The earliest recognition of fossil bones, whether they were dinosaurian, mammalian, or some other vertebrate, cannot be recounted, but it is clear that they were given serious attention at least since antiquity. Seized on as being the remains of giants, so began the link between fossils and what might be called gigantology, a fascination with large size that continues to this day.1
It was the bones of large fossil mammals, especially of extinct elephants, that originally attracted attention. Adrienne Mayor, in her tour de force treatment of the meaning of fossils in antiquity, noted many instances of massive bones discovered in such places as the islands of Sicily and Capri that were thought to be the remains of a race of giants.2
Discoveries and interpretations such as these continued to be made throughout the Middle Ages and the Renaissance—indeed, up until the end of the seventeenth century—whereby large fossil bones were generally thought to be the remains of human giants, dragons, or mythical monsters hearkening back to Greek mythology. In the skull of a Pleistocene dwarf elephant discovered in a Sicilian cave near Trapani in the fourteenth century, Giovanni Boccaccio (1313–1375), author of the Decameron, saw the face of the cyclops Polyphemus.3
As Christianity took hold across Europe, scripture (“There were giants in the earth in those days,” Genesis 6:4) and the existence of ancient behemoths became closely tied. Consequently, the practice of attributing fossil bones to giants—often as saints and other biblical personages—led to these remains being kept in churches, especially in Europe. Other interpretations fashionable at that time included ascribing animal fossils to real people. Thus, according to legend, the remains of Theutobochus (King of the Teutons, Cimbri, and Ambrones) were recovered at Lang-don, France, in 1613. The giant Theutobochus was the basis for considerable controversy, in which opposing physicians and surgeons attacked each other over the authenticity and determination of these remains. In the end, these bones proved to be those of a Miocene elephant called Deinotherium giganteum.4
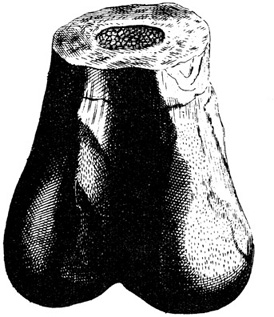
A more modern interpretation of fossil bones began to emerge with the rise of interest in comparative anatomy. During the seventeenth century, Robert Plot (1640–1696), curator of Oxford University’s Ashmolean Museum, was busy producing the first illustrated book of fossils from England.5 Among the plates was a figure of the lowermost part of a thighbone that looked very similar to that of humans, but was much greater in size (figure 5.1). Plot concluded that it “must have belonged to some greater animal than an Ox or Horse; and if so in all probability it must have been the Bone of some Elephant, brought hither during the Government of the Romans in Britain.”6 Another century passed, and Richard Brookes (dates unknown), also a natural historian from England, copied Plot’s figure into a compendium of natural history.7 In a caption to this illustration, Brookes now applied a name—Scrotum humanum. Although his designation was in apparent recognition of its general shape, Brookes was not persuaded that the fossil was an actual fossilized gigantic scrotum (although one French philosopher, Jean-Baptiste Robinet [1735–1820], apparently was convinced8). Instead, Brookes considered it, as Plot had before him, a portion of the thigh bone of a large animal. Although the original specimen is lost, we now know that this fragment was the lower end of a femur from the Middle Jurassic of Oxfordshire and that it must have belonged to some sort of large theropod dinosaur.9
In the seventeenth century, dinosaurs, and indeed deep time itself, had yet to be discovered; it took three people from England, the help of the great French comparative anatomist Georges Cuvier, and the first half of the nineteenth century to finally recognize them.10 William Buck-land (figure 5.2, left), Reader of Mineralogy, Reader of Geology, and then Canon of Christ Church College at Oxford University, sought to fuse geology and paleontology with the traditional Christian teachings of Noah’s flood and a divine Creator. A legendary eccentric, Buckland is also credited with the first scientific description of a dinosaur, a theropod that he named Megalosaurus, based on a fragment of a lower jaw with teeth and some postcranial bones from the Middle Jurassic Stonesfield Slate of Oxfordshire.11 Buckland’s contemporary, Gideon Mantell (figure 5.2, right), was trained as a physician, a career marked by considerable failure. It is Mantell’s other life pursuit, research on the paleontological riches of the English Weald, for which he is now remembered. As one of the world’s original dinosaur hunters, he energetically collected, swapped, and purchased teeth, isolated bones, and portions of a skeleton, from which he gave us the first-discovered herbivorous dinosaur, Iguanodon, from the Early Cretaceous of southeastern England.12
Figure 5.1. The first “named” dinosaur, Scrotum humanum, in actuality the lower end of a Megalosaurus femur. (Original plate from Brookes 1763)
Together, Buckland and Mantell recognized the unique nature of their material: the bones and teeth of reptiles whose great size eclipsed any reptile and most mammals known up to that time. Mantell, in particular, had the vision to impart animal form and habits to these fossil bones, as well as the intellectual courage to perceive that these were animals of the remote past, not relics of Noah’s flood. Deep time was about to be discovered.
Figure 5.2. William Buckland (1784–1856; left) and Gideon Algernon Mantell (1790–1852; right)
The actual recognition of Dinosauria, including Megalosaurus, Iguanodon, and an armored dinosaur known as Hylaeosaurus, was left in the hands of Sir Richard Owen.13 A comparative anatomist at the Royal College of Surgeons in London, Owen built on his personal insights and those of Mantell and Buckland and, with a flash of insight and no little political guile, hatched Dinosauria in April 1842.14 “The combination of such characters [in particular, a sacrum composed of more than two fused vertebrae] all manifested by creatures far surpassing in size the largest of existing reptiles, will, it is presumed, be deemed sufficient ground for establishing a distinct tribe or suborder of saurian reptiles, for which I would propose the name of Dinosauria.”15 This new group of reptiles called Dinosauria—the “fearfully great lizards”—was thought to consist of highly advanced terrestrial quadrupeds with the unmistakable character of enormous size.
From Owen’s day onward, the quintessential dinosaur, at least in the popular imagination, has been gigantic. These creatures were and still are the behemoths that pack the public into museum exhibits.16 With each thud of their feet, they have rumbled their way into books of all sorts, onto postage stamps and collecting cards, into children’s games (including the computer variety), and, of course, onto the big screen, from Gertie the Dinosaur (1912) through Jurassic Park III (2001).17
Dinosaur size records are set, only to be broken. Notable among the carnivores, the perennial favorite, Tyrannosaurus (or T. rex to its friends), a 12 m long predator from the Late Cretaceous of North America that may have tipped the scales at 7 metric tonnes, now has plenty of company among the great Mesozoic slayers.18 Carcharodontosaurus, a theropod from the middle Cretaceous of northern Africa, has been estimated to be as long as Tyrannosaurus, but it appears to have been slightly more massive,19 whereas Giganotosaurus from the Early Cretaceous of Argentina is thought by some to measure over 14 m and weigh up to 8 metric tonnes.20 Yet among the truly supergigantic, there’s nothing like the sauropods, which have long been known to be the largest of all terrestrial animals ever to have lived. For many years, the prize went to Diplodocus, reaching a length of 27 m,21 but this record has seen its challenges. In North America, there is Seismosaurus, an as-yet poorly known sauropod from the Late Jurassic of the southwestern United States, whose length has been variously estimated at upwards of 50 m.22 Not to be outdone, the Southern Hemisphere has offered up Argentinosaurus. Discovered in 1989, named in 1993, but with other aspects not yet fully published, this behemoth has also been estimated to be as much as 50 m long.23 These are clearly humbling animals.
