CHAPTER 10
MACROEVOLUTION
When we review evolutionary phenomena, we find that they can be assigned rather readily to two classes. One consists of all events and processes that occur at or below the level of the species, such as the variability of populations, adaptive changes in populations, geographic variation, and speciation. At this level one deals almost exclusively with populational phenomena. This class of phenomena can be referred to as microevolution. It was analyzed in Chapters 5–9. The other class refers to processes that occur above the species level, particularly the origin of new higher taxa, the invasion of new adaptive zones, and, correlated with it, often the acquisition of evolutionary novelties such as the wings of birds or the terrestrial adaptations of the tetrapods or warm-bloodedness in birds and mammals. This second class of evolutionary phenomena is referred to as macroevolution.
Macroevolution is an autonomous field of evolutionary study. The earlier advances in our understanding of this field were made by paleontologists and systematists. But in recent years molecular biology has made the most important contributions to the understanding of macroevolutionary change, and it continues to make astonishing advances.
From Darwin’s day to the present, there has been a heated controversy over whether macroevolution is nothing but an unbroken continuation of microevolution, as Darwin and his followers had claimed, or rather is disconnected from microevolution, as asserted by his opponents, and that it must be explained by a different set of theories. According to this view, there is a definite discontinuity between the species level and that of the higher taxa.
The reason why this controversy has not been fully settled is because there seems to be an astonishing conflict between theory and observation. According to Darwinian theory, evolution is a populational phenomenon and should therefore be gradual and continuous. This should be true not only for microevolution but also for macroevolution and for the transition between the two. Alas, this seems to be in conflict with observation. Wherever we look at the living biota, whether at the level of the higher taxa or even at that of the species, discontinuities are overwhelmingly frequent. Among living taxa there is no intermediacy between whales and terrestrial mammals, nor between reptiles and either birds or mammals. All 30 phyla of animals are separated from each other by a gap. There seems to be a large gap between the flowering plants (angiosperms) and their nearest relatives. The discontinuities are even more striking in the fossil record. New species usually appear in the fossil record suddenly, not connected with their ancestors by a series of intermediates. Indeed there are rather few cases of continuous series of gradually evolving species.
How can this seeming contradiction be explained? At first sight, there seems to be no method available to explain macroevolutionary phenomena by microevolutionary theories. But should it nevertheless be possible to expand the microevolutionary processes into macroevolutionary ones? And furthermore, can it be shown that macroevolutionary theories and laws are fully consistent with the microevolutionary findings?
The possibility of such an explanation was shown by a number of authors during the evolutionary synthesis, particularly by Rensch and Simpson. They successfully developed Darwinian generalizations about macroevolution without having to analyze any correlated changes in gene frequencies. This approach was consistent with the modern definition of evolution as a change in adaptedness and diversity, rather than as a change in gene frequencies, as suggested by the reductionists. To put it in a nutshell, in order to prove that there is an unbroken continuity between macro- and microevolution, the Darwinians have to demonstrate that seemingly very different “types” are nothing but the end points in a continuous series of evolving populations.
THE GRADUALNESS OF EVOLUTION
It is important to emphasize that all macroevolutionary processes take place in populations and in the genotypes of individuals, and are thus simultaneously microevolutionary processes. Whenever we study evolutionary change in living populations, we observe such gradualness. Let us consider drug resistance of bacteria. When penicillin was first introduced in the 1940s, it was amazingly effective against many types of bacteria. Any infection, let us say by streptococci or spirochetes, was almost immediately cured. However, bacteria are genetically variable and the most susceptible ones succumbed most rapidly. A few that had acquired by mutation genes that had made them more resistant survived longer and a few still had survived when the treatment stopped. In this manner, the frequency of somewhat resistant strains gradually increased in human populations. At the same time, new mutations and gene transfers occurred that provided even greater resistance. This process of inadvertent selection for greater resistance continued, even though ever stronger dosages of penicillin were applied and the period of treatment was prolonged. Finally, some totally resistant strains evolved. Thus by gradual evolution an almost completely susceptible species of bacteria had evolved into a totally resistant one. Literally hundreds of similar cases have been reported in the medical and agricultural (for pesticide resistance) literature.
Such gradual evolution can be observed wherever one looks. The history of our domestic animals and cultivated plants is a story of gradual evolution even though, in this case, it was effected by artificial selection. Furthermore, fossil-rich geological exposures have recently been found where one can follow a gradual, unbroken series of fossils that demonstrate a gradual change over time.
Even more convincing is the study of geographical speciation (see Chapter 9), in which we can follow how very distinct species by a populational process had gradually diverged from each other. Abundant evidence shows the gradual evolution even of genera. All of this is fully in agreement with Darwinian theory. But this inevitably poses the question, Why is this gradualness not fully reflected in the fossil record?
Darwin already had an answer and, as it turns out, it was indeed correct. He said that the seeming gaps in the fossil record are an artifact of the haphazard history of the preservation and recovery of fossils. He postulated that the available fossil record was an incredibly incomplete sampling of the actual formerly existing biota, and that it was this incompleteness that was responsible for the seeming gaps in an actually continuous development. All recent research has confirmed Darwin’s conclusions. Furthermore, two silent assumptions, both of them incorrect, have aggravated the difficulties.
SPLITTING VS. BUDDING
The first assumption was that evolution consists of a splitting of lineages, both of which subsequently diverge from each other at similar rates. Observation, as well as the theory of speciational evolution (see below), has shown that this assumption is not necessarily correct. Admittedly, such a splitting of lineages by dichopatric speciation does indeed occur. However, what is apparently far more frequent is that a new lineage buds off from the parental one by peripatric speciation and enters a new adaptive zone in which it evolves rapidly, while the parental lineage remains in its old environment and continues at the previous slow rate of change.
Let us assume, for instance, that the line leading to birds budded off one of the various lineages of archosaurs. This new avian lineage, exposed to the powerful selection pressures of the aerial way of life, changed very rapidly while the parental archosaurian lineage presumably hardly changed at all. That this is a common pattern of evolution is shown by the fossil record of almost any major taxon, but it is often overlooked in discussions of theory. The rapid change of the derived lineage as compared to the slowness of the parental one will undoubtedly be reflected by a gap in the fossil record represented by the period of the rapid changeover from the ancestral condition to the requirements of the new adaptive zone. Remarkably few paleontologists have given sufficient consideration to the fact that most new evolutionary lineages arise by budding rather than by splitting. And budding is usually achieved very simply by peripatric speciation. Sympatric speciation, likewise, is usually a budding process.
The second misconception held by most students of macroevolution was to think of evolution exclusively as a linear process in the time dimension. When they found a seeming gap in a linear fossil sequence, they assumed either the occurrence of a saltation or an incredible acceleration of evolutionary rate for a short period. Neither assumption fitted the theory of the evolutionary synthesis nor was it supported by credible evidence. Then how can these various discrepancies be explained? What is the explanation of such discontinuities?
Discontinuity
A clearer understanding of evolution was long delayed by a confusion of two meanings of the term discontinuity. One must distinguish between phenetic discontinuity and taxic discontinuity. A discrete difference among members of the same deme is a phenetic discontinuity. If different members of a mammalian deme have either two or three molars or members of an avian deme have either 12 or 14 tail feathers, it is a phenetic discontinuity. However, if the same difference distinguishes two species taxa from each other, it is a taxic discontinuity. Any discrete difference between two taxa, regardless of taxonomic level, is a taxic discontinuity.
Unfortunately, some typologically thinking evolutionists came to the erroneous conclusion that a phenetic discontinuity would in a single step lead to a taxic discontinuity. In reality a new phenetic discontinuity simply enriches the variation of a deme, producing polymorphism, and it requires a long process of selection to convert a phenetic discontinuity into a discontinuity between two taxa. But when and where is such an individual variation of a deme or group of demes converted into a taxic difference?
SPECIATIONAL EVOLUTION
This problem was solved by the students of speciation in living organisms. They showed that species taxa at a given time level not only have the linear dimension of time but also the geographical dimensions of longitude and latitude. Thus they are severely limited both in time and in space. Every species is, so to speak, on all sides surrounded by a gap. Yet it has complete continuity with the parental species from which it descended and the daughter species to which it is giving rise. Furthermore, most species of animals do not consist merely of a single more or less widespread contiguous population but rather are polytypic species, consisting of numerous local populations, many of which, particularly along the periphery of the species range, are more or less isolated from each other. This led to the theory of
speciational evolution (Mayr 1954), according to which isolated founder populations, established beyond the contiguous species range, may undergo a more or less profound genetic restructuring. This and the subsequent inbreeding of the new population may lead to the production of some unusual new genotypes and of new epistatic balances. Large populations are apparently more inert, less able to break the effects of multiple epistatic interactions than small, genetically impoverished populations. Such small populations are less constrained and able to make greater departures from the ancestral norm. This has been experimentally demonstrated by large and small
Drosophila populations (see
Fig. 6.4). At the same time, the founder population is exposed to new and increased selection pressure owing to the novelty of its new environment. As a result, such a population may rapidly become a different species (see Chapter 9). This theory was also independently arrived at by several botanists (Grant 1963). The chance that such a localized, isolated population, and the new species produced by such peripatric speciation, will be found in the fossil record is, of course, exceedingly small. Even though the continuity of populations during this process of speciational evolution is complete, it will appear in the scanty fossil record as a saltation and has been described as such. This is clearly a misinterpretation, since speciational evolution is at every step a gradual populational process.
