11
Pathogens and Parasites
Kate S. Hutson and Kenneth D. Cain
11.1 Introduction
There is an enormous diversity of pathogens and parasites that can infect aquatic organisms. Pathogens, as described here are infectious organisms (viruses, bacteria, and fungi) that cause disease and will harm the host or cell. Parasites may be considered pathogens if they cause disease in a host species, but a parasite may live on or within a host for all or part of its life but not cause clinical disease. Conditions associated with intensive monoculture of aquatic animals mean that only a limited diversity of disease causing agents can successfully propagate, proliferate and harm aquaculture stock. Host specificity is arguably the most important property of a pathogen or parasite because it can determine whether it has the potential to become established in aquaculture, although there is potential for the emergence of new pathogens and parasitic diseases or for free‐living organisms to switch to a parasitic life style. Aquaculture systems and biosecurity practices impact pathogen and parasite diversity and virulence by either promoting exponential growth or possibly eliminating conditions necessary for some species to survive. Disease management strategies are most effective if host contact with pathogens can be avoided. However, in many aquaculture systems fish are naturally exposed to endemic pathogens and other strategies aimed at preventing disease (e.g., vaccination) or reactionary methods (i.e., treatments) are needed to minimise the effects of specific pathogens.
This chapter provides an introduction to the diversity of infectious biological agents commonly encountered when farming aquatic animals and highlights specific and broader impacts of each group. Specific case studies are developed for particularly harmful pathogens and parasites in aquatic fish and invertebrates. Current approaches to reducing infection intensities are outlined for each group and potential human diseases associated with the production of aquatic animals are also considered. Plants grown in aquaponics systems are subject to many of the same pests and diseases that affect food crops which have been well documented in other sources, thus strategies to disease management are highlighted herein. The World Organisation for Animal Health (OIE) provides a current list of notifiable diseases (diseases that are required by law to be reported to government authorities) for molluscs, crustaceans and fish on their website and there are considerable economic costs attributable to a range of key parasites and pathogens in the world’s major marine and brackish water aquaculture production industries (e.g., Shinn et al., 2015). There are several text books dedicated specifically to aquatic animal diseases and disorders where further information can be sourced.
11.2 Viruses
Viruses are small particles that are infectious and require a host cell to replicate. Virus particles are referred to as virions and are made up of either DNA or RNA surrounded by a protein coat or capsid that provides protection for this genetic material. In some cases, viruses may be enveloped; meaning the virion has a lipid envelope usually acquired from the host cell membrane as it replicates and buds from the cell to form a new virus particle. Virus shapes can be complex and range from simple helical forms to icosahedral shapes. Most viruses can only be observed using electron microscopy. If special stains and markers are used, it is possible to visualise virally infected cells using light microscopy, but virus particles themselves are too small for this. To diagnose or detect viruses, they must be detected in tissue culture by infecting cell types (cell lines) they are able to replicate in. These cell lines are grown in the laboratory in nutrient media and when a sample is applied that contains a virus, the diagnostician will observe cell destruction and lysis under the microscope. This is usually referred to as cytopathic effect (CPE) of the virus. Viruses can infect many organisms and a range of cell lines have been developed from plants, insects, mammals, and fish. Some viruses (i.e., bacteriophages) can even infect bacterial cells and may have specificity to particular types of bacteria.
In aquaculture, viruses can spread through specific vectors (e.g., blood‐sucking parasites), via the faecal–oral route, physical contact and/or entering the body from food or water. Viral infections in animals may elicit an immune response that can eliminate the infecting virus. However, some viruses are effective at evading the host’s immune system and may even utilise part of the host cell to form a viral envelope as they replicate and bud from a cell. This makes it difficult for the host to recognise the virus particle as foreign and mount an immune response to neutralise it. Vaccines have been developed for a few select viral diseases that impact aquaculture, but their use is not currently widespread. Those that are available typically consist of a non‐virulent or killed virus, or some component of the virus of interest (e.g., protein or DNA) that is delivered to the animal by injection, immersion, or orally in the feed. Following a period of time (often temperature dependent) the host’s immune system responds to the viral ‘antigens’ and confers a level of acquired immunity that will result in the production of antibodies to the virus. These antibodies then provide specific protection and neutralization of the target viral pathogen if the animal comes into contact at a later time.
Viral pathogens are a significant challenge for aquaculture and can be devastating in hatcheries or when outbreaks occur at grow out sites. For most viral diseases, there are limited control or prevention options available. There have been hundreds of different viruses isolated from infected fish, but only a small percentage cause significant impact to aquaculture or are considered to be of regulatory significance and notifiable disease agents as designated by the OIE. As both freshwater and marine aquaculture expands globally, there are greater opportunities for the transmission and spread of aquatic viruses, and viral diseases will continue to cause substantial impact on aquaculture production. New and emerging viral pathogens impacting aquaculture are continually being discovered. One example is the recent discovery of tilapia lake virus (TiLV) found to be the cause of significant mortality in tilapia farms in Israel and Ecuador. In this chapter, we present a range of viral groups and specific viral agents that affect cultured as well as wild fish. Shrimp and prawn culture have also been heavily impacted by viral diseases (see sections 10.5.2.1 and 22.10). For example, white spot syndrome virus (WSSV) has decimated shrimp production in many regions due to its rapid spread and heavy mortality. It is important to note that not all viral groups and families are represented here and only a fraction of the many viral pathogens that impact aquaculture is described in this chapter. There are many books and reviews available that provide in depth coverage of these and other important viral pathogens of fish, crustaceans and molluscs.
11.2.1 Betanodaviruses
The genus Betanodavirus is in the family Nodaviridae. Viruses in this genus are linked to disease outbreaks in wild and cultured fish species, while the other genus in this family Alphanodavirus causes disease in insects. Many different species of marine and freshwater fish are susceptible to infection with Betanodaviruses and the disease is most commonly referred to as Viral Nervous Necrosis (VNN), but it is now recognised and has been renamed viral encephalopathy and retinopathy (VER) by OIE. This VER virus has been isolated from fish on all continents except South America; however, there has been one report on the identification of nodavirus positive samples from the brains of two freshwater aquarium species imported to South Korea from the Amazon. Nodavirus infections appear to be highly prevalent in areas where marine fish culture is widespread and have been linked to severe disease outbreaks primarily in larval and juvenile marine as well as freshwater fish. In Australia, VER was first detected in barramundi, and the virus has been isolated from over 40 species globally (Colorni and Diamant, 2014).
Nervous Necrosis Virus (NNV) species are small (25–30 nm) single stranded RNA viruses that are non‐enveloped and possess an icosahedral capsid (Colorni and Diamant, 2014). An important aspect of nodavirus infections is that they are transmitted vertically (from adults to their offspring) and horizontally (among individuals). It is likely that infected broodstock transmit VER virus to progeny during spawning or rearing. Clinical signs vary but the virus is known to affect nervous tissues and can cause erratic swimming, whirling, loss of equilibrium, and blindness; possibly due to its affinity for retinal tissue, which is a primary site of viral replication (Colorni and Diamant, 2014). Because surviving fish often become asymptomatic carriers and the virus impacts larval and juvenile fish, biosecurity is important. Outbreaks have been reported in European sea bass (Dicentrarchus labrax) in the 1990s, and the first report of VER virus in North America occurred in juvenile California white sea bass (Atractoscion nobilis) being cultured for population recovery efforts. Control measures for VER are limited and no commercial vaccine is available. Because fish that survive outbreaks can become carriers, the best method to reduce impacts from VER is to eliminate its presence through strict biosecurity and sanitation measures. Infected fish may need to be culled from the population and new fish should be quarantined and tested prior to mixing with the general population.
11.2.2 Birnaviruses
Viruses in the family Birnaviridae are non‐enveloped double‐stranded RNA (dsRNA) viruses with an icosahedral shape that infects fish and shellfish. The genus Aquabirnavirus comprises three species, the first being the type species Infectious Pancreatic Necrosis Virus (IPNV), the second being the marine aquabirnavirus (MABV) that infects yellowtail (Seriola quinqueradiata) and is referred to as yellowtail ascites virus (YAV), and the third being the Tellina virus (TV‐1) that was isolated from the marine mollusc Tellina tenuis. It should be noted that YAV has since been shown to infect a variety of marine hosts.
IPNV is one of the most significant finfish viruses in this family and causes the disease Infectious Pancreatic Necrosis (IPN). Although viral diseases were suspected in hatcheries in the early to mid‐1900s, it was not until the establishment of cell lines from specific fish species that IPNV and other fish viruses were identified. IPNV causes acute disease and is highly contagious, most often affecting very young salmonid fry. In some cases, mortality in the hatchery is up to 100% (Crane and Hyatt, 2011). IPNV is characterised as an aquatic birnavirus and since the time of its discovery similar IPN‐like viruses (often non‐virulent) have been found in many salmonid and non‐salmonid hosts in both fresh and sea water.
Interesting case reports have identified both non‐virulent forms of aquatic birnaviruses and virulent forms of IPNV that have impacted aquaculture facilities. In Australia, the first report of an aquatic birnavirus was from routine sampling of Atlantic salmon farms. This virus grew on a range of fish cell lines and reacted with commercial IPNV antisera; however, clinical disease associated with this was never observed and it was subsequently isolated from a range of other species in the marine environment. The first report of IPNV in Mexico came from a clinical outbreak in farmed rainbow trout (Oncorhynchus mykiss) where gross and microscopic pathology was consistent with IPN. Further characterisation identified this as the Buhl strain of IPNV.
The contagious nature of IPNV has been problematic and fish that survive a disease outbreak often become asymptomatic carriers that are able to horizontally transmit IPNV without exhibiting clinical signs of disease. The best control strategy for IPN or any viral disease affecting finfish is to avoid contact through proper biosecurity. It is not always possible to avoid contact; therefore, other management options such as vaccination are available in some countries.
11.2.3 Herpesviruses
Two significant fish viruses in the order Herpesvirales and the family Alloherpesviridae are Channel Catfish Virus (CCV) and Koi Herpesvirus (KHV). These viruses are highly contagious. CCV and KHV have resulted in substantial economic impact to the US catfish aquaculture industry and ornamental koi producers, respectively.
CCV is considered endemic in the USA and channel catfish virus disease (CCVD) emerged as a problem during the early years of commercial catfish farming. In the 1960s high mortalities in channel catfish (Ictalurus punctatus) were reported primarily in fry and fingerlings following introduction of fish from the hatchery to the fry ponds and in 1971, CCV was identified as a herpes virus. It is now present in nearly all regions in the US that produce catfish. There are no prevention or treatment options for CCVD, but there have been attempts to develop vaccines to CCVD. It was shown that a DNA vaccine could provide protection against CCVD; however, the use of this under practical conditions is limited by the need for injection delivery. In fish, CCV can persist in a carrier state and transmission of the virus is thought to be both vertical from the broodstock and horizontal through the water column. In catfish ponds, water temperature and other environmental stressors appear to play a role in disease occurrence and severity.
KHV (also known as cyprinid herpesvirus 3; CyHV‐3) has a more recent history compared to CCV as it was first reported in the UK in 1996. It has since been reported and confirmed in nearly all countries that produce koi or other common carp, except Australia and New Zealand. Interestingly, Australia is exploring the possibility that KHV could be used as a potential biological control agent against invasive common carp in areas where their populations have expanded. KHV is listed by OIE as a notifiable disease and this creates regulatory concern for aquaculture producers and koi growers. Once a fish becomes infected or survives KHV disease (KHVD) they are potential carriers of the virus.
There is no effective treatment for KHVD, however, an attenuated live vaccine was approved for the prevention of this disease. It has been shown to generate high antibody titres specific for KHV and to protect common carp or koi following challenge. One major issue is that import and export control regulations may not allow movement of KHV‐vaccinated fish. This relates to the live nature of the vaccine and the inability of diagnostic tests to differentiate between naturally infected or vaccinated fish. For the koi hobbyist or carp producer it is important to maintain strict biosecurity and prevent exposure of fish to KHV.
11.2.4 Iridoviruses
There are a number of viruses in the family iridoviridae that infect fish. A brief overview of the more significant species is provided here, but a critical review of fish iridoviruses is available in the literature (Whittington et al., 2010). Fish iridoviruses fall into three genera within this family, Megalocytivirus, Ranavirus, and Lymphocystivirus. There are also fish iridoviruses that are yet to be assigned to a genus, such as white sturgeon iridovirus (WSIV) and Erythrocytic necrosis virus (Whittington et al., 2010). Iridoviruses are icosahedral in shape (Figure 11.1), large in structure (120–300 nm) and may appear enveloped or non‐enveloped in some cases. They consist of a genome of double‐stranded DNA that is methylated in a way similar to viruses affecting vertebrates.
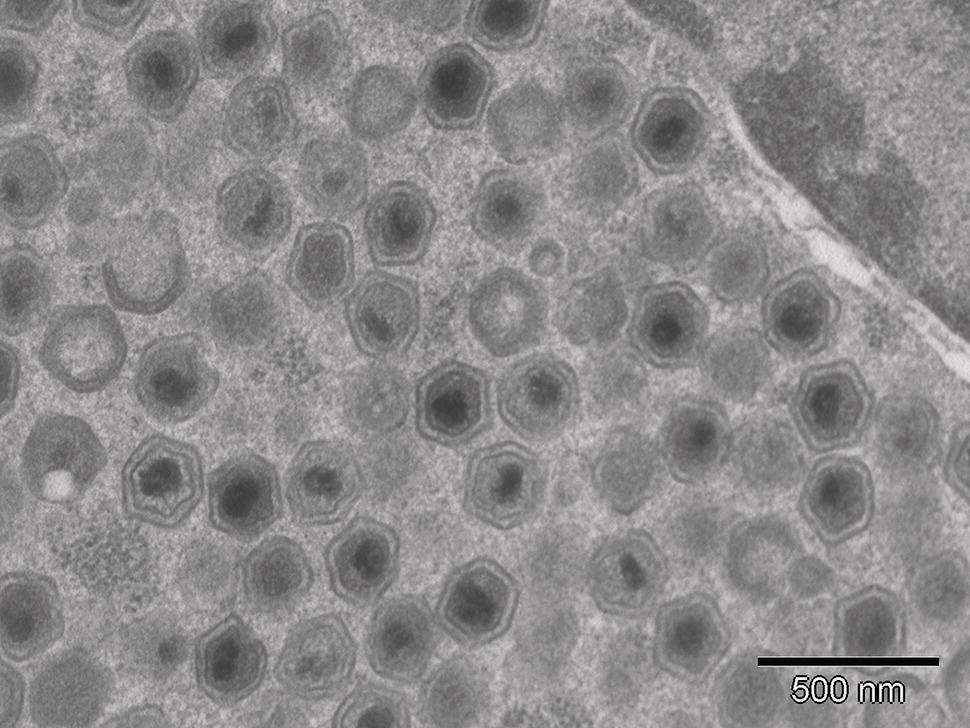
Figure 11.1 Electron micrograph of white sturgeon, Acipenser transmontanus, iridovirus (WSIV) particles showing the characteristic icosahedral shape.
Source: Reproduced with permission from Dr J. Drennan, Colorado Parks & Wildlife.
In general, ranaviruses and megalocytiviruses are important emerging pathogens for cultured and wild fish and affect fish in both marine and freshwater environments. Lymphocystis virus disease (LVD) affects fibroblastic cells in the skin and connective tissue resulting in superficial lesions on fish. LVD is known to affect more than 125 wild and cultured fish species in marine and freshwater and is widespread in distribution. Transmission is horizontal and incidence rates for this disease may be as high as 70%. It has been speculated that another iridovirus (WSIV) may be transmitted vertically as disease outbreaks have been observed in progeny from adult white sturgeon (Acipenser transmontanus) collected from the wild and spawned for conservation or commercial aquaculture programs. The potential that WSIV could be transmitted vertically was investigated following collection and disinfection of gametes from wild caught sturgeon. Vertical transmission did not occur but progeny from eggs incubated using a river water source all became infected. This and further cohabitation experiments clearly showed that WSIV was horizontally transmitted and highly contagious. WSIV does not cause a systemic infection and appears to be primarily localised to the epidermis of the skin, barbells, oropharynx, and gills of sturgeon. Although clinical signs vary, and limited pathology or tissue distribution is observed, haemorrhaging can occur, and mortality may be high in heavily infected fish due to secondary bacterial infection or possibly a wasting syndrome resulting in emaciation and eventual death (Figure 11.2).
