Nothing will ever happen to breast cancer unless it is politicized.
—Fran Kritchek, 1993
Breast cancer is a puzzle of enormous complexity. We don’t really have a clue as to what the factors are. What we’re trying to do is broaden the question to ask, what is wrong with a society that causes this?
—Susan Love, 1993
Barbara Balaban has been a breast cancer activist since the early 1990s, when, like many other educated women on Long Island, her frustration with the lack of an explanation for why she and others around her had gotten breast cancer pushed her into activism. As a breast cancer survivor and a social worker, Balaban had helped establish the Adelphi Breast Cancer Hotline and Support Program, which provided support groups for breast cancer patients. She was familiar with the literature from the National Cancer Institute and the American Cancer Society describing women who were at increased risk for breast cancer as being over fifty years of age, Jewish, having a poor diet (i.e., high in fat), never having had children, and with a family history of the disease. But the women who came in to the support program did not fit the “high-risk” profile. They were not necessarily older, they had good diets, and they had had children. Most had no family history. It struck Balaban that something else had to be going on.1
In the fall of 1990 the New York State Department of Health published a study of breast cancer on Long Island, which had been undertaken in response to community concern, and the results were announced at a press conference. Balaban was one of a handful of people who attended, and she was outraged that, as she put it, “they repeated the same old stuff about ‘high-risk groups.’” She recounts how, in the course of the presentation, the health department official displayed a map showing two “highincidence areas.” One was the Great Neck peninsula and the other was the Five Towns area, which consists of Woodmere, Lawrence, Hewitt, Hewitt Harbor, and Inwood. “I was familiar with these areas,” Balaban told me. “They are very heterogeneous, with the very, very wealthy in some areas and the very poor in others. It didn’t make any sense. I spoke up, saying this, and it got reported in the papers. This energized people to stand up and say, ‘Hey, we’ve got to get money for research and see that it gets done!’”
More than any other single event, this incident galvanized Balaban to become involved in advocacy for aggressive research into the causes of breast cancer. Within a year or two this came to mean focusing on environmental exposures as causes of the disease. As it was to play out in the course of the 1990s, the high-profile and highly charged issue of breast cancer and the environment reveals a complex web of reciprocal interactions between scientists, activists, politicians, and government agencies that fund research in this area. What studies were undertaken, how they got interpreted and reported in the press, and what the lay public thought was going on were all profoundly influenced by the activists’ conviction that something in the environment was causing breast cancer. There were schisms within the scientific community as well as within the activist community concerning what should be studied. Certain facts were distorted, and others were ignored because they did not fit with some groups’ agenda. Several isolated results from scientific studies were taken out of context and played up, adding to public concern and stimulating a wave of research studies that in the end provided little support for a role of hypothesized environmental factors in breast cancer. There was much confusion about how to interpret the studies that were carried out and much disappointment about their null results. Were the wrong culprits studied? Were the methods simply inadequate? Or was the whole idea that the environment played a role in breast cancer simply wrongheaded, as many in the scientific community believed? In the end, both scientists and activists learned important lessons from the early, rudimentary studies initiated in response to activist pressure, and in the longer term a much more sophisticated approach to the complex question of what causes breast cancer has emerged. For these reasons, the issue of a possible environmental contribution to breast cancer offers a rich instance of the interplay between science and the wider society.
Breast cancer is the most common cancer among American women, accounting for nearly one third of all cancers. It is also the second leading cause of cancer deaths in women. Over the period from 1940 to 1982 the incidence of breast cancer increased at an average rate of 1.2 percent per year in the United States, but between 1982 and 1986 its incidence rose more steeply, at a rate of 4 percent per year. Most of the increase in the mid-1980s was likely due to increased usage of mammography, but the reasons for the underlying rise over a longer period of time are unknown.2 Figure 3.1 shows breast cancer incidence among women less than age 50 and women aged 50 and above in the United States over a thirty-year period (based on data from nine of the National Cancer Institute’s SEER registries).3 At the same time, the incidence of breast cancer was increasing in countries that traditionally had very low rates, and it had become the most common cancer in women worldwide.
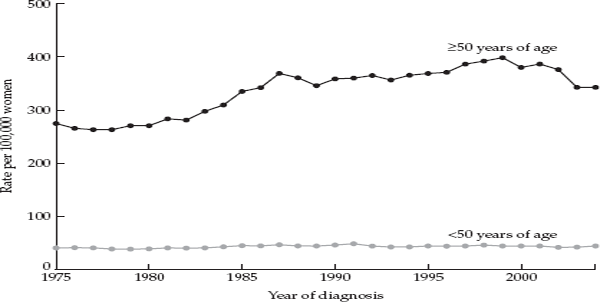
FIGURE 3.1 Trends in breast cancer incidence in women under 50 years of age and in women 50 and older, United States 1975–2004. Source: Ravdin et al., 2007.
Owing in part to the rising incidence rates and the rapid spread of mammography in the late 1980s and early 1990s, breast cancer became the focus of intense concern on the part of survivors of the disease, of women generally, and of politicians and scientists. Yet these developments alone cannot account for the dramatic upsurge of attention to this particular disease at that particular point in time. In the late 1980s the yearly number of new cases of breast cancer was roughly 180,000, and roughly 40,000 women died of the disease. In contrast, heart disease was responsible for about four-and-a-half times as many deaths per year in women, and lung cancer caused 50 percent more deaths in women. Yet neither of these more deadly diseases achieved anything like the visceral hold on women’s psyches that breast cancer did.
A number of characteristics of breast cancer help account for the powerful emotions it inspired. First, its impact is magnified because it afflicts women in all stages of life, and the prognosis is poorer in younger women. Furthermore, the disease attacks women in a part of their anatomy that is equated with femininity and beauty, as well as nurturance, and treatment was often radical and disfiguring. Another salient characteristic of the disease is the uncertainty surrounding both its clinical course and its etiology. There is no single overwhelming external cause identified in its development comparable to cigarette smoking, which accounts for roughly 90 percent of lung cancers, or even the handful of risk factors that account for the majority of cases of heart disease. Apparently, the firmer knowledge of the relationship of smoking and lung cancer is less anxiety-provoking than the uncertainty and ignorance surrounding the causes of breast cancer. In addition, breast cancer is a disease of women—less than 1 percent of breast cancer cases occur in men—whereas lung cancer and heart disease both have higher rates among men than women. Starting in the 1970s, the long-standing use of the radical mastectomy evoked a strong response from feminists, and subsequently breast cancer became a core issue in the women’s movement. These factors help account for the urgency and frustration surrounding the issue of breast cancer that reached their apogee in the late 1980s and early 1990s. Extensive media attention further amplified women’s fears of the disease. Although the majority of women who develop the disease survive, a diagnosis of breast cancer was viewed by many as a death sentence. As breast cancer became a high-profile disease, frustration and anger mounted at the lack of knowledge relevant to prevention.
KNOWN RISK FACTORS
As early as 1701, the Italian physician Bernardino Ramazzini had noted that breast cancer was more common among nuns than in married women, and he speculated that something about having children was protective. In the early twentieth century, physicians observed that women whose ovaries were removed at a young age had their risk of breast cancer reduced by half. And over the past 40 years, epidemiologic studies have consistently demonstrated that menstrual and reproductive factors influence a woman’s risk. These include an earlier age at onset of menstruation, later age at first full-term pregnancy, later age at menopause, having fewer, or no, children, and not breast-feeding. Each of these risk factors is associated with increased exposure to ovarian hormones, and particularly estrogen. Based on these findings, it became widely accepted that the greater the number of menstrual cycles a woman experiences during her lifetime, with exposure to high levels of estrogen and progesterone, the greater her risk of breast cancer. Being obese modestly increases the risk of breast cancer in postmenopausal women, and this is likely due to the conversion of androgens to estrogen in fat tissue, which becomes the major source of estrogen after menopause. In addition to these factors, a family history of breast cancer in a first-degree relative increases a woman’s risk by roughly twofold. A heritable factor underlying family history was identified in the mid-1990s with the discovery of the susceptibility genes, BRCA1 and BRCA2. While mutations in these genes carry a very high risk of breast cancer, because of their rarity, they account for only a few percent of breast cancers.