Eldredge and Gould (1972) have called this process “evolution by punctuated equilibria.” They pointed out that if such a new species is successful and becomes effectively adapted to a new niche or adaptive zone, it may subsequently remain unchanged for many hundreds of thousands if not millions of years. Such a stasis of a widespread populous species is widely observed in the fossil record.
HOW IMPORTANT IS SPECIATIONAL EVOLUTION?
The theory of speciational evolution was developed not as the result of theoretical considerations but strictly on the basis of actual observations. When studying a series of peripherally isolated populations of a species of birds, the present author noticed that the population that was most peripheral, and that was the product of a sequence of consecutive colonizations, was usually the most different. This observation was fully confirmed and strengthened by the studies of H. L. Carson, K. V. Kaneshiro, and A. R. Templeton on Hawaiian species of Drosophila. They showed that colonization of a different island or a different mountain range on the same island might result in a morphologically quite distinct new species, even in a genus with such a stable morphotype as Drosophila.
The majority of peripheral isolates, however, differ hardly, if at all, from the parental population. They will have only a rather limited life span and sooner or later become either extinct or merge again with the parental species. However, if we find a somewhat aberrant population in a species, it is almost invariably a far-distant peripheral isolate. This process of speciational evolution has also been referred to as “bottleneck evolution.” It may also occur in temporarily highly isolated and in relict populations.
For the new species to be truly successful it must be able to compete with larger, more diversified species. Distributional studies indicate that highly isolated island species in Malaysia and Polynesia are unable to invade the ranges of more widespread species in the West. To become successful when competing with parental and sister species, such founder populations have to increase in size and become more diversified. Such a development is possible for relicts in Pleistocene refuges that, after a change of conditions, can expand their range again and become widespread.
RATES OF EVOLUTIONARY CHANGE
Rates of physical processes, such as chemical reactions or radioactive decay, tend to be constant. This is not at all what we find when we study rates of change in evolution. The evolutionists G. G. Simpson and B. Rensch have been particularly emphatic in calling attention to the great variation in rates of evolution.
Chapter 9 described the high variability of rates of speciation. Equally variable is the rate of simple evolutionary change in phyletic lineages. At one extreme we find the so-called living fossils—certain species of animals and plants that have not visibly changed in more than 100 million years. This includes the horseshoe crab (Limulus; Triassic), the fairy shrimp (Triops), and the lampshell (Lingula; Silurian ). Equally long-lived genera have been found among plants: Gingko (dating to the Jurassic), Araucaria (probably Triassic), Equisetum (mid-Permian), and Cycas (Primo-Cycas; late Permian).
The complete standstill or stasis of an evolutionary lineage for scores, if not hundreds, of millions of years is very puzzling. How can it be explained? In the case of a living fossil, all the species with which it had been associated 100 or 200 million years ago had either changed drastically since that time or had become extinct. Why did this one species continue to prosper without any changes in its phenotype? Some geneticists thought they had the answer by ascribing it to normalizing selection, which culls all deviations from the optimal genotype. However, normalizing selection is equally active in rapidly evolving lineages. To explain why the underlying basic genotype was so successful in living fossils and other slowly evolving lineages requires a better understanding of development than is so far available.
Not only do species and genera differ from each other in their rate of evolutionary change, but so do entire higher taxa. Paleontologists have shown, for example, that mammals change over time far more rapidly than do bivalve mollusks. In part this difference may be an artifact of the taxonomic method. A bivalve shell has far fewer taxonomic characters than a mammalian skeleton and this discourages a more fine-grained subdivision of bivalve taxa. Yet, even in the most rapidly evolving lineages of animals, the evolutionary change per million years is usually astonishingly low.
We are, of course, fully familiar with the opposite, cases of extraordinarily rapid evolutionary change. This includes the acquisition of immunity to antibiotics in human pathogens and to pesticides among agricultural pests. It is very probable that human populations, living in areas endemic for Plasmodium falciparum malaria, have accumulated the sickle cell gene and other blood genes partially resistant to this Plasmodium in probably less than one hundred generations.
Rate of acquisition of lungfish characters after the origin of lungfishes. (A) Acquisition of new characters per million years. (B) Rate of approach to the final lungfish body plan per million years. Most of the reconstruction of the body plan of the new taxon takes place in the first 20 percent of its life.
Source: Simpson, George G. (1953). The Major Features of Evolution, Columbia Biological Series No. 17, Columbia University Press: NY.
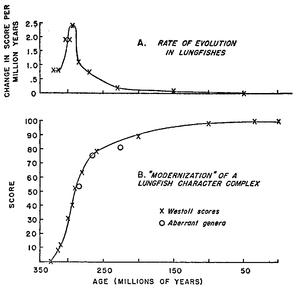
A phyletic lineage may experience slow and rapid periods of change. A well-known illustration of this phenomenon is the evolution of the lungfishes (Westoll 1949). The major anatomical reconstruction of this class of fishes took place in about 75 million years, while almost no further changes occurred in the ensuing 250 million years (
Fig. 10.1). Such a drastic difference between the rates of evolutionary change in young and mature higher taxa is virtually the rule. Bats originated from an in-sectivorelike ancestor within a few million years, but have hardly changed in basic body plan in the ensuing 40 million years. The origin of whales happened very rapidly, in terms of geological time, compared to the subsequent essential stasis of the new structural type. In all of these cases the lineage had shifted into a new adaptive zone and was for a while exposed to very strong selection pressure to become optimally adapted to the new environment. As soon as the appropriate level of adaptedness had been acquired, the rate of change was reduced drastically. The extreme variability of rates of evolution has been neglected by certain authors and this has led them to errors of interpretation.
How Does One Measure Rates of Evolution?
How long life has existed on Earth was long a complete mystery, and so was the date when the eukaryotes, vertebrates, or insects had originated. But now numerous concrete dates have been established. The oldest fossils (bacteria) are ca. 3,500 million years old, the Cambrian period began 544 million years ago, and the oldest australopithecine fossils are 4.4 million years old. How are these figures obtained?
Geology is the basic source. Many geological strata, particularly volcanic ashbeds or lava flows, contain radioactive minerals, the age of which can be determined by the measurement of their radioactive decay (see Box 2.1). There are now several methods for doing this and the accuracy of the most modern methods is very high.
An entirely different method is available to determine when the common ancestor of two living species lived: the so-called molecular coalescence method (see Box 10.1). It is based on the observation that all genes (molecules) change over time at rather uniform rates, and the two lineages derived from a common ancestor become over time more and more different from each other. If the common ancestor is represented by a fossil whose age was determined by geological methods, the average rate of molecular change can be determined accurately (using the molecular clock method). The reliability of this method depends on the constancy of the molecular change. Alas, there are all sorts of irregularities in molecular clock rates and to get reasonably reliable results one must test different materials. Noncoding genes are usually preferable to genes that are subject to changes due to selection. These difficulties are well illustrated by the inferred age of origin of the higher taxa (families and orders) of mammals and birds. The oldest fossils generally fall in the time range of 50–70 million years ago, with no earlier finds, even though there are excellent fossil deposits in the crucial period. According to the molecular evidence, these taxa must have originated already in the early Cretaceous, more than 100 million years ago. The cause of this discrepancy is still controversial. Did the molecular clock change its rate?
Box 10.1 The Coalescence Method of Age Determination
The molecular clock hypothesis states that for all evolutionary lineages there is a relatively constant rate of evolutionary change over time. More specifically, rather than there being a “global” universal rate for all molecules and evolutionary lineages, each molecule, DNA or protein, has a specific rate of evolution. If most mutations are neutral or almost neutral in their selective effects, and if this rate of mutation has not changed over time, then the rate of evolution of a particular molecule should be nearly constant over time permitting us to estimate the age of evolutionary lineages. However, some lineages, have been documented to have, for various reasons, faster rates of evolution than other lineages (e.g. rodents vs. primates). However, leaving this and other caveats aside, if molecules evolve at a constant rate they can be used as “time keepers” to calculate “lineage-specific” divergence times and to estimate the age of the nearest common ancestor of two species.
