You’re standing at one of those classic 1970s pinball machines—no doubt the “Bally 4 Million BC,” in keeping with our paleontological theme—and you pull back the plunger and release the ball on its initial trajectory. As the silver projectile cavorts with the zap and ding of the bumpers and the slap of the paddles, the score mounts. Eventually, the game ends when the ball drops into the machine and you cram another coin into the slot as you obsessively try to beat your previous score. Game after game, the itinerary of the pinball is different, guided or constrained, to be sure, by the rails, the paddles, and the bumpers, but the cascade of small variations in speed, angle of impact, or body jostles make every game unique. This improvisational dance goes to the heart of pinball’s appeal. Myriad small factors—such as scratches on the ball, the humidity of the machine, dust on the plunger, and the twist of the wrist—affect each and every pathway, even before the ball is unleashed. This unpredictability is the essence of historical contingency.
As much as it is a recipe for making pinball a challenge and enjoyment, historical contingency is also what holds our attention in the arts, especially in film. For example, Frank Capra’s 1946 film, It’s a Wonderful Life,1 has been invoked by Stephen J. Gould, the late Harvard professor, evolutionary theorist, and paleontologist, as a present-day metaphor for the role of chance in human history.2 In this Christmas favorite, George Bailey, depressed and suicidal over his inconsequential existence, is shown by an angel what his community would have looked like if it hadn’t been for all his good deeds over the years. In providing him with an alternative “what-if” version of his existence, the angel convinces George to return to his family and his way of life. Countless other films look at the other side of life’s singularity by exploring how seemingly insignificant events can unpredictably produce cataclysmic results. Of these, we especially like Terry Gilliam’s 1985 Orwellian extravaganza, Brazil. This film is one long nightmare of terrorist bombings, late-night shopping, true love, creative plumbing, and brainwashing, ultimately based on the intersection of a dead beetle and a typing machine.3 Instead of George Bailey, we have Sam Lowry, a harried civil servant in a convoluted and inefficient society, who escapes to dreams of flying, overpowering enemies, and spending eternity with the woman in his fantasies. At the same time, it is Gilliam’s colossal joke about chance and the unpredictability of the future. The small and apparently insignificant changes that are the lynchpins of both of these films become the catalysts for cascades of historical differences. It’s a Wonderful Life shows how a mere person can alter historical pathways, whereas Brazil does so by linking an unpredictable triviality to the overthrow of a man’s life. Change the initial conditions even slightly, and without apparent importance, and the path of history can be radically altered.
In coming to this place in our book, we now will reach past the dinosaurs of Transylvania to explore whether unpredictable events can have a uniquely important influence on the pattern of evolutionary history; we are going beyond the “who,” “what,” and “where” of the Transylvanian fauna to a framework that will allow us to explore “how” and perhaps even “why” questions. How did the creatures come to inhabit this part of eastern Europe? Who are the island immigrants, and are they a random sample of mainland faunas? If they indeed were dwarfs, how and why might they have become so?
Historical contingency—the unpredictability of life’s history from particular, often small-scale events—will take a prominent place as we build a foundation to answer these and other questions about the dinosaurs of Transylvania. Even one of the fundamentals of evolutionary processes—speciation—is fraught with its fair share of unpredictability. Speciation is the formation, through genetic divergence, of two or more descendant species from one ancestral species. This process is the motor for the diversification of life, and, when rendered in terms of cladogenesis (i.e., the generation of branches in a cladogram; see the discussion of cladistics in chapter 2), it forms the backbone for identifying the overall pattern of evolutionary history.4
Speciation is not just enhanced by, but, more importantly, it is dependent on a plethora of unpredictable conditions, particularly due to insularity. Contingency should be more common, and have greater effects, in situations of isolation; insularity should showcase historical contingency. What is crucial is reproductive isolation. When gene flow within a once-contiguous, interbreeding population is sufficiently interrupted, genetic divergence takes place between two or more portions of that original population. This divergence can occur in the same place as the ancestral population (sympatry), along the length of a geographically contiguous population (parapatry), or in geographic isolation from the ancestral population (allopatry).5 The evidence is extensive for allopatric speciation, which—despite some objections6—is generally regarded as the most influential theory of speciation. In addition to its prominence in evolutionary theory, we will be discussing it here in order to scrutinize the relationship of the Transylvanian fauna to geographic isolation.
The basis for allopatric speciation is the development of a geographic barrier separating an ancestral population into two subsequent descendant populations—think of a new mountain range or a river cutting through a formerly continuous population. If such barriers could split a species for a long enough period of time, gene flow between the two groups would cease and each would then evolve separately. Depending on the specifics, mutation, genetic drift, and/or natural selection would alter the genome of the two descendant populations over time. If these populations rejoined after they had diverged sufficiently, it is unlikely that they would successfully interbreed, either because mating would yield unviable or sterile offspring, or they might not recognize each other as members of the same species and hence would not interbreed. When the former applies, we speak of postmating or postzygotic isolating mechanisms, and, for the latter, premating or prezygotic isolating mechanisms. In either case, geographic isolation has led to reproductive isolation and thereby to speciation.
Preeminent among the cases of allopatric speciation are those involving peripherally isolated populations. Ernst Mayr, one of the twentieth century’s most influential evolutionary biologists, originally described the basis for speciation through peripheral isolation—sometimes called peripatric speciation—and argued that it was one of the most effective means and commonest way of producing two species where once there was one.7 Peripatric speciation involves the founding of a small second population—a few original individuals, or perhaps a single fertilized female in the most extreme case—through geographic isolation. The colonization of an island by a small population is the most cited example of what has been termed the founder effect.8 Should this group remain geographically separated from the main population for a sufficient period of time, they, too, will undergo the same divergence we’ve just discussed generally for allopatric speciation.
An especially important feature of peripatric speciation is that it would proceed very quickly. First, the founding population will carry only a small sample of the genetic reservoir of the ancestral population. Not only is there such random genetic loss, but a rare gene in the main population may also start being passed on with relatively high frequency in the founder population.9 These unpredictable sampling errors in gene frequencies—genetic drift—will be especially strong determinants of the subsequent genomes, so long as the population size remains small. Even though the chance of survival of this small peripheral population may be low,10 it is certainly not zero, and when the founder population increases to a less vulnerable size, natural selection will begin to take over, reflecting conditions that could be quite different from those of the more widespread population. In this way, the speciating population rapidly passes from one well-integrated and stable condition, through a highly unstable period, to another period of balanced integration.