However, these known risk factors were judged by epidemiologists to account for less than half of breast cancer cases, and individually they showed only modest associations with the disease. Furthermore, these factors were not amenable to modification. What other factors accounted for the large unexplained portion of breast cancer incidence? Diet, and particularly dietary fat, became a major focus of epidemiologic research on the causes of breast cancer in the 1980s. This was due to the strong observed international correlation between intake of dietary fat and breast cancer death rates, displayed in chapter 2. By the mid-1990s the highly publicized notion that dietary fat was an important risk factor had gained widespread acceptance, even though, by that time, large cohort studies had failed to support this relationship. Other potential risk factors that were examined included use of hair dyes, cigarette smoking, alcohol consumption, height and weight, and exposure to X-rays, but these showed either no relationship or only a weak relationship to the disease, or they accounted for only a small proportion of cases. After decades of research on the role of lifestyle factors assessed in adulthood on the risk of breast cancer, little was known that would permit women to reduce their risk of the disease.
At this juncture, in the late 1980s and early 1990s, three widely circulated ideas came together to focus attention on a link between breast cancer and the environment. These were: (1) the perception that there was an “epidemic” of breast cancer in certain areas of the country, such as Long Island, New York; Cape Cod, Massachusetts; and the San Francisco Bay area; (2) the idea that known risk factors could explain less than half of the disease incidence; and (3) the conviction among many survivors and the public generally that some form of environmental pollution must play a role. In actuality, there was no epidemic of breast cancer on Long Island, and there was little evidence to support a role for the environment as a major cause of the disease. Nevertheless, these beliefs became the driving force behind an extraordinary political campaign by breast cancer advocates on Long Island and elsewhere.
Attention to cancer rates was to play a key role in drawing attention to breast cancer as a neglected problem. But, as students of medicine and public health usually learn in their first class in preventive medicine, what constitutes a “high” rate is not always obvious. In the early 1990s, the New York State Department of Health published statistics indicating that breast cancer incidence rates in Nassau and Suffolk counties were slightly higher than those for the state as a whole. For the period 1978 to 1987 the breast cancer incidence rate in Nassau County was 103 cases per 100,000 women, or 16 percent higher than the state average of 89 cases per 100,000. In Suffolk County the rate was 97 cases per 100,000, or 9 percent higher. These rates are similar to rates in other affluent suburban communities elsewhere in the United States, and most epidemiologists believe that they have a straightforward interpretation, which I will come to in a moment. Furthermore, rates fluctuate, and, when data for the 1990s were published, the excess in Nassau and Suffolk had decreased. However, somehow, the figure of a 30 percent excess in breast cancer incidence on Long Island was cited, and once this figure gained currency, it proved virtually impossible to correct.4 The activists and the media continued to cite this figure in order to maintain a high level of public concern, even though scientists and federal officials who were familiar with the actual rates were alienated by this distortion of the facts. What national statistics showed was that breast cancer rates were generally higher in the northeast of the United States, and Long Island’s rate was in line with the region as a whole. There never was an epidemic of breast cancer on Long Island.5
To most epidemiologists, the fact that breast cancer rates are slightly higher on Long Island than the average for New York State is not surprising. The population of Long Island is more affluent and more educated than the norm, and these groups tend to have children at a later age and to have fewer children compared to less affluent women. Both of these factors are associated with higher breast cancer incidence. Use of hormone replacement therapy, which modestly increases the risk of breast cancer, and usage of mammography, which leads to a higher rate of detection of breast cancer, are also more common in women in higher income brackets.
The estimate that 50–70 percent of breast cancer could not be explained by known risk factors encouraged the activists in their belief that the “environment” must be responsible for a large part of the unexplained proportion.
The vastness of the scope of possible agents in the water, soil, air, and food that could conceivably make a contribution to breast cancer was equaled by the activists’ certainty that there must be an external cause and that that cause must be some form of pervasive pollution. But the existing evidence that some environmental factor or combination of factors played a major role in breast cancer was weak on the face of things. For one thing, breast cancer rates tend to be higher in higher income groups, which in general would be expected to be exposed to lower levels of pollution. Also, since the creation of the EPA and the passing of the Clean Air Act and the Clean Water Act in the early 1970s, air and water quality had improved greatly on Long Island and elsewhere. The use of DDT and PCBs had been banned in the United States in the early 1970s, and levels of these compounds in the environment and in human tissues had declined steadily over the following decades. Furthermore, Long Island was not more heavily polluted than many other areas of the country. Finally, animal evidence for the carcinogenicity of organochlorine compounds, including DDT and PCBs—which were to become a major focus of research in the 1990s—was weak, although the evidence for polycyclic aromatic hydrocarbons, or PAH, produced in the burning of fossil fuels, wood, and cigarettes, was somewhat stronger.6
Recognition of these facts should not have ruled out study of a relationship of environmental factors to breast cancer, which some scientists felt was justified. But it could have helped to correct fundamental misconceptions of the public and the breast cancer activists and to rein in unrealistic expectations for what conventional epidemiological studies carried out in places like Long Island could achieve. In spite of certain questionable assumptions, the activists had put their finger on a crucial issue, namely, how limited current scientific knowledge is concerning the causes of breast cancer. They were correct in emphasizing the fact that existing knowledge did not allow one to predict with any certainty who would develop breast cancer, much less how to prevent it.
In retrospect, it is striking how disposed the public was to believe that some form of environmental pollution—whether chemicals in the soil and water, radionuclides from nuclear reactors, or magnetic fields from power lines, or something else—must be involved in the development of breast cancer. But, from the beginning, there was a fundamental divergence between the beliefs of the lay public and scientists generally on this question. This can be seen in a Harvard survey from the mid-1990s in which lay women as well as scientists were asked for their ideas on what caused breast cancer. Fifty-six percent of lay women believed that “chemicals” in the environment played a role in the disease, whereas only 5 percent of scientists did.7 Most breast cancer researchers, including epidemiologists and basic scientists, were simply not receptive to the idea that the environment contributed to the etiology of the disease. Their skepticism stemmed from the fact that, if easier to study exposures like cigarette smoking, alcohol consumption, and hormone replacement therapy showed either no clear-cut relationship or, in the case of alcohol and hormone replacement therapy, only a very modest effect, the very much lower levels of exposure to chemicals in the environment were unlikely to show any relationship. Skepticism on the part of most scientists was to make it very difficult for researchers who felt that there was sufficient justification for carrying out studies to address a possible link to the environment. As one veteran researcher put it, proposals on this topic tended to be “derided” in federal grant review panels.8 Another reason for the skepticism of breast cancer researchers was that a number of studies had shown that most of the regional variation in breast cancer rates within the United States could be explained by the distribution of known breast cancer risk factors.9 In the early 1990s, in response to pressure from community activists, the federal Centers for Disease Control had also examined this question and had come to same conclusion.
LONG ISLAND AS LABORATORY
By the early 1990s, Long Islanders’ awareness of environmental pollution had been growing for two decades. Long Island is a densely populated coastal formation adjacent to New York City, which underwent rapid population growth and development starting in the 1940s. Over the thirty-year period between 1940 and 1970, the population of Nassau County increased by more than threefold and that of Suffolk County by almost sixfold. In the 1970s, there was widespread concern in the United States about environmental pollution and its possible link to cancer rates. Specific incidents like the Three Mile Island release of radioactive material and the evacuation of Love Canal in the late 1970s seemed to represent only the most extreme examples of a pervasive problem. On Long Island, concern about pollution focused on highly publicized well closings due to elevated levels of chemical pollutants, as well as on landfills, incinerators, and industrial sites. Long Island had been an important agricultural area until the 1950s and 1960s, and pesticides and herbicides, including DDT, chlordane, and dieldrin, had been widely used. Residents of Suffolk County also worried about emissions of radionuclides from the nuclear reactor at Brookhaven National Laboratories. Another source of pollution was the large volume of automobile and airplane traffic. In response to these fears about environmental pollution on Long Island, in 1990 the New York State Department of Health issued the report mentioned earlier, which confirmed the role of known risk factors but failed to demonstrate a clear link to environmental pollution.