To use the molecular clock in such a way requires the calibration of its “ticking rate.” This can be done through several means such as the fossil record (keeping in mind that the first occurrence of a fossil is always a minimum estimate for the age of this lineage) or through major vicariance events such as plate tectonics. Once the homologous gene A has been sequenced in, e.g., two species and the rate of evolution in this gene is known through prior calibration (let’s say 2% per million years) then knowing the percent difference in the DNA sequence of gene A between these two species permits the calculation of the age of their last common ancestor. In this example, if species 1 and 2 differed by 10% in their DNA sequence of gene A, then the common ancestor of these two species would be expected to have lived around 2.5 mya. It would have taken these two lineages this long to both diverge at a rate of 2% per million years to accumulate 10% difference in gene A.
Neutral Evolution
Molecular genetics has found that mutations frequently occur in which the new allele produces no change in the fitness of the phenotype. Kimura (1983) has called the occurrence of such mutations neutral evolution, and other authors have referred to it as non-Darwinian evolution. Both terms are misleading. Evolution involves the fitness of individuals and populations, not of genes. When a genotype, favored by selection, carries along as hitchhikers a few newly arisen and strictly neutral alleles, it has no influence on evolution. This may be called evolutionary “noise,” but it is not evolution. However, Kimura is correct in pointing out that much of the molecular variation of the genotype is due to neutral mutations. Having no effect on the phenotype, they are immune to selection.
SPECIES TURNOVER AND EXTINCTION
A striking observation made by paleontologists has been the steady change of biota from one geological period to the next. New species are added to the biota, while old ones disappear because they become extinct. Such extinction does not proceed at the same rate at all times, although a relatively low number of species usually go extinct in any given time span. This background extinction has been going on since the beginning of life (Nitecki 1984). The reason for it is that every genotype seems to have limits to its capacity for change and this constraint might prove fatal under certain environmental changes, particularly sudden ones. For example, the needed mutations may have failed to appear when there was either a change in climate or the sudden arrival of a new competitor, predator, or pathogen. Whenever a population is no longer able to reproduce enough offspring to replace losses from natural causes, it will become extinct. No organism is perfect; indeed, as Darwin already emphasized, an organism only has to be good enough to compete successfully with its current competitors. When an emergency arises, there may not be time enough to perfect an adequate genetic restructuring and extinction is the consequence. This steady extinction of individual species is due to biological causes in almost every case. Furthermore, it is observed in general that the smaller the population size of a species is, the more vulnerable it will be to extinction. However, occasionally a small population seems to be remarkably resistant to extinction.
Actual extinction should not be confused with pseudoextinction. This term, sometimes used by paleontologists, refers to the process by which a species may evolve into a different species and then be given a new name by paleontologists. The ancestral name thus disappears from faunal lists. However, the biological entity involved in this change of names has not become extinct and its seeming disappearance is simply due to a name change.
There are some cases when there was no obvious change in the Earth’s environment and yet a major group declined and became extinct. This was perhaps the case with the extinction of the trilobites. Not being able to come up with a better answer, paleontologists have suggested that they succumbed to the competition with the “physiologically superior” newly evolved bivalves. As plausible as this theory appears to be, the evidence for it up to now seems to be rather insufficient. Indeed, some paleontologists now attribute the extinction of the trilobites also to a climatic event.
COMPETITION
The supply of one or several of the resources needed by the population of a species may be limited. In such a case the individuals of this population may be competing with each other (intraspecific competition). Such competition is part of the struggle of existence. It may simply consist of a removal of the limited resources or consist of an actual interference of the competitors with each other. Furthermore, the ecological literature describes numerous examples of competition between individuals of different species. This involves not only similar species, but also competition for seeds between ants and small rodents in the deserts of the southwestern United States. If two species compete too seriously with each other, one of them will be eliminated. Such an occurrence illustrates the competitive exclusion principle, which states that two or more competing species cannot coexist indefinitely when they use exactly the same resources. Such differences may be rather subtle, because cases have been reported in the literature where it has not been possible to find any differences in resource utilization between two coexisting competing species. But such cases are rather rare. Normally competition is a major component of the selection pressure to which the individuals of a population are exposed. And competition between two species for a limited resource often seems to be the reason why one of the two became extinct.
MASS EXTINCTIONS
Quite different from the steady extinction of individual species are the so-called mass extinctions (Nitecki 1984), during which a large proportion of the biota is exterminated in a very short time on a geological timescale. Mass extinctions are due to physical causes. Most famous among them is the one at the end of the Cretaceous, which involved the extermination of the dinosaurs and of many other marine and terrestrial organisms. For a long time it was a puzzle as to what might have caused this catastrophic extinction, but, as suggested by Walter Alvarez, it is best explained as due to the impact of an asteroid on Earth 65 million years ago. The impact crater of this asteroid has now been discovered at the tip of the Yucatan Peninsula in Central America. The tremendous dust cloud produced by this impact resulted in a drastic drop in terrestrial temperature and in other adverse conditions, producing the extinction of a great proportion of the then existing biota. Although the dinosaurs among the Reptilia became extinct, other reptiles, such as turtles, crocodilians, lizards, and snakes, survived. Some insignificant and probably nocturnal mammals also survived and experienced in the Paleocene and Eocene a spectacular radiation, producing all the orders and many of the families of the now living mammals. The few survivors among the Cretaceous birds seem to have experienced a similarly explosive radiation during the first 20 million years of the Tertiary.
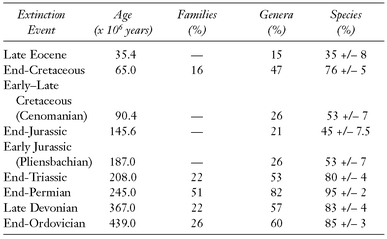
There have been several other mass extinctions since the origin of life on Earth, but those that happened since the origin of the animals (metazoans) are best documented (
Table 10.1). The most drastic of these other extinctions, apparently even more catastrophic than the Alvarez event, occurred at the end of the Permian and resulted in the estimated extermination of 95 percent of the then existing species. It was apparently not caused by an asteroid impact but by a change of climate or of the chemical composition of the terrestrial atmosphere. There have been three other major mass extinctions (in the Triassic, Devonian, and Ordovician periods), in which 76–85 percent of the then living species became extinct. We are now living in another era of mass extinction caused by humans through the destruction of habitats and the pollution of the environment.
Smaller mass extinctions have happened to specific groups of organisms. During a drought period in the Pliocene (ca. 6 million years ago), the softer C3 grasses in North America were largely replaced by harsh C4 grasses, which have three times as much silica content. Among the browsing horses, all species became extinct except those with the longest teeth.
The Pleistocene extinction of much of the mammalian megafauna of the large continents (including Australia) about 10,000 years ago seems to coincide with a climatic stress period, but also with the appearance of the first efficient human hunters. Presumably both factors contributed to the extinction. That humans were the cause of the extinction of many island faunas (Hawaii, New Zealand, Madagascar, and others) is well documented.
Natural selection, of course, is no protection against mass extinction. Indeed, there is a considerable probability that the successful survival through such an extinction event includes a considerable chance factor. Who, for instance, would have predicted at the beginning of the Cretaceous that the dinosaurs, at that time the most successful group of vertebrates, who occupied such a variety of ecological niches, would be completely exterminated 60 million years later by the Alvarez event? Other previously dominant groups of organisms that also became extinct at the end of the Cretaceous are many marine taxa, such as most nautiloids and the ammonites, both of whom had been previously highly successful organisms. No amount of natural selection succeeded in producing genotypes enabling them to survive.
Background extinction and mass extinction are drastically different in most aspects. Biological causes and natural selection are dominant in background extinction, whereas physical factors and chance are dominant in mass extinction. Species are involved in background extinction, and entire higher taxa in mass extinction. However, certain higher taxa are more susceptible to mass extinction than others. The two kinds of extinction should never be lumped in any statistical analysis of extinction.
MAJOR TRANSITIONS
In spite of its gradualness, macroevolution is characterized by numerous major inventions, which many authors consider to represent decisive steps in the advance of the living world. It begins with the inferred transitions involved in the origin of life and the development of the Prokaryotes. The evolution of life from the Prokaryotes to the most divergent animals and plants is the story of numerous such transitions, such as the rise of the Eukaryotes (with membrane-bonded nucleus, chromosomes, mitosis, meiosis, sex), symbiosis of cellular organelles, multicellularity, gastrulation, segmentation, specialized organs, improved sense organs, elaboration of a central nervous system, parental care, and cultural groups. Almost all of these steps seem to have contributed to the adaptedness of the phyletic lineages in which they occurred (Maynard Smith and Szathmary 1995).
