
At the time of Franz Nopcsa’s death in 1933, the Haţeg fauna was thought to include five dinosaurs, a bird, a crocodile, a turtle, and a pterosaur. Unfortunately, work in Transylvania went fallow after the First World War, when the defeated Austro-Hungarian Empire ceded Transylvania to Romania. It was not until the mid-1970s that the collection of vertebrate fossils resumed in the Haţeg Basin. In 1978, two teams came together to follow in Nopcsa’s footsteps. One was supervised by Dan Grigorescu from Universitatea din Bucureşti, and the other was originally organized by Ioan Groza, later supervised by one of us (Jianu) and then by us both, under the auspices of Muzeul Civilizaţiei Dacice şi Romane Deva.1 These two groups, plus a more recent joint expedition from Universitatea Babeş-Bolyai Cluj Napoca, Romania, and the Institut Royal des Sciences Naturelles de Belgique in Brussels (under the supervision of Vlad Codrea and Thierry Smith, respectively2) have ventured to the outcrops at Vălioara, Densuş, and the Sibişel Valley, as well as several new locations, this time in the Transylvania Depression—Jibou (Sălaj County) in the north and Oarda de Jos, Vurpăr, Bărăbanţ, Lancrăm, Sebeş, and Vinţu de Jos in Alba County, along the southeastern margin of the Apuseni Mountains north and east of the Haţeg Basin.3 These efforts have amassed several thousand new fossil specimens and added considerably to the diversity of the known assemblages. Several different kinds of dinosaurs are represented for the first time, as well as new bony fish, amphibians, mammals, lizards, and crocodilians. Along with this richer picture of the fauna has come a better understanding of the paleoecological context of the Haţeg Basin and other localities, as well as the evolutionary significance of this part of the world during the Late Cretaceous.4
The best-known members of the Haţeg and similar assemblages clearly are the dinosaurs. Nopcsa knew or named nearly all of them, including Telmatosaurus transylvanicus and Zalmoxes robustus among ornitho-pods, the armored Struthiosaurus transylvanicus, the sauropod Magyarosaurus dacus, and a theropod he referred to Megalosaurus, a poorly known form first identified from the Middle Jurassic of England.5 Given the Late Cretaceous age of these deposits, it is likely that many of these dinosaurs were among the last of their dynasties.
In this chapter, we hope to accomplish two things. First, we want to put members of the Transylvanian menagerie into their evolutionary or phylogenetic context. Second, we want to breathe life into the fragments of ancient bones from Transylvania and, thereby, get a meaningful picture of these beasts that once roamed the Transylvanian region. We begin by outlining a field of study called phylogenetic systematics, otherwise known as cladistics. Cladistics is used to establish who is more closely related to whom among a group of organisms. We also use it to understand the relationships of the dinosaurs and their Transylvanian cohorts along the way.
Fossils are the petrified remains of prehistoric life, something that has been recognized in the scientific community for three centuries, ever since the Danish scientist Nicholas Steno (1638–1686) first interpreted fossils as the vestiges of once-living creatures. Darwin understood that the links among these organisms constituted evolution, and he postulated a mechanism for the latter that depended not on divine design, but on the day-to-day action of environment on variable individuals within a population. He also understood that evolution, by its continual production of generations of descendants from earlier descendants, from still earlier descendants, and so on, back to primordial ancestry—in other words, diversification—was therefore hierarchical. It’s not for nothing that his canon—“descent with modification”—emphasized the hierarchical property of evolution. Others had already recognized this hierarchy in nature, most notably Carolus Linnaeus, the great eighteenth-century classifier of all organisms. His cataloging revealed that God’s creative hand had hierarchical tendencies, and all organismal taxonomies have had this structure thereafter. Darwinians and everyone since then have taken on Linnaeus’s practice of, if not his motivation for, identifying hierarchies in nature, because these nested sets of diversity conform to a single phylogeny, a single genealogy into deep time that documents the interrelatedness or connectedness of all life.
How, then, can we identify phylogeny’s hierarchy? We don’t have a written record, as we do for our personal family genealogies, but we do have the similarity of features possessed by organisms that underlie their evolutionary history. From Darwin’s time to the present, we have given special evolutionary significance to a particular class of similar features, called homologies, whose presence gives nature its hierarchical property. For a feature to be a homology, it must have evolved only once. How we determine whether a feature has a unique origin is a matter of comparison. Take, for example, the presence of retractile claws in mammals. Such claws are very anatomically and biomechanically similar to each other in all the mammals that possess them—cats, lions, tigers, and others. Here we’ve passed the first test of homology, the test of similarity. The second test is the congruence of a feature with phylogeny (i.e., with evolutionary history): can we tell if retractile claws evolved just once, instead of several times, in the groups of animals that have them? In our example, the answer is yes; all cats have retractile claws and, because we regard all of these animals as very closely related, we hypothesize that retractile claws evolved once in the group of mammals called Felidae.6 Said another way, this homology evolved once in the common ancestor of Felidae and then was passed on to its descendants. By the same token, if someone tells us that a given mammal is a felid, we would expect it to have had retractile claws, although they may have been subsequently lost. By their very nature, homologies evolve only once, and they therefore speak about the closeness of the relationship between two kinds of organisms.
By properly identifying a group of organisms as having a single common ancestor (a single origin) on the basis of its characters, we have begun determining whether that group is monophyletic. The other important aspect of monophyly is that the group contains all the descendants of this common ancestor. Seen in this way, Homo sapiens is monophyletic, felids are monophyletic, birds (Aves) are monophyletic, and Dinosauria is monophyletic. But Dinosauria without birds is not monophyletic—it leaves off some of the descendants of the common ancestor of all dinosaurs. This latter kind of grouping, known as paraphyly, is similar to leaving off Uncle Bob and his family from your family tree—you may want to, but the end result wouldn’t be a true reflection of your family history.

Figure 2.1. The founder of phylogenetic systematics (also known as cladistics), Willi Hennig (1913–1976)
The interdependence of homology and hierarchy forms the basis of what is known as cladistic analysis, a tool that is particularly well suited to reconstructing phylogeny. Cladistic methods, first developed by the German entomologist and founding father of phylogenetic systematics, Willi Hennig (figure 2.1), seek to establish the hierarchical nature of evolution by searching for the nested arrangement of organisms and the features they possess.7 This pattern is then portrayed on a branching diagram called a cladogram, with each collection of branches being referred to as a clade. The characters used to justify the branching pattern in a cladogram may have broad or limited distributions, such that some characters will diagnose more general relationships, while others will diagnose more restricted ones (figure 2.2). For example, Aves (modern birds) and Crocodylia are both diagnosed as archosaurs because of their brain-case and palate, general features that evolved once in the common ancestor of these two groups, although several aspects of these features may have later altered within the groups. More specific affinities, for example Homo sapiens and Australopithecus afarensis within Hominidae, can be assessed by identifying homologies—both possess features of the pelvis, femur, and knee that relate to their unique evolution of bipedality. In other words, a character may be specific to one group (i.e., bipedality in Hominidae), but general in a smaller subset of that group, because it is now being applied at a different position in the hierarchy.
