
Being a Short Interlude on Coelacanths
and Transylvanian Ornithopods
For evolutionary biologists of all sorts, 23 December 1938 was a very important day. It was then that a trawler called the Nerine put into port at the town of East London, located about 850 km to the east of Cape Town, South Africa. The skipper, Captain Hendrick Goosen, made a living fishing the nearby coastal waters of the Indian Ocean. Having made friends with Marjorie Courtenay-Latimer, the curator of a small local museum, he would often have the dockman call Courtenay-Latimer to come look over the Nerine’s catch and to take any unusual specimens she wanted for her museum. On this particular day, the Nerine entered port after trawling off the mouth of the nearby Chalumna River. When the dockman called Courtenay-Latimer, she took a taxi to the ship, delivered her Christmas greetings to the captain and crew, and went through that day’s catch for anything unusual. There, beneath a pile of rays and sharks on the deck, was, as she put it, “the most beautiful fish I had ever seen, five feet long, and a pale mauve blue with iridescent silver markings.”1 Although she had no idea what the fish was, she did know that it had to go back to the East London Museum at once. After convincing the taxi driver to allow the reeking, 5 ft fish to accompany her, he drove her and the specimen back to the museum.
Back in her office, Courtenay-Latimer tried to identify the bizarre creature (figure 6.1) by combing through the few reference books in her library. A picture of a long-extinct fish bore the greatest resemblance, particularly in the structure of the head and the trilobed shape of the tail. She made a crude sketch of her discovery and sent the drawing and a short description to Professor J. L. B. Smith, a chemistry professor at Rhodes University in nearby Grahamstown, who also was locally well known as an amateur ichthyologist. Unfortunately, Smith was away for the Christmas holidays and the consensus back home was going against her increasingly odiferous fish—the director of the East London Museum dismissed it as a common rock cod. In an effort to preserve the fish by mounting it, much of the viscera had been discarded and then lost, and, to the great disappointment of all concerned, even the photographs taken of the preparation were spoiled.
When Smith finally visited the East London museum on 16 February, he immediately identified the fish as a coelacanth, a group of fish thought to have gone extinct toward the end of the Cretaceous, some 80 million years ago. Called the “most important zoological find of the century,” this discovery made Smith and Courtenay-Latimer overnight celebrities. In 1939, the fish was named Latimeria chalumnae (after Courtenay-Latimer and the river near where it had been collected) by Smith.
The saga of the coelacanth continues. On 21 December 1952, Captain Eric Hunt, a British sailor operating in the waters off the Comorean island of Anjouan, was approached by two islanders carrying a hefty bundle. One of them, Ahamadi Abdallah, had caught a heavy, grouperlike fish by hand line, while the other—a schoolteacher named Affane Mohamed—thought the fish might be the fabled coelacanth. Hunt had the fish salted on the spot, then had it injected with formalin to preserve its internal organs, and cabled J. L. B. Smith in South Africa.
After some delay, which was laced with confusion and frustration, Smith managed to reach the Comoros and, when he saw the dead fish, he is said to have wept. It was indeed a coelacanth, this time with its organs intact and captured most probably near the creature’s actual habitat.
Thereafter, quite a number of coelacanths have been caught for study, first by French researchers and, after the Comoros Islands became independent in the 1970s, by various international groups of scientists. In addition, Latimeria has been studied in its natural habitat by direct observations and by videos obtained by submersibles diving in the waters off the Comoros Islands.2
A most remarkable chapter in the history of the study of modern coelacanths began on 30 July 1998. On this date, a population of these lobe-finned fish was discovered by American and Indonesian scientists about 10,000 km east of the Comoros Islands, off the coast of Sulawesi Island in Indonesia. When this coelacanth was first discovered, the only obvious difference between it and Latimeria chalumnae from the Comoros Islands was its color. The Comoros coelacanths are renowned for their steel blue color, whereas specimens from the Sulawesi population are brown. Described as a new species, Latimeria menadoensis, in 1999 by a host of Indonesians scientists and one Frenchman—L. Pouyaud, S. Wirjoatmodjo, I. Rachmatika, A. Tjakrawidjaja, R. Hadiaty, and W. Hadie—this discovery identifies living coelacanths as more widespread and abundant than previously assumed. Furthermore, it opens an incredible range of ecological, biogeographical, and evolutionary questions associated with these exceptional, albeit still very rare, living fossils.