There’s certainly a lot of dumb luck to peripatric speciation. Peripheral populations may be well adapted to their particular conditions, but, at the same time, their geographic separation from the main population is an unpredictable event. So are the details of genetic loss during separation, with the founders carrying a small, unrepresentative portion of the genome of the ancestral population. Chance also comes into play while the population remains small—gene frequencies are free to drift up and down randomly, without the controlling hand of natural selection. In this way, historical contingency is not just a possibility, but instead is a necessity in producing rapid speciation through isolation of the kind thought to exist in nature.
If anywhere in the world should reveal the power of contingency, it would be somewhere like Transylvania in the Cretaceous, where historical possibilities and pathways can be altered by chance events in the small, insular populations living there. Because of the geographic isolation of the place, coupled with its considerable tectonic flux (chapter 3), we should find an unusual agglomeration of animals and ecological relationships in Transylvania when compared with those more typical of the nearby North American and Asian landmasses, where the coastal plains were much more extensive, luxuriant, and tectonically quiet. Just who arrived in the Transylvanian region, and from where, are both far from predictable. Equally serendipitous are differences in the structure of predator-prey relationships, patterns of growth and development, intra-specific life histories, social structures, and evolutionary dynamics. When might these colonizers have arrived? Who interacted with whom? What changes in their features might have occurred? These are some of the questions we’ll be pursuing in our attempt to assess contingency in the history of the dinosaurs from Transylvania. Whatever answers we can bring to these questions, they must certainly be couched in the one thing we learned about the Transylvanian fauna in this and the previous chapter—its susceptibility to the vagaries of unpredictable evolutionary events due to its isolation.
In a shallow sea, a parcel of land becomes available for whatever terrestrial organisms come its way. Yet who’s to say which organisms? The issue of who successfully colonizes a new piece of ground is complicated by the size of the property; its proximity to other terrestrial regions; environmental differences in temperature, rainfall, and so forth; a species’ potential for transoceanic migration; which species populate neighboring regions; which species might already have arrived on the newly available land; and, above all, chance.
In this chapter, we will look at Transylvania as more than a place where dinosaurs once lived some 70 million years ago. With different eyes than we had at the beginning of this book, we now see more than a jumble of fossils, the remains of which have long been collected in the picturesque northern foothills of the Retezat Mountains of western Romania. First, by virtue of both the studies conducted by Franz Nopcsa and subsequent research efforts, we’ve gone well beyond merely recognizing various creatures from a collection of scattered bones found in western Romania: the duck-billed Telmatosaurus, the solidly built Zal-moxes, the long-necked Magyarosaurus, the armored Struthiosaurus, and the thus-far imperfectly understood predatory dinosaurs. Through paleobiological inference, these dinosaurs have become more than just entries in a Transylvanian faunal list. Second, we’ve used cladistics to put these Late Cretaceous denizens into their community of descent—siblings and cousins, as it were—with dinosaurs elsewhere in the world. Third, we’ve see them in as much of their complex terrestrial habitat as data and conjecture allow us to reproduce. Fourth, the small size of these dinosaurs—initially noted by Nopcsa—is now given a heterochronic context. Fifth, we’ve established that Transylvania was a special haven that existed in relative isolation from other terrestrial habitats at the end of the Cretaceous.
This understanding of the Transylvanian dinosaurs as not only once living, but also evolving creatures has provided us with the basis for exploring themes introduced earlier in this book: the paleogeographic relationships of the Cretaceous landforms of Europe, colonization and faunal balance in isolated environments, and the nature of body-size changes and life-history consequences. We are now at the logical place to add historical biogeography to this soup. We have given reasons for expecting that historical pathways should reflect the influence of chance events within the broad mediation of the laws of nature, rather than merely straightforwardly following the ever-present operation of these laws. Such unpredictability gives organisms the opportunity to circumvent the status quo—and the risks of extinction—and thereby expand their own evolutionary opportunities and innovations. Consequently, we will go out of our way in what follows to emphasize the theme of historical contingency, not only when interpreting the biogeographic history of the Transylvanian dinosaurs, but also as we integrate this information with their paleoecology, changing body sizes, life-history strategies, and phylogeny. Finally, we’ll let our Transylvanian dinosaurs wander on into the realm of evolutionary theory, in particular the role of chance in the regulation of diversity—the stuff of what is known as the Red Queen hypothesis.
Like organisms everywhere, the Transylvanian dinosaurs were the product of their individual histories. Obviously, one aspect of these histories is their arrival in the region of what is now western Romania. Nopcsa’s approach to where these dinosaurs (and the remainder of the Transylva-nian fauna) originated was to look solely within Europe. For example, he compared his Transylvanian hadrosaurid with Iguanodon from the rich faunas of the Early Cretaceous Wealden faunas of England, Belgium, and France,11 and why not—both Telmatosaurus and Iguanodon, although separated by 50 million years, were European members of Or-nithopoda. Likewise, Nopcsa directly compared the other members of the Transylvanian fauna with their European relatives from the Early Cretaceous as he attempted to understand how his peculiar dinosaurs arose. We agree that Nopcsa’s was a good and logical beginning, but why restrict ourselves to Europe? Why not expand the comparisons to all ornithopods throughout the world and evaluate their areas of origin using their phylogenetic relationships?
In order to build a global biogeographic history of the clades containing our Transylvanian dinosaurs, we will need to know not only about Iguanodon, Hypsilophodon, Hylaeosaurus, and Pelorosaurus from a Europe of earlier times, but a great many more dinosaurs from elsewhere. Now we’ve got to deal with the likes of Probactrosaurus, Gobisaurus, and Opisthocoelicaudia from Asia; Anabisetia, Gasparinisaura, and Saltasaurus from South America; Muttaburrasaurus, Leaellynasaura, Minmi, and Austrosaurus from Australia; Ouranosaurus, Valdosaurus, and Malawisaurus from Africa; and Tenontosaurus, Thescelosaurus, Pawpawsaurus, and Alamosaurus from North America. Bringing these additional taxa into our investigation, however desirable, should also give us pause, because of the uncertainties arising from the effects of patchy geographic coverage (now increased to worldwide scales) and additional aspects of stratigraphic incompleteness. What we don’t know is still more than we know, so any attempt to infer biogeographic patterns may be condemned to failure before we even begin. Nevertheless, we don’t intend to give up here without making an effort to reconstruct a history based on what we do know. In so doing, we turn again to the phylogenetic relationships of our Transylvanian dinosaurs, this time combining cladis-tic analysis with the global geographic occurrences of the groups within which they fall. This can be done by combining geographic data with phylogeny. If this approach sounds familiar, it should—we are again going to overlay a posteriori optimization of the geographic occurrences of the Transylvania taxa and their close relatives onto their phylogeny in order to reconstruct their biogeographic history (chapter 5). In this search for the source areas where particular dinosaurs evolved, we will rely not only on the paleogeographic reconstructions of this part of Europe through the Cretaceous (chapter 4), but also on any other landforms brought into consideration by the relationships of the taxa involved. This certainly means we will also have to take into account the paleogeo-graphic conditions and proximity of parts of Asia, South America, Africa, and Australia.