Figure 2.2. Cladogram of Archosauria (left) and Hominidae (right)
If things were as simple as this, the task of determining phylogeny would be a snap. We would seek the nested relationships of similarities in molecules, morphology, and behavior, then interpret them as homologies, and the hierarchical history of life would be revealed. All similarities would be homologies, from which we could then reconstruct phylogeny.8 However, nature, like history, is messy—she invents similarities that are not and cannot be homologies. Take, for example, the presence of eyes in both dolphins and squid. They are not considered as having evolved just once in the common ancestor of Vertebrata and Cephalopoda; given the other features and taxa that must be considered in this comparison, eyes must have evolved at least twice to account for their distribution. Said another way, these nonhomologous similarities, called homoplasies, are not congruent with a single origin during phylogeny. Homoplasies are those features that get in the way of discovering phylogenetic patterns, because they are produced by two or more events in evolutionary history.
Figure 2.3. A hypothetical example of how to determine the most parsimonious cladogram (see text for explanation)
Let’s take a final look at how characters can be identified as homologies and homoplasies, using a hypothetical example. We have six taxa (A–F) as well as two phylogenies of these taxa that we want to test, to see which one is best supported by the features we’ve hypothesized as being homologies. In figure 2.3, the cladogram on the upper left consists of two monophyletic groups (A–C and D–F), the first supported by three homologies (characters 4–6) and the second by two (characters 7–8). Within each of these groups there is another monophyletic clade, each supported by its own homologies (characters 1–3 for the first group and character 9 for the second).
The cladogram in the upper right implies dramatically different relationships from that in the upper left. All taxa, from A to E, are sequentially more closely related to taxon F. These relationships are supported at each level by single homologies (characters 10, 11, 12, and 13), each of which is different from those that produced the cladogram to its left. We can tell which tree is likely to be correct, given the data we have, by finding out which tree is shorter. In doing so, we must use not only the data that support a given cladogram, but also all the others that don’t support it. That is, we must also account for all the characters on each tree (i.e., by applying characters 10–13 to the left tree, and characters 1–9 to the right tree). We’ve done that for the cladograms in the lower row.
Because all characters must appear on the cladogram, conflict among features is bound to occur. These conflicts in cladograms and their character support are settled by letting the possible homologies fight it out among themselves. This rather bloodless battle is carried out in the arena of parsimony, otherwise known as Ockham’s Razor. Parsimony, originally articulated by the fourteenth-century Franciscan monk William of Ockham (in England), ensures that, in the case of cladistic analyses, the simplest or shortest cladogram is chosen. In following this principle, we therefore have to select the tree supported by the greatest number of homologies from those that we originally hypothesized. This is a requirement, since homologies, the sine qua non of close relationships, are represented only once on a cladogram, but homoplasies, by their very nature, are found multiple times on the cladogram. In other words, some of our original hypothesized homologies are forced to become homoplasies by the weight of evidence coming from the other characters. Depending on the situation, some characters will either evolve twice or evolve and then reverse back to their more primitive condition (shown with an asterisk). Once the distribution of all the characters is determined, their positions on each cladogram are counted up. The shortest tree, which we call the most parsimonious one because it maximizes the number of homologies, is the one on the left, with a length of 15 steps. It is four steps shorter than the one on the right, which takes 19 steps to account for all the evolutionary changes in the six taxa.
To understand the biology of extinct creatures (such as the denizens of ancient Transylvania) as best we can, it behooves us to do what every paleontologist must—fill in the missing bits of skeleton and teeth in a reasonable way to better understand the no-longer-living creatures we’re studying. We owe the means by which we meet this challenge to the founding father of dinosaur paleontology, Sir Richard Owen (figure 2.4), in his role as a comparative anatomist.9 Owen’s greatest achievement in this arena has become one of the most cited and mythologized of all episodes in paleontological history. In 1839, Sir Richard famously deduced the existence of an enormous flightless bird that once inhabited New Zealand, reconstructing the whole animal from a single fragment of a femur. The test of his construction—and the basis for his eminence as a comparative anatomist—came with the discovery, four years later, of well-preserved skeletal remains of the extinct moa, Dinornis maximus, a 3.5 m tall, ground-dwelling, flightless bird.10 Based not on arcane knowledge, witchcraft, manipulative card tricks, chicanery, or wild speculation, Owen’s achievement instead was due to years of studying the structure of a wide array of living and extinct animals, or what we now call comparative anatomy.

Figure 2.4. Richard Owen (1804–1892)
Like Owen, but also with insights that come from cladistics, we will use comparative anatomy to develop a better understanding of the Transylvanian dinosaurs, most of which are known from tantalizing and abundant, but fragmentary, material. As comparative anatomists, we will look for similarity in the sizes, shapes, and features of bones; the form and density of serrations on teeth; and anything and everything else that we can to better understand the creatures themselves. Of course, we can only infer so much from these features, but at least they’re something tangible with which to begin our assessment of the Transylvanian dinosaurs and their evolutionary legacy.
One last thing before tackling the Late Cretaceous inhabitants of Romania: what exactly are dinosaurs? The term Dinosauria, meaning “fearsomely great reptiles,” was coined a century and a half ago by Richard Owen for the agglomeration of what was then the poorly known, gigantic, prehistoric reptiles from the Mesozoic Era.11 Thanks to Harry Govier Seeley (figure 2.5), toward the end of the nineteenth century we have recognized that all dinosaurs are either saurischians (lizard-hipped) or ornithischians (bird-hipped).12 Said another way, Dinosauria (figure 2.6) is a monophyletic group, composed of Saurischia and Ornithischia and their common ancestor. We have known about each of these groups for as long as we have known about dinosaurs; the saurischian Megalosaurus and the ornithischian Iguanodon were discovered in England about 1820. Saurischia includes both the gigantic sauropods and the carnivorous theropods, familiar to museum-and movie-goers everywhere as the long-necked Apatosaurus and the fearsomely toothed Tyrannosaurus rex, respectively. The other major dinosaur group, Ornithischia, comprises a vast array of plant eaters that includes duck-billed, armored, and horned dinosaurs (such as Triceratops).
Figure 2.5. Harry Govier Seeley (1839–1909)
In all saurischian dinosaurs, the pubic bone slants down and forward. This is hardly a unique feature; so do the hips of many other extinct and living animals, including Homo sapiens. In fact, lizard hips are so common among land-living vertebrates that paleontologists must look to other features uniquely shared by these dinosaurs in order to recognize them as real, or monophyletic, evolutionary groups. Fortunately, several other characters are commonly used to unite this group (figure 2.7), including a hand with a large thumb and elongate second digit, and elongation of the neck, among other features.13 The various subgroupings of saurischian dinosaurs, the most important of which (for our narrative) are sauropods and theropods, coalesce on the basis of many additional features.
Ornithischians (figure 2.8), a diverse group of plant eaters, are known as the bird-hipped dinosaurs because their pubic bone is rotated backward, much like a bird’s, although the two conditions do not have the same origin and therefore are not homologous. One explanation advanced for this characteristic is that it evolved to provide more abdominal room for the fermentation of the plants they ate. Ornithischians share a number of other unique features, including a special bone (called the predentary) covering the tip of the lower jaws that gave extra strength to this region during biting.14 In addition, the back teeth were low, triangular, and well designed for chewing. Because these teeth were positioned away from the outer margins of the jaws, a space between them and the side of the face may have been enclosed by muscular cheeks to prevent food from falling out of the mouth while chewing. Finally, bundles of elongate ossified tendons formed a network along most of the vertebral column, providing additional support for the body, which was balanced at the hip joint during a bipedal stance. Ornithischian dinosaurs come in a variety of shapes and sizes, but most can be grouped as stegosaurs, ankylosaurs, pachycephalosaurs, ceratopsians, and ornitho-pods. Of these, only ankylosaurs and ornithopods have thus far been recovered from Transylvanian deposits.
