10
Disease Principles
Leigh Owens
10.1 Introduction to Disease
To put the impact of disease into perspective, it is interesting to evaluate the future of the human race and its needs. It has been estimated that for mankind to maintain its consumption of seafood at current levels, aquaculture needs to produce over 80 million tonnes (t) by 2030 in order to maintain current per capita consumption (Chapter 27). Thus, aquaculture will need to produce an additional 30 million t of seafood in less than a decade and a half. There is probably not enough land or suitable marine areas for this to occur without massive disruptions to multiple ecosystems. However, about 40% of all aquaculture production is lost to disease, as it is broadly defined below. So, by simply removing or limiting disease impacts, mankind could almost meet seafood requirements without changing any land utilising practices.
Disease can be defined as ‘any process that limits the productivity of a system’ and is one of the most serious factors in aquaculture (Kabata, 1985). Estimates of the economic cost of a disease outbreak are complicated by the complex interplay of numerous factors associated with a specific incident, which may range from direct production losses to socio‐economic impacts on livelihoods and industries associated with the primary producer. A recent review of economic costs attributable to a range of key parasite pathogens within the world’s major marine and brackish water aquaculture industries was presented by Shinn et al. (2015). It is estimated, for example, that the Asian shrimp industry lost at least USD 20 billion over the last decade as a result of disease. One of the most serious is white spot syndrome virus that typically results in an 80–100% loss of stock. Infections in 3907 hectares of shrimp ponds in the Mekong Delta, Vietnam in 2015 caused estimated losses of more than USD 8 million.
Diseases include both infectious (see Table 10.1) and non‐infectious (environmental, nutritional and genetic) problems. The non‐infectious diseases are solely due to management practices and are often limited to particular farms. However, infectious diseases have the potential to threaten whole industries and will therefore form the basis of this chapter.
Table 10.1 Some major pathogens of aquaculture species.
| Group | Genera, etc. |
| Viruses | Bacilliform viruses, herpesvirus, iridovirus, nodavirus, rhabdovirus, coronavirus, birnavirus |
| Bacteria | Rickettsiales, Aeromonas, Enterococcus, Flavobacterium, Flexibacter, Pseudoalteromonas, Pseudomonas, Streptococcus, Vibrio |
| Fungi | Aphanomyces, Branchiomyces, Lagenidium, Saprolegnia, Sirolipidium |
| Protozoa | Amoebae: Neoparamoeba |
| Flagellates: Hexamita, Ichthyobodo | |
| Ciliates: Ichthyophthirius, Trichodina | |
| Sporozoans: Bonamia, Loma, Marteilia, Perkinsus | |
| Helminths | Dactylogyrus |
| Nematodes | |
| Annelids | Polydora |
| Crustaceans | Fish ‘lice’: Isopods: |
| Fish ‘lice’: Branchiura: Argulus | |
| Copepods: Lernaea, Ergasilus, Mytilicola | |
| Crabs: Pinnotherids | |
| Gastropods | Pyramidellids |
10.2 General Principles of Infectious Diseases in Aquaculture
10.2.1 Interaction between Host, Pathogen and Environment
The Sneizko three rings, Venn diagram of the interactions between host (the aquaculture species), pathogen and environment is well known (Figure 10.1). It illustrates the fact that, to occur, most infectious disease is a three‐way interaction needing all components:
- pathogen;
- host;
- environment.
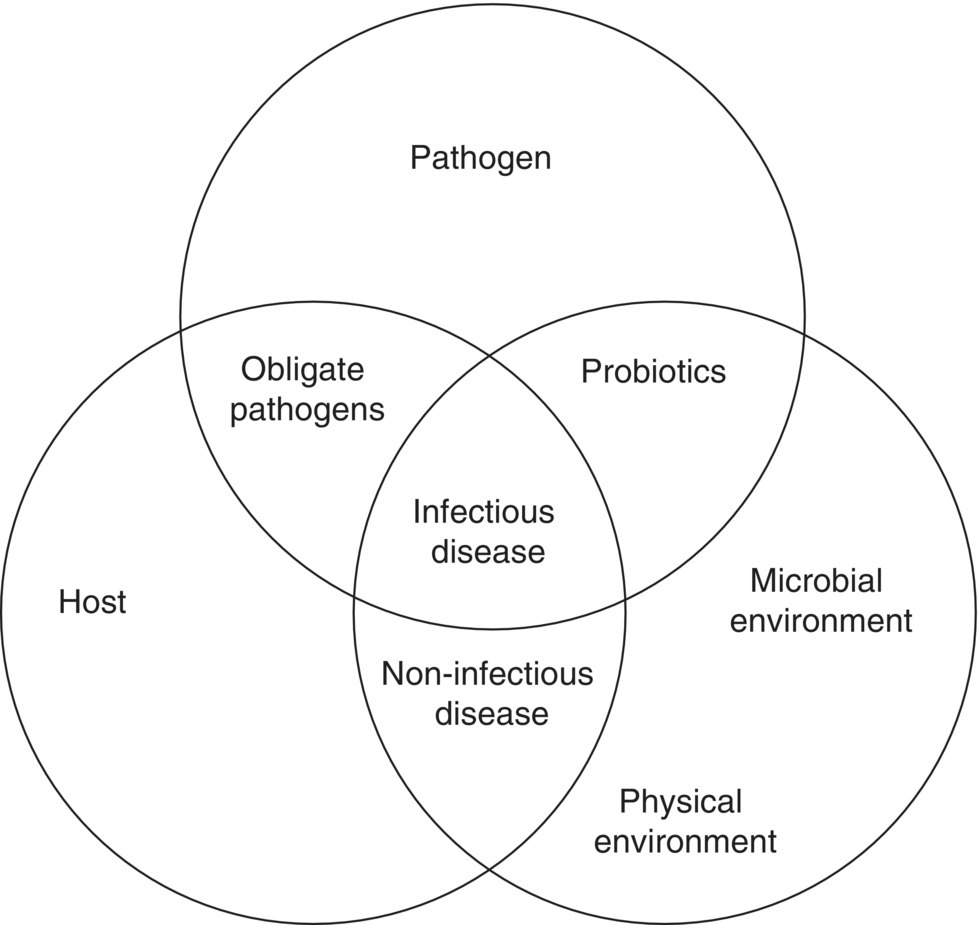
Figure 10.1 A modified Sneizko three‐ring model depicting the interaction between host, pathogen and the environment.
Various modifications of this model have been made to illustrate specific points. Non‐infectious disease is an interaction only between the host and environment. The area of overlap between pathogen and host represents obligate pathogens: the most threatening group as they do not need environmental stress to cause clinical disease. Epizootic Haematopoietic Necrosis Virus in redfin perch, and crayfish plague (Aphanomyces astaci) in signal crayfish are two such examples of obligate pathogens producing disease in the most pristine conditions.
For specific diseases of cultured species, the three‐ring model can be modified by changing the size of the rings to reflect the relative importance of the various components.
10.2.2 Density and Disease
The spread of pathogens is a density‐dependent process and is therefore affected by stocking rates. There is a relationship such that the greater the density, the smaller the distance between neighbours. This leads to a higher likelihood of pathogens crossing the distance between hosts in a viable state.
Immobile pathogens such as viruses, non‐motile bacteria, sporozoans and parasite eggs basically follow the diffusion laws and, therefore, in still water conditions a concentration gradient of the pathogens will form around an infected individual. Other pathogens such as bacteria, fungal zoospores, protozoa and metazoans generally have active but variable dispersal capabilities. As distance increases, fewer pathogens will be able to reach susceptible hosts to establish or continue a disease epidemic (outbreak). As there is natural attrition of pathogens in the environment, if the pathogen does not reach a susceptible host in a defined period of time, the chance of establishing a new infection is almost zero.