The evolution of dinosaurian gigantism is but one example of the general trend of animals becoming larger over time. Other well-known examples include horses, ratites (ostriches and their kin), and ammonites (long-extinct relatives to today’s pearly nautilus, which grew to diameters of about 2 m). Such evolutionary trends in increasing body size—commonly referred to as Cope’s Rule, after E. D. Cope’s recognition that evolution often results in phylogenetic size increases24—are thought to be due to an extension of growth tendencies already present in ancestral ontogenies. These extensions are known as peramorphs (“beyond shapes”).25 Peramorphosis implies that descendants reach new end stages (for example, a larger body size) by passing along this course of ancestral ontogeny, but then, instead of standing still, advance still further.
The production of peramorphs through an extension of growth has its opposite manifestation in the production of paedomorphs (“baby shapes”) through arrested growth.26 Said another way, paedomorphosis is the retention of ancestral juvenile characters into the adulthood of its descendants. Referred to by Ken McNamara (paleontologist and evolutionary biologist at the Western Australian Museum in Perth) as the Peter Pan syndrome, two modern organisms stand out as the most celebrated examples of arrested growth.27 The first is the axolotl (figure 5.3), otherwise known as the Mexican salamander Ambystoma mexicanum, which retains its larval characteristics into adulthood and thereby is able to reproduce while remaining in the form of an aquatic larva.28 The other modern epitome of paedomorphosis, as we shall see a little later, is ourselves—Homo sapiens. Furthermore, we encounter paedomorphs among dogs, horses, mice (including one named Mickey), finches, ratites, fish, insects, snails, and plants. They are even known from the fossil record, including the dwarfed dinosaurs from Transylvania.29
In chapter 2, we let Franz Baron Nopcsa introduce, for the first time, the notion of small dinosaurs from Transylvania in the context of his hypothesis that in the Cretaceous this region was an island. In his first synthesis of this subject, Nopcsa surveyed the Transylvanian fauna,30 emphasizing in particular the disparity in body size of its members: “While the turtles, crocodilians, and similar animals of the Late Cretaceous reached their normal size, the dinosaurs almost always remain below their normal size.”31 He observed that most of the Transylvanian dinosaurs hardly reached 4 m in length, and the largest (what was to become Magyarosaurus dacus) was a puny 6 m long, compared with a more representative 15–20 m for other sauropods. After this 1915 paper, Nopcsa’s fascination with body size shifted to dinosaurian giants, but in both cases he equated the evolution of both dwarfism and gigantism to changes in the endocrine system, such as in the size and function of the pituitary gland.32
Nopcsa viewed Transylvanian dwarfing as the body-size consequences of island evolution. Thus these dinosaurs joined the ranks of other insular organisms, both living and extinct, that have evolved into smaller and larger versions of their continental relatives: dwarfed crocodilians, giant moas, downsized island deer, enormous Galápagos turtles, dwarfed donkeys, huge Komodo lizards, small Bali tigers, pygmy elephants, and dwarfed hippos.33 After 1914, Nopcsa’s efforts to define “small” in dinosaurs ceased, and he simply concluded that the Transylvanian dinosaurs were “less than the usual colossal forms” that tended to be found elsewhere. Whereas such a statement is certainly true, it belies a more complex set of questions about the acquisition of small body size. Yes, these dinosaurs were smaller, but were they, and they alone, miniaturized from a larger ancestor?
Figure 5.3. The axolotl (Ambystoma mexicanum), a living salamander that reproduces as an aquatic form; in this way, it is a modern exemplar of paedomorphosis. (After Lamar 1997)
Why is it that all babies are born looking like Winston Churchill, and vice versa? To put it another way, why do the faces of both young and old humans alike tend to look more like the faces of short-faced chimpanzee and gorilla youngsters than these latter two juveniles do to their respective long-faced parents (figure 5.4)?34 These similarities and differences in proportions have been attributed to changes in different aspects of facial growth and, when given an evolutionary context, are described in terms of heterochrony. We have already seen heterochrony in action through the gigantic (peramorphic) tendencies of dinosaurs and the paedomorphosis of the axolotl. In the case of humans, gorillas, and chimps, it is the retardation of growth in the jaw region that gives us that youthful look. So to the degree that the pattern of growth in chimps and gorillas is considered the primitive condition for the immediate clade of primates that includes these two groups and us, then we (or rather our faces) represent the paedomorphic retention of juvenile features into adulthood.
In their book of the same name, Michael McKinney and Ken Mc-Namara define heterochrony (meaning “different time”) as the “change in timing or rate of developmental events, relative to the same events in the ancestor.”35 In other words, heterochrony seeks to link development with evolution. In Darwin’s world, where external forces held sway, there was little attempt to link ontogeny with phylogeny through his view of natural selection. Instead, it was principally the German scientific community, notably the nineteenth-century school of Naturphilosophie, that attempted to link developmental sequences with similar patterns found in evolutionary history.36 Ernst Haeckel (1834–1919), in his attempt to reconcile Darwin’s evolutionary mantra of “descent with modification” with emerging issues in developmental biology, coined his own slogan, “ontogeny recapitulates phylogeny,” now known as Haeckel’s Biogenic Law.37
The most fundamental reconsiderations of how development interconnects with phylogeny to produce heterochrony have been through Stephen J. Gould’s now-classic book, Ontogeny and Phylogeny,38 and a subsequent paper cowritten by him and an impressive cadre of evolutionary and developmental biologists: Pere Alberch, George Oster, and David Wake.39 Both publications stress how the features of organisms change with size during both ontogeny and phylogeny, and the paper sets forth the schema of heterochronic terminology to express these changes. The concept of heterochrony has seen an incredible growth in evolutionary biology in recent years.40 It is now used to explain, with great success, the differences in skull forms of domesticated dogs, the evolution of fossil sea urchins, the outrageously large antlers in the so-called Irish elk (actually a giant deer), and sexual dimorphism in the forked fungus beetle.41 Each of these examples of heterochrony, and many more, describes how ontogenetic changes form the basis for phylogenetic transformations.