Like Barbara Balaban, many breast cancer advocates on Long Island were enraged by what they saw as the failure of state and federal agencies, like the New York State Department of Health and the Centers for Disease Control, and the scientific community to acknowledge that they could not explain what was causing most breast cancers. Many activists had the impression that their neighborhoods contained more women with breast cancer and other cancers than should be occurring normally. In other words, they identified what they thought were clusters, even though it is notoriously difficult to determine whether an apparent cluster really has a common cause, or whether it is merely due to chance, since inevitably, some areas are going to have higher rates of cancer and some lower rates, simply due to chance.10
A number of breast cancer survivors undertook ambitious surveys in their communities producing detailed maps indicating homes where an occupant had been diagnosed with cancer. One theory put forward by a prominent activist held that breast cancer tended to occur in homes that were at the end of the water distribution system, suggesting that some pollutant in the water was accumulating in these homes. However, the assessment of geographical clusters requires sophisticated statistical techniques, and these lay efforts failed to produce interpretable results.
Spurred by their conviction that something was going on in their communities and inspired by the recent success of AIDS activists, breast cancer advocates on Long Island formed a number of organizations to raise consciousness in their communities regarding breast cancer and to press for increased government funding for research into its causes. The most influential of these organizations was One-in-Nine (the name referred to a woman’s chances of developing breast cancer in her lifetime). Many towns had their own breast cancer organizations, including Babylon, Huntington, West Islip, Brentwood, and Garden City, and these local organizations joined together to form the Long Island Breast Cancer Network. The women in these groups were extremely well-informed about breast cancer, were highly vocal and effective politically, and they had a well-defined goal. They wanted answers to the question of what had caused them, their relatives, and their neighbors to develop the disease and how they could prevent it in their daughters. In 1991 the National Breast Cancer Coalition was formed in order to lobby Congress for increased funding for research. The coalition held several meetings with scientists both in Washington and on Long Island to “brainstorm” about what could be done to address the gaps in knowledge about breast cancer. By bringing together prominent scientists concerned with breast cancer and advocates who had both a commitment to see progress in research and considerable political clout, these meetings provided the impetus for a large infusion of funds into breast cancer research. Barbara Balaban was one of the organizers of these meetings, and she recalls how excited and “energized” both the scientists and the activists were at the prospect of a concerted effort to address the neglected area of environmental contributions to breast cancer.11 Galvanizing speakers like the breast cancer surgeon and author Susan Love and the epidemiologist Devra Lee Davis helped articulate the activists’ sense of purpose and give it legitimacy in the eyes of politicians.12
To strengthen their case, activists made a highly effective comparison of the number of breast cancer cases with the number of AIDS cases over a twelve-year period and the amounts of money devoted to research on each disease. The comparison revealed that, in spite of its affecting seven times as many people as AIDS, breast cancer received only a third of the research funds devoted to AIDS.13
Once they had an action plan articulated by the National Breast Cancer Coalition, the activists started to lobby politicians to support increased funding for research on breast cancer. In the Senate, Tom Harkin of Iowa and Alphonse D’Amato, who was up for reelection from Long Island, became key supporters of increased funding for breast cancer, as did all of the five representatives from Long Island. Another key player was Phil Schiliro, an aide to Henry Waxman, the chairman of the House subcommittee on health and the environment. Schiliro was planning to run for a congressional seat on Long Island in 1992, and he realized the enormous power of breast cancer as an issue. It was Schiliro who, with input from Devra Lee Davis, drafted the legislation for a study of breast cancer on Long Island, which Waxman and D’Amato then introduced in Congress. After years of media attention to the supposedly high rates of breast cancer on Long Island and to concerns about the environment, the issue was now bipartisan and bicameral. When one congressman asked Barbara Balaban, “Why Long Island?” she responded, “This will be a model for the whole nation.”
THE CONGRESSIONAL MANDATE
In response to lobbying by Long Island activists and the support from powerful congressmen, the National Cancer Institute and National Institute of Environmental Health Sciences issued several requests for applications (RFAs), to stimulate researchers to investigate the reasons for the geographic variation in breast cancer rates within the United States.14 But an even more dramatic response came in the form of Public Law 103-43, which was passed by the U.S. Congress in June 1993. This unusual piece of legislation directed the head of the National Cancer Institute, in collaboration with the head of the National Institute of Environmental Health Sciences, to “conduct a case-control study to assess biological markers of environmental and other potential risk factors contributing to the incidence of breast cancer” in Nassau and Suffolk counties. The law went on to specify “the use of a geographic system to evaluate the current and past exposure of individuals, including direct monitoring and cumulative estimates of exposure to: 1) contaminated drinking water; 2) sources of indoor and ambient air pollution, including emissions from aircraft; 3) electromagnetic fields; 4) pesticides, and other toxic chemicals; 5) hazardous and municipal waste; 6) and such other factors as the director determines to be appropriate.”15 The full text of the law is shown in box 3.1.
Public Law 103-43 represents an uneasy marriage of science and politics. On the face of it, the law brought together the scientific community, the federal government, and the community activists in the common task of shedding light on the causes of breast cancer. In actuality, however, no matter how many community meetings were held on Long Island with the attendance of activists, politicians, government officials, and academic scientists, it is doubtful that the politicians or the activists on the one hand and the scientists on the other were ever really talking about the same study or really understood each other.
Several features of the law merit comment. First, here was the legislative arm of the government not only directing two institutes of the National Institutes of Health (NIH) to carry out scientific research to address a specific problem, but it was going so far as to dictate the type of study to be used—a case-control study. While a lot of science is a response to directed legislation, it is highly unusual for a congressional law to specify the design, the population, and the hypothesis of a study. One drawback of doing this is that it limits the investigators’ ability to respond with the best possible scientific conception.16
Box 3.1 Study of Elevated Breast Cancer Rates in Long Island Public Law 103-43, June 10, 1993
Sec. 1911. Potential Environmental and Other Risks Contributing to Incidence of Breast Cancer
(a) REQUIREMENT OF STUDY
(1) IN GENERAL—The Director of the National Cancer Institute (in this section referred to as the “Director”), in collaboration with the Director of the National Institute of Environmental Health Sciences, shall conduct a case-control study to assess biological markers of environmental and other potential risk factors contributing to the incidence of breast cancer in—
(A) the Counties of Nassau and Suffolk, in the State of New York, and
(B) the 2 counties in the northeastern United States that, as identified in the report specified in paragraph (2), had the highest age-adjusted mortality rate of such cancer that reflected not less than 30 deaths during the 5-year period for which findings are made in the report. [Schoharie County, NY, and Tolland County, CT]
(2) RELEVANT REPORT—The report referred to in paragraph (I)(B) is the report of the findings made in the study entitled “Survival, Epidemiology, and End Results,” relating to cases of cancer during the years 1983 through 1987.
(b) CERTAIN ELEMENTS OF THE STUDY—Activities of the Director in carrying out the study under subsection (a) shall include the use of a geographic system to evaluate the current and past exposure of individuals, including direct monitoring and cumulative estimates of exposure, to—
(1) contaminated water;
(2) sources of indoor and ambient air pollution, including emissions from aircraft;
(3) electromagnetic fields;
(4) pesticides, and other toxic chemicals;
(5) hazardous and municipal waste; and
(6) such other factors as the director determines to be appropriate.
(c) REPORT—Not later than 30 months after the date of the enactment of this Act, the Director shall complete the study required in subsection (a) and submit to the Committee on Energy and Commerce of the House of Representatives, and to the Committee on Labor and Human Resources of the Senate, a report describing findings made as a result of the study. [An amendment rescinded the 30-month deadline.]
(d) FUNDING—Of the amounts appropriated for fiscal years 1994 and 1995 for the National Institute of Environmental Health Sciences and the National Cancer Institute, the Director of the National Institutes of Health shall make available amounts for carrying out the study required in subsection (a).