Higher densities lead to genetic selection of mutant pathogens that are virulent:
- In the natural environment, a newly‐mutated pathogen does not ‘know’ where the next susceptible host will be encountered. In such a case, pathogens that slowly release progeny are selected for because this will give a random‐moving infected host a chance to spread the pathogen in the environment and to the next host. That is, a pathogen does not ‘want’ to destroy its current host, but rather allow it to function as close to normal as possible, thus increasing the chance of an encounter with another susceptible host.
- Under culture conditions the next host is very close. A mutant that uses all the available resources of a host to produce pathogenic progeny, no matter what the consequences to the host, will be selected for (i.e., a virulent organism). One of its large number of progeny is the most likely to infect the next host as opposed to a conservative, slow‐release adapted pathogen (1 above). Once a virulent, resource‐utilising pathogen arises by mutation, it is very quickly selected for in an aquaculture environment. A single mutation in a nucleotide that begins a codon for a single amino acid can change virulence from non‐virulent to highly virulent. This may contribute to the sequential rise of pathogen problems that beset so many aquaculture industries (Figure 10.2).
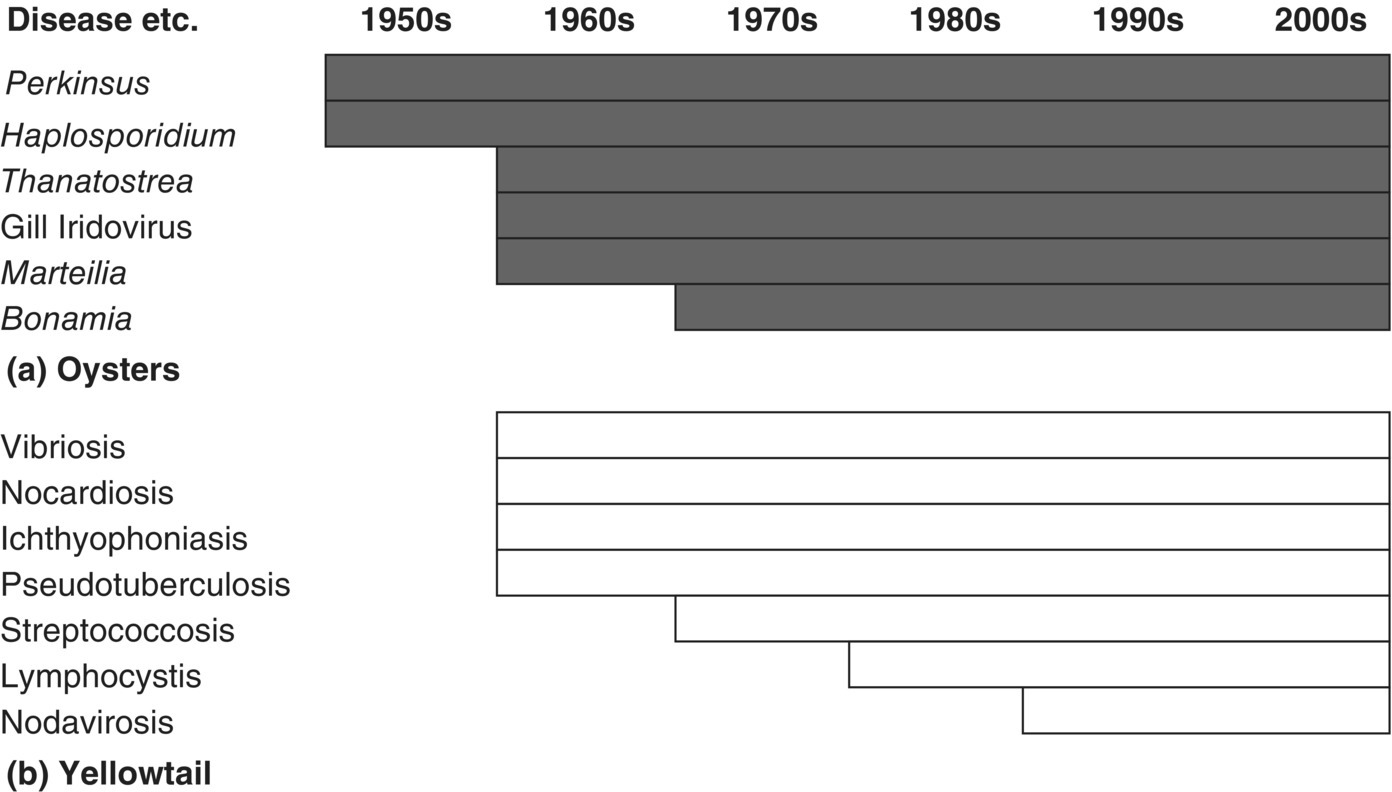
Figure 10.2 Sequential appearance of microbial diseases of cultured animals. The dates are approximate. (a) Atlantic oysters (Crassostrea virginica and Ostrea edulis), (b) Japanese yellowtail (Seriola quinqueradiatus).
10.2.3 The Effect of Aquaculture on Life Cycles of Pathogens
By stocking facilities with monocultures, aquaculture excludes both predators and competitors of the species being grown. A large number of the prey items of the cultured species are also excluded. Exclusion of cohabiting animals results in removal of intermediate hosts and definitive hosts from the aquaculture ecosystem. This effectively breaks the life cycle of many of the multi‐host helminths (e.g., digeneans and cestodes), which consequently have less of a role in disease in aquaculture than in wild populations. Sea cages are much less effective at breaking these life cycles than ponds or recirculation systems.
10.3 The Philosophy of Disease Control
Disease control in aquaculture is usually attempted on the assumption that an absence of pathogens is the desired state. However, the chance of beginning an aquaculture venture without any potential pathogens in the system is very slim and the question arises as to whether it is cost effective to achieve a pathogen‐free state. This ‘total elimination of pathogens’ strategy is the classic approach to disease control: the pathogenocentric approach. This may not always be the best strategy. If, for instance, the chance of reinfection from the local environment is very high, the infection starts to re‐establish immediately after control is completed. Clearly, the eradication philosophy is untenable in such areas.
There are a number of factors to consider when deciding on control measures:
- The cost of the control measure. Some pathogens make culture uneconomical in their presence and they must be totally removed from the culture system (e.g., highly pathogenic organisms like crayfish plague). Others might be self‐limiting and only reduce standing stocks by a small percentage (e.g., peritrichous ciliates on crustaceans). In this latter case, living with the pathogen is more cost effective than trying to eradicate it. In most cases, however, there are no accurate cost estimates of losses due to a pathogen. Rather, a general and often very inaccurate ‘feel’ for the costs associated with a disease agent are the best estimates available. However, this should not stop the aquaculturalist from trying to economically evaluate the cost of the control method and the downstream benefits. A classic example of the inaccuracy of the ‘feel’ of an impact of pathogens occurs in the freshwater crayfish industry of Australia. This industry has three ‘orphan viruses’ (viruses that cause no overt mortalities, so they are not considered important); Cherax bacilliform virus, Cherax giardiavirus and Cherax reovirus. Typically, crayfish with these viruses grow to 35 g in 7 months – standard practice. However, once these viruses were removed by hatchery rearing of surface‐sterilised eggs, the average weight of 6‐month‐old crayfish was 70 g!
- The likelihood of reinfection. Ideally, there should be almost no chance of the pathogen being re‐acquired from the environment or from wild stocks in the vicinity. Alternatively, infection with a pathogen and subsequent treatment will often allow the vertebrate immune system to be primed and thus further infections are limited, e.g., white spot (Ichthyophthirius multifilis) on fish. Sometimes, however, the environment is supersaturated with the pathogen from adjoining farms suffering the same problem and unless the farm can be isolated, treatment may be almost useless (e.g., vibriosis in Southeast Asian shrimp farms). In sea cage situations, wild stock will often congregate outside the cages where they can easily recontaminate caged stock that have been treated. Therefore, an understanding of the probability of reinfection is needed to correctly assess the control strategy. Again, this information is usually lacking.