Before we begin looking at the biogeographic history of Transylvania from a worldwide perspective, a word of caution is necessary. Inferences about global biogeographic histories can only be as good as the fossil record will allow, and the biases inherent in this record should be admitted from the start. Ours has to do with the skewed geographic sampling of dinosaurs throughout the world. For example, we know that North America, Asia, and Europe each contain about 28% of the number of world-wide locations during the Early Cretaceous, whereas the remaining continents—South America, Africa, and Australia—each contribute an average of 5% (not surprisingly, Antarctica has thus far contributed nothing). In the Late Cretaceous, North America dominates at 31%, followed by Asia at 24%, Europe at 18%, and South America at 13%. Africa, Australia, and Antarctica together total a measly 6%.12 Even though our knowledge of dinosaur distribution around the world is always on the rise (as witnessed by the abundance of media accounts), it is also dominated by the big three—North America, Europe, and Asia. Consequently, any search for the area of origin for any of the Transylvanian dinosaurs and their immediate clade is biased in favor of the big three (which constitute the supercontinent of Laurasia) and away from the Gondwanan landmasses, simply because of the number of fossil locations available to us at this point, rather than being based on real biogeo-graphic history. For now, there’s nothing we can do about this problem.
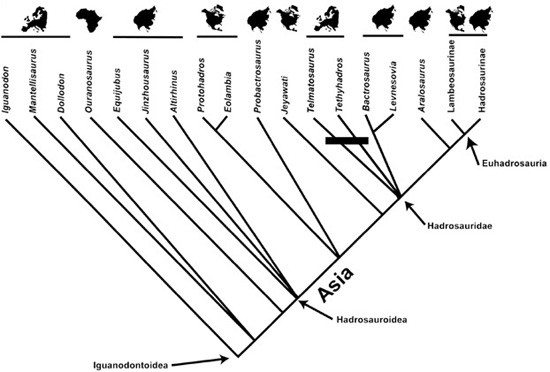
With this cautionary aspect to inferring biogeographic history in mind, we turn first to Telmatosaurus transsylvanicus. Nopcsa originally thought this dinosaur lay at the base of Hadrosauridae, a position that has been confirmed over the ensuing years, both through the discovery of many new hadrosaurids and other iguanodontians, and by the application of cladistic methods to understand the general shape of the group’s family tree (chapter 2). We’ve already used this information, plus strati-graphic data, to identify hadrosaurid ghost lineages and their duration while examining rates of character change (chapter 6).13 What might this approach additionally tell us about the source area for the lineage leading to Telmatosaurus and its arrival in what is now Transylvania?
The closest relatives of Telmatosaurus—among them, euhadrosau-rians, Bactrosaurus, and Levnesovia (chapter 2)—probably originated in Asia at some time during the latest Early Cretaceous (roughly 103 million years ago).14 When all of these locations are plotted on a cladogram (figure 7.1), it is unclear where the source area of Hadrosauridae was. It could have been either Asia or North America; many present-day studies support an Asian origin for Hadrosauridae.15 Telmatosaurus and Tethys-hadros (or their ancestor) can therefore be interpreted as having dispersed on their own to Europe. Similarly, forms such as Jeyawati and the small clade of Eolambia caroljonesa and Protohadros byrdi,16 the former from the early Late Cretaceous and the latter two from the earliest Late Cretaceous, represent independent migrations to North America from Asia.
We’d like to conduct a little thought experiment concerning the area of origin for hadrosaurids. In doing so, we will push the biogeographic distribution data as far as they allow, hoping that what they indicate about the area of origin for individual clades will not be outweighed by information about them not yet known to us, because it is hidden in their ghost lineages. If that is the case, we can then only look to future discoveries that sometimes, despite the odds, produce better phylogenetic resolution for whatever questions are under consideration (here, biogeogra-phy). Expanding the data envelope involves our attempt to resolve the previously unresolved relationships of Telmatosaurus, Tethyshadros, the clade of Bactrosaurus and Levnesovia, and Euhadrosauria, and looking to Europe for other close relatives.
Figure 7.1. A simplified cladogram of higher ornithopods that also includes geographic information, indicating Asia (or possibly North America) as the area of origin for Hadrosauridae and the dispersal of Telmatosaurus to Europe, as shown by the bar
Tethyshadros insularis: Only a few years ago, the prospect of Europe being the source area of any dinosaur clade was poor to nonexistent. Nevertheless, in the vicinity of Villaggio del Pescatore (along the Gulf of Trieste in northeastern Italy, near its border with Slovenia), a few fragmentary dinosaur bones were discovered in the 1980s, found in the eroding walls in an old abandoned limestone pit.17 Excavation of the site began in 1992, and by the end of the century this quarry, whose limestones date to the late Santonian (approximately 84 million years ago), had yielded a nearly complete skeleton (figure 7.2a) and the remains of three other individuals, all pointing to a small (4 m long) iguanodontian. These wonderful specimens were studied in detail in 2009 by the Italian paleontologist Fabio Dalla Vecchia,18 who noted that this dinosaur has many features uniting it with Hadrosauridae,19 lacks critical characters that unite Euhadrosauria, and has several features of the skull, dentition, hand, and pelvis indicating that it is not Telmatosaurus.
