Chapter 4. Chemicals for the Home Chemistry Lab
Chemical Names
It’s important for chemists to be able to identify a specific chemical unambiguously by its name or other designation. In the past, chemical naming was chaotic. Many chemicals had multiple names, and one name could refer to more than one chemical. Early names for specific chemicals, some of which originated with alchemists, have in recent years been largely superseded by modern, systematic names. Still, many of those older names are so ingrained that many chemists continue to use them.
For example, the following names and numbers all refer to the same inorganic compound, CuSO4 · 5H2O:
Systematic (IUPAC) name: copper(II) sulfate pentahydrate
Common name: cupric sulfate, copper(II) sulphate, cupric sulphate
Vernacular name: copper sulfate, copper sulphate
Archaic name: sulphate of copper, blue vitriol, vitriol of copper, copper vitriol, bluestone
CAS number: 7758-99-8
Systematic name
The systematic name, also called the IUPAC name, is the “official” name of a chemical, as determined by the detailed naming rules published by IUPAC (International Union of Pure and Applied Chemistry). Every systematic name identifies exactly one chemical.
Common name
IUPAC uses the term common name differently than you might expect. To IUPAC, a common name is not merely one that is used casually or in nontechnical conversation. IUPAC defines a common name for a substance as one that identifies that substance unambiguously but does not comply with IUPAC naming conventions.
For example, the common name cupric sulfate is unambiguous because it uses the older (and deprecated) form “cupric” to identify the bivalent copper (Cu2+) cation, which in the current IUPAC nomenclature is designed copper(II). Copper(II) sulphate is a common name rather than a systematic name because it uses the nonstandard “sulphate” spelling. Cupric sulphate is a common name rather than a systematic name for both reasons. Chemists, particularly those whose training predates the common use of IUPAC systematic names, frequently use common names for well-known compounds. The latest IUPAC standard approves the use of common names in addition to or instead of systematic names where the use of those common names would not lead to confusion.
Vernacular name
A vernacular name is one that is commonly used in trade or industry, but does not identify a substance unambiguously. For example, the vernacular name copper sulfate may refer either to copper(II) sulfate (CuSO4) or to copper(I) sulfate (Cu2SO4), which is also known under the common name of cuprous sulfate.
Archaic name
An archaic name predates organized naming schemes. Chemists no longer use archaic names, although many are still in common use among nonchemists. For example, artists and potters may use “blue vitriol” or “bluestone” to mean copper(II) sulfate, and might have no idea that copper(II) sulfate, cupric sulfate, and copper sulfate refer to the same compound. Knowledge of archaic names can be useful to a home chemist, because many useful chemicals are sold under archaic names. For example, if you need hydrochloric acid it’s useful to know that it’s sold in hardware stores under the archaic name muriatic acid.
CAS number
A CAS number unambiguously identifies a particular chemical. CAS numbers are assigned to any chemical that has been described in the literature by the Chemical Abstracts Service, a part of the American Chemical Society, but are used worldwide. As of late 2007, more than 30 million chemicals had been assigned CAS numbers, with about 50,000 new CAS numbers added every week. A CAS number comprises three numeric strings separated by hyphens, the first of up to six numbers, the second of two numbers, and the third a single check-digit. CAS numbers are assigned sequentially, and therefore have no inherent meaning. CAS numbers are used primarily for searching the literature.
Most chemists use both systematic and common names, depending on the chemical in question. Many IUPAC names for well-known compounds are seldom or never used. For example, not even the most pedantic chemist uses the IUPAC name dihydrogen oxide for water or the IUPAC name 1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione for caffeine. But IUPAC names for many chemicals are becoming more commonly used, particularly among younger chemists. For example, while many older chemists (and some younger ones) use the common names potassium ferrocyanide and potassium ferricyanide, many younger chemists (and some older ones) instead use the corresponding IUPAC names potassium hexacyanoferrate(II) and potassium hexacyanoferrate(III). Both naming styles are used interchangeably in this book, because it’s good to be familiar with both styles.
Chemical Grades
Chemical grades define standards of purity and suitability for use for particular purposes. In order of decreasing purity (roughly), here are the chemical grades that you should be aware of.
Primary standard grade and specialized ultrapure grades
Primary standard grade chemicals are analytical reagents of exceptionally high purity that are specially manufactured for standardizing volumetric solutions, preparing reference standards, and running lot analyses to determine the purity of production runs of other chemicals. Specialized ultrapure grade chemicals are sold under various brand names by laboratory chemical producers and are used for specialized purposes such as spectroscopy and trace metal analyses. These chemicals are of the highest purity attainable, are extremely expensive, and are overkill for any home laboratory.
Reagent ACS Grade
Reagent ACS Grade chemicals meet or exceed the current American Chemical Society standards for purity, and are the purest chemicals used routinely in laboratories. These chemicals are sufficiently pure for any but the most demanding uses.
Reagent grade
Reagent grade chemicals are of very high purity, but have not been subjected to the exacting testing and certification standards required for labeling as ACS Grade. Reagent grade or better chemicals are required for reliable quantitative analyses, and highly desirable for qualitative analyses.
USP grade, NF grade, or FCC grade
USP grade (United States Pharmocopeia), NF grade (National Formulary), or FCC grade (Food Chemical Codex) chemicals are manufactured in facilities that comply with current Good Manufacturing Practices (cGMP) standards and that meet the requirements of the USP, NF, or FCC, respectively. These chemicals are usually of a very high degree of purity, but may contain impurities that are insignificant for food or pharmaceutical use but may or may not be significant for qualitative or quantitative analyses and similar laboratory procedures. All of these grades of chemicals are suitable for general use in home laboratories.
Laboratory grade or purified grade
Laboratory grade chemicals are equivalent in purity to USP, NF, and FCC chemicals, but are not certified for pharmaceutical or food use. Purified grade chemicals are usually chemicals of high purity that were listed in earlier editions of the USP, NF, or FCC compendia, but are no longer listed in the current editions. Either of these grades of chemicals is suitable for general use in home labs.
Practical grade or chemically pure (CP) grade
Practical grade and chemically pure grade (CP grade) chemicals are of purity suitable for use in syntheses and other general applications, but are a step down in purity from laboratory or purified grade, and are not intended for analytical work. Either of these grades is acceptable for general use in home labs when purer grades are unavailable or unaffordable.
Technical grade
Technical grade chemicals are generally supplied in bulk quantities and are suitable for general industrial use, but should be used only as a last resort in a home chemistry lab.
Ungraded
Ungraded chemicals are produced for household, agricultural, and other uses where high purity is unimportant. The actual purity of ungraded chemicals can range from very poor to surprisingly good. To a large extent, the actual purity of these chemicals depends on the production process used to make them. For example, hydrochloric acid sold in hardware stores as “muriatic acid” for cleaning concrete is produced by reacting hydrogen and chlorine gases and dissolving the resulting hydrogen chloride gas in water, which may be ordinary tap water but is often distilled or deionized. Because there are few opportunities in the production process for the product to be contaminated, the muriatic acid you can buy in hardware stores is often of purity comparable to laboratory grade. Of course, by definition there are no guarantees of purity with ungraded chemicals.