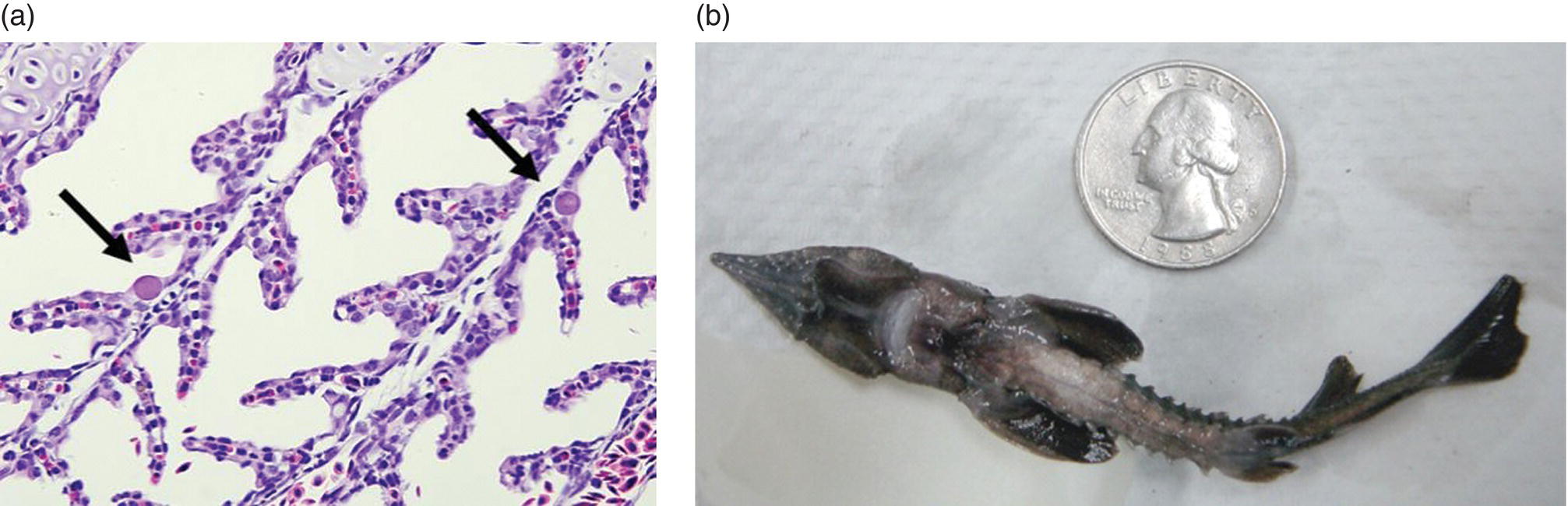
Figure 11.2 (a) WSIV infected cells in the gill lamellae of white sturgeon. Arrows indicate infected cells; and (b) juvenile white sturgeon with signs of emaciation due to lack of food intake following infection with WSIV.
Source: Reproduced with permission from Dr J. Drennan, Colorado Parks & Wildlife.
Epizootic haematopoietic necrosis virus (EHNV) was isolated in 1985 in Australia and represents the first iridovirus known to cause systemic infection and high mortality in finfish. It appears to be restricted to Australia, where large mortality events in wild redfin perch (Perca fluviatilis) and severe outbreaks in farmed rainbow trout (Oncorhynchus mykiss) have been reported. Due to its virulence and localization, the disease epizootic heamatopoietic necrosis (EHN) is notifiable to OIE. In general, redfin perch are highly susceptible and considered to be important carriers of the virus; however, occurrence of mortality in farmed rainbow trout highlights the potential risk should this virus spread outside endemic areas.
11.2.5 Orthomyxoviruses
Viruses in the family Orthomixoviridae consist of six genera. They include Influenza Virus A, B, and C, which infect humans, other mammals, and birds; Isavirus, which infects salmon; Thogotovirus, infecting insects and mammals, including humans; and Quaranjavirus, a new genus primarily infecting arthropods and birds. Infectious salmon anaemia virus (ISAV) is an orthomyxovirus in this family and considered the type virus in the Isavirus genus. ISAV is the causative agent of infectious salmon anaemia (ISA), a disease of Atlantic salmon (Salmo salar). This disease that initially affected farmed Atlantic salmon in Norway, causes a systemic condition resulting in severe anaemia in fish and is often linked to necrosis and haemorrhaging of internal organs. An outbreak may result in high cumulative mortality over time, but the disease may be more chronic in nature with low level daily mortality of 0.05–0.1%. ISA is considered a major disease impacting Atlantic salmon aquaculture and has been isolated from fish in Eastern Canada, the UK, Faroe Islands, and Chile. There are also reports of ISAV being discovered in tissues of farmed Atlantic salmon from the coast of British Columbia, Canada; however, only sequences from the highly polymorphic region (HPR) of ISAV variants have been identified by polymerase chain reaction (PCR) and to date ISAV has not been isolated in tissue culture. The lack of disease outbreaks and limitations in interpreting positive results from molecular detection methods brings into question the presence of this virus in this region. It is clear that identification of ISAV in British Columbia salmon farms would be particularly concerning since other salmonids including Pacific salmon (Oncorhynchus tshawytscha) can become infected. However, at this time, it does not appear that clinical disease develops in species other than Atlantic salmon, but it can be speculated that virulent strains of ISAV could emerge in Pacific salmon species if it were to persist in a carrier state.
Following infection from ISAV, fish have been shown to be resistant to re‐infection and develop a protective immune response. This has led to research on vaccine development and although the efficacy of such vaccines is not completely clear, there are vaccines available and these have been used in North America.
11.2.6 Rhabdoviruses
Within the family Rhabdoviridae there are six genera; however, those that infect fish are primarily from the genus Novirhabdovirus. Viruses in this genus are generally well characterised and infectious haematopoietic necrosis virus (IHNV) is the type species. Other fish rhabdoviruses were tentatively placed in the genus Vesiculovirus, but two new genera have been recently proposed as rhabdoviruses that are not congruent with Novirhabdovirus (Kurath, 2012). These include Sprivivirus of which spring viremia of carp virus (SVCV) is the type strain, and Perhavirus of which perch rhabdovirus is the type species. Rhabdoviruses of fish infect a broad range of host species and may have broad geographical distribution, including freshwater and sea water environments. Some of the most severe and contagious viruses impacting aquaculture and wild fish fall within this family. New fish rhabdoviruses continue to be discovered and due to their significance, there is continual work in the areas of diagnostics and development of new and better control/prevention methods.
Rhabdoviruses that infect fish are usually bullet‐shaped enveloped viruses with virions typically measuring approximately 75 nm wide and 180 nm long, replicate in the cytoplasm of cells and contain single‐stranded RNA encoding five to six proteins. Some rhabdoviruses are of particular concern in wild and cultured fish, cause diseases that are considered notifiable by OIE, and include SVCV, IHNV, and viral haemorrhagic septicaemia virus (VHSV). Of these, IHNV and VHSV have been extensively characterised and many sources are available in the literature that provide comprehensive descriptions and reviews (Kurath, 2012).
IHNV causes infectious haematopoietic necrosis (IHN) and was originally described following disease outbreaks in Sockeye (Oncorhynchus nerka) and Chinook salmon (Oncorhynchus tshawytsch) in the Pacific northwest USA in the 1950s. It has since been a significant concern in freshwater salmonid aquaculture (Figure 11.3) but can infect fish in both fresh and sea water environments. Outbreaks have occurred in Atlantic salmon farms in Canada, but IHN remains a primary concern for wild fish or hatchery‐reared Pacific salmon in North America as well as commercial rainbow trout farms in the USA and elsewhere. Originally considered a viral disease restricted to North America, it has since spread to European and Asian countries where salmonids are cultured. This disease affects young salmonids and an acute outbreak can result in mortalities up to 100%; however, most outbreaks are less severe and surviving fish may develop immunity to re‐infection. The best way to manage around IHN is to avoid contact but in areas where it is endemic this can be difficult. As with most diseases in fish, environment and stress during culture can impact the severity of an outbreak. Five genogroups are known to exist for IHNV and these have been linked to outbreak severity in different host species. The ability of fish to develop immunity to IHNV is well documented and research in the area of vaccine development has been substantial. The most effective vaccines against IHN are DNA vaccines that must be injected into fish. The first commercial DNA vaccine was developed and licensed by the Canadian Food Inspection Agency (CFIA) in 2005 and is available as a plasmid DNA‐based vaccine (Apex®‐IHN).

Figure 11.3 Severe exophthalmia in rainbow trout fry infected with IHNV.
Source: Reproduced with permission from G. Kurath.
Another important fish rhabdovirus is VHSV. This virus causes viral haemorrhagic septicaemia (VHS), considered the greatest disease problem in the European trout farming industry. This virus was originally thought to be restricted to Europe, but it is now clear that it has a broad host and geographic range and multiple strains of this virus have been described. In 1988 an interesting case occurred in Washington State where for the first time VHSV was discovered in returning adult Pacific salmon. This discovery and isolation of VHSV resulted in the compulsory euthanasia of millions of hatchery fish that where the progeny of infected adults. This occurred despite an actual disease outbreak and it was later found that this strain of VHSV was distinct from the European strain and appeared to be associated with a range of marine species such as herring (Clupea pallasii) and cod (Gadus macrocephalus). It is virulent to herring but unlike the European isolate it is avirulent in rainbow trout. In 2003, VHSV emerged in wild fish in the Laurentian Great Lakes in North America. Fish affected by VHS were dying in large numbers and washing up on shore in many areas. The number of fish and species affected was alarming with over 1823 cases and 19 fish species that tested positive for VHSV. This has led to further characterization of VHSV strains and now a series of four genogroups and additional subgroups exist, with the Great Lakes strain designated as genotype IVb.
11.3 Bacteria
Bacteria are categorised into a large domain of prokaryotic microorganisms. They are larger than viruses, are typically visualised using light microscopy, and may be rod‐shaped, spherical, or spiral‐shaped. Many bacteria are harmless or may even be beneficial to their host. They can be found in the environment, may be part of many animals' natural gut microbiota, or in some cases may invade an organism and cause disease. Bacterial disease outbreaks in aquaculture operations result in significant economic losses in both public (resource enhancement and stocking) and private sectors. In some cases, there are vaccines used to prevent bacterial disease outbreaks, and antibiotics delivered in feed have commonly been used to treat bacterial infections in fish and other animals. Such treatments have been important factors in disease management but represent risks due to the ability of bacteria to develop resistance to common antibiotic treatments. Infection to humans due to aquatic pathogens is relatively rare; however, bacteria are the most common aquatic pathogens found to cause zoonotic infections by transmission from fish or culture water to humans (see section 11.12).
In this section, an overview of select bacterial pathogens known to infect fish and cause significant economic impact for aquaculture is provided. It is not possible to cover all new and emerging bacterial pathogens of fish, crustaceans and shellfish here, but it should be noted that at this time there have been 13 genera of bacteria reported as pathogenic to aquatic animals. These include Gram‐negative pathogens such as Aeromonas, Edwardsiella, Flavobacterium, Francisella, Photobacterium, Piscirickettsia, Pseudomonas, Tenacibaculum, Vibrio and Yersinia; and Gram‐positive bacterial pathogens within the genera Lactococcus, Renibacterium and Streptococcus.
11.3.1 Aeromonas salmonicida
Furunculosis, caused by the Gram‐negative bacteria Aeromonas salmonicida was one of the earliest described fish diseases and was named due the formation of large boils (furuncles) just under the skin in clinically sick fish. First described in the late 1800s in cultured and wild fish in Europe, it is widely distributed and infects many salmonid and non‐salmonid species in marine and freshwater worldwide. Although the taxonomy of Aeromonas is not always agreed upon, there are four primary subspecies of A. salmonicida that are considered infectious. The ‘typical’ strain is considered A. salmonicida salmonicida primarily affecting salmonids, while the subspecies masoucida, achromogenes, and smithia are ‘atypical’ strains that affect salmonids, and A. salmonicida nova is an ‘atypical’ strain that is infectious for non‐salmonid fish.
Furunculosis is widely reported in commercial and ‘resource’ hatcheries in the USA that stock fish into public waters and is also known to impact wild fish. This disease became problematic in the Atlantic salmon industry due to outbreaks in smolts when they were moved from freshwater to seawater. When the disease affects young fish, mortality can be acute and clinical signs may be limited to darkening of fish, lethargy, and anorexia. The formation of haemorrhaged boils or furuncles may appear more often in fish that are chronically affected. If the furuncles rupture and release bacteria, toxins or necrotic cells, an increase in bacterial load can occur and thus an increase in the risk of horizontal transmission to other fish. As with other bacterial diseases of aquatic organisms, A. salmonicida is not considered by OIE as a notifiable disease; however, other regulatory agencies may restrict fish movement to non‐endemic areas (e.g., USA state or federal agencies acting under the U.S. Fish and Wildlife Service Aquatic Animal Health Program (FWS‐AAHP) Title 50 act).
Control of infection from A. salmonicida is most effective by preventing exposure. In areas where this pathogen is found in the environment, spring or ground water should be used as a water source for aquaculture facilities because they are typically free of most pathogens. If an open water source such as a river, lake, or marine environment is utilised then preventative measures such as vaccination should be incorporated if possible. Due to the long history of furunculosis, some of the earliest attempts at vaccinating fish were with A. salmonicida. Currently, there are a range of furunculosis vaccines commercially available. The use of these and other bacterial vaccines has nearly eliminated the need for antibiotic treatment in Norway’s Atlantic salmon industry. This is not the case everywhere and for some species and regions, antibiotic treatments are administered when fish show clinical signs of furunculosis. Due to the concerns over antibiotics, they should be used as a last resort.
11.3.2 Edwardsiella ictaluri
Enteric septicaemia of catfish (ESC) is caused by the Gram‐negative bacteria Edwardsiella ictaluri. This is considered one of the most severe bacterial diseases in the catfish industry in the USA and may account for 30% or greater losses. Cases of ESC are most often reported in the spring around May and June and also in autumn in September and October due to the bacterium’s preference for temperatures between 22 and 28 °C. In general, channel catfish (Ictaluri punctatus) are the primary species that is susceptible to ESC, but infection with E. ictaluri along with minor pathology has been reported in other species of catfish and non‐ictalurids. It has been a primary problem in the USA, but outbreaks, sometimes severe, have been reported in Australia, Indonesia, Japan, Thailand, and Vietnam.
Edwardsiella ictaluri infections can lead to acute or chronic forms of ESC. In the acute form the infection is systemic with bacteria moving into the blood stream. This causes septicaemia and results in ulcerations and necrosis in multiple organs. Haemorrhaging may appear around the mouth and operculum and along the base of the fins (Figure 11.4). Interestingly, in the chronic form, E. ictaluri may enter the olfactory organ or nasal passage and move into the skull and skin of the head to form a lesion, leading to a condition known as ‘hole in the head’.

Figure 11.4 Channel catfish (Ictalurus punctatus) showing external clinical signs of haemorrhaging near the mouth and base of the fins following laboratory challenge with E. ictaluri.
Source: Reproduced with permission from Dr B. LaFrentz.
Control of ESC in commercial catfish operations has relied on antibiotic treatment, and such treatments continue to be used to control losses due to this disease. There is a commercial immersion vaccine consisting of a live‐attenuated strain of E. ictaluri available in the USA. The potential to prevent major impacts due to ESC through vaccination is high, but some practical difficulties exist because delivery of the vaccine to fish when they are fully immunocompetent is often not possible. Catfish production in many cases is on a large scale and ponds are often stocked shortly after the fry hatch, which is usually the only time fish are handled until harvest and therefore the only time a vaccine can be administered.
11.3.3 Flavobacterium psychrophilum
Bacterial coldwater disease (BCWD) and rainbow trout fry syndrome (RTFS) are caused by Flavobacterium psychrophilum. This bacterial pathogen and a number of other bacteria in the Flavobacteriaceae family can cause disease in cultured and wild fish. Flavobacterium psychrophilum is one of the best‐known pathogens in this genus (along with F. columnare) due to the significant impact BCWD has on salmonid aquaculture worldwide. Nearly all salmonids are susceptible to F. psychrophilum and it has been reported to cause disease in a number of non‐salmonid species as well. Young fish are primarily affected, but when early hatched fry are infected, mortality may be greater than 50%. Although considered ubiquitous in the aquatic environment, it appears that F. psychrophilum can be transmitted both horizontally and in some cases vertically from parent to progeny (Cain and Polinski, 2014). It has been isolated from salmonid eggs and egg contents, and it has been shown that F. psychrophilum could survive inside fertilised eggs following nano‐injection, which resulted in high mortality in eyed eggs and early hatched fry due to F. psychrophilum. Such findings make broodstock selection critical and highlight the potential for this bacterium to persist within a hatchery environment.
Flavobacterium psychrophilum is a Gram‐negative bacterium with cell morphology consistent with other Flavobacterium species. Although strain differences exist, most bacterial cells are rod shaped (Figure 11.5) and range in size from 0.2–0.75 × 2–7 μm. This bacterium has gliding motility and a gliding mobility protein (GldN) may affect the bacterium’s ability to enter and infect cells.

Figure 11.5 Light micrograph or Flavobacterium psychrophilum cells showing long thin rod‐shaped bacteria.
Source: Reproduced with permission from Dr B. LaFrentz.