- An adequate assay for the pathogen. It must be possible to accurately identify the pathogen to be able to assess the effect of the control measures on the pathogen. In the first instance this relies on an accurate diagnosis and later on a sampling regime that will determine true positives (sensitivity) and true negatives (specificity) (Table 10.2). The sampling regime is constructed by assuming a certain prevalence which is the maximum allowable for that pathogen and the confidence level that is acceptable to the farmer. If the test is insensitive, the sample size or frequency of sampling must be increased to compensate for the low discriminatory ability of the test. If the test is non‐specific, then animals will be assessed as infected when they are not, and a pathogen control or treatment regime may be discounted when it had worked at the level required. The numbers needed for accurate sampling of low prevalences are large and few commercial ventures willingly part with the necessary numbers without monetary compensation or it being mandatory compliance to legislation.
Table 10.2 Relationship between a diagnostic test and the real presence of a pathogen.
| Result of test | Pathogen present | Pathogen absent |
| Positive | A | B |
| Negative | C | D |
| A + C | B + D |
Sensitivity (%) = A/(A + C) × 100.
Specificity (%) = D/(B + D) × 100.
10.4 Generalised Disease Management Techniques
The most important factor for the movement and introduction of pathogens to farms and, indeed on any geographical scale, is the movement of animals. This includes:
- live broodstock in particular;
- live larval forms for stocking;
- live alternative hosts;
- frozen carcasses for human consumption;
- aquaculture feeds; and
- bait.
The majority of new introductions of pathogens to uninfected systems are due to the unrestrained movement of contaminated animals. Sometimes this is unavoidable as aquaculture does not exist without either broodstock or live juveniles for stocking. However, the biosecurity of broodstock, the number one contaminator, has often been neglected and should be the first point considered. Thus, in Europe it is mandatory to have broodstock tested for a broad range of notifiable diseases (bacterial and viral) before transfer is permitted.
If pathogen‐free broodstock are not available, what is the pathogen status of the broodstock that is being used? For example, in marine finfish aquaculture and shrimp aquaculture, viral encephalopathy and retinopathy and white spot syndrome virus, respectively, are both spread vertically from broodstock to larvae and then distributed through infected postlarvae and juveniles to farms.
Whilst it is impossible to have strategies that will work for all pathogens, there are a number of procedures that can help limit pathogens within culture systems.
10.4.1 Batch Culture
Batch culture works on the ‘all in, all out’ principle. Continuous culture eventually suffers from the early batches acting as pathogen ‘factories' which contaminate the environment to levels where later batches cannot be raised (e.g., viral encephalopathy and retinopathy ‐ barramundi nodavirus). Furthermore, young animals are often susceptible to levels of a pathogen that only mildly affect older stages. This is due to a naïve immune response in young animals and, having fewer cells in a target organ, the animal is more compromised when cells lose their function due to a pathogen. Drying out and sterilisation of the culture system and associated items between batches stops the amplification in numbers of pathogens for later batches. This is widely practiced in hatcheries, but it is often not used in grow‐out systems, where individuals from a number of spawnings and of different ages may be mixed to stock to an optimum density. However, batch culture during grow‐out (single year class practice) and techniques to reduce pathogens between batches, such as drying out ponds and leaving ponds or sea cage areas fallow, are employed by the marine shrimp and salmon sea cage industries (see Chapters 22 and 17, respectively).
10.4.2 Incoming Water Treatment
Treatment of incoming water is essential in recirculating culture systems and more useful in hatcheries than grow‐out situations due to the sheer volume of water involved in the latter. Water treatment includes chemical sterilisation (chlorine, iodophores, ozone) and physical sterilisation (UV light) (see Chapter 4). All are greatly enhanced by the inclusion of good settlement ponds preceding the treatment. Particulate matter, producing high turbidity, offers a substrate, nutrition and protection for pathogens, particularly bacteria, which prefer to be attached to substrates. It has been shown that such settlement for nine days causes Vibrio bacteria to die out and be replaced by oligotrophic, less pathogenic, species (section 11.3.6).
The bodies of each generation of bacteria are used as nutrient for a progressively smaller biomass of bacteria, so that the numbers and biomass spiral downwards. This phenomenon is called ‘self‐cure’. Settlement ponds reduce the particulate load of incoming water. Sterilisation is most effective against obligate pathogens that cannot use an alternative life cycle to build up their numbers (e.g., viruses, rickettsia, chlamydia, sporozoans). However, aerosols arising from vigorous aeration are very common in hatcheries. They allow facultative pathogens to bypass the sterilisation processes, build up to threshold numbers and then cause problems. For example, bacteria in shrimp hatcheries have been shown to travel 8 m as aerosols to contaminate other tanks.
Chlorine is very dangerous to use as a sterilising agent in organically‐polluted waters due to the formation of chloramines. These are highly reactive, have a long half‐life and are not neutralised by chemicals used to remove free chlorine. Many farmers have neutralised free chlorine and subsequently watched in horror as their fry proceeded to die from chloramine toxicity when the water was used.
Ozone is a particularly useful sterilising chemical, especially for recirculation where its application can be tied into reduction‐oxidation (redox) measuring devices. When reduction potential is high, anaerobic bacteria easily produce electrons from electron‐donating substances and electron acceptors are relatively rare. These electrons are highly reactive and can cause cellular damage as a means of stabilising. At high oxidation potential, electron acceptors are abundant and free electron damage is low. For aquaculturists, oxidative conditions are preferable, and ozone produces such conditions. Ozone is also safe as it and its ozide derivatives have a very short half‐life and the final product, oxygen, is very useful.
10.4.3 Lower Stocking Density
By lowering the stocking density, the average ‘inter‐fish’ distance is increased and the probability of a pathogen reaching the next host is reduced on an exponential scale. On theoretical grounds, disease epidemics will fall to extinction unless a threshold number of hosts are present in a given area. Simplistically, each infected host must infect at least two other hosts as it succumbs, or the epidemic will not propagate. Furthermore, lowering stocking densities will also decrease the level of sibling‐interaction‐induced stress and competition for space and food.
10.4.4 Single Spawning Stockings
Differential growth is a good indicator of poor health in a captive population. Runts are very useful to screen for diseases as they are either stunted by pathogens, or behaviourally and nutritionally stressed by being at the bottom of a pecking order (Figure 10.3). Such stressed animals will also express pathogens. If a mixed spawning population is used to stock a culture system, the differential growth due to age, genetics or variations in hatching conditions will obscure pathogen‐caused, differential growth. Thus, stocking with a single spawning is of particular benefit for an aquatic pathobiologist. This technique is not quite as useful in many finfish species where size grading is a normal part of culture (e.g., eels, salmon and trout), but it works well for invertebrates (e.g., freshwater crayfish). This technique also highlights the problem of a very common practice amongst fish farmers. At harvest, most fish farmers will put the runts that are too small to meet market needs into a pond to allow them to grow to market size. This overlooks the most likely reasons for their failure to reach market size: they are compromised by having a disease. Therefore, in reality, the farmer is keeping a reservoir of diseased individuals on the farm to infect the next stocking.
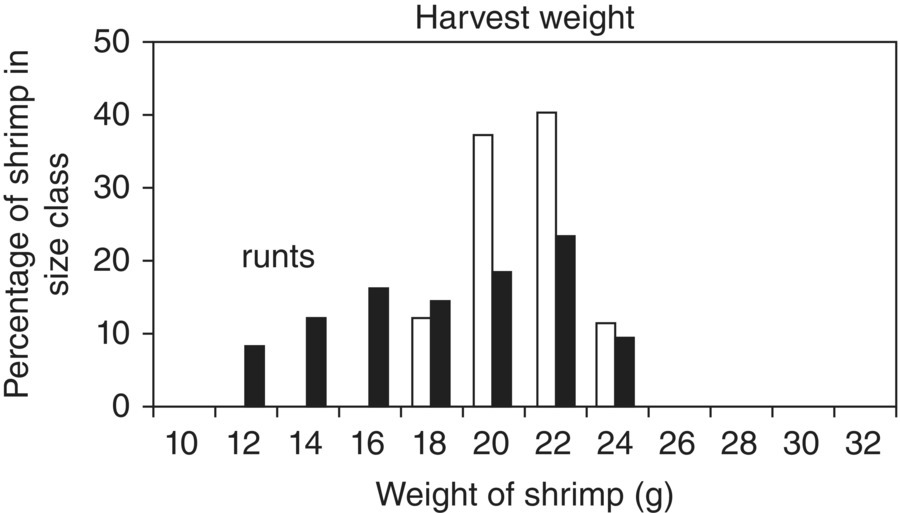
Figure 10.3 A typical proportion of runts in a population of shrimp carrying a suite of diseases. Note the spread of sizes of the infected shrimp (▪) across more size classes compared to uninfected shrimp (□).
