Chapter 9. Laboratory: Introduction to Chemical Reactions and Stoichiometry
There are many different types of chemical reactions. In this chapter, we examine the following reaction types:
Composition reaction
A composition reaction, also called a combination reaction, occurs when two or more reactants combine to yield one or more products. Composition reactions take the general form A + B → AB, where the reactants A and B may be elements or compounds and the compound AB is the resulting product. For example, if you heat a mixture of iron filings (A) and sulfur (B), those two elements react to form iron sulfide (AB).
Decomposition reaction
A decomposition reaction occurs when one reactant is broken down into two or more products, usually by the application of heat. Decomposition reactions take the general form AB → A + B, where the compound AB is the reactant and the products A and B may be elements or compounds. For example, if you heat sodium hydrogen carbonate (sodium bicarbonate or baking soda, NaHCO3), it breaks down into sodium carbonate (Na2CO3), carbon dioxide (CO2), and water (H2O). In this example, the general form AB → A + B is expanded to ABC → A + B + C or, more precisely, 2 ABC → A + B + C, where ABC is sodium bicarbonate, A is sodium carbonate, B is carbon dioxide, and C is water. The balanced equation for this reaction is:
2 NaHCO3 → Na2CO3 + CO2 + H2O
Displacement reaction
A displacement reaction, also called a replacement reaction, occurs when two or more reactant substances recombine to form two or more different products.
Single displacement reaction
In a single displacement reaction, a more active element displaces a less active element in a compound, forming a new compound that incorporates the more active element and releasing the less active element in elemental form. Single displacement reactions take the general form A + BX → AX + B, where A is the more active element, B is the less active element, and X is an anion (such as chloride, sulfate, or nitrate). For example, if you react sodium metal (Na) with hydrochloric acid (HCl), the more active sodium displaces the less active hydrogen, according to the following balanced equation:
2 Na + 2 HCl → 2 NaCl + H2
Double displacement reaction
In a double displacement reaction, also called a metathesis reaction, two compounds exchange elements or ionic species with each other. Double displacement reactions take the general form AX + BY → AY + BX, where A and B are cations and X and Y are anions. If AX and BY are both freely soluble and (for example) AY is insoluble, combining solutions of AX and BY results in a precipitate.
Any of these reaction types can be represented by stoichiometrically balanced equations. For example, the decomposition reaction of sodium hydrogen carbonate into sodium carbonate, carbon dioxide, and water can be represented as:
NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
or, in balanced form:
2 NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
The laboratory sessions in this chapter explore various aspects of these types of chemical reactions.
Laboratory 9.1: Observe a Composition Reaction
The reaction of iron and sulfur to form iron(II) sulfide is an example of the simplest type of composition reaction, one in which two elements react to form a compound. In its simplest form, the balanced equation can be represented as:
Fe(s) + S(s) → FeS(s)
In fact, it’s a bit more complicated. Although one iron atom can react with one sulfur atom to yield one molecule of iron(II) sulfide, solid sulfur exists as a molecule that comprises eight bound sulfur atoms. The balanced equation therefore becomes:
8 Fe(s) + S8(s) → 8 FeS(s)
But we’re not finished yet. The reaction of iron and sulfur is an example of a class of reactions called nonstoichiometric reactions. In a stoichiometric reaction, the proportions of the reactants are fixed. For example, exactly one mole of sodium (Na) reacts stoichiometrically with exactly one mole of chlorine (Cl) to yield exactly one mole of sodium chloride (NaCl), or two moles of aluminum (Al) react with six moles of hydrogen chloride (HCl, hydrochloric acid) to yield two moles of aluminum chloride (AlCl3) and three moles of molecular hydrogen gas (H2). In a nonstoichiometric reaction, the proportions of the reactants are not fixed relative to those of the product. In other words, different amounts of iron can react with different amounts of sulfur to yield a product in which the proportions of iron and sulfur may vary according to the available quantities of the reactants and the conditions under which the reaction takes place.