Figure 2.6. The skeleton of Herrerasaurus, indicating several of the unique features of Dinosauria: an elongate deltopectoral crest on the humerus; a brevis shelf on the postacetabular part of the ilium; an extensively perforated acetabulum; the tibia with a transversely expanded, subrectangular distal end; and the ascending astragalar process on the front surface of the tibia
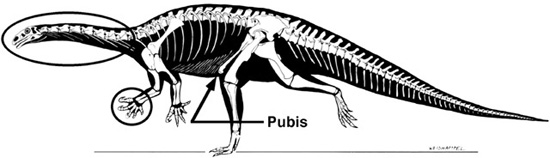
Figure 2.7. The skeleton of the prosauropod dinosaur Plateosaurus, indicating the lizard-hipped pelvis of saurischian dinosaurs, as well as several unique features that unite Saurischia: elongation of the neck, and a hand with a large thumb and an elongate second digit
Figure 2.8. The skeleton of the ornithopod dinosaur Camptosaurus, indicating several of the unique features that unite Ornithischia: the predentary covering on the tip of the lower jaw, the bird-hipped condition in which the pubic bone is rotated backward, and the presence of ossified tendons along the backbone
Sauropods of the Late Cretaceous of Europe are best known from the present-day French Pyrenees and northern Spain, particularly through the recent excavations led by Jean Le Loeuff, Eric Buffetaut, and other researchers at the Musée des Dinosaures in Espéraza, France,15 and field research at Laño in Basque County, conducted by Xabier Pereda-Suberbiola, José L. Sanz, and other French, Spanish, and Basque scientists. Less well known are the sauropods from the fossil beds farther to the east, thus far recognized only from the Haţeg Basin and the Transylvanian Depression in Romania. Nopcsa first reported finding sauropod bones here in 1902.16 Consisting almost entirely of isolated postcranial skeletal elements (mostly vertebrae and a few limb bones) of relatively small but presumably adult individuals (figure 2.9), Nopcsa referred these to Titanosaurus, a form otherwise known at that time only from India, Argentina, Madagascar, France, and England.17 Huene restudied Nopcsa’s sauropod material, renaming it Magyarosaurus and recognizing three species: M. dacus, M. transsylvanicus, and M. hungaricus.18 Recent research by Zoltán Csiki and others recognizes two species from Transylvania, the smaller and more numerous M. dacus and a larger, rarer titanosaur, Paludititan nalatzensis.19
Figure 2.9. Magyarosaurus dacus: reconstructions of the head (above), the left humerus (left below), the radius and ulna (middle below), and the femur (right below). Scale = 10 cm
From the outset, all of the sauropod material from Transylvania was considered titanosaurian in nature (box 2.1). The first-named of these titanosaurs, Titanosaurus indicus (described in 1877, although the taxon Titanosaurus is now a nomen dubium, or “doubtful name”),20 provided only a partial portrait of these giants, but later discoveries have helped refine our understanding of them.21 We now know that titanosaurs had arisen by the Late Jurassic and remained common in parts of the landmasses of the Southern Hemisphere (known as the supercontinent Gondwana) up through the end of the Cretaceous. Titanosaurs have been found in the southwestern United States, Europe, and Asia,22 but the best-known titanosaurs come from the Upper Cretaceous of Argentina and Madagascar (Saltasaurus loricatus and Rapetosaurus krausei, respectively).23
As is generally true of nearly all sauropods, the fossil record of titanosaurs consists mainly of disarticulated, dissociated, and often isolated material. Only a few relatively complete skeletons or skulls of any titanosaur are known.24 Despite this imperfect knowledge of the skeletal record, we can reasonably state that titanosaurs ranged from 7 to 30 m in length. However, M. dacus was probably no more than 5 to 6 m long as an adult,25 whereas the larger Paludititan nalaczensis from Haţeg may have been 12 to 14 m long.26 Similar to their other sauropod kin, titanosaurs must have looked like a cross between today’s elephant and giraffe, with a small head atop a long neck and a tail extended behind a rotund body supported on four sturdy legs (Plate III, bottom). Titanosaurs were unique among sauropods in one regard: their backs were covered in a pavement of bony armor.27
The skull of either of the Transylvanian titanosaurs, which would provide data critical to understanding their relationships and paleobiology, is all but unknown. We have no teeth, no jaws, and no facial skeleton from them. One small sauropod braincase was collected by the University of Bucharest field party from the Pui fossil locality (figure 2.10). It’s a relatively tall, boxy-looking affair, with plenty of openings for the cranial nerves used in the senses of smell, sight, hearing, balance, taste, and other sensory and motor functions. Through a large central hole in the back of the braincase (foramen magnum), the great rope of neurons called the spinal cord traveled the length of the animal to the tip of the tail, sending nerve impulses, for example, from the brain to the muscles and organs of the body and bringing back sensations from the skin to the brain about how warm or cold the weather was. The braincase is short and extremely deep. The bones are not strongly fused together, indicating that this specimen probably came from an immature individual. Perhaps the most interesting aspect of this braincase is the two short, oval swellings on the skull roof, which look vaguely like the bases of the antlers of deer. In addition, the specimen indicates that the openings of the nasal cavity were positioned high on the face, probably in front of the eyes, much like the arrangement seen in a number of other sauropod groups. Even though these openings are high on the skull, it is now thought that the nostrils themselves were to be found toward the front of the head.28
Figure 2.10. The braincase (right) of a juvenile titanosaur (possibly Magyarosaurus) from Pui, with a silhouette (left) indicating its position in the skull. Scale = 10 cm
To piece together other aspects of the biology of the Transylvanian titanosaurs, we must infer such characters from their close relatives.29 The long, chisel-shaped teeth of these sauropod relatives were restricted to the front portion of the jaws, with chewing limited to an up-and-down or perhaps a front-and-rearward movement of the jaws. Tooth morphology, and especially wear, indicate that these gigantic herbivores either nipped or stripped foliage, and it is doubtful that many of these animals fed selectively, given the construction of the jaws, the nature of the dentition, and body size. Instead, titanosaurs may have ground their food using gastroliths in their muscular gizzards. Whether Magyarosaurus and other titanosaurs browsed at high levels within the canopy or foraged a few meters above the ground is a matter of debate.30 Nearly all sauropods are now thought to have held their long necks nearly horizontal, except perhaps when occasionally feeding on high-growing foliage.
Despite having the smallest brains (relative to body size) of all dinosaurs, sauropod trackways indicate that these gigantic animals engaged in different kinds of social behavior, particularly gregariousness and probably migration (at least during part of the year). Some scientists have argued that it could not have been otherwise: a herd of sauropods would soon deplete its food sources in one area and then would have to move on to another in order to survive. These same trackways suggest that a migratory sauropod walked at rates of 20 to 40 km per day, although they may have been able to reach speeds of 20–30 km/hour for short bursts.
Defense in sauropods is obvious: large size confers the greatest deterrent against an attack by a predator, although not for the young, the very old, and the infirm. As for Magyarosaurus dacus, who these predators were is more than a little problematic.
