Chapter 21. Laboratory: Synthesis of Useful Compounds
Dr. Mary Chervenak Comments
When a pathogen infects one part of the plant, the tissue under attack notifies surrounding healthy plants of impending danger. The molecular messenger is methyl salicylate.
Laboratory 21.1: Synthesize Methyl Salicylate From Aspirin
Esters are a class of organic compounds. An ester comprises an organic or inorganic acid in which one or more hydroxy (OH) groups has been replaced by an alkoxy (O-alkyl) group. For example, the simplest ester, methyl formate (CHO-OCH3), is made up of formic acid (CHO-OH) in which the hydroxy group has been replaced by a methoxy group (-OCH3). Similarly, ethyl acetate (CH3CO-OCH2CH3) is made up of acetic acid (CH3CO-OH) in which the hydroxy group has been replaced by an ethoxy group (-OCH2CH3).
Although esters can be produced by many mechanisms, the most commonly used method is called esterification, which is a condensation reaction between an alcohol and an acid, typically in the presence of a strong acid catalyst, such as sulfuric acid. For example, ethyl acetate can be produced by reacting ethanol (ethyl alcohol) with acetic acid and isopropyl butyrate by reacting isopropanol (isopropyl alcohol) with butyric acid.
Esters were traditionally named by combining the name of the alcohol with the root name of the acid and adding “ate” as a postfix. Traditional names are still widely used by most chemists, particularly for the simpler esters. The IUPAC naming system uses the systematic names for the alcohol and root name of the acid, followed by “oate.” For example, the traditional name n-amyl acetate (n-amyl alcohol with acetic acid) is represented in IUPAC nomenclature as 1-pentyl ethanoate (1-pentyl alcohol, the systematic name for n-amyl alcohol, with ethanoic acid, the systematic name for acetic acid).
Esters typically have strong, often pleasant, scents and tastes, so many esters are used as flavoring and perfume agents, either individually or in combination. For example, the scent and taste of strawberries is due to the presence of (among others) methyl cinnamate, ethyl formate, ethyl butyrate, ethyl caproate, isobutyl acetate, and benzyl acetate.
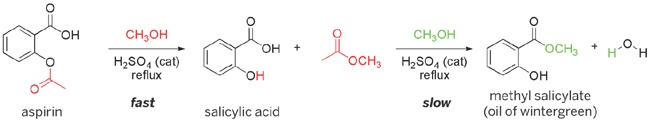
In this lab session, we’ll synthesize methyl salicylate, whose common name is oil of wintergreen. You might expect that we’d synthesize this compound by reacting methyl alcohol (methanol) with salicylic acid, and that is indeed one possible method. Instead, however, we’ll synthesize methyl salicylate by reacting methanol with aspirin, which is much easier to come by than salicylic acid. Aspirin is actually a substituted salicylic acid, called acetylsalicylic acid, which is itself both an acid and an ester.
Caution
Methanol is toxic (particularly to the optic nerve; eye protection is essential), extremely flammable, and has a flash point of only 11°C. (The flash point is the lowest temperature at which a flammable liquid can form an ignitable mixture in air.) Have a fire extinguisher handy and avoid all open flames. Concentrated sulfuric acid is extremely corrosive. Methyl salicylate is toxic, irritating, and penetrates the skin. Do not taste the methyl salicylate produced in this lab, and be cautious when you test its odor. Wear splash goggles, gloves, and protective clothing.
Procedure
This lab is broken into three parts. In Part I, we’ll synthesize methyl salicylate. In Part II, we’ll isolate and purify the product. In Part III, we’ll determine the density and freezing point of our product.
Before you begin this lab (or, indeed, any organic synthesis), you should calculate the theoretical yield for the reaction. We’ll react 13.0 g of aspirin with excess methanol to form methyl salicylate. Enter the actual mass of aspirin you use to 0.01 g on line A of Table 21-1. The formula weight of aspirin is 180.160 g/mol. Based on the actual mass of your aspirin sample, calculate the number of moles of aspirin to be reacted and enter that value on line B of Table 21-1. In the presence of an acid catalyst, one mole of salicylic acid reacts with one mole of methanol to produce one mole of methyl salicylate. (One mole of aspirin reacts with two moles of methanol to produce one mole of methyl acetate in addition to one mole of methyl salicylate.) The formula weight of methyl salicylate is 152.1494 g/mol. Calculate the theoretical yield of methyl salicylate in grams, and enter that value on line C of Table 21-1. The density of methyl salicylate is 1.1825 g/mL. Using this density and the theoretical yield in grams, calculate the theoretical yield of methyl salicylate in mL and enter that value on line D of Table 21-1.