Three products are possible, which may occur in relatively pure form or as a mixture. All three of these products occur naturally as minerals, and can be produced in the lab by adjusting the proportions of the reactants and the reaction conditions.
Troilite
Troilite is the most common natural form of iron(II) sulfide. It has the formula FeS, and contains stoichiometrically equivalent amounts of iron and sulfur.
Pyrrhotite
Pyrrhotite has the general formula Fe(1–x)S, which indicates that it contains a stoichiometric deficiency of iron and therefore a stoichiometric excess of sulfur. In other words, even though there is insufficient iron present to react stoichiometrically with all of the sulfur present, the iron that is present reacts with all of the sulfur to form a nonstoichiometric form of iron sulfide in which the proportions differ from the stoichiometric form.
Caution
This experiment uses the hot flame provided by a gas burner. Be careful with the flame, and have a fire extinguisher readily available. The reaction may be vigorous and throw sparks that may ignite flammable materials. The sulfur may ignite and emit toxic and choking sulfur dioxide fumes, so perform this experiment under a fume hood or strong exhaust fan or do it outdoors. (Even without ignition, this reaction smells really, really bad.) The product, iron sulfide, is pyrophoric when in powdered form, which means that it may ignite spontaneously. Wear splash goggles, gloves, and protective clothing.
Mackinawite
Mackinawite has the general formula Fe(1+x)S, which indicates that it contains a stoichiometric excess of iron and therefore a stoichiometric deficiency of sulfur.
In this lab, we’ll prepare iron(II) sulfide using quantities of reactants that are stoichiometrically balanced. Our product should therefore be primarily the FeS (troilite) form of the compound, with perhaps minor amounts of the other two forms as contaminants. We’ll use a slight excess of sulfur, because unless the reaction occurs under an inert atmosphere, it’s common for the heat of the reaction to ignite the sulfur, converting some of it to sulfur dioxide gas.
Procedure
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Set up your tripod stand in the sand bed with your gas burner set up to direct the hottest part of its flame at the center of the ring.
Weigh the tin can lid to 0.01 g and record its mass on line A of Table 9-1.
Center the tin can lid flat on the tripod ring.
Weigh out 3.0 g of iron filings and 2.0 g of sulfur to within 0.01 g and record the masses on lines B and C of Table 9-1.
Calculate the number of moles of iron filings and sulfur, and enter those values on lines D and E of Table 9-1.
Determine the limiting reagent from the values you calculated in the preceding step. Assume that one mole of iron reacts with one mole of sulfur to form one mole of FeS, calculate the expected mass of FeS and enter that value on line F of Table 9-1.
Combine the iron filings and sulfur in the center of a folded sheet of ordinary paper and flex the paper back and forth until the two chemicals are well mixed.
Pour the mixed iron and sulfur into a small pile in the center of the can lid. Try to make the pile as compact as possible. If the material is spread out, it’s likely that the sulfur will catch fire and burn off to sulfur dioxide gas before the reaction between the iron and sulfur begins.
If you are not performing this experiment outdoors, turn on the fume hood or exhaust fan. (And you will probably regret doing this indoors, even with an exhaust fan.)
Light the gas burner, and make sure that it’s centered under the pile of iron and sulfur. At first, nothing appears to happen. As you continue heating the mixture, some of the sulfur begins to melt. Soon after, you should notice a glow begin to develop in the mixture as the iron and sulfur begin to react. Once that glow becomes obvious, turn off the gas burner and allow the reaction to proceed to completion on its own. See Figure 9-1.
Caution
Sulfur dioxide is a toxic gas with a sharp, choking odor. You’ll certainly get a whiff of it when you do this experiment. It will probably remind you of the smell produced by firecrackers. If too much sulfur dioxide is produced or the reaction becomes too vigorous, stop the reaction by gripping the tin can lid with the tongs and dropping the lid and reaction mixture into the pail of water. Evacuate the area until the exhaust fan clears the air.
Once the reaction has completed, allow several minutes for the iron(II) sulfide and the equipment to cool.