Figure 5.4. Neoteny and the human face. The left column (a) of three skulls represents the ontogenetic changes in the facial skeleton of a chimpanzee, while the right column (b) of two skulls represents a newborn and an adult human. The superimposed grids indicate how much change occurs in the skulls during ontogeny. Note the similarity of the faces of a newborn chimpanzee and a newborn human, the similarity of newborn and adult humans, and the dissimilarity between an adult human and an adult chimpanzee. (After Starck and Kummer 1962)
So what is this thing we call heterochrony? We began talking about the subject by introducing paedomorphs and peramorphs, the two ontogenetic patterns that are linked between ancestors and descendants. A paedomorph is an ontogenetically less well-developed descendant than its ancestor. That is, reduced or arrested development will produce a paedomorph. In contrast, if its development goes beyond that of its ancestor, a descendant is considered to be a peramorph. Peramorphs are produced by increased development. A sequence of successive paedomorphs is known as a paedomorphocline. Likewise, a continued trend of peramorphs is called a peramorphocline. Each of these states—paedomorphosis and peramorphosis—is the product of three growth processes: (1) the timing of trait formation, (2) the cessation of its development, and (3) the rate at which it is acquired (figure 5.5).
A change in the time that the growth of an organ or structure starts development, relative to others, results in either predisplacement (if the change is earlier) or postdisplacement (a later change). Predisplacement is seen in the nasal horns of the large, early Tertiary mammals known as titanotheres, in which earlier development results in peramorphosis.42 In contrast, postdisplacement involves the delayed onset of growth as, for example, is seen in the timing of the cellular differentiation of fins and limbs in vertebrates.43 In this way, later starters produce the paedomorphic condition.
When the development of a structure ceases also has an effect on the final body form of an organism. Earlier cessation/offset in a descendant, rather than in its ancestor, produces what is known as progenesis. Progenesis is thought to have altered the ancestral developmental program of arthropods and reduced the number of segments and limbs in insects, the paedomorphic descendants of their common ancestor with the multisegmented, multilegged centipedes and millipedes.44 Hypermorphosis produced by delayed offset—the extension of growth beyond that of an ancestor—has peramorphically produced large dogs such as Irish wolfhounds, Great Danes, and St. Bernards.45
Changes in the rate of growth can produce either acceleration, when growth rate is increased, or neoteny, when growth rate is retarded. Neoteny—that is, less shape change and a decrease in complexity—is thought to be behind why adult human faces look much like those of youngsters: the rate of growth of the face is decelerated, compared with that seen in ancestral ontogenies, to produce the paedomorphic human facial profile.46 Acceleration, by contrast, can be seen in the growth of the bones in the digits of the hand in bats, acting to produce the peramorphic scaffolding of their special kind of airfoil for flight.47
Figure 5.5. Heterochrony, its two manifestations (peramorphosis and paedomorphosis), and the six processes that produce them. (After McKinney and McNamara 1991)
As we have briefly introduced the concepts here, when descendants go beyond the size and shape of their ancestors, we have a pattern known as peramorphosis. And when descendants fail to achieve the size and shape of their ancestors, due to arrested growth, we have paedomorphosis. Although peramorphs and paedomorphs can be more-or-less easily recognized by allometric comparisons of size and shape, the processes that produce them (e.g., neoteny, progenesis, postdisplacement, etc.) are more difficult to discern, because they require a measure of actual ontogenetic timing. For living organisms, there is no barrier to establishing when various life-history events—the timing of protein expression, cell differentiation and interaction, hormone secretion, sexual maturity, and so on—take place (although this information is rarely available). The question is, can we do this with fossils?
We are fortunate to be living in a time when the insides of fossils are as important—and are nearly as easily seen—as their outsides are. Bone in thin-section is beginning to reveal how dinosaurs lived, reproduced, metabolized, and grew in ways for which, a generation ago, we had hardly a clue. For our purposes here, bone in thin-section appears to be able to allow us to calibrate anatomical changes in real time. This burgeoning research is called skeletochronology.48
How does it work? First, we know that bone growth can be episodic, and this ebb and flow is laid down in the bone itself, much like tree rings.49 These lines, found in both extant and extinct vertebrates, reflect disruptions in growth: periods of rapid growth followed by slowdowns or cessation. More simply put, growth lines—what scientists call lines of arrested growth (LAGs)—appear as thin, circumferential, avascular regions when bone is seen in thin-section. LAGs are thought to be annual, representing yearly histories of seasonality and ecological stress.
Provided that they are annual, LAG counts give an estimate of the longevity of a particular individual. When these are combined from individuals of different sizes within a single species—say, Tyrannosaurus rex—such longevity estimates, coupled with size data or mass estimates, can be used to make the classically sigmoidal age-versus-size curves. From such studies, we know that at least some of the extinct dinosaurs (e.g., ornithopods, theropods, and sauropods) grew quite rapidly, perhaps as much as is seen in modern birds.50 Yet how did these rates vary phylo-genetically among close relatives? By reconstructing and comparing the growth curves of closely related forms, it is possible to bring skeleto-chronologies to bear on real-time heterochronic problems. This has indeed been done for T. rex and other tyrannosaurids, Janenschia and other sauropods, the prosauropod Plateosaurus, the ceratopsian Psittacosaurus, and, perhaps most relevant here, a new species of dwarfed sauropod from the Late Jurassic of Germany.51
Preliminary thin-section work relating to when particular developmental stages occur during the lifetime of dinosaurs has been done on some of the ones from Transylvania. Ragna Redelstorff, Zoltán Csiki, and Dan Grigorescu conducted histological studies of a series of femora from Telmatosaurus transsylvanicus, to test whether the largest among them (464 mm long) come from small adults (i.e., dwarfed taxa) or from juveniles of more normal-sized taxa.52 Histologically, these femora exhibit up to eight lines of arrested growth, and the outermost layer of the largest femur is very thin and avascular, indicating that growth had either slowed down considerably or ceased. Such bone microstructure indicates that the largest femora belong to fully adult dwarfed individuals, not juveniles of much larger forms. The reduction in body size was presumably caused by a slowdown of their growth rate.
A decidedly different bone histology is encountered in Magyarosaurus dacus.53 Thin-sections were again made of different sizes of long bones (femora 346–545 mm long, humeri 223–488 mm long) but, unlike the situation in Telmatosaurus, the overwhelming majority of the cortex in each of these sections is extensively remodeled by secondary bone. There is almost no preservation of primary bone that could record lines of arrested growth, even in the smallest individuals. Koen Stein and his coworkers noted that the intense secondary remodeling in the bones of Magyarosaurus is closest to that of other individual sauropods of very late histological ontogenetic stages. Although lacking a thin avascular outer layer that would indicate reduction or cessation of growth (as in Telmatosaurus, above), the early appearance of secondary remodeling in Magyarosaurus suggests an early onset of sexual maturity and a short lifespan.