Part I: Synthesize Methyl Salicylate
Perform this part of the lab under an exhaust hood or in a well-ventilated area. The reaction produces a strong odor of methyl salicylate and methanol vapor. Make certain that there are no open flames or other ignition sources nearby.
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Transfer about 60 mL of methanol to the 125 mL Erlenmeyer flask. Add 13.00 g of aspirin to the flask and swirl or stir the contents until the aspirin tablets dissolve. (Aspirin tablets contain binders and other inactive ingredients that may not dissolve in methanol, which is no cause for concern.)
Add about 10.0 mL of concentrated sulfuric acid to the reaction vessel and swirl to mix the solutions.
Clamp the flask to a ring stand and partially immerse it in a hot water bath at about 60°C.
Allow the reaction to proceed, stirring the reaction mixture occasionally, for 60 minutes. As the methyl salicylate forms, you’ll notice its distinct wintergreen odor. Keep the reaction mixture at about 60°C, adding water to the bath if necessary to keep its level up. The level of the liquid in the reaction vessel will decrease as methanol evaporates from the flask. Add more methanol as needed to keep the liquid in the reaction vessel near its original volume.
After 60 minutes, stop adding methanol to the flask. Increase heat slightly to bring the liquid in the reaction flask to a gentle boil. Boil the solution long enough to vaporize most of the remaining methanol.
When enough methanol has boiled off to reduce the volume of liquid in the reaction vessel to about half its original volume, remove the flask from the water bath and set it aside to cool.
Part II: Isolate and Purify the Product
The brown liquid in the reaction vessel is a complex solution that contains methanol, crude methyl salicylate, sulfuric acid, unreacted aspirin, and other impurities. Methyl salicylate is freely soluble in methanol, but only very slightly soluble in water. We’ll take advantage of that differential solubility to extract most of the water-soluble impurities from the crude product.
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Pour the contents of the reaction flask into the sep funnel.
Add about 50 mL of ice-cold tap water to the sep funnel, cap the funnel, and agitate the contents vigorously for 30 seconds.
Dr. Mary Chervenak Comments
Generally, sep funnels need to be vented after vigorous shaking. Even if gas isn’t generated, it’s good practice. The sodium bicarbonate wash in step 6 will definitely have to be vented after shaking. Enough gas can be generated to blow the stopper off the back of the sep funnel.
Allow the contents of the sep funnel to separate into two layers. The aqueous layer contains nearly all of the sulfuric acid and most of any other water-soluble impurities. (Make sure you know which layer is which.) Separate the two layers, and transfer the aqueous layer to the 250 mL beaker.
Do a second washing by repeating steps 3 and 4.
Add about 50 mL of sodium bicarbonate solution to the sep funnel, cap the funnel, and agitate the contents vigorously for 30 seconds. (See previous note on venting.)
Allow the contents of the sep funnel to separate into two layers. The aqueous layer contains an excess of sodium bicarbonate and a small amount of sodium sulfate produced by the neutralization of any sulfuric acid that remained in the organic layer. Separate the two layers, and transfer the aqueous layer to the 250 mL beaker.
Neutralize the sulfuric acid solution in the 250 mL beaker with sodium carbonate or sodium bicarbonate and flush the neutralized solution down the drain with plenty of water. Retain the organic layer in the sep funnel, which contains the crude methyl salicylate.
Part III: Determine Density and Freezing Point of the Product
Our product is a few mL of semi-pure methyl salicylate. If we had the equipment needed for microscale distillations, we could further purify our raw product by distillation. We don’t have that equipment, so we’ll test our product as-is to determine its density and freezing point.