10.4.5 Specific Pathogen‐Free Broodstock
Most pathogens are more virulent to the younger stages of a host. By producing offspring from broodstock free of specific pathogens, the offspring have a good chance of growing to a non‐susceptible size before being infected and thus a crop can be produced even in an area where disease regularly affects animals. This can also work if all life stages are equally susceptible, but by late infection of the host, the crop can be grown to harvest before the disease has a chance to establish. This is the approach taken for Infectious Hypodermal and Haematopoietic Necrosis Virus in marine shrimp (Litopenaeus vannamei) culture.
Use of the words ‘specific pathogen‐free’ (SPF) is greatly misunderstood by farmers and scientists alike who, in their minds, hear the words ‘free of all diseases’. The term SPF should be followed by the pathogens that the host has been tested for and found to be free of as part of the definition to clear up misinterpretations.
10.4.6 Stress Reduction
Stress is often used as an excuse for problems when no other logical explanation is available. Despite this nebulous use of the concept of stress, it does have a real physiological basis and consequences (Pickering, 1981). Unfavourable conditions lead to an adaptive response and a new level of homeostasis is achieved. If this is not achieved, then exhaustion follows along with the over‐production of the stress hormones (including, in fish, the corticosteroids cortisol, cortisone, corticosterone, 11‐deoxycortisol and adrenocorticodrophic hormones). High plasma levels of corticosteroids:
- induce lymphopaenia;
- reduce phagocytosis;
- reduce access of lymphocytes to inflammatory sites;
- deplete vitamin C reserves and consequent wound repair; and
- increase protein catabolism (gluconeogenesis) leading to muscle wasting, low antibody and collagen synthesis, which again restricts wound repair.
Levels of the stress hormones do not correlate well with levels of stress as some fish pass through the high levels of secretion to a new, highly‐stressed state with no secretion of the hormones. The two most practical ways of limiting stress are to double the aeration, thus alleviating any oxygen stress that may be occurring, particularly during hot summers, and to lower stocking density as mentioned above. In marine shrimp ponds, it has been found that keeping the morning dissolved oxygen (DO) >4.5 ppm is the single biggest factor that is able to limit viral mortalities and keep survival at 85%.
10.4.7 Vaccination
Vaccination basically works on the premise that an immunological memory exists and that prior exposure to a pathogen will allow a stronger and quicker immune response. This technology is treated in detail in Chapter 12.
10.5 Major Diseases
The following rendition gives a broad brush to commonly‐occurring disease problems experienced in major aquaculture industries. The diversity of infectious biological agents in aquaculture is treated in detail in Chapter 11.
10.5.1 Molluscs
10.5.1.1 Bivalves
Worldwide, protozoan parasites are the most significant cause of losses to bivalve industries. This predominance of protozoan parasites is reflected in a guide to diseases for the mollusc farmer (Elston, 1990). Of the 11 ‘Notable Oyster Diseases' described in this guide, seven are caused by protozoans:
- Perkinsus marina;
- Haplosporidium nelson;
- Haplosporidium costalis;
- Bonamia mackini;
- Bonamia ostrea;
- Marteilia refringens; and
- Hexamita nelson.
Although this guide to diseases is based primarily on the experiences of the North American and European cultured bivalve industries, similar diseases affect bivalve industries around the world.
For example, the haplosporidian parasite Marteilia sydneyi causes QX disease in Sydney rock oysters (Saccostrea glomerata) in Australia. A freshwater flushing of rivers usually brings on an outbreak of the diseases (epizootic) with deaths in about six weeks. The digestive gland is attacked, first in the Leidig tissue and later the epithelium. The gland becomes pale yellow and watery, and gonadal condition may be greatly reduced. No control is known, but growers try not to have oysters on their leases over the wet summer months. One intermediate host appears to be a polychaete that scavenges the tissues of dead and dying oysters, although further intermediate hosts cannot be excluded. Marteilia refringens causes similar problems in Ostrea edulis in France and Spain, with infection occurring first in summer and most deaths in winter. At least one intermediate host, the copepod Paracartia grani, has been implicated. As the parasite can be transmitted from oysters to P. grani, but not vice versa, further hosts are likely, and at least five species of copepod have been reported to be parasitised by M. refringens.
The Bonamia group of intracellular parasites cause problems worldwide by infecting both the cupped and flat oysters (section 11.5.2). They are characterised by causing mortality in oysters 3 years or older when the oysters enter the female stage of their life cycle. Bonamia roughleyi causes winter mortality in Sydney rock oysters and requires high salinity (30–35 ‰). Small yellow to brown pustules occur on the gills, palps and mantle. Bonamia mackini occurs in North America and causes similar problems in the Pacific oyster (Crassostrea gigas).
Bonamia ostreae is a major problem in the cultured flat oysters of Western Europe. It appears that it was originally taken to France with shipments of oysters from western USA, similar to the route of the iridovirus, Oyster Velum Viral Disease (OVVD). Bonamia exitiosa has also caused local problems in New Zealand bluff oysters (Ostrea chiliensis), where it destroyed over 80% of the industry over a six‐year period. In Australia in 1991, the Victorian flat oyster industry also lost 90% of its production to Bonamia. Early this century, boats took live bluff oysters from New Zealand to Hobart and then to Melbourne, because the local fisheries had collapsed. Speculation suggests that this translocation spread the Bonamia from New Zealand to Australia, but the question of what first caused the collapse of these oyster industries is unclear. The occurrence of Bonamia in Western Australia suggests that the parasite is native to Australia.
Bonamia first infects the tissues below the gut and later invades the haemocytes that are involved in resorbing unspent gonadal material, especially the ovaries. Bonamia is a density‐dependent disease. Reducing the stocking density and growing the oysters on hanging lines to keep them off the bottom should allow marketable crops to be grown in areas where Bonamia is endemic.
It is not just protozoans that cause disease in molluscs, however, viruses and bacteria are also involved.
A herpes virus (131 nm diameter) has been associated with larval mortalities in Pacific oysters in northern New Zealand. Feeding ceased between days 3 and 4, and 60 to 100% mortality occurred in days 7–11. The cells infected were the fibroblasts and presumptive phagocyte precursors, which displayed enlarged, marginated nuclei with both intranuclear and cytoplasmic inclusion material. Herpes viruses have been reported from oysters in the north‐eastern USA, northern Wales and northern France, with the Ostreid herpes virus OsHV‐1 being one of the more prevalent and damaging diseases of oysters in Europe. Predisposing conditions for all herpesvirus‐related disease included elevated temperatures and crowding.
Iridoviral infections cause disease of the larval velum in Pacific oysters in western USA (OVVD), and gill necrosis and haemocytic diseases in adult Crassostrea angulata and, lately, Pacific oysters in France. There has been speculation that the same iridovirus is responsible for all diseases and was imported into France from the USA with Pacific oysters.
Bacterial infections have from time to time caused mortality in Tasmanian oyster hatcheries. Vibrio (13 strains), Alteromonas (10 strains), Pseudomonas (8 strains) and Flavobacterium (3 strains) were all involved. Five of the Vibrio and two of the Alteromonas strains caused mortality at 107 and 105 bacteria/mL, respectively, with the lower doses taking longer. All strains were lethal at 108 bacteria/mL. Bacterial virulence for oysters has been strongly associated with a low molecular weight (500–1000 kDa), heat‐stable toxin. The toxin stops cilia movement (ciliostasis), which in turn reduces feeding and swimming in larvae, and inhibits the cleaning function of the gills of older oysters.