Industry-specific grades
Many industries have industry-specific grading systems for chemicals, but the only one you’re likely to encounter is photo grade. A photo grade chemical is one that contains only impurities that do not interfere with the intended use of the chemical in photographic processing. Photo grade chemicals may contain other impurities, which may or may not interfere with other uses. In general, photo grade chemicals are comparable in purity to laboratory grade or purified grade, and most are suitable for use in a home chemistry lab.
The best advice is to buy the purest chemicals you can afford, always keeping an eye on the intended use of those chemicals and the price differential between grades. If you have to control costs by buying only some chemicals in high-purity grades, we recommend that you buy purer grades of frequently used primary chemicals such as mineral acids, sodium hydroxide, and so on. Avoid anything less than laboratory/purified grade if you can possibly do so.
Chemical Risk Factors and Safety Advice
Any chemical can be dangerous, given the right (or wrong) conditions. People have died from acute water toxicity, which is to say from drinking too much water. Still, some chemicals are obviously more hazardous than others. Government and industry groups have created various labeling systems to warn users of the type and degree of danger associated with chemicals that present greater than normal hazards for storage, handling, and use. The following sections describe the safety information and hazard labeling systems you’re most likely to encounter.
Material Safety Data Sheets (MSDS)
The MSDS (Material Safety Data Sheet) for a chemical presents pertinent safety information in a structured format that includes the following categories:
Product Identification
Composition/Information on Ingredients
Hazards Identification
First Aid Measures
Fire Fighting Measures
Accidental Release Measures
Handling and Storage
Physical and Chemical Properties
Stability and Reactivity
Toxicological Information
Ecological Information
Disposal Considerations
Transport Information
Regulatory Information
Other Information
Most laboratory supply houses publish an MSDS for each chemical they carry, or at least for each chemical that presents any significant hazard. The MSDS may be produced by the vendor or by the company that actually made the chemical.
The MSDS should be your primary source of safety information about each chemical you buy. If no MSDS is provided, search the Web for an MSDS for that chemical. Read the MSDS for every chemical, even those that present no special hazard. Print a copy of the MSDS for each chemical that presents a significant hazard, and file it in your laboratory binder. Decide conservatively which MSDS sheets to print. For example, although you probably don’t need a printed copy of the MSDS for sodium bicarbonate or even ethanol, you probably should keep printed copies of the MSDS sheets for strong acids and bases, oxidizers, and poisons.
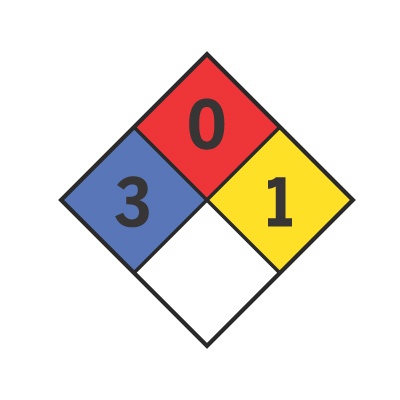
NFPA 704 Fire Diamond
The NFPA (National Fire Protection Association) rates various chemicals to identify the specific types and degrees of risks they present to firefighters and other emergency personnel. Rated chemicals are assigned an NFPA 704 fire diamond. The fire diamond helps emergency personnel quickly and easily to identify special procedures, precautions, and equipment necessary to minimize the danger the chemical presents.
The four sections of the fire diamond specify the type of risk in four categories: health (left), flammability (top), reactivity (right), and special codes for unique hazards (bottom). The three top sections contain a number from zero to four that specifies the degree of that risk. The bottom section may contain a symbol that represents the unique danger, if any. Although it is not required, the four sections of the NFPA 704 fire diamond are usually color-coded.
Health (Blue)
4. | Very short exposure may cause death or major residual injury (e.g., hydrogen cyanide gas) |
3. | Short exposure may cause serious temporary or residual injury (e.g., carbon tetrachloride) |
2. | Intense or continued but not chronic exposure may cause temporary incapacitation or possible residual injury (e.g., pyridine) |
1. | Exposure may cause irritation but only minor residual injury (e.g., potassium carbonate) |
0. | Exposure offers no hazard beyond that of ordinary combustible material (e.g., sodium chloride) |
Flammability (Red)
4. | Rapidly or completely vaporizes at normal temperature and pressure or is readily dispersed and will burn readily (e.g., butane gas, magnesium dust) |
3. | Can be ignited at normal ambient temperatures (e.g., gasoline) |
2. | Must be moderately heated or exposed to relatively high ambient temperatures before ignition occurs (e.g., fuel oil) |
1. | Must be preheated before ignition can occur (e.g., olive oil) |
0. | Not flammable (e.g., calcium carbonate) |
Reactivity (Yellow)
4. | Readily detonates or explosively decomposes at normal temperature and pressure (e.g., nitroglycerin) |
3. | May detonate or explosively decompose from a strong initiating force or severe shock, if heated under confinement, or in contact with water (e.g., benzoyl peroxide) |
2. | Undergoes violent chemical change at high temperature and pressure, reacts violently with water, or may form explosive mixture with water (e.g., phosphorus) |
1. | Normally stable, but may become unstable at high temperature and pressure (e.g., calcium metal) |
0. | Normally stable even when exposed to fire and is not reactive with water (e.g., boric acid) |
Special Hazard (White)
W or WATER – Reacts with water in an unusual or dangerous manner (e.g., sodium metal)
OX or OXY – oxidizer (e.g., potassium permanganate)
Form Matters
NFPA 704 fire diamonds and similar safety warnings may apply only to a specific form of a chemical. For example, aluminum, magnesium, or zinc metal lumps present little or no fire hazard, but those same metals in the form of dust are severe fire hazards. Don’t make the mistake of judging the safety hazards of one form of a chemical based on data for another.
Although NFPA 704 defines only these two special hazards, other self-explanatory symbols are sometimes used unofficially. For example a strong acid may be flagged ACID, a strong base BASE or ALK (for alkali), and either may be flagged COR or CORR (for corrosive). More than one unique hazard may be listed in this section. For example, potassium chromate is both an oxidizer and a corrosive, so the white diamond may include both OX or OXY and COR or CORR.
Note that the NFPA 704 fire diamond is designed to warn firefighters and other emergency personnel, who may have to deal with very large quantities of a chemical in a burning building. For that reason, the NFPA 704 fire diamond may exaggerate the danger that a particular chemical represents in the very different environment of a home chem lab. A 5,000 gallon tank of acetone might explode during a fire and devastate an entire neighborhood; a 25 mL bottle of acetone might cause some excitement if it catches fire, but isn’t likely to cause much damage if you have a fire extinguisher handy.
Risk Phrases (R-phrases) and Safety Phrases (S-phrases)
Some chemicals are labeled with Risk Phrases (R-phrases). R-phrases are published in European Union Directive 2001/59/EC, which defines the “[n]ature of special risks attributed to dangerous substances and preparations.” Although R-phrases have official standing only within the EU, they are commonly used worldwide to define the specific risks associated with particular chemicals. The following list includes all currently defined R-phrases. (Missing R-phrase numbers indicate phrases that have been deleted or replaced by another R-phrase.)