Cases of BCWD and/or RTFS are widespread and include most salmonid producing countries. It is widespread in the USA and European trout industries, and F. psychrophilum has been reported in trout and other species in a number of countries, including Australia where it was reported in Atlantic salmon during the freshwater stages of production. There have been a large number of F. psychrophilum strains isolated and such strains can have varying levels of virulence. Although mortality may be high from BCWD or RTFS, commercial aquaculture also experiences losses due to growth and performance impacts as well as increased levels of deformities (resulting in a lower grade product) following an outbreak. In the USA, public hatcheries that rear steelhead (ocean run rainbow trout) and salmon for stocking purposes are also heavily impacted by BCWD, and this disease causes greater overall losses in these facilities than any other fish disease (Cain and Polinski, 2014).
Clinical signs of BCWD/RTFS will vary depending on the species and age of fish infected. There are acute forms of the disease that cause septicaemia and high mortality, but lingering chronic infections may also occur. Fish infected with F. psychrophilum exhibit behavioural changes such as spiral swimming or lack of feeding response. Internally, this bacterium has an affinity for the spleen but it can be isolated from many organs and tissues depending on the severity of infection. Externally, a range of clinical signs may appear including frayed fins, exophthalmia, dark pigmentation, or haemorrhages on the skin or bases of fins. In some cases, the caudal peduncle region may be severely eroded (Figure 11.6), which relates to this disease originally being referred to as ‘peduncle disease’.

Figure 11.6 Laboratory challenged rainbow trout showing severe erosion in the dorsal musculature at the site of Flavobacterium psychrophilum injection.
Source: Reproduced with permission from Dr B. LaFrentz.
Currently, control of BCWD/RTFS relies on good fish culture practices and antibiotic treatment following confirmation of an outbreak. However, there has been extensive work aimed at developing an effective vaccine to prevent and/or limit disease impacts. Due to the small size and the production requirements for species such as rainbow trout, the key to a successful vaccine has been creating one that can be mass delivered to very young fish. Recently, a live‐attenuated F. psychrophilum strain that provides protection following immersion vaccination has been developed. This shows great potential for commercial development, and more recent improvements in the formulation have improved efficacy in the laboratory and in the field. Other alternative control strategies may include the use of natural gut bacteria that can be incorporated into feed as probiotics. This has been shown to reduce mortality from BCWD following feeding of two bacterial strains (Enterobacter C6‐6 and C6‐8) isolated from the gut of healthy rainbow trout. Further work has demonstrated that reduced mortality is due to the expression of an anti‐microbial peptide by the Enterobacter.
11.3.4 Flavobacterium columnare
Flavobacterium columnare is the causative agent of columnaris and has historically been a major disease in warmwater aquaculture; however, columnaris is emerging as a significant problem in salmonid aquaculture with infections increasing in recent years. A wide range of species are affected by columnaris, and in the catfish industry, it has been estimated that infection by F. columnare resulted in mortality and 39% losses in 2009. Clinical disease occurs most often in young fish and transmission of this pathogen is considered horizontal. Good water quality is important as outbreaks can be linked to stress associated with poor environmental conditions. Temperature plays an important role in infection with occurrence in channel catfish being most prevalent at temperatures between 25–32 °C (Lio‐Po and Lim, 2014). In wild adult salmon in the Pacific northwest of the USA, columnaris has been an issue during migration from the ocean to spawning grounds since many of the reservoirs they must pass through have elevated water temperatures in spring and summer months.
Similar to other Flavobacteria, F. columnare is Gram‐negative and represented as a long rod‐shaped bacterium. One characteristic that gives columnaris its name relates to the tendency of these bacteria to form ‘hay stacks’ or columns in wet mounts of the gills or other infected tissues (Figure 11.7). Clinical signs of disease often include frayed fins, skin lesions that may appear yellowish in colour or in some cases depigmented (Figure 11.8), or gill damage due to colonisation of bacteria (Figure 11.9). It has been suggested that acute mortality results more often when F. columnare is associated with the gills (Lio‐Po and Lim, 2014), but infections can become systemic and result in high mortalities and in some cases minimal pathology to infected organs. To prevent or control columnaris, it is critical to maintain fish in optimal conditions. Should an outbreak occur and represent primarily a systemic infection, then antibiotic treatment with medicated feed may be beneficial. External and gill associated infections may require application of chemical therapeutants, such as potassium permanganate to the water (Lio‐Po and Lim, 2014). Currently, in the USA there is a commercially available vaccine aimed at prevention of columnaris in channel catfish; however, it is not clear how widespread its use is or if this live‐attenuated vaccine is efficacious in other fish species.
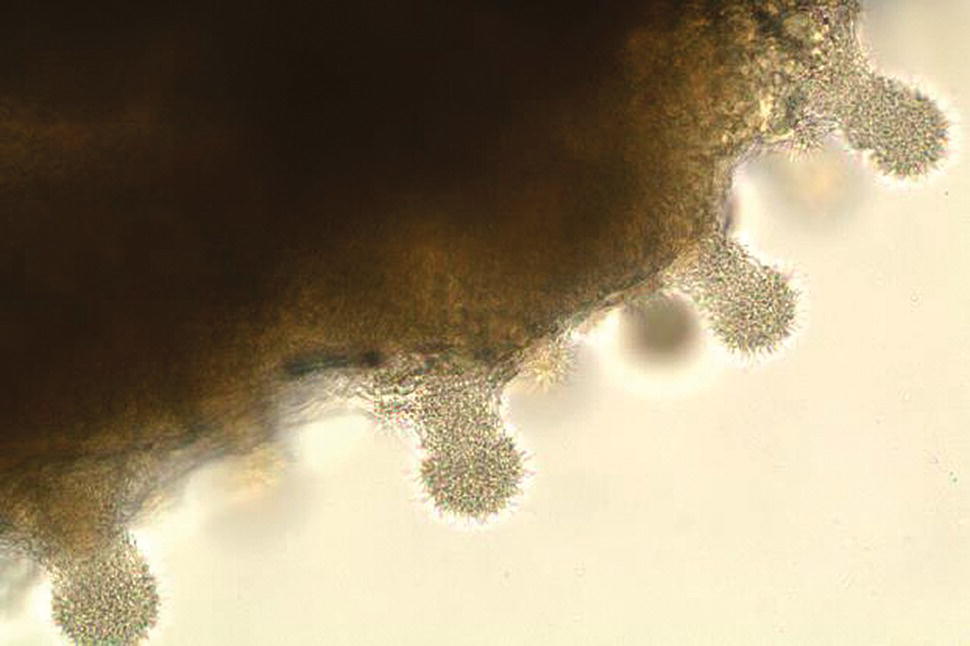
Figure 11.7 Columns or ‘hay stacks’ formed by Flavobacterium columnare visible in a wet mount of infected gill tissue.
Source: Reproduced with permission from Dr A. E. Goodwin.

Figure 11.8 Depigmented lesions following laboratory challenge of tilapia Oreochromis sp. with Flavobacterium columnare.
Source: Reproduced with permission from Dr B. LaFrentz.

Figure 11.9 Gill necrosis following laboratory challenge of rainbow trout with Flavobacterium columnare.
Source: Reproduced with permission from Dr B. LaFrentz.
11.3.5 Piscirickettsia salmonis
Salmonid rickettsial septicaemia (piscirickettsiosis) caused by the intracellular Gram‐negative bacteria Piscirickettsia salmonis is a disease that impacts fish primarily in seawater. It infects salmonids and was first reported in coho salmon in Chile. This disease has had the greatest impact on salmonid aquaculture in Chile, but P. salmonis has been found in a number of countries including Europe, North America, and Australia, where a rickettsia‐like organism was isolated in Tasmania. In Chile, cases resulting in up to 90% mortality of coho salmon were reported in the 1980s with clinical disease occurring between 6–8 weeks post transfer to seawater cages. Transmission of P. salmonis appears to be primarily horizontal, but it has been suggested that vertical transmission can occur.
The intracellular nature of P. salmonis makes isolation and confirmation of disease more difficult. Typically, piscirickettsiosis is diagnosed following histological examination and immunohistochemistry of cells infected with P. salmonis. However, it can be isolated using fish cell lines in a similar manner to virus isolations. Cytopathic effect due to P. salmonis is evident on CHSE‐214 cells when incubated for up to two weeks at temperatures of 15–18 °C. Other serological and molecular methods such as PCR are available to confirm infection.
Fish that are affected by P. salmonis may be lethargic, go off feed, become dark in colour, and have pale gills. Internally, the posterior kidney and spleen may be enlarged, and the liver may develop nodules that if ruptured will result in crater‐like lesions. In some species, the nervous system can be affected, and fish will swim in an irregular manner.
Prevention and control options for piscirickettsiosis have been limited in the past and the use of antibiotics appears to have been less successful due to the intracellular nature of P. salmonis. Vaccination has shown promise and commercial vaccines have recently become available. Although the efficacy of these vaccines is still in question, it is clear that prevention of an outbreak through methods such as vaccination offer the best opportunity to manage this disease in aquaculture. Good management practices including low density stocking of pens and removal of mortalities early in an outbreak are important. Other considerations include potential screening of broodstock and rejection of eggs from fish that are positive for infection.
11.3.6 Vibrio spp.
Vibriosis is caused by a number of bacterial species in the family vibrionaceae and affects both coldwater and some warmwater species of fish, crustaceans and molluscs in marine or brackish water. It should be noted that many species of Vibrio cause disease in aquatic organisms, but the focus here will be primarily on the most common species causing vibriosis and include Vibro anguillarum (also referred to as Listonella anguillarum), V. ordalii, and V. salmonicida (also referred to as Aliivibrio salmonicida). Vibrio anguillarum had major economic impacts on salmonid aquaculture prior to the implementation of commercial vaccines, which can be highly effective against vibriosis. Mortality in Atlantic cod (G. morhua) aquaculture has also been reported due to V. anguillarum, and early reports from Chile indicated outbreaks with moderate mortality of up to 8% due to V. ordalii. Vibrio salmonicida has been shown to accumulate rapidly in the blood and colonise the intestine, which is thought to result in release and spread in the environment. However, overall losses and impact to commercial aquaculture can be kept low if vaccination procedures are implemented effectively.
Vibrio spp. can be diagnosed easily as it grows readily on or in standard culture media. Differentiation at the species level is more difficult but biochemical tests and PCR based assays are available. When fish succumb to Vibrio a range of external and internal clinical signs can be observed. Mortality in unvaccinated fish is usually high and infection results in a bacteraemia with fish showing signs of lethargy, dark colouration, reduced feeding response, and possible petechial haemorrhaging near the belly and base of the fins. Anaemia is common and multiple organs can be impacted.
As mentioned, vaccination is the preferred method of prevention for vibriosis; however, if outbreaks occur it is important to remove moribund and dead fish quickly and diagnose early in case antibiotic treatment is needed. As more species are reared in marine environments, there is concern that new problems involving vibriosis will develop and require additional strategies for control and prevention.
11.3.7 Yersinia ruckeri
Enteric redmouth (ERM) caused by the Gram‐negative bacterium, Yersinia ruckeri is a major cause of disease in aquaculture worldwide. ERM was first described in rainbow trout in the 1950s in the Hagerman Valley of Idaho, USA and referred to as Hagerman red mouth disease. This disease may also be referred to as yersiniosis in areas outside the USA and primarily impacts rainbow trout. Movements of fish and eggs are thought to have contributed to early spread of Y. ruckeri, and it was found in Canada shortly after initial isolation in Idaho. In the mid‐1980s it was reported in Europe and has since been found in Norway, Denmark, UK, France, Germany, Italy, South Africa, and Australia. One difficulty linked to ERM is that multiple varieties of Y. ruckeri strains or biotypes exist and may be linked to disease severity in salmonids. Outbreaks can cause significant impact and surviving fish can become asymptomatic carriers. However, similar to vibriosis, ERM is generally preventable by the use of commercial vaccines. Prior to vaccine development, it was believed that ERM caused up to 35% loss and an economic impact of approximately USD 2.5 million in Idaho’s Hagerman Valley.
ERM is readily confirmed through culture of Y. ruckeri in general media followed by biochemical tests and or other confirmatory serologic methods or PCR. This bacterium is motile and measures 0.5–0.8 × 1.0–3.0 μm. Although it impacts rainbow trout and other salmonids at colder temperatures (generally 15 °C or below) growth is optimal between 22–25 °C. As mentioned, different strains or biotypes exist. The Type I (Hagerman strain) is usually considered the most virulent. It has been found that the biotype of Y. ruckeri can be linked to vaccine efficacy and understanding this is important when developing vaccination programs. Separate biotypes have been found in Europe and North America, and recent atypical biotypes found in Australia were linked to high mortalities in Atlantic salmon (Bridle et al., 2012).
Acute infections with Y. ruckeri in young fish result in heavy losses as it causes a septicaemic infection. More chronic infections may appear in larger fish where clinical signs can include dark colouration, blindness, and lethargy. Ulceration and haemorrhaging in the mouth are classic signs and reflect the name ‘red mouth’. This is observed regularly in rainbow trout but infections in Atlantic salmon may not produce this classic sign. Often, ERM results in a septicaemic condition where internal clinical signs may include bloody ascites, enlarged spleens, as well as intestinal and muscular haemorrhaging.
Some antibiotics have been used to control mortality following ERM outbreaks. However, the most effective means of prevention and control for ERM is through the use of commercially available vaccines. Development of commercial immersion ERM vaccines was considered the single most important management tool for the trout industry in Idaho when introduced, and clearly limited the potential impact of ERM early on. Although vaccines are effective, it is important to implement good fish culture practices and minimise environmental stressors that would contribute to reduced disease resistance in any aquaculture situation.
11.3.8 Renibacterium salmoninarum
Renibacterium salmoninarum is a Gram‐positive bacterial pathogen that causes bacterial kidney disease (BKD), which was first described in Scotland in 1930. This disease is considered a problem in salmonids but has been reported in non‐salmonids such as ayu (Plecoglossus altivelis) and Pacific hake (Merluccius productus), and it was found that sablefish (Anoplopoma fimbri) developed clinical disease when experimentally challenged with R. salmoninarum. Most salmonid producing countries in Europe, Asia, as well as North and South America have reported BKD outbreaks in farmed and/or wild fish. At this time Ireland, Australia, and New Zealand are considered BKD free. Renibacterium salmoninarum is transmitted both horizontally and vertically. This poses serious problems in aquaculture programs where broodstock are highly valuable. To manage the problem of vertical transmission, resource enhancement hatcheries in the Pacific north‐west, USA have implemented an effective screening program for migrating adult Pacific salmon collected for hatchery production. Kidney samples from each female broodfish are taken at the time of spawning and infection levels are quantified using a standardised enzyme‐linked immunosorbent assay (ELISA). If fish test positive with moderate or high levels of R. salmoninarum the fertilised eggs from that female are culled from the population. This has resulted in substantial reduction in overall incidence of BKD in these hatchery programs.
Renibacterium salmoninarum is considered a coryneform bacterium that is a non‐motile short rod measuring 0.3–1.0 × 1.0–1.5 μm. It is an intracellular bacterium and standard culture methods for identification are problematic as it grows slowly under most conditions, taking from 8–12 weeks to produce colonies at 15 °C. Improved culture methods can shorten this to as few 5–7 days. This may improve diagnosis of R. salmoninarum in the future; however, current confirmation of infections relies on serological or molecular methods.
Cases and reports of BKD are widespread and in general juvenile salmonids are severely impacted by BKD. However, adult fish can develop clinical disease in both freshwater and marine environments. Both wild and hatchery produced Pacific salmonids and Atlantic salmon can be affected by R. salmoninarum. Disease severity increases when smolts are transferred from freshwater to seawater most likely due to the physiological stresses of the smoltification process. Coho salmon smolts infected with R. salmoninarum experienced much higher mortality when held in seawater compared to siblings maintained in freshwater.
Clinical BKD may include a number of signs including darkening of the skin, lethargy, exophthalmia and abdominal distention. Spawning fish with heavy infections may show haemorrhaging at the base of their fins and internally can exhibit classic white‐grey granulomatous lesions in the kidney. The bacterium can be isolated from other organs as well, and in some cases the infection can move into the musculature where tissue destruction and necrosis may occur.
Prevention of BKD is best achieved through avoidance of exposure to R. salmoninarum. For broodstock that may be potential carriers, it is important to utilise the screening and egg culling methods described above. Movement of fish or eggs into facilities should require prior inspection of fish or eggs to determine disease status. If R. salmoninarum is endemic to a region, it is possible that outbreaks could occur. This may require antibiotic treatment of infected fish; however, few antibiotics have been successful. Use of erythromycin is common, but overall it has been relatively ineffective in young fish. A vaccine for BKD is commercially available and developed for Atlantic salmon. This is a live vaccine, but interestingly it consists of the bacteria Arthrobacter davidanieli, which is a closely‐related soil bacteria that provides cross‐protection when fish are injection‐vaccinated.
11.3.9 Streptococcus spp.
With the expansion of tilapia (Oreochromis spp.) aquaculture globally, one of the major disease problems has involved streptococcal septicaemic infections. Although most prevalent in freshwater fish, infections have also been reported in both wild and farmed marine fish. In freshwater, the following bacterial species appear to be the most problematic; Streptococcus agalactiae, S. iniae, S. ictaluri, S. difficile, and S. shiloi.