Finally, there is Zalmoxes robustus. As before, with Telmatosaurus, Redelstorff and her coworkers studied the histology of a series of femora (164–355 mm long) to test whether the largest of these represent dwarfed adults or juveniles of normal-sized taxa.54 The long-bone histology reveals a slow growth rate in Zalmoxes, indicated by the high number (15) of narrow-spaced lines of arrested growth. However, vascular canals in the cortex open onto the bone surface, which indicates that growth had not plateaued at the time of death. Thus it is likely that the existing material of Zalmoxes represents bones not yet fully grown, and this ornithopod can probably be characterized by an extreme slowdown of its growth rate and an extended growth period.
We began this chapter describing the ways in which dinosaurs have pushed the envelope of body size, and, in so doing, they have provided a heterochronic context for their enormity. Although peramorphosis appears to be the main driving force in the evolution of nonavian dinosaurs,55 several examples of paedomorphosis have also been identified in this group,56 and these claims have led us to consider the importance of heterochrony more generally in dinosaurian evolution. Nopcsa’s notion that the dinosaurs from Transylvania were dwarfed could well be equivalent to seeing them as an assemblage of heterochronic Peter Pans. To this end, we have begun exploring both ontogenetic and phylogenetic aspects of both size and shape changes in the hadrosaurid Telmatosaurus, as well as in the titanosaurian sauropod Magyarosaurus and the primitive ornithopod Zalmoxes.
Optimization is our tool of choice when placing characters in their phylogenetic context. Once we have an explicit, well-supported cladogram for a particular group of organisms, we can then use this tree to determine the most parsimonious distribution of other aspects of this taxon, be it adaptation, coevolution, historical biogeography, biomechanics, or ecology. The use of phylogenetic trees as the basis for determining the most parsimonious sequences of evolutionary transformations for these additional characters—known as character optimization—does not help build trees, but instead is used for evaluating the behavior of other features on trees that are already constructed.57 This a posteriori method has been used to evaluate historical biogeographic relationships, to infer soft-tissue anatomy in extinct vertebrates, and to project the function and behavior of living organisms into the past.58
Let’s look at some simple examples of how a posteriori character optimization works. Figure 5.6a illustrates four taxa with a known relationship, all of which also share the same character (here we go with geographic distributions—they are all known from area A—but the characters to be optimized might be soft anatomy, biomechanics, physiology, or other features not included in the original cladistic analysis). Where is the ancestor of taxon 1 and taxon 2 (node 5) likely to have come from? Of course, it could be anywhere, but the most parsimonious interpretation is that this ancestor shared the same locale (area A) as its two descendants. When further relationships are considered (taxa 3, 4), since they all come from area A, then it’s obvious that the ancestors (nodes 6, 7) are also inferred to have come from area A. Once we’re at the base of the tree (character generalization), we then, in figure 5.6b, double check our ancestral characters back up the tree to resolve any ambiguities that may be left (character optimization). In this case, there is none—all of the nodes have A as their ancestral area. In other words, the evolution of this clade would be considered to be endemic to area A.
Yet what if there is a mixture in where the members of a clade come from? In figure 5.6c, half of the taxa (1–4), those to the right, are from area A, while those from the other half (5–8), on the left, are from area B. Generalizing down the tree, we know that the ancestors reconstructed at nodes 9, 10, and 11 would also have been found in area A, using the same logic we applied when confronting the situation in figure 5.6a. But what about the ancestral area at node 12—is it area A or B? Clearly something happened here, but we don’t yet know what it is. In truth, it could be either one, so we register it as “?A/?B” and then move down the tree yet another step to node 13. Here, we compare the area for taxon 6 and that for node 12. Area B is held in common with node 12, so we infer that the ancestral area at node 13 is B. Thereafter, down the tree, area B is inferred to the ancestral areas at nodes 14 and 15.
Figure 5.6. A posteriori character optimization (see text for explanation)
In order to resolve what’s going on at node 12, in figure 5.6d we optimize the characters back up the tree. The ancestral area at nodes 15, 14, and 13 is clearly B. We then compare node 13 with taxon 5 to resolve the ambiguity that we encountered during the generalization phase at node 12. Area B is held in common between node 13 and taxon 5, so we infer that the ancestral area at node 12 is B. With all remaining ancestral areas already identified as A, figure 5.6e shows that the shift from area B to area A occurred between nodes 12 and 11.
These examples have been simple and direct, but you can imagine how strenuous it would be to do this by-hand effort for clades with many members from disparate geographic regions. This problem is even more acute for sets of cladograms that are equally the most parsimonious ones. Fortunately, a posteriori optimization of characters on a tree is more easily accomplished by using any of the available numerical cladistic algorithms, such as PAUP, MacClade, and Winclada.59
Combing through the drawers of fossil bones and teeth, often in dusty, dark basement rooms in museums and universities, is very much like being in the field. You never know what you might discover next. Perhaps it will be some bone that indicates something new, something different, or something overlooked by the people who had studied it before.
Imagine yourself inside the Hungarian Geological Survey building, with its picturesque blue-and-white checked ceramic roof and rooms with vaulted ceilings, being turned loose among the collection of fossils by Dr. László Kordos, curator of these bones and teeth. Here, amid the pleasant smells of pipe smoke and hearty coffee, is where the considerable fossil remains from the Late Cretaceous of Transylvania are stored.
Our trek through these specimens yields the usual assortment: isolated vertebrae and limb bones, trays of teeth, and indeterminate scraps of bone. No surprises there—we’ve seen the likes of these before, and it’s easy to assign them to particular kinds of dinosaurs. Then, from one of the drawers, we find something totally unexpected. According to the label, these teeth are supposed to belong to Telmatosaurus, but they certainly don’t look like any sort of hadrosaurid teeth we’ve seen before. They’re relatively wide, somewhat like the teeth of Iguanodon, and they don’t display the typical strongly developed, vertical ridge of other hadrosaurids; instead, there are several low ridges on the enameled side of each tooth (figure 5.7, middle). Excitedly, we think we’ve discovered a new form of dinosaur, previously overlooked by Nopcsa and all who have come after him. At least that’s our first reaction. However, we must be sure whereof we speak, so it’s off to the literature. There, in black-and-white on plate VI, figure 3, in Nopcsa’s 1900 monograph on Telmatosaurus, are these same teeth: he had clearly recognized that they belonged to this hadrosaurid and, furthermore, that this lower dentition had a different morphology than do the teeth in the upper jaws.60 Disappointed and chastened, we are nevertheless enlightened and perplexed at the same time. What are these teeth of Telmatosaurus telling us?