We know that the density of pure methyl salicylate is 1.1825 g/mL, so the density of our product will give us some idea of its purity. That’s not sufficient, however. Even if the density of our product is very close to 1.1825 g/mL, for all we know our product might be a small amount of methyl salicylate mixed with a large amount of some impurity that has the same density. The freezing point of our product will give us a better idea of its purity. Pure methyl salicylate freezes (or melts) at –8.3°C. Furthermore, the sharpness of the freezing/melting point gives a good indication of a product’s purity. Pure substances tend to freeze/melt very sharply, at one particular temperature. Impure substances freeze/melt more gradually, over a range of a few degrees.
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Weigh a clean, dry, empty 50 mL beaker and record its mass to 0.01 g on line E of Table 21-1.
Draw up as much as possible of your product into the 10.0 mL Mohr or serological pipette. Record the volume of product (if possible, to 0.01 mL) on line F of Table 21-1. (If you somehow produced more than 10 mL of product, use as close as possible to a 10.00 mL sample.)
Transfer the measured product to the 50 mL beaker. Reweigh the beaker and record the combined mass of the beaker and methyl salicylate to 0.01 g on line G of Table 21-1. Subtract the mass of the empty beaker from the combined mass of the beaker and sample, and enter that value to 0.01 g on line H of Table 21-1. Divide the mass of the sample (line H) by the volume of the sample (line F) to determine the density of the sample. Enter that value on line I of Table 21-1.
Transfer as much as possible of the sample to a test tube. Immerse the test tube in the ice bath, and use a thermometer (carefully) to stir the contents of the test tube. When the temperature of the sample falls to 0°C, check the contents of the tube frequently to see whether solid crystals of methyl salicylate have begun to form. (You’ll need to remove the tube from the ice bath and examine it against a strong light to detect the first formation of crystals.) Record the temperature at which crystals just begin to form on line J of Table 21-1.
Continue cooling the contents of the tube until the methyl salicylate freezes solid. (Leave the thermometer embedded in the sample.) Record that temperature on line K of Table 21-1.
Remove the test tube from the ice bath and allow it to begin warming. Record the temperature at which the frozen sample first begins to liquefy on line L of Table 21-1.
Allow the tube to continue warming. Stir gently with the thermometer, and record the temperature at which the sample again becomes completely liquid on line M of Table 21-1.
Disposal
The methyl salicylate produced in this lab is not safe for human consumption under any circumstances. It contains numerous impurities, some of which may be toxic. (Or perhaps I should say even more toxic than methyl salicylate itself.) Do not taste it or allow it to contact your skin. Discard it by flushing it and all other waste solutions from this lab down the drain with plenty of water. Alternatively, you can use your crude methyl salicylate in a scent candle by mixing it with paraffin.
Item | Value |
A. Mass of aspirin | __________. ____ g |
B. Moles of aspirin (A/[180.160 g/mol]) | ___.________ mol |
C. Theoretical yield of methyl salicylate (B · [152.1494 g/mol]) | __________. ____ g |
D. Theoretical yield of methyl salicylate (C/[1.1825 g/mL]) | ______._____ mL |
E. Mass of empty 50 mL beaker | __________. ____ g |
F. Volume of methyl salicylate sample | ______._____ mL |
G. Mass of beaker + methyl salicylate sample | __________. ____ g |
H. Mass of methyl salicylate sample (G – E) | __________. ____ g |
I. Density of methyl salicylate sample (H/F) | _______. ____ g/mL |
J. Temperature at which crystals begin to form | ______.____°C |
K. Temperature at which sample freezes solid | ______.____°C |
L. Temperature at which sample begins to melt | ______.____°C |
M. Temperature at which sample is fully melted | ______.____°C |
Review Questions
Laboratory 21.2: Produce Rayon Fiber
In this lab session we produce rayon, the first practical artificial fiber. We use the word “artificial,” because rayon is neither a natural fiber nor a synthetic fiber. What other option is there? Half and half. Rayon, first produced commercially in 1899, is actually a semisynthetic or reconstituted form of the natural polymer cellulose.
Rayon was first produced commercially using the cuprammonium process, a process that is still in limited use today but has been largely supplemented by alternative processes that are more environmentally friendly. In the cuprammonium process, which we use in this lab session, solid cellulose (such as paper or wood chips) is dissolved in Schweizer’s Reagent, a coordination compound of copper(II) hydroxide in aqueous ammonia. The cellulose is then reconstituted by reacting the tetraamminecopper(II) hydroxide solution with an acid, to neutralize the ammonia and destroy the coordination compound that makes cellulose soluble. The cellulose precipitates as a solid.