R1: | Explosive when dry |
R2: | Risk of explosion by shock, friction, fire or other sources of ignition |
R3: | Extreme risk of explosion by shock, friction, fire or other sources of ignition |
R4: | Forms very sensitive explosive metallic compounds |
R5: | Heating may cause an explosion |
R6: | Explosive with or without contact with air |
R7: | May cause fire |
R8: | Contact with combustible material may cause fire |
R9: | Explosive when mixed with combustible material |
R10: | Flammable |
R11: | Highly flammable |
R12: | Extremely flammable |
R14: | Reacts violently with water |
R15: | Contact with water liberates extremely flammable gases |
R16: | Explosive when mixed with oxidizing substances |
R17: | Spontaneously flammable in air |
R18: | In use, may form flammable/explosive vapor-air mixture |
R19: | May form explosive peroxides |
R20: | Harmful by inhalation |
R21: | Harmful in contact with skin |
R22: | Harmful if swallowed |
R23: | Toxic by inhalation |
R24: | Toxic in contact with skin |
R25: | Toxic if swallowed |
R26: | Very toxic by inhalation |
R27: | Very toxic in contact with skin |
R28: | Very toxic if swallowed |
R29: | Contact with water liberates toxic gas |
R30: | Can become highly flammable in use |
R31: | Contact with acids liberates toxic gas |
R32: | Contact with acids liberates very toxic gas |
R33: | Danger of cumulative effects |
R34: | Causes burns |
R35: | Causes severe burns |
R36: | Irritating to eyes |
R37: | Irritating to respiratory system |
R38: | Irritating to skin |
R39: | Danger of very serious irreversible effects |
R40: | Limited evidence of a carcinogenic effect |
R41: | Risk of serious damage to eyes |
R42: | May cause sensitization by inhalation |
R43: | May cause sensitization by skin contact |
R44: | Risk of explosion if heated under confinement |
R45: | May cause cancer |
R46: | May cause heritable genetic damage |
R48: | Danger of serious damage to health by prolonged exposure |
R49: | May cause cancer by inhalation |
R50: | Very toxic to aquatic organisms |
R51: | Toxic to aquatic organisms |
R52: | Harmful to aquatic organisms |
R53: | May cause long-term adverse effects in the aquatic environment |
R54: | Toxic to flora |
R55: | Toxic to fauna |
R56: | Toxic to soil organisms |
R57: | Toxic to bees |
R58: | May cause long-term adverse effects in the environment |
R59: | Dangerous for the ozone layer |
R60: | May impair fertility |
R61: | May cause harm to the unborn child |
R62: | Possible risk of impaired fertility |
R63: | Possible risk of harm to the unborn child |
R64: | May cause harm to breast-fed babies |
R65: | Harmful: may cause lung damage if swallowed |
R66: | Repeated exposure may cause skin dryness or cracking |
R67: | Vapours may cause drowsiness and dizziness |
R68: | Possible risk of irreversible effects |
R-phrases may be combined. For example, one of the R-phrases listed for copper(II) sulfate pentahydrate is R36/38. Combining R36 (Irritating to eyes) and R38 (Irritating to skin) yields a description for R36/38 of “Irritating to eyes and skin.”
Whereas R-phrases define specific risks, Safety Phrases (S-phrases) define specific safety precautions that should be used to avoid injury. The following list includes all currently-defined S-phrases. (Missing S-phrase numbers indicate phrases that have been deleted or replaced by another S-phrase.)
S1: | Keep locked up |
S2: | Keep out of the reach of children |
S3: | Keep in a cool place |
S4: | Keep away from living quarters |
S5: | Keep contents under ... (appropriate liquid to be specified by the manufacturer) |
S6: | Keep under ... (inert gas to be specified by the manufacturer) |
S7: | Keep container tightly closed |
S8: | Keep container dry |
S9: | Keep container in a well-ventilated place |
S12: | Do not keep the container sealed |
S13: | Keep away from food, drink and animal feedstuffs |
S14: | Keep away from ... (incompatible materials to be indicated by the manufacturer) |
S15: | Keep away from heat |
S16: | Keep away from sources of ignition — No smoking |
S17: | Keep away from combustible material |
S18: | Handle and open container with care |
S20: | When using do not eat or drink |
S21: | When using do not smoke |
S22: | Do not breathe dust |
S23: | Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) |
S24: | Avoid contact with skin |
S25: | Avoid contact with eyes |
S26: | In case of contact with eyes, rinse immediately with plenty of water and seek medical advice |
S27: | Take off immediately all contaminated clothing |
S28: | After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) |
S29: | Do not empty into drains |
S30: | Never add water to this product |
S33: | Take precautionary measures against static discharges |
S35: | This material and its container must be disposed of in a safe way |
S36: | Wear suitable protective clothing |
S37: | Wear suitable gloves |
S38: | In case of insufficient ventilation wear suitable respiratory equipment |
S39: | Wear eye/face protection |
S40: | To clean the floor and all objects contaminated by this material use ... (to be specified by the manufacturer) |
S41: | In case of fire and/or explosion do not breathe fumes |
S42: | During fumigation/spraying wear suitable respiratory equipment (appropriate wording to be specified by the manufacturer) |
S43: | In case of fire use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add “Never use water”) |
S45: | In case of accident or if you feel unwell seek medical advice immediately (show the label where possible) |
S46: | If swallowed, seek medical advice immediately and show this container or label |
S47: | Keep at temperature not exceeding ...°C (to be specified by the manufacturer) |
S48: | Keep wet with ... (appropriate material to be specified by the manufacturer) |
S49: | Keep only in the original container |
S50: | Do not mix with ... (to be specified by the manufacturer) |
S51: | Use only in well-ventilated areas |
S52: | Not recommended for interior use on large surface areas |
S53: | Avoid exposure — obtain special instructions before use |
S56: | Dispose of this material and its container at hazardous or special waste collection point |
S57: | Use appropriate containment to avoid environmental contamination |
S59: | Refer to manufacturer/supplier for information on recovery/recycling |
S60: | This material and its container must be disposed of as hazardous waste |
S61: | Avoid release to the environment. Refer to special instructions/safety data sheet |
S62: | If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label |
S63: | In case of accident by inhalation: remove casualty to fresh air and keep at rest |
S64: | If swallowed, rinse mouth with water (only if the person is conscious) |
Like R-phrases, S-phrases can be combined to better define the appropriate safety measures for a particular chemical.
Hazard Pictograms and Letter Symbols
Many packaged chemicals display one or more of the following pictograms and/or letter symbols to alert the user to specific hazards posed by that chemical.
These pictograms are intended to provide only general guidance. For additional information about the specific hazards involved and the steps required to handle the chemical safely, read the MSDS or other detailed safety information.
Safe Chemical Handling
It’s important to treat your laboratory chemicals with respect and to handle them safely. But it’s also important to realize that everyone routinely deals with many potentially dangerous chemicals, and that injuries occur only when those chemicals are mishandled.
For example, your bathroom medicine cabinet may contain isopropanol (flammable), ethanol (flammable), acetone nail polish remover (flammable), acetaminophen (toxic), tincture of iodine (toxic, flammable), and various prescription drugs (toxic). That can of crystal drain cleaner under the sink is almost pure sodium hydroxide (corrosive, toxic). Your automobile contains several gallons of gasoline (flammable), and its battery contains sulfuric acid (corrosive, toxic). If you use a wire brush to clean the terminals on your car battery, that powder you’re scraping off is lead sulfate (toxic). You probably have a gallon of chlorine bleach (toxic, corrosive, oxidizer) in your laundry room, and the shelves in your basement shop probably hold cans of paint thinner (flammable), turpentine (flammable), and perhaps a gallon of concentrated hydrochloric (muriatic) acid (toxic, corrosive) for cleaning concrete. Your garden shed is probably full of insecticides (toxic) and that 50-pound bag of 34-0-0 fertilizer is actually pure ammonium nitrate (oxidizer, explosive). There may even be an old container of arsenic-based rat poison (toxic). Old thermometers contain mercury (toxic), as do fluorescent tubes. And so on.