Figure 5.7. The lower teeth of Iguanodon (left), Telmatosaurus (middle), and Maiasaura (right). Scale = 1 cm
In order to answer this question, we need to look at the teeth of a wide array of iguanodontian ornithopods, such as Iguanodon, Ouranosaurus, Camptosaurus, and Tenontosaurus, and try to understand how they changed during the process of tooth replacement. Morphologically, the squat, lozenge shape of the teeth of these forms is regarded as the primitive condition for the Iguanodontia. As these ornithopods grow during their lifetime, their “baby” teeth are rather small, but, as these teeth are replaced throughout life, they become proportionately much larger. Concomitantly, the number of teeth necessary to fill up the jaws doesn’t increase very much, compensating instead by an increase in tooth size during ontogeny. Take Iguanodon, for example (figure 5.7, left). As it grows, its teeth get much larger as they are replaced (a 300% increase in tooth size during ontogeny), but the number of tooth positions remains roughly the same (about a dozen tooth positions from juvenile to adult).
Compared with this more primitive system of replacement, hadrosau-rids do something entirely different. In these ornithopods, the dentary teeth increase in size only slightly during ontogeny. With adult teeth nearly the size of the “baby” teeth, it is the enormous increase in the number of tooth positions in the jaws that compensates for increasing jaw size as the animal gets larger during ontogeny. For example, Maiasaura hatchlings have a dentary dentition consisting of approximately 10 tooth positions filled by 6 mm wide teeth, whereas adults have jaws containing 40–45 tooth positions filled by 8 mm wide teeth (figure 5.7, right). Compared with the primitive ornithopod condition, hadrosaurids have added teeth like crazy, expanding not only the length of the dentition as the jaw grows, but also providing the possibility of having a greater number of closely packed replacement teeth at one time. In this way, the classic hadrosaurid dental battery is created.61
Figure 5.8. Telmatosaurus as a dwarf. The solid silhouette represents an iguanodontian ornithopod of ancestral body size; the open silhouette represents Telmatosaurus. Scale = 2 m
Fitting Telmatosaurus into this picture, our Haţeg hadrosaurid is little different morphologically from the somewhat more basal iguanodontians Ouranosaurus or Iguanodon, except in two respects—its smaller body size (figure 5.8) and the size and shape of its teeth (figure 5.9). The upper teeth of Telmatosaurus are narrow, diamond-shaped, and equipped with a single, centrally placed ridge; in other words, they are most like the juvenile condition seen in non-hadrosaurid iguanodontians. Its lower teeth, in contrast, are wider, asymmetrical, and bear several low ridges, making them intermediate between those of other hadrosaurids and more primitive iguanodontians. These teeth, too, were small, but they most resemble the shape of adults of non-hadrosaurid iguanodontians.
How this baby-toothed condition arose in hadrosaurids can be evaluated by optimizing body size and tooth development onto the cladogram of Iguanodontia (figure 5.10). Optimization is an a posteriori method for identifying the most parsimonious sequence of character changes by reference to an explicit phylogenetic tree. As we learned in chapter 2, the majority of hadrosaurids belong to two major subgroups, the hollow-crested lambeosaurines and the flat-headed, nasal-arched, solid-crested hadrosaurines, which together form the group called Euhadrosauria. Several hadrosaurids, Telmatosaurus prominent among them, are positioned below this clade of euhadrosaurians, but above non-hadrosaurid iguanodontians such as Ouranosaurus and Iguanodon.62 When body and tooth size are optimized on this cladogram, they fall out as a peramorphocline from the base of Iguanodontia to the base of Hadrosauridae. At this nexus, something happens to this ever-increasing relationship: body size trends reverse themselves, and the basal members of the hadrosaurid clade then undergo downsizing, not only in body size, but also in the size of their teeth. Thereafter, the evolutionary history of tooth size is decoupled from that of body size—as body size trends become peramorphic again, the teeth remain miniaturized, like those of juveniles.
Figure 5.9. The relationship between dentary tooth width and tooth row length for both non-hadrosaurid iguanodontians and hadrosaurids. Note that Telmatosaurus transylvanicus plots among juvenile hadrosaurids and not far from the juveniles of non-hadrosaurid iguanodontians.

Figure 5.10. A cladogram of higher iguanodontian ornithopods, indicating the dwarfing event leading to Telmatosaurus and Tethyshadros. The bar immediately below Hadrosauridae indicates the acquisition of miniaturized maxillary teeth.
In summary, we regard the small adult size seen in Telmatosaurus as the trigger for the evolution of the dental battery of duck-billed dinosaurs, the hallmark of Hadrosauridae. By arriving at adulthood having a smaller body size than its ancestors did, Telmatosaurus was assured that its teeth would resemble those in the younger ancestral stages. This juvenilization of their teeth amounts to arrested growth, otherwise known as paedomorphosis. Thereafter, euhadrosaurians increased in body size, but retained their juvenile dentitions into adulthood. This juvenile dentition, which was organized into a closely packed battery of miniaturized teeth with a complex occlusal surface, was passed on to their descendants.63 In this way, the Peter Pan syndrome provided the means by which the duckbills developed their complex dentitions.
Being the largest of all terrestrial organisms, sauropods assuredly packed a lot of weight onto their limbs. Standing still, an average adult Apatosaurus had to withstand 10 metric tonnes on its forelimbs and 24 metric tonnes on its hind limbs and, to amble across the Mesozoic countryside, these same limbs would have to have been built to withstand double or triple these loads.64
The stresses on the limbs of sauropods smaller than Apatosaurus obviously would have been less. This would also have been the case for juveniles and subadults of Apatosaurus and of youngsters of other species. Does that mean that the limb bones of adults should display different scaling properties when compared with those of a growth series? Because Magyarosaurus adults are the smallest among sauropods, we were interested to find out.
At a length of 5–6 m, and weighing in excess of 750 kg, Magyarosaurus is certainly among the smallest of all known sauropods (figure 5.11), as originally noted by Nopcsa in 1915.65 Although there are no complete or even partially articulated skeletons, this sauropod is known from abundant material representing nearly the entire skeleton, all collected from the Upper Cretaceous rocks of Transylvania.
In addition to gaining a reasonably good understanding of the anatomy of Magyarosaurus, we are also coming to learn more about its position in the evolutionary history of sauropods. Since their original discovery and the fulsome years of exploration in the American West, the excavations at Tendaguru (Tanzania), the finds in Sichuan (China), and the discoveries from Patagonia in South America, the phylogeny of sauropods was anything but clear. However, thanks to a number of recent phylogenetic analyses, their internal relationships are becoming much better understood.66 For our purposes, it is important to remember that Magyarosaurus is a titanosaur, a clade of sauropods that includes Rapetosaurus, Saltasaurus, and Malawisaurus, and it is more closely related to Brachiosaurus and its relatives than it is to other sauropods such as Diplodocus (chapter 2). Together, all of these sauropods play an important role in providing the evolutionary context for understanding the heterochronic significance of Magyarosaurus.