Some diseases affect the oyster's shell and not the soft tissues. The shell may be much weakened structurally in extreme cases, thus exposing the oyster to predation. In lesser infections there is malformation of the shell, making the oyster less presentable or unacceptable to the market, which is as good as killing it from the viewpoint of the industry.
There is a shell disease caused by a fungus that grows as filaments through the shell, weakening the shell and causing dark raised 'warts' on the inner surface of the shell. It occurs in the European flat oyster, where it has caused heavy mortalities in some regions. Similarly, boring sponges, Cliona species, riddle the shells of some bivalves, including clams and pearl oysters, with very deleterious effects on marketability. However, Cliona is apparently not a problem with table oysters.
Boring polychaete worms (Polydora species) invade the shells of oysters, some other benthic bivalves (e.g., scallops) and even abalone. They have a widespread distribution, including Norway, France and Australia. Polydora is responsible for the use of intertidal stick culture of Sydney rock oysters in northern New South Wales. These 'mudworms' bore through the shell, causing blisters on the inner surface. By culturing oysters in the intertidal zone, the racks dry out during low tides and newly‐settled polychaete larvae are killed. Polydora also affects abalone farms and benthic mussels, however, rafted mussels are almost free of infection.
10.5.1.2 Other Molluscs
There is a large family of ectoparasitic gastropods, the Pyramidellidae and some parasitise oysters and other bivalves (Figure 10.4). Pyramidellids are small gastropods which suck the blood or other tissue fluids of the host by using a long, penetrating proboscis. The host has less energy for growth in mild infections. In heavy infections, the host may die of tissue and fluid loss. The pyramidellid, Boonea impressa, is an important parasite of the American oyster.

Figure 10.4 Pyramidellid parasite (Turbonilla sp.) on a juvenile giant clam (Tridacna gigas) during ocean nursery culture. Note the pyramidellids’s long white proboscis that it is using to pierce and suck fluid from the mantle edge of the clam.
Source: Reproduced with permission from John Lucas.
Epizootics have been observed in cultured pearl industries. There were considerable long‐term mortalities in black‐lip pearl oysters (Pinctada margaritifera) in French Polynesia during the late 1980s, but no causative agent was identified. In the silver‐lip pearl oyster (P. maxima) industry in Australia, a bacterium, Vibrio harveyi, was isolated from the haemolymph of dying oysters. Cold water temperatures (19°C) apparently predisposed the pearl oysters to infection and crowded transportation with very little water circulation allowed the bacteria to establish. More recently rapid die‐off of spat due to an unknown disease, likely to be of viral etiology has caused serious concern in the Australian pearl oyster industry. Of some interest, is the association of V. harveyi with mortalities of scallop spat in temperate, central New South Wales.
A protozoan parasite, Perkinsus, has been associated with mortalities of pearl oysters and abalone (section 11.5.3). When water temperatures are elevated, the cellular immune system of the abalone cannot encapsulate and destroy the Perkinsus. Treatment consists of lowering the water temperature by 8°C in a holding tank. This will stop even a progressing disease outbreak in two days. A related species, Perkinsus marinus, has caused kills of table oyster in southeastern USA during summer. More significantly a herpes virus causing ganglioneuritis is also a major problem in abalone fisheries in Taiwan (Haliotis diversicolor supertexta) and in the state of Victoria (Haliotis laevigata and H. rubra) on the south coast of Australia. The virus can cause in excess of 90% mortality within two weeks, and is of major concern in wild stocks and fisheries as well as aquaculture. The major diseases of abalone are covered in detail in section 25.7.
There are several families of decapod crustaceans which include species that live within the mantle cavities of large bivalves, such as large clams, mussels and oysters. These are species of the shrimp family Pontoniidae and the crab family Pinnotheridae (section 11.11.4). These crustaceans often live in pairs within the host. They usually occur on the gills, where they feed from the host’s food grooves. They cause mechanical damage to the host’s gill tissue. There is no evidence that these parasites cause mortality, but they have some minor adverse effect on the host.
10.5.2 Crustaceans
10.5.2.1 Marine Shrimp
Viruses have caused hatchery mortalities and considerable grow‐out problems in marine shrimp culture. The most devastating virus known to date is whitespot syndrome virus (WSSV). It started in 1993 in China where it destroyed almost USD1 billion worth of Fenneropenaeus chinensis. When infected kuruma (Marsupenaeus japonicus) prawns were imported from China and Korea to Japan, the virus was spread with devastating consequences. Further spread of WSSV to Taiwan, then to Thailand and the rest of southeast and central Asia ensued. Later the virus was moved to Texas, USA, presumably via frozen commodity shrimp. Central and South America followed, so by 1999 only the Philippines, Australia and Oceania were known to be free of this virus. Unfortunately, illegal movement of broodstock from the Indo‐Malaysian archipelago to the Philippines infected that country. In late 2016, there was an outbreak in southeast Queensland, Australia, where the disease was detected in seven prawn farms as well as wild prawns in the region. On a regional scale, the spread of this virus was always with live animals or frozen commodity shrimp. Interestingly, other rod‐shaped viruses have been described from crabs, yet crabs do not seem as to be as susceptible to the virus as shrimp. The original outbreak of this virus coincided with the upsurge in the practice of feeding raw crustaceans, particular mud crabs (Scylla species), to broodstock shrimp as a maturation diet.
Shrimp stocks have become partially resistant to WSSV so that carrier animals are common. Carriers can be detected biochemically using nested polymerase chain reactions. The most highly infected animals are detected at the first stage of the reaction and are discarded. Should these infected progeny be stocked, the risk of crop failure is 95%. Progeny of broodstock that are positive on the second stage of the reaction and those that are negative (ideally) are used to stock shrimp ponds. The risk of crop failure from these shrimp is 31%. The risk of crop failure is enhanced by stresses (osmotic, pH, oxygen) associated with the wet season. Failure rate might be around 19% during the dry season, but it may leap to 70% with onset of the wet season. These same crop failures, which are enhanced by the wet season, occurred in Australia, where they were called Midcrop Mortality Syndrome (MCMS). Stresses from infections with other pathogens also greatly affect the outcome of an infection with WSSV.
Taura syndrome virus has been responsible for widespread mortalities in Litopenaeus vannamei in the Americas after initial outbreaks near the Taura River, Ecuador. Taura syndrome was initially believed to be due to the toxicity of fungicides used to control black sigatoka disease on banana crops. The washing of the fungicides into the waterways allowed it to accumulate in the shrimp ponds. The fungicides were very similar to crustacean moult inhibitors and this is believed to be the mode of action. A massive lawsuit against the European chemical companies that produced the fungicides was launched for compensation. Subsequently, cell‐free bioassays and electron microscopy unequivocally demonstrated the presence of a picornavirus that produced the characteristic hypodermal, buck‐shot lesions and mortalities. The disease seems to be most aggressive in L. vannamei so that L. stylirostris had become an alternative crop in infected areas. Unfortunately, survivors of the epizootic are chronic carriers and infected L. vannamei have been introduced to Taiwan, China and Indonesia.
Monodon‐type baculoviruses (MBV) can infect all species of shrimp, although their ability to produce disease differs between shrimp species. MBV has been implicated as one of the causative agents of the mass mortalities that swept through Taiwanese shrimp farms in 1987/88 and resulted in the crash of that industry. This may not be correct as yellow head ronivirus (initially called a baculovirus then a rhabdovirus in the literature) (YHV), which was undiagnosed at the time, seems to have had a very prominent role. All life stages of the host after mysis 1 are susceptible to MBV, but it is a disease of hatcheries rather than later stages, which are largely asymptomatic when infected. In an Australian study, MBV was found in 8 of a total of 13 monitored hatcheries for P. monodon. However, it has been largely managed out of existence in P. monodon, but it has been a major problem with the establishment for aquaculture of Fenneropenaeus merguiensis. Control measures, developed in Tahiti, involve separating floating eggs from the spawning female, surface sterilising them and then using the phototactic response of the nauplii to separate the larvae from the egg shells and moribund larvae.
