16
Carps
Sena De Silva and Qidong Wang
16.1 Introduction
The family Cyprinidae comprises a diverse and widely distributed group of mostly freshwater fish. Only two marine cyprinid species exist, and neither are of commercial interest. Cyprinids are collectively referred to as carps, barbels and minnows. Some use the term ‘carp’ to refer to the entire family, although common usage usually restricts the term to describe only the larger cyprinid species. The common name ‘barbel’ refers to cyprinids mostly in the genus Barbus. ‘Minnows’ comprise the smaller cyprinids, although in many places the term is loosely used to refer to many small fishes—cyprinid or not. Many cyprinids, such as danios (Danio spp.) and barbs (mostly Puntius spp.), are popular ornamental aquarium fish (Chapter 26), and zebrafish (Danio rerio; Figure 16.1) are widely used as a simple vertebrate model for research in developmental biology, toxicology, oncology and gene function. Mostly in the USA, cyprinid ‘minnows’ such as golden shiner Notemigonus crysoleucas, fathead minnow Pimephales promelas and goldfish Carassius auratus are grown in aquaculture to provide bait for angling. However, the focus of this chapter is fish grown for human food and for simplicity’s sake we will refer to all cyprinids used in food fish aquaculture as carps.

Figure 16.1 Zebrafish are one of the most important cultured fish in the world. These small cyprinids (approximately 4 cm total length) are widely used in biological and medical research.
Source: Oregon State University 2013. Reproduced under the terms of the Creative Commons Attribution Share Alike license, CC‐BY‐SA 2.0, via Flickr.
Carps are native in North America (northern Canada to southern Mexico), Africa and Eurasia, but were absent from South America, Australasia and Madagascar. Distinguishing features of the group are the presence of pharyngeal teeth in one to three rows, with not more than eight teeth in any one row; lips that are usually thin and an upper jaw that is usually bordered only by premaxillae. The Cyprinidae includes about 275 genera and more than 2400 species, although taxonomy is in a constant state of flux. The greatest diversity of the group occurs in Asia. Cyprinids include one of the largest freshwater fishes, Catlocarpio siamensis, which may reach 3 m in length, and some of the smallest, with maximum lengths less than 5 mm.
Carps include many species that have been widely introduced—often with negative consequences—outside their native ranges either accidentally or for varying purposes. The common carp is native to Europe and Asia but has been introduced to every continent except Antarctica as a food fish or as a species for angling. Feral common carp are regarded as pests in Australia, most of the USA, and many other countries due to its fecundity, feeding habits and resulting negative impacts on native fisheries. Koi are varieties of common carp that were bred for dramatic coloration in Japan in the 1800s and have been widely distributed throughout the world for display in ornamental ponds and aquaria (Figure 16.2). Similarly, selective breeding of the Prussian carp Carassius gibelo in China more than a thousand years ago produced the goldfish, which is perhaps the most widely distributed aquarium fish in the world. In many places, koi and goldfish have escaped into the wild and are considered a nuisance species. Grass carp have been introduced as a biocontrol agent for weed control into the USA, New Zealand and other countries. Grass carp are controversial outside their native range as society tries to balance the usefulness of the fish for weed control against the potentially negative impacts on native ecosystems. In countries where introduced cyprinids are considered as invasive or nuisance species, management plans for their eradication have been developed. Identification of certain fish species as undesirable often shows considerable cultural bias: common carp and tilapias, for example, are considered as invasive pests in some countries whereas salmonids introduced outside their native range are almost never categorised in that way. The bias against carps in some countries must be viewed in the context of the tremendous contribution of cyprinids to global food production.

Figure 16.2 Koi in the Berlin Aquarium, Berlin Zoological Gardens.
Source: Diether 2008. Reproduced under the terms of the Creative Commons Attribution Share Alike license, CC BY‐SA 3.0, via Wikimedia Commons.
Carp aquaculture is extraordinarily important. Carps are major sources of animal protein for hundreds of millions of people in Asian, African and European countries. Carp aquaculture also provides an impetus or starting point for general aquaculture development in many countries. Global carp aquaculture production in 2014 was almost 28 million t, accounting for 65% of all inland finfish aquaculture, 56% of total finfish aquaculture, and almost 40% of total animal aquaculture production.1 Carp aquaculture production has increased significantly since records have been kept, and increased more than twofold from 2000 to 2014 (Table 16.1). Interestingly, the percentage contribution of carp aquaculture to total inland freshwater finfish production has remained essentially unchanged since 1980.
Table 16.1 Global annual aquaculture production of the nine most important (by weight produced) carp species and global annual production of all cyprinid species.
| Annual production (million t) | |||||||
| Common name | Species | 1970 | 1980 | 1990 | 2000 | 2010 | 2014 |
| Grass carp | Ctenopharygodon idella | 0.093 | 0.156 | 1.054 | 2.976 | 4.362 | 5.537 |
| Silver carp | Hypophthalmichthys molotrix | 0.271 | 0.449 | 1.134 | 3.035 | 4.100 | 4.968 |
| Common carp | Cyprinus carpio | 0.242 | 0.365 | 1.134 | 2.410 | 3.421 | 4.159 |
| Bighead carp | Hypophthalmichthys nobilis | 0.125 | 0.199 | 0.678 | 1.428 | 2.587 | 3.253 |
| Catla | Gibelion (Catla) catla | 0.031 | 0.087 | 0.235 | 0.602 | 2.977 | 2.770 |
| Rohu | Labeo rohitus | 0.031 | 0.090 | 0.245 | 0.733 | 1.133 | 1.670 |
| Wuchang bream | Megalobrama amblycephala | 0.029 | 0.045 | 0.162 | 0.446 | 0.652 | 0.783 |
| Black carp | Mylopharyngodon piceus | 0.018 | 0.271 | 0.038 | 0.149 | 0.424 | 0.557 |
| Mrigal | Cirrhinus cirrhosus | 0.011 | 0.046 | 0.160 | 0.552 | 0.302 | 0.415 |
| Total cyprinid aquaculture | 0.907 | 1.573 | 5.628 | 13.859 | 23.110 | 27.882 | |
Carps are a diverse group of fish and many species have traits that make them good candidates for aquaculture. The United Nations Food and Agricultural Organization lists 49 species or species groups in its 2016 FishStat J database for global aquaculture production. However, six species (grass carp, silver carp, common carp, bighead carp, catla and rohu), each with an annual production of more than 1.5 million t in 2014, constitute 91% of total carp aquaculture. Production of three additional species—Wuchang bream, black carp and mrigal—represents an additional 6% of total carp aquaculture. As such, 97% of carp aquaculture is represented by nine species.
China and India dominate cyprinid aquaculture production. The importance of carp aquaculture in these two countries stems initially from the fact that they comprise the native ranges of the Chinese and Indian carps that constitute the backbone of cyprinid aquaculture (Table 16.2). China alone contributes about 70% to global cyprinid production. Importantly, it is evident that countries such as Bangladesh, Myanmar, Indonesia, Laos and Vietnam have adopted cyprinid aquaculture to a significant extent, further emphasising the importance of the group in global aquaculture. Throughout China, Southeast Asia and the Indian Subcontinent, carp aquaculture is a growing contribution to food fish production and, therefore, food security.
Table 16.2 The global total and the top ten countries for aquaculture production of cyprinids for selected years. The percent contribution to the global total from each country is given in parentheses.
| Country/ Territory | 1990 | Country/ Territory | 2000 | Country/ Territory | 2013 |
| Total cyprinid | 5 621 860 | 13 793 350 | 26 410 010 | ||
| China | 4 093 124 (72.8) | China | 10 788 565 (78.2) | China | 18 809 593 (71.2) |
| India | 628 157 (11.2) | India | 1 703 357 (12.3) | India | 3 737 358 (14.2) |
| Russian Fed. | 252 296 (4.5) | Bangladesh | 458 978 (3.3) | Bangladesh | 1 076 604 (4.1) |
| Indonesia | 131 725 (2.3) | Indonesia | 224 868 (1.6) | Myanmar | 789 160 (3.0) |
| Ukraine | 80 801 (1.4) | Myanmar | 93 948 (0.7) | Indonesia | 473 355 (1.8) |
| Romania | 34 400 (0.6) | Russian Fed. | 63 154 (0.5) | Viet Nam | 394 565 (1.5) |
| Taiwan | 26 818 (0.5) | Brazil | 54 566 (0.4) | Iran | 167 883 (0.6) |
| Iran | 26 254 (0.5) | Thailand | 54 482 (0.4) | Pakistan | 144 881 (0.5) |
| Poland | 22 200 (0.4) | Ukraine | 30 835 (0.2) | Russian Fed. | 98 385 (0.4) |
| Uzbekistan | 21 948 (0.4) | Iran | 27 500 (0.2) | Laos | 79 950 (0.3) |
The remarkable development trend of inland aquaculture in Myanmar, considered to have been pioneered by rohu aquaculture, is depicted in Figure 16.3. This development occurred in less than 20 years and rohu culture accounts for over 60% of national production. Rohu culture in Myanmar originally focused exclusively on local markets with a small quantity of fresh fish on ice exported to Bangladesh. Over time rohu exports from Myanmar increased up to 64 000 t. The development of an export market for rohu has essentially exploited a niche market of expatriate Indian and Bangladeshi communities, at first in Middle East and later in Europe. Development of the Myanmar export market for rohu—which are normally gutted, cleaned, glazed and frozen whole—has created many employment opportunities for rural females in processing plants (Figure 16.4), with significant impacts on the socio‐economic conditions of poor communities in developing countries.
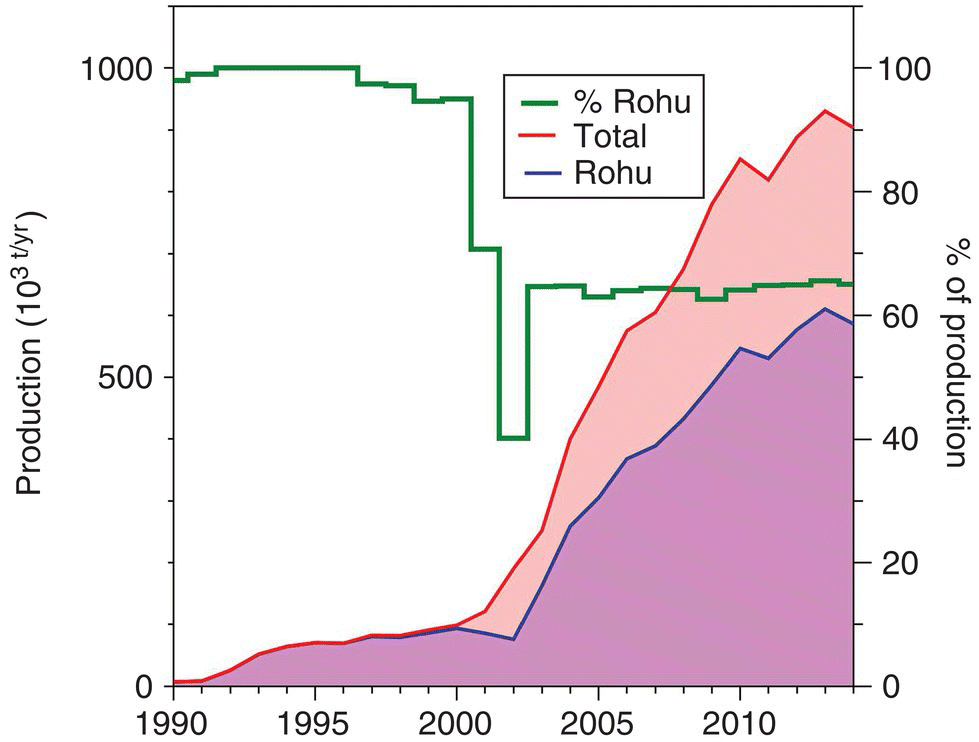
Figure 16.3 Trends in the development of inland aquaculture and the role of rohu in Myanmar aquaculture.
