
Jöns Jacob Berzelius (1779–1848).
Building from the work of John Dalton and Humphry Davy, the Swedish chemist Jöns Jacob Berzelius took a major step towards the modern understanding of atoms, molecules and chemistry. Because opposite electrical charges attract one another, he realized that if electrolysis of, for example, water broke it down into hydrogen (attracted to the negatively charged cathode) and oxygen (attracted to the positively charged anode), then water itself must consist of a combination of hydrogen and oxygen atoms held together by some form of electrical attraction – oxygen atoms with an overall negative charge and hydrogen atoms with an overall positive charge (in modern terminology, such charged atoms are called ions).

In order to investigate further, Berzelius carried out a series of experiments in which he measured the proportions of different elements present in various compounds. His declared aim was ‘to find the definite and simple proportions in which the constituents of inorganic nature are bound together.’ The archetypal example involved taking an oxide of iron and heating it in a stream of hydrogen gas, so that all of the oxygen is extracted and combined with hydrogen to make water; he carried out similar experiments with other metal oxides. By comparing the total weight of the oxide at the start of the experiment with the final weight of iron at the end of the experiment, he knew what weight of oxygen was combined with that weight of iron in the compounds. As well as investigating other metal oxides, Berzelius studied the proportions of oxygen to sulphur in two oxides of sulphur, now known as sulphur dioxide and sulphur trioxide. And in order to present his results in a clear, easily understood fashion, he invented the system of identifying elements by the initial letter of the name, or Latin name; if there might be confusion between two elements whose names began with the same letter an extra letter was added (for example, carbon became C, so calcium became Ca; Sulphur became S, so silicon became Si).
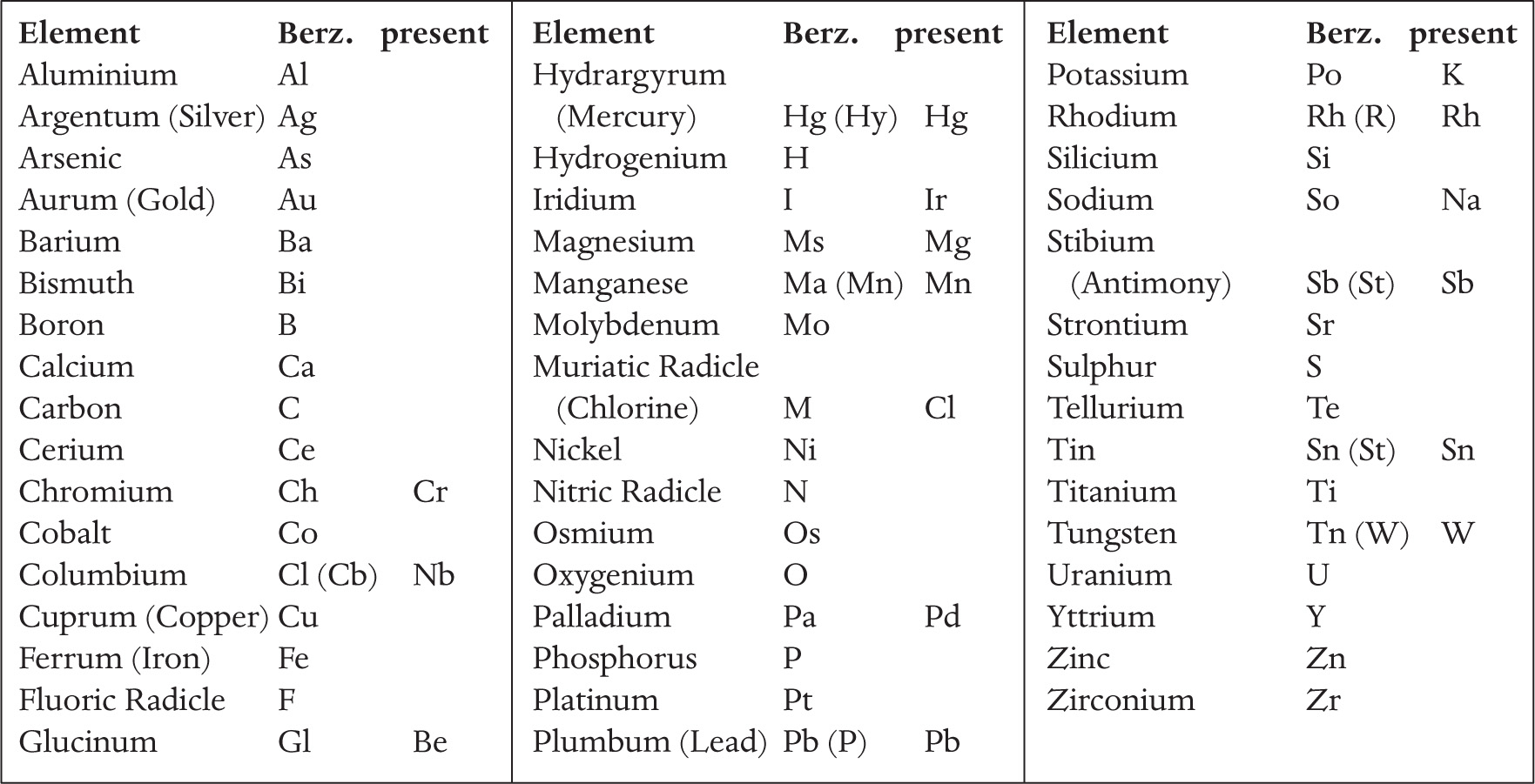
In order to turn all of this into a system for representing the structure of molecules, Berzelius had to know the relative weights of atoms of different elements. Because hydrogen is the lightest element, he set the weight (or mass) of hydrogen as one unit, and worked out the atomic weights of other elements in proportion. At first, there was some confusion because chemists