Once the lid and iron(II) sulfide have cooled, weigh them to 0.01 g and record the combined mass on line G of Table 9-1. Calculate the actual mass of the product by subtracting line A from line G and record this mass to 0.01 g on line H of Table 9-1.
Assuming that the product is pure FeS, calculate the percent yield by dividing the actual yield (line H) by the theoretical yield (line F) and multiplying by 100. Enter this value on line I of Table 9-1.
Use the spatula to scrape the iron(II) sulfide into your mortar. The product is a fused mass of lumps. Use the pestle to crush the lumps into a coarse powder.
Use the magnet to test the iron(II) sulfide for ferromagnetism (attraction to a magnet). If the reaction has proceeded to completion and there is not an excess of elemental iron present, the product should not be attracted to the magnet.
Sulfur does not react with hydrochloric acid under normal conditions. Iron reacts with hydrochloric acid to form hydrogen gas. Iron(II) sulfide reacts with hydrochloric acid to form hydrogen sulfide, which has an intense odor of rotten eggs. Test for the presence of iron(II) sulfide by adding a few sand-size grains of your product to a test tube that contains about 1 mL of concentrated hydrochloric acid and checking for the odor of hydrogen sulfide. (Note that concentrated hydrochloric acid itself has a strong, distinctive odor, but the odor of hydrogen sulfide is quite different.) Don’t put the test tube under your nose to check for hydrogen sulfide. Instead, hold the test tube well away from your face and use your hand to waft the odor from the test tube toward your nose.
Caution
In addition to smelling awful, hydrogen sulfide gas is extremely toxic—more so than hydrogen cyanide. Hydrogen sulfide is particularly pernicious, because the human nose rapidly becomes desensitized to its odor. That means it’s possible to be exposed to a lethal concentration of hydrogen sulfide without noticing a particularly strong odor. Use only a few tiny grains of iron(II) sulfide, and limit your exposure to hydrogen sulfide to a short, wafted sniff. Never put the test tube under your nose.
Disposal
The waste material from this lab can be ground to powder and flushed down the drain with plenty of water.
Item | Data |
A. Mass of tin can lid | ______.______ g |
B. Mass of iron filings | ______.______ g |
C. Mass of sulfur | ______.______ g |
D. Moles of iron filings | ____.________ moles |
E. Moles of sulfur | ____.________ moles |
F. Expected mass of FeS | ______.______ g |
G. Mass of tin can lid + product | ______.______ g |
H. Actual mass of product (G – A) | ______.______ g |
I. Percent yield (100 · H/F) | ______.______ % |
Review Questions
Laboratory 9.2: Observe a Decomposition Reaction
The refractory (using heat) decomposition of sodium hydrogen carbonate to sodium carbonate, carbon dioxide, and water is a typical decomposition reaction, in which a compound reacts to form two or more other elements and/or compounds. The balanced equation for this reaction is:
2 NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
I didn’t choose this example decomposition reaction arbitrarily. The refractory decomposition of sodium bicarbonate to sodium carbonate is the second most important decomposition reaction used in industrial processes, and is crucial to the world’s economy. This reaction is the final step in the Solvay process, which is used to produce about 75% of the world’s supply of sodium carbonate, also known as washing soda or soda ash. (The remaining 25% is mined or extracted from brine.) Worldwide production of sodium carbonate is more than 40 billion kilograms annually, about 7 kilograms for every person on the planet. Sodium carbonate is an essential component of glass, and is also used for hundreds of other purposes, including processing wood pulp to make paper, as an ingredient in soaps and detergents, as a buffer, and as a neutralizer.
Unlike many decomposition reactions, which are essentially irreversible, the refractory decomposition of sodium hydrogen carbonate is easily reversible, and in fact a considerable amount of sodium hydrogen carbonate is produced commercially by this reverse reaction.
Na2CO3(s) + CO2(g) + H2O(g) → 2 NaHCO3(s)
Carbon dioxide gas is bubbled through a saturated solution of sodium carbonate and reacts to form sodium hydrogen carbonate, which is less soluble than sodium carbonate. The sodium hydrogen carbonate precipitates out, and is isolated by filtration.