The reasons for the choice of a case-control study are clear. This type of study can be carried out in a much shorter time and is also much less expensive than the main alternative, the cohort study design. Interestingly, a number of scientists, including the National Cancer Institute’s project director for the Long Island Breast Cancer Study, Dr. Iris Obrams, felt that a case-control study was the wrong approach and favored a cohort study.17 However, while cohort studies have a number of strengths, they are not superior to case-control studies for reconstructing exposures that occurred in the past.
Second, the scope of the specific topics to be addressed within the law was vast, and the text of the law provided no ranking of the different topics in terms of importance. There was also no discussion of the rationale behind including specific items on the list and no discussion as to what the focus of the efforts should be. The reason for this is that the government was making funds available for research on the broad question of environmental exposures and breast cancer and leaving it up to the scientists applying for funding to make a convincing case in their proposal for what specific questions should be addressed and how. Scientists are used to this “mechanism” of the federal government for stimulating research. But the diffuse “laundry list” of potential environmental hazards undoubtedly reinforced the activists’ conviction that many aspects of their environment were a problem. In reality, what the diffuse list reflects is the fact that almost nothing is known about a possible contribution of the environment to breast cancer.
Third, as written, the law required the use of an overarching geographic information system (GIS), a high-powered, computerized system, to supply the framework for estimating individual exposure to the wide array of exposures of interest. However, the use of a GIS in the study of chronic diseases of long latency, such as breast cancer, was, and still is, in its infancy. In addition, there was a huge problem of missing data. (Data on levels of various pollutants in the air, water, soil, and food throughout Long Island over the decades were generally not available.) The GIS, which was sold to the activists by the National Cancer Institute as the ultimate methodology for coming to grips with the health effects of environmental pollution, was in effect a concept without the necessary data to deliver on its promise. Thus, a central requirement of the mandate was totally unrealistic and unachievable within the envisioned period of performance. More than ten years after the enactment of the public law, a first-generation GIS for Long Island was still under development.18
It should also be noted that while the language of the law was both broad and ambitious, at the same time, the stated objective was cautiously worded and modest. It was not to “determine” or “identify” “the causes of breast cancer on Long Island” or “the causes of the elevated rates of breast cancer on Long Island,” but merely “to assess biological markers of environmental and other potential risk factors contributing to the incidence of breast cancer.” This carefully crafted technical language is highly revealing and goes to the heart of the difference in “culture”—and hence understanding—of the community activists and the politicians on the one hand and the scientists on the other.
Although epidemiologists’ ultimate objective is to identify new causes of disease, in reality, what they work with are entities that can be carefully defined and measured and correlated with the occurrence of the disease. Thus, the scientists were well aware that all they had to work with were “biological markers of environmental and other potential risk factors.” And they were also well aware of the need for multiple, independent studies of the same question to clarify a hypothesized association. No single study was likely to pinpoint and confirm a particular exposure as a cause of disease. And, given the problems of accurately assessing long-term exposure, even a large number of studies that suffer from the same limitations, can be inconclusive. Finally, the scientists were painfully aware that the ability to investigate a given question hinged on the availability of adequate methodological tools. Most crucially, the ability to investigate specific hypothesized exposures depends on the availability of validated markers of exposure. (Many exposures may merit investigation, but unless one has a marker of long-term exposure, studies are likely to be inconclusive). These considerations, with their implied limitations on what could be studied, were difficult for the community activists to appreciate and to accept. Where the scientists were talking about “markers of environmental and other risk factors,” the activists were thinking “causes” and were anticipating dramatic and useable knowledge.
Another aspect of the legislation merits comment. In order to broaden political support for the bill, two other counties—Tolland County, Connecticut and Schoharie County, New York—were included in the final legislation. Both had the highest breast cancer mortality rates in their respective states in the last year for which statistics were available at the time the legislation was being drafted. However, both counties have extremely small populations, and cancer incidence rates in small populations tend to be unstable. In fact, when data became available for the following years, Schoharie County showed the lowest breast cancer mortality of all sixty-eight counties in New York State. Nevertheless, the researchers were obligated under the law to conduct a study of breast cancer in that county, even though over a period of several years, they only succeeded in identifying fewer than a dozen cases of the disease—far too few to obtain meaningful results.
Finally, it should be noted Congress did not appropriate any additional funds to carry out the studies on Long Island. The money designated by Public Law 104-43 had to come out of the existing NIH budget. In other words, every dollar spent on the Long Island Breast Cancer Study Project, as it came to be known, was at the expense of other competing NIHsponsored projects.
Enactment of the 1993 law represented an extraordinary victory for the Long Island breast cancer activists. Through their persistence and political resourcefulness, they had managed to obtain coveted federal funding for a major scientific study on Long Island. But, owing to constraints imposed by the legislation and the large gulf in understanding separating them from the scientists, it was inevitable that the actual study designed and implemented by scientists would fall far short of their expectations. It is hard to convey to the lay public just how limited any individual study of this kind is owing to methodological limitations and the difficulties of obtaining accurate information on exposures at different periods of life. Each study can only address in a highly simplified form a small number of relationships that are isolated from a vast universe of possible relationships. If one measures exposure at the wrong time of life, one may fail to find an important relationship that exists. This is why it is necessary to carry out many different types of studies in different locations and in different populations. But it is also difficult for the lay public to accept just how incremental progress in a difficult area such as effects of environmental pollution on human disease is.
The activists’ expectations of what this high-profile, government-sponsored study could achieve were not tempered by any familiarity with the painfully slow process of epidemiologic research on chronic diseases of long latency or with the considerable practical and technical limitations affecting research in this area. They viewed the study as their creation since it was owing to their efforts that the law was passed and the funding made available. The fact that the study was to draw participants from their communities encouraged the activists in their belief that it would turn up an explanation for why many of them had contracted breast cancer and that it would enable their daughters to avoid the disease in the future. In retrospect, it is easy to see that the activists’ hopes for a dramatic breakthrough in the understanding of the causes of breast cancer were bound to be disappointed.
THE LONG ISLAND STUDY
A number of different scientific projects were funded under the aegis of Public Law 104-43, and these are collectively referred to as the Long Island Breast Cancer Study Project (LIBCSP). Responding to the congressional mandate for a study, in 1994 a group of New York City and Long Island researchers from major area medical centers submitted a detailed application for a large case-control study to the National Cancer Institute and the National Institute of Environmental Health Sciences. This effort was led by Dr. Marilie Gammon of Columbia University’s School of Public Health, who had experience carrying out large collaborative studies on breast cancer. After a lengthy peer review process, including a “site visit” at Columbia by a panel of scientists and extensive written critiques of each component of the study, which needed to be responded to, the proposal was funded. The objective was to enroll every newly diagnosed breast cancer case occurring in female residents of Nassau and Suffolk Counties during a one-year period (August 1, 1996, to July 31, 1997). For each case a woman with no history of breast cancer was randomly selected as a “control.” In the end, roughly 1,500 cases and 1,550 controls were recruited to the study. Each participating woman provided extensive information on her personal history, collected by means of an in-person interview and self-administered questionnaires; in addition, most women provided a blood and a urine specimen. Because of its size and prominence, the Columbia study itself came to be referred to as the LIBCSP.
The study had two major objectives. The first was to determine whether levels of organochlorine compounds were higher in the blood of breast cancer patients than in the blood of women without breast cancer. These compounds included the pesticides DDT (and its major breakdown product DDE), chlordane, and dieldrin, as well as polychlorinated biphenyls (PCBs). PCBs are compounds found in coolants and lubricants in transformers, capacitors, and other electrical equipment.
The second objective was to determine whether exposure to a class of compounds known as polycyclic aromatic hydrocarbons (or PAH) produced by the burning of fossil fuels and other organic materials was associated with increased risk of breast cancer. This hypothesis was formulated to address the question whether air pollution from heavy automobile traffic on Long Island as well as consumption of grilled foods contributes to incidence of the disease. PAH can bind to DNA forming “adducts,” and the resulting DNA damage is thought to play a role in initiating cancer. The plan for the study envisaged measuring these adducts in the blood of women with and without breast cancer.
How strong was the rationale for focusing on organochlorine compounds and PAH as causes of breast cancer? The answer appears to be: not very strong.