The point is this: we are all exposed regularly to potentially hazardous chemicals, but awareness of the dangers and proper handling minimizes the risk of injuries. Use common sense precautions when handling chemicals. Wear gloves and protective clothing when handling chemicals, and always wear splash goggles. If you are working with powdered chemicals, wear a respirator mask. Have a fire extinguisher and first-aid kit handy.
Respect your chemicals. Do not fear them.
Incompatible Chemicals
Some chemicals are incompatible with each other, in the sense that combining those chemicals may result in a violent or dangerous reaction such as intense heat, fire, explosion, or release of a toxic gas. In other cases, the reaction may not be immediately obvious. For example, by combining some chemicals you may unintentionally create a shock-sensitive explosive such as a peroxide or a fulminate, which might later detonate unpredictably.
Of course, sometimes such incompatible chemicals are intentionally combined to cause a reaction, but it’s important that you avoid doing so unintentionally. If you’re expecting spectacular results, you can take steps to deal with them safely. If you’re not expecting a violent reaction, the results can be tragic.
Table 4-1 is a matrix that lists general incompatibilities between classes of chemicals. The matrix is not exhaustive. For example, the matrix lists organic acids, organic poisons, organic solvents, and water reactive compounds as incompatible with oxidizers, but there are many other substances that react violently with some or most oxidizers. Similarly, not every chemical in a particular category reacts badly with every chemical in an incompatible category. For example, many inorganic poisons do not react at all with many organic poisons. Still, within its limitations, this matrix provides useful guidance.
Although it is by no means exhaustive in either column, Table 4-2 lists some of the specific chemical incompatibilities that are most likely to be encountered in a home chem lab. The nature of the hazard varies. For example, acetone and hydrogen peroxide in the presence of an acid catalyst react to form the hideously dangerous acetone peroxide, an explosive that has been used by terrorists. Glycerol bursts into flame in contact with potassium permanganate. Cyanides (including complexes like ferricyanides and ferrocyanides) react with strong mineral acids to form toxic hydrogen cyanide gas. And so on.
Deadly Chemicals
All of that said, there are chemicals that scare any sane chemist silly. Some chemicals are so toxic that literally one drop contacting your skin may be lethal, as may inhaling even a slight amount. (Dimethyl mercury comes to mind.)
That’s why it’s so important not to mix chemicals randomly or to use them other than as recommended. For example, potassium ferricyanide is used in some experiments in this book. Despite the presence of “cyanide” in the name, that chemical is of relatively low toxicity and is relatively safe to store and handle. However, if you heat potassium ferricyanide to decomposition or expose it to strong mineral acids, it produces extremely toxic hydrogen cyanide gas.
Acids (Inorganic) | Acids (Organic) | Acids (Oxidizing) | Bases (Alkalis) | Oxidizers | Poisons (Inorganic) | Poisons (Organic) | Solvents (Organic) | Water Reactives | |
Acids (Inorganic) |
| STOP! |
| STOP! |
| STOP! | STOP! | STOP! | STOP! |
Acids (Organic) | STOP! |
| STOP! | STOP! | STOP! | STOP! | STOP! |
| STOP! |
Acids (Oxidizing) |
| STOP! |
| STOP! |
| STOP! | STOP! | STOP! | STOP! |
Bases (Alkalis) | STOP! | STOP! | STOP! |
|
|
| STOP! | STOP! | STOP! |
Oxidizers |
| STOP! |
|
|
|
| STOP! | STOP! | STOP! |
Poisons (Inorganic) | STOP! | STOP! | STOP! |
|
|
| STOP! | STOP! | STOP! |
Poisons (Organic) | STOP! | STOP! | STOP! | STOP! | STOP! | STOP! |
|
|
|
Solvents (Organic) | STOP! |
| STOP! | STOP! | STOP! | STOP! |
|
|
|
Water Reactives | STOP! | STOP! | STOP! | STOP! | STOP! | STOP! |
|
|
|
Acetic acid | Chromic acid, ethylene glycol, hydroxyl compounds, nitric acid, perchloric acid, permanganates, peroxides |
Acetone | Concentrated nitric/sulfuric acid mixtures, hydrogen peroxide, strong bases |
Ammonium nitrate | Acids, chlorates, combustible organic powders, flammable liquids, metal powders, nitrites, sulfur |
Charcoal (activated) | Calcium hypochlorite, oxidizers |
Chlorates | Acids, ammonium salts, combustible organic powders, metal powders, sulfur |
Chlorine | Acetylene, ammonia, benzene, butadiene, butane, hydrogen, metal powders, methane, propane, sodium carbide, organic solvents |
Copper | Acetylene, hydrogen peroxide |
Hydrocarbons | Bromine, chlorine, chromic acid, fluorine, sodium peroxide |
Ferrocyanides, ferricyanides | Acids |
Hydrogen peroxide | Acetone, alcohols, aniline, chromium, copper, iron, flammable liquids, metals and metal salts, nitromethane, organic materials, nitrates |
Hypochlorites | Acids, activated carbon/charcoal |
Iodine | Acetylene, anhydrous or aqueous ammonia, hydrogen |
Nitrates | Sulfuric acid |
Nitric acid | Acetic acid, aniline, brass, chromic acid, copper, flammable liquids and gases, heavy metals, hydrocyanic acid, hydrogen sulfide |
Nitrites | Acids |
Oxalic acid | Mercury, silver |
Potassium chlorate | Sulfuric acid and other strong acids |
Potassium permanganate | Benzaldehyde, ethylene glycol, glycerol, sulfuric acid |
Silver | Acetylene, ammonium compounds, fulminic acid, oxalic acid, tartaric acid |
Sodium nitrite | Ammonium salts |
Sulfides | Acids |
Sulfuric acid | Chlorates, perchlorates, permanganates |
The best ways to avoid dangerous chemical incompatibilities are to store chemicals safely and with regard to their possible interactions; to study the MSDS for each chemical you purchase, paying particular attention to listed incompatibilities; and to use the minimum quantities of chemicals necessary to complete each experiment. Finally, NEVER MIX CHEMICALS RANDOMLY.
Storage Color Codes
The storage color code system devised by J. T. Baker and shown in Table 4-3 has become an industry standard. This system assigns each chemical one of five storage color codes, with or without a stripe, which indicates the primary storage consideration for that chemical.
In theory, a chemical with a particular color code can safely be stored with other chemicals that have the same color code. In practice, there are many exceptions to that rule. For example, although both sulfuric acid and sodium hydroxide may be coded white for corrosive, that does not mean that they can be safely stored together. Strong acids and strong bases must be stored separately from each other.