In this lab, we’ll produce sodium carbonate from sodium hydrogen carbonate via a decomposition reaction.
Caution
This experiment uses heat. Be careful with the heat source and when handling hot objects, and have a fire extinguisher readily available if your heat source uses flame. Wear splash goggles, gloves, and protective clothing.
Procedure
This laboratory has two parts. In Part I, we’ll do qualitative testing to determine the products of the reaction. In Part II, we’ll do some semi-quantitative testing to verify that the mass of the solid product (sodium carbonate) closely matches the mass we would expect from examining the balanced equation for the reaction.
Part I
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Add sodium hydrogen carbonate to the test tube until it is about one quarter full.
Light your gas burner (or alcohol lamp).
Using the test tube holder or clamp, hold the test tube so that the flame heats that part of the test tube that contains the sodium hydrogen carbonate. Try to play the flame evenly over the entire sample. (Using a clamp mounted on a ring stand rather than a test tube holder makes the following steps easier, because it frees both hands.)
After you have heated the sample for at least 15 to 30 seconds, ignite a toothpick or wood splint and insert the burning end into the top of the test tube. If the flame or ember is snuffed out (Figure 9-2), that’s a good indication that the reaction is producing carbon dioxide as expected. If the splint continues to burn, continue heating the sample until enough carbon dioxide is produced to displace the air inside the test tube and try using the splint again.
As you heat the sample, you should notice a liquid condensing on the cooler inside upper surface of the test tube, at first as fogging and later as actual droplets. Touch a blue cobalt chloride test strip to this liquid. Does it turn pink? If so, that confirms that the liquid is water.
Part II
In Part II, we’ll determine the mass loss that occurs when sodium hydrogen carbonate is heated to decompose it to sodium carbonate. In theory, all of the mass loss is attributable to the outgassing of carbon dioxide and water during the heating process, and the remaining mass should represent only sodium carbonate. In practice, things can be a bit more complicated. Sodium carbonate exists in four hydration states: anhydrous (Na2CO3), the monohydrate (Na2CO3 • 1H2O), the heptahydrate (Na2CO3 • 7H2O), and the decahydrate (Na2CO3 • 10H2O). If we don’t heat the sample strongly enough, it’s possible that some of the water produced by the decomposition will be retained by the sodium carbonate as water of hydration. Accordingly, we’ll heat our sample at a high enough temperature and for long enough to ensure that it is completely converted to anhydrous sodium carbonate.
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Place the covered crucible in the clay triangle, and adjust the height of the supporting ring to put the bottom of the crucible in the hottest part of the flame. Make sure that the crucible lid is positioned to allow gases to be vented. Heat the empty crucible and lid strongly for at least a minute to drive off any water and volatile contaminants present.
After allowing them to cool completely, weigh the crucible and lid and record the mass to 0.01 g on line A of Table 9-2.
Add approximately 5.0 g of sodium hydrogen carbonate to the crucible, reweigh the crucible and lid, and record the mass to 0.01 g on line B of Table 9-2.
Subtract the empty mass of the crucible and lid from the combined mass of the crucible, lid, and contents, and record the mass of the sodium hydrogen carbonate to 0.01 g on line C of Table 9-2.
Place the covered crucible in the clay triangle, make sure that the crucible lid is positioned to allow gases to be vented, and heat the crucible strongly for 15 minutes.
After allowing the crucible, lid, and contents to cool completely, reweigh the crucible, lid, and contents and record the mass to 0.01 g on line D of Table 9-2.
Determine the mass of the product remaining in the crucible by subtracting the mass of the empty crucible and lid from the combined mass of the crucible, lid, and contents after heating. Record the mass of the sodium carbonate to 0.01 g on line E of Table 9-2.
Calculate the mass loss attributable to outgassing of carbon dioxide and water vapor by subtracting the mass of the sodium carbonate product (line E) from the mass of the sodium hydrogen carbonate reactant (line C). Enter the mass loss to 0.01 g on line F of Table 9-2. Calculate the mass loss percentage by multiplying F by 100, then dividing by C. Enter the result on line G.