The first hypothesis hinged on the finding that some organochlorine compounds can mimic the effects of estrogen, and, as we have seen, estrogen is widely believed to play a critical role in the development of breast cancer, although the precise mechanism is not understood. By the early 1990s, the possibility that “endocrine disruptors” in the environment might be responsible for a wide range of effects in wildlife and humans had become a topic of serious concern.19 Thus, the study focused on organochlorine compounds because they are widespread in the environment, measurable levels are found in biological fluids in many Americans, and they persist in the body for many years. However, comprehensive assessments of the available animal and human evidence bearing on the carcinogenicity of DDT, PCBs, and other organochlorine compounds that appeared in the mid-1990s concluded that these compounds were unlikely to affect the risk of breast or endometrial cancer “in any but the most unusual situations.”20 These reports noted that evidence from the handful of small case-control studies did not indicate the existence of a consistent risk.21 In addition, women with relatively heavy exposure from occupational settings did not appear to be at increased risk. Finally, if DDT were an important cause of breast cancer, the marked decline in DDT levels over recent decades should have led to a reduced incidence of breast cancer, which is not the case.22
The rationale for the second major hypothesis concerning PAH was that experimental evidence indicated that chemicals produced in the combustion of organic material were mammary carcinogens in animals. Therefore, higher exposure to these compounds present in tobacco smoke, charcoal-broiled foods, and air pollution might show an association with human breast cancer. The researchers argued that the use of PAH-DNA adducts had the virtue of providing both a marker of exposure and a marker of the individual’s ability to repair damage caused by these compounds. But the external reviewers of the grant were far from being convinced of the ability of this component to produce informative results. A major problem with the hypothesis that PAH play an important role in the development of human breast cancer is the failure of a large number of studies to show a consistent effect of cigarette smoking on breast cancer, since smoking and eating charbroiled food are much more significant sources of PAH than air pollution. In addition, PAH-DNA adducts only provide an intermediate-term marker of exposure. They may reflect exposure over recent months but not exposure years earlier, when breast cancer was likely to be initiated. It is also noteworthy that the level of PAH-DNA adducts do not show the kind of strong and consistent relationship to lung cancer risk that one sees with smokers’ reports of the number of cigarettes they smoked per day.23
A number of other hypotheses were included in the Columbia University proposal, but it is fair to say that these two were responsible for its being funded. (Another major hypothesis—that exposure to electromagnetic fields in the home increased the risk of breast cancer—was the focus of a linked study carried out at the State University of New York at Stony Brook. This study will be described in chapter 4.)
In spite of the LIBCSP’s relatively weak hypotheses and its methodological limitations, both NCI and the lead investigators indulged in the kind of public relations promotion of the study that could not fail to encourage unrealistic expectations. According to Iris Obrams, at the time NCI’s chief of extramural programs in epidemiology and director of the Long Island project, the study represented “an opportunity for groundbreaking epidemiologic research that may serve, ultimately, as a research model for the nation. When the project is finished, we hope to be in a position to give solid preventive advice not only to women on Long Island, but to women all over the country.”24 Commenting on the package of studies to be carried out under the umbrella of the Long Island Breast Cancer Study Project, a staff writer for the Long Island newspaper Newsday wrote: “Many of the studies will be groundbreaking—either because they will be the first, among the largest and most rigorous or because they are taking a different approach. And, taken together, they should provide an in-depth look at the impact of the environment on breast cancer on Long Island, as well as potentially offer insights into the disease for all scientists and doctors.”25 And Marilie Gammon, the lead researcher of the large case-control study, offered the following: “This study is the envy of the nation. Every women’s group wants it, and Long Island has it.”26 Although the euphoria was understandable, given the hard work on the part of all parties in obtaining funding for the study, these statements from people in a position to know better betrayed a naïve expectation that this one set of studies with highly focused objectives and limited methods was going to deliver a breakthrough. It was as if, in the flush of launching the study, both NCI and the researchers succumbed to the naïve expectations of the activists.
The scientific project within the LIBCSP, which the reviewers judged to be of greatest interest, was the organochlorine component. And the major reason for this was that Mary Wolff of the Mount Sinai Medical School, a highly respected toxicologist who had done extensive work on organochlorine compounds, was the lead investigator on that project. Together with Paolo Toniolo, a New York University epidemiologist, Wolff had recently published a study purporting to show a dramatic association between DDT/ DDE exposure and breast cancer.27 This study appeared at a crucial juncture—April 1993—in the mounting concern over environmental pollution and breast cancer. Published in the prestigious Journal of the National Cancer Institute, it seemed to provide solid evidence in support of the breast cancer activists’ contention that the environment on Long Island played a role in their developing breast cancer. In addition, it strengthened the position of epidemiologists making a case for focusing on the environment and led to the conduct of numerous studies of DDT and PCBs by other investigators.
Wolff and Toniolo had used stored blood samples from New York University’s Women’s Health Study—a cohort study designed to investigate the role of diet and hormones in the development of cancer—to measure DDT, its main metabolite DDE, and PCBs in breast cancer cases and in women without breast cancer. The Women’s Health Study had stored blood specimens obtained from 14,290 women enrolled between 1985 and 1991. From this cohort, the investigators selected 58 women who had developed breast cancer in the one to six months following enrollment and 171 matched control women from the cohort without breast cancer. The authors reported that, after adjustment for potential confounding factors, women with the highest blood level of DDE had a statistically significant fourfold increased risk of breast cancer compared to women with the lowest levels. In contrast, no significant association of elevated PCB levels with breast cancer was observed. In the discussion section of the paper, the authors wrote, “Our data suggest that organochlorine residues and, in particular, DDE are strongly associated with breast cancer risk.”28 And their closing sentence drove home the significance of their results: “Given the widespread dissemination of organochlorines in the environment, these findings have immediate and far-reaching implications for public health intervention worldwide.” A similar sentence concludes the abstract of the paper, which is all that many people read.
Looked at in hindsight, perhaps the most striking feature of the Wolff and Toniolo paper is the rather substantial gulf between the limited data presented by the authors and their far-reaching claims for their potential significance. Among the study’s strengths were its somewhat larger number of cases compared to previous studies and the authors’ ability to take into account potential confounding factors. Another apparent strength was its prospective design—the fact that blood samples were collected from apparently healthy women before the diagnosis of breast cancer. However, the selection of cases diagnosed within one to six months of enrollment makes it likely that the cases already had breast cancer when their blood was drawn, raising a question about whether the levels measured in cases were truly reflective of their predisease levels. Furthermore, bloods were obtained in the late 1980s and early 1990s, and, therefore, organochlorine levels might not be indicative of what the levels were two decades or more earlier, before DDT use was banned.
Although the number of cases was larger than in the handful of previous studies, fifty-eight cases is still small when one wants to look for a dose-response relationship and when one has to take into account a variety of confounding or modifying factors.
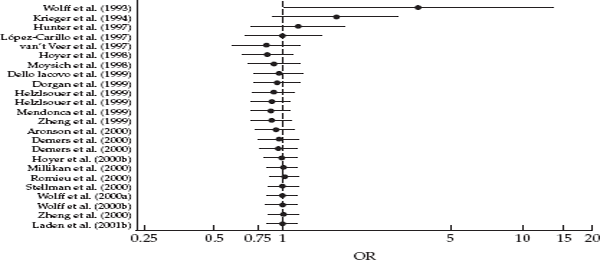
A look at the key results demonstrates how an influential conclusion can hinge on tenuous data. In fig. 3.2, taken from their paper, the authors presented graphs for both DDE and PCBs, showing how a woman’s blood level of each compound affected her risk of breast cancer. Blood levels of both compounds were partitioned into five levels, or “quintiles,” and odds ratios for breast cancer were computed for quintiles 2–5 relative to the lowest quintile (Q1). The authors contrasted the results for the two compounds. In describing the PCB results (panel B), they noted that, in spite of the fact that two levels (Q2 and Q3) were significantly elevated, the “flattening out” at higher levels did not suggest a dose-response relationship. In contrast, they reported a significant increasing linear trend with increasing DDE level (panel A). But comparison of the two panels reveals that the two curves are quite similar. In both graphs the middle point (Q3) represents the maximum, with Q4 and Q5 showing a relative decline. In the DDE data, only Q3 has a statistically significant odds ratio, and the same flattening out occurs at the highest exposure levels. In addition, when the data were categorized more finely into ten levels of exposure, the odds ratio for the highest “decile” (90th percentile) was not substantially higher than the odds ratio for the highest quintile of exposure. This actually provides further evidence of a lack of a dose-response relationship. All of this suggests that the results in panel A could merely be a chance finding based on small numbers.