The Origin of Evolutionary Novelties
Some of Darwin’s critics readily admitted that an existing structure could be improved by use and disuse or by natural selection, but how could such processes produce an entirely new structure? They would ask, for instance: “How can the origin of wings in birds be explained by natural selection?” Having a small wing, they said, would be of no selective advantage, being useless for flight. Natural selection cannot operate until an already functioning structure is present. Actually, this claim is only a half-truth, because an already existing structure can, by a behavioral shift, assume an additional function that can eventually modify the original structure into an evolutionary novelty. There are two different pathways by which an evolutionary novelty can be acquired: by an intensification of function or by the adoption of an entirely new function (Mayr 1960).
Intensification of Function. In ordinary gradual evolution, most descendant taxa differ from their ancestors only quantitatively. They may be larger, of faster locomotion, more cryptically colored, or differing by some other incremental difference. Nevertheless, the end stages of gradual evolutionary change are often so different from their earliest ancestors that they seem to represent a major saltation. Let us consider the anterior extremities of mammals as an example. Normally, they are adapted for walking, but in moles and other subterranean mammals they are adapted for shoveling earth; in some arboreal mammals, such as monkeys and apes, they are adapted for grasping; in aquatic mammals they become swimming paddles or flukes; and finally in bats they are converted into wings. In all of these cases, except the last one, only a magnification of an existent potentiality is involved. This is what evolutionists refer to as an intensification of function.
Perhaps the most spectacular instance of an intensification of function is presented by the eye. Darwin was puzzled by how such a perfect organ could have evolved gradually. The study of the comparative morphology of organisms has revealed the answer. The simplest, the most primitive stage of the series leading to an eye is a light-sensitive spot on the epidermis. Such a spot is of selective advantage from the very beginning, and any additional modification of the phenotype that enhances the functioning of this light-sensitive spot will be favored by selection. This would include the deposition of pigment around the light-sensitive spot, also any thickening of the epidermis leading up to the development of a lens, of muscles to move the eye, and other accessory structures, but most importantly, of course, the development of a retinalike photosensitive neural tissue.
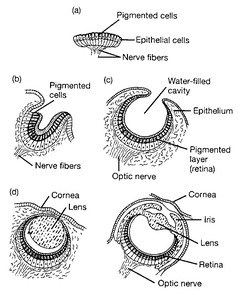
Photosensitive, eyelike organs have developed in the animal series independently at least 40 times, and all the steps from a light-sensitive spot to the elaborate eyes of vertebrates, cephalopods, and insects are still found in living species of various taxa (
Fig. 10.2). They include intermediate stages and refute the claim that the gradual evolution of a complex eye is unthinkable (Salvini-Plawen and Mayr 1977). Most photosensitive organs of the invertebrates lack the perfection of the eyes of vertebrates, cephalopods, and insects, but their origin and subsequent evolution were nevertheless helped by natural selection. As long as a variant was superior, it was favored, with multiple slight advantages reinforcing each other.
Every individual possesses scores, perhaps even hundreds of very slight differences from other members of his or her population. Some observers have felt that these differences would be too slight to be favored by natural selection. This view ignores that many slight advantages can compound and have the effect of one large advantage. Such slight advantages accumulate in the course of generations and thus play an increasing role in evolution. A slight accumulation of pigment and a light-sensitive spot, for example, might not be a special target of selection, but might be favored by survival together with several other equally slight advantages in a phenotype.
The origin of eyes in 40 branches of the evolutionary tree was always considered to be an independent convergent development. Molecular biology has now shown that this is not entirely correct. A regulatory master gene (called Pax 6) has recently been discovered that seems to control the development of eyes in the most diverse branches of the tree (see Chapter 5). However, this gene occurs also in taxa whose species have no eyes. Pax 6 is apparently a basic regulatory gene, presumably involving some other functions in the nervous system. Molecular biology has discovered a number of other such basic regulatory genes whose existence in some cases goes back to a time before the major animal phyla had branched. When survival is favored by the acquisition of a new structure or other attribute, selection makes use of all available molecules already present in the genotype.
Stages in the evolution of eyes among molluscs. (a) A pigment spot; (b) a simple pigment cup; (c) the simple optic cup found in abalone; (d) the complex lensed eye of the marine snail and of the octopus.
Source: Evolutionary Analysis 2nd ed. by Freeman/ Herron, copyright © 1997. Reprinted by permission of Pearson Education, Inc. Upper Saddle River, NJ.
That a structure like the eye could originate numerous times independently in very different kinds of organisms is not unique in the living world. After photoreceptors had evolved among animals, bioluminescence originated at least 30 times independently among various kinds of organisms. In most cases, essentially similar biochemical mechanisms were used. Virtually scores of similar cases have been discovered in recent years, and they often make use of hidden potentials of the genotype inherited from early ancestors.
Change of Function. Is intensification of function the only way in which complex new organs are acquired? The answer to this question is “No!” There is indeed a second process for such an acquisition, particularly stressed by Darwin, Anton Dohrn, and A. N. Sewertzoff: the acquisition of new organs by the change of function of an existing structure. Such a change requires that this structure is able to perform both the old and the new function simultaneously. For instance, the gliding wing of primitive birds was eventually used also for flapping flight. There are numerous cases of evolutionary novelties that can be explained in this manner. The swimming paddles of Daphnia were originally antennae (sense organs) and still function as such, but they are now also used as locomotory structures. Lungs in fishes have been converted into swim bladders and extremities in arthropods have acquired a whole series of new functions. In many cases, what happens is better described as a new ecological role rather than a new function. A structure that is able to adopt a new function is said to be preadapted for such a shift. Preadaptation is a purely descriptive term and does not imply any teleological forces.
All the more spectacular origins of new structures or habits in the history of organisms were due to a change of ecological role. Such shifts splendidly illustrate the opportunism of evolution. As stated in Jacob’s (1977) principle of tinkering, any existing structure may be used for a new purpose.
A change of function may also play a role in some cases of speciation. It is possible, particularly in the case of sympatric speciation, that a factor favored by sexual selection takes on the new role of a behavioral isolating mechanism.
Any change of function event simulates a saltation, yet it is actually a gradual populational change. It affects at first only one individual within a population and becomes evolutionarily significant only if it is favored by natural selection and spreads gradually to the other individuals of the population and then to the other populations of the species. Hence, even evolution by change of function is a gradual process.
ADAPTIVE RADIATION
Whenever a species acquires a new capacity, it acquires, so to speak, the key to a different niche or adaptive zone in nature. The branch of reptiles that invented the feather and subsequently the capacity to fly conquered an enormous adaptive zone. As a result, birds now have about 9,800 species as compared to only 4,800 species for all the mammals and 7,150 species of living reptiles. The structural type that we call “insect” is particularly successful, having given rise to several million species. However, all attempts by birds to conquer water have been only mildly successful. There are about 150 species of ducklike birds, and a few grebes (20), auks (21), and loons (4), while the penguins, the most water adapted of all aquatic birds, have only 15 species—thus only two percent of all species of birds are aquatic. A considerable number of species of mammals have succeeded in becoming leaf-eaters, but only a few birds, most successfully the hoatzin, succeeded in conquering that niche. No amphibian succeeded in adapting to salt water.
The History of Life: A Story of Adaptive Radiation
The success of a phyletic lineage to establish itself in numerous different niches and adaptive zones is called adaptive radiation. It is conspicuous in most of the higher taxa of organisms. Reptiles, without abandoning their basic structure, evolved into crocodilians, turtles, lizards, snakes, ichthyosaurs, and pterosaurs; mammals produced mice, monkeys, bats, and whales; and birds evolved into the niches of hawks, storks, songbirds, ostriches, hummingbirds, and penguins. Each of these groups has carved out its own suite of niches in nature, without any major change in the ancestral structural type.
Actually, the entire ascent of life can be presented as an adaptive radiation in the time dimension. From the beginning of replicating molecules to the formation of membrane-bounded cells, the formation of chromosomes, the origin of nucleated eukaryotes, the formation of multicellular organisms, the rise of endothermy, and the evolution of a large and highly complex central nervous system, each of these steps permitted the utilization of a different set of environmental resources, that is, the occupation of a different adaptive zone.
Disparity
The diversity of the living world takes many forms. It may express itself purely quantitatively as in the large colonies of ants and termites, or in the number of species in a family, like the weevils among the beetles (and the order of beetles as a whole), and of course in the enormous biomass of prokaryotes. But diversity may also express itself in the degrees of difference, the number of strikingly different types of organisms. And here evolution has produced a real surprise. In the rise of the metazoans (animals), one would expect that soon after their appearance in the fossil record they would consist of a series of rather similar orders that would become increasingly more dissimilar to each other in the course of time. Yet the facts are astonishingly different from this assumption! When the metazoans appeared as fossils about 550 million years ago (admittedly they must have already existed for ca. 200 million years), they included four to seven bizarre body plans that soon became extinct. All the other Cambrian phyla survived, and what is quite unexpected, without a major revolution of the basic body plan. If we look at individual phyla, the same situation is encountered. The living classes of arthropods are already found in the Cambrian with the same body plans. But again there are a handful of strange types of arthropods in the Cambrian that do not exist today. I agree with those who conclude from this evidence that the variety of realized body plans was greater in the Cambrian than it is now. Furthermore, no fundamentally new body plan has originated in the 500 million years since the Cambrian.