Source: Data from FAO 2015.

Figure 16.4 A processing facility for rohu in Myanmar. Processing is usually limited to descaling and removing gills and viscera.
Source: Reproduced with permission from Sena De Silva, 2017.
16.2 Biology of the Important Carps in Aquaculture
This chapter deals primarily with the culture of common carp and the Chinese and Indian major carps. These fish are riverine, typically being found in large river systems. The food habits of the various species differ considerably (Table 16.3), which is the fundamental reason why these fish are often grown together in polycultures rather than alone in monoculture like so many other aquaculture species. That is, the different fish co‐cultured together feed on particular types of food—such as snails, zooplankton, phytoplankton or larger aquatic plants—and are complementary rather than competitors with respect to food resource utilisation. Carp polyculture is a highly efficient culture strategy that makes full use of the wide variety of foods available in aquatic habitats. Food habits of the major carp species also differ between life stages; for example, grass carp fry and early fingerlings are zooplanktivorous but the fish becomes herbivorous and feed on larger aquatic plants as they grow.
Table 16.3 Feeding habits of important carps used in aquaculture.
| Species | Feeding habit |
| Common carp | Omnivore; predominantly a grazing bottom‐feeder. |
| Grass carp | Herbivore; grazing on succulent emergent, submersed, or floating aquatic plants. |
| Silver carp | Omnivore; water‐column filter‐feeding on phytoplankton and zooplankton. |
| Bighead carp | Omnivore; water‐column filter‐feeding principally on zooplankton. |
| Black carp | Molluscivore/omnivore; grazing primarily on snails, mussels and clams. |
| Wuchang bream | Herbivore, grazing on filamentous algae and succulent aquatic plants. |
| Catla | Omnivore; water‐column filter‐feeding principally on zooplankton. |
| Rohu | Omnivore; water‐column filter‐feeding and periphyton grazing. |
| Mrigal | Omnivore; predominantly a grazing bottom‐feeder. |
Carps are very fecund—depending on species, females usually produce 75 000 to 300 000 eggs/kg body weight—and most of the cultured carp species attain sexual maturity in their third year of life. In the wild, they spawn once per year, generally with the onset of monsoonal floods. It is generally accepted that the interaction of a large number of factors associated with flooding is responsible for bringing about ovulation and spawning of Indian major carps under natural conditions. One cue that seems to be common among the Chinese and Indian major carps seems to be a sudden increase in water flow. The spawning of Indian major carps may be synchronised with the phase of the moon during the floods. In the case of Chinese major carps, it is believed that temperature and photoperiod provide primary cues for maturation. Chinese and Indian major carps have not been known to spawn naturally in lake waters, nor under captive conditions without hypophysation (section 16.3). All the Chinese and Indian major carps are single spawners in the wild, in that during any one spawning season the female sheds all her mature oocytes within a very short period.
All the major carps grow to about 1 m in length, and generally the Chinese major carps grow to a larger size than the Indian major carps. For example, it is not uncommon for silver and bighead carp to grow up to 1.5 m in length and exceed 10–20 kg in weight. Under culture conditions, however, all except broodstock are harvested in their second or third year, often at weights of 1–2 kg.
16.2.1 Common Carp
Common carp (Figure 16.5) are native to Europe and Asia but have been introduced throughout the world for fish culture and angling. Appearance is variable. Body shape ranges from elongated to deep and stocky. The body is fully scaled with relatively large scales, although natural mutations produce scaleless fish (leather carp) or fish with only a few scales (mirror carp; Figure 16.6). The scale edges are more darkly pigmented, giving the fish a cross‐hatched appearance. Colours vary from silvery grey to reddish bronze. The subterminal mouth has two obvious barbels on each side at the corners of the mouth, with shorter barbels at the tip of the snout. The fish may grow to more than 40 kg in nature, but usually grow to 5–15 kg. Common carp biology and culture are summarised in the dated, but still useful, monograph by Billard (1999).

Figure 16.5 Common carp from Lake Enghien les Bains.
Source: ASSOPECHE 2011. Reproduced under the terms of the Creative Commons Attribution Share Alike license, CC BY‐SA 4.0, via Wikimedia Commons.

Figure 16.6 Common carp (left) and the irregularly scaled, mirror carp mutation (right).
Source: Reproduced with permission from Karelj 2011, via Wikimedia Commons.
Common carp tolerate a wide range of environmental conditions, which explains their wide distribution and favoured status as a fish for aquaculture. They live in waters with temperatures ranging from near freezing to more than 35 °C; optimum water temperature for growth and propagation is 20–30 °C. Common carp survive at salinities above 10‰ but grow best in fresh waters with salinities below 5‰. They can survive for long periods at dissolved oxygen levels near 1 mg/L, or lower, but like most warmwater fish, grow best when dissolved oxygen concentrations are near saturation (see Chapter 4).
Wild common carp spawn in lakes, streams and rivers from late spring through early summer in temperate regions, when water temperatures rise to 18–24 °C. Rising water levels that flood littoral regions also stimulate spawning. Spawning takes place in shallow littoral areas with slow‐moving or stagnant water and abundant weeds which serve as spawning substrate. Fertilised eggs stick to the spawning substrate and hatch in 3–5 days, depending on water temperature. One female common carp may produce 100 000 to 300 000 eggs/kg body weight.
Larval fish eat zooplankton and consume an ever‐increasing variety of foods as they grow. Juveniles and adults are opportunistic omnivores, feeding on zooplankton, aquatic insects, detritus of plant origin, and a variety of benthic organisms. Larger fish may eat fish eggs, small fish and crayfish. Adults are notorious for disturbing and suspending sediments during foraging.
Common carp used for aquaculture may be produced using volitional spawning in ponds or tanks provided with a spawning substrate (submersed aquatic plants, palm fronds, etc.). However, most cultured fish are spawned under controlled conditions using hormone injection to induce and synchronise spawning. Larval fish are raised in ponds fertilised with organic material (manures) or synthetic fertilisers to promote appropriate zooplankton communities (section 9.9). Fish may be fed a supplementary diet of nut or cereal meals as they grow. Fry may be grown to fingerlings in the same pond or thinned and transferred to another pond for growth to fingerlings.
Ongrowing to market size takes place in a wide variety of systems, ranging from extensive pond polycultures based on natural food production (Figure 2.4) to intensive monocultures in tanks and cages using commercial pelleted feeds. Common carp are often the primary species raised in semi‐intensive pond polycultures with Chinese and Indian major carps or tilapia. In these systems, common carp are fed farm‐made or commercial feeds. Wastes excreted by the primary species promote abundant phytoplankton and zooplankton growth that are then grazed upon by secondary carp or tilapia species. Common carp may grow to more than 2 kg in one year in feed‐based systems in tropical or subtropical regions.
16.2.2 Chinese Major Carps
Several hundred cyprinid species are endemic to China and neighbouring areas but only a few are important in aquaculture. These are referred to as ‘major carps’ based on potential for growth in culture and importance in food production. The Chinese major carps (sometimes called Asian major carps) include grass carp, silver carp, bighead carp, black carp, Wuchang bream and mud carp (Cirrhina molitorella). Common carp and Crucian carp (Carassius carassius) are sometimes included on this list by virtue of their importance in Asian aquaculture. The three most important Chinese major carps—those with total 2014 aquaculture production exceeding 1 million t—are the grass, silver and bighead carp.
16.2.2.1 Grass carp
Grass carp are native to large rivers in eastern Asia, from northern Vietnam to northern China and southeast Russia. It has been widely introduced and is present on every continent except Antarctica. The fish was introduced as a food fish in many Asian countries but was introduced as a biocontrol agent to control aquatic weeds in Europe, the USA, New Zealand, Australia and elsewhere. Grass carp have strong, torpedo‐shaped bodies (Figure 16.7); large, clearly defined scales; and a terminal mouth with no barbels. They are brownish olive dorsally, shading to bronze on the sides and white ventrally. Grass carp can grow to more than 35 kg in the wild. Grass carp biology is summarised by Cudmore and Mandrak (2004).

Figure 16.7 Grass carp (approximately 15 kg) harvested from a lake in the USA. This fish is much larger than those grown in aquaculture, which are usually harvested at less than 2 kg.
Source: Reproduced with permission from David Cline, 2017.
Grass carp are hardy fish, with environmental tolerances similar to common carp. They tolerate water temperatures from near freezing to about 35 °C, with an optimum range for growth of 20–30 °C. Feeding activity declines sharply below about 10–15 °C. Salinities up to 10–12‰ are tolerated, but fish grow best and are healthiest below about 5‰. Grass carp are relatively tolerant of low dissolved oxygen concentrations and can tolerate brief exposure to oxygen concentrations as low as 0.2 mg/L.
Grass carp mature at 2–8 years depending on their environment. Longer maturation periods are typical of cooler waters. Adult grass carp migrate to the upper reaches of large rivers to spawn. Spawning is stimulated when water temperatures reach 15–20 °C and river flows increase rapidly, corresponding to typical spring and early summer conditions in native rivers. Other spawning stimuli may also be involved. Males and females congregate in groups and females release eggs into flowing water where they are immediately fertilised. Females may release 75 000–150 000 eggs/kg body weight.
Fertilised eggs float downstream and hatch in 2–4 days. Larvae continue to drift downstream as the yolk sac is absorbed over a 2–3‐day period, after which fry begin feeding on zooplankton. After about 3 weeks, feeding habit becomes increasingly herbivorous, with increasing proportions of filamentous algae, succulent aquatic plants and plant‐based detritus. By 2 months the diet is almost exclusively plant‐based, although larger zooplankton, aquatic insects and even small fish can contribute to the diet throughout life. Adults prefer succulent aquatic plants with soft leaves but will consume almost any plant when preferred food items are scarce. Under aquaculture conditions, fish may eat plants growing naturally in the pond or may be fed terrestrial or aquatic plants harvested from nearby land or water bodies and then processed to some degree, usually by simply chopping into smaller pieces. Increasingly, however, cultured grass carp are fed artificial feeds such crop by‐products or manufactured pelleted feeds.