Streptococcal organisms are small Gram‐positive bacteria that may occur in chains consisting of 0.3–0.5 μm cocci. They can be isolated from various organs and cultured on different nutrient media. They are considered non‐motile and those associated with infections in fish have been divided into four primary groups based on specific characteristics.
Outbreaks have been reported in many fish species, but cumulative mortalities in tilapia have been reported as high as 50–60%. Clinical infection results in a variety of external signs including, but not limited to exophthalmia and petechial haemorrhaging near the operculum, anus, mouth, and fins. Internally, the intestinal tract, pyloric caeca, and liver may show petechial haemorrhaging. Once systemic, bacteria can move to various organs including the liver, heart, kidney, stomach, intestine, brain, eyes, and musculature. Outbreaks with S. agalactiae have been reported to cause mass mortality in tilapia. Streptococcal infections can also be a problem in catfish culture, and rainbow trout have been experimentally infected.
Prevention and control of streptococcal infections is difficult; however, it is important to maintain good water quality and minimise stress in an aquaculture setting. Removal of moribund or dead fish is important and should an outbreak occur, there have been reports of antibiotics such as enteroflaxin and erythromycin‐doxycycine being effective (Tung et al., 1985). In recent years, work has been carried out to develop vaccines to protect fish from major disease impacts. In tilapia, vaccines have been shown to be efficacious when injection delivered, and antibody mediated protection appears to be important. A commercial vaccine consisting of inactivated S. agalactiae (Biotype 2) is available for cultured fish but must be delivered via injection. Caution should be taken when handling fish suffering from streptococcal infections as some Streptococcus spp. are considered zoonotic and can cause infections in humans, which typically happens due to injuries to the skin while handling or preparing fish for cooking (see section 11.12).
11.4 Fungi
Fungi are considered eukaryotic organisms and are often referred to as water molds or oomycetes. They are microscopic, filamentous, absorptive organisms that function as decomposers in ecological systems. They reproduce both sexually and asexually and can produce toxins that may be harmful to animals. These water molds may be referred to as pseudo‐fungal organisms and cause diseases such as saprolegniasis and branchiomycosis, which are discussed in this section.
11.4.1 Saprolegniasis
Saprolegnia disease (saprolegniasis) can develop and impact cultured and wild fish or their eggs/embryos. In general, Saprolegnia is considered an opportunistic pathogen that feeds on necrotic tissue or organic debris. When conditions are optimum Saprolegnia can cause infection in just about any fish species at any life stage. However, infections are most prevalent in aquaculture facilities during egg incubation or early larval rearing or may become a problem in pre or post spawning salmonid broodstock.
Saprolegnia are classified as fungal‐like organisms and with filamentous hyphae, sporulation, and a classic ‘cotton tuft’ like appearance. However, some differences separate them from true fungi and place them more closely taxonomically to heterokonts, which include organisms such as diatoms and brown algae. Presumptive diagnosis may rely on visualization of cotton‐like tufts and identification of non‐septate filamentous hyphae. Confirmation to species level is difficult by microscopy, but genetic sequencing based on PCR identification is becoming more common. In most cases Saprolegnia are rarely diagnosed to the species level since treatment procedures are identical for all. Nevertheless, common species that are known to infect fish or fish eggs include S. parasitica, S. diclina, and S. ferax (see Cain and Polinski, 2014).
In general, Saprolegnia is opportunistic and can parasitise at temperatures ranging from 2–35 °C. In most aquaculture operations, S. parasitica is considered the most prevalent and concerning. Costs associated with this pathogen are estimated to be in the tens of millions of dollars in salmon and catfish culture facilities annually. Although S. parasitica is more common, S. diclina and S. ferax are considered more pathogenic than S. parasitica to Atlantic salmon eggs during incubation. New aquaculture species such as burbot (Lota lota), which require temperatures below 4 °C for egg incubation are highly susceptible to Saprolegnia (Figure 11.10). It was shown that without administration of chemical treatments, mortality of both eggs and larvae due to Saprolegnia was near 100%.

Figure 11.10 Characteristic hyphae shown attached to incubating burbot eggs. Eggs clearly infected with Saprolegnia appear cloudy and are dead, while visibly uninfected eggs appear healthy with developing embryos inside.
Source: Reproduced with permission from Dr M. Polinski.
Infection with Saprolegnia in fish will often start at the site of a previous injury or even a lesion resulting from other infections. Saprolegnia will manifest and appear as cotton‐like tufts on the external surface of the lesion of a fish or will rapidly spread once an infection is established on the surface of eggs. If fish are immunocompromised in any way as a result of predisposing stress or other factors, infection with Saprolegnia may be enhanced (Cain and Polinski, 2014).
Saprolegnia are considered ubiquitous in most freshwater environments and therefore fish or eggs will likely be exposed in an aquaculture facility. Infections can be limited in fish by minimizing stress and maintaining optimum culture conditions. Handling can cause physical injury, which predisposes fish to fungal type infection at these sites. For incubating eggs or newly hatched larvae, Saprolegnia may develop if nutrients are present; therefore, it is important to clean all organic material including dead eggs or egg shells as often as possible. Standard procedures in most facilities utilise routine treatments of eggs (and possibly larvae) with approved chemicals such as hydrogen peroxide, formalin, or in some cases sodium chloride. For fish, such as adult salmon, that may be heavily infected at sites where skin abrasions and damage occur (common if utilizing wild broodstock returning to spawning grounds), it is important to administer treatments as soon as possible. Malachite green was an early historic treatment and was quite effective; however, it is no longer allowed as it was found to have carcinogenic and toxicological effects. In the US, formalin and hydrogen peroxide are the most common chemicals used to treat saprolegniasis.
11.4.2 Branchiomycosis
Branchiomyces sanguinis and B. demigrans infections cause a condition known as ‘gill rot’. Branchiomycosis is known to impact cultured fish and has been linked to severe losses in tilapia in Israel and in farmed catfish. This condition can be linked to poor environmental conditions that affect gill health and provide an environment that favours colonisation of Branchiomyces on the gills. This condition occurs at temperatures between 25–32 °C and may manifest within 2–4 days following exposure if predisposing stressors are present.
To diagnose and distinguish between B. sanguinis and B. demigrans, gill preparations should be examined under light microscopy. B. sanguinis infects the lamellar capillaries and is characterised by a (0.2 μm) thin hyphal wall and spores of 5–9 μm in diameter, while B. demigrans is found in the parenchyma of the gills and is thicker with walls of 0.5–0.7 μm and spores measuring 12–17 μm. Gill rot may result in high mortalities and clinical signs of disease include those typical of most gill problems. These include lethargy and gulping of air at the surface of the water. Examination of fish may show striated gills with pale areas of necrosis due to infection.
Unlike Saprolegnia it does not appear that Branchiomyces are ubiquitous in the environment and movement of infected fish should be prevented. Prevention of disease is directly linked to good management practices that maintain high water quality and minimise other stressors in an aquaculture setting. Infected fish are considered carriers of these pathogens and should be separated from non‐infected fish. Treatment with formalin or copper sulphate may be effective at controlling mortality due to branchiomycosis, but to limit the spread of these pathogens all dead fish should be disposed of appropriately and ponds dried and disinfected.
11.5 Protozoans
Protozoans are among the most significant parasite problems in bivalve and finfish aquaculture industries where open water sources are utilised. They comprise a diverse group of unicellular eukaryotic organisms, many of which are motile. Protozoans range from 10 to 52 micrometers, but can grow as large as 1 mm, and can be observed using a microscope. The protozoan cell has one or more nuclei, a set of cellular organelles and special organelles serving vital functions such as locomotion, food intake or invasion of the host organism. Some parasitic protozoa have life stages alternating between proliferative stages and dormant cysts. As cysts, protozoa can survive harsh conditions, including exposure to extreme temperatures or harmful chemicals, or long periods without access to nutrients, water, or oxygen. At present there are no commercial vaccines available for any fish‐parasitic protozoa. This section provides an overview of the most common groups that occur in aquatic animals including flagellates, amoebae, haplosporidians, apicomplexans, microsporans and ciliates (Figure 11.11).
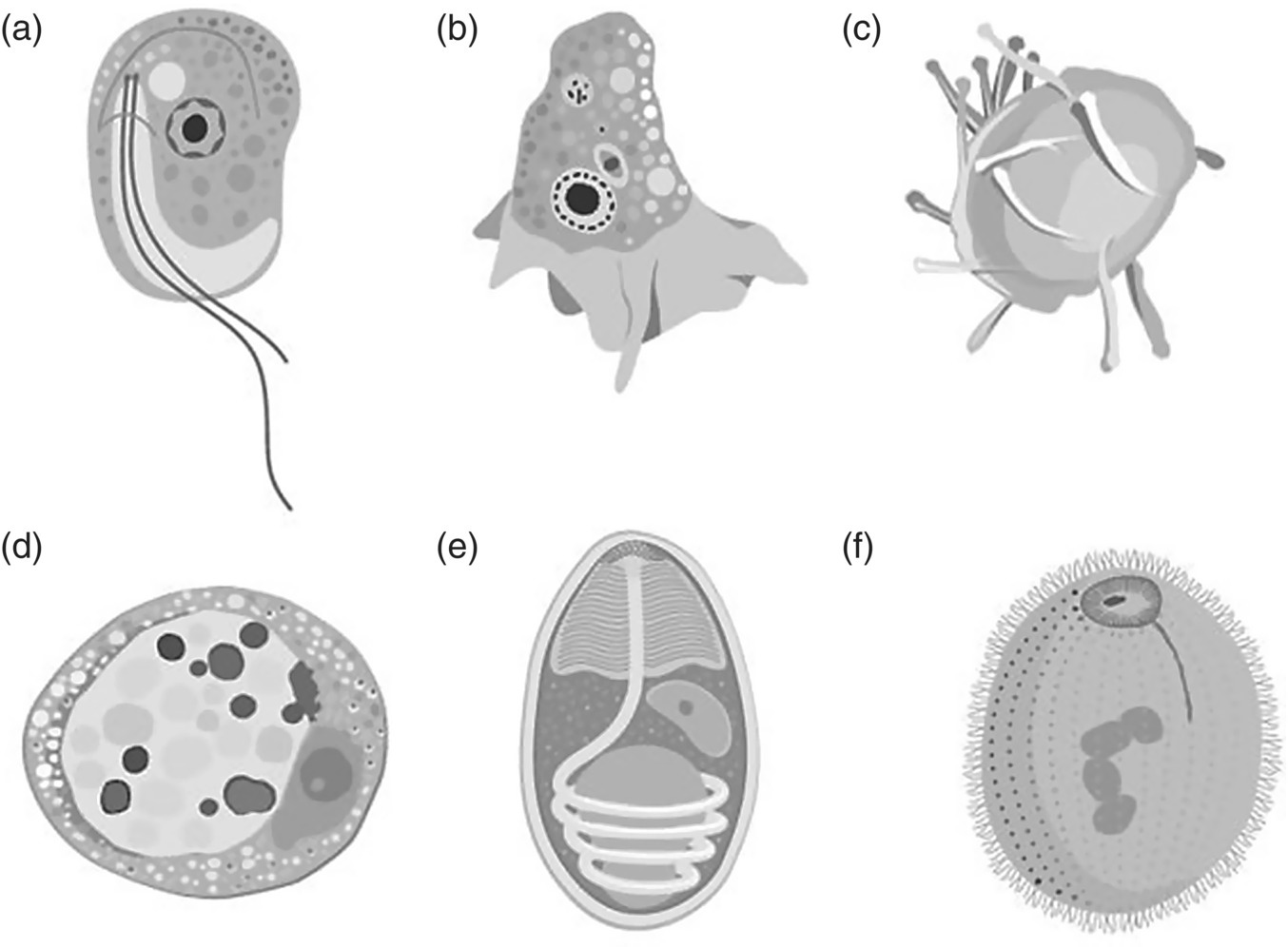
Figure 11.11 Schematic diagram of representative protozoa that may be present in invertebrate and finfish aquaculture. (a) flagellates (e.g., Icthyobodo); (b) amoebae (e.g. Paramoeba); (c) haplosporidians (e.g., Bonamia); (d) apicomplexans (e.g., Perkinsus); (e) microsporidia (e.g., Thelohania); and (f) ciliates (e.g., Cryptocaryon).
Source: Reproduced with permission from Dr Kate Hutson, graphics by Eden Cartwright, Bud Design.
11.5.1 Mastigophora (Flagellates)
The subphylum Mastigophora includes the dinoflagellates, blood parasitic trypanosomatids and ectoparasitic bodonids (Figure 11.11a). This group is characterised by elongate flagella (singular: flagellum) which undulate to propel the cell through liquid environments. Flagella are ‘whip‐like’ extensions of the cell membrane with an inner core of microtubules. Most parasitic flagellates have a simple, one‐host life cycle and reproduce by longitudinal binary fission.
The dinoflagellate Amyloodinium ocellatum is a dangerous agent of marine aquaculture fishes, causing fatal epidemics worldwide. Outbreaks can occur rapidly and result in 100% mortality within a few days. Amyloodinium has a direct but tri‐phasic life cycle. The parasites feed as stationary trophonts on the epithelial surfaces of the skin and gills. Trophonts attach to fish with an attachment disc that has filiform projections (rhizoids) that embed deep into the epithelial tissue of the host. After reaching maturity the trophont detaches from the fish skin and forms a reproductive cyst, the tomont, in the substrate. The tomont divides forming multiple free‐swimming individuals (dinospores) that can infect a new host. Under optimal conditions that parasite can complete its life cycle in less than one week. The severe disturbance of the parasite on the epithelia can lead to infected fish rubbing their body against objects or hard surfaces and osmoregulatory problems. Infections can result in hyperventilation, anorexia and mass mortality. The free‐swimming dinospore is suceptible to certain drugs, but trophonts and tomonts are relatively resistant, making eradication challenging. Infection can be diagnosed by observing parasites through light microscopy and molecular diagnostic tools have been developed.
Ichthyobodosis is an important parasitic disease that has caused severe loss among ornamental and farmed fish worldwide. Infections by members of the genus Ichthyobodo (Figure 11.11a) have been reported from more than 60 different host species in freshwater and seawater. The disease is caused by heavy infections on the skin and gills. In the past, infections have commonly been associated with a single variable species, Ichthyobodo necator. However, molecular studies have revealed that the genus Ichthyobodo consists of several different species. Two life stages are known, a kidney‐shaped free‐swimming stage and a sessile pyriform state which penetrates the epithelial cell lining. Infected fish may show discoloration of the skin and hyperventilate. Osmoregulation of fish hosts may be impaired due to destruction and fusion of gill lamellae and can cause considerably morbidity and possibly mortality. Both life stages are susceptible to formalin and oxidising agents.
Trypanosomes are parasites of invertebrates and fishes worldwide in freshwater and marine environments. They are normally found in the blood system, on the gills or in the digestive system. These parasites have a complex life cycle, with several development stages within the intestinal caecae of an intermediate leech host. Leeches may transfer blood trypanosomes (e.g., Cryptobia spp.) when they bite fish, although some species may be transmitted directly between hosts. Blood smears on stained slides show the presence of these parasites using light micropsy. Anaemia may result due to haemolysins released from the parasites; lipids and proteins that cause lysis of red blood cells by destroying their cell membrane. Young fish tend to be more vulnerable and mortality can result from an infection.
To avoid infection by parasitic flagellates there should be high filtration and treatment of receiving water. Leeches and other blood‐sucking parasites such as gnathiid isopods can serve as vectors of flagellates that infect the blood system and should be eradicated to reduce transmission. External flagellates may be treated with formalin, hydrogen peroxide or hyposalinity (for marine species) and some anaesthetics can cause flagellates to detach. High or low temperatures or salinity may inhibit multiplication of flagellates but may not be feasible for the host organism.
11.5.2 Sarcodina (Amoebae)
Most species of amoebae are free‐living although a small number are parasitic in animals. In aquaculture amoebae can be problematic for crustaceans, echinoderms and fish. Amoebae exhibit locomotion by the formation of pseudopodia (false feet) or by distinct protoplasmic flow. Movement is also used by many species to engulf and ingest food items by phagocytosis. Amoebae are extremely robust and survive under a wide range of challenging environments. They reproduce by binary fission or multiple fusion.
Paramobae perurans (the causative agent of amoebic gill disease) has become an issue for Atlantic salmon farming worldwide and affects a range of farmed marine fish species. Presently amoebic gill disease is a major issue for salmonid aquaculture in Australia, Ireland, Scotland, Norway and the USA with 10% to 82% mortality (see section 17.8). The severity of the disease is largely influenced by high salinity and high temperature. Clinical signs include respiratory distress and lethargy, which are associated with grossly visible gill lesions. The case definition of amoebic gill disease is based on histology, the presence of hyperplastic lesions and associated amoebae. The disease causes characteristic changes in the gill tissue, including severe hyperplasia of lamellar epithelium and an inflammatory response which leads to mortalities if left untreated.