made the assumption that water consists of equal numbers of hydrogen and oxygen atoms, written as HO in the new notation. But it became clear that everything fitted together better if there were two hydrogen atoms in each molecule of water, so that each hydrogen atom weighed half as much as previously thought. Berzelius would have written the revised formula for water as H2O; but the convention was soon altered to its modern form, H2O. These studies also give the oxides of sulphur that Berzelius studied their names, from the proportions of oxygen found in the different molecules – sulphur dioxide is SO2 and sulphur trioxide is SO3.
There is one other important difference between the modern chemical conventions and those of Berzelius. Instead of defining the basic unit of weight, or mass (the atomic mass unit), as the mass of a single hydrogen atom, it is now defined as one-twelfth of the mass of an atom of the most commonly occurring form (isotope) of carbon, known as carbon-12. However, this is a subtle distinction that is only really important for chemists and physicists.
In 1818, Berzelius published a table giving the chemical compositions of nearly two thousand compounds, and the atomic weights (actually measured relative to the weight of an oxygen atom in this case) for 45 of the 49 elements that were known at the time. He had determined 39 of these atomic weights himself, with the other six being determined by his students. There were only so many elements to list in this way because in the course of their experiments Berzelius himself and his students had identified many ‘new’ elements, including cerium, selenium, thorium, lithium, vanadium, and several of the elements known as rare earths. In 1819, he wrote that ‘every chemical combination is wholly and solely dependent on two opposing forces, positive and negative electricity, and every chemical compound must be composed of two parts combined by the agency of their electrochemical reaction’.12
But even Berzelius still thought that the chemistry of living things was different from the chemistry of non-living things and involved a ‘vital force’. He coined the term ‘organic compound’ to refer to the molecules built around carbon chemistry that seemed to be uniquely involved in biological chemistry, with the rest being termed ‘inorganic’. The beginning of the end of this chemical misconception occurred just ten years after Berzelius published his table of atomic weights (see here).