Grass carp spawn naturally only in large rivers, and will not spawn in ponds, tanks, or raceways without hormone injection. Nearly all grass carp used in aquaculture are therefore produced using hatchery‐based artificial propagation, with occasional use of eggs collected from wild sources. Artificial propagation relies on injecting mature broodfish with an inducing hormone (usually pituitary extract or luteinising hormone‐releasing hormone analogue, LHRHa; see section 16.3). Eggs are stripped from the female, fertilised, and incubated in tanks or jars with water circulation to keep eggs suspended. Larvae are held in the hatchery for 3–5 days until the yolk sac is absorbed and then transferred to small ponds fertilised with organic material (manures) to promote zooplankton growth. Natural foods may be supplemented with soybean cake, grain by‐products or finely ground aquatic plant fodders. Fry are usually thinned and transferred to fingerling‐rearing ponds for further growth after a variable length of time (weeks to months) in the fry nursery pond. Length of time in nursery and fingerling ponds and the desired fingerling size for ongrowing to market‐sized fish varies depending on climate and custom.
A variety of systems are used for grow‐out to market size. The most common methods include semi‐intensive polyculture in ponds and intensive polyculture or monoculture in cages placed in reservoirs or large lakes. Grass carp are an esteemed food fish in China (and elsewhere) and increasingly they are the primary species in pond polycultures. In the past, most cultured grass carp were fed lightly processed, chopped plant material (aquatic plants or terrestrial grasses) but use of high‐quality manufactured feeds is becoming common. Secondary species in polyculture include silver carp, bighead carp or Indian major carps, depending on the country. Grass carp are usually the primary (or only) species when grown intensively in cages. If other fish are used, such as Wuchang bream or silver carp, they are added to cages at much lower numbers than grass carp. In India, grass carp often are a relatively minor part of polyculture systems comprising Indian major carps and silver carp. Desired size at harvest is 1–3 kg depending on country and market. Fish are usually marketed locally as fresh fish.
16.2.2.2 Silver carp
Silver carp are native to large rivers in eastern China. The fish has been introduced to at least 90 countries in Asia, Europe, Africa and North and South America. Introductions were most often made for aquaculture, but fish were also introduced as a biological agent for phytoplankton control in eutrophic waters. Accidental introductions have also occurred. Silver carp biology is reviewed by Kolar et al. (2005).
Silver carp are deep‐bodied, laterally compressed fish with a scaleless abdominal keel running from the pectoral region to anus. Adult coloration is dark grey dorsally shading to silver laterally and ventrally. Barbels are absent from the terminal mouth. Eyes are set well forward and below the angle of the jaw (Figure 16.8). They may grow to more than 1.2 m and 40 kg in natural habitats. The gill rakers are highly modified into a sponge‐like filtering apparatus. Mucus produced by the epibranchial organ helps consolidate filtered material on the gill rakers and the overall result is a highly effective food filtering and concentrating process.

Figure 16.8 Silver carp (approximately 1 kg) harvested from a ‘composite’ polyculture pond in India.
Source: Reproduced with permission from Les Torrans, 2017.
Within their native range silver carp live in rivers and backwater lakes that are connected to large rivers. When introduced outside their native range, they adapt well to a variety of habitats including rivers, streams, reservoirs, lakes and ponds, but require flowing water for reproduction. Temperature tolerance ranges from near freezing to more than 35 °C, with optimum growth temperatures of 24–30 °C. Feeding activity slows below 15 °C and ceases at about 10 °C. Salinity tolerance appears to be less than grass or common carp, with tolerance to 7.5–10‰ and best growth below 4‰. The fish also does not appear to be as tolerant of low dissolved oxygen as common carp.
Silver carp mature at 2 years in warm climates and 5 years, or more, in cooler waters. Spawning activity is initiated when water temperatures reach 18–25 °C and river levels rise, corresponding to spring and early summer conditions in rivers within their native range. Adult fish migrate upstream and congregate in groups. Females release eggs into flowing water and eggs are immediately fertilised. Each female may release 100 000–200 000 eggs/kg body weight. The semi‐buoyant eggs drift downstream and hatch in 2–4 days. After larvae absorb their yolk sac, currents sweep them into slower‐moving backwater areas and fry begin feeding on small zooplankton. Over 2–3 weeks feeding becomes increasingly phytoplanktivorous, and juvenile and adult fish feed primary on larger phytoplankton, with lesser amounts of zooplankton and suspended detritus. Food is captured using a highly efficient filter feeding process. Particles as small as 10 µm, or less, can be captured by the fine gill rakers and sponge‐like raker net.
Silver carp will not spawn in ponds, tanks, or raceways without hormone injection. Eggs are stripped from the female, fertilised, and incubated in running‐water hatching jars, vats or tanks with enough water circulation to keep eggs suspended. Larvae are held in the hatchery for 3–5 days until the yolk sac is absorbed and then transferred to small ponds fertilised with organic material (manures) to promote zooplankton growth. After 14–21 days, fry may be thinned and moved to fingerling‐rearing ponds for 3–6 months of further growth.
The anatomy and feeding habits of silver carp are such that they do not feed to a significant degree on supplemental feeds. Their unique filter‐feeding habit makes them ideal for polyculture and silver carp are almost always used in systems with other fish. Silver carp polyculture systems consist of extensive and semi‐intensive ponds as well as intensive cages and net pens. They may be grown in extensive ponds fertilised with organic material or synthetic fertilisers, in combination with other filter feeders such as bighead carp, Wuchang bream, Indian major carps or tilapia. In more intensive systems, silver carp are a secondary species, with grass carp or common carp being the primary species. The primary culture species are fed some type of farm‐made or commercial prepared feed, and wastes produced by the primary species stimulate phytoplankton blooms that are eaten by filter‐feeding silver carp. The fish is mainly consumed locally and marketed alive or fresh.
16.2.2.3 Bighead Carp
The native range of bighead carp is similar to silver carp, although the range of bighead carp does not extend as far north. Bighead carp have been widely introduced throughout the world for aquaculture or as a biocontrol agent for remediation of eutrophic waters. Naturally reproducing populations are known to be established in many countries in Asia, Europe and North and South America. The fish is similar in appearance to silver carp; it is deep‐bodied, laterally compressed, with a smooth, scaleless abdominal keel running between the pectoral fins to anus. Adult coloration is grey dorsally shading to creamy white ventrally, sometimes with darker blotches on the sides (Figure 16.9). The head appears disproportionately large for the body. Eyes are well forward, somewhat below the angle of the jaw and are oriented more ventrally than those of the silver carp. Bighead carp can reach 40 kg in natural habitats but are usually harvested at 1–2 kg in culture.

Figure 16.9 Bighead carp (approximately 5 kg) harvested from a naturally reproducing population in the Illinois River, USA.
Source: J. Amberg, U.S. Geological Survey.
Reproductive behaviour, environmental tolerances, and general mode of feeding are similar to the closely related species, silver carp (Kolar et al., 2005). Bighead carp adapt well to a variety of freshwater habitats but require flowing water for reproduction; they will not spawn in ponds, tanks, or raceways without hormone injection. Spawning methods are similar to those described for silver carp. For aquaculture purposes, the important distinctions between the two species are slightly larger prey selection by bighead carp during filter‐feeding and consumer preference for bighead carp over silver carp in China and most other Asian countries (Dey et al., 2005).
Unlike in silver carp, the gill rakers of bighead carp are not fused at the base into a sponge‐like filtering apparatus. As such, bighead carp are not as effective as silver carp at retaining small particles during filter feeding. Bighead carp are therefore considered primarily zooplanktivorous whereas silver carp are considered phytoplanktivorous, although both species are highly opportunistic and considerable dietary overlap exists.
Bighead carp are grown primarily in pond polycultures, filling a slightly different feeding niche than silver carp. They are almost always a secondary species, feeding on zooplankton and larger phytoplankton that are produced as a natural by‐product of fertilisation or feeding practices for the primary culture species, which may be grass carp, common carp, or others. Bighead carp are mostly sold as fresh fish and consumed locally.
16.2.2.4 Black carp and Wuchang Bream
Black carp and Wuchang bream (also called blunt snout bream and Wuchang fish) both contribute more than 0.5 million t to global aquaculture. Black carp are native to large rivers in eastern China and outwardly resemble grass carp in appearance, but are dusky‐grey rather than bronze‐coloured. The fish has been widely introduced throughout the world for aquaculture or as a biocontrol agent for snails or other molluscs. Black carp spawn naturally only in large rivers and will not spawn in captivity without hormone injection. Larvae and small juveniles feed on zooplankton, but as they grow the diet switches to snails, mussels and clams. The molluscan diet provides a unique feeding niche that can be utilised in polyculture. Black carp are a highly revered food fish in China and are grown mainly as part of pond polyculture systems where it is a minor species with grass or Crucian carp. The relative high prices paid for black carp has stimulated interest in emphasising black carp production. The fish will accept pelleted artificial feeds and in some areas it is now farmed as the principal fed species in polyculture, with silver or bighead carp as the minor species utilising plankton communities stimulated by waste nutrients excreted by black carp. Black carp are harvested and transported live to wholesale markets locally or regionally, often in major cities.
Wuchang bream are also a valuable and esteemed fish in China. They are native to the Yangtze River basin in China. Wuchang bream inhabit lakes and slow‐moving waters and migrate into rivers to spawn. They are herbivorous in nature but in culture they are usually fed farm‐made or commercial feeds as the primary species in pond or pen polyculture systems together with other carps as secondary culture species.
16.2.3 Indian Major Carps
Indian major carps comprise a handful of cyprinid species that are endemic to the Indian subcontinent and are important aquaculture species because of their relatively fast growth to a desirable market size. The major carps are in the genera Labeo, Gibelion (Catla) and Cirrhinus, and, more specifically, the term usually refers to the species catla, rohu and mrigal. Major carps are important aquaculture species in India but are also produced throughout the subcontinent and Southeast Asia. Although Indian and Chinese major carps are often grown together in countries of the subcontinent, temperature tolerance is an important distinction between the two groups of fish. The Chinese carps evolved in a temperate climate and tolerate an extremely wide range of water temperature—from near freezing to more than 35 °C. Indian carps evolved in a tropical climate and cannot tolerate temperatures below about 15 °C and have a slightly higher optimum temperature for growth and a slightly higher critical maximum temperature near 40 °C. This limits aquaculture of Indian major carps to tropical regions.