In the late 1990s, the two of us, along with Jason Mussell, then a graduate student at the Johns Hopkins University, decided to approach growth and development in these sauropods by examining the size and shape of the humerus and femur. We collected a variety of measurements from our specimens and analyzed the significance of these data, using both statistics and phylogeny. For our statistical analyses, we assembled one series consisting solely of adults and another that combined growth stages drawn from a mixture of 25 titanosaurs and brachiosaurs that are closely related to Magyarosaurus.67 By focusing on the long bones of these sauropods, we sought patterns of heterochrony using young and adult individuals, in this case in terms of the biomechanics of long-bone design. A limb bone must obviously be built to withstand, without fracturing, the great loads that pass down their length. A bone’s ability to resist this kind of load is proportional to the area of its cross-section, so for sauropods, long-bone proportions are particularly sensitive to changes in body size. Naturally, we wanted to see just what kind of humeral and femoral dimensions Magyarosaurus and other sauropods, young and old, have relative to each other, by examining them in a way that reflects their strength. This again is a exercise in scaling, so we set about determining the relationship between the length of these long bones and their midshaft widths, dividing the sauropod sample into individuals thought to be fully adult, and other individuals that could be assembled into a growth series from smallest to largest. Age classing of humeri and other bones is often difficult with ever-growing animals such as dinosaurs. However, as we have already seen in the case of Telmatosaurus and the other ornithopods we compared it with, it was possible to get an idea about the approximate maturity of an individual sauropod. The features we looked at were the degree of fusion of the bones of the braincase and vertebrae, the maturity of the joint surfaces of the limb bones, overall body size, and (sometimes) anecdotal comments in the literature. In nearly all cases, the largest individuals of a species were identified as adults.
Figure 5.11. Magyarosaurus as a dwarf. The solid silhouette represents a titanosaurian sauropod of ancestral body size; the open silhouette represents Magyarosaurus. Scale = 3 m
Figure 5.12. The relationship of humeral length and width (midshaft diameter) in titanosauroid sauropods. The interspecific regression of adults is indicated in the graph on the upper left. The regression of the composite growth series is indicated in the graph on the upper right. The combined adult and growth regressions—with Magyarosaurus superimposed on these lines—are indicated in the graph at the bottom.
What we report here comes from our analyses of the humerus, although roughly the same applies to the femur. When we plotted the data from the sample composed exclusively of adults, we got a reasonable linear relationship between humeral length and midshaft diameter (figure 5.12). Then, from our assembled growth series, we obtained another line with a similar slope to that of the adult sauropods, but which plotted slightly beneath the latter.68 This means that the humerus of a growing sauropod would be narrower than the same length of humerus for an adult. We then added data from Magyarosaurus specimens, and put a few other titanosaurs to the mix, to see how closely they resembled either the adult or the growth samples. Magyarosaurus plotted closest to the growth-series line, rather than to the adult line. Beyond mere inspection, the Magyarosaurus data are also statistically more similar to the growth series than to the adult sample.69 Therefore, we concluded that Magyarosaurus humeri appear to be more similar to those of subadults than to adults of other taxa.
Figure 5.13. A simplified cladogram of titanosauriform sauropods, showing two dwarfing events—one in Magyarosaurus and another in Rocasaurus—as indicated by the bars. (Modified after Curry Rogers and Forster 2001)
To judge whether these statistical insights had a phylogenetic context, we optimized body size at adulthood onto a cladogram of Magyarosaurus and its fellow titanosaurs.70 Figure 5.13 represents one such cladogram of these sauropods.71 Here we can see that small size evolved in the lineage leading to Magyarosaurus (as well as that leading to Rocasaurus). As did Telmatosaurus, Magyarosaurus joins Peter Pan’s merry band by retaining juvenile features into adulthood—that is, by paedomorphic dwarfing.
At approximately 3–5 m long, Zalmoxes does not strike one as an extremely small iguanodontian dinosaur (figure 5.14). Nonetheless, the more relevant questions are: Is Zalmoxes bigger or smaller than its closest known relative, Rhabdodon from the Late Cretaceous of France and Spain? How do these two ornithopods compare with their next closest relatives, the iguanodontian ornithopods? And thereafter?
Zalmoxes certainly doesn’t appear to be especially different in its proportions from most other basal iguanodontians. The skull isn’t particularly shorter than that of Tenontosaurus, and the details of its construction don’t cry out for heterochronic interpretations. However, the postcranial skeleton appears to be more robust and heavy, and we would have been remiss if we didn’t pursue all heterochronic connections related to changing body size and skeletal proportions. Consequently, we needed to look at how the likes of Zalmoxes, Rhabdodon, Tenontosaurus, Orodromeus, and Hypsilophodon grew.
We approached growth and development in these ornithopods much as we had with Magyarosaurus, although we chose to examine only the femur, since it was much better represented in our sample of bones than the humerus.72 Measuring the length and midshaft width of these femora, we then analyzed the significance of this set of data, again using both statistics and phylogeny. However, unlike our analyses of Magyarosaurus, where we had to cobble together a single, generalized growth series for titanosaurs, virtually all of the ornithopods that we wanted to compare with Zalmoxes are known from adults, subadults, and even juveniles, so we had direct information on the growth patterns of each of these taxa. The other significant difference from our Magyarosaurus analyses was that we had a much better understanding of the phylogenetic history of ornithopods than we did for titanosaurs and other sauropods (chapter 2). This, in fact, provided us with a detailed, well-tested template for the comparisons we wanted to make.
Figure 5.14. Zalmoxes as a dwarf. The solid silhouette represents a basal iguanodontian ornithopod of ancestral body size; the open silhouette represents Zalmoxes robustus. Scale = 3 m
We began our work by rounding up as large a sample as possible of skeletal elements for both species of Zalmoxes. We selected the femur (as noted above), which, in the case of Z. robustus, turned out to represent samples from seven individuals with femora distributed from about 20 to 40 cm in length, and, for Z. shqiperorum, three individuals with femora ranging from 28 to 45 cm. Dimensions measured from several femoral landmarks were then used to produce growth curves for the Zalmoxes sample. The same data were collected for a selection of ornithopods that are thought to be closely related to Zalmoxes: Rhabdodon, Tenontosaurus, Orodromeus, and Hypsilophodon. These growth curves were then compared pairwise with each other.73
The overall pattern of growth for Zalmoxes—treated here in terms of both species (Z. robustus and Z. shqiperorum)—and for closely related ornithopods is quite similar (figure 5.15). Indeed, when we compared growth curves between any two of these ornithopods (say, between Orodromeus and Tenontosaurus), they were statistically indistinguishable from each other. The same applies to comparisons involving the two species of Zalmoxes, as well as comparisons with Rhabdodon—their growth curves are virtually identical. This overarching trend among these ornithopods indicates that the femur changed its proportions in the same fashion, regardless of whether it belonged to Hypsilophodon, Tenontosaurus, or Zalmoxes. The largest individuals, then, constitute the only difference among the samples. For the biggest Tenontosaurus or Rhabdodon, the femur grew to a length of nearly 60 cm. Right behind that comes Zalmoxes shqiperorum, with a femur length of just over 45 cm. Z. robustus, with a femur length of 37 cm, is smaller still, whereas Orodromeus and Hypsilophodon have the shortest femora (both between 15 and 20 cm long).