The solution to this puzzling problem will have to be supplied by developmental biology. Development in the recent phyla is rigidly canalized by hox genes and numerous other regulatory genes. There are indications that this regulatory system has considerably tightened since the Cambrian. Hence, at the time of the origin of the metazoans, the constraining power of the regulatory system was apparently still very rudimentary. Seemingly rather minor mutations might have produced totally novel structures. This “freedom of construction” was lost as the regulatory machinery was increasingly perfected and now, hundreds of millions of years later, different feeding types of cichlid fishes can still originate, but all are still cichlid fishes. To say that the body plans of the living fauna display the same disparity as that of the Cambrian is simply not true. And yet the contrast between the innovativeness of the Cambrian fauna and the conservativeness of the body plans of the living fauna is no longer an insolvable puzzle when the recent findings of developmental molecular biology are duly considered.
COEVOLUTION
Whenever two kinds of organisms interact with each other, let us say a predator and its prey, or a host and its parasite, or a flowering plant and a pollinator, each will exert a selection pressure on the other. The result is that they will coevolve. For instance, the prey may develop better escape mechanisms that force the predator to improve its attack capacity. Much of the process of evolution occurs through such coevolution.
The pollinators of the flowers of plants, whether they are butterflies, other insects, birds, or bats, are adapted to the flowers of their host plants and these flowers, in turn, evolve in such a way as to make the pollination more successful. Darwin conducted a fascinating study of the adaptations of orchids for pollination. All cases of symbiosis or mutualism found in nature are subject to such coevolution due to natural selection.
Plant species protect themselves against herbivores by the production of all sorts of toxic chemicals, such as alkaloids, which make them unpalatable to potential herbivores. The herbivores then develop detoxifying enzymes to overcome this problem. In response, the plants develop new chemicals for their protection. The herbivores then have to develop again the appropriate detoxifying enzymes to combat these new toxins. Such a series of back and forth interactions has been referred to as an “evolutionary arms race,” and there are an almost infinite number of such arms races among organisms. Marine snails, for instance, protect themselves against snail-eating crabs by evolving stronger shells as well as all sorts of structural elaborations of the shell that make it more difficult for the crabs to crush them. The crabs, in turn, develop stronger claws, which induces the snails to grow even tougher shells, and so on.
Obviously it is not the best evolutionary strategy for a pathogen to wipe out its host. Indeed, there should be a premium on the evolution of less virulent strains. It is sometimes possible to observe such an evolution taking place. When, for instance, the myxomatosis virus was introduced into Australia to control the escalating population of rabbits, the most virulent strains of this virus killed their host rabbits so quickly that there was no time for the virus to be transmitted to another rabbit. As a result, most of the highly virulent strains became extinct. Rabbits attacked by less virulent strains survived longer and provided the source for infecting other rabbits. Eventually, much less virulent strains of the virus evolved that killed only a certain percentage of the rabbits while most survived. At the same time, the most susceptible rabbits were killed off and populations of rabbits evolved that were less susceptible to the myxomatosis virus.
Most European infectious diseases currently exist in a similar steady state. Over many millennia, the European populations have become somewhat resistant to these human diseases and mortality is relatively low. This was not the case, however, with foreign populations that first came in contact with the Europeans after 1492. All over the world, but particularly in the Americas, the native populations were ravaged by epidemics caused by European infectious diseases, particularly smallpox. The native population of the Americas, which was estimated to have been 60 million when Columbus first landed in the Bahamas, had crashed to 5 million only 20 years later. These diseases were so deadly because the Native Americans had not coevolved with them. They were left defenseless when the pathogens spread through their populations.
Internal parasites, such as cestodes, trematodes, and nematodes, tend to become gradually host specific after they have colonized a new host, and from that point on they evolve together with their host. Whenever the host splits into two species, the parasite in due time will do the same. As a result, it is sometimes possible to construct a phylogenetic tree of the parasite that parallels that of the host. There are exceptions, because once in a while a parasite may be able to jump to an entirely different lineage of hosts. What is true for internal parasites is equally true for external ones, such as lice, feather lice (Mallophaga), and fleas.
SYMBIOSIS
In the discussion of evolution, not nearly enough attention is paid to the overwhelming role of symbiosis. Symbiosis is the collaboration of two different kinds of organisms in producing a system of reciprocal helpfulness. Lichen, a system consisting of a fungus and an alga, is an oft-cited case of symbiosis. It is apparently widespread among bacteria, resulting in the evolution of entire bacterial communities, for instance, among soil bacteria, in which different kinds of bacteria produce different metabolites useful to other species.
All insects that feed on plants and plant juices have intracellular symbionts that produce enzymes needed for the digestion of the plant material. Blood-sucking insects likewise often have intracellular symbionts facilitating the digestion of blood.
The most important event in the history of life on Earth, the production of the first eukaryotes, was apparently initiated by the symbiosis between a eubacterium and an archaebacterium, leading eventually to the formation of a chimaera between these two kinds of bacteria. Additional events led to the incorporation of symbiotic purple bacteria in the new eukaryote to form the mitochondria, and in plants to the symbiotic incorporation of cyanobacteria into the cell to become chloroplasts. Other cellular organelles are also symbionts (Margulis 1981; Margulis and Fester 1991; Sapp 1994).
EVOLUTIONARY PROGRESS
Evolution means directional change. Since the beginning of life on Earth and the rise of the first prokaryotes (bacteria) 3,500 million years ago, organisms have become far more diversified and complex. A whale, a chimpanzee, and a giant sequoia are surely very different from a bacterium. How can this change be characterized?
The answer most frequently given is that current life is simply more complex. On the whole this is indeed true, but it is not universally true. Many phyletic lineages demonstrate simplifying trends, and this is particularly true for various kinds of specialists such as cave animals and parasites. But surely, it will be said, evolution shows progress. Are not vertebrates and angiosperms (flowering plants) more highly evolved, more progressive, than “lower” animals and plants, and bacteria? We have already analyzed this claim and shown how difficult it is to apply the designations “higher” and “lower.” In fact, the prokaryotes, as a whole, seem to be as successful as the eukaryotes. Yet, every step in evolution, generation after generation, that eventually led to rodents, whales, grasses, and sequoias took place, so to speak, under the control of natural selection. Does not this lead by necessity to a steady improvement, generation after generation, of every phyletic lineage? The answer is “No,” because most evolutionary changes are dictated by the need to cope with current temporary changes of the physical and biotic environment. Hence, considering also the enormous frequency of extinction and the occurrence of regressive evolution, it is inevitable that one must reject the notion of universal progress in evolution. However, a different answer can perhaps be given when one looks at single lineages at particular moments of their evolution. There are a considerable number of phyletic lines that one could well call progressive during the period of their greatest flowering.
DOES SELECTION LEAD TO PROGRESS AND ULTIMATELY TO PERFECTION?
In the eighteenth century it was widely believed that the world was perfectly designed by God, and that even where such perfection had not yet been achieved, he had instituted laws that would ultimately lead to it. This belief reflected not only the thinking of natural theology but also the optimism of the Enlightenment, as well as the teleological thinking (finalism) that was so widespread in that period. Lamarck’s theory of evolution, for instance, postulated a steady rise toward perfection. Modern evolutionists reject the idea that evolution is able ultimately to produce perfection. Yet most of them believe that some sort of evolutionary progress has occurred since the beginning of life. The gradual change over time from bacteria to unicellular eukaryotes, and finally to flowering plants and higher animals, has often been referred to as progressive evolution. Such terminology has been used particularly often with reference to man as the end stage of a series leading from reptiles through primitive mammals to placentals and finally to monkeys, apes, and hominids. At one time the idea was almost universally held that man was the culmination of Creation and that anything was progressive that led in the direction of man’s perfection.
Doesn’t the series from bacterium to man indeed document progress? If so, how can such seemingly progressive change be explained? In recent years a number of books were published debating the existence or validity of evolutionary progress. There is great dissension on this question because the word “progress” has so many different meanings. For instance, those who adopt teleological thinking will argue that progress is due to a built-in drive or striving toward perfection. Darwin rejected such a causation and so do modern Darwinians, and indeed no genetic mechanism was ever found that would control such a drive. However, one can also define progress purely empirically as the achievement of something that is somehow better, more efficient, and more successful than what preceded it. The terms “higher” and “lower” have also been criticized. For the modern Darwinian it is not a value judgment, but “higher” means more recent in geological time or higher on the phylogenetic tree. But is any organism “better” by being higher up on the phylogenetic tree? Progress, it is claimed, is indicated by greater complexity, more advanced division of labor among organs, better utilization of the resources of the environment, and better all-around adaptation. This may be true to some extent, but the skull of a mammal or bird is not nearly as complex as that of their early fish ancestors.