Figure 7.2. (a) Tethyshadros insularis; (b) a maxilla and dentary referred to Pararhabdodon isonensis; and (c) a dentary of the Fontllonga hadrosaurid. Scale = 100 cm (a), 5 cm (b, c). ([a] after Dal Sasso 2003; [b] after Casanovas et al. 1999a; [c] after Casanovas et al. 1999b)
Pararhabdodon isonensis: The wooded mountains of northeastern Spain, which rise to their greatest height as the Pyrenees, shelter the region of Catalonia, the original home of Salvador Dalí. There, in central Lleida Province, halfway between Barcelona on the Mediterranean and the small, landlocked country of Andorra, is one of the most prolific areas for the discovery of dinosaur bones, footprints, and egg nests.20 Our particular interest in the dinosaurs from Lleida is focused on the village of Isona, for here, in 1985, the remains of P. isonensis were recovered from rocks of Maastrichtian age, like those of Transylvania.21 It was originally described by María Lourdes Casanovas-Cladellas and José Vicente Santafé-Llopis (Institut de Paleontologia “M. Crusafont,” Sabadell), and Albert Isidro-Llorens (Institut Guttman, Barcelona). Based on post-cranial elements, this new species was thought to be a close relative of an individual estimated to be about 6 m in length (figure 7.2b).22 Most recently, it has been reexamined by Albert Prieto-Márquez and Jonathan Wagner.23
Pararhabdodon clearly is a member of Hadrosauridae (the dentary, unfortunately preserved without teeth, indicates the presence of small teeth arranged in a dental battery, and the deltopectoral crest [not illustrated] is large and angular in profile). Additional material of Pararhabdodon has been described from the Maastrichtian in the Upper Aude Valley of southern France, some 150 km to the northeast, over the Pyrenees, from Isona. Both the Spanish and the French specimens indicate that Pararhabdodon is not Telmatosaurus or Tethyshadros. It is either a non-euhadrosaurian hadrosaurid24 or a very basal lambeosaurine.25
The Fontllonga jaw: A second specimen from Spain, thus far consisting of a small dentary (figure 7.2c), was also recently discovered in Lleida Province, in beds contemporary with those of Isona, but 25 km to the south. Known as the Fontllonga hadrosaurid,26 this form cannot be referred to either Telmatosaurus, Tethyshadros, or Pararhabdodon. Its small teeth, organized into a dental battery that continues all the way to the front of the jaws, bear an asymmetrically placed median ridge. These features indicate that the Fontllonga hadrosaurid fits between Telmato-saurus and Euhadrosauria.27
So, by our count, that’s three basal hadrosaurids and near-hadrosaurids from Romania, Italy, Spain, France, and European Russia. Given the tree topology resolving Telmatosaurus, Tethyshadros, an Asian clade (Lev-nesovia and Bactrosaurus), and Euhadrosauria, we now have before us the possibility that Europe was the source area for all of Hadrosauridae (figure 7.3). This interpretation, of course, depends on the resolution of these new European forms, but if the phylogenetic interpretation we’ve outlined here is more or less correct, it has the effect of shifting hadro-saurid origins in the Early Cretaceous from the broad coastal plains of Asia or North America in the Early Cretaceous to the much smaller, more isolated, and therefore much more evolutionarily volatile landmasses of Europe.
In order to examine the other dinosaurs from Transylvania in the same way, we need to travel to southern France, northern Spain, and westcentral Hungary. The region extending from the sprawling vineyards of Provence, along the Mediterranean coast in the east, to the green meadows and deep forests of the valley of the Garonne and the foothills of the Pyrenees, in the west, has produced one of the best Late Cretaceous records of dinosaurs in all of Europe (chapter 4).28 Numerous taxa—including possible dromaeosaurid and avialan theropods, nodo-saurids, ornithopods (Rhabdodon, Pararhabdodon, and thus-far indeterminate hadrosaurids), and titanosaurian sauropods—have been collected from the approximately 20 localities in this region. These faunas of southern France are presently under study by Jean Le Loeuff at the Musée des Dinosaures in Espéraza and Eric Buffetaut at the Laboratoire de Géologie, École Normale Supérieure, in Paris.
Figure 7.3. A simplified cladogram of higher ornithopods that includes a tentative placement of new hadrosauroid discoveries, indicating the area of origin of Hadrosauridae in Europe and the dispersal of remaining hadrosaurids to North America and Asia
In Spain, Laño has one of the most important terrestrial vertebrate assemblages to be discovered in recent years. Located 70 km south of the warm waters of the Bay of Biscay, near the city of Vitoria (and therefore between the European and Iberian tectonic plates), Laño is a small village in the Burgos region of the Basque Country (Euskal Herria in native Euskeran). This site, in an abandoned sand quarry, was discovered in the 1980s and worked by Humberto Astibia and his coworkers from the Euskal Herriko Unibertsitatea in Bilbao, as well as by numerous researchers from Spain and France since then.29 Over the past 20 years, some 40 species have been recognized, among them new fish, squamates, turtles, crocodilians, dinosaurs, and pterosaurs, all of which are slightly older (late Campanian) than the dinosaur fauna from Transylvania. Thus far, the Laño dinosaurs consist of the euornithopod Rhabdodon priscus, some poorly preserved hadrosaurids, indeterminate theropods, and Li-rainosaurus astibiae, one of the best-known sauropods from Spain.30
Finally, in Hungary, north of Lake Balaton, central Europe’s largest freshwater lake, this part of the forested Transdanubian highlands, known as the Bakony Mountains, were once home to surface bauxite mining. Iharkút is one of these places. Discovered in 2000 by Attila Ősi and András Torma, this site has now produced fish, amphibians, squamates, crocodilians, pterosaurs, and dinosaurs that are 10-15 million years older than the other Late Cretaceous faunas in Europe. Among the dinosaurs are thus-far indeterminate theropods; abundant material of a nodosaurid ankylosaur called Hungarosaurus tormai (figure 7.4a); teeth and a femur indicating a rhabdodontid that appears to be different from both Zalmoxes and Rhabdodon (figure 7.4b-e); and, most recently, Bagaceratops kozmai, a very important and unexpected neoceratop-sian. Conspicuous in their absence from Iharkút are sauropods and hadrosaurids.
We turn from these localities first to Zalmoxes, Rhabdodon, and the Iharkút rhabdodontid. Because all are known only from Europe and nowhere else, we draw the conclusion that this clade is endemic to southern Europe (more precisely, the central part of the northern Neotethyan region) no later than about 80 million years ago. Prior to that, the record of the iguanodontian clade, the closest relative to the clade of Zalmoxes and Rhabdodon, dates from the Late Jurassic (the age of Camptosaurus and Dryosaurus; probably near the boundary between the Kimmeridgian and Tithonian, approximately 151 million years ago) of western North America. These dinosaurs indicate that the geographic source area of the lineage leading to Zalmoxes, Rhabdodon, and the Iharkút rhabdodontid was North America (figure 7.5).31
Figure 7.4. (a) A left scapulocoracoid of Hungarosaurus tormai; (b) a tooth from a rhabdodontid dentary; (c) a rhabdodontid maxillary tooth; (d) a rhabdodontid maxillary tooth; and (e) a left rhabdodontid femur, in caudal view, all from the Late Cretaceous Iharkút fauna of Hungary. Scale = 5 cm (a), 10 mm (b), 3 mm (c); 6 cm (d); 5 cm (e). ([a] after Ősi 2004; [b-e] after Ősi et al. 2003)
Likewise, the lineage leading to Struthiosaurus, presently known from both Transylvania and Franco-Iberia, dispersed from North America to Europe no later than the Late Jurassic (figure 7.6). The three species of Struthiosaurus have, as their successive closest relatives, well-known nodosaurids from places such as Utah, Montana, Wyoming, and Texas in the United States. On the basis of this distribution, it’s a good bet that the common ancestor of Struthiosaurus hails from North America, and that its migration to Europe resulted in a modest, within-species endemic radiation in the Late Cretaceous. Hungarosaurus, the best-known member of the Hungarian Iharkút fauna,32 probably is a second migrant from North America to Europe. However, the two may constitute the product of a single dispersal to Europe; we just can’t tell at the moment.