As their teeth and powerful jaws attest, theropods were the supreme predators of the Mesozoic. They included the likes of Tyrannosaurus, Carcharodontosaurus, and Giganotosaurus, all vying, at up to 13 m long, for the distinction of being the largest of all terrestrial carnivores (figure 2.11). Theropods were also among the smallest—though no less dangerous—of the dinosaurs: Velociraptor, Troodon, and Compsognathus were all less than 2 m long. Through the evolutionary connections between Deinonychus and Archaeopteryx (box 2.2), these creatures of the past have given us living birds.31
Late Cretaceous theropods are best known from fossils recovered in North America, Asia, Africa, and South America; those from Europe—consisting mostly of isolated teeth, vertebrae, and various limb bones32—offer intriguing glimpses into theropod diversity, but their features do not pinpoint species relationships very well. Theropods represent the most diverse component of the vertebrate biota of the Late Cretaceous of Transylvania, but elusively so—their remains, known from a meager dozen or so limb elements and teeth, are the most poorly known of the entire fauna. Consequently, paleontologists have juggled and reassigned fossil fragments to a number of different kinds of theropods. Some of these fragmentary Transylvanian fossils, as well as new collections of theropod remains, are only now beginning to be sorted out.
Figure 2.11. The large and the small among predatory dinosaurs: reconstructions of Giganotosaurus (above) and Velociraptor (below), with a human included for scale. (After Coria and Salgado 1995)
Nopcsa described the first “conventional” (i.e., nonavian) theropod—Megalosaurus hungaricus—from the Late Cretaceous of Transylvania, although the small isolated teeth on which it was based came not from the Haţeg Basin, but from older (possibly Santonian33) coal outcrops in the Borod Basin, some 150 km to the northwest.34 Nopcsa attributed these teeth to Megalosaurus with some trepidation, fully aware that in many other species assignments to this genus, all that was usually meant was “moderately large theropod from Europe.” Similar to most of these uncritical determinations, Megalosaurus hungaricus is now regarded as an indeterminate theropod—one, to make matters worse, whose original remains unfortunately are now lost.
The first theropod from the Haţeg Basin was originally thought to be a bird, rather than one of the more conventional theropods, such as Megalosaurus or Allosaurus. Elopteryx nopcsai, described in 1913 by Charles W. Andrews, an ornithologist at the British Museum (Natural History) in London, was based on the top end of a femur and the lower end of a tibiotarsus (the product of fusion between the shin bone—the tibia—and the upper ankle bones, a feature universally found in birds, but also, as we know today, in some nonavian theropod dinosaurs). Andrews originally identified E. nopcsai as a Late Cretaceous pelican.35 Andrews’s interpretation was seconded by Kálmán Lambrecht, a friend of Nopcsa and himself a renowned paleo-ornithologist. He also referred three new tibiotarsi to Elopteryx.36 In 1975, two researchers at the British Museum (Natural History), ornithologist Colin J. O. Harrison and paleontologist Cyril A. Walker, reexamined the available specimens of E. nopcsai and determined that they really came from three different forms. One was Andrews’s original Elopteryx, but they also allotted one of the tibiotarsi to another form called Bradycneme draculae and another two to a second form, Heptasteornis andrewsi, both classified as owls (figure 2.12).37 Elopteryx returned to the stage in 1981 with newly discovered material, the lower end of a femur that was described by Dan Grigorescu from the University of Bucharest and Eugen Kessler from Muzeul Tării Crişurilor Oradea (Secţia de Ştiinţe ale Naturii) in Oradea, Romania; these two paleontologists argued again that Elopteryx was a pelican.38 Regardless, this new specimen turns out to belong to a juvenile Telmatosaurus and thus doesn’t help in determining the affinities of the other theropod fragments.39
Although they never garnered much attention in the studies of paleo-ornithologists, nevertheless things were changing for Elopteryx, Bradycneme, and Heptasteornis. W. Pierce Brodkorb, of the University of Florida, was the first to cast doubt on the avian affinities of these forms in 1978, suggesting instead that they represented small nonavian theropods of uncertain affinity.40 Thereafter, many paleontologists followed Brodkorb’s lead in excluding these three Haţeg forms from Aves, suggesting instead that they were troodontids or dromaeosaurids, based on their size and the age of the beds from which they were recovered.41 We will question whether these assignments make sense, but first we will recount some exciting new theropod discoveries made in the 1990s.
The first time a portion of a theropod skull, a small one, was unearthed from a rock outcrop in the Sibişel Valley was in the early 1990s.42 The two elements of the skull roof (figure 2.13) suggested that we had something new among the Transylvanian dinosaurs. So we compared our new find with other theropods, proposing that the new Haţeg skull fragments came from a dromaeosaurid (we ended up calling it the “Romanian Raptor”), with its greatest similarity to Saurornitholestes lang-stoni, known from the Late Cretaceous of North America.43
Figure 2.12. The top end of the femur of Elopteryx nopcsai (top), the bottom end of the tibia of Bradycneme draculae (bottom left), and the bottom end of the tibia of Heptasteornis andrewsi (bottom right). Scale = 2 cm
A more recent analysis of all of this theropod material—as well as of abundant, newly collected specimens by Zoltán Csiki and Dan Grigorescu,44 and by Vlad Codrea, Thierry Smith, and coworkers45—has advanced Romanian dinosaur paleontology in two ways. First is an improved understanding of the fauna through the study of the new microfaunal collections—small teeth and bones—from several sites in the Haţeg Basin. From these, the Late Cretaceous fauna includes dromaeosaurids, a small troodontid theropod, and several curious small forms that are compared with the enigmatic Euronychodon, Richardoestesia, and Paronychodon, all known from elsewhere in Europe and North America at the end of the Cretaceous (figure 2.14). Second is a reevaluation of Elopteryx, Bradycneme, and Heptasteornis and their evolutionary relationships with other theropods. Although none of the Haţeg forms appears to be diagnostic at the species or generic level, Elopteryx nopcsai possesses features that suggest that its affinity lies within Maniraptora, the large theropod group that includes Deinonychus, Velociraptor, and birds. Elopteryx is clearly not a Late Cretaceous pelican, but a maniraptoran (possibly a dromaeosaurid,46 such as Velociraptor and Deinonychus, or a troodontid,47 like Saurornithoides). Nor are Bradycneme draculae and Heptasteornis andrewsi Late Cretaceous owls. Instead, B. draculae appears to be an indeterminate maniraptoran, and H. andrewsi is thought to be an indeterminate alvarezsaurid.
Figure 2.13. A reconstruction of the head of the Haţeg dromaeosaurid (top; restored after Dromaeosaurus), and a dromaeosaurid skull roof in dorsal view (bottom right), with a silhouette (bottom left) indicating its position in the skull. Scale = 3 cm
Figure 2.14. Theropod teeth from Transylvania: from a dromaeosaurid (left), a troodontid (middle), and Euronychodon (right). Scale = 3 mm. (After Csiki and Grigorescu 1998)
Thus far absent from the Haţeg assemblage is any evidence of a large dinosaurian predator, commonly at the top of the food chain in nearly all Mesozoic terrestrial fauna.48 The closest to this pinnacle is a single dorsal vertebra (5 cm in diameter) with pleurocoels, which represents no more than a medium-sized theropod.49 Instead, most theropod material from Transylvania consists of small teeth belonging to several kinds of small (2 m long), yet aggressive killers roaming the Haţeg region in the Late Cretaceous (Plate IV, top). Troodontids were also small predators, with long, slender, lightly built skulls that housed a large brain and very large eyes (positioned in such a way as to give these dinosaurs binocular vision) and was equipped with many small, but sharply recurved teeth. Alvarezsaurids, a group known principally from the Late Cretaceous of Mongolia, Argentina, and the United States, were small (about 1 m long), cursorial theropods with exceedingly diminutive, but robust fore-limbs and long, gracile hind limbs.50 The phylogenetic position of alva-rezsaurids within Theropoda is presently controversial: they have been placed within or just outside Avialae or as the sister group of Ornithomimosauria. Sauropods such as Magyarosaurus and other herbivorous inhabitants of the Transylvanian region surely would have feared an attack by any or all of these predators. The form and lifestyles of Euronychodon, Paronychodon, and Richardoestesia are much more problematic. We know almost nothing about any of these theropods beyond their small size and the details of their sharp teeth. Nor can we say much about their affinities. Euronychodon has been thought by some paleontologists to be a primitive ornithomimosaur, and by others to be an indeterminate coelurosaur or even an indeterminate theropod;51 Paronychodon may be a troodontid; and Richardoestesia, thus far, stands as a tetanuran of no known affinities. These are really only guesses, and there is little hope of understanding the evolutionary and ecological significance of these three small theropods without better material than we presently have.