Global production of catla and rohu exceeded 1 million t in 2014 and these two species are described below. Mrigal (Figure 16.10) production was about a quarter that of rohu, but its bottom‐feeding habit makes it a valuable component of some polyculture systems with the other two Indian major carp species (which feed in the water column) and in so‐called ‘composite’ polyculture systems with Chinese major carps. However, harvest difficulties associated with the mrigal’s bottom‐dwelling habit make it the least preferred of the Indian major carps for aquaculture.

Figure 16.10 Mrigal (approximately 0.5 kg) collected from a reservoir in India.
Source: Reproduced with permission from Dibakar Bhakta, 2017.
16.2.3.1 Catla
Catla are native to rivers of the Indo‐Gangetic plains of northern India, Pakistan, Bangladesh and Myanmar. The fish has been introduced into waters throughout the subcontinent. The body is stout and laterally compressed, with a large head and terminal, up‐turned mouth with no barbels (Figure 16.11). The eyes are large and placed well forward. The fish is grey dorsally shading to silvery white, with large, prominent scales. Catla are considered to be the fastest growing of the three major Indian carps. They may grow to more than 35 kg in the wild but are usually grown only to 1–2 kg in culture.

Figure 16.11 Catla (approximately 1.5 kg) collected from Ukai reservoir in Gujarat, India.
Source: Reproduced with permission from Dibakar Bhakta, 2017.
Environmental tolerances are not well known. The fish survives at temperatures of about 17 to 40 °C, with an optimum temperature for growth near 30–32 °C. Catla grow well at salinities up to at least 5 ‰. Catla appear to be somewhat less tolerant of low dissolved oxygen concentrations than common carp.
Catla mature in 2 years and are stimulated to migrate upriver to spawn when rivers rise during the monsoon season and water temperatures are above 25 °C. Fish congregate and breed in littoral areas. Females may produce 100 000 to more than 200 000 eggs per kg body weight. The semi‐buoyant eggs drift downstream and hatch in 18–24 hours, depending on water temperature. Larvae begin to feed on small zooplankton 3–4 days after hatching. Prey size and diversity increases as fish grow but catla remain essentially planktivorous throughout life, feeding opportunistically in near‐surface waters on a variety of suspended matter, including zooplankton, colonial algae and detritus.
Catla spawn naturally only in rivers and reproduction stimulated by environmental cues such as rising water temperatures and increased river flows. In the past, brood catla were induced to spawn in flooded fields (called ‘bundhs’) where water levels and flow were manipulated during the monsoon season to simulate river conditions. Eggs were collected from the bundhs by filtering outflow water through fine netting. However, most catla are now produced in hatcheries using hormone injections to induce spawning.
Catla are appreciated as a food fish in India and other countries, and a variety of systems are used in catla aquaculture (Ramakrishna et al., 2013). Most growers in India use a 3‐stage system of nursery, juvenile culture and ongrowing to market size. Nursery ponds are small (0.05–0.25 ha; 1‐m‐deep) and are prepared by liming and fertilisation (usually with poultry or cow manure) to stimulate zooplankton communities. Postlarvae are usually offered a supplemental mash feed consisting of powdered groundnut, rice bran, cottonseed meal, and other by‐products—either alone or in mixtures. Fry are grown for 20–30 days and then transferred to fingerling growout ponds, which are larger (0.4–4 ha) and deeper than nursery ponds. Fertilisation and supplemental feeding practices are similar to those used in nursery ponds Fingerlings may be grown at high densities (up to 0.3 to 1 million fry/ha) to produce a 20–25 g/fish in 2–4 months or grown at lower densities to produce large numbers of ‘yearlings’ of 50 to 125 g/fish after 10–12 months.
The widest variety of culture practices occurs at the ongrowing stage. For example, at least a dozen different ongrowing systems using catla and rohu have been used in the state of Andra Pradesh, in southeastern India (Ramakrishna et al., 2013). The use of catla in these various pond polycultures is a consistent feature, but size and number of fingerlings stocked, culture intensity and the relative mixture of fishes vary widely. Catla are often grown in semi‐intensive, fertilised pond systems as a secondary species with rohu (as the primary species), and perhaps mrigal, usually with supplemental feeding of mash feeds made from mixtures of de‐oiled rice bran, groundnut cake, cottonseed cake or similar ingredients. However catla (and rohu) are also grown in polyculture with Chinese major carps, common carp, tilapias, striped catfish (Pangasianodon hypophthalmus), freshwater prawns (Macrobrachium rosenbergii) and even in low‐salinity polycultures of penaeid shrimp. Of note, commercial pelleted feeds are commonly used to grow striped catfish, with catla or rohu feeding on natural productivity stimulated by the wastes generated from feeding catfish.
Catla are harvested at 1–2 kg and marketed mostly as fresh fish. Markets are usually local or regional but may be quite some distance from the production site. For example, much of the Indian major carp production in Andra Pradesh is traded throughout India. Fish are washed, packed in crushed ice and shipped in insulated trucks or vans through the country.
16.2.3.2 Rohu
The native range of rohu is similar to that of catla and the fish has been introduced for aquaculture throughout the subcontinent and in several countries in Asia, Africa and Oceania.
Rohu are handsome, moderately elongated fish with conspicuous scales (Figure 16.12). The mouth is relatively small and subterminal, with a snout projecting beyond the mouth. The eyes are lateral, dorsal of the midline. The caudal fin is deeply forked, and the fish is greyish dorsally, shading to silvery on sides. Rohu grow slower than catla but faster than mrigal in culture. Rohu may grow to more than 40 kg in rivers.

Figure 16.12 Rohu (approximately 0.75 kg) from an aquaculture pond in India.
Source: Reproduced with permission from Les Torrans, 2017.
Environmental tolerances, spawning behaviour and early life history of rohu are similar to those of catla. Larvae begin feeding on zooplankton but food selection becomes more opportunistic as fish get older. Adult rohu feed in the water column on phytoplankton, zooplankton, submerged aquatic vegetation and detritus.
Rohu, like catla, spawn naturally only in rivers. Although rohu can also be induced to spawn in flooded fields (bundhs) manipulated to simulate river conditions, most rohu are produced in hatcheries using hormone injections to induce spawning.
Rohu aquaculture is broadly similar to that used for catla, with a three‐stage system of nursery ponds, juvenile culture ponds and final ongrowing to marketable fish. Natural food production stimulated by pond fertilisation, provides much of the nutriment at all stages, although supplemental feeding with powdered groundnut, rice bran, cottonseed meal and other grain and seed by‐products is nearly universal. Rohu ongrowing is always conducted as part of a pond polyculture systems with catla, catla and mrigal, or in ‘composite’ culture with other Indian major carps, common carp, grass carp and silver carp. In India, consumer preference for rohu relative to the other species has led to emphasis on rohu as the principal species in these polyculture systems. For example, in Andhra Pradesh, rohu production is emphasised by growing the fish in a simple, two‐species combination with catla, with rohu constituting more than 70% of the harvest biomass (Ramakrishna et al., 2013). Rohu are harvested at 1–2 kg and marketed locally or distributed as iced, fresh fish regionally (Figure 16.13).

Figure 16.13 A harvest of rohu transported to a central location for transport to a processing facility in Myanmar.
Source: Reproduced with permission from Sena De Silva, 2017.
16.3 Artificial Propagation
As for many cultured fish species, the critical breakthrough in carp aquaculture was the development of techniques for artificial propagation of the major species. Before this, carp culture depended on the collection of natural seed for stocking. In fact, specialised fisheries developed in the flood plains of major rivers in mainland China and India to collect the natural seed during the spring and early summer spawning runs. In the case of catla and rohu, seed could also be collected from flooded fields that were manipulated to simulate river conditions.
The traditional and most commonly used technique of induced spawning in carps is injection of either a) crude extract of the pituitary gland of common carp (or from other mature fish species that are phylogenetically close to carp) or b) partially purified human chorionic gonadotropin (hCG). Use of hormones and/or analogues for inducing spawning is referred to as hypophysation. Details of the hormonal control of reproduction in fish and artificial spawning induction are given in Chapter 6 (section 6.2.1; Figure 6.1). The use of various techniques for spawning induction of Indian and Chinese major carp was originally summarised in the still‐useful text by Jhingran and Pullin (1985).
Initially, high efficiency of ovulation was achieved using hCG either alone or in combination with carp pituitary extracts. This protocol was replaced by the use of gonadotropin‐releasing hormone analogue (GnRHa) to stimulate reproduction. But GnRHa alone is not entirely effective and has to be accompanied by the administration of a dopamine receptor blocker. A better understanding of neuroendocrine regulation of gonadotropin secretion has led to the development of new effective techniques for induced ovulation and spawning of cultured fish species. Basically, modern techniques of inducing ovulation and spawning use a combination of drugs, one of which blocks the inhibiting action of dopamine within the neurohormonal systems (the Linpe method; section 6.2.1). Details of various effective combinations of GnRHa (LHRHa, luteinising hormone‐releasing hormone analogue) or sGnRHa (salmon GnRHa)] and a dopamine antagonist (e.g., domperidone) for induced ovulation and spawning of Chinese major carps are summarised in Table 16.4. The Linpe method is known to be more effective in many ways, ensuring a high rate of ovulation, consistency between broods, complete ovulation and that the time lag between injection and ovulation is short and predictable.
Table 16.4 A summary of the Linpe method of ovulation and spawning of cultured carp in China.
| Treatment | |||||
| Species | Temperature (°C) | Domperidone (mg/kg) | LHRHa (µg/kg) | sGnRHa (µg/kg) | Time to ovulation (h) |
| Silver carp | 20–30 | 5 | 20 | – | 8–12 |
| 5 | – | 10 | 8–12 | ||
| Mud carp | 22–28 | 5 | 10 | – | 6 |
| Grass carp | 18–30 | 5 | 10 | – | 8–12 |
| Bighead carp | 20–30 | 5 | 50 | – | 6–8 |
| Black carp | 20–30 | 3 | 10 | – | 6–8 |
| 7 | 15 | – | 6–8 | ||
Ovulation and spawning by the Linpe method do not influence the subsequent reproduction cycles of the same brood fish. The Linpe method uses synthetic drugs that are cheaper and more stable and, because only one injection is needed, brood fish are stressed to a much lesser extent.
Spawning induction of carps in India has been undertaken using commercially available kits (marketed under the trade name ‘Ovaprim™’), which utilise the Linpe method.
Another important advance in induced spawning of the major carps has been the development of the ability to spawn brood fish twice in a calendar year. A second spawning is now achieved for most major carps and is commonly practised. This development has enabled farmers to maintain fewer brood fish and has enhanced seed availability almost year round. This has almost completely eliminated the need for dependence on natural seed.