Infectious hypodermal and haematopoietic necrosis virus (IHHNV) has all but destroyed the aquaculture industry based on Litopenaeus stylirostris in the Americas. Interestingly, as L. stylirostris was not susceptible to Taura virus, IHHNV‐resistant strains made a temporary comeback as an alternative crop to L. vannamei before the virus mutated and started killing L. stylirostris. Intranuclear inclusions were prominent throughout all tissues except hepatocytes. However, the inclusions from Australian shrimp did not cross‐hybridise with an IHHNV gene probe that was 90% specific for the American IHHNV strain. A Philippine IHHNV strain did cross‐hybridise with the American gene probe suggesting that the Australian IHHNV is a different strain. Nor did the gene probe react with lymphoidal parvovirus that may be a variant in expression of IHHNV. Similarly, an American‐produced hepatopancreatic parvovirus (HPV) gene probe based on virus from Korean Fenneropenaeus chinensis did not cross‐react well with the Indonesian and Australian shrimp strains nor with HPV from Malaysian freshwater prawns (Macrobrachium rosenbergii). HPV has not been found to be a problem in the aquaculture of Australian Penaeus monodon, even though it is common in wild stocks of Australian banana shrimp (Penaeus merguiensis) and causes 28% losses in farmed P. merguiensis. However, recent information about Penaeus monodon in Thailand demonstrated HPV to cause severe stunting and therefore considerable loss of income to farmers.
Spawner mortality virus (SMV) (a parvo‐like virus) in conjunction with gill associated virus (ronivirus) has caused major losses in P. monodon broodstock and grow‐out. This Midcrop Mortality Syndrome (MCMS) has become a major grow‐out problem in northern Australia with most farms losing 50% of their stock. Around A$44 million was estimated to have been lost over the years 1995–97. The disease outbreak is slow in its onset and shrimp grow to around 12–15 g before dying. Much time, labour, feed and money are thus invested in a crop which will be devastated if not harvested. A symptom of MCMS is the presence of sick, weak and reddened shrimp at the edge of grow‐out ponds. The number of days the stock is in ponds is a major risk factor with the probability of epizootics increasing dramatically after 120 days. Partial harvests to decrease stocking density allow some animals to be grown beyond 120 days.
Bacteria are still a major constraint to hatchery production with very few batches of postlarvae being able to be produced without antibiotics. Strategic use of antibiotics during larval and early post‐larval development (nauplii VI to protozoea I and again at mysis III to postlarvae I) is usual. The Vibrionaceae predominate in isolations from shrimp hatcheries with 8 of 37 strains producing significant mortality in larval bioassays. Vibrio species, including V. harveyi and V. tubiashi, and Photobacterium damselae are involved. On a worldwide basis, the non‐sucrose utilising bacteria (those producing green colonies on thiosulphate citrate bile salts sucrose agar, TCBS) are often involved in disease in crustaceans. Application of sucrose can alter the bacterial balance favouring the less pathogenic bacteria and hence reduce losses.
V. harveyi has been involved in massive mortalities in Ecuador and major mortalities in two grow‐out farms in Australia. In one study in Australia, over 50% of the bacteria at the completion of grow‐out were V. harveyi and V. alginolyticus. Strains of V. harveyi in Australia and the Philippines have been shown to have very strong antibiotic resistance with one virulent Australian strain having a plasmid coding for four different antibiotic resistances. V. penaecida has been associated with mortalities in ponds in Japan (Marsupenaeus japonicus) and New Caledonia (Litopenaeus stylirostris); and V. nigripulchritudo has been associated with mortalities in ponds in New Caledonia.
In the early years of shrimp culture, the fungi Lagenidium callinectes and Sirolipidium were frequently involved in causing larval mortality, especially in hatcheries where there was excessive use of antibiotics. Lobster eggs have been shown to have a surface commensal Pseudoalteromonas species, which protects against fungi. Antibiotics inhibit this commensal allowing the fungi to become established. A Pseudoalteromonas species has also been found associated with shrimp eggs and so the mechanism is presumed to be similar. Use of the agent orange‐based herbicides has effectively controlled fungi in the hatchery.
While the fungus Fusarium solangi is present in grow‐out ponds in Australia, it is only seen when moulting frequency is slowed due to cool water temperatures or another disease problem. It is thus a good indicator of retarded growth. Similarly, the peritrich ciliate protozoans also indicate suboptimum conditions and, at their highest fouling rates on the shrimp gills, can cause death by suffocation if any further stress is added. Peritrich ciliates need high organic loads to feed. So, by lowering the amount of uneaten food with water changes or by restricting supplemental feeding, the peritrich numbers can be reduced within a week.
10.5.2.2 Freshwater Crayfish
The European noble crayfish (Astacus astacus) industry, which was a major aquaculture industry, has been decimated by a fungus, Aphanomyces astaci. Furthermore, in 1986/87 the crayfish industry in Turkey was also devastated by the introduction of this fungus across the natural barrier of the Bosphorus Straits. This led to the introduction into Europe of the signal crayfish (Pacifastacus leniusculus) from the USA; a freshwater crayfish that is highly resistant to the fungus.
WSSV has recently been identified in the Louisiana crayfish‐ranching industry infecting red swamp crayfish (Procambarus clarkii). Whilst the route of introduction of WSSV is unknown and since crustaceans in the Gulf of Mexico waterways are not known to be infected with the virus, commodity shrimp are suspected.
With the exception of the devastating crayfish ‘plague' in Europe and WSSV in USA, freshwater crayfish are generally considered to be relatively disease free. This reflects the extensive culture methods used for crayfish, whereby only very low stocking densities (5 /m2) are used. With the development of more intensive culture methods, disease will become more of a problem. A list of pathogens already documented from farmed crayfish in Australia is presented in Table 10.3. The most important agents causing mortalities so far are:
- the rickettsia Coxiella cheraxi;
- Cherax baculovirus/ bacteraemia; and
- bacterial erosion of the eyeballs associated with Aeromonas hydrophila and A. sobria.
Table 10.3 Pathogens found in farmed Australian freshwater crayfish.
| Type | Pathogen |
| Viruses | Cherax bacilliform virus, Cherax giardiavirus, parvovirus, reovirus, picornavirus |
| Bacteria | Coxiella cheraxi, Aeromonas hydrophila, A. sobria, Citrobacter freundii, Klebsiella pneumoniae, Plesiomonas shigelloides, Pseudomonas, Shewenella putrifaciens, Streptococcus |
| Microsporidia | Agmasoma (= Thelohania), Vavraia parastacida |
| Fungi | Achlya, Mucor, Psorospermium |
| Temnocephalids | Craspedella spenceri, Diceratocephala, Didymorchis, Notadactylus, Temnocephala dendyi, T. minor |
| Ciliates | Corthunia, Epistylus, Lagenophrys, Tetrahymena, Vorticella, Zoothamnium |
The rickettsia‐ and the baculovirus have been introduced into at least Ecuador and the baculovirus into the USA with trans‐shipments of crayfish from Australia. Emerging pathogens include microsporidiosis in Western Australia and a systemic parvovirus in the redclaw crayfish, Cherax quadricarinatus.
10.5.3 Finfish
10.5.3.1 Carp
As outlined in Chapter 16, the carp and their relatives are the largest group of finfish or shellfish produced from aquaculture. They made up around 30% of global finfish and shellfish production in 2015, and two‐thirds of all finfish and shellfish from freshwater. The Disease section in Chapter 16 (section 16.6) refers the reader to a monographic treatment of diseases in carps and their relatives by Hoole et al. (2001).
10.5.3.2 Salmonids
The finfish group that dominates the aquaculture literature on a worldwide basis is the salmonids because, unlike carp, they are cultured in so many countries around the world. Over 20 viral diseases are recognised in salmonids and approximately half are considered to have moderate to high virulence.