Figure 7.5. A simplified cladogram for basal ornithopods that also includes geographic information, indicating North America as the area of origin of Ornithopoda, and the subsequent dispersal to Europe of the lineage leading to Zalmoxes and Rhabdodon
From its phylogenetic position and its geographic distribution, Mag-yarosaurus shared Late Cretaceous Europe with at least two other ti-tanosaurs, Lirainosaurus from Spain and Ampelosaurus from France (figure 7.7). Ampelosaurus, the best represented of these European ti-tanosaurs, is now known from abundant isolated material pertaining to nearly the entire skeleton and, more recently, from an articulated skele-ton.33 Lirainosaurus is nearly as well known, from the majority of a single skeleton. For a long time, titanosaurs were thought to originate somewhere in the southern continents (Gondwana), but recent phylo-genetic studies suggest that the jury is out on their place of origin.34 Nevertheless, Magyarosaurus, Ampelosaurus, and Lirainosaurus seem to be the result of speciation within Europe (figure 7.8). In addition to this European source area, there are two migrations: one to South America, involving Rocasaurus and Saltasaurus, and the other, for Malawi-saurus and Rapetosaurus, to Africa.
Figure 7.6. A simplified cladogram for the nodosaurid ankylosaurs that also includes the dispersal of Struthiosaurus and Hungarosaurus to Europe, as indicated by the bar
Other than the dinosaurs, biogeographic affinities can be worked out for several other Transylvanian taxa, including Allodaposuchus, Kalloki-botion, and kogaionids. Based on its phylogenetic position,35 Allodapo-suchus appears to have descended from a Euramerican common ancestor shared with Hylaeochampsa, another basal eusuchian from the Early Cretaceous of England. This relationship, and their stratigraphic occurrences, indicate a long ghost lineage (55-60 million years) for Alloda-posuchus. Furthermore, its distribution appears to be the result of insular dispersal combined with the Euramerican breakup. What is more significant is the possibility that, with Allodaposuchus, Hylaeochampsa, and Iharkutosuchus (a eusuchian from the Late Cretaceous of Hungary)36 all having a European distribution, crown-group crocodilians may also have dispersed from Europe to North America sometime during the Early Cretaceous.37 The phylogenetic position of the Transylva-nian turtle Kallokibotion is somewhat controversial. In some studies, it has been placed as a basal cryptodiran most closely related to Tretoster-non.38 The lineage leading to Kallokibotion therefore must have had its origin by the earliest Cretaceous (the oldest occurrence of the Tretoster-non clade). Because it represents the Late Cretaceous terminus of an old phylogenetic lineage, Kallokibotion, with a 70 million year ghost lineage, constitutes an endemic relict in Europe, due to the breakup of Euramerica. Other analyses have situated Kallokibotion outside crown-group Testudines.39 This more basal position does not alter the hypothesis of European endemicity. However, shifting its position more basally within Testudinata drastically increases the ghost lineage duration leading to Kallokibotion (by about 20-25 million years, from the Middle Jurassic to the Maastrichtian). Finally, kogaionid multituberculates are known solely from the Late Cretaceous of Transylvania and the Paleocene of France, Spain, Belgium, and Romania.40 Optimization of the distribution patterns reveals that this clade of mammals had a European origin, stemming from a broader Euramerican distribution, sometime during the Early Cretaceous.41
Figure 7.7. (a) A dorsal vertebra of Lirainosaurus astibiae from the Late Cretaceous of Spain, in right lateral view; and (b) a dorsal vertebra of Ampelosaurus atacis from the Late Cretaceous of France, in right lateral view. Scale = 5 cm. ([a] after Sanz et al. 1999; [b] after Le Loeuff 1995)
Figure 7.8. A simplified cladogram for basal ornithopods that also includes geographic information, indicating the dispersal of Magyarosaurus, Lirainosaurus, and Ampelosaurus to Europe
Other Late Cretaceous localities in Europe lend themselves to similar interpretations for the other Transylvanian dinosaurs. Do these Spanish, French, and Hungarian faunas support, or perhaps alter, our earlier biogeographic interpretations for other Transylvanian dinosaurs, such as in the case of Telmatosaurus? We can’t say. We know far less about the biogeographic dynamics of the Transylvanian theropods, because we know next to nothing about the details of their respective phylogenetic relationships.42 How they will turn out, we don’t yet know; only future discoveries and analyses will tell.
Clearly, we have a long way to go to grasp the full biogeographic dynamics of the dinosaurs from Transylvania and elsewhere in Europe. Yet, from what can be discerned so far, there is growing evidence that the biogeographic history of the Transylvanian taxa is more complex than meets the eye, and certainly much more so than Nopcsa had imagined. Here’s what our present data tell us. Two lineages endemic to Europe—one leading to Struthiosaurus and the other to Rhabdodon-tidae—diversified into small clades there, one recognized at the species level (within Struthiosaurus) and the other at the generic level (within Rhabdodontidae). They all became extinct in Europe slightly before or at the end of the Cretaceous, without giving rise to new taxa. They were the headstones marking their respective clades, never to tread elsewhere in the world.
For the titanosaur clade of Magyarosaurus, Rapetosaurus, Malawi-saurus, and Nemegtosaurus, only the first-named remained in Europe, whereas the second two migrated to Africa, and the last-named to Asia. Thus Europe acted as a partial venue of diversification, with Magyarosaurus, the last titanosaur from this part of the world, becoming extinct just before the end of the Cretaceous.
If our resolution of the cladogram is correct, then Telmatosaurus stands apart from these Transylvanian dinosaurian dead ends. First, this individuality was the product of a single ancestral migration to Europe by a North American or Asian iguanodontian. Second, this ancient invader likely gave rise to a modest hadrosaurid diversification, in order to account for their ultimate Transylvanian, Italian, and Franco-Iberian distribution in Europe. However, according to their phylogeny, the legacy of this European nexus of primitive hadrosaurids also spilled back into North America and into Asia. The great radiation of euhadrosau-rians came with their migration out of Europe, with important consequences for their anatomy, development, and evolutionary dynamics, which we will examine below.