After hormonal treatment, broodstock are put into spawning ponds in a ratio of three males to two females. They usually spawn at dawn following a second injection of hCG or pituitary extract and are removed from the spawning ponds after spawning. The floating eggs are moved from the spawning pond, by movement of the water through the tank, into a collection box. Alternatively, the brood fish may be stripped and a dry fertilisation performed. The process of stripping gametes from brood fish and dry fertilisation is described in detail in Chapter 15 (section 15.3.3 and Figure 15.3). This method is becoming increasingly common in major carp culture.
Specially designed spawning tanks are now commonly used for spawning, hatching of fertilised eggs fry production of Chinese carps (Figure 16.14). They are usually circular or elliptical cement tanks about 1.2–1.5 m deep, containing 50–60 m3 of water. The tank bottom usually slopes towards the centre, where an outlet leads to an egg collection chamber. Incoming water is directed to create a circular flow within the pool at a rate of 200–400 L/s.

Figure 16.14 A commonly used cylindrical spawning tank cum hatching tank for carps (as well as other species) known as the ‘Chinese design’. By removing the polythene nets and concrete stairs it could be converted into a spawning tank.
Source: Reproduced with permission from Sena De Silva, 2017.
Fertilised eggs are transferred into incubation tanks or hatching pools which are circular, 3.5–4.0 m in diameter and 1 m deep. Water flow is maintained at approximately 0.2–0.3 m/s. Eggs are usually incubated at a density of around 700 000–800 000 eggs/m3. Under these conditions, a hatching rate of about 80% is achieved. In China, 150 000–200 000 eggs are incubated in 150‐L clay jars or in funnel‐type incubators with vertical water movement. After 4–5 days, when the larvae have absorbed the yolk sac, they are removed to nursery ponds.
Glass hatchery jars are also commonly used for hatching both Indian and Chinese major carp eggs. The jars are generally about 13 cm in diameter and 60 cm long, with conical bases. Each jar is supplied with water up through its conical base to create vertical water movement. The basic concept of all hatchery designs for major carps is to provide a water current of sufficient strength to maintain the eggs in the water column and to remove metabolic waste products.
Common carp and crucian carp are important to Chinese aquaculture and technical advances in the type of facilities used in artificial propagation of these species have been achieved in the last two decades. In the case of common carp different modes of operation may be used for producing viable larvae (Figure 16.15).
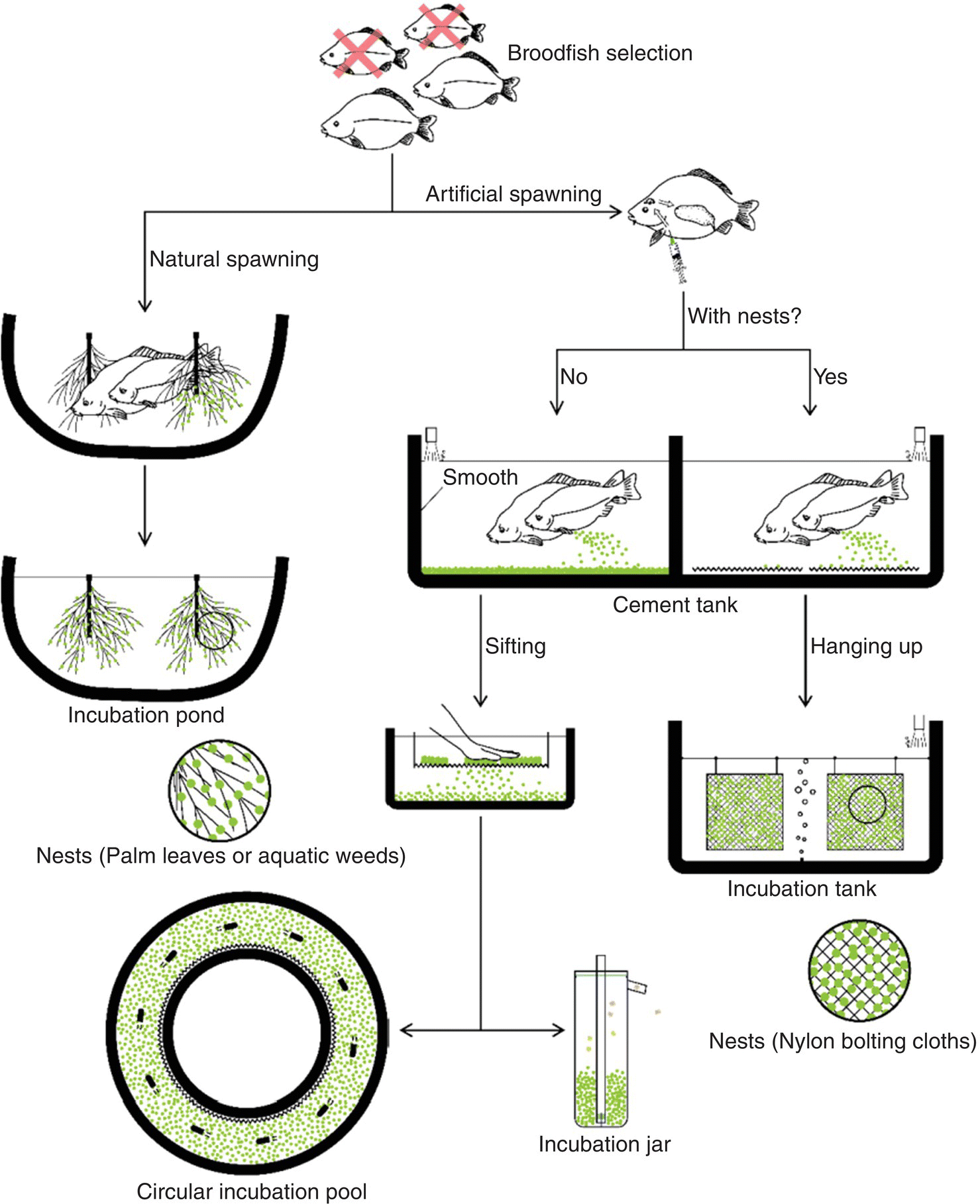
Figure 16.15 Different modes of Common carp artificial propagation in China.
Source: Reproduced with permission from Dr X. Hu, 2017.
The recent emphasis on the development of culture of native species has led to concerted efforts to artificially propagate cyprinid species that are of value to certain countries in Asia. In this regard the development of artificial propagation techniques for two, high‐valued Malaysian mahseer species, Tor tambroides and T. douronensis, stand out (Ingram et al., 2007). The above trend is being linked to introduce acceptable broodstock management procedures in order to maintain the genetic diversity of wild stocks and for conservation purposes.
16.4 Nutrition
Of the commonly cultured cyprinid species, the nutrient requirements of common carp are best known. This is to be expected because it was one of the earliest species to be cultured and examined experimentally. The nutrient requirements of most Chinese and Indian major carps are incompletely documented when compared to our understanding of cultured salmonids and many other fish. In part, the relative lack of research is due to the manner in which most carps are cultured. That is, most carp are grown extensively or semi‐intensively in ponds, and derive much of their nutriment from natural foods. As such, there has been little need to understand their exact nutrient requirements and basic nutritional research on the group has lagged behind that of other cultured fish. Only recently has emphasis shifted, for some species, to feeding commercial feeds that have been compounded and formulated, and research on nutrient requirements of selected carp species is increasing. But with recent controversies on the use of fishmeal and fish oils in aquaculture (see Chapter 5) there has developed a significant awareness on the use of these ingredients in feeds for all aquatic animals, including carps.
The dietary protein requirement of the major carps has been investigated. Most of these investigations have been carried out with fry and fingerling stages, and there is considerable variation in the results of different investigators. This is mostly a result of variations in experimental protocol. Based on available information, the dietary protein level that results in maximum growth of major carps is 45% and the economically optimal dietary protein content is 31% (Webster and Lim, 2002; see also section 8.7.3 and Figure 8.2).
The protein, amino acid and carbohydrate requirements of common carp are known (National Research Council, 2011) and partial information is available for catla and rohu (Webster and Lim, 2002). The dietary fatty acid requirements of cultured cyprinids are not well known. Indeed, apart from the early work on common carp which demonstrated that this species requires equal amounts of dietary linoleic acid (18:2n‐6) and linolenic acid (18:3n‐3), no studies have been conducted on the fatty acid requirements of carps. It is plausible that the originally identified requirement is true for all carps and this conforms to the basic notion that freshwater fish require the two fatty acids (section 8.9.7). They have the capability to elongate and desaturate these to longer‐chain polyunsaturated fatty acids, such as eicosapentaenoic acid (20:5n‐3), docosahexasenoic acid (22:6n‐3) and arachidonic acid (20:4n‐6), among others.
Information available on the requirements of other nutrients is scant and all the nutrient requirements for any one species of major carps are not known. This situation is changing rapidly however, as production intensification has made formulated feed use increasingly common for some species, including common carp, grass carp, Crucian carp, and even the mollusc‐feeding black carp. The increased emphasis on intensive, feed‐based culture has stimulated considerable research into the nutrient requirements of certain major carps.
16.5 Culture Practices
16.5.1 Larval Rearing
Induced cyprinid spawning is discussed in section 16.3. After the eggs are fertilised, hatching occurs in 2–3 days at 23–27 °C. The yolk sac continues to provide nourishment for a further 3 days or so, at which time larvae require an exogenous food supply. It is desirable, if not essential, to expose the larvae to an external food source before yolk sac resorption is complete. This involves removing larvae from the hatchery jars and introducing them into a fry‐rearing facility. The young hatchlings, which mostly move vertically, tend to change to a horizontal movement, which indicates their readiness to ingest food particles.
Larval rearing in carp culture has two distinct phases:
- Rearing postlarvae to the fry stage—usually carried out in nursery ponds or in hapas (fine mesh enclosures) suspended in ponds or channels
- Rearing fry to fingerling stage—most effectively done in well‐fertilised rearing ponds.
It is, however, not uncommon to combine the above two stages in one pond.
Preparation of nursery and rearing ponds often involves sowing a short‐term crop of a leguminous plant (e.g., beans, clover), and ploughing and levelling the pond once the plant crop has grown to 6–10 cm. This process is known as green manuring and is believed to enhance pond productivity. In most instances, unwanted organisms in the ponds are eradicated using a biodegradable toxicant or quicklime. This procedure is carried out at least a fortnight before stocking. Commonly used toxicants are derris powder (4–20 mg/L), oilcake of the plant Bassia latifola (mahua oilcake; 200–250 mg/L), tea‐seed cake (525–675 kg/ha); or quicklime (CaO; 900–1000 kg/ha).
The next stage is to prepare the pond with a view to ensuring a good production of small zooplankters, such as rotifers, which provide a food source for growing larvae. Ponds are often treated with either organic or inorganic fertilisers. The quantity of manure to be used is related to the toxicant used earlier. For example, if mahua oilcake was used, a dose of dry cow manure at the rate of 5000 kg/ha two weeks before stocking and a similar dose 1 week after hatching are desirable. However, with toxicants that have no fertiliser value, doses of 10 000–15 000 kg/ha initially, and 5000 kg/ha later, are desirable. These manuring doses are sufficient for 1–2 million larvae/ha.