Infectious salmon anaemia virus, ISAV, is currently the most economically damaging disease in Atlantic salmon aquaculture worldwide and is the first of the diseases classed on the List One of the European Commission’s fish health control regimen. List One diseases require eradication of all infected stock as part of the control measures. ISAV, a member of the family Orthomyxoviridae, is a single stranded RNA virus that infects blood cells causing anaemia. Symptoms include pale gills, liver and spleen inflammation and necrosis, and circulatory failure as the system becomes ‘clogged’ with dead cells. Initially discovered in Norway during the 1980s, ISAV is now found in Scotland, Canada, the Faroes, and is devastating the Chilean aquaculture industry (for further information see section 11.2.5).
Pancreas disease (PD) caused by the salmonid alphavirus (SAV) was first discovered in Scotland in 1976. Initially associated with poor performance or ‘runts’, attributed to loss of centroacinar cells in the pancreas rather than overt mortality, the last decade has seen a resurgence of a more acute form of the disease. There are at least nine subtypes of SAV that can cause mortality. With significant losses in Norway, Scotland and Ireland, the Chilean government moved to block the import of salmonid eggs in November 2009 to prevent introduction of the disease.
The Aquabirnavirus, e.g., infectious pancreatic necrosis virus (IPNV), and similar viruses are found worldwide (see section 11.2.2). These viruses have a great propensity for replicating or surviving in hosts other than fish. These other hosts include bivalves, crayfish, marine shrimp, leeches and the intestines of fish‐eating birds. Therefore, they are very difficult to eradicate. Primarily a disease of young freshwater salmonids, IPNV now increasingly causes significant mortalities in marine farmed post‐smolts, with outbreaks usually occurring within 8 weeks of seawater transfer. Control in post‐smolts by early vaccination has had some effect, but control in young freshwater fish by this method is impractical. Such is the economic significance of this disease that the first genetically selected lines of Atlantic salmon with genetic markers for resistance to IPNV are available commercially in Norway.
Infectious haematopoietic necrosis virus is one of the most studied as it infects a range of salmon species including Atlantic salmon, rainbow trout, sockeye salmon, chinook salmon and coho salmon. While this rhabdovirus is endemic to the north‐eastern Pacific it has been spread across the northern Pacific as far south as China and Taiwan, eastern USA, France and Italy. It primarily affects fish less than 6 months old and, as it is found in the sexual secretions of both male and female broodstock, it has ready access to young fish for infection.
Viral haemorrhagic septicaemia (VHS) has a broad host and geographic range and multiple strains of this virus have been described (see section 11.2.6). It only grows at temperatures below 16°C so its geographic range is limited and losses can be ameliorated by increasing temperature. Recently, VHS has been found in wild Southern Californian pilchards and mackerel that are used extensively as bait and fodder for other aquaculture industries such as bluefin tuna.
Bacteria are major pathogens of cultured salmonids. Antibiotics have been widely used to control outbreaks, but they are becoming less and less acceptable in food animals due to concerns about antibiotic resistance spreading to pathogenic bacteria of humans. Bacteria in the sediments of abandoned aquaculture sites have been assayed for antibiotic resistance 10 years after the culture operation ceased and they were found to still have major resistance against oxytetracycline. This type of problem has led to the production of effective vaccines. The 30% rise in Atlantic salmon production in Norway is due to control of furunculosis caused by Aeromonas salmonicida subsp. salmonicida, and winter disease caused by Vibrio salmonicida. In fact, A. salmonicida is probably the most important finfish pathogen in all marine aquaculture industries (see section 11.3.1). Whilst many of the original causes of generalised septicaemias in salmonids have been successfully controlled through vaccination, emerging infections with intracellular bacteria are proving more difficult to control. Bacterial Kidney Disease (BKD) caused by Renibacterium salmoninarum has been an intracellular pathogen of note throughout salmonid aquaculture for decades (see section 11.3.8). In the last 15 years, rickettsia‐like infections caused predominantly by Piscirickettsia salmonis have also become prevalent in major Atlantic salmon farming nations (see section 11.3.5). Most recently, significant mortalities associated with members of the bacteria genus Francisella have occurred in salmonid aquaculture in Norway, Canada and Chile. Whilst the fish isolates (F. noatunensis and F. asiatica) do not appear to be pathogenic to humans, interest has been high as the closely‐related F. tularensis almost invariably causes fatal diseases in humans.
Septicaemia caused by Lactococcus garvieae is a major problem in rainbow trout in Europe, Asia, South Africa and southern Australia. These pathogens appear to evade host macrophages as part of their colonisation strategy. L. garvieae is encapsulated (coated with a polysaccharide) reducing the ability of the lytic complement pathway to lyse the cells and impeding phagocytosis. Hence, macrophage engulfment is compromised, and host antibody production is slowed.
Of the parasitic infections of farmed salmonids, perhaps the two most significant are sea lice (see section 11.11.3) and proliferative kidney disease (PKD) (see section 11.6). Infestations by calagid copepods of the genera Lepeophtheirus and Caligus are probably the most prevalent pathological and environmental concerns facing Atlantic salmon farmers in the dominant producing countries. Treatment is generally via chemicals. Treatment by bathing in organophosphates was phased out in the early 1990s with pyrethroids becoming licensed as replacements. Latterly, treatment has been via feed with emamectin benzoate, although resistance is emerging and biological solutions are being sought. The causative agent of PKD was first identified as a myxozoan parasite, Tetracapsuloides bryosalmonae, and is probably the most significant parasitic disease of salmonids (particularly trout) in Europe and North America causing up to 90% mortality in infected stock. Severe wild fish kills have also been reported. Treatment with malachite green was favoured until it its use was banned. The fungicide fumagillin has been trialled in both the USA and UK with some positive indications. Survivors of infection tend to be resistant to reinfection thus control by vaccination may be possible in the future.
Kent and Margolis (1995) outlined the diseases caused in seawater‐reared salmonids by parasitic protozoans. There are numerous and diverse protozoans that parasitise aquatic vertebrates in freshwater and marine habitats and these cause various levels of debilitation (see section 11.5). For example, the amoeboid protozoan, Neoparamoeba species, is an opportunistic pathogen that infects the gills of fish under some conditions (see section 11.5.2). On a coho salmon farm in Washington State, Kent and Margolis (1995) observed 25% mortality that was attributed to Neoparamoeba species. The amoebae appeared on marine farms and were quickly eradicated from the fish gills by exposing them to freshwater, e.g., towing the fish cages from seawater into freshwater or by treating the fish in freshwater baths. However, the Neoparamoeba are developing resistance to the freshwater treatment and it is only now effective for about 30 days. A parasitic amoeba, Neoparameoba perurans caused problems at the outset of the salmonid sea cage industry in Tasmania, Australia. Fish in their first year in seawater develop a proliferative gill disease especially if sea cages are fouled or water temperature is above 15°C. Amoebic gill disease (AGD) may kill up to 2% of fish per day if untreated and represents the major disease problem of Tasmanian farmed salmon. Chemical baths are largely ineffective but freshwater baths of a few hours, or moving the whole sea cage to freshwater, is reasonably effective. Once the cycle is broken, the immune system may prevent further heavy infestations. Unfortunately, there is strong evidence that the freshwater treatment is becoming less effective. Instead of a one‐off bath, bathing frequency has been increased to every few weeks during the warmer weather, incurring enormous cost to the industry. Research into vaccination against this parasite is underway.
The salmonid aquaculture industry in Australia merits some attention as these animals are farmed at the extreme upper limit of their temperature tolerance. Most culinary experts prefer rainbow trout to Atlantic salmon and accordingly the Australian sea cage industry started with rainbow trout. Enterococcus (previously Streptococcus) biovar I killed 30% of rainbow trout in freshwater and as high as 60% in marine waters associated with the acclimatisation stress. Therefore, the industry swapped en masse to Atlantic salmon, which is highly resistant to this bacterium.