Item | Data |
A. Mass of crucible and lid | ______.______ g |
B. Mass of crucible, lid, and sample (before heating) | ______.______ g |
C. Mass of sodium hydrogen carbonate (B – A) | ______.______ g |
D. Mass of crucible, lid, and sample (after heating) | ______.______ g |
E. Mass of sodium carbonate (D – A) | ______.______ g |
F. Mass loss (C – E) | ______.______ g |
G. Mass loss percentage (100 · F/C) | ______.______ % |
Review Questions
Laboratory 9.3: Observe a Single Displacement Reaction
In a single displacement reaction, a more active element displaces a less active element in a compound, forming a new compound that incorporates the more active element and releasing the less active element in elemental form. The reaction of an active metal with an acid is a common type of single displacement reaction in which the metal is oxidized to a cationic oxidation state and the hydrogen present in the acid is reduced to elemental hydrogen. The fizzing and bubbling that occurs when an active metal comes into contact with a strong acid is caused by the emission of gaseous hydrogen.
A nonchemist might believe that the metal “dissolves” in the acid. That’s not true, of course. When we dissolve table salt or sugar in water, for example, no chemical reaction occurs. We can recover the solute unchanged, simply by evaporating the solvent. But when we “dissolve” a metal in an acid, evaporating the liquid does not allow us to recover the metal unchanged. Instead, the metal has been converted to a salt by a single displacement reaction.
Different metals have different reactivity, as shown in Table 9-3. The most active metals, such as lithium and potassium, react (sometimes explosively) even with cold water to release hydrogen gas. Less active metals, such as magnesium and aluminum, do not react with cold water, but do react with steam, particularly if the metal is finely divided and the steam is superheated. Still less active metals, such as cobalt and nickel, do not react with water in any form, but do react with acids to release hydrogen gas. Finally, the least active metals, from antimony and bismuth to platinum and gold, do not react even with acids under normal circumstances. (Even platinum and gold do react with aqua regia, a mixture of concentrated hydrochloric and nitric acids.)
Another common type of single displacement reaction is the reaction of a solid metal immersed in a solution of a salt of another metal. For example, an iron (or steel) nail immersed in a solution of copper sulfate becomes plated, because the copper ions are reduced to metallic copper while the iron metal is oxidized to iron ions. If sufficient metallic iron is available, eventually all of the copper ions are reduced, leaving metallic copper in a solution of iron sulfate.
In this laboratory, we react aluminum metal with hydrochloric acid to form aluminum(III) chloride and hydrogen gas. The balanced equation for this reaction is:
2 Al(s) + 6 HCl(aq) → 2 AlCl3(aq) + 3 H2(g)
We’ll observe the reaction and test for the presence of hydrogen gas by using a burning splint. We also react iron metal with a solution of copper sulfate to form iron(II) sulfate and copper metal. The balanced equation for this reaction is:
Fe(s) + CuSO4(aq) → Cu(s) + FeSO4(aq)
Caution
Concentrated hydrochloric acid is corrosive and emits hazardous fumes. Keep the test tube pointed away from you and in a safe direction in case the reaction proceeds too vigorously. Spilled acid can be neutralized with an excess of solid sodium bicarbonate (baking soda). Hydrogen gas is extremely flammable. Although only a small amount of hydrogen is generated in this experiment, it may “pop” when ignited, startling you and causing you to drop the test tube. Do not sniff the test tube during or after the reaction, because the heat of the reaction liberates irritating hydrogen chloride gas. Perform this experiment outdoors, or under an efficient fume hood or exhaust fan. Wear splash goggles, gloves, and protective clothing.
Procedure
This laboratory has two parts. In Part I, we’ll observe a single-displacement reaction in which aluminum metal displaces hydrogen from hydrochloric acid. In Part II, we’ll observe another single-displacement reaction in which iron metal displaces copper ions from a solution of copper sulfate.