Fertilisation with a mix of organic and inorganic fertilisers is undesirable, as more often than not it results in harmful plankton blooms. Despite the early preparation of the ponds, undesirable predatory insects such as water spiders and water skaters may colonise the ponds. Therefore, the ponds have to be regularly treated for insect control, particularly before stocking. Jhingran and Pullin (1988) recommend any one of the four treatments:
- spraying an emulsion of 56 kg of mustard or coconut oil and 18 kg of washing soap per hectare;
- spraying an emulsion of 56 kg of mustard oil and 560 mL of Teepol (detergent) per hectare;
- a 0.01 ppm dose of pure gamma isomer of benzene hexachloride dissolved in ethyl alcohol; or
- application of 0.25–3.0 ppm organophosphate such as fumadol, sumithion or diptrex.
The prepared ponds are stocked when it is certain that a substantial zooplankton population (particularly small zooplankters such as protozoans and rotifers) is established. Abrupt changes in quality and temperature between hatchery water and nursery water are avoided when stocking. Stocking is best done in the evening, which hopefully gives the larvae sufficient time to acclimatise themselves before any possible predation. The stocking rate depends on the proposed management practice and, if the following conditions are met, a stocking rate of 1 million fry/ha can be used:
- continued and repeated fertilisation to produce and maintain good plankton production;
- supplemental feeding; and
- equipment to remedy oxygen deficiencies that may occur.
Carp post‐larvae are voracious grazers. Supplemental feeding and manuring, when carried out concurrently, result in better survival and growth. The commonly used supplemental feeds in carp culture are rice bran and oilcakes of peanut (groundnut), coconut and mustard in India. These are also used in China, together with soybean milk and meal, and egg‐yolk paste. It is rare for juvenile carp culture to be based on complete formulated feeds, except in the case of common carp culture in some countries. Feeds are often dispersed as a crude mix, in either dry (e.g., meals or pellets) or moist form. A summary of commonly used feeds and feeding schedules for fry of Chinese carps is shown in Table 16.5. However, it should be noted that in the case of common carp, which is cultured fairly intensively on a small scale in ponds in Israel and in cages in China, formulated diets (pellet feeds) are used.
Table 16.5 Some feeds and feeding rates used for Chinese carp fry and fingerling rearing.
Source: Jingran and Pullin (1988). Reproduced with permission from Worldfish.
| Country | Species | Pond area (m2) | Depth (m) | Fish length (mm) | Age (days) | SD (per m2) | Feed | Feeding rate |
| China | Bighead, grass carp | – | 0.5–1.0 | Up to 20 | Up to 30 | 100 | Egg yolk paste or soybean milk + peanut cake after 10 days | 1 egg/2500–7500 fry/day or milk from 300–500 g beans/ 50 000 fry/day |
| All species | – | 0.5–1.0 | 23.1 | Up to 30 | – | Soybean meal | 45 kg/5000 fry/month | |
| Hong Kong | All species | 1000 | 0.8 | 8–30 (3 mg to 1 g) | Up to 25–30 | 150 | Soybean milk and peanut cake meal | 100 kg soybean milk or 200 kg peanut cake meal/month |
| All species | 1400 | 1.0 | 31 (1.5 g) | 30–70 | 35 | Peanut cake, rice bran or soybean cake | Start at 1.5 kg/day, build up to 5 kg/day |
The fish in rearing ponds are harvested with sieve nets when they reach 4–6 cm. Periodical harvesting may be carried out to avoid overcrowding. Often, rearing postlarvae to fingerlings is undertaken in larger, earthen ponds and polyculture is practised. Stocking densities of fingerlings in polyculture range from 100/m2 to 2500/m2 with a mean of about 800/m2 (Table 16.6). Size at harvesting usually ranges from 7–20 cm, with grass and black carps tending to be the largest.
Table 16.6 Examples of stocking rates and size at harvesting of carp fingerling in polyculture (gc, grass carp; bc, black carp; sc, silver carp; bhc, bighead carp; cc, common carp; wb, Wuchang bream.
| Stocking density (×100/m2) | Size at harvesting (cm) | ||||||||||
| gc | Bc | sc | bhc | cc | wb | gc | bc | sc | bhc | cc | wb |
| 4–6 | – | 20–25 | – | – | – | 13–15 | – | 8–10 | – | – | – |
| 10–25 | – | 4–5 | – | – | – | 8–13 | – | 11–13 | – | – | – |
| 2–4 | – | – | 8–12 | – | – | 16–20 | – | – | 11–13 | – | – |
| 4–6 | – | – | 15–20 | – | – | 13–15 | – | – | 8–10 | – | – |
| 10–25 | – | – | 4–5 | – | – | 8–13 | – | – | 11–13 | – | – |
| – | 5–6 | 4–5 | – | – | – | 13–15 | 13 | – | – | – | |
| – | 5–6 | – | 4–5 | – | – | – | 13–15 | – | 11–13 | – | – |
| 1 | – | – | 4 | 5–6 | – | 13 | – | – | 13 | 8–10 | – |
| 1 | – | – | 0.08–0.1 | – | 15–20 | 16–20 | – | – | 0.25–0.5 kg | – | 7 |
16.5.2 Ongrowing to Market Size
Three distinctive features characterise culture practices commonly used for ongrowing major carps to market size (Ramakrishna et al., 2015; Wang et al., 2015):
- ponds are the most commonly used culture system;
- practices are predominantly semi‐intensive, relying on natural foods supplemented with some type of feed; and
- polyculture is almost always used.
Cage culture in reservoirs is also common, particularly in China. Carps are also grown as a component of ‘culture‐based fisheries’ which is essentially a form of stock enhancement. In culture‐based fisheries, reservoirs or lakes are fertilised and stocked with various fish or crustaceans which utilise natural productivity for growth. Major carps are important contributors to culture‐based fisheries (section 16.8) wherever it is practised.
In the past, carp culture was often integrated with other poultry, duck, or swine farming wherein waste from animal production were used to fertilise fish ponds. Integrated farming, despite its appeal as an extraordinarily efficient farming system, is rapidly disappearing (Edwards, 2015) due to the emphasis on maximising profits from the aquaculture component as well as public health and quality‐control concerns. Indeed, a recent governmental decree in China—the global leader in aquaculture production and carp culture—bans integrated fish‐livestock farming.
Polyculture is thought to have originated in China, when various combinations of species with widely different food habits were cultured together, e.g., black carp (feed on snails), grass carp (feed on coarse vegetable matter), silver carp (feed on phytoplankton), bighead carp (feed on zooplankton and omnivorous) and mud carp (a bottom scavenger). A typical species combination used in a polyculture practice with an approximate indication of the niches occupied by each of the species is shown in Chapter 2 (Figure 2.4). The number of species used, and the ratio of each species, varies from region to region. Polyculture, apart from ensuring that most of the food resources in the system are efficiently utilised, offers other advantages, including higher yields, reduced incidence of infectious diseases and better growth rates of some species than in monoculture. Polyculture maximises the synergistic fish–fish and fish–environment relationships and minimises antagonistic relationships.
Despite various experimental findings in both China and India, farming activities tend to depend on the indigenous species of each country. This trend is primarily influenced by the preferred consumer acceptance of indigenous species. In China and India, where carp culture is the predominant form of fish culture, two or three species of either Chinese or Indian carps are polycultured. In these polyculture systems, the dominant species in China is silver carp, and in India it is rohu. The actual culture practices vary from region to region and country to country. The primary variables are the size at stocking, stocking density, fertilisation regimes, and the nature and quantity of supplementary feeds. In Chinese systems, for example, fingerlings are generally stocked at a size of about 15–20 g (>10–12 cm total length). In Andhra Pradesh, India, rohu are stocked in grow‐out ponds when they are more than 2 years old (between 80 and 100 g), as it is believed that this is when they approach their maximum growth rate.
Since about 1990 farmers and scientists in the main carp‐producing countries have developed country‐ or region‐specific culture protocols. This is best exemplified in Andhra Pradesh, a coastal state in south‐east India. In this state, many different combinations of fish have been used in polyculture but currently the most common approach is to co‐culture only two species of Indian major carps, catla and rohu (rohu being the dominant species at about 80% of production). Ponds often exceed 1 ha and are generally stocked at a density of 5000 fish/ha with 6‐ to 12‐month‐old (100–150 g) juveniles. Ponds are fertilised with poultry manure and inorganic fertiliser, and are provided with supplementary feed, often consisting of simple mixtures of rice bran (de‐oiled) and oilcake (mustard, peanut). The feed mixture is suspended in perforated polythene bags from bamboo poles at a number of locations in the ponds (20–25 poles/ha), from which the fish soon learn to feed. In this region, production averages about 8000 kg/ha with a range of 5300–14 620 kg/ha. Fish are harvested when they are over 1.5 kg.
Another, less common, approach to carp culture in India uses both Chinese and Indian carps. This concept was termed ‘composite fish culture.’ The basic species combination in Indian composite polyculture are catla, rohu, mrigal, silver carp, bighead carp and common carp. When stocked at a density of 5000/ha (120–250 kg/ha) the annual yield was nearly 9 t/ha when ponds are fertilised, and fish are provided simple supplemental feed, such as a mixture of rice bran and oilcake.
In China, polyculture is practiced with Chinese carps in conjunction with common carp. There are also significant differences in regional culture practices within China. The most important difference is the dominant species in polyculture systems. For example, grass carp is the main species used in southern China, whereas silver and bighead carp dominate in central China. There has been a trend towards increasing the proportion of grazing fish—such as grass carp, black carp and Wuchang bream and a corresponding decrease in filter‐feeding fish such as bighead and silver carp. This change in culture emphasis is attributable to changing food preferences, with silver and bighead carp becoming less popular, especially with the growing Chinese urban population. There is also an increasing tendency to use Chinese major carps in low stocking numbers in conjunction with the culture of species of high economic value, such as crayfish (Procambrus clarkii) and mitten crab Eriocheir sinensis (Wang et al., 2015). These practices are relatively new and also provide improved water quality and subsidiary income.
The wide range of culture practices adopted in carp culture, within and between regions, makes it almost impossible to assess the potential yield from any one practice. A summary of practices and production from a survey of 348 fish farms in Jiangsu Province is presented in Table 16.7. Farms in the survey were classified as belonging to one of four models based on the primary and secondary species in the system. Production of common carp in one system ranged between 14 250–17 475 kg/ha, and the total yield of all species combined was 18 000–18 750 kg/ha—a very high production for a pond system relying mostly on primary production to support fish production. A detailed study on carp farming systems in Andhra Pradesh, India, was conducted by Ramakrishna et al. (2013) and reported average annual production from several production systems used in the region. As described above, most ponds were stocked with rohu and catla and operated semi‐intensively with supplemental feeding. Average annual production was about 6000–10 000 kg/ha.