The most effective treatment for P. perurans is a fresh water bath for two hours which alleviates pathological signs of infection. Gills are usually scored based on macroscopic examination to indicate the necessity for bath treatment, but there are limitations with this method because it is based on subjective interpretation and experience. Baths are stressful to fish and impact production, because fish need to be starved prior to bathing. There is evidence that a percentage of amoebae can survive and recover from a fresh water bath as they are able to form vacuoles to separate and then expel influxes of freshwater (Lima et al. 2015). Excluding caged salmon from upper cage depths where free‐living P. perurans tend to be more abundant could be an effective management strategy to reduce the speed at which initial infections occur. Use of cleaner fish as a biocontrol may be limited as many species are susceptible to amoebic gill disease.
11.5.3 Haplosporidia
The protist phylum Haplosporidia comprises over 40 described species with representatives infecting a range of marine mollusc hosts with some found in freshwater. They produce spores without the complex structures found in similar groups (such as polar filaments or tubules), but the spore stage has ornamentation consisting of tails or wrappings (Figure 11.11c). Haplosporid spores have a single nucleus and an opening at one end, covered with an internal diaphragm or a distinctive hinged lid. After emerging, it develops within the cells of its host, usually a marine mollusc or annelid. They develop within the digestive system and undergo internal budding to produce multicellular spores. The dynamics of haplosporidians in their hosts is seasonal and depends on environmental parameters.
Bonamiosis is a lethal infection of the haemocytes of flat oysters caused by Haplosporidia in the genus Bonamia (see section 10.5.1.1). This intrahaemocytic protozoan quickly becomes systemic with overwhelming numbers of parasites coinciding with the death of the oysters. Infection in oysters rarely results in clinical signs of disease and often the only indication of the infection is increased mortality. In some cases, the disease is accompanied by yellow discoloration and lesions on the gills and mantle, but in most cases infected oysters appear normal. Lesions can be detected by histology and may occur in the connective tissue of the gills, mantle, and digestive gland. Although the life cycle is unknown, it has been possible to transmit the disease experimentally in the laboratory by cohabitation or inoculation of purified parasites. Bonamia ostreae has been associated with considerable devastation of oyster populations. It was first observed in France in 1979 and caused substantial destruction of Ostrea edulis populations before spreading through much of Atlantic coastal Europe.
Similarly, outbreaks of Haplosporidium nelsoni along the mid‐Atlantic coast of the USA have devastated oyster populations and caused significant economic disruption of coastal communities. Oyster mortality associated with outbreaks can exceed 90%, producing significant financial losses for oyster industries. Haplosporidium nelsoni is believed to have been introduced to the USA from Asia. It infects the Pacific oyster (Crassostrea gigas) in Asia, Europe and the west coast of the USA and the eastern oyster (C. virginica) along the east coast of North America and Canada. Mortalities are usually highest in the summer months, and also increase in higher salinity waters. The disease reduces the feeding rates of infected oysters and reduction in stored carbohydrates inhibits normal gametogenesis in the spring, with a reduction in fecundity.
11.5.4 Apicomplexa (Sporozoans)
The phylum Apicomplexa is a large group of protozoan parasites with over 8000 species described as parasites of invertebrate and vertebrate hosts. Apicomplexans have a special cell organelle, the apical complex, which facilitates invasion of the host cell (Figure 11.11d). They undergo cyclic development involving three divisional processes: merogony, gamogony and sporogony. Cell division can occur by fission or endogeny. Fish apicomplexans are divided into two major groups; coccidia are primarily intestinal parasites, while haematozoa are blood parasites which have stages in fish with spore formation in leeches or gnathiid isopods. Species are usually differentiated based on the morphology of the spore, also termed the oocyst.
Several species of the genus Perkinsus are responsible for causing disease in molluscs including oysters, mussels, clams and abalone worldwide. Perkinsus olseni is the only species known to cause disease in the Asia–Pacific region and occurs in abalone, clams and pearl oysters. It was first described from the abalone, Haliotis rubra, in southern Australia. Perkinsus olseni is included on OIE’s list of notifiable diseases because infection can cause widespread mass mortality. Clusters of Perkinsus cells near the surface of the abalone appear as a white nodule or micro‐abscess in the foot and muscle. This develops to form a brown spherical pustule up to 8 mm or more in diameter. Transmission is direct from host to host and all life stages are infective, with parasite cells released from the host following host death and decomposition. Prevalence is highly variable depending on host and environmental conditions, but it is often 100%, as determined by histology or PCR. Infections can impede respiration and other physiological processes including growth and reproduction, and stress from high temperatures is believed to exacerbate the disease. Adaptive immunity is not known in oysters or other molluscs, so they cannot be immunised against Perkinsus species. Selective breeding of disease‐resistant strains may confer some protection against infection.
11.5.5 Microsporidia (Microsporans)
Microsporidia or microsporans are obligate intracellular parasites which lack mitochondria and form small unicellular spores. Microsporans proliferate in host tissues by merogony (asexual division) followed by sporoblastogenesis and sporogony (spore formation). Mature spores contain a unique coiled polar tube which everts forcibly to inject the infective sporoplasm into host cells (Figure 11.11e). Infections may be disseminated throughout the tissues or they may cause focal lesions, inflammation and granulomas.
Microsporan species in the genus Thelohania (family Thelohaniidae) cause ‘cotton‐tail’ disease in aquatic crustaceans. Infections have been detected in most freshwater crayfish species, including wild and cultured marron (Cherax tenuimanus), yabbies (Cherax destructor) and redclaw (Cherax quadricarinatus). Heavily infected muscles become white in appearance and are unmarketable. Mildly infected crustaceans may be stunted in growth, while heavy infections may be fatal. Transmission is assumed to be direct, via water‐borne transport of infective spores and/or ingestion. In infected individuals, mature spores may penetrate adjacent cells, injecting infective sporoplasm which subsequently divides and ultimately forms new spores. Heavy infections can be detected macroscopically by visual examination of crayfish tails which are opaque in appearance, rather than translucent and clear. Alternatively, infections can be diagnosed by microscopic detection of cysts and spores in squash preparations or histology of the muscle. The most successful prevention of infection is to drain culture ponds, lime them and dry them before restocking. There are no drugs currently available to treat infestations.
11.5.6 Phylum Ciliophora
Ciliated protozoa are among the most common external parasites of fish but can also infect invertebrates. Most ciliates have a simple life cycle and divide by binary fission. Ciliates can be motile, attached, or found within the epithelium. Ciliates living in or on fishes range from harmless ecto‐commensals to dangerous parasites in fish aquaculture. With few exceptions, ciliates possess cilia at some stage of their life cycle. Asexual reproduction occurs by transverse binary fission. About 150 species occur in fish, most as ectoparasites causing fouling, irritation and local lesions and sometimes penetrating wounds. Some are endoparasites and can damage of the tissues or organs. Ciliates can irritate the surface cells, penetrate into deep tissue layers and ingest the cell debris produced.
Some of the most important ciliates in captive freshwater fishes include Ichthyophthirius multifiliis, Chilodonella spp., the trichonids (including Trichodina, Trichodinella, Tripartiella, and Vauchomia spp.) and Tetrahymena species. In fish confined to ponds, tanks or aquaria, ciliates that live freely in the water column, such as Trichodina spp. and Chilodonella spp., can form dense populations on fish resulting in morbidity and mortality. Chilodonella spp. have a voracious appetite for living cells and use a specialised mouth organ, the cytostome, to graze on bacteria, diatoms, filamentous green algae and cyanobacteria present on biofilm substrates of fish gills and skin.
In marine environments, Cryptocaryon irritans and scuticociliates are considered among the most problematic ciliate parasites. Cryptocaryon irritans cause marine white spot disease in tropical and subtropical marine teleost fish (Fig 11.11f). It commonly occurs in public aquaria and food fish farming and can rapidly proliferate and severely impair the physiological functions of its host’s skin, eyes and gills. Cryptocaryon irritans has a quadriphasic life cycle including four developmental stages; the theront, trophont, protomont and tomont. The trophont penetrates the host epidermis and obtains nutrition by feeding on sloughed cells. Subsequently, the parasite leaves a small wound and visible white spot or nodule where each parasite encysts. It becomes a free‐swimming protomont when it exits the host and moves along the substrate for a few hours. The protomont adheres to the hard surface, sheds its cilia and encysts forming the reproductive stage tomont, which divides over 3–28 days. After several divisions, the tomont ruptures, releasing about 300 free‐swimming theronts which must find a suitable host within 48 h or they will die.
Scuticociliates are aggressive and invasive minute ciliates that are known to cause disease in marine fishes, including sea horses, flounders, turbots, bass, tunas, and crustaceans. In southern bluefin tuna (Thunnus maccoyii) sea cage culture in Australia, fish infected with scuticociliates exhibited atypical swimming behaviour followed by rapid death in winter. It is hypothesised that the parasites initially colonise olfactory rosettes and then ascend to the olfactory nerves to eventually invade the brain where they cause encephalitis. Species of the scuticociliate Mesanophrys live as scavengers on the exoskeletons of crabs and lobsters, but if they find a break in the exoskeleton they invade the haemocoel, multiply and kill the host.
11.6 Myxozoans
Myxozoans are obligate parasites that use invertebrate and vertebrate hosts as part of their life cycle and can occur in marine and freshwater environments. They are found principally in the muscle, brain and gall bladder of fish. Myxozoa form complex valved spores with polar capsules containing extrudible filaments which are used for attachment to host cells (Fig. 11.12a, b). Their development involves multicellular differentiation, which does not conform to the unicellular definition of the protozoans, and recent molecular studies confirm that myxozoans are cnidarians (Chang et al., 2015). Their transmission requires a developmental phase in an invertebrate host, although rare cases of direct fish to fish transmission have been reported. Myxospores released from the fish can survive in the aquatic environment for more than one year. When ingested by an oligochaete (in freshwater) or a polychaete (in the sea), they invade the worm’s intestinal tissues where they proliferate. Infected annelids release tri‐radiate actinospores that float in the water. Actinospores penetrate the surface of the fish on contact and migrate to the site where the sporogonic plasmodium develops. Infections by myxozoans may be asymptomatic, but some may cause tissue hyperplasia, unsightly cysts, erosive and necrotic lesions, enzymatic lysis of fish tissue (i.e., myoliquefaction), deformities and even death. The most notorious species are known from the genera Myxobolus, Sphaerospora, Ceratomyxa, Hexacapsula, Kudoa, Unicapsula, Henneguya, Enteromyxum and Tetracapsuloides. Some fish‐borne myxozoan species (i.e., Kudoa spp.) can cause food poisoning in humans (see section 11.12).
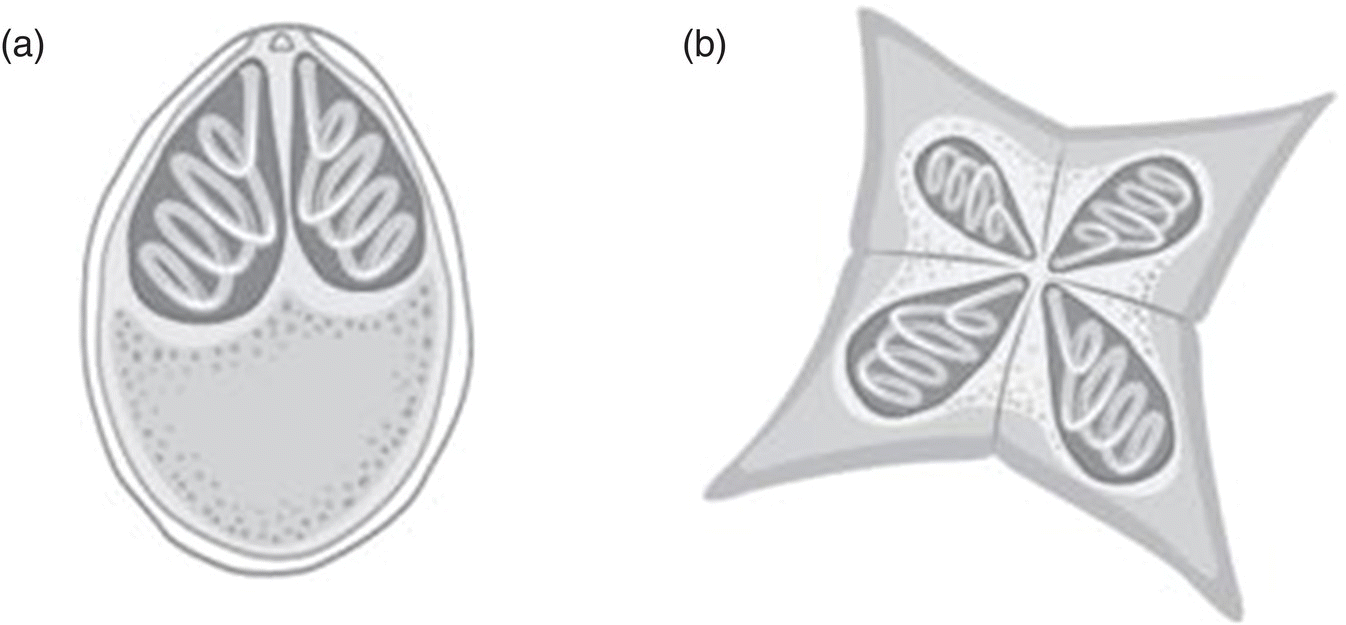
Figure 11.12 Schematic diagrams of representative myxozoa that may be present in finfish aquaculture. (a) Myxobolus sp.; (b) Kudoa sp.
Source: Reproduced with permission from Dr Kate Hutson, graphics by Eden Cartwright, Bud Design.
‘Whirling disease’ is an ecologically and economically debilitating disease caused by Myxobolus cerebralis in commercially reared salmonids. Whirling disease afflicts juvenile fish (fingerlings and fry) and causes skeletal deformation and neurological damage. Fish ‘whirl’ forward in an awkward, corkscrew‐like pattern instead of swimming normally, find feeding difficult, and are more vulnerable to predators. The parasite has a complex life cycle, alternating between salmonid fish and the oligochaete host, Tubifex tubifex. The parasite infects its hosts during its free‐swimming triactinomyxon stage when appendages on the parasite pierce the skin of salmonids and sporoplam is injected into the host, where it migrates through nervous tissue to areas of cartilage around the brain. There it matures into an M. cerebralis spore containing the classic polar filaments found in the myxozoans (Figure 11.12a). Mortality rates may be up to 90% in infected populations, and those that do survive can develop deformities due to spores residing in their cartilage. Dead fish act as a reservoir for the parasite, which may be released into water and ingested by susceptible T. tubifex, thus removing mortalities promptly is important for managing fish health.
Proliferative kidney disease (PKD) also causes significant losses among salmonid (Pacific salmon and rainbow trout) populations in Europe and western North America. The parasite that causes PKD, Tetracapsuloides bryosalmonae, was named in reference to its two known hosts – bryozoans and salmonids. Various freshwater bryozoans are susceptible to infection and release spores into the surrounding water where they can infect fish. Massive numbers of spores can be produced from relatively small volumes of bryozoans. The disease is often seasonally dependent occurring at water temperatures above 15 °C. Parasites are initially prominent in the blood sinuses of the kidney and provoke a strong inflammatory response. Mortality in uncomplicated cases of PKD is generally 20% or less but often secondary pathogens or unfavourable environmental conditions coincide with peak periods of PKD and mortalities can reach 95–100%. In fish that have experienced a full clinical episode of the disease, a strong acquired immunity develops. No vaccines have been developed to control PKD.
Kudoa spp. can occur in the muscle, kidney, ovary and brain of a variety of marine aquaculture fishes (Figure 11.12b). Infections of the skeletal muscle are the most common. In British Columbia, Canada, annual costs to the Atlantic salmon aquaculture industry due to K. thyrsites infections in the muscle can reach millions of dollars. Infection may be characterised by visible cysts, which can make fillets unsightly, unappetising, and therefore unmarketable (Figure 11.13), or alternatively they may be microscopic intracellular pseudocysts. Parasite‐derived enzymes degrade the flesh post‐mortem, making fillets soft and watery. Recent evidence suggests that human consumption of fish flesh infected with Kudoa can cause food poisoning (see section 11.12). Infection prevalence can be monitored within affected farms by PCR or examining fillets for manifestation of soft‐flesh, but there are no external clinical signs to identify and cull fish infected with Kudoa in the skeletal muscle. Kudoa yasunagai infects the brain of various important marine aquaculture fishes including Japanese sea bass (Lateolabrax japonicas), sea bream (Pagrus major), olive flounder (Paralichthys olivaceus), tiger puffer (Takifugu rubripes), yellowtail (Seriola spp.), and Pacific bluefin tuna (Thunnus orientalis). Clinical signs of infection include abnormal swimming behaviour and skeletal curvature. In Japan, mortalities of juvenile kingfish Seriola lalandi in net cages occurred approximately two months after being transferred from hatchery facilities, with more than 50% of fish developing scoliosis and abnormal swimming behaviour including whirling or lying at the bottom of cages. Symptomatic fish had difficulty feeding and suffered from damaged skin from swimming into the cage net. To date, there is no effective control method for Kudoa spp. in aquaculture. Managing the production schedule to avoid seasonal abundance of infective parasites may be possible, but further information is needed on the life cycle (i.e., identity of the invertebrate host), seasonality and infection dynamics.
