicin) that would specifically bind to a receptor in animals that warns for burning pain, deterring the animals from eating the fruit and destroying the seeds. Birds, however, that do not have the grinding molars of a mammal have TRPV1 receptors, but capsaicin does not bind to these genetically distant receptors. Thus birds, with a more delicate way to pick and swallow the seed without injury, can spread and disperse the seeds after traveling though the gut.
An interesting study on this problem tested the consumption of fruit and chilies by mice, rats, and birds. All three animals ate the hackberry fruit, while neither the cactus mouse nor the packrat consumed the capsicum fruit. Yet, thrashers (bird) readily ate the hot pepper fruit. Moreover, the seeds eaten by the birds were passed and germinated the same as nonconsumed seeds. However, the rodents did not pass intact seeds, and no plants grew from the nonintact seeds that passed through either the rat or the mouse [12]. It was also found that the birds spread the seeds under its tree where shade helped the plant to grow. This study illustrates that capsaicin is an effective deterrent of ineffective seed dispersers, which increases the evolutionary fitness of the plant.
Another set of studies by the same scientist (Dr. Joshua Tewksbury, University of Washington) highlights a second chemical defensive and evolutionary reason for the capsaicinoids [13]. Recall that the placenta produces capsaicin and some of the compound coats seeds attached to the placenta. Several pathogenic microbes, including fungus, are able to grow inside the pods of punctured or damaged chilies. These fungal plant pathogens produce several compounds that are toxic to the plant host. Since the bite of a foraging insect will leave a wound within which a fungus can grow and bring about disease to the plant, if capsaicin could either detract insects from biting or fungus from growing, the compound would then serve a second chemical plant warfare purpose.
Looking at plants in Bolivia, Argentina, and Paraguay, Dr. Tewksbury and his colleagues found an interesting directional gradient of capsaicinoid-producing plants. There were more plants in the southwest than northwest by more than two thirds. Interestingly, the number of insect bites in both groups of plants was the same, indicating that capsaicin was not a deterrent to the insects. However, plants that contained capsaicinoids were half as likely to be infected and rotting due to fungus when compared with those plants with less capsaicin. This study suggests that capsaicin protects seeds from the pathogenic fungus.
Thus, if you want to know “why heat?,” the answer is clearly chemical defense. Capsaicin stops the molared mammals from eating and grinding the seeds into oblivion, and the “heat” slows fungal infection, thus sparing seeds from rot. With some humans, however, this evolutionary protection mechanism seems to be lost. We can postulate that we have adapted to the spice as it inhibits fungus on our food, but the organoleptic pleasure of spices is lost when eating a habanero. In fact, one-third of humans seek out the pain of eating chili peppers each day. This is nothing new as ancient civilizations in the Old and New World included peppers in food and drink. Clearly our innate aversion to a key biology response to pain has somehow been short-circuited. Psychologists have attempted to understand the reason behind the phenomenon of chili consumption. Two hypotheses have been proffered. The first is the social and learned behavior of cultures that consume peppers. Simply put, Mom, Dad, and the relatives eat it and the kids learn to like it too. The second is a “benign masochism.” Like the thrill of danger and our fondness for roller coasters, chili heat presents a unique human activity, similar to a trip through a haunted house or a scary amusement park ride. Whatever hypothesis is true for the chili lovers of the world, these pepper heads will continue to fight to claim to have eaten the hottest chili, despite the obvious reasons for avoiding it.
11.5 MEDICAL USES OF HERBS AND SPICES
There are plenty of nonculinary uses of herbs and spices listed in social media outlets, on the internet, or in your great, great grandmother’s book of wisdom. Historically, herbs and spices had a distinct antimicrobial activity that kept food from spoiling, which was critical when refrigeration space was nonexistent or at a premium. In addition to fighting microbial growth, several herbs and spices have antioxidant activity, which preserves the food (and the person ingesting the food). There are plenty of medical uses of some compounds to decrease blood pressure, fight cancer, and reduce depression. Let’s learn more examples of these medical uses in the following; you may have some medicine in your spice rack without having known it!
11.5.1 Antimicrobial Activity
Prior to refrigeration, people who lived in warm, tropical climates struggled to maintain safe stores of meat and vegetables, much more than those in the cooler climates. Taking advantage of the natural resources growing in these tropical areas, the herbs and spices added to the foods during curing and cooking provided more than an organoleptic purpose. They were added to ensure that the food was safe to eat! In fact, nearly half of the recipes for meat in tropical climates included spices, while less than 5% of those dishes from cooler climates called for the spice. Does this explain the flavoring of Midwestern foods versus those in the southwest corners of the United States? Maybe! Very low amounts (0.05–0.1%) of some herbs and spices inhibit the microbes that cause salmonella, cholera, and food poisoning. Specifically, linalool, eugenol, myristicin, cinnamic aldehyde, allyl isothiocyanate, and vanillin inhibit 50–75% of bacterial growth as compared with nontreated control samples! While the exact mechanism by which the compounds in herbs and spices inhibit bacterial growth is unknown, some evidence points to interference with the bacterial membrane continuity or inactivation of DNA and RNA needed for cell growth and division.