Part I
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Transfer about 5 mL of concentrated hydrochloric acid to one of the test tubes in your test tube rack.
Ignite a toothpick or wood splint, and hold it just above the mouth of the test tube to verify that no flammable gas is present.
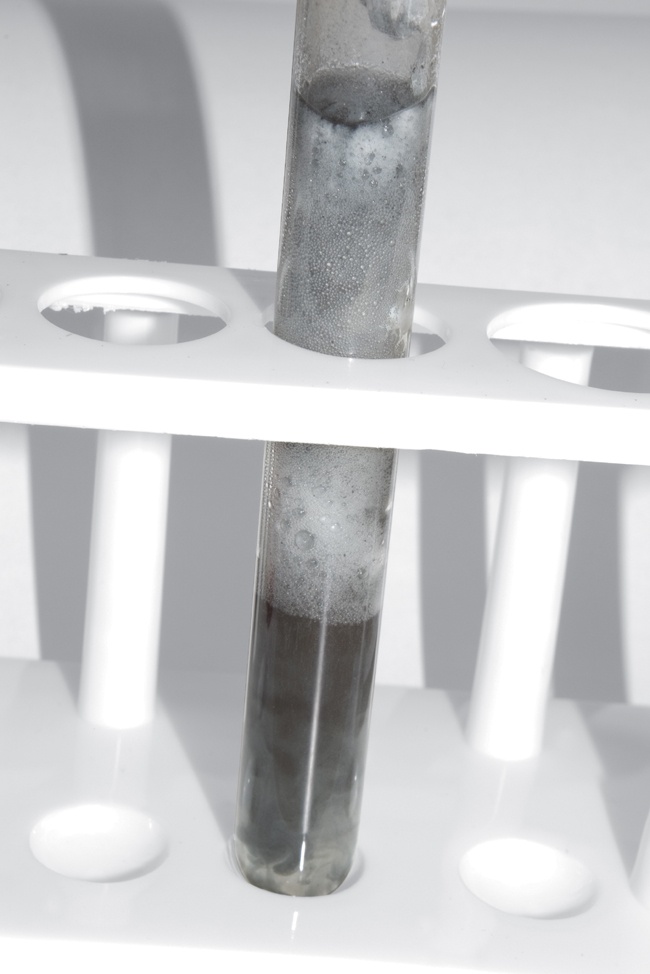
Weigh about 1 g of aluminum granules, and transfer them to the test tube with the hydrochloric acid. (Depending on the fineness of the aluminum metal, the reaction can be quite vigorous, so make sure that the mouth of the test tube is pointing in a safe direction in case liquid is ejected.) It takes a moment for the hydrochloric acid to remove the aluminum oxide that coats the aluminum metal. After this oxide coating is removed, the reaction proceeds vigorously (Figure 9-3).
Ignite another toothpick or wood splint and hold the burning end just above the top of the test tube. The hydrogen gas produced by the reaction ignites. (Don’t be startled if the gas ignites with a noticeable pop; the small amount of hydrogen gas produced by this reaction is harmless.)
Part II
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Weigh about 2.5 g of copper sulfate pentahydrate and add it to a test tube in the rack.
Transfer about 10 mL of water to the test tube, and swirl or shake the test tube until the copper sulfate dissolves completely.
Use the sandpaper or emery board to clean the surface of the nail thoroughly. Remove all oxidation and leave a shiny, bright surface.
Tie the thread around the head of the nail, and carefully submerge the nail in the solution of copper sulfate, keeping the thread outside the tube.
After 15 minutes and then again every 15 minutes, withdraw the nail, and observe and note any visible changes, and then return it to the copper sulfate solution. Continue this until no further change is evident (Figure 9-4).
Review Questions
Laboratory 9.4: Stoichiometry of a Double Displacement Reaction
In a double displacement reaction, two compounds exchange elements or ionic species with each other. Double displacement reactions take the general form AX + BY → AY + BX, where A and B are cations (positively charged ions) and X and Y are anions (negatively charged ions). Note that the reactants combine in a fixed proportion—1:1 in this case—and that the products are also in a fixed proportion, again 1:1 in this case.