In summary, the common ancestors of the immediate clades of Zal-moxes (with Rhabdodon) and Struthiosaurus (with Hungarosaurus?) migrated to Europe from North America, Telmatosaurus dispersed outward from Asia or North America, and Magyarosaurus differentiated itself from other European titanosaurs, all probably in the Early Cretaceous or Late Jurassic (chapter 4). If so, then each colonization took place as continental configurations were becoming ever more complex. Beginning in the Early Jurassic, northwestern Africa and eastern North America had drifted apart to form the beginnings of the North Atlantic Ocean, but this separation occurred at what today would be the eastern seaboard of the United States to the west and Morocco and the adjacent coastline to the east. The northernmost part of this proto-Atlantic Ocean was only just beginning to open, whereas the southern part, between Africa and South America, would not open until several million years later. Thus intermittent land connections must have existed before and into the Early Cretaceous (about 100 million years ago)—between what is now Labrador in northeastern Canada, Greenland in the middle, and Europe to the east—through a dense array of large islands. Thereafter, the terrestrial habitats associated with these landmasses came and went as the tectonics of the region changed and the sea level fluctuated. At the same time, possible dinosaurian dispersal from Asia was also limited, by sporadic land bridges across the Polish Trough (a large but intermittent seaway running southeast from the present Baltic Sea and across Ukraine), and by the West Siberian Sea and Turgai Straits (extending north of the present-day Caspian Sea to the paleo-Arctic region),43 both of which existed from the Middle Jurassic to the Oligocene.44 To the south was a string of islands of various sizes that stretched from what is now central Asia to Anatolia.
The time periods appear to fit the hypothesis, so our conjecture that the initial introduction of the ancestors of Telmatosaurus, Zalmoxes, and many of the rest of the Transylvanian dinosaurs from North America or Asia occurred sometime in the Late Jurassic through the Early Cretaceous seems to be warranted, at least based on current evidence. But why was it these taxa and not a different set of migrants? Where are diplodocid sauropods, allosaurid and therizinosauroid theropods, and stegosaurs, all of which were present in North America and Asia over this stretch of time? Why the gamut of dead ends and success stories for the Transylvanian dinosaurs themselves? These questions are easy to ponder, but hard to answer. Is there any reason why the ancestors of the Transylvanian dinosaurs should have been more likely to leave North America or Asia to end up in Europe than any of those left behind? Why should the ancestry of Telmatosaurus also have spawned the great eu-hadrosaurian radiation elsewhere in the world, but that of Struthiosau-rus, Zalmoxes, and Magyarosaurus remained so barren and restricted? We’re hard pressed to explain these patterns (either individually or collectively) as the results of some common property that enhanced their ability to migrate or colonize, a characteristic that the “left-behinds” wouldn’t have possessed. The travelers certainly don’t appear to have been more honed by natural selection for long-distance, multienviron-mental journeys—which would have increased the probability of colonization—than the other dinosaurs of those times. Instead, who stayed and who went is unpredictable, reflecting historical contingency. Only by chance, and not by choice, did the progenitors of Telmatosaurus, Zal-moxes, and the rest manage to enter the mosaic of microcontinental movement and of submerging and emerging landmasses that is now Europe. Being in the right place at the right time was no more than luck.
In this geographic and ecological context, we now return to our miniaturized Transylvanian dinosaurs. For these heterochronic dwarfs, small things (literally and figuratively) can lead to big things in unpredictable ways. A miniaturized dinosaur, free of the constraints imposed by a large body size, has more versatility to adapt to a changing environment by means of evolutionary innovations.45 Using several examples from Transylvania, we will examine how, when, and why particular features of these dinosaurs were either predictable or the random consequences of their changing statures, as well as consider how they may fit into the evolutionary history of their respective clades.
As we saw in chapter 5, Telmatosaurus, Magyarosaurus, and possibly Struthiosaurus were identified as being dwarfed, downsized from the primitive condition of their respective clades. In addition, Zalmoxes robustus (but not Z. shqiperorum) may have been dwarfed. However, the Transylvanian theropods are roughly the same size as elsewhere; the same applies to the region’s crocodilians, turtles, and mammals. We also noted the possibility of dwarfing in some of the Transylvanian pterosaurs, but certainly not in Hatzegopteryx, one of the largest among the large azhdarchid pterosaurs.
By integrating this cast of characters with their evolutionary relationships, and with their geographic and stratigraphic distributions, we can now begin to explore the biogeographic dynamics by which they achieved their dwarfed status. Sometime in the Early Cretaceous (or earlier), the ancestor of each of the Transylvanian lineages crossed the northern realm between North America or Asia and the mosaic of large and small landmasses that formed what is now Europe. We suspect that these migrants were no smaller than their closest stay-at-home relatives—since fasting endurance is proportional to body size,46 only large individuals would have been able to travel long distances without risking starvation, famine-induced disease, or susceptibility to predators.
Some of these migrants colonized the isolated, rapidly changing patches of terrestrial habitats in Europe. This region—isolated as it was from the grand coastal plains from which they came—was a place of serendipity, the product of the chance arrivals of various plants and animals. What were once more-or-less stable, evolutionarily well-honed ecosystems in North America and Asia appear to have been stitched together more by chance than by design as they developed in Europe. One hallmark of the kind of isolated habitats we envision here is the taxo-nomic and trophic imbalance of the European faunas.
Things now are starting to get really interesting, once each migrant had become established in these new habitats. Whereas those who traveled to Europe seem to be a random sample from their ancestral area (although they needed to be large enough in body size initially), selection appears to have favored downsizing. Very quickly, being large was hardly as advantageous as it had been before and during the journey to Europe. Many attempts have been made to account for dwarfing in isolated organisms. For example, large body size requires lots of resources—which were relatively plentiful in the now-forfeited coastal plains of Asia and North America, but perhaps critically rare in the new homeland. From this metabolic perspective, it would no longer pay to be big. Instead, it is reasonable to expect that the size reduction in these animals, living in restricted European habitats, was due to selection for small body size, based on resource limitations.47
From an ecosystem perspective, downsizing could also have been related to a release from predator pressure that comes from the chance jumbling up of colonizing predators and prey (chapter 5). The randomness of which predators arrived and evolved in Transylvania and elsewhere in Europe and which ones did not would have unpredictably altered the predator-prey relationships in these new regions. Selection for large body size as an antipredatory device, which may have existed in Asia and North America, would have been out of whack in Europe. It has been argued that with a loss of or decline in body-size selection, the distribution of body sizes in prey may evolve into an equilibrium that is not mediated by predator pressure—large prey get smaller and small prey become larger when they’re not using size to escape from their usual predators.48
Even though both of these ecological explanations of dwarfing are good enough by themselves, we argued in chapter 5 for the Roth model of downsizing: dwarfing was the product of selection based on life histories in an isolated setting.49 In this model, only a few individuals ever disperse to any isolated region. The resulting population bottleneck entails an immediate loss of genetic variation in the organisms colonizing the newly invaded environments. This small population is usually doomed to extinction through the consequences of genetic drift. However, in order to reduce the probability of extinction, a premium is placed on the invaders to increase their population size as rapidly as possible. Options on how this can be achieved are limited, but selection for early (precocial) sexual maturation—one aspect of what is called r selection—may be the colonizers’ best chance for success.50 By moving up the timing of its sexual maturity, an organism will be in a better position to increase its presence in the environment more quickly than its slower-developing ancestors and competitors. While this is applicable for whatever the quality of the colonized environment, it is even more effective in increasing survival rates in an ecologically unstable environment.51 In addition, precocial species are also better at exploiting patchy resources, and they disperse across their new habitats earlier than their more slowly developing competitors do.