The best known of all of these small theropods are the dromaeosau-rids, fast-running, bipedal predators with a large, sharply curved claw on each hind foot. The best known of the Transylvanian dromaeosaurids is the newly discovered Balaur bondoc (“stocky dragon” in archaic Romanian). B. bondoc presently consists of a partially complete, articulated skeleton from the Sebeş Formation near Sebeş (a second specimen is known from the Densuş-Ciula Formation at Tuştea).52 Unlike many other dromaeosaurids, this theropod is peculiar in having short forelimbs, highly fused hands and distal hind limbs, a very retroverted pubis, and dual claws capable of extreme hyperextension on each foot, presumably for grasping or disemboweling prey (figure 2.15). Similar in size to contemporary Laurasian dromaeosaurids, B. bondoc is presently the best known theropod from the Late Cretaceous of Europe. It is thought that dromaeosaurids in general, and perhaps B. bondoc in particular, may have hunted in packs, dispatching their prey by leaping upon them, raking them open with their deadly toe claws, and using their rigid tail to maintain balance.
Figure 2.15. Left lateral view of what is known of the skeleton of Balaur bondoc. Scale = 10 cm. (After Csiki et al. 2010)
Ankylosaurs are one of the two major groups of ornithischians with bony plates on their back (the other being Stegosauria), and nature lavished a full suit of armor on them.53 Ankylosaurs were probably experts in hunkering down in self-defense.
The first-discovered ankylosaur was Hylaeosaurus. One of the original members of Owen’s Dinosauria, it was not clear, at the time of its discovery in 1832, what this bizarre animal truly looked like.54 Nor was it much clearer to Franz Nopcsa when the armored dinosaur—Struthiosaurus transylvanicus—was first discovered in the Haţeg Basin in 1912. At that time, few good ankylosaur specimens had been uncovered anywhere in the world. It took several more years and many discoveries for paleontologists to conclude that these were lumbering quadrupeds covered by a shell-like armor of bony plates and spines across the neck, back, and tail. With these discoveries, paleontologists also determined that ankylosaurs are principally formed into two groups: nodosaurids, with their great shoulder spines, and club-tailed ankylosaurids (box 2.3).
The story of Struthiosaurus is as intricate and complex as that of the Transylvanian theropods. It begins in 1871, when Emmanuel Bunzel, an Austrian physician and avid fossil collector, described new vertebrate material recovered from the Gosau Beds of Muthmannsdorf, near Vienna.55 One specimen consisted of a small braincase fragment that he thought was rather birdlike; Bunzel named the specimen Struthiosaurus austriacus (“Austria’s ostrich-reptile”). However, Harry Govier Seeley first identified the ankylosaurian nature of Struthiosaurus in 1881.56 Thereafter, when Nopcsa studied the ankylosaur material from the Haţeg Basin, he compared it closely with S. austriacus and the other ankylosaurs from Europe. On the basis of these studies, Nopcsa considered the Haţeg ankylosaur to be generally the same as Bunzel’s Struthiosaurus, yet sufficiently different to merit a new species designation, Struthiosaurus transylvanicus.57
S. transylvanicus is currently known from skull elements (the brain-case and portions of the skull roof and cheek region), as well as vertebrae, the shoulder girdle, and dermal armor belonging to at least two individuals collected from the Sânpetru Formation in the Haţeg Basin (figure 2.16). There is new material of S. transylvanicus, collected at Oarda de Jos and Vurpăr by researchers from Universitatea Babeş-Bolyai Cluj Napoca, which should provide additional information on the anatomy of this nodosaurid.58 All of these specimens are relatively small, but they are thought to be from adult individuals, suggesting that members of this species grew to not much more than 2 m in length. What we know about Struthiosaurus is due in large part to studies in the 1990s of S. austriacus by Xabier Pereda-Suberbiola, a Basque dinosaur paleontologist also working in Paris, and Peter M. Galton, a paleontologist and anatomist at the University of Bridgeport in Connecticut.59
The skull of S. transylvanicus is robustly built: most of the holes at the back of the head, through which the jaw muscles could bulge, are reduced or closed, the back surface of the skull is fused into a single unit, and the top of the head is covered with armor. Although their placement and appearance are yet unknown, armor plates also covered the back of the body, and a large spine projected from its shoulder. In addition, the outer surface of the shoulder girdle bears a prominent ridge (pseudo-acromion), where strong muscles were attached to move the large and muscular forelimb.
Paleontologists have relied on these and other features of Struthiosaurus to provide clues to its kinship and lifestyle (Plate IV, bottom). It is clearly a nodosaurid, based on the scapula having a pseudoacromion that is displaced downward and backward toward the shoulder joint and ends in a knoblike projection.60 Nopcsa thought that there may have been teeth in the front of the upper jaws (known as premaxillary teeth), unlike more derived nodosaurids such as Edmontonia and Panoplosaurus, whose premaxillary teeth are absent. If this is true, Struthiosaurus would assume a relatively primitive position in nodosaurid phylogeny.
Figure 2.16. The Transylvanian nodosaurid ankylosaur, Struthiosaurus transylvanicus (above), and a reconstruction of its head (below). (Struthiosaurus from an original plate from Nopcsa 1929a; restored head after Pawpawsaurus and Edmontonia)
Given its size and posture, we assume Struthiosaurus was a very low-browsing feeder, foraging no more than a meter above the ground. The relatively narrow and scoop-shaped front of the upper and lower jaws of other nodosaurids (but not yet known in Struthiosaurus) was probably overlaid with a horny covering (called a rhamphotheca), also seen in living turtles and birds, and suspected in many ornithischian dinosaurs. The shape of this region of the head suggests that these animals were somewhat selective feeders, plucking or biting at particular kinds of leaves and fruits with the sharp edge of the rhamphotheca.61
Food, once cropped, was apparently chewed through a combination of simple up-and-down puncturing and fore-and-aft grinding. Beyond this suggestion, which we deduce from the pattern of wear on the teeth, ankylosaur mastication is puzzling. For example, the triangular teeth of both nodosaurids and ankylosaurids do not appear particularly well suited to a diet of plants; they are small, not very elaborate, and less tightly packed together in the jaws than the teeth of other ornithischian dinosaurs. On the basis of these simple teeth, Nopcsa thought that Struthiosaurus instead fed on insects,62 although no one today doubts that its diet was dominated by plants. An extensive bony palate closed off the oral cavity from the nasal cavity, allowing these ankylosaurs to chew and breathe at the same time. Moreover, deeply inset tooth rows suggest the presence of deep cheek pouches to keep food from falling out of the mouth. The jaw bones themselves were relatively large and strong, although lacking enlarged areas for muscle attachment. Every jaw feature except the teeth suggests that ankylosaurs were adept chewers.