In addition to the bioactive compounds in herbs and spices already discussed, there is a wealth of scientific literature that focuses on the role of herbs and spices in cancer. Some compounds that cause or promote the formation of cancer tumors must first be chemically altered by enzymes in the liver, such as the P450 enzyme system. Compounds in pepper, rosemary, turmeric, and cinnamon stop this process by inhibiting several of the P450 enzymes, thus potentially preventing an accumulation of cancer-causing material. Once formed, tumors begin with uncontrolled growth and spread throughout the body in a process called metastasis. Turmeric decreases the signal from the tumor cell that induces its growth. Curcumin and capsaicin inhibit several activation signals to the tumor cell and inhibit the proteins that support cancer cell metastasis. Many herbs and spices also have components that may compete with estrogen for its receptor in human breast cancer cells. Thus, some herbs and spices may reduce the incidence of or progression of estrogen-dependent breast cancers.
Although all of these important effects that are imparted by herbs and spices on human health are interesting, they must be treated with a dose of cautious skepticism. Herbs and spices are familiar and considered “natural”; thus many are drawn to use a herbal treatment to achieve a positive health outcome to their ailment. Unfortunately to date, few clinical applications for herbs and spices have been found. Many studies have been performed in culture dishes of bacterial or animal cells, and some work in animal models such as mice or rats show promise; however the transfer of the cell or animal model effect into humans has largely failed. One example is a study that found that cinnamon extracts suppressed the growth of a bacterium (Helicobacter pylori) that is the major cause of stomach cancer and some esophageal cancers. Unfortunately, patients with the bacteria who were given high doses of cinnamon extract did not experience an altered bacterial growth or cancer outcome. However, not all of the studies have yielded disappointing results. The curcumin compound found in turmeric blocks inflammation and other enzymes involved in cancer. Early clinical (phases I and II) trials suggest there is an anticancer activity to the compound that has successfully translated to the patient.
Another danger is the thinking that “natural” herbs and spices automatically make them safe. We have already seen examples of the strong side effects of nutmeg and the potential health hazard of high doses of cassia cinnamon. There are many other stories that could fill a book about both the positive and the negative role of herbs and spices. Care and caution with healthy skepticism are always a good spice to include in your decision-making process.
REFERENCES
- [1] Drummond, J.C. and Wilbraham, A. (1939) The Englishman’s Food: A History of Five Centuries of English Diet. J. Cape, London.
- [2] Woodman, M. (1978) Food and Cooking in Roman Britain. Corinium Museum, Cirencester.
- [3] McGee, H. ed. (2004) On Food and Cooking. Simon and Schuster, Inc., New York.
- [4] He, F.J., Li, J. and Macgregor, G.A. (2013) Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ, 346: f1325.
- [5] Stolarz-Skrzpek, K., et al. (2011) Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA, 305: 1777–1785.
- [6] Vallverdú-Queralt, A., Regueiro, J., Martínez-Huélamo, M., Rinaldi Alvarenga, J.F., Leal, L.N. and Lamuela-Raventos, R.M. (2014) A comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 154: 299–307.
- [7] Potter, T.L. Fagerson, I.S. (1990) Composition of coriander leaf volatiles. J. Agric. Food Chem. 38(11): 2054–2056.
- [8] McGee, H. (2010) Cilantro Haters, It’s Not Your Fault. The New York Times. Available at http://www.nytimes.com/2010/04/14/dining/14curious.html (accessed on November 16, 2015).
- [9] Mauer, L. and El-Sohemy, A. (2012) Prevalence of cilantro (Coriandrum sativum) disliking among different ethnocultural groups. Flavour 1: 8.
- [10] Nicholas, E., Shirley, W., Chuong, B.D., Amy, K.K., Joyce, Y.T., Joanna, L.M., David, A.H. and Uta, F. (2012) A genetic variant near olfactory receptor genes influences cilantro preference. Flavour 1: 22.
- [11] Scoville, W.L. (1912) Note on capsicums J. Am. Pharm. Assoc., 1(5): 453–454.
- [12] Tewksbury, J. and Nabhan, G. (2001) Seed dispersal: directed deterrence by capsaicin in chillies. Nature 412: 403–402.
- [13] Tewksbury, J., Reagan, K., Machnicki, N., Carlo, R.T., Haak, D. and Penanaloza, A. (2008) Evolutionary ecology of pungency in wild chilies. PNAS 105: 11808–11811.