Under this life-history approach, r selection, in the context of colonizing isolated regions, acts primarily on accelerating sexual maturity—a good colonizer is one that can quickly return to a healthy population size after the migrational bottleneck. Interpreted this way, dwarfing is not the direct consequence of selection, but instead is a tagalong, a predictable consequence of selection for those life-history parameters that relate to colonizing abilities.52
For our Transylvanian dinosaurs, early sexual maturity certainly accounts for the evolution of their small body sizes, especially in the context of their arrival in Europe. By means of their early maturation, they would have been better off than their larger ancestors during this time of population bottlenecking, simply by being able to recover better and increase more quickly in population size. These factors probably created a feedback loop with the other features that favor downsizing, such as limited space and resources and new predator-prey relationships. Taken together, the resulting morphology is an animal smaller than its ancestors.
How long it may have taken for this dwarfing to occur is hard to reconstruct from the fossil record. In other vertebrates, for instance in the dwarfed hippos of Crete, it is thought that dwarfing was achieved within a millennium, or even shorter periods of time.53 We don’t know if this time frame also applied to the Transylvanian taxa, due to our lack of information (concealed by their long ghost lineages). Nevertheless, we can reasonably conjecture that the isolation of these dwarfed populations, over whatever interval of time, not only reduced their genetic variability (by reducing the influx of expected variation, due to their migration), but also probably accelerated the divergence of the dwarfing populations from their mainland stocks.
Living as dwarfs in isolation may not have been all it’s cracked up to be, at least in terms of evolutionary legacy. For all but one of the Transylvanian dinosaurs, we are looking at the final pulse of their immediate clades. The likes of Zalmoxes and Struthiosaurus, in their isolated outpost within Europe, died out at the end of the Cretaceous without giving rise to other taxa. Magyarosaurus was part of only a small evolutionary radiation. Only in Telmatosaurus can we identify a link between dwarfed isolates and their subsequent migration and high levels of diversification. If we want to examine the great radiation of euhadrosaurian species in North America and Asia from non-hadrosaurid iguanodontians, we’ve got to go through Telmatosaurus and its European allies. In doing so, we hope to explain how this European occurrence of basal hadro-saurids is an important, but historically contingent factor in the rise of Euhadrosauria.
The construction of the hadrosaurid dentition is distinct from that of more primitive iguanodontians such as Tenontosaurus, Dryosaurus, Camptosaurus, and Iguanodon, so much so that it has been considered a hallmark for Hadrosauridae (chapters 2 and 5). Instead of having a dentition composed of a single large functional tooth and one replacement tooth per tooth position, hadrosaurids had hundreds of small functional and replacement teeth in each jaw, interlocked to form a complex dental battery that is ornate by anyone’s criterion (figure 2.21). Each tooth was composed of various kinds of dentine tissue and contained much less of the harder and more resistant enamel; yet, when worn down, this dentition provided a long, roughened surface to grind up tough plants, a dentition more effective at breaking down fibrous leaves than the teeth present in their ancestors. Teeth were continually replaced, such that this chewing surface was always present throughout the animal’s life. When the teeth in both the upper and lower jaws were worn, the opposing occlusal surfaces formed a complex arrangement of enamel and dentine, wonderfully designed to grate and rasp their way through the toughest plant material in ways not available to hadrosaurid precursors.
This dental battery certainly was a remarkable trophic invention, one that improved hadrosaurids’ chewing efficiency over the more primitive condition of mastication occurring among ornithopods. Surely it qualifies as a true adaptation—that is, an organismal feature whose origin and maintenance was the product of natural selection.54 Viewed in terms of the Cretaceous rise and diversification of flowering plants, a dental battery makes sense from such a Darwinian perspective: new kinds of plants provide new challenges (i.e., ecological problems and opportunities) for contemporary herbivores, which then select for those variants among iguanodontians that have an incipient dental battery (i.e., biotic solutions to these problems and opportunities). Using these arguments, it is always tempting to take an adaptationist perspective, rendering the rise of the hadrosaurids in terms of their coadaptation or coevolution with angiosperms.55
However attractive this inference is, we’re not particularly persuaded by this adaptationist explanation, whereby the evolution of the hadro-saurid dental battery was directly mediated by natural selection. We think it appropriate to look first at alternative explanations, using all the tools, perspectives, and information we now have at hand. In particular, that means an examination of dwarfing and migration to the isolated regions of Europe.
Dwarfing within the lineage of iguanodontians that gave us Telmato-saurus and the other basal hadrosaurids of Europe brought about the possibility of adding a few more teeth in the tooth row and another replacement tooth per position—the beginnings of an incipient dental battery. With this more complex dentition, made up of small teeth placed in small jaws, it is possible that these dwarfed hadrosaurids altered what they fed upon. As has been hypothesized for dwarfed, island-dwelling elephants, the Transylvanian dwarfs are likely to have experienced a decrease in their total metabolic requirements, coupled with a reliance on higher-quality food, assumptions based on their reduced body sizes and decreased population size.56 Whether the European ancestors of Telmatosaurus subsisted on a diet higher in nutrient-rich seeds and fruits than their larger North American and Asian relatives is unknown, but this may be anticipated, since dietary selectivity in many living animals is known to increase with smaller body size.57
Whatever the diet of these miniaturized hadrosaurids, their downsized stature—a consequence of early maturation—is likely to have promoted their success in the more restricted terrestrial habitats of Europe. Nonetheless, according to their phylogenetic history, the descendants of these animals migrated from Europe (most likely first to North America, but an Asian migration is also a contender here), going from a suite of environments that promoted small size back to a region of great coastal-plain openness. As far as we can tell, this out-of-Europe migration happened once, to produce what we now call euhadrosaurians (chapter 2).58 Once they were “back home,” their small size could not have been as advantageous as it had been in their restricted European habitats. These dwarf descendants would have again faced a great array of small and large predators (dromaeosaurids, tyrannosaurids) already present in North America and Asia. It’s possible that through the kinds of predator pressure their more distant ancestors experienced, they regrew to their previous larger sizes as a way to defend against predation. Whatever the case, these euhadrosaurians once more reached 10 m or more in length.