Perhaps the paradox of unsophisticated teeth set in strong, cheek-bound jaws can be understood by looking not at how ankylosaurs chewed food before swallowing, but at the other end of the animal, where much of the plant digestion must have been accomplished by gut fermentation. What the teeth couldn’t accomplish mechanically, the gut could, by breaking down the roughly chopped leaves by chemical means. A deep, broadly rounded rib cage circumscribing an enormously expanded abdominal region indicates that the digestive tract was huge.
Nopcsa’s interest in Struthiosaurus extended beyond how and what it ate to how it moved and how its small brain controlled the movement of its great mass. We know the brain size of this Transylvanian nodosaurid from a latex cast of the brain cavity that Nopcsa had made for his detailed study of the beast. Measuring less than 50 ml, the brain of Struthiosaurus was very small, even by dinosaur standards—only sauropods had smaller brains for their body size. There were no intellectual giants here, nor was athleticism their strong point. In addition, Struthiosaurus, like other ankylosaurs, were among the slowest moving of all dinosaurs. According to Australian paleontologist Tony Thulborn’s calculations,63 these armored dinosaurs walked at a leisurely pace of about 3 km/hour and probably ran no faster than 10 km/hour, about the average running speed of an elephant among living animals. Struthiosaurus was plodding, to be sure, but certainly not without defenses. It probably was able to stab at predators and competitors alike by firmly planting its hind limbs, ducking its head, and rolling its strong shoulders, with their formidably long spines, forward. Otherwise, Struthiosaurus hunkered down, relying on a suit of bony armor to protect it from packs of troodontid or dromaeosaurid theropods.
Zalmoxes, named for the Dacian god of the underworld and immortality who was famous in Romanian lore for living, teaching, and healing from his subterranean crypt, was one of two sorts of Transylvanian ornithopods. Our account of Zalmoxes and how it fits into the ornithopod scheme of things begins in 1897, when Nopcsa first recognized some distinctive bones and teeth recovered from localities along the banks of the Sibişel River.64 These he called Mochlodon, because of their similarity to ornithopod specimens from the Gosau Beds of Austria (the same locality that yielded the bones of Struthiosaurus austriacus) that Seeley had named Mochlodon in 1883.65 Rhabdodon priscus, another Late Cretaceous dinosaur from southern France originally described in 1869 by Philippe Matheron, a geologist from Marseille,66 was also similar to the Haţeg Mochlodon material, but Nopcsa initially regarded them as different kinds of ornithopods. By 1915, however, he was considering the possibility that the two were from the same species, perhaps being male and female. Since Rhabdodon was the earlier find, Nopcsa conceded Matheron’s claim of priority and renamed the Mochlodon material from Transylvania Rhabdodon, for Matheron’s animal.67
Once Rhabdodon was properly named to Nopcsa’s satisfaction, where did it belong on the ornithopod family tree? Nopcsa was the first to advocate particular affinities for this Haţeg ornithopod in 1902. Comparing it with other ornithopods, he noted that Rhabdodon seemed to be more primitive than Iguanodon (from the Early Cretaceous of Europe), but more closely related to Camptosaurus (from the Late Jurassic of North America). Thereafter, Rhabdodon was either included in a group that contained Iguanodon (either Iguanodontidae or the more encompassing Iguanodontia) or shifted to the group of small ornithopods called Hypsilophodontidae.68
Figure 2.17. Skull elements of Zalmoxes, one of the Transylvanian ornithopods (left) and a reconstruction of its head (right). (Skull elements from an original plate from Nopcsa 1904)
Although Nopcsa regarded the proper parentage of Rhabdodon as solved, from the perspectives of both taxonomy and evolutionary relationship, we were far from convinced when we started examining the remains of this beast. So, working closely with Zoltán Csiki (Universitatea din Bucureşti) and David Norman (Sedgwick Museum at Cambridge University), we tried to bring the animal Nopcsa ended up calling Rhabdodon (figure 2.17) into its present-day context. We combed through dusty museum drawers across Europe, trying to compare our Romanian specimens with material of Rhabdodon from France and Spain, and Mochlodon from Austria, as well as with other specimens from England and North America (Camptosaurus, Hypsilophodon, and Tenontosaurus, among others), integrating this trove of data using cladistic analyses (box 2.4).
Here’s what we discovered during the course of our research. The specimens from France and Spain that have been referred to as Rhabdodon probably all belong to the same genus and species, a conclusion that was confirmed by material and analyses by Marie Pincemaille and her colleagues at Université Montpellier in the late 1990s (figure 2.18).69 This creature naturally retains the name proposed in 1869 by Matheron, Rhabdodon priscus. However, our work also identified two species from Transylvania, both of which are now known from various localities in the Haţeg Basin and several other locales—Vurpăr, Oarda de Jos, Jibou, Bărăbanţ, Lancrăm, Sebeş—in the Transylvanian Depression.70 In fact, the two Transylvanian species appear more closely related to each other than either is to Rhabdodon from France and Spain. We considered this a prime reason for coining a new name, and in 2003 we settled on Zalmoxes. The two species were called Zalmoxes robustus and Zalmoxes shqiperorum (“shqiperorum” honoring Nopcsa’s love of the northern Albanian tribes).71 Of the two, Z. robustus is quite well known, based on upwards of 300 specimens, whereas Z. shqiperorum is known from a nearly complete skeleton from Nălaţ-Vad, as well as a few dozen bones and teeth from elsewhere in the Haţeg Basin. It is further known from the Şard Formation at Vinţu de Jos on the southern margin of the Transylvanian Depression,72 and from the Jibou Formation near Jibou along its northern margin.73
So Zalmoxes has now received its name, but how is it related, phylogenetically, to other ornithopods? Our cladistic analyses clearly indicated that its closest relative was Rhabdodon. Together, Zalmoxes and Rhabdodon form a clade called Rhabdodontidae, which is most closely related to the group of ornithopods known as iguanodontians, the latter including animals such as Tenontosaurus, Iguanodon, hadrosaurids, and many other forms.
Now that we can recognize Zalmoxes as distinct from Rhabdodon and other ornithopods, we can also begin to understand its form and lifestyle (Plate V, top). The bones suggest a moderate-sized ornithopod (3–4 m long). Its body was stocky, it had a bipedal stance, and its back was held nearly horizontally, with its long muscular tail counterbalancing the front half of the body at the hip joint. The features of the bones of the fore- and hind limbs suggest that these regions were well muscled, whereas the ribcage seems more barrel-shaped than in other ornitho-pods. Limb proportions indicate that Zalmoxes was probably not a particularly fast runner, scurrying along at no more than 25 km/hour.74 Although it would be great if we had numerous trackways of Zalmoxes to test these calculations, there is a track site in the Vurpăr Formation near Sebeş (the only footprints known from the Late Cretaceous of Transylvania) that consists of two hind footprints thought to have been made by Zalmoxes. These tracks indicate a more leisurely walking speed of less than 7 km/hour.75
Figure 2.18. Skeletal reconstructions of Zalmoxes (above) and Rhabdodon (below). Scale = 100 cm. (Rhabdodon after Garcia et al. 1999)
The skull of Zalmoxes is relatively large but compact, with a short face (figure 2.19). Some individuals have a stout transverse crest above the eyes, perhaps a sign of sexual dimorphism or a sign of age. The narrow, toothless beak was probably covered in life with a sharp rhamphotheca (as also discussed for Struthiosaurus), and was thus likely to have been able to cut through tough foliage and fruits. Both the upper and lower jaws were strongly built, containing at least ten large, closely packed teeth. Patterns of tooth wear reveal that Zalmoxes chewed its food well, using a transverse grinding motion. The teeth are recessed from the sides of the face, suggesting—as in the case of Struthiosaurus and other ornithischians—that cheeks covering this region may have prevented food from slipping out of the sides of the mouth.