Figure 5.15. The relationship between femoral length and midshaft diameter in basal ornithopod dinosaurs. Included are growth series obtained from Orodromeus makelai, Hypsilophodon foxii, Tenontosaurus tilletti, Rhabdodon priscus, Zalmoxes shqiperorum, and Z. robustus. The line represents the regression of femoral length and midshaft diameter for the combined sample of all species.
As far as this simple scaling analysis goes, both species of Zalmoxes appear to grow their femora just like other ornithopods. One of them (Z. shqiperorum) does so to the degree exhibited by the existing sample of Tenontosaurus, but the other (Z. robustus) is known from femora that are greater in length than those of Orodromeus and Hypsilophodon, but smaller than those of Rhabdodon, Tenontosaurus, and Z. shqiperorum. Is this pattern one of successive peramorphosis in the clade leading to Tenontosaurus and the larger species of Zalmoxes, or of paedomorphosis leading to Orodromeus and Hypsilophodon? Or is it a mixture of both? Without full-grown adults from this taxon (see the discussion above on bone histology), we really can’t tell when Zalmoxes stopped growing. This ornithopod slowed down its growth rate and had an extended growth period, but it had not yet achieved its largest size. To see what this eventuality entailed in a phylogenetic context, we again optimized these size data onto the cladogram for basal euornithopods (figure 5.16). We can see that the more primitive trend within this part of the clade consists of a peramorphocline from basal euornithopods such as Orodromeus through more highly positioned taxa like Hypsilophodon, Tenontosaurus, Rhabdodon, and Zalmoxes shqiperorum. However, downsizing appears to occur in Zalmoxes robustus within the clade represented by the two last-mentioned taxa, all other thing being equal. If this is true, then we may have another Peter Pan paedomorph in Z. robustus.
Figure 5.16. A cladogram of basal ornithopods, showing the possible dwarfing event leading to Zalmoxes robustus, as indicated by the bar
Positive evidence exists for dwarfism in Telmatosaurus, Magyarosaurus, and perhaps Zalmoxes robustus. What of the other members of the Transylvanian fauna? The ankylosaur Struthiosaurus and the unnamed pterosaur are quite a bit smaller than expected, compared with their phylogenetic compatriots, but we’re not yet certain whether they are represented by mature adult material or by subadult specimens. In contrast, the dromaeosaurid and troodontid theropods, although small, are no smaller than their close relatives in Asia and North America. Only Hatzegopteryx, poorly known though it may be, is large, but with an estimated wingspan of 12 m, it may turn out to be among the largest of all the azhdarchid clade.74 Turning to the Transylvanian crocodilians, turtles, and mammals, they are neither more diminutive nor more sizeable than what would be expected, based on their evolutionary cohorts. Thus this triumvirate of turtles, mammals, and crocodilians don’t seem to be playing the heterochrony game.
In the end, then, we have within the Transylvanian fauna a handful of cases of dwarfing through paedomorphosis, a roster of normal-sized animals, and an example of gigantism by way of peramorphosis. Maybe you’ve been expecting universal dwarfing among the animals found in Transylvania, but why should all of these forms have gotten small? We’re more than happy that paedomorphic downsizing can be identified in as many Transylvanian groups as it has—for here we have an important evolutionary signal. Nopcsa knew that such body sizes, compared with those elsewhere in the world, flew in the face of the basic tenets of dinosaur evolution, where large size reigns supreme, so he sought a causal basis for all of the changes in body size among dinosaurs.
As Nopcsa saw it, the evolution of both large and small dinosaurs came about through some sort of neo-Lamarckian mechanism. For him, this inheritance of acquired characteristics was mediated by diseases or other physiological disturbances.75 For example, he looked to such endocrine diseases as acromegaly, in which hyperfunction of the pituitary gland leads to gigantism and a thickening of the bones in humans, to account for an increase in body size. His evidence for acromegaly was the enlarged pituitary fossa found in dinosaur braincases.
Other explanations of dinosaurian gigantism, even up to the 1950s, took a decidedly non-Darwinian perspective. Otto H. Schindewolf, one of the twentieth century’s great macroevolutionary paleontologists, devoted much of his long career to defending his theory of typostrophy. Schindewolf sought to understand evolutionary patterns as being internally regulated: groups of organisms, like individuals, go through phases similar to birth, childhood, adulthood, old age, and death as they evolve, and their extinction therefore is inevitable, predicated on this “evolutionary lifecycle.”76 In dealing with dinosaur extinction, he argued that these animals were driven to a sort of “racial senescence” that accompanied their growth to an exceedingly large size, as were other organisms, such as ammonites. How else to account for the “inevitable” poor design of dinosaurs? “It’s quite likely that these gigantic forms were no longer able to carry their enormous body weight around on land. They lived like amphibians, submerged in swamps and lagoons where water lightened the load and made locomotion easier.”77
The then-popular notion of dinosaurs as evolutionary failures because of their extinction at the end of the Cretaceous masked, for Schindewolf, just how these animals attained their gigantic size. He was forced to search for an ecological context, in which these animals were in their senescent phase. We now know that dinosaurs were not particularly relegated to the swamps, nor do taxa have well-defined “life stages,” but Schindewolf was right to seek evolutionary and ecological explanations for the changes in body size in dinosaurs. For example, their large size has been related to their physiology, in particular to the ways in which these animals regulate temperature: either through mass homeothermy (stable internal temperatures due to their large body’s low surface area / volume ratio) and endothermy (warm-bloodedness based on metabolic heat generation).78
Size increases through phylogeny not only accompany dinosaur evolution, but are also notoriously common elsewhere in the fossil record, so much so that they have been generalized under the rubric of Cope’s Rule, named after the prominent American neo-Lamarckian evolutionary biologist and vertebrate paleontologist Edward Drinker Cope (1833–1897).79 Ironically, its counterpart, evolutionary dwarfism, has received much less attention and is mostly centered on insular dwarfing, where changes in body size—downsizing in particular—are very common.80
Nopcsa viewed dwarfing as a consequence of body-size effects through island habitation; in other words, Transylvanian dinosaurs evolved to a smaller size as they became geographically isolated on an island in the Neotethyan Sea. There is ample indication that Transylvania, at the end of the Cretaceous, was a remote terrestrial region, a relatively small, isolated landmass revealed by the receding sea. This insular environment surely must have been large enough to accommodate viable populations of a variety of dinosaurs and other vertebrates, but apparently it was not so large as to support sauropods and ornithopods, at least in their normal, quite large body sizes.