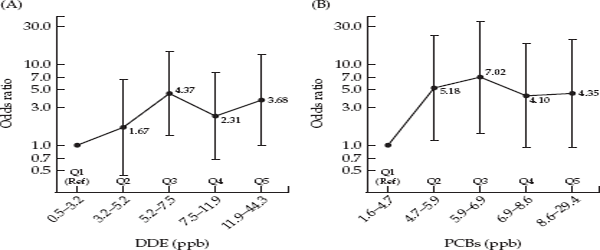
FIGURE 3.2 Odds ratios for breast cancer by quintiles of DDE and PCB concentrations in serum. Source: Wolff et al., 1993.
While the authors mentioned the small number of cases and the short interval between blood draw and diagnosis in their discussion, these qualifications were given short shrift compared to the emphasis on the strength of the observed relationship and its implications. Given these aspects of the study, and the problem of using a one-time measurement of DDE in midlife to characterize a woman’s exposure in the past, Wolff and Toniolo’s claim of a “strong association” and of its important implications for “public health intervention worldwide” was questionable, to say the least.
However, in spite of their study’s weaknesses, Wolff and Toniolo’s dramatic results galvanized researchers and the federal government to undertake larger studies of organochlorine compounds and breast cancer. The editorial that accompanied the article referred to it as a “wake-up call for further urgent research,”29 and the National Cancer Institute and the National Institute of Environmental Health Sciences set up special programs to encourage research on DDT and other organochlorine compounds and breast cancer. In the eleven years following publication of the Wolff and Toniolo paper, approximately thirty epidemiologic studies were published on this topic.30
Overwhelmingly, the many new and larger studies showed no association of DDT/DDE or PCB exposure and risk of breast cancer. A 2002 review of the accumulated literature by researchers at the American Cancer Society concluded that: “With rare exceptions, there is consistent evidence from the many methodologically sound studies of no association between levels of persistent organochlorine compounds, notably DDE and PCBs, and breast cancer.”31 Furthermore, a meta-analysis that included 22 studies of DDT exposure and breast cancer reported no elevation in risk of breast cancer (summary odds ratio = 0.97, 95% CI 0.87-1.09), and the authors were unable to explain elevated findings like those of Wolff and Toniolo. Interestingly, the cumulative plot showing the summary estimate with the publication of each new study shows that the summary odds ratio is elevated in the Wolff study but thereafter declines remaining around 1.0, with increasingly narrowing confidence intervals as the weight of more studies is added to the meta-analysis (fig. 3.3). The authors suggested that Wolff and Toniolo’s result might just be a chance finding, concluding that, “Overall, these results should be regarded as strong evidence to discard the putative relationship between p,p’-DDE and breast cancer risk.”32
Among the new studies were updated results from the NYU Women’s Health Study published in 2000. In the new analysis, based on an average of two-and-a-half years of follow-up (from blood collection to diagnosis) and 148 breast cancer cases and 295 matched controls, Wolff, Toniolo, and colleagues wrote: “We found no association of DDE, PCBs, or their half-lives with risk of breast cancer in this cohort study . . . our results do not support a relation between DDE or PCB levels and breast cancer in a prospective cohort of New York City women.”33
FIGURE 3.3 Cumulative meta-analysis of studies of DDE exposure and breast cancer. Source: Lopez-Cervantes et al., 2004. Lines on either side of the black circles give the 95 percent confidence interval. The wide confidence interval of the Wolff et al. study reflects its small sample size. As more studies are added to the metaanalysis, the summary odds ratio approaches 1.0, and the width of the confidence interval becomes narrower.
When the long-awaited results of the Long Island study were finally published in 2002, they too showed no association of blood levels of DDE, chlordane, dieldrin, or of the four most common PCB congeners with breast cancer and no indication of a dose-response relationship.34 Furthermore, there was no suggestion of an effect of exposure in subgroups of women who might be at increased risk: those who had not breastfed, were overweight, were postmenopausal, or were long-term residents of Long Island.
In retrospect, the significance of the 1993 paper by Wolff and Toniolo is that it shows how even highly competent scientists can get carried away by a result and that the scientific community can then react with insufficient skepticism. Not surprisingly breast cancer advocates latched onto this study, since here were two well-known scientists implying that their results provided strong evidence of an environmental exposure on breast cancer. Of course, it is only human to want to come up with positive findings and to have them be meaningful. But for this reason, it is important to be aware that it is only too common for a small, early study of a new question to show an intriguing result. When the initial study is followed by larger and more carefully executed studies, it is often the case that the initial finding is not borne out.35 This has happened in an early study indicating that coffee drinking was associated with pancreatic cancer,36 in a study suggesting that consuming beverages containing caffeine could lead to benign breast disease,37 and in early studies linking fat intake with increased breast cancer risk38—to cite but a few instances of this pattern. When the stakes are high—both in terms of the public’s desire for knowledge and of researchers’ drive to obtain meaningful findings—the danger of getting carried away and committing the cardinal sin of “believing one’s hypothesis” is at its greatest.
REACTIONS TO THE LONG ISLAND STUDY
Well before the results of the LIBCSP became known, the breast cancer advocates who had lobbied so aggressively for it had become disillusioned with the project. In part, this was because a considerable portion of the funds from the National Cancer Institute earmarked for research on Long Island had nothing to do with the environment but were to be used to promote mammography and to study medical records. But the main reason for their disaffection was the fact that the case-control study was narrowly focused on only a small number of compounds measured in blood and that these compounds for the most part had been taken out of use decades earlier. There were many other compounds that the activists wanted to see included within the scope of the study, and they hoped that incriminating evidence would lead to statewide bans on additional chemicals. In short, they wanted a much more comprehensive study. However, for the many additional compounds the activists were concerned about, there was simply inadequate information available of their carcinogenicity in animals.39 In addition, there was no suitable human biological marker that would provide an indicator of long-term exposure. This became the major source of dissatisfaction among the activists. By the time the results were published, they were prepared for the null findings and felt that the LIBCSP had done little to address their concerns.
Publication of the Long Island results in 2002 was greeted with criticism and postmortems from a number of quarters. The Long Island newspaper Newsday published a special series of articles delving into the study’s history and attempting to explain what went wrong.40 The New York Times published an article by the science reporter Gina Kolata entitled “The Epidemic That Wasn’t.”41 One of the figures interviewed by Kolata, Dr. Deborah Winn, the head of extramural epidemiology at the National Cancer Institute, was not exactly supportive of the study that her institute, along with the NIEHS, had spent thirty million dollars on. When asked if the study was based on a false premise—since the breast cancer incidence rates on Long Island were not as high as they had been made out to be—Winn responded, “You’re not going to get me to answer that question.”42 Also interviewed by Kolata was Michael Bracken, a professor of epidemiology at Yale University, who was even more critical of the study. According to him, it should never have been undertaken: “It is an example of politicians jumping on the bandwagon and responding to the fears of their local population without really thinking through what is going on in science.” Such a study, he said, “is not so much science as a political response.”43 While this is clearly true, it should also be said that the Long Island study was not inferior to similar studies that were carried out elsewhere in the United States.