Figure 6.1. The icon of living fossils, Latimeria chalumnae
Latimeria has dual significance in evolutionary biology. First, in the 1930s, extinct coelacanths were thought to be the direct ancestors of the tetrapods (land-living animals, which also include humans), and living coelacanths thus provided a wealth of new information on their non-skeletal anatomy, ecology, and—once living coelacanths had finally been observed in their natural habitat—their behavior. These rare glimpses into their biology have forced evolutionary biologists to reassess the closeness of the relationship between coelacanths and tetrapods, and we now know instead that lungfish hold such a position. Nevertheless, evolutionary biologists remain equally intrigued by Latimeria chalumnae through its eminent position as the icon of all living fossils.
What exactly is a living fossil, and why does Latimeria qualify? Along with horseshoe crabs, bowfin fish, and opossums, coelacanths are often cited as living fossils, but few scientists have agreed on the precise meaning of the term.3 Darwin introduced the phrase “living fossils” for forms that are the result of a long survival of lineages and remarkably slow evolutionary rates, both of which he attributed primarily to an absence of ecological competition.4 Since then, the concept of living fossils often took on an adaptational flavor. For example, Delamare-Deboutteville and Botosancanu envisioned living fossils as organisms that, by virtue of the narrowness of their adaptation, are restricted in their ability to change over time.5 In contrast, Simpson considered them to be characterized by broad adaptation.6 Adaptations aside, most assessments of living fossils agree that they share the following: they have survived for a relatively long time—measured in terms of geologic periods—and they exhibit a plethora of primitive characteristics that suggest that they have undergone little evolutionary change over this period of time.
The Transylvanian fauna were what formed the backbone of Nopcsa’s ideas in paleobiology and evolutionary theory, among them the connection between body size and habitat area (i.e., dwarfs and islands) and the evolutionary processes that mediated this relationship (endocrine disease and neo-Lamarckian inheritance). It also revealed to Nopcsa that his dinosaurs represented a depauperate assemblage of relicts of a much richer European fauna from earlier Cretaceous times. Although he never discussed this relictual nature of the Transylvanian dinosaur fauna using Darwin’s term, living fossil, in what follows, we are going to claim that—taken from the perspective of 70 million years ago—at least one member of the Transylvanian fauna can be regarded as a paleontological example of a living fossil. This is not an oxymoron—we really do mean living fossil, in the sense that it is separated by a long interval of time, and is little transformed, from its closest relatives or its potential ancestor. We will also show that other members of this region’s fauna changed at much more normal rates.
Evolutionary rates come in two varieties: taxonomic rates and rates of character change. In extinct organisms, taxonomic rates have generally been estimated from the first occurrence of a particular taxon, its temporal duration, and its diversity, and these rates are often expressed using survivorship curves.7 Rates of character change typically have been evaluated as changes in the size and shape of a feature (e.g., tooth-crown height) over a given time interval, although rates of change among character complexes have also been analyzed.8
Here we’re going to try something a little different: instead of measuring rates of character change from what has come directly from the fossil record, we’re going to calculate these changes on the basis of what we don’t have. In order to calculate the differences in rates of character change in some of our Transylvanian dinosaurs and to analyze their significance—in other words, to look for the presence of living fossils in the Late Cretaceous—we must also come to terms with their ghosts.
Our quest to understand the how and why of differences in evolutionary rates among the Transylvanian dinosaurs requires us to estimate their rates of morphological change, and to calculate these rates we need to know not only the time period, but also the historical pattern of common descent. As we have learned in chapter 2, the latter is what cladograms are all about: portraying the closeness of the relationships of different organisms. In so doing, cladograms also provide information about the relative sequence of evolutionary events—the mutual divergence of new lineages—that produced these organisms. As originally noted by Willi Hennig and later elaborated by Mark Norell, a vertebrate paleontologist and systematist at the American Museum of Natural History in New York City, it is axiomatic from evolutionary and cladistic theory that monophyletic sister taxa must have separated from each other at the same time through the same evolutionary splitting event.9 It is this historical continuity between paired sister taxa through their common ancestor at splitting events, when combined with temporal information from the stratigraphic record, that allows us to determine the minimal age of splitting and then use it as a “clock” to extend the minimal age of each group beyond the information that comes from stratigraphy alone.