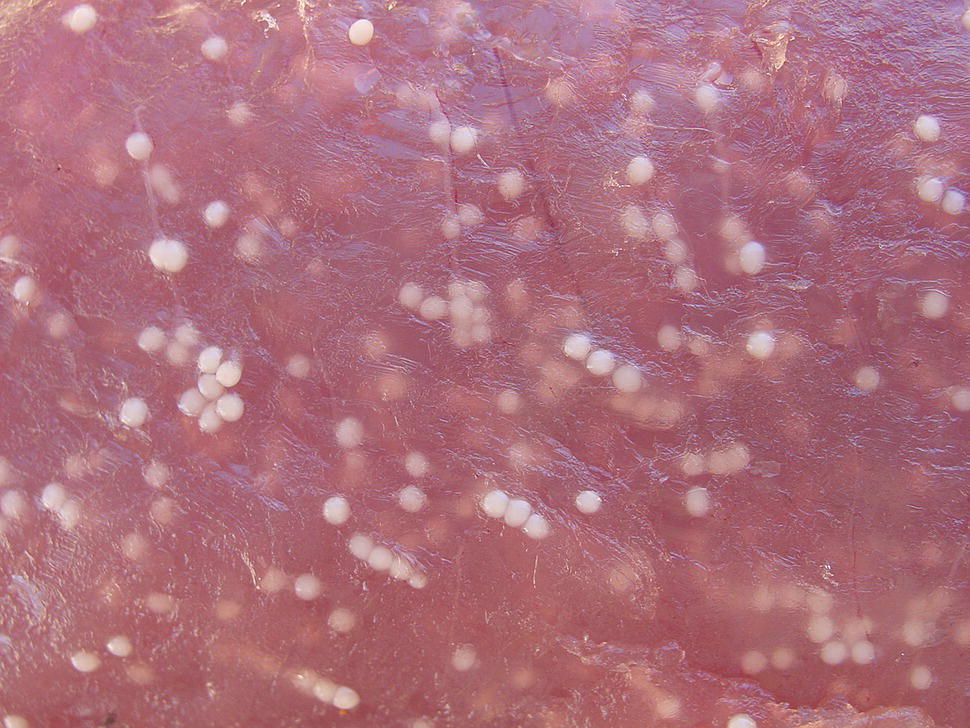
Figure 11.13 A wild captured tuna, with obvious white cysts in the muscle, in this case evidence of infection with the parasite Kudoa.
Source: Reproduced with permission from Dr R. D. Adlard.
11.7 Platyhelminths
Although some turbellarians have adopted a parasitic life style, there are three major classes of platyhelminths that are entirely parasitic. Two of these, the tapeworms (Cestoda) and the flukes, (Digenea), are endoparasites and the third group, the Monogenea, comprise a range of skin and gill parasites of fishes.
11.7.1 Turbellarians
Turbellarian worms are predominantly free‐living members of the Platyhelminthes but include several parasitic or predatory species, some of which are relevant to aquaculture. Most turbellarians differ from all other platyhelminths in that they lack an oral sucker, move in a gliding fashion using multi‐ciliated epithelial cells, and having a direct life cycle. Most forms reproduce sexually, and, with a few exceptions, all are simultaneous hermaphrodites.
Paravortex spp. are viviparous turbellarians known from molluscs and fish and can cause diseases characterised by host death, acute focal dermatitis, and secondary infections of Vibrio. Infections of Paravortex have been observed in hatchery‐reared stonefish, Inimicus japonicus, fry and cage‐cultured sea perch, Lateolabrax spp., greater amberjack, Seriola dumerili and red sea bream Pagrus major in Japan and commercial clams, Ruditapes decussatus.
Polyclads are carnivorous micropredators that pose a considerable threat to cultured invertebrates including sponges, corals, mussels, edible and pearl oysters, scallops and giant clams. Efforts to culture acroporid corals in aquaria can be severely compromised by polyclad flatworms which, if not removed, can eat entire colonies (Figure 11.14a). Their occurrence in culture facilities warrants careful scrutiny because live flatworms are camouflaged against live tissue. The Acropora‐eating flatworm, Amakusaplana acroporae, exhibits relatively high fecundity and lays multiple batches of capsules on the coral skeleton (Figure 11.14b), with each capsule containing up to seven embryos. The polyclad flatworm Stylochus matatasi can reach nearly 6 cm and have been associated with the death of giant clams, Tridacna gigas. Although they can sometimes be observed on the mantle of clams, they can also damage the inside of the mantle cavity and may not necessarily be detected until after a clam has died.

Figure 11.14 (a) The Acropora‐eating flatworm, Amakusaplana acroporae, can eat entire coral colonies in culture. (b) Worms lay multiple batches of small brown coloured capsules on the coral skeleton, with each capsule containing up to seven embryos.
Source: Reproduced with permission from Kat Dybala, Kate Rawlinson and Jonathan Barton.
Temnocephalans are generally considered as ectosymbionts rather than parasites. They are relatively small (0.5–1 mm) flatworms and lay their eggs in gills and on the shell of freshwater crustaceans. Adult worms move over the surface of the crayfish, eating algae and other microfauna. Although they are very common on freshwater crayfish, they do not pose any considerable health risk to the animal, however, large numbers of adhesive eggs laid on the surface of crustaceans may lead to rejection or poor prices at market.
11.7.2 Cestodes
Numerous cestode species can cause disease in fish, while fish‐borne cestodes in Diphyllobothrium can cause disease in humans (see section 11.12). Cestodes, commonly called tapeworms, are hermaphroditic endoparasitic flatworms characterised by the presence of the scolex (the head) which attaches the worm to the host. A string of segments (proglotids) with sex organs is found beneath the scolex. They feed by absorbing nutrients over their tegument because they have no mouth or intestine. Cestodes are oviparous and have complex life cycles (i.e., they require more than one host to complete their life cycle). The adult worms produce eggs which are delivered to the environment with the faeces of the host. An intermediate host (usually a crustacean) ingests the egg or free‐swimming larvae (coracidica) and becomes infected. Some tapeworms exhibit a two‐host life cycle, while others may have three hosts, where the second intermediate host is typically a fish.
The Asian tapeworm, Schyzocotyle acheilognathi is an important pathogenic cestode of aquaculture in Asian cyprinid fish. The parasite is indigenous to East Asia, but has spread rapidly throughout the world through the ornamental fish trade. It exhibits a simple two‐host life cycle involving common copepod species as an intermediate host. Once established, it may endanger native fish populations. The parasite attaches to the gut wall using its scolex where it can cause severe damage to the intestinal tract, including pressure necrosis and haemorrhage. Heavily infested fish exhibit reduced growth, loss of condition and mortality. Indeed, 100% mortality can occur in infected hatchery‐reared carp. Triaenophorus spp. include other pathogenic cestode species in Eurasia, causing epidemics and mortality in juvenile rainbow trout in hatcheries and rearing ponds. This parasite causes unsightly infestation in the muscles of the fish and can impact marketability.
Most aquaculture facilities use local natural water bodies as their source of water, and these can be naturally infected with tapeworms. To reduce infestation by cestodes, the water should be filtered (e.g., screen or sand filters) or alternatively ground water could be used if available. The use of low‐value fish in the diet can potentially introduce second intermediate contaminated fish, so alternative feeds such as an extruded pellet diet can be used. An anthelmintic, praziquantel, originally synthesised to treat endoparasitic flatworm infections of mammals may have an effect on fish cestodes but will not kill necessarily kill the eggs or free‐swimming larvae.
11.7.3 Trematodes
Trematodes exhibit complex life cycles and the adult stage is parasitic in vertebrates. Few adult trematode species are known to cause considerable harm to their definitive fish host with the most notable exceptions being mostly extra‐intestinal parasites such as the aporocotylid blood flukes and the cyst‐forming didymozoids. Metacercariae (encysted larvae) infection can cause mortalities in farmed fishes with subsequent economic loss (e.g., Bolbophorus damnificus; see section 19.3.4) and ocular diplostomiasis (infection of the eye by metacercariae of Diplostomum spp.) can cause blindness in farmed salmonids and catfish. Some metacercariae in fisheries and aquaculture products (fish and shellfish) are a source of infections in humans and domestic animals (see section 11.12).
Trematodes are hermaphrodites and most adults are dorso‐ventrally flattened with a sucker around the mouth and an additional ventral sucker. Gastrointestinal trematodes release eggs with the host faeces that hatch into free‐swimming, sometimes ciliated, miracidia. The miracidia invade a suitable mollusc host where asexual reproduction takes place. Specificity of individual trematode species to the molluscan host appears to be quite restricted, usually to a species. Trematodes undergo part or all of their larval development in molluscs with the exception of marine blood flukes (aporocotylids) which complete their larval development in terebellid polychaete annelids. In some cases, trematode sporocysts occupy the gonad and cause partial or full castration of the intermediate host. Cercariae emerge and may have a tail or other locomotory device to assist in infection of the second intermediate host where they encyst as metacercariae. Trematode life cycles can be broken in aquaculture where it is feasible to exclude intermediate molluscan hosts or polychaetes.
Aporocotylid trematodes or ‘blood flukes’, cause lethal infections in cultured salmonids and cyprinids in freshwater, and scombrids, carangids, sparids and tetradontids in marine environments. Detection of adult flukes can be made in the blood vessels, while thin shelled eggs can be detected in the gills using microscopy or histology. Adult parasites release eggs into the fish’s vascular system which may be sequestered in the gill, heart, kidney, liver, spleen, pancreas, or other organs, where they cause inflammation and decrease the physiological and mechanical efficiency of these organs. Freshwater and marine blood flukes use either gastropods or terebellid polychaetes as the intermediate host, respectively (Figure 11.15). Blood flukes are unusual because their life cycle lacks a second intermediate host and an encysted metacercaria. Thus, the life cycle depends on the proximity of definitive and intermediate hosts to the free‐swimming infective stages of the parasite. Freshwater snails easily establish in culture systems and in pond farms, while the polychaetes may proliferate on supporting materials on sea cages (i.e., in the biofouling) or in sediment. Manipulating the proximity of the fish host to its fluke’s intermediate host is desirable, and several marine blood fluke life cycles for tuna have been resolved recently which will assist to develop appropriate management methods (e.g., Cribb et al., 2011; Fig. 11.15).
Figure 11.15 Schematic diagram showing the life cycle of Cardicola forsteri infecting farmed southern bluefin tuna, Thunnus maccoyii. Adult blood flukes in the circulatory system lay eggs which lodge in the gills. Eggs embryonate and hatch to release micracidia that seek the second intermediate host (terebellid polychaete). Asexual reproduction takes place in terebellid polychaetes, where infective cercariae develop in sporocysts.
Source: Reproduced with permission permission Dr. Kate Hutson, graphics by Eden Cartwright, Bud Design.
Infection of farmed fish by trematodes can be managed by minimizing interactions between farmed stock and wildlife (namely molluscs or polychaete worms). For example, metacercarial infections can be prevented when fish‐eating birds are excluded using nets and/or water filtration eliminates snail populations. Fallowing or farming sea caged fish in deeper water likely reduces interactions with intermediate hosts that live on or in sediment. Oral administration of praziquantel is effective against blood fluke infections in tuna, although does not seem to affect eggs.
11.7.4 Monogeneans
Monogeneans are ubiquitous in finfish aquaculture. They infect captive ornamental and food fishes in fresh or marine environments and can also infect elasmobranchs and aquatic turtles. Commonly termed ‘flukes’, they are primarily ectoparasites and have a characteristic posterior attachment organ called a haptor. The Monogenea can be generally divided into two subclasses based on the complexity of the haptor: the Monopisthocotylea have one main part to the haptor, often with hooks or a large attachment disc, whereas the Polyopisthocotylea have multiple parts to the haptor, typically clamps. Monopisthocotyleans tend to live on the gills, skin and fins where they feed on epithelial tissue and include species that cause harmful infections in aquaculture (e.g., species in Gyrodactylus, Pseudodactylogyrus, Dactylogyrus, Neobenedenia and Benedenia). Polyopisthocotyleans are almost exclusively gill‐dwelling blood feeders and heavily infected fish suffer from anaemia (e.g., infections from species in Discocotyle, Heterobothrium and Zeuxapta).
Monogeneans have a direct life cycle (they require only a host to complete their life cycle) and most are oviparous – they produce eggs that are released and hatch in the aquatic environment (Figure 11.16). Monogeneans are hermaphrodites and are capable of reproducing in isolation. Eggs laid by parasites can bear long filamentous strings that catch on aquaculture infrastructure (nets, filters and the substrate) so that the infective larvae, or oncomiracidia, hatch close to fish. The egg casing is physically strong, but a detachable lid or operculum permits escape of the oncomiracidium. Most species have oncomiracidia that are equipped with rows of cilia, which enable them to swim and directly attach to a new host. Once attached, they typically migrate over the body to the final site of attachment. At high water temperatures parasite generation time is reduced, rapidly causing numbers to increase and if unmanaged, infections may kill captive fish.
Figure 11.16 Schematic diagram showing the life cycle of Neobenedeniagirellae infecting farmed cobia, Rachycentron canadum. Adult Neobenedenia attached to the body surface of fish lay eggs into the water column which may become entangled in sea cage netting. Following a short embryonation period (usually 5–8 days at 25 °C), eggs hatch in close proximity to fish. Ciliated oncomiracidia can swim and attach to fish where they feed on epithelium and grow to sexual maturity.
Source: Reproduced with permission from Dr Kate Hutson, graphics by Eden Cartwright, Bud Design.
Outbreaks of Neobenedenia species have caused particular devastation in marine fish aquaculture. Neobenedenia (considered here as a collection of potentially undifferentiated species) has been reported from around the globe in multiple aquaculture industries including tilapia (Orechromis spp.), grouper (Epinephelus spp.), jacks (Seriola spp.), puffer fish (Takifugu rubripes), barramundi (Lates calcarifer) and cobia (Rachycentron canadum; Figure 11.16). Neobenedenia are dorsoventrally flattened, oval‐shaped and adults range from 2–7 mm. A single worm is capable of producing ~3300 eggs in its lifetime. Live worms are almost entirely transparent, but bathing in dechlorinated fresh water kills them and turns specimens white. Haptoral sclerites (sclerotised hook structures) penetrate host epidermis and injure fish skin and large populations of worms grazing on fish can erode the epithelium. In some circumstances, oncomiracidia predominantly recruit to the eye and can cause blindness. Infestations appear to irritate fish so that they rub their body or ‘flash’ against structures or other fish, presumably in an attempt to dislodge attached parasites. Lesions may worsen from secondary infection (bacteria, viruses and fungi) of parasite‐inflicted wounds.
Some monogeneans are viviparous (e.g., Gyrodactylus) – they develop the embryo in their uterus, give birth to the daughter which is fully developed and already carrying the next generation in the uterus. This mode of reproduction, hyperviviparity, has been colloquially termed ‘Russian Doll’ and permits exponential population increases on susceptible hosts. Parasites are spread from direct physical contact between hosts or alternatively they can detach and survive for a few days before infecting a new host. Transmission from dead hosts to live hosts may also occur. Gyrodactylus salaris, a freshwater parasite of salmonids, is believed to have been introduced with infected salmon from Sweden to Norway based on high demand for salmon for stocking and experimental purposes (Johnson and Jensen, 1991). The parasite was new to Norwegian stocks and fish were incredibly susceptible, with the parasite spreading to at least 46 rivers in Norway resulting in catastrophic ecological and economic problems. Economic loss related to diminished fish stocks, loss of angler tourism and ongoing surveys and management measures. Gyrodactylus‐infected rivers are periodically treated with the indiscriminate pesticide/piscicide rotenone prior to salmon runs to increase spawning success, but this treatment is only possible in short rivers with favourable biological and geographic conditions.
Attention to farm husbandry can reduce monogenean infections on livestock. On sea cage farms eggs laid by oviparous species can entangle on nets and other fouling material (Figure 11.16) so regular cleaning or changing of nets can reduce egg load. The frequency of net changes should increase in summer temperatures when eggs hatch rapidly. Treatment of monogenean infections commonly involves fresh water, hydrogen peroxide or formalin baths. The eggs are generally resistant to short exposure to these chemicals due to their proteinaceous shell, but they are susceptible to desiccation. Praziquantel has been tested against a range of monogenean infecting fish with promising results, although if delivered orally, highly medicated feed may be unpalatable to some fishes. Administration of bath or in‐feed treatments requires strategically timed dual delivery for optimal results to kill adult parasite populations on fish, followed by a second treatment to kill immature parasites that have recruited as larvae from eggs around the farm. Cleaner organisms (fish, shrimp) show considerable promise for reducing infection intensity of monogeneans, but the risks of co‐culture need thorough examination.