After almost a decade of carrying out high-profile research on Long Island, Marilie Gammon has a highly nuanced view of how scientific issues can become distorted and politicized when the anxiety of the public is aroused. But she also has keen insights into how, within science itself, there are “fashions” and political pressures. She has an appreciation for the paradoxical nature of the Long Island Breast Cancer Study Project and forthrightly acknowledges that it would never have been funded if it had not been mandated by congressional legislation. On the other hand, even though the study owed its existence to lobbying by activists, Gammon believes strongly that it was justified both on scientific grounds and as a response to the intense level of public concern. As to the scientific grounds, she points to animal experiments that suggested that organochlorine compounds and polycyclic aromatic hydrocarbons (PAH) could play a role in breast cancer. Gammon noted that scientists routinely disagree about how compelling the evidence from specific animal studies is and how it relates to human beings. But she felt that the existing studies were sufficiently suggestive to merit carrying out epidemiologic studies. As she put it, “if you actually look at the data—the laboratory research—there is some hint that something might be going on. . . . Scientifically, I felt that there was enough biological evidence there to make us really take a serious look at what was going on.”44 But she also makes a totally separate argument that, when the public is deeply concerned about a public health question, researchers who, after all, are supported by taxpayers’ dollars have some obligation to attempt to resolve the issue. As she put it, “It’s hysteria in a lot of ways—you could label it that. But on the other hand, you could say, ‘Well, why don’t we find out once and for all?’ And we can eliminate it if that’s really true. So I felt that, even though it was politically-motivated and it wouldn’t have happened without politics, I still think it was valuable. . . . Without the politics, it would never have happened. And I don’t think that’s wrong, I guess is what I’m saying.”
Gammon went on to argue that to acknowledge the fact that the study was politically motivated does not mean that its quality wasn’t high. On the level of the hypotheses that were proposed and the methods used to test those hypotheses, she feels that she and her collaborators got the science right and that this was acknowledged by her peers.
By deciding to undertake research on a topic that was subject to great public concern and one that was not taken seriously by the majority of mainstream cancer researchers, Gammon had put herself in a thankless position. She made heroic efforts to keep the activists on Long Island informed of the study’s progress by giving dozens of talks to community groups and meetings of the Long Island Breast Cancer Network. She tried to correct erroneous beliefs (like the alleged thirty percent excess in breast cancer incidence on Long Island) and to temper their more unrealistic expectations. She tried to convey to them the very real limitations in doing this kind of research. But in spite of her efforts, over time, the activists became increasingly dissatisfied by what this one study could accomplish. Commenting on the gulf between the mindset of the researchers and that of the activists, Gammon said:
I think that it was harder for them because they expected that if we did the study we would find the right answer. And the truth of the matter is that’s not how science works. And in epidemiology it’s magnified because it takes a lot longer. You go to the lab and you work for three months and you get an answer. You go into the field and you work for three years, and you still don’t have an answer, right? Epidemiology is magnified by whatever percent because it takes so long to do the research. The women wanted a SWAT team. They wanted us to come in and measure everything. We didn’t know how. We don’t know how to measure everything. They wanted Dustin Hoffman in The Hot Zone coming in and measuring every window sill. We don’t have that. It’s a fantasy that we know how to do all that. We don’t.45
Looking back on the 1993 publication by Wolff and Toniolo, which had provided a major justification for her Long Island proposal, Gammon was refreshingly willing to engage in self-criticism, acknowledging that Mary Wolff and later she herself had gotten carried away:
I think that our mistake in what we did initially was in assuming that Mary’s [risk estimates] could even be that high. Because there’s no risk factor for breast cancer that carries that kind of odds ratio. Family history is what—two? So, what were we thinking! That was totally short-sightedness. I remember half-way through the study I turned to Mary and said, ‘What were we thinking!’ She looked at me and said, ‘You’re right! That’s impossible, you’re right!’ When you see an odds ratio of four you get carried away.46
As we have seen, large epidemiological studies of DDT and PCBs were initiated in the mid-1990s due to the heightened concern about an environmental contribution to breast cancer and, in part, to the provocative results of the article by Wolff and Toniolo. Once a question achieves high visibility and the government puts out requests for applications, researchers flock to address it. However, what needs emphasizing is that when a topic like organochlorines is catapulted into the spotlight, important scientific considerations can be lost sight of. One such issue was the fact that, due to the ban imposed on the manufacture of DDT and PCBs in the United States in the 1970s, levels of these compounds in human tissues and in the environment had shown a dramatic decline over the past several decades. This decline can be tracked in the studies carried out in the United States. A California study used stored blood samples from the mid-1960s; in the 1993 paper, Wolff and Toniolo used stored bloods from the late 1980s to early 1990s; and both the LIBCSP data and another Long Island study used blood samples from the mid-to-late 1990s. And you can see the levels fall from the earlier to the later studies. An important consequence of the declining levels of these compounds over time, which received little attention in most grant proposals, was that the statistical power to detect a dose-response relationship, which depends on having an adequate spread of exposures, was reduced. As Steven Stellman of Columbia University’s Mailman School of Public Health put it, “As the range of exposures gets compressed lower and lower, you are getting more noise and less signal.”47
But a more serious problem confronting all of the studies is one that, again according to Stellman, is glossed over by most researchers involved in this work. This is the assumption that a single measurement of blood or adipose tissue levels of DDT or PCBs at one point in time is sufficient to characterize an individual’s lifetime exposure. This assumption was crucial to the rationale for carrying out the epidemiologic studies initiated in the 1990s. At the site visit for the LIBCSP in 1994, Mary Wolff had made the argument that because blood levels of DDT are “in equilibrium” with adipose tissue levels, blood levels can provide an “historical window” on exposure earlier in life at a period relevant to the development of breast cancer. This argument, however, is open to question. Stellman, a physical chemist turned epidemiologist, who has had a long involvement in studying compounds like dioxin and DDT, emphasizes that a one-time measurement of such compounds in midlife can be highly misleading. To illustrate, he frequently borrows an example that was originally used by the Institute of Medicine to criticize the Air Force Health Study of pilots who sprayed dioxin-contaminated Agent Orange in Vietnam.48 Stellman posits three women who participated in the Long Island study and provided blood samples in the mid-1990s. Suppose that all three women have “low” levels of DDT/DDE in their blood at the time the study is conducted. If the researchers had had one or more measurements from ten, twenty, or thirty years earlier on these same women, when levels of DDT in food and the environment were considerably higher, the results might be quite different. It is altogether possible that in samples taken decades earlier one of the three women might have a “low” value, the second might have an “intermediate” value, and the third might have a “high” value. This is because, owing to individual differences in metabolism and personal history, women may eliminate DDE from their bodies at different rates. Weight loss, breast-feeding, and physical activity may all increase the rate at which these compounds are excreted. Thus, if measurements made later in life and close in time to diagnosis, are used to evaluate the relationship of DDE to breast cancer risk, this may lead to a failure to detect a relationship. Even though many different studies have been conducted in different populations and using different study designs (both case-control and cohort), they may all suffer from the same fatal flaw. This is another way in which the rationale provided by the researchers for conducting the Long Island study—namely that a contemporaneous measurement of DDT/DDE and other organochlorine compounds in blood can provide an accurate indication of exposure decades earlier—was greatly overstated.
Stellman took this issue one step farther. He pointed out that certain researchers have recognized the inadequacy of a single measurement in midlife, and this has led them to address the problem by using the current measurement to extrapolate backward in time using a “pharmacokinetic model.” However, not only does the half-life of a compound like DDT (that is, the amount of time it takes the body to excrete half of the body burden) vary between different individuals, but it even varies in the same individual from one time to another. Stellman concluded, “That very observation completely destroys the fundamental assumption of a constant half-life that is the basis of the extrapolation. So, most of the extrapolation that is done now is completely illegitimate. No self-respecting chemist would accept that for one minute.”49
The implication of these limitations of a one-time measurement of DDT or PBCs in midlife and decades after peak exposure is that the generally null results of these studies do not rule out a possible relationship.