Critics of the concept of progress have pointed out that in some ways bacteria are at least as successful as vertebrates or insects, and therefore why should vertebrates be considered progressive over prokaryotes? The decision as to who is right depends largely on what one considers to be progress.
If one looks at the evolutionary series, one cannot deny that some recently evolved taxa have adaptations that were particularly successful for survival. Warm-bloodedness, for instance, permits an organism to cope more successfully with climate and weather fluctuations than is possible for ectotherms. A large brain and extended parental care permit the development of culture and its transmission from generation to generation (see below). Each of these advances has been the result of natural selection, with the survivor having had an advantage over the nonsurvivors. In this descriptive sense, evolution was clearly progressive in certain phylogenetic lineages. It was as progressive as the development of the modern motor car from such early types as Ford’s Model T. Each year the manufacturers of motor cars adopted new innovations and these were then exposed to the selection pressure of the market. Many models with certain innovations were eliminated; the successful ones formed the basis for the next level of innovation. As a result, the cars improved from year to year, becoming safer, faster, more durable, and more economical. Surely the modern car represents progress. If we consider a modern car as representing progress over the Model T Ford, we are equally justified to call the human species progressive compared to lower eukaryotes and prokaryotes. It all depends on how we interpret the word “progressive.” However, Darwinian progress is never teleological.
Many definitions of evolutionary progress have been offered. I particularly like one that emphasizes its adaptationist nature: Progress is “a tendency of lineages to improve cumulatively their adaptive fit to their particular way of life, by increasing the number of features which combine together in adaptive complexes” (Richard Dawkins, Evolution 51(1997): 1016). For other definitions and descriptions of progress, see Nitecki (1988).
The incorporation of symbiotic prokaryotes evidently was a highly progressive step by the first protists, resulting in the immensely successful empire of the eukaryotes. Other progressive steps have often been cited: multicellularity, the development of highly specialized structures and organs, endothermy, highly developed parental care, and the acquisition of a large, efficient central nervous system. The “inventors” of each new progressive step were also highly successful and this contributed to their ecological dominance. Indeed, the gist of every selection event is to favor individuals that have succeeded in finding a progressive answer to current problems. The summation of all of these steps is evolutionary progress.
To continue my analogy, the development of the motor car by no means displaced all other modes of transportation. Walking, the horse, the bicycle, the railroad, they all still coexist with the motor car, all being used under certain circumstances. Nor did the invention of the airplane make the railroad or the motor car obsolete. It is the same with organic evolution. Rather primitive prokaryotes still survive more than 3 billion years after their first appearance on Earth. Fish still dominate the oceans and, except for humans, rodents are more successful in most environments than primates. Also, as shown by cave inhabitants and by parasites, evolution is often retrogressive. However, it is quite legitimate to refer to the series of steps from the prokaryotes to eukaryotes, vertebrates, mammals, primates, and man as progressive. Each step in this progression was the result of successful natural selection. The survivors of this selection process have been proven to be superior to those that were eliminated. The end product of all successful so-called arms races can be considered to be examples of progress.
BIOSPHERE AND EVOLUTIONARY PROGRESS
Most accounts of the history of life on Earth are written as if the environment had been constant, but actually it was not. In particular, there was a drastic change in the composition of the atmosphere. At the time when life originated (ca. 3.8 billion years ago), the atmosphere was reducing, consisting presumably of some mixture of methane (CH4), ammonia (NH3), molecular hydrogen (H2), and water vapor (H2O). There was hardly any free oxygen, and whatever was produced by cyanobacteria disappeared quickly in various sinks, among which the oxidation of iron to iron oxide was the most conspicuous. This led to the deposit of the so-called banded iron formation. The supply of oxidizable iron in the world’s oceans was exhausted ca. 2 billion years ago. The continuing production of free oxygen by cyanobacteria quickly converted the anoxic atmosphere into an oxygen-rich atmosphere and this contributed to the evolution of a rich fauna of multicellular animals. It is believed that the so-called Cambrian “explosion” of new animal types was assisted by the simultaneous enrichment of the atmosphere by oxygen.
The evolutionary changes of the biota during the last 550 million years have greatly affected the composition of the atmosphere. Most important have been the conquest of land by plants (beginning about 450 million years ago), the development of rich angiosperm forests with their capacity to consume CO2, and the evolution of detritus-consuming bacteria.
Vernadsky (1926) was the first to point out the ongoing coevolution between oxygen-producing and oxygen-consuming organisms, as well as the changes in the biota in response to gradual as well as cataclysmic changes in the environment, such as mass extinctions. Organisms can respond to changes of the environment only if they can quickly produce the appropriate variants needed by natural selection. If they do not, they become extinct. Oxygen is not the only element in very active interchange with organisms. Others include calcium (chalk, limestone, corals, shells) and carbon (coal, oil). Changes in the world’s climate have of course also had great evolutionary effect, particularly glaciations and correlated changes in the course of ocean currents, particularly around Antarctica.
HOW CAN WE EXPLAIN TRENDS IN EVOLUTION?
Often when paleontologists compare related organisms in succeeding strata they discover “trends.” For example, the later descendants may be increasingly larger than their ancestors. This trend toward increased size is very widespread among animal lineages and is known as Cope’s Law. A trend may be described as a directional change in a feature in a phyletic lineage or in a group of related lineages. For instance, in a study of horse evolution during the Tertiary, it was discovered that there was a tendency for a reduction in the number of toes, so that the modern horse has only a single one of its original five toes. At the same time, in certain lineages of horses there was a tendency in the molar teeth to become higher and to continue growing throughout life. This is referred to as hypsodonty. Trends such as these were discovered in ammonites, trilobites, and virtually all types of invertebrates. An increase in brain size, not only in primates, is a widespread trend in the evolution of Tertiary mammals. A trend in one specially favored character (e.g., hypsodonty in horses) may result in trends in various correlated characters. In other words, a particular trend may be nothing but the by-product of a trend in a different character, such as body size.
Some paleontologists were puzzled by the seeming linearity of some of these trends. Selection, they claimed, is far too haphazard a process to account for such linearity. This argument, however, overlooks that any evolutionary change in a series of organisms is subject to severe constraints, as shown by the constraints on an increase in the size of the teeth of a horse exerted by the size of the body. There is, for example, a severe constraint on body size in flying organisms, which is why the flying taxa of vertebrates (bats, birds, pterosaurs) are only a fraction of the size of their largest terrestrial relatives. Furthermore, almost all trends are not consistently linear, but change their direction sooner or later, sometimes repeatedly, and they may even totally reverse their direction.
In the days when teleological thinking was widespread, trends were interpreted as evidence for intrinsic tendencies or drives. This was used as the major evidence for a rather popular school of evolutionists who believed in teleological orthogenesis (see Chapter 4). The almost lawlike progression in some of these trends was interpreted by this school as being incompatible with Darwin’s natural selection. Subsequent research, however, has shown that there is no such conflict. No support for the existence of intrinsic evolutionary trends was ever found and trends can be explained quite confidently by the Darwinian model with due consideration of constraints. It is now quite evident that all observed evolutionary trends can be fully explained as being the result of natural selection.
Correlated Evolution
An organism is a carefully balanced, harmonious system, no part of which can change without having an effect on other parts. Let us consider the increase in the size of teeth in horses. This change requires a larger jaw, and in turn a larger skull. To carry the larger skull, the entire neck has to be reconstructed. The larger new skull has an effect on the rest of the body and in particular on locomotion. This means that in order to acquire larger teeth virtually the whole horse must to some extent be reconstructed. This has been confirmed by a careful study of the anatomy of hypsodont horses. Also, since the whole horse had to be reconstructed, the change could occur only gradually and slowly over many thousands of generations. Many lineages of horses with low molar teeth failed to come up with the required genetic variation for hypsodonty and became extinct.
The shift from the quadrupedal locomotion of a lizardlike reptile to bipedalism and flight in birds initiated a considerable restructuring of the body plan: a compacting of the whole body to have a better center of gravity, the development of a more efficient four-chambered heart, restructuring of the respiratory tract (lungs and air sacs), endothermy, improved vision, and an enlarged central nervous system. The acquisition of all of these adaptations was a matter of necessity. Details, however, are often dictated by constraints and the availability of genetic variation.