A chemical that has a striped color code requires individual consideration, and often must be stored separately from other chemicals. For example, many suppliers color-code sodium, potassium, calcium and other alkali metals and alkaline-earth metals red with a stripe. The red indicates that the metal is flammable, and the stripe indicates that special storage requirements apply. (In this case, the metals must be kept away from liquid water, with which they react violently.) Similarly, some suppliers color-code strong mineral acids white (corrosive) and strong bases white (corrosive) with a stripe to indicate special storage considerations (in this case, that they must be stored separately from acids).
Chemical Allergies
Some people are allergic to substances that are generally considered to be “safe.” For example, some people react very badly to the latex used in some protective gloves.
Before an allergic reaction can occur, you must be exposed at least once to the allergen, but the number of exposures required before an allergic reaction results is unpredictable. For example, I knew a man who was convinced he was immune to poison ivy. He’d handled it with bare hands dozens of times over more than 20 years, and had never had the slightest reaction to it. Until one day he did.
Contact allergies are particularly problematic in a chem lab, and for that reason you should wear gloves when handling any chemical, even one that is generally considered benign and that you have handled many times before with no adverse reaction. Otherwise, the next time you handle it you may regret not wearing gloves.
Furthermore, the color code assigned to a chemical indicates only the primary storage consideration for that chemical. For example, a chemical coded yellow (oxidizer/reactant) may also be quite toxic (blue), while a chemical coded white (corrosive) may also be quite flammable (red). Different suppliers may code the same chemical differently. For example, Fisher Scientific codes concentrated nitric acid yellow (oxidizer/reactant), and other suppliers code it white (corrosive) or white with a stripe (corrosive with special storage considerations; nitric acid reacts with almost anything).
Storage Code | Notes |
| White: Corrosive. May harm skin, eyes, and mucous membranes. Store separately from flammable and combustible materials. |
| Yellow: Oxidizing and/or reactive. May react violently with air, water, or other substances. Store separately from flammable and combustible materials. |
| Red: Flammable. Store separately only with other flammable reagents. |
| Blue: Toxic. Hazardous to health if inhaled, ingested, or absorbed through skin. Store separately in secured area. |
| Green: Presents no more than moderate hazard in any of categories above; use general chemical storage. |
| Gray: Presents no more than moderate hazard in any of categories above; use general chemical storage. (Used by Fisher Scientific instead of green.) |
| Orange: Presents no more than moderate hazard in any of categories above; use general chemical storage. (Obsolete color code; superseded by green.) |
Stripes: A reagent that is incompatible with other reagents of the same storage code color; store separately. |
Storage codes may understate the danger. Violent reactions can occur between two chemicals for which the color codes offer little hint of danger. For example, potassium permanganate (yellow), a strong oxidizer, bursts into flames if it contacts glycerol (green). For that particular combination, yellow plus green equals extreme red. Many suppliers code such chemicals individually with a STOP icon, upraised palm icon, or similar indicator that some special storage requirement applies to that chemical.
Conversely, storage codes may exaggerate the danger, particularly for the small quantities typically stored and used in home labs. For example, some suppliers color code 5% acetic acid white (corrosive). That 5% acetic acid is exactly the same chemical and in the same concentration as the white vinegar you can buy at the supermarket.
Use the color coding on chemical bottles as a starting point rather than as an absolute guide. Study the MSDS for each chemical carefully, and pay particular attention to listed incompatibilities with other chemicals. If you are storing only small amounts of chemicals (say, 100 g or mL or less), it’s probably sufficient just to segregate them on different shelves or in different cabinets. If you are storing larger quantities, pay correspondingly greater attention to proper storage.
Proper Disposal of Used and Unneeded Chemicals
Many home chemists dispose of all chemical wastes simply by flushing them down the drain with lots of water. That’s an acceptable practice for many common laboratory chemicals, but not for chemicals that are particularly toxic or hazardous to the environment.
Lock Them Up
If there is even the slightest chance that children or pets may gain access to your stored chemicals, secure the storage cabinets and/or the lab itself with a sturdy lock. Keep your chemicals locked up except when you’re actually using them.
Proper disposal of such hazardous chemicals is subject to an incredible maze of federal, state, and local laws and regulations. Fortunately, in practice many of these laws and regulations apply only to commercial and industrial users. For example, regulations for a particular chemical may come into play only if you are disposing of 10 kilograms or more of that chemical per month. Home chemists usually work with at most a few hundred grams of any particular chemical, so check your local hazardous waste disposal laws and regulations to determine the threshold that applies.
Before you choose a disposal strategy for your own home lab, check the web site of your local or state environmental affairs department to determine which laws and regulations apply to you. Most communities provide some means for free disposal of residential hazardous waste. Some communities hold periodic hazardous waste days, when hazardous waste can be placed separately at the curb for pickup. Others maintain hazardous waste disposal centers where you can drop off containers of hazardous waste. Take advantage of these services, and follow any labeling or other requirements they specify.
Disposal of Common Laboratory Chemicals
Safe disposal of chemicals raises two questions that are not necessarily synonymous. First, you have to determine whether it is safe to dispose of a particular chemical by a particular means. Second, if it is safe, you have to determine whether it’s legal.
Here are some general guidelines for disposing safely of common laboratory chemicals. Note that these procedures describe my own practices, which may or may not be lawful where you live. Check before you use these procedures:
Small amounts of most flammable organic solvents (acetone, alcohols, ethers, and so on) can safely be disposed of by taking them outdoors and allowing them to evaporate. Obviously, do this in an area that is not near a flame and not accessible to children or animals. For larger amounts, place the solvent in a sealed container labeled with its contents and take it to the nearest hazardous waste disposal site.
Mineral acids (hydrochloric, sulfuric, nitric, and so on) and most organic acids (formic, acetic, and so on) can be disposed of safely by neutralizing them with sodium bicarbonate (baking soda) until the fizzing stops. The resulting sodium salts (chloride, sulfate, nitrate, acetate, and so on) can safely be flushed down the drain with lots of water. If the acid is concentrated, pour it into ten times its volume of water to dilute it before you neutralize it. Alternatively, add a few drops of phenolphthalein solution to the dilute acid and add aqueous sodium hydroxide or sodium carbonate until the solution turns pink, indicating that the acid has been neutralized.
Strong bases such as sodium hydroxide and potassium hydroxide can be disposed of safely by diluting them, if necessary, and then treating them with a dilute solution of hydrochloric acid. The resulting chloride salts (for example, sodium chloride—common table salt—and potassium chloride) are innocuous and can safely be flushed down the drain. To neutralize the base, add enough phenolphthalein solution to the aqueous base solution to give it a noticeable pink color, and then add dilute acid until the pink color disappears.
Solutions that contain large amounts of free bromine or iodine are hazardous to the environment, although the small amounts that home chemists work with are generally innocuous. To safely dispose of bromine or iodine solutions, add sodium thiosulfate solution until the waste solution turns colorless. This converts the free bromine or iodine to bromide or iodide ions, which are safe to flush down the drain.
With the exception of heavy metal ions, described in the following section, most cations are reasonably benign and can safely be flushed down the drain. “Safe” cations include aluminum, ammonium, bismuth, calcium, cerium, cesium, cobalt, gold, hydrogen, iron, lithium, magnesium, manganese, potassium, rubidium, sodium, strontium, and tin.