A ghost lineage is that part of an evolutionary tree for which there is no fossil record, but which must have existed because of the continuity through time between all of the ancestors and their descendant.10 In order to identify the existence of ghost lineages in a clade and thereby calculate their durations, we obviously need to know the clade’s phylogeny. That is, we must have as complete, as fully resolved, and as well-tested a cladogram as possible for the group in which we’re interested. All of the taxa in the cladogram should be monophyletic, without unresolved putative ancestors. When these aspects of a cladistic analysis are fulfilled, the resulting cladogram is the best we can expect from the most parsimonious treatment of the data. In addition, we must have the best data on the stratigraphic distribution of the various members of this clade. Anything less than all of what is currently available, on as precise a form as possible, will reap as much error in ghost lineage analyses as it would in an improperly constructed cladogram. If these aspects are kept in mind, ghost lineage analysis can meld information from phylogeny and stratigraphy to provide a measure of the quality of the fossil record.
To assess how well a set of first appearances in the fossil record corresponds to the prediction of specific phylogenetic hypotheses, we will use a method that has been called the Sum of Minimum Implied Gaps (SMIG).11 SMIG analysis pits the first occurrence of a taxon in the fossil record against its relationships, as determined by cladistic analysis, in the following way. Suppose dinosaurs X and Y are each other’s closest relatives (figure 6.2). As a result, they shared a common ancestor that is not shared by dinosaur Z or any other life forms. From different sources of information, we know that dinosaur X comes from rocks dated 100 million years ago and dinosaur Y comes from 125-million-year-old rocks. Since ancestors must come before descendants, the ancestor of X and Y has to be at least 125 million years old. We have thus discovered a ghost lineage leading to dinosaur X, amounting to 25 million years of not-yet-sampled history—its ghost lineage duration (GLD). The same follows for the ghost lineage of 5 million years leading from the common ancestor of dinosaur Z and the common ancestor of dinosaurs X and Y GLDs are obtained by subtracting the earlier age (the earliest stratigraphic occurrence of the form that lacks a ghost lineage) of one taxon from the later age (the earliest stratigraphic occurrence of the form with the ghost lineage) of its sister taxon.
Short GLDs imply that there is not a great deal of missing history in the stratigraphically calibrated cladogram, while long GLDs indicate the opposite. The ghost lineages for the dinosaurs from Transylvania are of the 10-million-year magnitude, similar to those calculated for other dinosaur taxa. To provide a broader perspective, the ghost lineages for horses and humans are on the order of several million years.12 This difference between dinosaurian GLDs, on the one hand, and those of horses and humans, on the other, indicates not only that considerable time is missing in dinosaurian history in general, but also that the quality of the fossil record for at least some of the dinosaurs in the Mesozoic is significantly less than that for many of the mammals in the Cenozoic. Do these temporal lapses make it easier to speculate more and be less critical about the history of the Transylvanian fauna? We certainly hope not, and in chapter 7 we will take up this issue of the quality of the fossil record we might expect from a small, isolated region enveloped in the widespread tectonic dynamism that is the Late Cretaceous of eastern Europe.
Figure 6.2. Determining ghost lineages and their duration. The diagram in the upper left indicates the phylogenetic relationship of three dinosaur species (dinosaurs X, Y, and Z), while the diagram in the upper right provides the stratigraphic distribution of these same species. Together, the stratigraphic calibration of this dinosaur phylogeny is provided in the lower diagram. Ghost lineages are indicated by shaded rectangles in this lower diagram (see text for a further explanation).
By identifying ghost lineages and estimating their durations, we now have the time part of the equation for calculating rates. Now all we need are the number of characters that change over these intervals. For that information, we return to Courtenay-Latimer’s favorite fish, the coelacanth.
Latimeria is considered to be a living fossil not only because of its extensive removal in time from its last known relatives in the Cretaceous, but also because it bears a very close resemblance to those ancient fish. That is, it hasn’t changed very much over the course of its history. On the other hand, other living creatures (say, teleost fish) with an ancestry coeval with that of Latimeria have changed far more dramatically, both in terms of their taxonomic diversity and their morphology, than have coelacanths—the evolutionary rates of the telosts are profoundly greater than that of Latimeria. This difference between the coelacanth and the teleost conditions is one that involves rates of morphological change, a subject that we now want to explore, from the perspective of coelacanths and—naturally—the dinosaurs of Transylvania.