Sometimes the development of one aspect of the phenotype may have unexpected consequences for other parts of the body. This is well illustrated by evolution among the reptiles. Two major subdivisions of the Reptilia are recognized: the Synapsida, with one temporal skull opening, and the Diapsida, with two openings. The turtles, without any temporal opening, were believed to be an old group that had originated before the development of any temporal openings. Molecular analysis, however, has shown that the turtles are diapsids, related among living reptiles to the crocodilians. Apparently they lost the skull openings during the acquisition of the carapace as part of a general reduction of all openings to the outside. This, incidentally, also shows how drastically a taxonomic character may change its value during evolution.
Complexity
Many early evolutionists were convinced that evolution advanced steadily toward ever greater complexity. Indeed, the prokaryotes, which represented life on Earth for more than 1 billion years, are far less complex than the eukaryotes, which evolved subsequently. But among the prokaryotes there is no indication of ever increasing complexity in the long period of their existence. Nor does one find any evidence for such a trend among the eukaryotes. To be sure, multicellular organisms are, on the whole, more complex than the protists, but at the same time numerous evolutionary lineages are found among both plants and animals that evolved from complexity to greater simplicity. The skull of a mammal, for instance, is far less complex than that of its placoderm ancestors. Wherever we look, we find simplifying trends as well as trends toward greater complexity. Parasites are, on the whole, notorious for their many physical and physiological simplifications. All theories that postulated the existence in all organisms of an intrinsic trend toward greater complexity have been thoroughly refuted. There is no justification in considering greater complexity to be an indication of evolutionary progress.
MOSAIC EVOLUTION
Organisms never evolve as types; there is always a greater selection pressure on some properties than on others, and these attributes then evolve faster than the others. In the evolution of man, for instance, there are enzymes and other proteins that have not changed in six or more million years, and are therefore still identical with those of chimpanzees or even earlier primate ancestors. Other primate properties of hominids have changed drastically, with the central nervous system changing the most. The Australian Platypus has hair and suckles its young with milk and has other characteristics of primitive mammals, but lays eggs, like reptiles, and has some “dead-end” specializations, like a poison spur and a duckbill. This uneven rate of evolution of different properties of an organism is called mosaic evolution , and it may create difficulties for classification. The first species of a new branch of a phylogenetic tree will have acquired a single derived key character but may agree in everything else with its sister species. Darwinian taxonomists usually classify such a species with its sister species with which it agrees in most of its characters. A Hennigian cladist, however, may assign it to a new clade.
The fact that the evolution of different components of the phenotype of an organism may to some extent be independent of each other provides great flexibility for evolving organisms. To successfully enter a new adaptive zone, an organism might have to change only a limited component of its phenotype. This is well illustrated by Archaeopteryx, which in many respects (e.g., teeth, tail) is still a reptile, even though it has the feathers, wings, eyes, and brain of a bird. Mosaic evolution is even more strikingly demonstrated by the highly different rates of evolution of different proteins and other molecules.
Not knowing how to explain mosaic evolution, geneticists long ignored it. Now a theory of “gene modules” has been proposed, in which the concerted action of certain groups of genes (“modules”) has been postulated. Such modules can, to some extent, evolve rather independently of each other.
PLURALISTIC SOLUTIONS
Evolution is an opportunistic process. Whenever there is an opportunity to outcompete a competitor or to enter a new niche, selection will make use of any property of the phenotype to succeed in this endeavor. Several different solutions are usually available for any challenge by the environment.
Flying was invented by vertebrates three different times, but the wing of each flying taxon—birds, pterosaurs, and bats—is different. Even more different are the wings of different kinds of insects, for instance, dragonflies, butterflies, and beetles, although all of them seem to be derived from a single ancestral flying type.
Pluralism is characteristic of all aspects of the evolutionary process. Genetic variation is replenished in most eukaryote species by sexual reproduction (recombination), whereas in the prokaryotes it is replenished by unilateral gene transfer. Reproductive isolation is effected in most higher animals by prezygotic isolating mechanisms (e.g., behavior), and in others by chromosomal incompatibilities, sterility, or other postzygotic factors. Speciation usually occurs for geographic reasons in terrestrial vertebrates, but it is sympatric in certain groups of fishes and perhaps in plant-host-specific groups of insects. There is a very reduced amount of gene flow in some species, while others disperse so easily that the entire species is virtually panmictic. Furthermore, some families have many actively speciating genera, while others have only a few old monotypic genera.
In view of this rampant pluralism, at the level of both micro- and macroevolution, it is advisable to exercise great caution when applying the findings for one group of organisms uncritically to others. Findings made in one group of organisms do not necessarily refute different findings made in another group.
CONVERGENT EVOLUTION
Convergent evolution is a phenomenon that convincingly illustrates the power of natural selection. The same ecological niche or adaptive zone is often filled on different continents by exceedingly similar, but entirely unrelated organisms. The opportunity provided by the same adaptive zone results in the evolution of similarly adapted phenotypes. This process is called
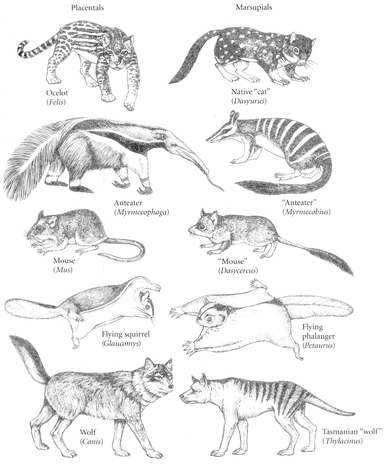
convergence. The most famous case is that of the Australian marsupials. These indigenous mammals have, in the absence of placental mammals, produced types analogous to placental mammals in the northern continents. The northern wolf is matched by the Tasmanian wolf, the placental mole by the marsupial mole, the flying squirrel by the marsupial phalanger, and there are other less close analogs: a mouse, a badger (wombat), an anteater. (
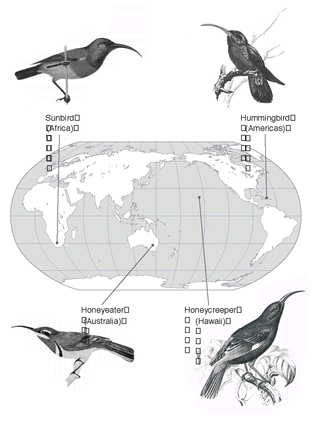
Fig. 10.3). Species adapted to subterranean life (and convergently similar) have independently evolved in four different orders of mammals and among the rodents in eight different families (Nevo 1999). Such cases of convergent evolution are not exceptional, but are actually quite widespread. To mention a few others: the American and the African porcupine, the New World vultures (Cathartidae, related to storks) and the Old World vultures (Accipitridae, related to hawks), and the nectar-feeding birds—hummingbirds (Trochilidae) in the Americas, sunbirds (Nectariniidae) in Africa and southern Asia, honeyeaters (Meliphagidae) in Australia, and honeycreepers (Drepanididae) on Hawaii (
Fig. 10.4). Any knowledgeable zoologist would be able to list several pages of such cases of convergent evolution.
Convergent evolution of vertebrates in the ocean produced sharks, porpoises (mammals), and the extinct ichthyosaurs (reptiles). Convergent developments have occurred in many animal taxa, but they also occur among plants. The various kinds of cactus in America are paralleled by analogs among the Euphorbiaceae of Africa (
Fig. 10.5). Convergence illustrates beautifully how selection is able to make use of the intrinsic variability of organisms to engineer adapted types for almost any kind of environmental niche.
Convergent evolution of Australian marsupials (right) and placental mammals (left) on other continents. Each pair is similar in form and lifestyle.
Source: A View of Life by Salvador E. Luria et al. Copyright © 1981 Benjamin Cummings. Reprinted by permission of Pearson Education, Inc.
Independent evolution of nectar-feeding adaptations in four songbird families: sunbird (Nectariniidae), hummingbird (Trochilidae), honeyeater (Meliphagidae), and honeycreeper (Hawaiian finches, Drepanididae).
Sources: Honeycreeper (Hawaii), Wilson, S.B. and Evans, A.H. (1890–1899). Aves Hawaiienses: The Birds of the Sandwich Islands; Honeyeater (Australia), Serventy, D.L. and Whittell, H.M. (1962). Birds of Western Australia (3rd ed.) Paterson Brokensha: Perth; Sunbird (Africa), Newman, K. (1996). Newman’s Birds of Southern Africa: The Green Edition . University Press of Florida: Gainesville, FL. Reprinted by permission of Struik Publishers of Cape Town, South Africa and Kenneth Newman; Hummingbird (Americas), James Bond (1974) Field Guide to the Birds of the West Indies. HarperCollins Publishers.
POLYPHYLY AND PARALLELOPHYLY
In the pre-Darwinian days of classification, convergent groups were often combined into a single taxon owing to their similarity. Such a taxonomic assignment is known as polyphyly. Recognition of such a polyphyletic taxon was in conflict with Darwin’s demand that every taxon should be monophyletic, that is, should consist exclusively of descendants of the nearest common ancestor. Darwinian taxonomists broke up such polyphyletic taxa and placed the parts with their nearest relatives. The combination of whales and fishes was such a polyphyletic taxon that was later rejected.