Yersinia ruckeri serovar III (the Australian strain) causes some problems mainly in young Atlantic salmon particularly when water temperatures are above 15°C. Some fish may develop the pathogonomic ‘red mouth’ due to haemorrhagic lesions and in chronic cases, bilateral exophthalmia and anorexia may predominate. The bacteria can be spread in the faeces of carrion feeding raptors. Vibrio anguillarum serovar C or 01 has been isolated from Tasmanian rainbow trout and Atlantic salmon. Clinical signs include hyperaemic prolapsed rectum, congestion at the bases of the fins and reddened ulcers on the flanks. The muscles may contain enclosed lesions and severe peritonitis can occur. Overseas, virulence in V. anguillarum has been linked to a mostly plasmid‐encoded siderophore (iron sequestering) system with the most virulent strain known having four separate siderophores. Both of these conditions can be controlled by efficient vaccines.
The typical, non‐motile strain of Aeromonas salmonicida (subsp. salmonicida) usually forms large boils or furuncles in salmonids. This obligate pathogen exists in Victoria as the atypical, non‐salmonid infecting, motile strain in goldfish Carassius auratus. The bacterium seems to have been imported to Victoria from Japan in 1974. Despite there being a restriction on the movement of goldfish from Victoria to Western Australia and Tasmania, there was an isolation of a typical strain of A. salmonicida from flounder in Tasmania. Similarly, a silver perch farm in New South Wales was quarantined due to this bacterium.
Since the eradication of infectious haematopoietic necrosis virus during the initial outbreak, only three viruses have been found in Australian salmon. The chum salmon Aquareovirus was isolated from ovarian fluid of Atlantic salmon and it apparently only causes disease in fry of chum and chinook salmon. This virus has more significance as a trade barrier for exclusion of competing Australian products by importing countries than as a disease threat. Infectious Pancreatic Necrosis virus was isolated from sea‐caged Atlantic salmon displaying pinhead.
10.5.3.3 Tropical and Subtropical Finfish
Amongst the farmed fish that have shown substantial increases in production in recent years in tropical and subtropical freshwater are tiliapia and catfish species. The diseases of tilapia and their management are outlined in section 18.9. The diseases of channel catfish, Ictalurus punctatus, in the southern USA are outlined in section 19.3.4. The diseases of pangasids largely mirror those found in ictalurids including enteric scepticaemias caused by Edwardsiella ictaluri and motile Aeromonads, particularly of the A. hydrophila group. In Vietnam the pangasid hatchery industry is becoming more advanced. Control of infection is largely by antibiotics, but resistant in both these diseases is not uncommon. Vaccination with live attenuated E. ictaluri has proved effective in the USA, but introduction of a live agent is generally poorly accepted by farmers due to concerns over reversion to virulence under farm conditions. The A. hydrophila group of organisms present a particularly intriguing challenge for vaccination due to their high intraspecific diversity and range of different species, subspecies and subgroups that cause disease. With demand of upwards of 100 million doses of effective vaccine in Vietnam alone, research into these diseases is intense.
Epizootic Ulcerative Syndrome (EUS) is caused by the fungus Aphanamyces invadans. EUS came into prominence in the 1980s as a devastating pathogen of snakehead and other fish of southeastern and central Asia. Snakehead fish are used for protein supplementation in the diets of the rural poor. Destruction of these fish led to widespread malnutrition, starvation and socioeconomic disruption of subsistence lifestyles. After many competing hypotheses on the aetiological agent including rhabdoviruses and Aeromonas hydrophila bacteria, A. invadans was identified by a group of researchers in Australia. In the fullness of time, EUS has been traced back to early outbreaks in the 1970s in Australia and Papua New Guinea. The earliest outbreak was in Japan in 1970. One interesting outbreak occurred only in the fishpond at the Sri Lankan international airport, where presumably a passenger had thrown some fish scraps from a meal into the pond. Lately, EUS has become a problem in the establishment of the jade perch (Scortum barcoo) as an aquaculture species.
Barramundi (Lates calcarifer) is cultured throughout tropical Asia and is the only fish seriously cultured in the tropics of Australia. Whilst most farms have intermittent problems, the most common pathogens are Cytophaga johnsonae causing saddle back, which may lead to dirty yellow ulcers and white spot from Cryptocaryon irritans in sea cages (see section 11.5.6; Figure 10.5). Problems are associated with the onset of cooler temperatures, usually in May. Streptococcus iniae is emerging as the most important disease in barramundi in estuarine seacage operations, freshwater ponds and in recirculation systems (see section 11.3.9). The initial episode starts after displacement of soil in the watershed where the barramundi are grown. Losses approach 30% and are not ameliorated by winter water temperatures. Also, Vibrio harveyi is emerging as major enteric disease in barramundi hatcheries.

Figure. 10.5 Barramundi (Lates calcarifer) infected with ‘white spot’ (Cryptocaryon irritans).
Source: Reproduced with permission from Professor Bob Lester.
Politically, there have been major problems associated with nodaviruses which cause viral encephalopathy and retinopathy of larval barramundi, and kill young, crowded larvae, especially in later batches through a hatchery. The growing of barramundi outside their natural range in the Murray‐Darling watershed, southeastern Australia, and the potential impact of the nodavirus on the endemic freshwater fish have been hotly debated. A similar, if not identical virus, has been found in barramundi in Tahiti and Singapore, and in most fish species (about 30 species at present) around the world. Almost all fish raised in marine hatcheries end up being infected with this virus, once the correct tools for investigation have been used.
Other pathogens seen from time to time include lymphocystis virus associated with fouled sea cages, vibriosis, epitheliocystis, and Epieimeria. Experimental infections with Bohle iridovirus, a systemic Ranavirus have shown barramundi to be acutely susceptible in both fresh and marine waters. Total mortalities usually occur within 10 days.
Other warm water species of note include the various species of grouper farmed throughout Asia. Nodaviruses are the major issue in larvae, fry and juvenile fish with broodstock screening and hatchery biosecurity the major means of control currently. Although experimental vaccines appear to hold promise in larger fish (Chapter 12), protection of fry is more problematic. A combination of vaccination of larger juveniles and broodstock, then carefully managed hatchery biosecurity appears to be most promising means of control in the future. Bacterial diseases of warm water marine finfish include Photobacterium damselae subsp. piscicida, vibriosis caused by a number of different Vibrio species, and streptococcosis caused by Streptococcis iniae and S. agalactiae. As the major proportion of aquaculture developments is in warm water, it is here that new diseases are most likely to arise as significant problems for the future.
10.6 Summary
- The nature of aquaculture and the best anti‐disease technologies are the direct nemeses of each other.
- Aquaculture is about maximising profit by growing as many animals as possible in the smallest possible volume of water, whereas anti‐disease methodologies include keeping culture densities as low as possible.
- As profit is the major goal in aquaculture and culture densities maximised because of this, disease problems are amplified.
- Because of the above, aquatic pathobiologists will always be in demand and the challenges faced make a very interesting and rewarding career path for students.
References
- Elston, R.A. 1990. Mollusc Diseases: Guide for the Shellfish Farmer. Washington Sea Grant Program, University of Washington Press, Seattle.
- Hoole, D., Bucke, D., Burgess, P., Wellby, I. 2001. Diseases of Carp and Other Cyprinid Fishes. Fishing News Books, Oxford.
- Kabata, Z. 1985. Parasites and Diseases of Fish Cultured in the Tropics. Taylor and Francis, London.
- Kent, M.L., Margolis, L. 1995. Parasitic protozoa of seawater‐reared salmonids. Aquaculture Magazine, 21, 64–74.
- Pickering, A.D. (Ed.) 1981. Stress and Fish. Academic Press, London.
- Shinn, A.P., Pratoomyot, J., Bron, J.E., Paladini, D., Brooker, E.E., Brooker, A.J. 2015. Economic costs of protistan and metazoan parasites to global mariculture. Parasitology, 142, 196–270.