Most anions are harmless, particularly in small quantities and low concentrations. “Safe” anions include acetate, bicarbonate, bisulfite, borate, bromate, bromide, carbonate, chlorate, chloride, cyanate, iodate, iodide, nitrate, nitrite, perchlorate, periodate, permanganate, phosphate, silicate, stannate, sulfate, sulfite, thiocyanate (sulfocyanate), thiosulfate, titanate, tungstate, and vanadate. Unless there is another reason not to (such as the presence of a toxic cation or anion complex), solutions of these anions can safely be flushed down the drain with copious amounts of water.
Private Sewage Systems
It’s safe to dispose of relatively benign chemicals by flushing them down the drain only if your drain connects to a sanitary sewer system, which quickly dilutes any chemicals you flush. If you use a septic tank or other means of sewage disposal, you might want to think twice about flushing chemicals into it. It’s difficult to know for sure whether a particular chemical will “poison” a septic tank, so the best practice is to avoid flushing any chemical unless you are absolutely certain that it’s safe to do so. Instead, neutralize waste chemicals as described in this section and take them to a waste disposal site or other location where they can be safely disposed.
Disposal of Heavy-Metal and Other Toxic Compounds
Although many common laboratory chemicals can be safely flushed down the drain, it is unacceptable to flush chemicals that contain heavy-metal ion species or contain other very toxic and/or persistent species. Treat any waste that contains organic compounds as hazardous unless you are certain that it is not, and treat any waste that contains heavy-metal ion species as toxic.
Fortunately, heavy-metal waste can be converted into safer forms, which can then be disposed of by taking them to a hazardous waste disposal center. Your goals in pretreating heavy-metal compounds for disposal are to convert hazardous soluble heavy-metal ions to much less hazardous insoluble solids, and to minimize the volume of solid waste that will require proper disposal. Here are the guidelines I use, although again, you must verify that these methods are legally acceptable in your own jurisdiction.
Barium
Soluble barium salts are extremely toxic and hazardous for the environment. Before disposal, treat any solution that contains soluble barium salts with an excess of a soluble sulfate salt, such as sodium sulfate. The barium ions precipitate as insoluble barium sulfate, which can be filtered, dried, and (including the filter paper) added to a hazardous waste container for later proper disposal. The filtrate contains few barium ions, and can safely be flushed down the drain.
Chromium
Soluble salts with chromium cations or chromium anion complexes (chromates, dichromates) are extremely toxic and hazardous to the environment. Chromium(III) cations can be precipitated by excess sulfate ions as insoluble chromium(III) sulfate. The much more toxic chromium(VI) cation can be reduced to chromium(III) with an excess of hydrogen peroxide and then precipitated as chromium(III) sulfate. Chromate and dichromate anions can be treated by adding a stoichiometrically equivalent amount of a soluble lead(II) salt, such as lead(II) nitrate or lead(II) acetate, which precipitates the chromium ions as insoluble lead(II) chromate. In any case, filter the precipitate and dispose of it (including the filter paper) in a hazardous waste container. The filtrate contains few chromium ions, and can safely be flushed down the drain.
Copper
Soluble copper salts are moderately toxic and moderately dangerous for the environment. Precipitate copper(I) ions by adding an excess of potassium iodide to form insoluble copper(I) iodide. Precipitate copper(II) ions by adding excess sodium carbonate or sodium hydroxide to form insoluble copper(II) carbonate or copper(II) hydroxide. Filter, and dispose of the filtrand and filter paper in a toxic waste container. The filtrate contains few copper ions, and can safely be flushed down the drain.
Lead
Soluble lead salts are extremely toxic and dangerous for the environment. Precipitate lead ions by adding an excess of sodium carbonate to form insoluble lead carbonate. Filter, and dispose of the filtrand and filter paper in a toxic waste container. The filtrate contains few lead ions, and can safely be flushed down the drain.
Silver
Soluble silver salts are toxic and dangerous for the environment. Precipitate silver ions by adding excess potassium iodide to form insoluble silver(I) iodide. Filter, and dispose of the filtrand and filter paper in a toxic waste container. The filtrate contains few silver ions, and can safely be flushed down the drain.
Chemicals Used in This Book
Any home chemistry lab needs a good selection of chemicals. When I started this book, I had no real idea what I needed, so I ordered a wide variety of potentially useful chemicals. I knew that some of those chemicals would end up not being used in any of the lab sessions in the book, and that I might need more of some chemicals than I originally ordered. So I kept careful track of what chemicals I used for the various labs and in what quantities.
Table 4-4 lists the chemicals required to complete all of the laboratory sessions in this book, except those in Chapter 19, Chapter 20, Chapter 21, and Chapter 22. With the exception of a few items that must be purchased from specialty chemical suppliers, all of the chemicals are readily available from local sources such as the supermarket, drugstore, and hardware store. Table 4-5 lists the additional chemicals needed to complete the laboratory sessions in the final four chapters.
The storage, risk, and safety information in Table 4-4 and Table 4-5 is neither authoritative nor definitive. For chemicals that list “none” under R(isk)-phrases and/or S(afety)-phrases, an MSDS for that chemical explicitly listed the chemical as nonhazardous and/or not requiring any safety precautions. For chemicals for which the R-phrases and/or S-phrases column is empty, no information was available. Note well: the absence of safety information does not indicate that a chemical is safe, nor does the absence of safety information indicate that no special safety measures are required. Always check a current MSDS for every chemical before you use it.