The proportions are not always 1:1. For example, copper(II) sulfate reacts with sodium hydroxide to form copper(II) hydroxide and sodium sulfate, according to the following balanced equation:
CuSO4(aq) + 2NaOH(aq) → Cu(OH)2(s) + Na2SO4(aq)
or, looking at the ionic species:
Cu2+(aq) + SO42-(aq) + 2Na+(aq) + 2OH-(aq) → Cu(OH)2 (s) + 2Na+(aq) + SO42-(aq)
Note that on both sides of both equations the number of molecules (or moles) of the cationic species (copper and sodium) and the anionic species (sulfate and hydroxide) are the same and that the total net charge is zero.
In this lab, we’ll react solutions of copper(II) sulfate and sodium hydroxide to produce copper(II) hydroxide and sodium sulfate. Knowing the mass of the copper(II) sulfate we used and after determining the masses of the copper(II) hydroxide and sodium sulfate products, we’ll examine the stoichiometric relationship of the reactants and products and determine whether our balanced equation accurately represents the actual reaction.
To illustrate that reactions are useful for practical purposes as well as learning purposes, we’ll use the copper(II) hydroxide produced by this reaction as one of the reactants needed to synthesize a new compound that we’ll use in a later laboratory session. This compound, tetraamminecopper dihydroxide, [Cu(NH3)4](OH)2, or Schweizer’s Reagent for short, has the interesting property of being able to dissolve cellulose (for example, wood pulp, cotton, paper), and is used to produce rayon and other semisynthetic fibers.
Caution
Copper sulfate is moderately toxic. Sodium hydroxide is corrosive and caustic. Aqueous ammonia is corrosive and irritating. Wear splash goggles, gloves, and protective clothing.
Procedure
This laboratory has two parts. In Part I, we’ll react solutions of copper(II) sulfate and sodium hydroxide to produce copper(II) hydroxide and sodium sulfate, determine the masses of the products, and calculate the stoichiometric relationships for the reaction. In Part II, we’ll use the copper(II) hydroxide we produced in Part I to synthesize Schweizer’s Reagent.
Part I
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Weigh about 24.97 g of copper(II) sulfate pentahydrate, and record the mass to 0.01 g on line A of Table 9-4.
Calculate the number of moles of copper(II) sulfate represented by this mass, and record the number of moles on line B of Table 9-4.
Transfer the copper sulfate to a 150 mL beaker, add about 100 mL of water, and stir until the copper sulfate dissolves completely.
Weigh about 8.00 g of sodium hydroxide, and record the mass to 0.01 g on line C of Table 9-4.
Calculate the number of moles of sodium hydroxide represented by this mass, and record the number of moles on line D of Table 9-4.
Transfer about 10 mL of water to the second 150 mL beaker, and add the sodium hydroxide to the water with constant stirring or swirling. (Caution: this reaction is extremely exothermic.)
With constant stirring, pour the sodium hydroxide solution into the beaker of copper(II) sulfate solution. Rinse the sodium hydroxide beaker two or three times with a few mL of water and transfer the rinse water to the beaker of copper sulfate solution to make sure that you’ve transferred all of the sodium hydroxide (Figure 9-5).
Rinse the empty beaker thoroughly and set up your funnel holder and funnel with the empty beaker as the receiving container.
Fanfold a piece of filter paper, weigh it, record its mass to 0.01 g on line E of Table 9-4, and place the filter paper in the funnel.
Stir or swirl the beaker that contains the copper(II) hydroxide to make sure as much as possible is suspended in the solution, and then pour the solution into the filter funnel, catching the filtrate in the empty 150 mL beaker. Rinse the reaction beaker two or three times with a few mL of water and transfer the rinse water into the filter funnel to make sure all of the copper(II) hydroxide is retained by the filter paper.