We hope by now that it is obvious that the Transylvanian fauna includes a number of dinosaurs of small adult body size. The region in which these diminutive forms lived was an insular habitat, which leads us (in the same way as Nopcsa) to look for an explanation for our dwarfed Transylvanian dinosaurs by examining the relationship between body size and isolation.
The pattern of island dwarfing among mammals has been termed the Island Rule by Chicago evolutionary biologist Leigh Van Valen, who noted that it “seems to have fewer exceptions than any other ecotypic rule in animals.”81 The epitome of the Island Rule must assuredly be the dwarfing of insular elephants commonly present in the Pleistocene. These diminutive proboscideans are interesting—and relevant—for two reasons. First, among animals with living relatives, they most closely approximate dinosaurs in terms of body mass. However, even more importantly, their pattern of dwarfing is well enough understood, thanks to V. Louise Roth, for us to explore our own patterns of dwarfing in Transylvania. Roth, an evolutionary biologist at Duke University, conducts research on both large and small elephants, particularly in terms of their anatomy, feeding habits, growth, ecology, reproduction, fossil record, and evolution. Armed with this information, she developed a multi-faceted proboscidean model that relates interspecific competition, predation, and subsequent population densities, in order to explore what is causally behind the Island Rule.82
Roth’s model begins by taking up the events of island colonization. Getting to these islands is clearly a function of their remoteness from the adjacent mainland, and surviving such a migration depends on a combination of good luck and preparedness. In the case of nonhuman migrants, preparedness is a matter of body size. As it turns out, large individuals are more likely to be successful in surviving overseas dispersal and colonization than small individuals; hence, the founding population on an island may be of relatively large body size.83 This larger initial body size may be counterintuitive, but it provides a means for surmounting the problems of breaking through the barrier of isolation.
At this point, the largish elephants of Roth’s model have survived dispersal and are now island residents. Consider, though, that this new home base for the elephants must be much more confining than their previous range on the mainland. With this more restricted land area comes an ever-greater exploitation—and the eventual deterioration—of existing food supplies as the isolated populations reach high densities and the elephants suffer from increased overcrowding. Smaller body size is favored under these conditions and, according to Roth’s model, is most likely to arise when individuals reach reproductive maturity at earlier—and therefore smaller—stages. This connection between reproductive maturity and body size among dwarfed insular elephants again focuses our attention on the role of heterochrony and, in particular, on paedomorphosis. This shift of sexual maturity to younger and therefore smaller individuals is the most important connection with individual fitness.
So far, so good—the dwarfed elephant model provides some similarities with the Transylvanian dinosaurs. The paleogeography of the Tethyan realm during the Cretaceous indicates that terrestrial habitats were relatively well isolated from each other and often limited in area. Thus conditions in Transylvania are consistent with the geographic and ecological underpinnings of the elephant model.
We likewise argue that small size in Telmatosaurus, Magyarosaurus, and perhaps other Transylvanian forms appears to be favored under conditions of decreasing food supply, due to increased population densities in restricted habitats, just as it appears to be the case in the dwarfed, island-dwelling elephants. In this way, the dwarfing of Transylvanian dinosaurs would have arisen through paedomorphosis: individuals reaching reproductive maturity at earlier, smaller stages.
From a faunistic perspective, the dinosaurian herbivores are dwarfed relative to their close relatives elsewhere in the world, whereas the few predators known from Transylvania are not much different in body size from their closest relatives. In terms of the elephant model, these observations are not surprising. The lack of large individuals among the herbivores strongly suggests that immigration to this restricted area must have taken place considerably earlier than the latest Cretaceous. (The time interval for isolation and colonization of the Transylvanian region will be discussed further in chapters 6 and 7.)
As for body size among the Transylvanian theropods, as we have indicated, there were no behemoth, Tyrannosaurus-like predators living in this region in the Late Cretaceous. This, too, is not a surprise. The isolated habitats of the kind we envision for Transylvania were probably capable of supporting a lesser quantity and diversity of predators, mainly ones that had smaller home ranges; and, of these, those with smaller bodies fit the more restrictive Transylvanian ecological conditions better than large-bodied predators.
The downward trend in body size among large herbivores, which Roth’s model links with limited island resources, may also be driven by differences in predation between the mainland and an island. We know that large mammalian predators, though present on the mainland, were absent from islands inhabited by dwarfed elephants during the Pleistocene, and the same appears to have been true for most of the dinosaurian predators of the ancient Transylvanian region.84 Lower levels of predation on islands may act as a permissive factor, allowing large herbivores to evolve into smaller, perhaps more optimal sizes, since large size would not be selected as a deterrent to predators. In addition, this rationale for insular dwarfism may also help explain the evolution of gigantism among many small mammals and other animals on islands. Take, for example, the case of the modern giant rats on the Lesser Sunda Islands in the South Pacific.85 Such rat gigantism—a nearly half meter long rodent, without the tail that gives new meaning to The Princess Bride’s “rodent of unusual size”86—may be viewed as evolving closer to its optimal body size in the absence of the usual roster of predators from the mainland. Similar to the counter-case of island downsizing among large animals, very small size is no longer necessary as a defense against predators, producing, as a consequence, an increase from a smaller to a larger body size.87 This embellishment to the Island Rule provides an argument for there being an optimal intermediate body size that organisms would be free to evolve toward when predator pressures are significantly altered, as they would be on an island.88
Whether dwarfism in Magyarosaurus, Telmatosaurus, and maybe Zalmoxes was spurred by limited resources or by an alteration and perhaps a reduction in predation, these small dinosaurs beg for a hetero-chronic and ecological explanation. If Roth’s elephant model applies to the Transylvanian dwarfs, and we believe that it does, the adaptive significance of dwarfing is its immediate relationship with life-history strategies such as accelerated sexual maturation (i.e., paedomorphic heterochrony), rather than it being a development based on morphological advantages alone. As Gould put it, the “timing of reproduction is an adaptation in itself, not merely the consequence of evolving structure and function.”89 The morphological consequences of paedomorphosis—juvenile morphology—come along initially as biological baggage: a consequence of downsizing due to isolation. Thus, for example, it is not the retention of a juvenile dentition and the formation of a dental battery in Telmatosaurus that is the primary focus of selection. Instead, the overarching selection is for a small body—that is, paedomorphic dwarfism, based on the ecological particulars of the isolated region of Transylvania.