The rates of morphological evolution through geologic time, and, co-incidentally, about coelacanth evolution, have recently been investigated by Richard Cloutier, a phylogenetic systematist in the Laboratoire de Biologie Évolutive at the Université du Québec à Rimouski, Québec, Canada. Because these rates are based on history, Cloutier began his analysis with the construction of a cladogram for actinistian fish (the group to which coelacanths belong), providing the shape of the evolutionary tree but not the timing of the branching events (figure 6.3a). Cloutier then calibrated his cladogram with the stratigraphic distribution of the actinistians he was analyzing. For example, because the closest relative of Latimeria is a coelacanth known as Macropoma from the Cretaceous of Europe, the duration of the ghost lineage leading to the former is approximately 80 million years (figure 6.3b). Based on his cladistic analysis, Cloutier had, at hand, all the character changes that took place along each branch of the tree (figure 6.3c). In fact, there were 101 changes distributed among the 17 branching events leading from the bottom of the tree to present-day Latimeria. Cloutier was then able to estimate the rates of morphological change through geologic time for these fish. We will take the same approach as Cloutier, using phylogeny and stratigraphy to estimate evolutionary rates for some of the Transylvanian dinosaurs. In so doing, we will address another of Nopcsa’s claims about Transylvanian dinosaurs, namely, their supposed primitive nature and their rates of character evolution.
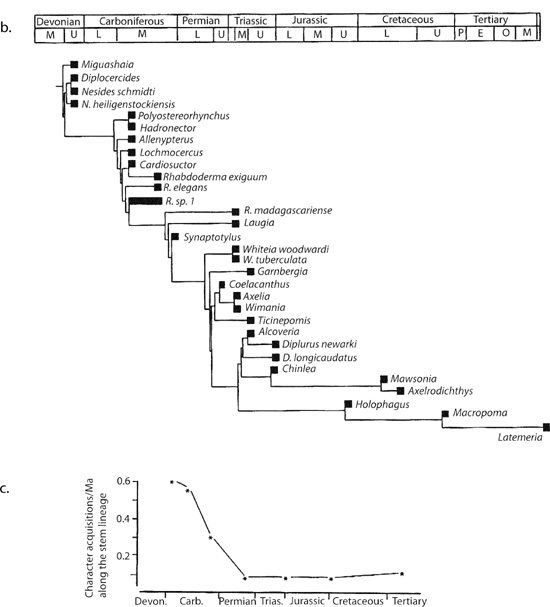
Figure 6.3. (a) A cladogram of actinistian fish; (b) ghost lineages determined from the actinistian cladogram and the stratigraphic distribution of fossil actinistians; and (c) the rate of morphological change, calculated as the number of character changes for each ghost lineage divided by the ghost lineage duration. (After Cloutier 1991)
“The Haţeg fauna turns [out] to be nothing else than the poor remains of an older and richer but less known fauna. Thus we have not only to deal with a primitive fauna, but with one in which the genera are reduced in number whereas individuals are abundant.”13 With these words, Nopcsa tied not only dwarfing, but also aspects of character evolution, to his emerging view of the Haţeg region as a Late Cretaceous island. When he identified the Haţeg assemblage as a relict fauna of dwarfs, Nopcsa was arguing that Europe was moving from a more continental to a more insular geography during the Cretaceous, complete with increases in extinction and a slowing down of evolutionary rates. He would also have argued that the further back in time we go, there would have been more habitable area in Europe, and therefore a greater diversity of the kinds of dinosaurs, than we met with in the later Transylvanian fauna. As we will discuss in some detail in chapter 7, the geographic distributions of the closest relatives of the Transylvanian dinosaurs are most often not European, strongly suggesting that we should look elsewhere for their ancestral regions of origin. Now, however, it is important to address the rates of character evolution that Nopcsa used to characterize the Haţeg dinosaurs as relicts.