11.8 Nematodes
Nematodes, or round worms, are long, slender and cylindrical unsegmented endoparasites tapered at each extremity. They infect freshwater, marine and brackish fish species. Some nematode genera include species that can spread between animals and humans, e.g., Anguillicola, Philometra, Skrjabillanus and Anisakis. Pathology in fish normally occurs within the intestines but nematodes can be found in all organs. Nematodes have separate sexes and exhibit complex life cycles. Adults have to complete their life cycle in a specific bird, fish or mammal species while their larval stages may be able to survive in a large variety of intermediate hosts. Any disruptions to these cycles prevent the development of the adult nematodes, therefore cultured fish with low connectivity to the natural environment (such as land based recirculating systems) are less likely to develop nematode infections. Few studies have examined the potential effects of nematodes on the health condition or fitness of fish hosts, but humans may become infected with nematodes if consumed in raw or undercooked seafood (see section 11.12). Nematodes have the potential to limit market value of fish through consumer attitudes.
One of the most invasive nematode species is Anguillicoloides crassus which is strictly parasitic to eel species in the genus Anguilla. This parasite is known to infect six eel species and has been recorded from 46 countries in four continents (Lefebvre et al., 2012). Human‐mediated transfers for aquaculture are suspected to be the reason for the rapid invasion of this species around the globe. Losses have been reported in wild and farmed eels and the parasite is considered among the main threats to the survival of European and American eel species. It has been reported in South East Asia, Europe, the USA and Africa, but so far has not been reported in Australian eels, probably as a result of strict import legislation. The parasite causes severe pathology in the hosts’ swim bladder including lesions, inflammation, haemorrhaging and fibrosis (Figure 11.17). Eels infected with nematodes typically exhibit reduced swimming performance, swim near the surface and are emaciated.
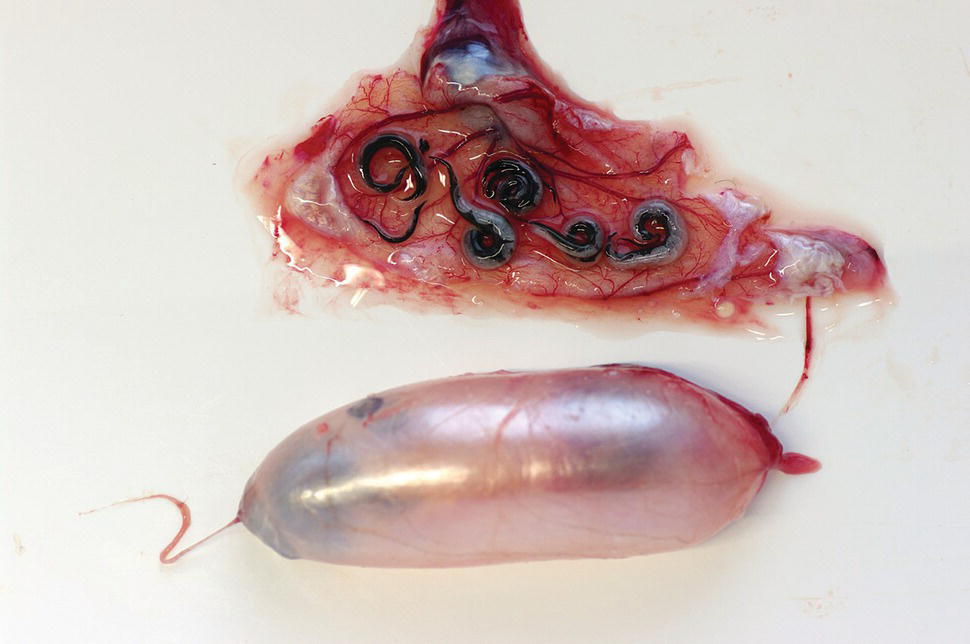
Figure 11.17 One of the most invasive nematode species is Anguillicoloides crassus which parasitises the swim bladder of eel species in the genus Anguilla.
Source: Reproduced with permission from Dr S. Klimpel and Dr S. Emde.
Anguillicoloides crassus is trophically transmitted – it depends on predator–prey interactions to complete its life cycle. Eggs leave the swim bladder via the pneumatic duct and pass through the intestine and hatch in water. Alternatively, they may hatch internally. Newly hatched second‐stage larvae attach to the substratum by their caudal extremity and wiggle intensively, presumably to stimulate predation by aquatic invertebrates. They can survive and remain infective for days. A range of crustacean species may serve as intermediate hosts, including copepods and ostracods. Once ingested, that larvae invade the haemoceoel where they grow and moult. Eels become infected when they eat infected aquatic organisms, including paratenic fish hosts (a host is not necessary for the development of a particular species of parasite, but serves to maintain the life cycle and can promote dispersal). Once ingested by the eel, the parasite passes through the intestinal wall and migrates to reach the swim bladder wall within one week (Figure 11.17).
Disrupting the life cycle is one of the best preventative strategies for reducing nematode infections in aquaculture systems. This can be achieved by filtering incoming water sources or adding chemicals to eliminate intermediate crustacean hosts. Clean feed sources should be maintained, such as an extruded pellet diet. There is no effective drug against fish nematodes but drugs such as flubendazole, levamisole, mebendazole, trichlorphon and triclabendazole have been trialed with varying results.
11.9 Acanthocephalans
Acanthocephalans or ‘spiny headed worms’ comprise at least 500 species of endoparasites of freshwater and marine fish, although only a few species are reported to be problematic in aquaculture. They live in the host intestine where they attach using a retractable proboscis covered in numerous hooks. They have no mouth or intestine and absorb nutrients from the lumen. Worms have separate sexes and a complex life cycle. Adult female worms living in the intestine of fish copulate and eggs are passed with the host faeces into the aquatic system. Eggs are ingested by a free‐living intermediate crustacean host (e.g., amphipod or isopod) which becomes infected. When the definitive fish host ingests the crustacean, the parasite develops to the adult stage in the fish intestine.
Epidemics caused by acanthocephalans have been reported in aquaculture hatcheries, particularly in salmonids and eels. The most well‐known genera include Acanthocephalus, Echinorhynchus, Pomphorhynchus and Neoechinorhynchus. The attachment of the proboscis to the mucosa elicits an inflammatory response at the site of attachment. Heavy infestations may cause lethargy, morbidity and deformation of the spinal column and may result in reduced host absorption of nutrients from the intestine. Prevention of intermediate hosts into aquaculture systems will break the life cycle.
11.10 Leeches
Leeches (Phylum Annelida) are segmented worms in the Subclass Hirudinea. Some medicinal species are farmed for use in human medicine in a quirky aquaculture industry called Hirudiculture. Not all leeches are bloodsuckers and many leeches encountered living freely in ponds and rivers are predators. Leeches can infect a variety of aquaculture organisms including crustaceans, molluscs, fish, frogs and soft‐shelled turtles (note ‘oyster leeches’ are turbellarians) and occur in freshwater, brackish and marine environments (Kearn, 2004). As micropredators, or temporary parasites, they may leave their host after feeding and not reattach to a new host until the last meal has been digested. In addition to crawling, where the anterior and posterior suckers are used for locomotion, some leeches may swim. Leeches are negatively phototactic, which prompts them to seek out the benthos where they rest attached to vegetation or submerged infrastructure. When hungry, they become sensitive to water turbulence and shadows generated from passing fish. The leech then stretches out like a rod and performs ‘searching’ movements by swaying the body. Sensory structures including simple eyes, papillae and sensilla also enable them to find prey or hosts.
When contact is made with a potential host the oral sucker is attached and the posterior sucker released from the substrate. Some leeches also have adhesive secretions that play a role in cementing them to the surface of the host. Leeches are simultaneous hermaphrodites and adults will detach from the host in order to lay cocoons on a chosen substrate, including aquaculture structures such as moorings and nets. Cocoons contain a ring‐shaped compartment that is effectively sealed from the environment which protects the developing embryo (Kearn, 2004). Cocoons are usually adhered to the substrate and each typically contains a single egg. Hatching is temperature dependent and young leeches can survive for a week or more before their first blood meal.
Leeches attach to their host using anterior and posterior suckers and use their jaws to gain access to blood. They may prevent clotting while feeding by injecting saliva that inhibits the host’s clotting enzyme, thrombin. Leeches can ingest several times their own weight in blood at one meal and digestion is slow, which enables the leech to survive for up to several months without the host. Some leech species feed mainly from the fins, but feeding has been reported to occur all over the body surface, including the gill chamber. Clinical symptoms of leech infection include anaemia, lethargy, body discolouration, fish scale loss, and frayed fins and restless swimming. Severe infestations of leeches render aquatic animals unmarketable due to unsightly clusters of worm‐like parasites, frayed fins, haemorrhages and swelling at attachment and feeding sites (Figure 11.18). Excessive blood loss probably occurs with intense and prolonged infestations and can result in mortality of aquaculture stock. Moreover, leeches can serve as a vector for other parasites and pathogens, including bacteria, viruses, flagellate trypanosomes and digeneans.

Figure 11.18 The marine leech, Zeylanicobdella arugamensis, has been associated with mortalities of juvenile barramundi, Lates calcarifer.
Source: Reproduced with permission from D. B. Vaughan.
The marine leech, Zeylanicobdella arugamensis, has been associated with mortalities of juvenile and adult grouper (Epinephelus coioides) in the Philippines and mortality in grouper and barramundi fingerlings Lates calcarifer reared in sea cages in Malaysia. In some instances, between 80–100% of fish in sea cages may be infected (Kua et al., 2014). Formalin bath treatments (50 ppm) are effective for managing leeches attached to fish, while draining and drying out of pond facilities desiccate leech cocoons and render them unviable. Alternatively, removable hard substrates can be introduced and periodically removed so that cocoons cemented to them can be destroyed. Biological controls, such as cleaner fish, may be feasible.
11.11 Crustaceans
Parasitic crustaceans have great economic importance as agents of disease in wild and farmed fish populations. These parasites can affect host survival and cause unsightly changes in the flesh. Among them, the copepods are most dominant in aquaculture.
11.11.1 Branchiurans
Branchiurans, commonly called fish lice, are temporary ectoparasites. Most branchiurans occur in fresh water, but a few species infect the skin of marine fish. Of the 150 or so species, about 125 belong to the genus Argulus. Argulids range from 2 to 30 mm in length, with females typically larger than males, and most can be observed by naked eye on the surface of fish (Figure 11.19). They exhibit a flattened, disc‐shaped body with a prominent pair of compound eyes. They bear a pair of conspicuous ventrally‐directed suckers (modified first maxillae) which is the principle mechanism of attachment. Each sucker is able to move independently of the other and the parasite is able to move in a fast ‘walking’ motion over the host’s body. Parasites typically attach to the caudal peduncle, flank, caudal fin and pectoral fins with few occurring in the buccal or gill cavities.

Figure 11.19 A rainbow trout with a severe infestation of Argulus on the body surface.
Source: Reproduced with permission from Dr C. Williams, Environment Agency, UK.
Transmission is direct since they are excellent swimmers and will actively seek fish and attach themselves. During the day, Argulus have been shown to hover, almost motionless in aquaria before advancing towards potential hosts. At night, the parasite switches to an active, widely searching strategy, actively swimming in long straight lines. Fish infected with Argulus are more likely to obtain additional fish lice because infected individuals swim erratically, providing greater attraction for hovering parasites. The suckers used for principal attachment are supplemented by a variety of hooks and spines. Indeed, the ventral margins of argulids are typically covered in backwardly directed spines. Argulus attaches itself on the host facing into the current so that these spines and hooks engage in the host’s skin and prevent it from sliding backwards or being dislodged. Unlike copepods, argulids do not retain fertilised eggs in strings or sacs but leave the host and attach their eggs to hard surfaces, such as stones or aquaculture infrastructure. The eggs are laid one at a time, side by side, in columns. The stage that hatches from the egg is immediately parasitic and searches for a host on which it will mature.
Once attached to the host, they are believed to either pierce the host’s skin and suck blood and other internal fluids, or feed on mucus and skin sloughed off by the host. Infested fish become lethargic, cease feeding, lose condition and may try to remove parasites by rubbing against the substrate. In chronic infections the skin becomes opaque, ulcerative lesions develop and the fins become frayed. Mortalities are usually associated with hundreds of argulids per fish, likely as a result of a breakdown in epithelial integrity. Argulids have been implicated in the transfer of viruses and other parasitic organisms.
Argulus species have been recognised as pests of farmed trout in Europe and carp in China for centuries. The most notorious species include A. coregoni and A. japonicus. Argulus japonicus is believed to have been introduced with aquarium fish from Asia and has now invaded the world. It primarily infests goldfish and other cyprinids, but has also been found on a range of other fish hosts. Argulus coregoni primarily infects salmonids but can also be found on cyprinids and other hosts. Rainbow trout, Onchorhynchus mykiss, sampled at a commercial fish farm in Central Finland in 2002 exhibited 100% infection prevalence with a range between 4 and 1309 A. coregoni individuals per fish (Bandilla et al., 2005).
To prevent introduction of argulids in aquaculture, incoming water can be filtered, and incoming fish quarantined. In ponds, removable hard substrates such as wooden slats can be introduced and periodically removed so that eggs laid on them can be destroyed. Anti‐crustacean substances or insecticides are commonly used to treat fish, but argulids rapidly develop resistance to chemical treatments. Their temporary parasitic lifestyle could potentially enable them to avoid predation by cleaner fish, although small fish are known to ingest argulids.
11.11.2 Isopods
Parasitic isopods comprise three major groups including the cymothoids, epicaridians and gnathiids. Most isopods seen on aquaculture fish are cymothoids (Family Cymothoidae). Cymothoids are primarily ectoparasitic on marine fish in warm waters but occur occasionally in temperate systems. Their legs form grappling hooks for attachment to external surfaces or in the mouth cavity of their hosts. The female permanently attaches to the host and releases eggs into a brood pouch or ‘marsupium’ before they embryonate, hatch and undergo two moults before being released. After a short swimming period they need to find a fish host, or they will die. Attached parasites use a mouth cone to tear at the fish’s flesh and pierce into the tissue to penetrate blood vessels or sinuses. It has been speculated that cymothoids may transmit viral diseases or allow viruses to enter through damaged tissue from attachment or feeding activity. Cymothid isopod‐inflicted mortalities in mariculture facilities are common and have been reported from important farmed fishes including, but not limited to, seabass (Dicentrarchus labrax), gilthead sea bream (Sparus aurata), barramundi (Lates calcarifer), catfish (Mystus gulio) and the goby (Oxyurichthys microlepsis). Infections of hatchery‐reared barramundi (Lates calcarifer) in the branchial and anterodorsal regions by Cymothoa indica resulted in skin lesions and have been associated with lowered growth rates and mortality. Parasites may be introduced to fish through wild zooplankton used as food, consequently, infection could be reduced by filtering wild zooplankton to remove the infectious swimming larvae of C. indica, or by using alternative live feeds.
Parasitic isopods of Bopyroidea and Cryptoniscoidea (commonly referred to as epicarideans) are unique in using crustaceans as both intermediate (pelagic copepod) and definitive hosts (both benthic and pelagic species). Among epicarideans, nearly all species in Bopyroidea are ectoparasitic on decapod hosts. The energy burden they impart on their host can have dramatic changes for the host’s reproductive capability. Indeed, female cultured giant freshwater prawns Macrobrachium malcolmsonii, infected with Probopyrus buitendijkibut in the gill chamber do not produce roe. When the parasites are removed females recover.
Commercially significant mortalities of eels and mullet have been associated with infection by gnathiid isopods. Gnathiids are temporary parasites of fishes. Only the ‘praniza’, the larval stage, parasitise the gills and body of fish while the adults are free‐living. Fishes confined in cages can be attacked by a large number of pranizae at night. Within 2 to 4 hours, the larva's modified mid‐gut becomes engorged with host fluid and the isopod leaves its victim. Consequently, some aquaculture farms may be oblivious to the impact of infections. Gnathiid infections should not be underestimated because they serve as vectors of some fish blood protozoa and may also spread filarial nematodes.
Scavenging cirolanid isopods have also been reported to inflict mortalities in some captive fishes including barramundi (Lates calcarifer) stocked in sea cages. Cirolanids are not parasitic, so infestations are likely to result only when fish are compromised by other injuries or disease.
11.11.3 Copepods
Copepods, commonly called fish lice, have considerable economic importance in marine and freshwater fish aquaculture. Commonly called fish lice, copepods are one of the most diverse groups of metazoan ectoparasite of fishes and also infect a wide range of invertebrates. They are typically small and inconspicuous but are extremely abundant. Some copepods are mesoparasites (partly embedded in their host) with highly modified anchoring structures (e.g., Lernaea cyprinacea) while caligid copepods have a cephalothorax that forms a broad shield that acts like a suction cup to hold the louse on to the fish. Copepods have free‐swimming and parasitic life stages. When attached to fish hosts they feed on mucus, epidermal tissue and blood, and untreated infections can result in host death, elevated stress, immunosuppression, secondary infections and increased risk of predation. Copepods may also be carriers of bacteria and viruses, probably obtained from their attachment to and feeding on contaminated fish.