As far as we can tell, this size-increase phase equally affected just about all of their body proportions (as exemplified by Telmatosaurus). All, that is, but their dentition (chapter 4). Rather than scaling up to a bigger size (like the rest of the body), the teeth remained small, not much larger than those of hatchlings and youngsters. In other words, euhadro-saurians retained their dwarfed, juvenilelike dentitions into adulthood. In this way, hadrosaurids appear to have decoupled the increase in their overall body size from their dentition. Mediated by their European dwarfing phase, the teeth of these North American and Asian hadrosaurids, as they rebounded to a larger body size, paedomorphically remained small. Simply add more of these miniaturized teeth (i.e., baby teeth) along the length of their jaws and as replacement teeth, and voilá, the hallmark dental battery for which Euhadrosauria is famous had been created.
Having a dental battery must have counted for something among all hadrosaurids. It is retained in all of the nearly 50 species of euhadrosau-rians presently known worldwide, never deviating in its fundamental construction—small teeth, with three or more teeth per tooth position. Once formed, this dental battery became one of the most stable of all euhadrosaurian anatomical systems. From an engineering perspective, this dentition appears to have been a great invention, permitting the complex mastication of plant material. Once this structural complexity, and therefore better chewing efficiency, was achieved, natural selection would have acted to maintain the hadrosaurid dental battery. Only in this sense can the stability of the hadrosaurid dental battery be considered an adaptation, and we certainly wouldn’t be surprised if this was the case.59 However, the origin of dental batteries cannot be judged from the same perspective. This evolutionary innovation arose not as the primary object of selection, but instead as a serendipitous creation. Hadrosaurid dental batteries came about from the transformation of the dentition of the numerous, more basal iguanodontians to that of Telmatosaurus and other hadrosaurids, not for their properties related to chewing, but through the sequellae of paedomorphic dwarfing.
This notion of contingency in the evolution of hadrosaurid teeth may have its mirror in what we know about hadrosaurid reproductive biology. From work by Jack Horner of the Museum of the Rockies in Montana, we know that hadrosaurids such as Maiasaura and Hypacrosaurus— much larger forms than Telmatosaurus—laid large clutches of relatively small spherical eggs (between 16 and 22 eggs in a clutch, with the egg volume ranging from 900 to 4,100 cm3) and cared for the small (approximately 1% of adult body mass), immature hatchlings for some length of time until the juveniles left the nest (figure 7.9).60 Parental care of immature offspring is usually selected for in populations at or near the carrying capacity of the environment, what is known as the K life-history strategy. The logical opposite of r strategy that we previously discussed, K strategy is to be expected in animals of large adult body size inhabiting stable environments of the kind envisioned in western North America and Asia during the Late Cretaceous.61 K selection is also associated with small clutch size and parental care. In this way, the parents are able to invest a great deal of their energy promoting the survival of a few offspring. Humans, for example, are regarded as K strategists. However, a large clutch size is more consistent with an r strategy, in which the parents invest in abundant offspring, but do not care for them. Among vertebrates, sea turtles are an excellent example of this strategy. They lay plenty of eggs in the sand, but care for them not at all. After climbing out of their eggs, the hatchlings must fend for themselves.
Figure 7.9. A lambeosaurine hadrosaurid nest containing 18 eggs, from the Two Medicine Formation (Upper Cretaceous) of western Montana. Scale = 10 cm. (After Horner and Dobb 1997)
Hadrosaurids exhibit both r and K strategies in their reproductive biology. Is this a paradox, requiring an explanation in which natural selection is driving life-history traits in polar-opposite directions? Not necessarily, if we interpret these reproductive features along the lines of our schema of a European dispersal and dwarfing at the base of the hadrosaurid family tree. Recall that early maturation and its consequent reduction in body size make for good colonizers. In addition, selection on individuals in populations well below the carrying capacity of their environments favors the early and rapid production of large numbers of young. This aspect of r strategies makes good sense in such isolated terrestrial realms as Transylvania in the Cretaceous. Large clutch size is likely to have evolved in the context of colonizing the unpredictable, often severely fluctuating Neotethyan terrestrial habitats. The large clutch size of small hatchlings that we see in euhadrosaurians would then be primitively inherited from the isolated origin of the group, an evolutionary holdover from more stressful times. The K strategies (stable habitat, large body size, parental care) would then have evolved after hadrosaurids left Europe for North America and Asia. Now known as euhadrosaurians, these creatures evidently maintained their earlier r-selected reproductive strategies in much the same way as they ended up with their miniaturized dentitions.
In sum, unpredictability abounds in the details of what we’ve presented here. If we are correct about how the downsizing of the Transylva-nian dinosaurs was accomplished, then it would have been impossible to predict from their North American or Asian ancestors which taxa would make the journey and, once there, which ones would have been successful in the restricted environments of Europe. These colonizers then became dwarfed, not so much from a direct selection for body size, but instead as the consequence of selection for early sexual maturity.
Populations eking out a living in isolation, even with their odd genetic sampling and high levels of speciation, usually end up as evolutionary dead ends. This may have been true for the Transylvanian dinosaurs—Europe was their last stop. However, Telmatosaurus and its closest European relatives were the progenitors of the euhadrosaurians on the larger adjacent continental landmasses. Their reintroduction into Asia and North America (the postdwarf stage) has its own contingency overprint, with the same iffy-ness of dispersal and success or failure in these descendant regions. In this group, the effects of isolation on life histories indirectly produced evolutionary novelties, such as the hadrosaurid dental battery. These features were then bequeathed to descendant species that evolved into a diverse array of larger animals. In other words, the period of r selection experienced by the basal taxa of Hadrosauridae, which was related to their early sexual maturity in an uncrowded insular habitat, supplied a pool of dwarfed morphology that could be used as the raw material for the subsequent radiation of these herbivores. If true, then here we encounter one of the few examples of isolated populations successfully recolonizing, radiating, and dominating the biota in a broad continental setting.62
Our ability to identify serendipitous historical conjunctions—changes in features, resulting from a different interactive context—provides us with the means to suggest alternatives to explanations that involve immediate selection for biological features. Such momentary shifts in context can have huge consequences, some fatal, others with no effect at all, and still others that are spectacularly successful. Whatever the case, these shifts and their consequences cannot be predetermined, or even be strictly predictable; they can only be understood in a historical sense.63