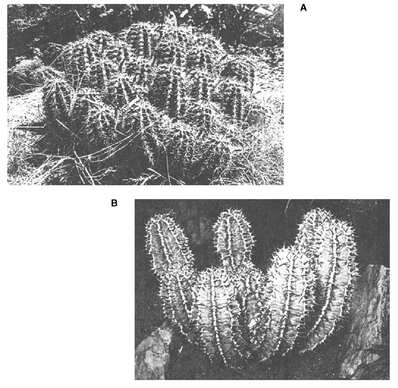
Parallel evolution of similar arid country adaptations in (a) American cactuses and (b) African euphorbs. (From Starr et al. 1992.)
Source: Photographs copyright © 1992, Edward S. Ross. Reprinted by permission.
Convergence must be carefully distinguished from
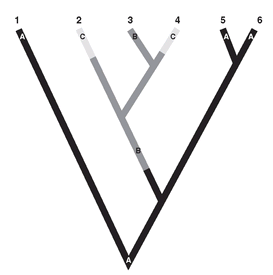
parallelophyly, which designates the independent emergence of the same character in two related lineages descended from the nearest common ancestor (
Fig. 10.6). For instance, stalked eyes occur independently and irregu-larly in various lineages of acalypteran flies, because all of these lineages have inherited from their common ancestor the genotypic capacity for the production of such eyes. But this propensity has been realized in only some of the lineages. Many if not most cases of
homoplasy are caused by such parallelophyly. In a reconstruction of phylogeny, not only the phenotype must be considered but also the ancestral genotype and its phenotypic potential.
Parallelophyly. The independent evolution of similar phenotypes (2, 4) owing to the inheritance of the same propensity in the common ancestral genotype (3).
A Case Study: The Origin of Birds
The greatest current controversy in phylogeny will perhaps be settled by invoking parallelophyly; it concerns the origin of birds. There is no argument over the conclusion that birds derived from the archosaurian lineage of the diapsid reptiles. But when this happened is the argument. As far back as the 1860s, T. H. Huxley called attention to the remarkable similarity of the avian skeleton to that of certain reptiles and concluded that the birds had descended from dinosaurs. Later, other authors postulated a much earlier origin, but recently the dinosaur origin has been proclaimed by the cladists with such vigor that at present it seems to be the most widely accepted explanation of the origin of birds. Indeed, the similarity of the pelvis and legs between birds and certain bipedal dinosaurs is astonishingly close (see
Fig. 3.6).
However, the arguments of their opponents are also very persuasive. The fossil chronology seems to be in conflict with the dinosaur theory. The particular bipedal dinosaurs that are most birdlike occurred in the later Cretaceous, some 70–100 million years ago, while Archaeopteryx, the oldest known fossil bird, lived 145 million years ago. Archaeopteryx has so many advanced avian characters that the origin of birds must be placed considerably earlier than the late Jurassic, perhaps in the Triassic, but no birdlike dinosaurs are known from that period. Furthermore, the digits in the dinosaurian hand are 2, 3, 4 while in the avian hand they are 1, 2, 3. Also, the anterior extremities of the birdlike dinosaurs are very much reduced and in no way preadapted to become wings. It is quite inconceivable how they could have possibly shifted to flight. These are only a few of the numerous facts in conflict with a Cretaceous origin of birds from a dinosaurian ancestry. The argument will probably not be fully settled until more Triassic fossils are found.
ARE THERE LAWS OF EVOLUTION?
This is a question that physicists and philosophers like to ask. To answer it, one first needs to decide what one means by the word “law.” The kind of laws characteristic of the physical sciences, which can be stated in mathematical terms and have no exceptions, are sometimes also encountered in functional biology. Mathematical generalizations can often be applied to biological phenomena, like the Hardy-Weinberg equilibrium relating to the distribution of alleles in populations. By contrast, all so-called evolutionary laws are contingent generalizations, and thus not equivalent to the laws of physics. Evolutionary “laws,” such as Dollo’s Law of the irreversibility of evolution or Cope’s Law of an evolutionary increase in body size, are empirical generalizations, with numerous exceptions, and are quite fundamentally different from the universal laws of physics. Empirical generalizations are useful for ordering observations and in the search for causal factors. Rensch (1947) made a particularly helpful contribution to this subject in pointing out that evolutionary “laws” are greatly restricted in time and place and therefore do not satisfy the traditional definitions of scientific laws.
CHANCE OR NECESSITY?
For years there has been a rather heated controversy over whether chance (contingency) or necessity (adaptation) is the dominant factor in evolution. Enthusiastic Darwinians tended to ascribe every aspect of a living organism to adaptation. They argued that in every generation there is a drastic culling of each population, sparing on the average only two of the hundreds, thousands, or in some cases even millions of offspring of each set of parents. Only the most perfectly adapted individuals, they would claim, could pass through this ruthless process of elimination. Those who uphold adaptation as the dominant force in evolution have indeed a strong argument.
Unfortunately, some of the strict adaptationists forgot that natural selection is a two-step process. To be sure, selection for adaptedness is paramount at the second step, but this is preceded by a first step—the production of the variation that provides the material for the selection process, and here stochastic processes (chance, contingency) are dominant. And it is this randomness of variation that is responsible for the enormous, often quite bizarre diversity of the living world. Let us consider two cases. The first is the enormous diversity of the unicellular eukaryotes (“protists”). Margulis and Schwartz (1998) recognize in this kingdom no fewer than 36 phyla of mostly unicellular eukaryotes, many of them parasitic. These include such utterly diverse organisms as amoebas, radiolarians, foraminifera, sporozoans, Plasmodium, zooflagellates, ciliates, green algae, brown algae, dinoflagellates, diatoms, Euglena, slime molds, and chytridiomycota, to mention just a few of the better-known ones. Another specialist recognized as many as 80 phyla. Many of them are strikingly different from each other, and for some of them, it is still argued whether they should not rather be classified with fungi, plants, or animals. Does it really require that many different body plans for unicellular eukaryotes to be well adapted?
The diversity among the multicellular organisms is even more astonishing. Not only do we have multicellular “protists” like the brown algae, but the differences between and within the three rich multicellular kingdoms, the fungi, plants, and animals, are even more overwhelming. Did they need all of these differences in order to be well adapted? Let us look at the bizarre types in the Burgess shale fauna. One cannot escape the suspicion that many of them were due to mutational accidents that were not eliminated by selection. Indeed, I sometimes wonder whether the elimination process is not sometimes a good deal more permissive than is usually assumed. Furthermore, one must not forget that chance always plays a considerable role even at the second step of evolution, that of survival and reproduction. And not all aspects of adaptedness are tested in every generation.
Or let us look at the 35 or so living phyla of animals. They are the survivors of the 60 or more body plans that existed in the early Cambrian. When one studies their differences, one does not get the impression that they are necessities. Many or even most of their unique characteristics may have had their origin in a developmental accident that was tolerated by selection, while the seeming failure of those that became extinct may have been the result of a chance event (like the Alvarez asteroid extinction event). S.J. Gould (1989) made such contingencies a major theme in Wonderful Life, and I have come to the conclusion that here he may be largely right.
One can conclude from these observations that evolution is neither merely a series of accidents nor a deterministic movement toward ever more perfect adaptation. To be sure, evolution is in part an adaptive process, because natural selection operates in every generation. The principle of adaptationism has been adopted so widely by Darwinians because it is such a heuristic methodology. To question what the adaptive properties might be for every attribute of an organism leads almost inevitably to a deeper understanding. However, every attribute is ultimately the product of variation, and this variation is largely a product of chance. Many authors seem to have a problem in comprehending the virtually simultaneous actions of two seemingly opposing causations, chance and necessity. But this is precisely the power of the Darwinian process. e
Can we also apply this conclusion to man? Some of the most enthusiastic promoters of the principle of contingency have claimed “Man is nothing but an accident.” This conclusion is, of course, in complete conflict with the teachings of most religions, which consider man the pinnacle of Creation or the end point of a long drive toward perfection. The success—at least in terms of population growth and expanding range—of mankind in the last 500 years would seem to demonstrate how well man is adapted. On the other hand, if the making of man had been a deterministic process, why did it take 3,800 million years to produce? The species Homo sapiens is only about a quarter million years old, and prior to that time our ancestors were in no way outstanding within the animal kingdom. No one could have predicted that a defenseless, slow-moving biped should become the pinnacle of Creation. But one of the australopithecine populations somehow acquired the brain power to survive by its wits. One can hardly avoid considering this more or less of an accident, but it wasn’t a pure accident because every step in the change from an australopithecine to Homo sapiens was furthered by natural selection.