Sea lice cause multi‐million‐dollar losses to the salmon farming industry worldwide. They are well adapted to potential low host densities in wild environments and exhibit high fecundity and specialised dispersal stages, hence in confined, captive environments with high densities of fish they can cause severe epidemics. The most infamous species, Lepeophtheirus salmonis, has two infective larval stages (the chalimus stages) which attach to fish using a frontal filament. This mode of attachment is essential to maintain connection to the host, otherwise they would fall off during subsequent moulting to pre‐adult stages. Adult females can produce six to eleven broods of paired egg strings that contain 300 to 1000 eggs, with potential for multiple paternity in a single egg sac. Egg development is closely related to seawater temperature with development taking longer at colder temperatures. Eggs hatch while still attached to the female, releasing a planktonic copepodid stage that is susceptible to the speed and direction of currents. Local conditions impact whether or not there is re‐infection of the site or contamination from neighboring farms. In Chile, Atlantic salmon (Salmo salar) and trout (Oncorhynchus mykiss) are highly susceptible to infections of the caligid copepod Caligus rogercressyi which naturally occurs on several native marine fishes that occur near farms. Female parasites produce two egg strings containing 29 eggs each but can produce up to 11 pairs of strings in their short lifetime of approximately seven months.
In most countries, strict lice control regimes have been put in place to reduce the release of salmon louse larvae from aquaculture facilities into the environment to negate negative impacts on wild migratory salmonids and other farms. This usually involves ‘treatment triggers’, whereby a certain number of female lice on fish warrant synchronised delousing in the area. The cost of managing salmonid sea lice in 2013 was USD 80 million in medicines. However, parasites show resistance or are developing resistance against most chemicals in use. This is largely because of limitations in dose efficiency because treatments do not result in 100% of parasites being killed. Parasites that survive treatments respond quickly given high fecundity, short generation times and efficient dispersal. The industry is beginning to accept that the battle against drug resistance cannot be won because drug development takes time and parasite populations respond too quickly. There is now research emphasis in determining the feasibility of vaccine development against sea‐lice following elucidation of the salmon louse genome (Torrissen et al., 2013). Meanwhile, some farms in Scotland, Norway, and Ireland use cleaner fishes (Labridae and Cyclopteridae) as a biological control as they will pick off and consume sea lice from infected salmon. The successful use of cleaner fish depends on providing ample shelter and clean cages, as cleaner fish readily seek alternative feed sources if the nets are overgrown. Several million wild‐captured cleaner fishes are routinely used in Norway, but there are now multi‐million‐dollar research programs into farming certified disease free labrids to supply farms. The use of cleaner fishes reduces or avoids the need to use parasiticides and the farmed fish can be harvested without drug residues.
11.11.4 Pea Crabs
Pea crabs are small parasitic crabs (Malocostraca: Pinnotheridae) that live as parasites in the mantle cavity of bivalves (e.g., oysters, clams, mussels), abalone, inside the test of sea urchins and in the rectum of sea cucumbers. Pea crabs are completely reliant on their host for shelter and food. Adult female pea crabs have a soft‐shelled exoskeleton and are oval in shape with their eggs tucked under their abdomens, giving them an almost spherical, pea‐shaped appearance. Adult males have a hard, chitinous exoskeleton and are generally smaller than the female. Female pea crabs spend their entire adult lives within a single host, while adult males will leave their host in order to find a mate. A pheromone‐based mate location system is likely used by pea crabs to reduce the risks associated with the location of females. Male pea crabs attempt to enter a mussel hosting a female crab by stroking the mantle to increase mussel valve gape.
Pea crabs can cause problems for bivalve aquaculture through reduced growth rates and end‐consumer complaints. Interestingly, the prevalence of pea crabs in pearl oysters can be as high as 85%, without apparent harm to the host. In contrast, the pea crab Nepinnotheres novaezelandiae infects approximately 5% of farmed greenlip mussels, Perna canaliculus, in New Zealand, and causes 30% reduction in meat yield of infected individuals which equated to estimated loss of USD 2.16 million annually (Trottier et al., 2012; Figure 11.20). There are no known preventative methods for pea crab infections, although the prevalence of infection is higher in shallower water, indicating that moving mussel farms into deep water offshore could reduce the incidence of infection.

Figure 11.20 The pea crab, Nepinnotheres novaezelandiae, infects approximately 5% of farmed green‐lip mussels, Perna canaliculus, in New Zealand.
Source: Reproduced with permission from Dr. O. Trottier.
11.12 Fishborne Zoonotic Agents and Aquaculture
Human contact with and consumption of fishes and shellfish presents potential disease risks from bacterial pathogens and metazoan parasites. Of the large numbers of species of pathogens and parasites that infect aquaculture organisms, only a few cause illnesses in humans. Nevertheless, the reported incidence of fish‐borne zoonoses has increased in recent years because of increased raw or undercooked fish consumption (e.g., ‘sushi’ and ‘sashimi’), improved diagnosis, and growth in aquaculture development and the international seafood market. Negative impacts on human health from ingestion of pathogens and parasites may be prevented by freezing or heating infected fresh meat. Indeed, current European Union legislation requires that all fishery products from finfish or cephalopods that are intended to be consumed raw must be frozen before consumption. This includes cold smoked fish where the smoking process does not achieve a core temperature of 60 °C for at least one minute. This section provides a brief overview of primary human zoonoses including bacteria and metazoan parasites associated with fisheries, aquaculture and ornamental aquaria. For further information please consult reviews by Lima dos Santosa and Howgate (2011), and Gauthier (2015).
There is substantial epidemiological and molecular evidence to support classification of bacteria species Clostridium botulinum, via ingestion, and Streptococcus iniae, Mycobacterium spp. and Vibrio vulnificus via inoculation, as fish‐borne zoonoses (Gauthier, 2015). A variety of other bacteria have been reported as potential fish‐borne zoonotic agents, but evidence is limited and few molecular genetic analyses have been made to link fish and human strains. Clostridium botulinum, which occurs in the intestines of marine and freshwater fish species worldwide, has a potent paralytic neurotoxin which induces paralysis in humans. Disease in fishes from C. botulinum has been reported in earthen pond culture of salmonids and catfishes and human botulism has been associated with consumption of contaminated fish products, notably smoked fish in northern temperate regions of the world. Streptococcus iniae has been reported in a variety of fish hosts and has been implicated in outbreaks with cellulitis related to processing and handling raw or live fishes in the Unites States, Southeast Asia, Canada and Hong Kong. Similarly, Mycobacterium spp. cause granulomatous inflammation of human skin, also known as ‘fisherman’s finger’. Antibiotic therapy is generally effective for mycobacteria, although surgical excision of lesions may be required. Vibrio spp. are widely distributed in marine and estuarine environments and can cause serious disease in cultured fishes. Vibrio vulnificus has been reported in humans that have handled fish, including eels. Infection is associated with gastroenteritis, septicemia and wound infections and is of particular concern because of high case fatality rates (3.6%). Vibrio cholera, which produce cholera toxin, has also been implicated in human disease outbreaks associated with the consumption of shellfish and has been reported in water used to house or transport ornamental fish.
Fish protozoans are not known to be infective to humans, but there is emerging evidence that myxozoan species can cause food poisoning. Since the year 2000, western regions of Japan have reported a foodborne disease that causes vomiting and diarrhea within several hours after ingesting olive flounder (Paralichthys olivaceus), tuna (Thunnus spp.) or amberjack (Seriola dumerili). In October 2010, a particularly large outbreak was reported among individuals who consumed olive flounder sashimi that had been raised in aquaculture systems. Microscopic examination revealed that the fish were infected with Kudoa septempunctata in the muscle. Since then, other wild and farmed fishes associated with instances of food poisoning have been examined and confirmed positive for Kudoa species providing mounting evidence that these parasites are the most likely cause of the diarrhea outbreaks.
Infection from cestodes belonging to the genus Diphyllobothrium can be transmitted to humans by consumption of raw or undercooked freshwater and marine fishes. The incidence of human infection with Diphyllobothrium tape worms has been increasing in urban areas of Japan and in European countries and recent estimates indicate that approximately 20 million individuals could be affected. Wild salmonids, which harbour the plerocercoid larva, appear to be the major transmitter of this disease, although Diphyllobothrium larvae have also been reported in cultivated salmonids in several countries. Diphyllobothrium exploits fish as its second intermediate host and infection in humans is incidental with the natural definitive hosts including birds, bears, seals, cats and dogs. The adult worm attaches to the mucosa of the ileum. Most infections are asymptomatic, but sometimes infections are associated with nausea, vomiting, abdominal pain, diarrhea, and discharge of the parasite’s main body (strobila), which can be as long as 12 metres! A recent surge of clinical cases highlights a change in the epidemiological trend of this tapeworm disease from one of rural populations to a disease of urban populations worldwide who eat seafood as part of a healthy diet.
The World Health Organization and the Food and Agriculture Organization of the United Nations estimated that more than 18 million people were infected with fish‐borne zoonotic trematodes in 2002. Human infection by trematodes is particularly important in developing nations where many people are dependent on freshwater fish as the major source of protein. Liver flukes include three pathogenic species in the family Opisthorchiidae including Clonorchis sinensis (endemic to Japan, China, Taiwan and Southeast Asia), Opisthorchis viverrini (endemic throughout Thailand, the Lao People's Democratic Republic, Vietnam and Cambodia) and O. felineus (distributed throughout Europe). The life cycle involves a freshwater snail in which asexual reproduction takes place and freshwater fishes (particularly cyprinids) are intermediate hosts. Fish‐eating mammals, including humans, dogs and cats, act as definitive hosts, in which sexual reproduction occurs. Adult parasites attach to the bile duct in humans and can lead to obstruction when there are high burdens. O. viverrini and C. sinensis are capable of causing cancer of the gall bladder and/or its ducts. Poor sanitation practices and inadequate sewerage infrastructure can facilitate the spread of the parasite eggs in human faeces into bodies of fresh water.
Anisakiasis is a disease caused by infection of larval ascaridoid nematodes whose normal definitive hosts are marine mammals. Freshwater and marine fishes the second intermediate hosts and to acquire an infection they must consume infected prey. The risk of infection from farmed fish can be substantially reduced by using extruded pellet diets. In addition, gutting fish soon after they are caught allegedly prevents migration of nematode parasites to muscles. The disease currently affects over 2000 people per annum worldwide with most cases noted in Japan and sporadic cases in most other parts of the world. Worms can only survive for a limited period in the gastrointestinal tract of humans following ingestion. Acute gastric infections are manifested by gastric pain, nausea and vomiting within hours of ingesting raw infected seafood. In chronic cases this may last from several weeks to two years. Onset of intestinal anisakiasis occurs within seven days following ingestion with severe pain in the lower abdomen, nausea and vomiting. Removal of worms by gastrofibrescope is used for acute infections, but partial resection may be required in chronic infections.
11.13 Aquaponics
Plants grown as part of aquaponic systems are subject to the same pests and diseases that affect field crops, although they are less susceptible to soil‐borne agents. Plants can absorb and concentrate chemicals used to treat parasites and infectious diseases of fish and invertebrates held in recirculated systems, so therapeutics should be avoided. It should also be kept in mind that salt water, which is commonly used to treat parasitic diseases of freshwater fish, is deadly to plants. There are several alternative approaches to reduce plant pest and diseases including biological controls, disease‐resistant cultivars, barriers, traps and manipulation of the environment.
11.14 Summary
- Viral outbreaks cause significant mortality and substantial economic impact to aquaculture operations. Outbreaks have dramatically impacted commercial and resource enhancement aquaculture due to heavy losses and or regulatory requirements mandating the eradication of fish infected with specific viruses of concern. New and emerging viral pathogens of aquatic species are continuously being discovered and characterised, and it is critical that aquaculture operations develop and adhere to strict biosecurity practices to minimise the risk of viral outbreaks.
- Bacterial diseases cause significant economic impact due to mortality or performance issues in fish that survive an outbreak. The majority of bacterial diseases in aquaculture are caused by Gram‐negative bacteria; however, some infections with Gram‐positive bacteria result in significant impact. Future control methods will need to rely on vaccines and other non‐antibiotic methods which will require substantial research investments to overcome challenges associated with aquaculture vaccine development.
- Protozoans are among the most significant parasite problems in bivalve and finfish aquaculture industries. They comprise a diverse group of unicellular eukaryotic organisms, many of which are motile. Some parasitic protozoa have life stages alternating between proliferative stages and dormant cysts which can survive harsh conditions. At present there are no commercial vaccines available for any fish‐parasitic protozoa.
- Metazoan parasites are extraordinarily diverse, with various routes of invasion. Species with direct life cycles are most frequently observed in aquaculture, although where farm environments provide suitable habitat for intermediate hosts, parasites with complex life cycles may be common. The most successful strategy to reduce metazoan parasite infestations is to break the parasite’s life cycle through strategically timed treatments.
- Parasites can serve as a vector for other pathogens and parasites such as bacteria and viruses. This typically occurs in blood feeding parasites such as leeches and crustaceans, which may have the capacity to infect several individual hosts during their life time and facilitate disease spread.
- Human contact and consumption of fishes and shellfish presents potential disease risks from bacterial pathogens and metazoan parasites. The reported incidence of fish‐borne zoonoses has increased in recent years. Negative impacts on human health from ingestion may be prevented by freezing or heating infected fresh meat.
References
- Bandilla, M., Hakalahti, T., Hudson, P.J. and Valtonen, E.T. (2005). Aggregation of Argulus coregoni (Crustacea: Branchiura) on rainbow trout (Oncorhynchus mykiss): a consequence of host susceptibility or exposure? Parasitology, 130, 169–76.
- Bridle, A.R., Koop, B.F. and Nowak, B.F. (2012). Identification of surrogates of protection against yersiniosis in immersion vaccinated Atlantic salmon. PLoS ONE, 7, e40841. doi: 10.1371/journal.pone.0040841. Epub.
- Cain, K.D. and Polinski, M.P. (2014). Infectious diseases of coldwater fish in fresh water. In: Diseases and Disorders of Finfish in Cage Culture. 2nd ed. Eds. Woo and Bruno. CABI, Boston, MA 342 pp.
- Colorni, A., and Diamant, A. (2014). Infectious diseases of warmwater fish in marine and brackish waters. In: Diseases and Disorders of Finfish in Cage Culture. 2nd ed. Eds. Woo and Bruno. CABI, Boston, MA 342 pp.
- Crane, M. and Hyatt, A. (2011). Viruses of Fish: An Overview of Significant Pathogens Viruses, 3, 2025–463.
- Cribb, T.H., Adlard, R.D., Hayward, C.J., Bott, N.J., Ellis, D., Evans, D. and Nowak, B.F. (2011). The life cycle of Cardicola forsteri (Trematoda: Aporocotylidae), a pathogen of ranched southern bluefin tuna, Thunnus maccoyi. International Journal for Parasitology, 41, 861–870.
- Gauthier, D.T. (2015). Bacterial zoonoses of fishes: a review and appraisal of evidence for linkages between fish and human infections. The Veterinary Journal, 203, 27–35.
- Johnson, B.O. and Jensen, A.J. (1991). The Gyrodactylus story in Norway. Aquaculture, 98, 289–302.
- Kearn, G.C. (2004). Leeches, live and lampreys. Springer Publishing, The Netherlands 423 pp.
- Kurath G. (2012). Fish novirhabdoviruses. In: Dietzgen RG, Kuzmin IV (eds) Rhabdoviruses: molecular taxonomy, evolution, genomics, ecology, host‐vector interactions, cytopathology and control. Caister Academic Press, Wymondham, p. 89−116.
- Lefebvre, F., Wielgoss, S., Nagasawa, K. and Moravec, F. (2012). On the origin of Anguillicoloides crassus, the invasive nematode of anguillid eels. Aquatic Invasions, 7, 443–453.
- Lima dos Santosa, C.A.M. and Howgate, P. (2011). Fishborne zoonotic parasites and aquaculture: a review. Aquaculture, 318, 253–261.
- Lima, P.C., Taylor, R.S. and Cook, M. (2015). Involvement of contractile vacuoles in the osmoregulation process of the marine parasitic amoeba Neoparamoeba perurans. Journal of Fish Diseases, 39, 629–637.
- Shinn, A.P., Pratoomyot, J., Bron, J.E., Paladini, G., Brooker, E.E. and Brooker, A.J. (2015). Economic costs of protistan and metazoan parasites to global mariculture. Parasitology, 142, 196–270.
- Torrissen, O., Jones, S., Asche, F., Guttormsen, A., Skilbrei, O.T., Nilsen, F., Horsberg, T.E. and Jackson, D. (2013). Salmon lice – impact on wild salmonids and salmon aquaculture. Journal of Fish Diseases, 36, 171–194.
- Trottier, O., Walker, D. and Jeffs, A.G. (2012). Impact of the parasitic pea crab Pinnotheres novaezelandiae on aquacultured New Zealand green‐lipped mussels, Perna canaliculus. Aquaculture, 344–349, 23–28.
- Whittington, R.J., Becker, J.A. and Dennis, M.M. (2010). Iridovirus infections in finfish – critical review with emphasis on ranaviruses. Journal of Fish Diseases, 33, 95–122.