Figure 2.19. A reconstructed skull of Zalmoxes robustus. Scale = 10 cm. (After Weishampel et al. 2003)
We don’t know much about the sociality, growth and development, and reproductive behavior of Zalmoxes. On these matters, the fossil record has been silent. We do know that this ornithopod is probably the most common element within the Transylvanian assemblages, but whether it was truly gregarious or merely appeared frequently amid the fauna is unknown. Similarly, there are no data to suggest colonial nesting and parental care—or rule it out. For these aspects of a dinosaur’s life, we need to turn to the Transylvanian hadrosaurids.
The duckbills of the Late Cretaceous—members of Hadrosauridae—were among the most diverse forms of plant-eating dinosaurs, known principally from North America and Asia (figure 2.20). Many hadrosaurids are known from well-preserved fossils, including those of embryos, hatchlings, juveniles, teenagers, and adults. From these specimens, and from abundant nesting sites, we are learning more about parental care of offspring, group nesting, and the rapid growth rates that appear to characterize these ornithopods.
As we have noted previously, the first dinosaur described from the Haţeg Basin was a new genus and species of hadrosaurid dinosaur that Nopcsa named Telmatosaurus transsylvanicus. Virtually the entire skull and the majority of the postcranial skeleton of T. transsylvanicus, based on more than 100 cranial and postcranial elements, are known from a number of individuals of various body sizes collected at numerous localities in the Haţeg Basin and Transylvanian Depression.76 At an adult length of about 5 m, Telmatosaurus was one of the smallest hadrosaurid dinosaurs (Plate V, bottom), much smaller than hadrosaurids elsewhere in the world or their more distant relatives (such as Iguanodon), both of which ranged upwards of 10 m in length.77 We will return to this issue of body size and its evolutionary significance in chapter 6.
Even though the original material of Telmatosaurus that Nopcsa described in 1899 was severely crushed, we were able to compare it with other, subsequently discovered specimens and thereby reconstruct what the skull must have looked like in three dimensions (figure 2.21). What emerged from the reconstruction is a long, somewhat horselike cranium, reminiscent of Iguanodon and other hadrosaurids.78 The front of the snout (the premaxilla) is narrow, toothless, and crenulated, most probably supporting a rhamphotheca. Both the upper and lower jaws contain as many as 30 vertical positions for the teeth, far more toothy than the jaws of non-hadrosaurid iguanodontians, but considerably less so than in most other hadrosaurids. Both the upper and lower sets of teeth consisted of hundreds of functional and replacement teeth, interlinked to form dental batteries (figure 2.22). Such a complex arrangement of teeth, coupled with well-developed jaw muscles and a unique masticatory system, would have made short work of the toughest vegetation.79 Behind the head, the skeleton of Telmatosaurus looks much like that of both more primitive iguanodontians (such as Ouranosaurus and Iguanodon) and other hadrosaurids (like Brachylophosaurus and Maiasaura).80 However, its body was smaller than these taxa, and therefore not unduly bulky. Similar to other ornithopods (including Zalmoxes), the long tail, stiffened and strengthened by crisscrossing bony tendons, acted to counterbalance the front half of the body.
Figure 2.20. Diversity of skull morphology in hadrosaurids: Gryposaurus (above left), Edmontosaurus (above right), Saurolophus (below left), and Corythosaurus (below right). Scale = 10 cm. (After Weishampel and Horner 1990)
In 1993, Telmatosaurus was subjected to cladistic analyses to see where it fit within hadrosaurid evolution (box 2.5).81 Based on this work, which has been borne out in subsequent studies,82 Telmatosaurus appears to fit best as the sister group of both lambeosaurines and hadrosaurines. In 1993, Weishampel et al. called this latter clade Euhadrosauria, but most paleontologists have followed a more historical path, considering hadrosaurines and lambeosaurines as the sole members of Hadrosauridae; this makes Telmatosaurus a non-hadrosaurid hadrosauroid. In either taxonomy, the relationships remain the same: Telmatosaurus is a (or perhaps the most) primitive outsider to all other hadrosaurids. We will discuss the significance of the position of Telmatosaurus with respect to ornithopod evolution, especially in relationship to its small body size, later in this book.
Figure 2.21. A reconstruction of the skull of Telmatosaurus transsylvanicus (left) and a reconstruction of its head (right). Scale = 10 cm
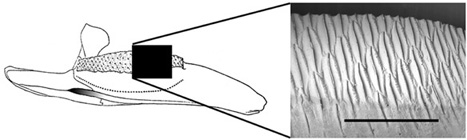
Figure 2.22. A hadrosaurid dental battery (left), indicating the complex interlocking pattern of replacement teeth (right). Scale = 5 cm
Large ornithopods in general were not particularly fleet of foot, and Telmatosaurus was no exception. Based both on hadrosaurid trackways and on limb proportions, these bipedal forms probably were able to reach a top speed of 15–20 km/hour during a sustained sprint, but at slower speeds and at rest, they apparently assumed a quadrupedal posture. In this position, Telmatosaurus most likely browsed no higher than a meter or so above the ground, probably using its forelimbs to grasp at leaves and branches in order to bring the foliage closer to its mouth.
From Gryposaurus (with its arched snout), to Saurolophus (with its supracranial spine), to Parasaurolophus (with its hollow, plumelike crest), these dinosaurs stand out from the crowd with their wild headgear. In 1975, Jim Hopson of the University of Chicago examined the functional significance of these cranial adornments. He argued that the arches, spikes, hollow crests, and other cranial decorations evolved in the context of complex intraspecific social behavior. These crests were used in intraspecific aggression and display, both visual and vocal; in courtship displays; and in mate rivalries. Crests and the rest presumably helped hadrosaurids recognize kin, avoid enemies, display to each other and to members of different species, communicate with offspring, and establish social hierarchies—an amazing evolutionary achievement that placed hadrosaurids in the top ranks of complex social behavior among dinosaurs.
As a basal member of the group, Telmatosaurus may have something to tell us about the origin of these aspects of hadrosaurid sociality. New Telmatosaurus material indicates that this dinosaur had a pair of sinuous ridges that ran along the sides of the snout. Though less dramatic than the headdresses of more evolved hadrosaurids, these ridges would have made its face more visually interesting than those of more primitive ornithopods. Were the ridges parts of a display apparatus, perhaps a progenitor to the exhibitionists yet to come? Were these sigmoidal ridges sexually dimorphic? We don’t yet know. Nor can we say with certainty whether Telmatosaurus was territorial, or engaged in parental behavior. The only other aspect of behavior known in this Transylvanian hadrosaurid is an ever-so-brief glimpse into its early growth and development, which comes from the discovery of embryo or baby bones associated with some eggs unearthed in the late 1980s.
Nests and nesting horizons are among the most exciting dinosaur discoveries in Romania over the past decade and a half. The first announcement of Haţeg Basin eggs came in 1989, through work near the village of Tuştea by researchers from Universitatea din Bucureşti.83 Another site, Toteşti-baraj, was discovered by the joint expedition from Universitatea Babeş-Bolyai Cluj Napoca, Romania, and Brussels’ Institut Royal des Sciences Naturelle de Belgique in 2000.84 The eggs within these nests seem to be laid in a somewhat organized, curvilinear fashion.85 In life, the eggs would have been nearly spherical, approximately 15 cm in diameter, and nearly a liter in volume (figure 2.23). On average 2.4 mm thick, the eggshell is covered with an irregular pattern of small, hemispheric tubercles. This locality has thus far produced forty eggs distributed in eleven different nests. It is not certain what the conformation of each nest is, but the eggs themselves, although deformed, also appear to be subspherical in shape, with a diameter of about 15 cm.