We turn first to the Transylvanian hadrosaurid Telmatosaurus (chapter 2). The sole species, T. transsylvanicus, is well diagnosed, recognized by a suite of characters that are not found in any other dinosaur. In addition, it is positioned basally among Hadrosauridae, prior to the split of the latter into hadrosaurines and lambeosaurines.14 This phylogeny of higher iguanodontian ornithopods, when calibrated against stratigraphy, indicates that there is a long ghost lineage between Telmatosaurus and the minimal age of its common ancestor with the remaining hadrosaurids, a GLD that is roughly 35 million years long (figure 6.4).15
Figure 6.4. A diagram of the ghost lineages of Telmatosaurus and its close relatives, Euhadrosauria, Levnesovia, Bactrosaurus, Tethyshadros, and Jeyawati (above); and the rates of character changes corresponding to these ghost lineages (below)
GLD calculations for the two species of Zalmoxes follow the same approach (figure 6.5). Because Z. robustus and Z. shqiperorum are found in the same Sânpetru and Densuş-Ciula beds of Transylvania, they have a coeval stratigraphic distribution, and no ghost lineages can be identified between the two. The same applies to Rhabdodon and Zalmoxes—both are known from the Campanian and Maastrichtian, and therefore only a short ghost lineage can be identified here, perhaps upward of a few million years. However, when these two ornithopods are tethered to Iguanodontia (their immediate sister group), we get our first glimpse of a ghost lineage leading to Rhabdodontidae. The earliest known iguanodontians (Dryosaurus and Camptosaurus from the Late Jurassic) are older than both Rhabdodon and Zalmoxes, so the ghost lineage leads from the common ancestor of all involved to that of Zalmoxes and Rhabdodon. With stratigraphic calibration, this amounts to a GLD of more than 80 million years.
Figure 6.5. A diagram of the ghost lineages of Zalmoxes and Rhabdodon and their close relatives, Dryomorpha, Tenontosaurus, and Anabisetia (above); and the rates of character changes corresponding to these ghost lineages (below)
These GLDs clearly indicate that a good deal of the tangible history of these lineages is thus far invisible to us. Such a pity, this gob of spit in the face of every paleontologist trying to make sense of the evolutionary significance of fossils (paraphrasing Henry V. Miller in Tropic of Cancer16). Bothersome though they may be, long ghost lineages could be all we’re ever going to get, because they’re the most likely expected consequence of the ephemeral terrestrial habitats that existed during the Cretaceous in Europe (chapter 4). The patchiness of available land in time and space drastically decreases the probability that nonmarine sediments will contribute to the geologic and fossil records, which in turn reduces our ability to sample many of the Transylvanian lineages. In other words, it’s our previous bugbear of European isolation in the Cretaceous again, where terrestrial habitats must have been rare, at best, over the long run. GLDs may shorten as we discover new taxa whose phylogenetic position fits within these long ghost lineages, but these improvements will probably be hard won for places such as Europe in the Cretaceous.
Such a long ghost lineage might suggest that Telmatosaurus was a Late Cretaceous living fossil—its latest Late Cretaceous occurrence (approximately 68 million years ago) stands in contrast with its closest phylogenetic relationships, dating back to the late Early Cretaceous (some 103 million years ago). But long expanses of time do not make a living fossil. What about the rate of evolutionary change over this ghost lineage? We can calculate the minimum rate of character changes accumulated along ghost lineages from Telmatosaurus to its most recent common ancestor with the remaining hadrosaurids. These transformations amount to those features that mark a species as being unique. Returning to figure 6.4, Telmatosaurus is currently diagnosed by five characters, which had to evolve during the 35 million years of its ghost lineage.17 That’s one character every 7 million years, a very slow evolutionary rate indeed. A number of closely related iguanodontians evolved at much higher rates. For four of these taxa, GLDs range from 5.4 to 28.5 million years, during which approximately two to six character transformations took place. That amounts to approximately three characters per million years for the Levnesovia/Bactrosaurus clade. Interestingly, the number of characters per million years for Tethyshadros, the other taxon from the Late Cretaceous of Europe, is higher (4.75 characters per million years), between that of the Levnesovia/Bactrosaurus clade and Telmatosaurus.
Zalmoxes is another dinosaur from Transylvania for which there is good phylogenetic and stratigraphic information. As we learned earlier, there are two species of Zalmoxes in Transylvania, the more common Z. robustus and the rarer Z. shqiperorum. Figure 6.5 shows that these two species are most closely related to Rhabdodon priscus, as members of Rhabdodontidae. Rhabdodontidae is then closely related to the great clade of ornithopods called Iguanodontia, which includes Tenontosaurus, Dryosaurus, Camptosaurus, and Iguanodon, as well as Telmatosaurus and the remaining hadrosaurids (chapter 2). Thereafter, more primitive relationships are with Anabisetia and a clade consisting of Thescelosaurus and Parksosaurus.
Ghost lineages for this suite of dinosaurs were determined in the same way as we did for Telmatosaurus. With a GLD of more than 80 million years, rhabdodontids are separated by approximately 20 characters from their common ancestor with Iguanodontia. That’s one character every 4 million years, slightly less than twice the rate of character evolution in the Telmatosaurus lineage.