Like Stellman, Mary Wolff also feels in retrospect that the work on organochlorines and breast cancer lacked a strong rationale. She told me that, “frankly you know in the U.S. today all of these exposures are so low, there’s just not many heavily exposed people.” Furthermore, while she thought that there was evidence suggesting that these compounds might have effects on reproductive health, the same was not true for cancer. Referring to the studies that were done in the 1990s, she said, “Between the susceptibility factors and the decline over time, I’m not sure that these studies are possible. I’m certainly not interested in doing them.”50
***
Publicity about increasing breast cancer rates in the late 1980s and early 1990s had provided an impetus for societal concern, activism, and research into possible environmental causes of the disease. Activists who focused on a possible role of environmental pollution tended to give little weight to evidence that increased use of mammography and hormone replacement therapy accounted for some proportion of the increase during the 1980s and 1990s. Then, in 2003, national statistics showed an abrupt downturn in breast cancer incidence rates after a six-decade rise (see fig. 3.1). From 2002 to 2003, the incidence of all breast cancers decreased by 7 percent, whereas that of estrogen receptor positive cancers (that is breast cancers that are fueled by estrogen—the most common type in post-menopausal women) decreased by 12 percent.51 The dramatic reversal in breast cancer rates appeared to mirror the precipitous decrease in the use of hormone replacement therapy that followed the widely publicized results from the Women’s Health Initiative in the summer of 2002, indicating that HRT use increased the risk of breast cancer and heart disease. In the six months following publication of the Women’s Health Initiative findings, prescriptions for hormone replacement therapy fell by 38 percent. So far, the evidence linking the decrease in HRT use with the decline in breast cancer is purely ecological, and it remains to be seen whether the decrease in incidence persists over a longer period of time and whether it affects mortality. Other possible factors contributing to the downturn include mammography and use of selective estrogen receptor modulators (SERMs) like tamoxifen and raloxifene to prevent breast cancer in women at high risk.52
Whatever the full explanation of the downturn in breast cancer incidence, the unexpected change serves to underscore the primary importance of lifestyle factors and preventive strategies in the development of breast cancer. One person who was not surprised by the apparent correlation between the drop in breast cancer incidence and the abrupt decrease in use of HRT was V. Craig Jordan, the vice president and scientific director of the medical science division at the Fox Chase Cancer Center in Philadelphia. For over thirty years, Jordan has played a major role in documenting the effects of estrogen-blocking drugs on breast cancer. As a result of his work and that of others, it is known that tamoxifen, which blocks the effects of estrogen in the breast, reduces the risk of breast cancer in women by about 40 percent.53 While Jordan points out that removing postmenopausal hormones would not be expected to eradicate estrogen receptor-positive breast cancers, he thinks it is possible that “many subclinical cancer cells may never grow inside a woman’s breast if she has no estrogen around to fuel that fire.”54 And, when asked about a possible role of chemicals like DDT or chemicals in plastics that can mimic estrogen in causing breast cancer, his response helps to put this possibility in perspective: “There are a group of compounds like DDT that are byproducts of industry and are in our environment. They can affect cells in the laboratory and can affect the reproduction of animals, but in really huge doses. There is an effect, but does it cause an increase in cancer? I personally don’t think there is enough around to do that. A pinch of estrogen in the environment is very small compared to the gallons in a woman’s body.”55
***
In retrospect, the focus on specific environmental pollutants without giving adequate weight to what was known about breast cancer can be seen for what it was—a response to intense public concern based on a number of fundamental misconceptions. Also in retrospect, the Centers for Disease Control were correct in their judgment that the breast cancer rates on Long Island did not justify the carrying out of a “crash” study there to identify the environmental causes of breast cancer. By responding to the alarm on Long Island with just such a crash research program, politicians, individual scientists, and the federal agencies only validated and encouraged the activists’ certainty that the environment must be a major culprit. The politicization of breast cancer led initially to the carrying out of studies based on weak hypotheses and inadequate methods, which were greatly oversold by their sponsors. When the results of the studies were published, the activists felt betrayed by the failure to uncover some dramatic and useable knowledge about how to prevent breast cancer.
Much of the difference in perspective between mainstream breast cancer researchers and the activists comes down to how each group defined “environment.” To the activists, “the environment” was restricted to chemical and other forms of pollution in the air, soil, water, and food. What concerned them most were pesticide and other chemical residues, products of combustion of fossil fuels, heavy metals, and electromagnetic fields. In contrast, since the beginnings of cancer epidemiology in the 1960s, scientists have defined the environment in the broadest possible terms to include diet, exogenous hormones, and lifestyle behaviors, such as smoking, alcohol consumption, physical activity, body weight, and occupational and other exposures. In this view, the environment includes any agent that can affect the internal milieu, not just “pollution.” For scientists, environment is simply contrasted with genetic makeup. To ignore, as the activists did, what was known from decades of research into the relationship of reproductive and hormonal factors to breast cancer, was to ignore an important part of the scientific picture. If the initial studies carried out in response to public pressure had a conceptual failing, it was the assumption that a single measurement of markers of exposure close to the time of diagnosis could shed meaningful light on the risk of breast cancer.
However one judges the initial response to concern about the environment, the cooptation of science by politics was short-lived. Collectively, these studies were able to provide reassurance that organochlorine compounds and PAH—at least as measured in midlife—did not play any substantial role in breast cancer. But their deeper significance is that, once conducted, they made clear the need for more sophisticated approaches to come to grips with the complex biology of breast cancer and possible scenarios whereby environmental exposures might contribute to the risk of disease. This could only be achieved by acknowledging the complexity of breast cancer and of the environment, properly defined.
A decade after the peak of alarm concerning environmental causes of breast cancer, discourse about the causation of this complex disease has become much more sophisticated. One major change is that through organizations like the National Breast Cancer Coalition breast cancer activists have become much more knowledgeable about the science relating to breast cancer and, in an unprecedented development, community representatives participate on an equal basis with scientists in the peer review process for the Army Breast Cancer grants. This means that, for the first time, breast cancer survivors and activists feel that they have a direct voice in the research process devoted to advancing knowledge of how to prevent the disease. On another level, due to the many workshops and consensus meetings that have taken place in the past ten years, there is a greater awareness of the need for a unified view of breast cancer that incorporates what is known from different disciplines, including epidemiology, genetics, endocrinology, toxicology, and molecular biology. Increasingly, researchers are turning their attention to events that occur in the first two decades of life before the breasts are fully developed. And prospective studies are seen as indispensable to document exposures and their effects on early development. In addition, researchers are focusing more on “intermediate endpoints,” that is, earlier landmarks preceding the clinical diagnosis of breast cancer, such as increased mammographic density and benign breast disease. Finally, genetic factors affecting susceptibility and the role of gene-environment interactions have become a major focus of research.
Findings regarding environmental exposures need to be integrated into the evolving and highly complex picture of the determinants of breast cancer risk. This means that possible effects of residues from pesticides, products of combustion, heavy metals, or other pollutants, or synthetics, such as bisphenol A, styrene, and other compounds of interest are to be evaluated in the context of their effects on hormone levels, growth factors, receptors, and genes involved in susceptibility to breast cancer. In this new climate, it is less likely that an isolated finding lacking credible grounding in the biology and epidemiology will get the kind of attention from the media and from scientists that was devoted to organochlorine compounds.
Box 3.2 Major Take-Home Points
Much is known about factors that influence the risk of developing breast cancer, but this knowledge cannot be used to accurately predict who will develop the disease and who will not. Furthermore, most known risk factors for breast cancer are not susceptible to prevention.
Within the United States, breast cancer rates are higher in the Northeast and the San Francisco Bay area. This geographic variation in rates is largely explained by sociodemographic and behavioral characteristics. Breast cancer rates tend to be higher in higher income women, who are more likely to have a late age at first birth and fewer children and are more likely to have used hormone replacement therapy. All of these characteristics are associated with increased risk.
In the early 1990s, breast cancer activists became alarmed at what they believed were abnormally high rates of breast cancer in places like Long Island, Cape Cod, and the San Francisco Bay area. Some activists were convinced that some form of environmental pollution was responsible for their having gotten the disease, in spite of a variety of considerations that weighed against this possibility.
A large, federally funded study was undertaken on Long Island to determine whether exposure to organochlorine compounds (including DDT) or combustion products was more common in women who had had breast cancer compared to women who were free of the disease. The results obtained after seven years showed no evidence that any of the hypothesized exposures were related to breast cancer risk. The activists were disappointed at the “null” findings.
The results of the Long Island study and those of other similar studies do not preclude the possibility that some environmental exposures could make a contribution to breast cancer. However, there are reasons to believe that any role of pollution is small relative to known risk factors.
Breast cancer is a complex disease, and current research is attempting to unravel how early events and exposures during and after puberty, as well as genetic susceptibility, can affect risk in addition to known risk factors.