Figure 2.23. A reconstruction of the dinosaur nest from Tuştea. Scale = 15 cm. (After Grigorescu et al. 2010)
In addition to their macroscopic features, still more information can be discerned about these eggs under the microscope (figure 2.24). At a magnification of about 80 power, the eggshell reveals its mineral structure. The eggshell is calcite, organized into structural units that vary characteristically among different egg-laying species. Circa the early 1990s, Karl Hirsch and Konstantin Mikhailov erected a classification scheme for these structural units.86 They identified basic types, each broken down into subgroupings to produce a taxonomy of names that trip up the most dexterous tongue: dendrospherulitic, angusticanalicu-late, Laevidoolithidae. Still, it’s often difficult to attribute these categories to particular kinds of egg-laying animals, in large part because we generally lack a direct connection between eggs and moms. However, some truly remarkable occurrences—embryonic remains preserved in fossil eggs—have proven to be a Rosetta Stone. Consequently, we know that eggs found in Montana, which fall within the Mikhailov/Hirsch category termed Spheroolithidae, were laid by the hadrosaurid called Maiasaura, because they were found with the embryonic or hatchling bones of this dinosaur.87 On a similar basis, we know that oviraptorid theropods laid elongatolithid eggs, and, based on discoveries in Argentina made in 1998, titanosaur sauropods laid megaloolithid eggs.88
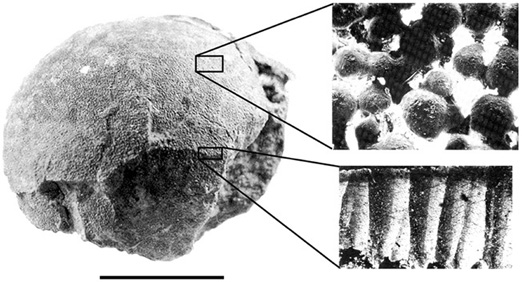
Figure 2.24. One of the eggs recovered from Tuştea (left), indicating the surface texture (above right) and a shell cross-section (below right). Scale = 10 cm. (After Weishampel et al. 1991)
Based on their architecture, the Tuştea eggs likewise belong to the Megaloolithidae category, sharing similarities in microstructure, pore organization, size, and shape with some of the eggs from southern France. The only principal difference is in nest structure: the French megaloolithid eggs apparently were laid in sweeping curves instead of curvilinearly.89 Although no embryonic remains associated with these eggs have ever been recovered in France, they are thought to have been laid by a titanosaur sauropod, an animal of the right size to have laid such an egg and one common in the Late Cretaceous fauna of the region. Realizing that we also had sauropods in the Haţeg fauna, at first we suggested, in 1990, that these eggs may have been laid by one or the other titanosaur from Transylvania.90
Even more significantly, the bones of either full-term embryos or newborn hatchlings (technically called perinatal remains) were also discovered at the same site, in fact from the same bedding planes that yielded the Tuştea egg clutches. Consisting of partially articulated skeletons and additional associated remains, the specimens can be identified with confidence as those of a hadrosaurid (most probably Telmatosaurus), even though the joint surfaces of the bones are very porous and the surface texture of their shafts is immature (figure 2.25).91 Their proximity to the Tuştea eggs suggested that the perinatal remains belong with the eggs; that is, the female who laid the eggs would have been the mother of the babies whose small bones we found. This question of who laid the Tuştea clutches has brought some consternation to dinosaur “egg-ologists”: the structure of the Tuştea eggs resembles that of titanosaur sauropods (thereby implicating Magyarosaurus as the egg-layer), whereas the perinatal fossils come from a hadrosaurid (suggesting that the parent was Telmatosaurus).92 This conflict might be solved if we found identifiable embryonic remains preserved inside the Tuştea eggs and not alongside them. Some scanning electron microscopic photographs have been taken,93 but they are far from conclusive.
This conundrum—titanosaur or hadrosaurid eggs—has been indirectly solved by discoveries made halfway around the world, in the badlands of Patagonia in the southern half of Argentina.94 It was here, at a site known as Auca Mahuevo, that an extensive nesting ground—covering more than a square kilometer and littered with tens of thousands of large, unhatched eggs—was discovered in 1997 by an expedition led by Luis Chiappe from the Natural History Museum of Los Angeles County, Lowell Dingus from the American Museum of Natural History in New York, and Rodolfo Coria from the Museo Municipal Carmen Funes in Plaza Huincal, Argentina. Unlike the Haţeg clutches, those from Argentina were organized into clusters of between 15 and 34 eggs. Most spectacularly, and of particular relevance here, was the fact that a high proportion of these eggs contained embryonic skeletons, some with the impressions of embryonic skin! Moreover, these imprisoned embryos, many of them in near articulation, possess nearly complete skulls that clearly belong to titanosaur sauropods.
Figure 2.25. Perinatal hadrosaurid remains from Tuştea: a distal femur (left) and a proximal tibia (right). Scale = 10 mm. (After Weishampel et al. 1993)
The importance of Auca Mahuevo to our Transylvanian research is at least threefold. First, we have an unambiguous association of eggs with embryos. Indeed, they are the first known embryonic remains of sauro-pods. Thus the question of who laid these clutches has been answered with absolute certainty: titanosaurs. Second, the incredible geographic extent of the nesting horizons at Auca Mahuevo speaks strongly for colonial nesting and maybe for gregarious behavior in these and perhaps other titanosaurs elsewhere in the world. Finally, these titanosaur embryos provide the opportunity to discover the details of early stages of sauropod growth and development, a subject we will take up in chapter 7.
Returning to Haţeg, what we have learned from Auca Mahuevo is that the clutches of these Transylvanian eggs were most probably laid by one or the other titanosaur from the fauna. From the sediments surrounding the nests, we known that the eggs were buried under a thin layer of fine sand. From the construction of the eggshell, we can calculate the rate of water–vapor exchange, absolutely critical during embryonic incubation, and we can conclude that, for optimal conditions, the humidity of the nest should have been between 85% and 95%, and the eggs would have hatched in 50–60 days.95 Not a bad picture of nesting paleoecology with only data from pores, shell thickness, and sedimentology to go by.
The perinatal bones known from Haţeg, on the other hand, may have washed into the titanosaur nesting area, but from very close by, because their porousness and delicacy would have made long-distance transport impossible. These small bones belong to a hadrosaurid, probably Telmatosaurus transsylvanicus, and reveal different aspects of how this ornithopod may have grown and developed. Because hadrosaurids (including Telmatosaurus) are skeletally immature when compared with other perinatal dinosaurs, it appears that they hatched at an underdeveloped, dependent stage. Jack Horner, the Museum of the Rockies (Bozeman, Montana) paleontologist whose work has revolutionized our understanding of dinosaur growth and development, has argued that these immature hatchlings would have been nest-bound while they matured.96 This extended parental care, necessary to promote their survival, is a condition ecologists call altriciality.97 Telmatosaurus was almost certainly an altricial dinosaur, and, given its primitive position among hadrosaurids, it could represent the turning point in the origin of this hadrosaurid life-history strategy. We will have more to say about this connection in chapter 7.