How do these character change rates stack up against those of other dinosaurs? In answer, we’ve tracked the number of character changes for the ghost lineages of several other, closely related taxa within Ornithopoda in a similar fashion. For example, the ghost lineage of Tenontosaurus (Early Cretaceous of the western interior of the United States) comes out to one character every 3 million years. Other rates within Ornithopoda generally fall out around 1 million years for each character change, although for the Argentinean Anabisetia the figure is more like 3.25 million years (what this elevated number means is unclear—is it because these ornithopods are themselves living fossils, or because there are lots more of these kinds of dinosaurs yet to be discovered in South America?). Other dinosaur groups for which these estimates have been made show a similar pattern.18 For thyreophorans (remember, this group includes, among other taxa, stegosaurs and ankylosaurs like our own Struthiosaurus), ceratopsians, sauropods, and nonavian theropods, on average it takes slightly over 3 to about 4.25 million years for a character to change. Interestingly, for avian theropods the rate climbs to one character change per 200,000 years. It is difficult to say whether this shift is real, reflecting a change in the life-history strategies and evolutionary dynamics of these small theropods, or if, instead, it is a product of the intense work that has gone into understanding their phylogenetic relationships. In either case, future discoveries are bound to make this an exciting realm of dinosaur evolutionary biology.
Returning to our treatment of the Transylvanian dinosaurs, the rate of evolution in the lineage leading to Zalmoxes is similar to that found both in its immediate clade and in other dinosaurs elsewhere in the world; whereas it is much slower—about the same rate as the living fossil, Latimeria19—in the lineage leading to Telmatosaurus. Without good phylogenetic analyses for titanosaurid sauropods, nodosaurid ankylosaurs, and dromaeosaurid theropods, it’s not yet possible to calculate the evolutionary rates of Magyarosaurus, Struthiosaurus, and the Romanian raptor.
Even without rates for these remaining Transylvanian taxa, what we know from Zalmoxes and Telmatosaurus suggests that Nopcsa’s faunistic view of the Haţeg assemblage—as primitive and impoverished by an ever-dwindling habitable “Europe”—is probably incorrect. Even though we have sampled only two taxa, their evolutionary rates, determined through phylogeny and stratigraphy, are definitely far from uniform and slow: character transformations leading to the hadrosaurid Telmatosaurus creep as laggardly as those leading to Latimeria, whereas their pace is much more normal in the ghost lineage leading to Zalmoxes. Late Cretaceous life in Transylvania, and the evolution that preceded it, was much more complicated—and richer for it—than Nopcsa had originally imagined.
We’ve now moved our understanding of the Transylvanian dinosaurs a step farther forward. In some (if not all) cases, they were neither the faunistic residue nor the uniformly phylogenetically arrested descendants of earlier European biotas. Each, as might be expected, arose independently, and they happened to find themselves living together and being preserved together in the uppermost Cretaceous rocks of Transylvania. Some dinosaur species had evolved a great deal relative to their ancestors, whereas others hardly evolved at all. As with Latimeria—the icon for all living fossils, with its restricted deepwater habitats off the coasts of the Comoros Islands in the western Indian Ocean and Sulawesi Island in the South Pacific—the isolation of the Transylvanian dinosaurs produced at least one Late Cretaceous living fossil—the slowly evolving Telmatosaurus. At the same time, Zalmoxes took another road, one that involved more normal rates of evolution. Nonetheless, many more questions remain to be asked than we have answered thus far during our exploration of evolutionary rates. For example, what drove the differences in the evolutionary rates of character change among the Transylvanian dinosaurs? From what we determined earlier in this chapter, these differences are not merely an effect of respective ghost lineages, but may instead be a function of the timing of a creature’s arrival in the Transylvanian region. If it is historical biogeography and not ghost lineages that are relevant here, then we should look to regional changes in European terrestrial environments, and also to what was happening on other continental landmasses. In addition, we should ask why the isolated Transylvanian region included a primitive member of one clade and not of others. Depending on the connections enabling colonization of the Transylvanian region over time, this area may have been available for early or late members of contemporary clades distributed elsewhere. Or maybe there is some sort of ecological effect, in terms of how resources or local habitats were available to and used by the Transylvanian dinosaurs. In our next chapter, we turn to these issues of historical biogeography and colonization, their relationships to body size and faunistics, and, ultimately, to the interplay of contingency and selection as they are played out in evolutionary history.