Table 16.7 Data on stocking and harvesting of four different polyculture farming models based on primary and other species used in each of the practices in Jiangsu Province.
Source: Data are from the unpublished 2006 Master’s Degree dissertation ‘Pond Culture Technology Investigation and Study in North Jiangsu’ by Yongguang Luo, Nanjing Agriculture University). Models 1–4 Reproduced with permission from Pr Y. Luo, Nanjing Agriculture University.
| Stocking | Harvest | |||
| Species | Size (g/ind.) | Density (ind./ha) | Size (kg/ind.) | Yield (kg/ha) |
| Model 1 | ||||
| Crucian carp | 50 | 22 500–30 000 | 0.35–0.45 | 7500–12 250 |
| Silver carp | 50–100 | 2250 | 1–1.5 | 2025–2625 |
| Bighead carp | 100–167 | 750 | 1.5–2 | 1225–1350 |
| Wuchang bream | 50 | 1500 | 0.45 | 600–650 |
| Total | 12 250–18 000 | |||
| Model 2 | ||||
| Grass carp | 167–250 | 3750 | 2.5 | 7500–8250 |
| Silver carp | 50–100 | 2250 | 1–1.5 | 4050–5250 |
| Bighead carp | 100–167 | 750 | 1.5–2 | 2250–2700 |
| Crucian carp | 50 | 4500 | 0.33 | 2250–3000 |
| Total | 11 625–13 500 | |||
| Model 3 | ||||
| Common carp | 100–125 | 18 000–22 500 | 0.9 | 14 250–17 475 |
| Silver carp | 50–100 | 2250 | 1–1.5 | 2025–2625 |
| Bighead carp | 100–167 | 750 | 1.5–2 | 1125–1350 |
| Grass carp | 250 | 300 | 3 | 625–750 |
| Total | 18 000–18 750 | |||
| Model 4 | ||||
| Wuchang bream | 50 | 18 000 | 0.6–0.75 | 9750–12 000 |
| Silver carp | 50–100 | 2250 | 1–1.5 | 2025–2625 |
| Bighead carp | 100–167 | 750 | 1.5–2 | 1125–1350 |
| Crucian carp | 50 | 3000–4500 | 0.35 | 900–1500 |
| Total | 14 1 250–17 250 | |||
Not all carp are grown in extensive or semi‐intensive pond polycultures and exceptions to this paradigm are becoming more common. Common carp and grass carp, for example, are often intensively cultured in ponds and cages in China, as is the case of rohu in Myanmar. A typical carp farm in China can be rather large and ponds often have supplemental aeration using paddlewheel aerators (Figure 16.16). In some areas, cage culture of carp is relatively common. One example is in West Java, Indonesia, where common carp are grown intensively in cages in three reservoirs. The cage systems used in Indonesia—and being adopted elsewhere— are interesting. They are locally referred to as ‘apis dua’ and are multi‐layered systems consisting of an inner cage with the primary (fed) species surrounded by a much larger cage with one or more secondary species that feed on feed wastes produced by the primary species or natural foods swept through the cage by currents (Wang et al., 2015). For example, a possible combination might be common carp fed pelleted feed in the inner cage and Nile tilapia in the outer cage.

Figure 16.16 A typical farm for carps in Hubei Province, China. Note the grass planted on the banks of each pond, which is used for feeding grass carp.
Source: Reproduced with permission from Sena De Silva, 2017.
16.5.3 Food and Feeding
16.5.3.1 Natural Food Availability
In view of the fact that the great bulk of carp culture is semi‐intensive, increasing the availability of natural food types in the culture systems plays a crucial role in enhancing yields. As pointed out earlier, the most common method used for increasing natural food supply in carp ponds is through the application of inorganic fertilisers and/or organic manures. The commonly used organic manures include cow dung, poultry litter and pig dung, and the inorganic fertilisers are superphosphate and ammonium sulphate.
There have been many studies conducted on the effects of fertilisation and manuring in carp polyculture practices. However, apart from the fact that such practices result in increased algal production, it is impossible and impractical to make a set of general conclusions from the findings. This is for a number of reasons, the foremost among these being:
- many different fertilisation and/or manuring regimes are used;
- many different stocking densities and species combinations are used; and
- responses to fertilisers and other culture practices vary with climate, water source, soil type and other physicochemical factors.
Pond fertilisation is discussed in more detail in Chapter 4 (section 4.4.2) and Chapter 9 (section 9.9) and a summary of the principles of pond fertilisation, including carp ponds, is presented by Mischke (2012).
16.5.3.2 Supplementary feeds
The supplementary feeds used in carp culture are diverse. Most supplementary feeds are simple mixes of agricultural by‐products, which are readily available at a relatively low cost. The most common of these are brans of rice and wheat, often mixed with cakes or meals of various oilseeds such as mustard, canola and soybean. Most farmers tend to use some sort of supplementary feed, which could be either a single ingredient or a mixture of two or three, at most. The quantity of feed as well as the amount of individual ingredients used in the feed mixes could vary greatly. Obviously, this is an area that needs further research, which in the long run could reduce feed use and thereby increase profitability. In addition, it could also lead to improved water quality in the ponds and cleaner pond effluent. This trend is indicative of a potential constraint to expansion of culture activities due to increasing competing demands for the same food ingredients from other animal husbandry activities and from other users.
In Chinese polyculture, a wide range of ingredient mixes is also used as feed—the type and quantity often being dictated by availability and price. Soybean meal, sesame cake, silkworm pupae powder and canola meal are more commonly used in major carp farming systems in China.
As mentioned above, the use of manufactured pelleted feeds has become common in many types of carp aquaculture. For example, Chiu et al. (2013) surveyed 351 farms in three provinces of China (Hainan, Shandong, and Zhejiang) to evaluate production practices and feed use. Most farms engaged in some form of carp polyculture and more than 95% of the farms used commercial, manufactured feeds to some degree.
16.5.3.3 Feeding
In general, feed management is relatively poor in carp culture. The main reason for this is that practices often depend on supplementary feeds, which are simple mixes of agricultural by‐products. Perhaps the only exception is the feeding practice adopted in common carp farming in eastern Europe and in some parts of Asia. A wide range of feeding practices are used by carp farmers, from simple hand broadcasting to tying perforated bags containing food to sticks in the pond and allowing the stock to obtain feed through the perforations. It is almost impossible to assess the food lost in the latter practice, and indeed it is possible that the food has a greater effect as a fertiliser than providing direct nutrition to the fish. All of the above aspects, particularly in relation to Indian major carp culture systems are described by Ramakrishna et al. (2013).
Mixed feeding schedules, i.e., use of different feeds at different feeding times, have been found to have beneficial effects on growth and food cost reduction, and a reduced discharge of nitrogen and phosphorous, without compromising growth performance. This concept was originally developed for Nile tilapia but has been found to be effective for carps also. However, and rather unfortunately, the experimental findings have not been extended to on‐farm practices, and as such missed the opportunity to reduce feed costs and nutrient levels in the effluent.
16.5.4 Harvesting
Pond‐cultured carps are harvested when individual fish reach a weight of about 1–2 kg unless they are destined for a specialised market. Harvesting in most instances is done by seining and is labour intensive. Major carp culture ponds are rarely flow‐through and are rarely completely drainable. These factors together with the large size of ponds make harvesting by seining almost an imperative. Major carps are generally marketed fresh. It is not uncommon to retain portions of the catch, live, in temporary net pens, to minimise market saturation within a short period of time. This practice is followed in most rural areas when the distances to population centres are high and the total production in an area does not justify transportation to such centres.
16.6 Diseases
Carps, like most fish, are susceptible to a variety of infectious (viral, bacterial, fungal and parasitic) as well as environmentally induced disorders caused by poor water quality (Chapter 4). A somewhat dated, although still useful, comprehensive monograph on carp diseases, diagnostic procedures and treatments is provided by Hoole et al. (2001) and readers are recommended to refer to this rather extensive work.
16.7 Genetic Improvement
As described in Chapter 7, apart from the genetic improvement of salmonid stocks and, more recently, Nile tilapia and channel catfish, genetic improvement of cultured fish and shellfish species has lagged far behind that of farmed terrestrial animals. There is a gradual change however, with an increasing emphasis on the genetic improvement of cultured groups in Asia (Nguyen, 2016).
Some of the cyprinid species, in particular common carp and crucian carp, have been domesticated for centuries. Domestication and consequent selection have resulted in the development of a number of strains, generally selected for aesthetic purposes rather than for production characteristics useful in aquaculture, such as growth rate, meat yield and tolerance to diseases and environmental stressors. Examples of this are discussed in section 7.2.1 in relation to selection for scale type in common carp.
In view of the importance of common carp to Chinese aquaculture there had been a concerted effort to produce strains that possess improved traits that are important in aquaculture (Penman et al., 2005). In China, a number of varieties of common carp have been identified and used in breeding programs. For example, the Xingua red carp C. carpio singuonenis is a common carp variety used in culture for hundreds of years. Beginning in the 1970s, a program of mass selection has increased growth rate by about 10% per generation and the improved strain has been used as the parent strain for several other important varieties. Similarly, a cold‐resistant strain of Chinese Purse red carp was produced by hybridising Heilongjiang common carp and Purse red carp C. carpio wuyuanensis. Systematic selective breeding of F1 sibs produced a variety that retained the cold resistance of the Heilongjiang carp but also tolerance to hypoxia and desirable red colour of the Purse carp. An important intraspecific hybrid—the Jian carp, C. carpio var. jian) was produced in a complex program of family selection. The Jian carp grows about 50% faster than either of the two parental strains and is perhaps the single most widely‐used common carp variety in Chinese commercial aquaculture.
Genetic resources of Asian and Indian carps are reviewed in depth by Penman et al. (2005). This synthesis included genetic resources and improvement programs in India, China, Bangladesh, Thailand and Vietnam and summarised the status of genetics research up to the time of publication. Nguyen (2016) more recently reviewed the status of carp genetic research. Since about 2000, there has been considerable progress in certain areas of genetics research. Selective breeding programs in China have produced superior strains of common carp and breeding programs for other major carps exist in India and other countries where carp aquaculture is important. Differences in production traits clearly exist among strains of the same species and genetic improvement programs are usually based on strain comparisons in field studies and mass selection to improve performance of superior strains. For example, common carp strains selected for improved performance are widely used in China. There has also been interest in producing interspecific hybrids to produce populations with heterosis for important production traits or to produce sterile populations. More than 100 different hybrids have been made in China among common carp, Crucian carp and various Chinese major carps. Likewise, more than 40 hybrids have been produced in India among common carp, Chinese major carps and Indian major carps. Promising hybrids have, for example, been produced between rohu and catla that have superior meat quality and faster growth in culture.