Squirt a few mL of water onto the retained copper(II) hydroxide in the filter paper to rinse as much of the soluble sodium sulfate as possible into the receiving container.
Carefully remove the filter paper from the funnel and set it aside to dry. You can speed drying by placing the filter paper and product under an incandescent lamp or by drying it gently in an oven at the lowest heat setting.
Weigh the evaporating dish and record its mass to 0.01 g on line F of Table 9-4.
Place the evaporating dish on the hotplate, transfer as much of the filtrate as the dish can comfortably contain, and turn the hotplate on, set to low heat. As the solution in the dish evaporates, transfer more of the filtrate until all of it has been transferred to the dish. Finally, rinse the receiving beaker with a few mL of water, and transfer the rinse water to the evaporating dish. When the liquid in the evaporating dish is nearly gone, watch the dish carefully and continue heating until the solution has been evaporated to dryness.
While you are waiting for the copper(II) hydroxide and sodium sulfate to dry, calculate the expected masses for both of them based on the initial masses of the copper(II) sulfate and sodium hydroxide. (Remember that one mole of copper(II) sulfate reacts with two moles of sodium hydroxide, and remember to determine the limiting reagent before you calculate the expected masses.) Enter the expected masses of copper(II) hydroxide and sodium sulfate on lines G and H of Table 9-4, respectively.
When the copper(II) hydroxide is dry, weigh the filter paper and product, and record their mass to 0.01 g on line I of Table 9-4. Subtract the mass of the filter paper (line E) from the combined mass of the filter paper and product (line I) to determine the mass of copper(II) hydroxide, and record that value to 0.01 g on line J of Table 9-4.
When the sodium sulfate is dry, weigh the evaporating dish and product, and record their mass to 0.01 g on line K of Table 9-4. Subtract the mass of the evaporating dish (line F) from the combined mass of the evaporating dish and product (line K) to determine the mass of sodium sulfate, and record that value to 0.01 g on line L of Table 9-4.
Calculate the actual percent yields of copper(II) hydroxide and sodium sulfate, and enter those values on lines M and N of Table 9-4, respectively.
Item | Data |
A. Mass of copper sulfate pentahydrate | ______.______ g |
B. Moles of copper sulfate pentahydrate | ____.________ moles |
C. Mass of sodium hydroxide | ______.______ g |
D. Moles of sodium hydroxide | ____.________ moles |
E. Mass of filter paper | ______.______ g |
F. Mass of evaporating dish | ______.______ g |
G. Expected mass of copper(II) hydroxide | ______.______ g |
H. Expected mass of sodium sulfate | ______.______ g |
I. Mass of filter paper + copper(II) hydroxide | ______.______ g |
J. Actual mass of copper(II) hydroxide (I – E) | ______.______ g |
K. Mass of evaporating dish + sodium sulfate | ______.______ g |
L. Actual mass of sodium sulfate (K – F) | ______.______ g |
M. Percent yield of copper(II) hydroxide (100 · J/G) | ______.______ % |
N. Percent yield of sodium sulfate (100 · L/H) | ______.______ % |
Part II
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Transfer as much as possible of the dried copper(II) hydroxide from the filter paper to a clean 150 mL beaker.
Transfer approximately 70 mL of 6 M aqueous ammonia to the beaker, and stir until all of the copper(II) hydroxide has reacted with the ammonia to form tetraamminecopper dihydroxide (Schweizer’s Reagent). If all of the copper(II) hydroxide does not react, add a bit more aqueous ammonia and stir until no copper(II) hydroxide remains on the bottom of the beaker (Schweizer’s Reagent, Figure 9-6).
Transfer the Schweizer’s Reagent to a storage bottle. Label the bottle with its contents and date it. (If you prefer, you can evaporate the solution to dryness and store the tetraamminecopper dihydroxide as crystals.)
Disposal
The sodium sulfate produced in Part I can be stored for later use, or can be disposed of with household waste. If you did not complete Part II, you can store the copper hydroxide in an airtight container for later use. (If exposed to air, the blue copper hydroxide is gradually oxidized to black copper oxide.)