Chemical | Quantity | Storage Code | R(isk)-phrases | S(afety)-phrases |
Acetic acid, 17.4 M (glacial) | 100 mL | R10 R35 | S23 S26 S45 | |
Acetone1 | 125 mL |
| R11 R36 R41 R66 R67 | S9 S16 S26 |
Alka-Seltzer tablets | 6 |
| none21 | none21 |
Aluminum, granular, turnings, or shot | 25 g |
| none21 | none21 |
Ammonia, aqueous, 6 M (household) | 125 mL | R20 R21 R22 R34 R36 R37 R38 R41 R50 | S26 S36 S37 S39 S45 S61 | |
Ammonium acetate2 | 25 g |
| R36 R37 R38 | S26 S36 |
Ammonium chloride3 | 25 g |
| R22 R36 | S22 |
Ammonium nitrate4 | 40 g |
| R8 R20 R21 R22 R36 R37 R38 | S17 S26 S36 |
Calcium carbonate antacid tablets | 3 |
| R36 R37 R38 | S26 S36 |
Celery | 1 stalk |
| none21 | none21 |
Charcoal, activated | 25 g |
| R36 R37 | S24 S25 S26 S36 |
Club soda | 200 mL |
| none21 | none21 |
Copper(II) sulfate5 | 250 g |
| R22 R36 R38 R50 R53 | S22 S60 S61 |
Dishwashing liquid | 25 mL |
| none21 | none21 |
Ethanol, 70%6 | 1 L |
| R11 R20 R21 R22 R36 R37 R38 R40 | S7 S16 S24 S25 S36 S37 S39 S45 |
Food coloring dyes, assorted | 1 |
| none21 | none21 |
Gasoline | 100 mL |
| not defined22 | not defined22 |
Glycerol (glyercin)7 | 25 mL |
| none21 | S26 S36 |
Hydrochloric acid, concentrated8 | 250 mL | R23 R24 R25 R34 R36 R37 R38 | S26 S36 S37 S39 S45 | |
Iron filings | 25 g |
| none21 | none21 |
Iron shot | 100 g |
| none21 | none21 |
Iron or steel nail (6d to 12d) | 6 |
| none21 | none21 |
Lead metal shot9 | 5,000 g |
| R23 R25 | none21 |
Lemon | 4 |
| none21 | none21 |
Lighter fluid | 25 mL |
| not defined22 | not defined22 |
Magnesium sulfate10 | 50 g |
| none21 | S22 S24 S25 |
Milk, homogenized | 25 mL |
| none21 | none21 |
Mineral oil | 25 mL |
| R36 R37 R38 | not defined22 |
Oxalic acid11 | 25 g | R21 R22 | S24 S25 | |
Petroleum ether (ligroin) | 100 mL |
| R11 R20 R21 R22 R45 R65 | S45 S53 |
Phenolphthalein (powder) | 1 g |
| R36 R37 R38 | S26 |
Polystyrene foam, rigid | 35 g |
| R36 R37 | none21 |
Potassium hydrogen tartrate12 | 25 g |
| none21 | none21 |
Potassium permanganate13 | 25 g |
| R8 R22 | S17 S26 S36 S37 S39 S45 |
Sand | 25 g |
| none21 | none21 |
Sodium acetate14 | 25 g |
| none21 | S22 S24 S25 |
Sodium bicarbonate | 50 g |
| none21 | none21 |
Sodium bisulfite | 25 g |
| R20 R22 R36 R37 R38 | S26 S36 |
Sodium borate (borax) | 25 g |
| R22 R36 R37 R38 R62 R63 | not defined22 |
Sodium carbonate15 | 100 g |
| R36 R37 R38 | none21 |
Sodium chloride16 | 500 g |
| R36 | S26 S36 |
Sodium hydroxide17 | 100 g | R35 | S26 S37 S39 S45 | |
Starch | 25 g |
| none21 | none21 |
Sucrose18 | 450 g |
| none21 | none21 |
Sulfur19 | 25 g |
| R36 R37 R38 | not defined22 |
Sulfuric acid20 | 100 mL | R23 R24 R25 R35 R36 R37 R38 R49 | S23 S30 S36 S37 S39 S45 | |
Talcum powder | 25 g |
| none21 | none21 |
Vegetable oil | 25 mL |
| none21 | none21 |
Vinegar, white distilled | 250 mL |
| none21 | none21 |
Notes:
You can produce ammonium acetate by neutralizing clear household ammonia with distilled white vinegar and evaporating to dryness.
You can produce ammonium chloride by neutralizing clear household ammonia with hardware store muriatic acid and evaporating to dryness.
Pure ammonium nitrate fertilizer from the lawn and garden store is fine.
Pure copper sulfate is sold as root killer by hardware stores.
Drugstore rubbing alcohol is often 70% ethanol (ethyl alcohol), but check the label to make sure that it’s not isopropanol (isopropyl alcohol).
Glycerol under that name or as glycerin is available in drugstores.
Hardware stores sell hydrochloric acid as muriatic acid. Make sure that the acid concentration is listed as 30% or higher.
Lead metal shot is sold by sporting goods stores for use in reloading shotgun shells.
Pure magnesium sulfate is sold by drugstores as Epsom salts.
Pure oxalic acid is sold by hardware stores as wood bleach (check the label to make sure).
Pure potassium hydrogen tartrate is sold by grocery stores as cream of tartar.
Pure potassium permanganate as crystals or a solution is sold by some pet stores for aquarium use.
You can produce sodium acetate by neutralizing a sodium hydroxide solution with distilled white vinegar and evaporating to dryness.
Hydrated sodium carbonate is sold as washing soda; anhydrous sodium carbonate as soda ash.
You can substitute noniodized salt such as popcorn salt, kosher salt, or rock salt.
Some hardware stores sell pure sodium hydroxide as crystal drain cleaner (check the label to make sure that it’s pure).
Sucrose is table sugar.
Lawn and garden stores sell sulfur under that name.
Auto supply stores sell 35% sulfuric acid as battery acid.
“None” means that I was able to locate risk and safety information for these substances and that no R-phrases and/or S-phrases were provided.
“Not defined” means that I was unable to locate published risk and/or safety information that included specific R-phrases and/or S-phrases for these substances. The absence of these R- and/or S-phrases does not indicate that no risks exist or that no safety measures should be taken. Refer to the container label for risk and safety information.
For most chemicals, Table 4-5 lists a minimum quantity of 25 g or 25 mL, even if that amount is significantly more than is actually needed for the lab sessions, because those quantities are the smallest typically offered by specialty chemical suppliers. Although some suppliers offer chemicals in smaller quantities, packaging and labeling costs are substantial even for small amounts, so it often costs little or no more to buy 25 g of a chemical than only a gram or two. Table 4-5 lists smaller recommended quantities for a few relatively expensive chemicals (such as iodine, ninhydrin, and silver nitrate) that are required in small amounts.
Compare prices among several suppliers. Quite often, a particular supplier has high prices for some chemicals and low prices for others.
Chemical | Quantity | Storage Code | R(isk)-phrases | S(afety)-phrases |
Aluminum nitrate | 25 g |
| R8 R36 R37 R38 R41 | S17 S26 S27 S36 S37 S39 |
Ammonia, aqueous, 15 M (~30%) | 125 mL | R20 R21 R22 R34 R36 R37 R38 R41 R50 | S26 S36 S37 S39 S45 S61 | |
Ammonium molybdate | 25 g |
| R36 R37 R38 | not defined |
Ammonium oxalate | 25 g |
| R20 R21 R25 R34 | not defined |
Aspirin | 25 g |
| none | none |
Barium chloride | 10 g |
| R20 R21 R25 R36 R37 R38 | S45 |
Barium hydroxide | 10 g |
| R20 R21 R22 R34 R41 | S26 S28 |
Barium nitrate | 25 g |
| R8 R20 R25 R37 | S28 |
Calcium nitrate | 25 g |
| R8 | not defined |
Chloroform | 25 mL |
| R20 R22 R38 R40 R48 | S36 S37 |
Chromium(III) nitrate | 50 g |
| R8 R20 R21 R22 R36 R37 R38 | S17 S26 S27 S36 S37 S39 |
Cobalt(II) chloride | 5 g |
| R25 R40 R42 R43 | S22 S36 S37 S39 S45 |
Cobalt(II) nitrate | 25 g |
| R8 R22 R40 R43 R50 R53 | S17 S36 S37 S60 S61 |
Copper(II) nitrate | 25 g |
| R8 R20 R22 R36 R37 R38 | not defined |
Formaldehyde, 37% to 40% | 25 mL |
| R10 R26 R27 R28 R34 R40 R41 R43 | not defined |
Hydrogen peroxide, 3% | 1 mL |
| none | S26 S36 |
Iodine crystals | 5 g | R21 R23 R25 R34 | S23 S25 | |
Iron(III) nitrate | 25 g |
| R8 R36 R38 | S26 |
Iron(II) sulfate | 25 g |
| R22 R36 R37 R38 | S26 S36 S37 S39 |
Lead nitrate | 50 g |
| R8 R20 R21 R22 R33 R36 R37 R38 R60 R61 | S17 S36 S37 S39 S45 S53 |
Manganese(II) sulfate | 25 g |