Caution
Concentrated aqueous ammonia is corrosive and produces strong irritating fumes. Sulfuric acid and sodium hydroxide are corrosive. Copper sulfate is moderately toxic. Wear splash goggles, gloves, and protective clothing.
Procedure
Perform this lab under an exhaust hood or in a well-ventilated area to dissipate the fumes of concentrated aqueous ammonia.
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Weigh out about 25 g of copper sulfate pentahydrate and transfer it to the 250 mL beaker. Add about 100 mL of water and swirl or stir until the copper sulfate dissolves completely. (This is a nearly saturated solution; you may warm the solution to dissolve the copper sulfate faster.)
Transfer about 50 mL of water to the 250 mL Erlenmeyer flask and sufficient crushed or chipped ice to bring the total volume to about 125 mL. Weigh out about 8 g of sodium hydroxide and transfer it in small portions, with swirling, to the flask. (Caution: this process is extremely exothermic. Add the sodium hydroxide slowly.)
With both solutions at about room temperature, pour the sodium hydroxide solution into the copper sulfate solution, with stirring. The solution immediately turns a milky powder-blue color as the two solutions react to form a precipitate of insoluble copper(II) hydroxide.
Rinse the 250 mL Erlenmeyer flask immediately and set it aside to drain.
Set up your filter funnel, using the 600 mL beaker as the receiving container.
Pour the blue mixture from the beaker into the filter funnel. The blue precipitate is voluminous, so you may have to use several pieces of filter paper to get all of it. (I used 11 cm filter paper, and it took me three filtrations to capture all of the precipitate. If you use 15 cm filter paper, you may be able to get all of the precipitate in one filtering pass.)
When the filter paper fills with precipitate, allow the remaining filtrate to drain into the receiving vessel and then wash the filtrand with about 10 mL of water and allow the water to drain.
Transfer the filtrand, filter paper and all, to the 250 mL Erlenmeyer flask. If you weren’t able to capture all of the precipitate on one piece of filter paper, repeat steps 7 and 8 until you have transferred all of the precipitate to the flask.
Measure about 70 mL of concentrated aqueous ammonia, and add it to the 250 mL Erlenmeyer flask with swirling. (The copper(II) hydroxide in the flask reacts with the aqueous ammonia to form tetraamminecopper(II) hydroxide, also known as Schweizer’s Reagent.)
Including the pieces you used to do the filtration, add pieces of filter paper to the flask to a total of 5 pieces of 11 cm filter paper or 3 pieces of 15 cm filter paper. (The filter paper provides the cellulose that will be dissolved by the tetraamminecopper(II) hydroxide.)
Stopper the flask and set it aside to allow the tetraamminecopper(II) hydroxide to dissolve the filter paper. Swirl the flask periodically, and allow the reaction to proceed for at least 24 to 48 hours.
Prepare about 300 mL of about 1.5 M sulfuric acid solution in the 600 mL beaker. (Caution: this process is extremely exothermic. Add the acid slowly to the water with stirring.)
Pour the cellulose solution from the 250 mL Erlenmeyer flask into the 250 mL beaker. If there is undissolved filter paper in the flask, discard it.
Fill the syringe with the cellulose solution, submerge the tip of the syringe in the sulfuric acid bath, and slowly expel a stream of cellulose solution into the sulfuric acid. Refill the syringe and repeat until you have transferred all of the cellulose solution to the sulfuric acid bath. The threads and clumps of solid material that appear are rayon.
Carefully pour off the liquid into a waste container, retaining the raw rayon. Rinse the rayon several times with tap water, discard the rinse water, and allow the rayon to dry.
Disposal
The waste solution produced in this lab session is a solution of copper sulfate in sulfuric acid. Neutralize this solution by adding sodium carbonate or sodium bicarbonate until all of the copper precipitates as blue copper carbonate. Discard the supernatant solution of sodium sulfate by flushing it down the drain with plenty of water. You can retain the crude copper carbonate for later use or dispose of it with household solid waste.