Despite considerable recent research activity in genetic improvement and demonstration that considerable gains can be made by using improved germplasm or hybrids, there has not been widespread adoption in the commercial sector. Penman et al. (2005) and Nguyen (2016) both conclude that increased efforts to transfer technology to the commercial sector need to be a priority for aquaculture development in carp‐producing countries.
16.8 Culture‐Based Fisheries
Culture‐based fisheries are, in essence, a form of stock enhancement where fish are added to a water body that is incapable, for one reason or another, of sustaining a fishery. For example, a water body may not have suitable spawning areas and regular stocking is required to replenish populations. In the traditional view of stock enhancement, the resulting fishery is commonly accessible. What distinguishes culture‐based fisheries from traditional stock enhancement is individual or collective ownership and management of the fishery. As such, culture‐based fisheries can easily be defined as a type of aquaculture.
Culture‐based fisheries are usually established in relatively small water bodies although larger lakes and reservoirs partly or completely managed as culture‐based fisheries are increasingly common. In most cases culture practices are limited to stocking of juvenile fish that use the natural productivity of the waterbody for growth. Occasionally, however, productivity is enhanced by fertilisation. Culture‐based fisheries use existing water resources and therefore do not compete for land and water with other uses. Culture‐based fisheries are also ecologically efficient because they use water non‐consumptively and have minimal input of resources, such as feed.
Culture‐based fisheries are considered to have very high potential to contribute to food resources, particularly in the light of increasing demand for primary resources such as land and water. They are often recognised as an important avenue for increasing inland fish production, particularly in developing countries (De Silva, 2016).
Apart from all of the above, culture‐based fisheries have significant relevance for carp culture, because the majority of such fisheries are based on Chinese and Indian major carps, occasionally augmented with tilapia and other minor species. The variety of feeding niches offered by the various carps, and their generally low trophic level, provides opportunities for efficient use of natural food produced in ponds and lakes. Also, the fact that the Chinese and Indian major carps need flowing water to reproduce offers an advantage in some situations; that is, the absence of natural reproduction means that populations can be carefully managed for optimal fish production relative to the waterbody’s potential productivity.
Culture‐based fisheries in China are the most developed in the world. This fishery is confined to small‐ and medium‐sized reservoirs throughout the country. Estimated production from culture‐based fisheries in China have increased from about 100 000 t in 1981 to more than 3.3 million t in 2012 (Wang et al., 2015). Annual productivity is estimated at about 1800 kg/ha, which is amazing for extensive systems that rely only on seed addition (De Silva, 2016).
Culture‐based fishery practices in China are based primarily on stocking grass carp, bighead carp, silver carp and common carp. In addition, species such as the Wuchang bream, black bream (Megalobrama terminalis) and mud carp may be used. In southern Asia, culture‐based fisheries are primarily based on a combination of Chinese and Indian carps, the latter being predominant. Success of the culture‐based fishery practices in China are based on the following:
- Consideration (at the planning stage of reservoir construction) of those factors that enhance fishery production;
- Relatively large and uniform size of fish at stocking;
- Minimising the number of escapees;
- A staggered but complete harvesting of the stock; and
- Adopting marketing strategies that minimise an oversupply of fish within a narrow time‐frame.
Wang et al. (2015) and De Silva (2016) review developments in culture‐based fisheries in China and elsewhere and point out the changes in culture‐based fisheries practices following restrictions of using fertiliser in open water bodies in China. These changes include, for example, a shift to higher‐valued species such as Chinese mitten crab and Mandarin fish Siniperca chuatsi.
16.9 Conclusions
This chapter highlights the importance of carp culture, some of the key features of carp culture practices, and the potential of carp culture as a food source. Living standards are generally increasing throughout the world, and it is often suggested that the demand for carp will decline as a result. However, production trends do not support that contention. To the contrary, in light of increasing environmental concerns related to the culture of carnivorous fish species, it may be that carp culture could become even more important in the future. In this regard China, the main producer of cultured carp species, continues to improve the carp culture techniques, including reducing the environmental impact of production (such as nutrient pollution associated with aquaculture facility effluents).
One of the major constraints to further intensification of carp culture is increasing competition for supplementary food sources, which are primarily agricultural by‐products. As such, a concerted effort may be required to develop suitable feeds and to develop more prudent strategies of feed management. Apart from yield increases through intensification and better pond culture practices, popularisation and development of culture‐based fisheries using carp species appears to have the greatest potential to augment inland fish production. This is particularly important to developing countries, thereby making available a good‐quality source of animal protein, at an affordable price, to the poorer sectors of the community. It is also important to note that selective breeding for faster growth, disease resistance and cold‐ and warm‐tolerance of selected carp species is proceeding at many research centres in China, India and elsewhere. It is possible that such strains will become available to farmers in the not too distant future and will help maintain economic viability of carp farming. It certainly will be hard to replace the role of carps as a group of fish possessing so many desirable attributes for farming.
16.10 Summary
- Carps (family Cyprinidae) are the most commonly cultured fish in the world. Total carp production in 2014 was almost 28 million t, accounting for 65% of all inland finfish aquaculture, 56% of total finfish aquaculture, and almost 40% of total animal aquaculture production. Carps are grown throughout the world, but China and India dominate cyprinid aquaculture production.
- Many carp species have traits that make them good candidates for aquaculture but six species (grass carp, silver carp, common carp, bighead carp, catla and rohu), each with an annual production of more than 1.5 million t in 2014, constitute 91% of total carp aquaculture. Three additional species—Wuchang bream, black carp and mrigal—represents an additional 6% of total carp aquaculture. As such, 97% of carp aquaculture is represented by nine species.
- Although the important carps in aquaculture are very fecund, they do not spawn in captivity without using hormones to induce spawning.
- Feeding habits of the major carp species vary considerably, including opportunistic bottom‐feeding omnivores (common carp and mrigal), opportunistic water‐column planktivores (rohu and catla), water‐column filter feeders (bighead and silver carp), aquatic plant herbivores (grass carp) and molluscivores (black carp). The variety of feeding habits provides opportunities for polyculture where fish with complimentary feeding habits are co‐cultured together to make efficient use of the wide variety of foods available in aquatic habitats.
- Most carp aquaculture is conducted in ponds using various combinations of fish in polyculture. Typically, there is a primary species (for example, common, Crucian, or grass carp) that is fed a supplemental diet of agricultural by‐products or pelleted manufactured feed. Feeding waste stimulates production of natural foods that support growth of secondary species (for example, the filter‐feeding bighead or silver carp). Increasingly, however, carp aquaculture relies on simpler intensive systems, such as monocultures, based on pelleted commercial feeds.
- Carps are also cultured in cages and pens in lakes and rivers and in culture‐based fisheries. Culture‐based fisheries are a form of stock enhancement where water bodies are periodically stocked with juvenile fish which grow by using the natural productivity of the water. The fishery is owned and managed individually or collectively.
References
- Billard, R. (1999). Carp: Biology and Culture. Springer‐Praxis, Chicester.
- Chiu A., Li L., Guo S., et al. (2013). Feed and fishmeal use in the production of carp and tilapia in China. Aquaculture, 414, 127–134.
- Cudmore, B. and Mandrak, N. E. (2004). Biological synopsis of grass carp (Ctenopharygodon idella). Canadian Manuscript Report of Fisheries and Aquatic Sciences 2705, Fisheries and Oceans Canada, Burlington, Ontario. http://www.dfo‐mpo.gc.ca/Library/286222.pdf [accessed March, 2017]
- De Silva, S. S. (2016). Culture‐based fisheries in Asia are a strategy to augment food security. Food Security, 8, 585–596.
- Dey, M. M., Rab, M. A., Paraguas, F. J. et al. (2005). Fish consumption and food security: a disaggregated analysis by types of fish and classes of consumers in selected Asian countries. Aquaculture Economics and Management, 9, 89–111.
- Edwards, P. (2015). Aquaculture environment interactions: past, present and future likely trends. Aquaculture, 47, 2–14.
- Hoole, D., Bucke, D., Burgess, P. et al. (2001). Diseases of Carp and Other Cyprinid Fishes. Fishing News Books, Oxford.
- Ingram, B., Sungan, S., Gooley, G. et al. (2005). Induced spawning, larval development and rearing of two indigenous Malaysian mahseer, Tor tambroides and T. douronensis. Aquaculture Research, 36, 1001–1014.
- Jhingran, V. G. and Pullin, R. S. V. (1985). A Hatchery Manual for the Common, Chinese and Indian Major Carps. ICLARM Studies and Reviews. No. 11. Asian Development Bank and International Centre for Living Aquatic Resources Management, Manila. https://ideas.repec.org/b/wfi/wfbook/2861.html [Accessed March, 2017].
- Kolar, C. S., Chapman, D. C., Courtenay, W. R. et al. (2005). Asian Carps of the Genus Hypophthalmichthys (Pisces, Cyprinidae) – A Biological Synopsis and Environmental Risk Assessment. United States Fish and Wildlife Service, Washington DC. [http://digitalcommons.unl.edu/natlinvasive/5/] [Accessed December, 2016].
- Mischke, C. C. (Ed). (2012). Aquaculture Pond Fertilisation: Impacts of Nutrient Input on Production. Wiley Blackwell, Ames.
- National Research Council. (2011). Nutrient Requirements of Fish and Shrimp. National Academies Press, Washington DC.
- Nguyen N.H. (2016). Genetic improvement for important farmed aquaculture species with special reference to carp, tilapia and prawns in Asia: achievements, lessons and challenges. Fish and Fisheries, 17: 483–506.
- Penman, D. J., Gupta, M.V. and Dey M.M. (2005). Carp Genetic Resources for Aquaculture in Asia. WorldFish Center, Penang, Indonesia. http://www.worldfishcenter.org/content/carp‐genetic‐resources‐aquaculture‐asia [accessed March, 2017].
- Ramakrishna, R., Shipton, T. A. and Hasan, M. R. (2013). Feeding and Feed Management of Indian Major Carps in Andra Pradesh, India. FAO Fisheries and Aquaculture Technical Paper 578. Food and Agricultural Organization of the United Nations, Rome. http://www.fao.org/docrep/019/i3146e/i3146e00.htm [accessed March, 2017].
- Ravi, J. and Devaraj, K. V. (1991). Quantitative essential amino acid requirements for growth of catla, Catla catla (Hamilton). Aquaculture, 96, 281–291.
- Wang, Q., Cheng, L., Liu, J. et al. (2015). Freshwater aquaculture in PR China: trends and prospects. Reviews in Aquaculture, 7, 283–302.
- Webster, C. D. and Lim, C. (2002). Nutrient Requirements and Feeding of Finfish for Aquaculture. CABI Publishing, Wallingford.