| R20 R21 R22 R36 R37 R38 R40 | S26 S36 |
Methanol | 125 mL |
| R11 R23 R24 R25 R39 | S7 S16 S24 S36 S37 S45 |
Nickel(II) nitrate | 30 g |
| R8 R20 R21 R22 R36 R37 R38 R45 | not defined |
Ninhydrin powder | 1 g |
| R22 R36 R37 R38 | S26 S28 S36 |
Nitric acid, concentrated (15.8 M) | 100 mL |
| R8 R23 R24 R25 R34 R41 | S23 S26 S36 S37 S39 S45 |
Potassium bromide | 25 g |
| R20 R21 R22 R36 R37 R38 | S22 S26 S36 |
Potassium chromate | 25 g |
| R23 R24 R25 R45 R47 | not defined |
Potassium hexacyanoferrate(II) | 25 g |
| R32 R52 R53 | S50B S61 |
Potassium hexacyanoferrate(III) | 25 g |
| R20 R21 R22 R32 | S26 S36 |
Potassium iodide | 25 g |
| R36 R38 R42 R43 R61 | S26 S36 S37 S39 S45 |
Potassium nitrate | 25 g |
| R8 R22 R36 R37 R38 | S7 S16 S17 S26 S36 S41 |
Potassium thiocyanate | 25 g |
| R22 R36 R37 R38 | not defined |
Seawater | 200 mL |
| none | none |
Silver nitrate | 5 g |
| R22 R34 R36 R37 R38 | S26 S45 |
Sodium hypochlorite (laundry bleach) | 125 mL | R20 R21 R22 R34 R41 | S1 S2 S28 S45 S50 | |
Sodium nitrite | 25 g |
| R8 R25 R50 | S45 S61 |
Sodium phosphate, tribasic | 25 g |
| R34 R36 R38 | S22 S26 S27 S36 S37 S39 |
Sodium sulfate | 25 g |
| none | none |
Sodium sulfite | 25 g |
| R20 R21 R22 R36 R38 R40 | S22 S26 S36 |
Sodium thiosulfate | 25 g |
| R36 R37 R38 | S26 S36 |
Strontium nitrate | 25 g |
| R8 R36 R37 R38 | S26 |
Sulfuric acid, 98% (18.4 M) | 100 mL | R23 R24 R25 R35 R36 R37 R38 R49 | S23 S30 S36 S37 S39 S45 | |
Vitamin C tablet, 500 mg | 1 |
| none | none |
Zinc metal (powder, granular, mossy) | 25 g |
| R11 | S7 S8 S43 |
Zinc nitrate | 25 g |
| R8 R20 R21 R22 R34 R36 R37 R38 | S17 S22 S26 S36 S37 S39 |
Shipping Hazardous Chemicals
Some of the chemicals used in this book are classified as hazardous for shipping purposes. The rules and regulations for shipping hazardous chemicals are incredibly complex and change frequently. It’s almost a full-time job to keep up with them. The specific chemical, its form (granular, powder, chunks, and so on), and the quantity in question all have a bearing on whether that chemical can be shipped by a particular method. The upshot is that it can be extremely expensive to ship even small quantities of some chemicals.
Commercial, industrial, and university laboratories deal with this problem by ordering large quantities at one time, consolidating orders, and resigning themselves to paying high transportation charges. Those aren’t realistic options for most home labs. There are four ways to avoid these high shipping charges:
Don’t use the chemical. In some cases, you can substitute another chemical that is available without hazard surcharges. Other times, you can make the chemical yourself from precursors that are not subject to shipping surcharges. (Note that some of these chemicals are listed as hazardous for a very good reason; you may want to avoid them entirely for safety’s sake. On the other hand, some of them, such as acetone and alcohols, are no more hazardous than many chemicals in your medicine cabinet or garden shed.)
Buy the chemical locally. If you live in a large city, there are probably numerous commercial chemical supply houses within easy driving distance. Even if you live in a small town, you can find many hazardous chemicals at a hardware store (e.g., hydrochloric acid, sodium hydroxide, acetone and other ketones), drugstore (e.g., ethanol, isopropanol), pool supply store (e.g., potassium permanganate), agricultural supply store (e.g., potassium nitrate, ammonium nitrate, calcium nitrate), auto parts store (sulfuric acid), and so on.
Chemicals that require hazardous shipping surcharges in their solid or concentrated forms are often available as dilute solutions without surcharges. Although the price is very high per gram of chemical, if you need only a small amount of the chemical, this can be the least expensive way to get it. For example, rather than ordering solid barium nitrate and paying hazard surcharges, you might find you can order 50 mL of 0.1 M barium nitrate solution for $2.50 without hazard surcharges. That solution contains only 1.3 g of barium nitrate, but that may be all you need. This, incidentally, is also a cost-effective way of ordering any chemical that you need in very small quantities, whether or not the solid or concentrated chemical is subject to surcharges.
Buy the chemical from a vendor that does not charge extra shipping for hazardous materials. (The vendor still has to pay those surcharges to the shipping company, but they simply absorb them as a cost of doing business.)
For some chemicals, notably nitric acid, option 4 is often the only economical choice. There are at least three good sources that do not charge hazardous shipping surcharges on most or all chemicals:
ScienceKit (http://www.sciencekit.com) does not charge hazardous material shipping surcharges on any of the broad range of chemicals it carries. ScienceKit does not sell any chemicals to individuals, but they will sell any chemical they stock to a recognized home school (you must provide evidence that your home school is a member of an official home-schooling group) and to businesses (a copy of your business license or IRS EIN suffices as proof). As of this writing, as strange as it sounds, your luck in ordering chemicals from ScienceKit may depend on what time of day you call. If you call before 5:00 p.m. EST, you connect to their New York group, which flatly refuses to sell chemicals to anyone but public schools and universities. If you call after 5:00 p.m. EST, you connect to their California group, which will happily sell chemicals to you as long as you can prove that you’re a recognized home school or business. And all you need to prove that you’re a business is a copy of an Employee Identification Number (EIN), which the IRS will issue to you upon request even if you have no employees.
Elemental Scientific (http://www.elementalscientific.net) sells a broad range of chemicals to individuals, most without any hazardous shipping surcharges. They do this by limiting the quantities they offer on their price list to amounts that can be shipped normally. For example, one chemical may be listed only in 1-ounce quantities, another in 1- and 4-ounce quantities, and still another in 1-, 4- and 16-ounce quantities. As long as you order only one of each chemical in a size that appears on the price list, there are no hazardous shipping surcharges. The only exceptions are chromic acid, chromium trioxide, 30% hydrogen peroxide, nitric acid, and sodium peroxide, which require a surcharge, and carbolic acid (phenol), barium chlorate, barium hydroxide, barium nitrate, barium peroxide, calcium carbide, calcium and sodium metal, ethylene dichloride, magnesium and zinc metal dust, mercuric iodide, mercuric oxide, mercurous chloride—all of which require a poison pack in addition to the hazardous shipping surcharge.
Home Science Tools (http://www.homesciencetools.com) sells a reasonably broad range of chemicals in small sizes, typically 30 g or 30 mL, to anyone, including individuals, without any hazardous shipping surcharges. HST doesn’t offer nitric acid, but does carry such things as concentrated hydrochloric and sulfuric acids, barium compounds, and numerous oxidizers.