8
Nutrition and Feeds
Lou D’Abramo
8.1 Introduction
The predicted demand for animal protein in 2050 derived from an estimated global population of 9.1 billion represents 470 million tonnes (t) of animal meat production, an increase of 200 million t from that in 2009 (FAO, 2009). A large proportion of the animal protein demand will emanate from developing countries, stimulated by relocation to cities and a corresponding increase in per capita income. Meeting this surge in demand in meat consumption will probably fall to increases in cultured freshwater and marine seafood production (aquaculture) as capture fishery landings have essentially remained unchanged for more than 20 years (Figure 1.5). During the period 1994‐2004, the annual increase of aquaculture animal production exceeded that of terrestrial animal food production by 5% (Duarte et al., 2009). Over the past fifty years, growth in the supply of fish has exceeded world population growth and continues to gain importance as a source of protein and other essential nutrients (FAO, 2014). Aquaculture continues to make an ever‐increasing proportional contribution to meeting animal protein demand, having achieved over 50% of the total (by volume) of aquatic food products. Due to limited land and freshwater resources, the greater increases in production may be realised through the marine environment (only a small percentage of possible areas is being used) whereby aquaculture will continue to be a noteworthy contributor to meeting the consumptive global demand for animal protein. Within the context of the future value and need for both intensive and resourceful animal agricultural production systems (NRC, 2015), aquaculture is poised to offer the most efficient source of sustainable management practices, as defined by environmental, economic and sociological components.
A principal component of meeting the environmental and economic sustainability criteria for freshwater, estuarine, and marine aquaculture is knowledge of nutrient requirements combined with effective production and management of feeds and feeding practices. In the majority of aquaculture enterprises, the cost of feed is the primary operational (variable) cost. Hence, knowledge of the nutrition of the farmed organisms, including nutrient requirements, digestibility of feed ingredients, and when and how much to feed is critical to success. As production systems continue to invoke sustainable management practices, knowledge and understanding of the nutritional requirements and nutritional physiology of farmed aquatic species are critical to producing environmentally and economically sustainable feeds, which are either nutritionally complete or supplemental, depending on the culture system. Recognising this need, the National Research Council (NRC) published specific research recommendations and complementary research priorities in nutrition to meet required levels of animal protein production and global food security brought on by increases in global population (NRC, 2015). This source of guidance is based on the presumption that freshwater, marine and estuarine production systems will need to become more intensive, and, in most cases, requiring nutritionally complete feeds.
The level of knowledge of the nutrition of aquatic animals is considerably lower than that of terrestrial species such as beef cattle, swine, or poultry. However, during the past three decades, knowledge of the nutrition of fish and crustacean species has notably increased as evidenced by the number of research publications and scientific journals that specifically address or highlight aquatic animal production. A large portion of the knowledge of aquatic animal nutrition is primarily founded in species of fish, but information about crustaceans, molluscs, and echinoderms is gradually increasing. Over the past 50 years, two notable attempts toward synthesis of the existing knowledge of nutrient requirements of fish and shrimp have been published (NRC, 1993, 2011). Two other publications have provided a compendium of knowledge about nutrient requirements, feed formulation, and feeding of fish (Webster and Lim, 2002) and crustaceans (D’Abramo et al., 1997).
8.2 Energy Consumption and Partitioning (Bioenergetics)
The allocation of energy moving through an organism (Figure 8.1) is often described through a bioenergetics diagram that shows the flow of energy as losses with some ultimately remaining for the allocation of growth. In an aquaculture enterprise, achieving the lowest cost per unit of protein produced is accomplished through the allocation of the greatest amount of consumptive energy to growth. Energy is not a nutrient but is essential for the performance of physiological function and for growth. The source of energy is the ingested food (IE) and the units of energy are expressed as joules (J) or traditionally as calories. Energy that is potentially available to an organism via the consumption of food is found in three macronutrients: protein, carbohydrates, and lipids which contain 23 kJ, 17 kJ and 38 kJ, respectively, /g ingested. These are termed physiological fuel values. After the food (energy) is consumed, some is lost in the form of faecal energy (FE), because organisms lack the ability to digest all of the macronutrients that are consumed. Some of the remaining energy, called metabolisable energy (ME), is used to perform three energy‐requiring physiological activities. These activities include:
- basal metabolism;
- heat increment (HE); and
- voluntary activity (VE).
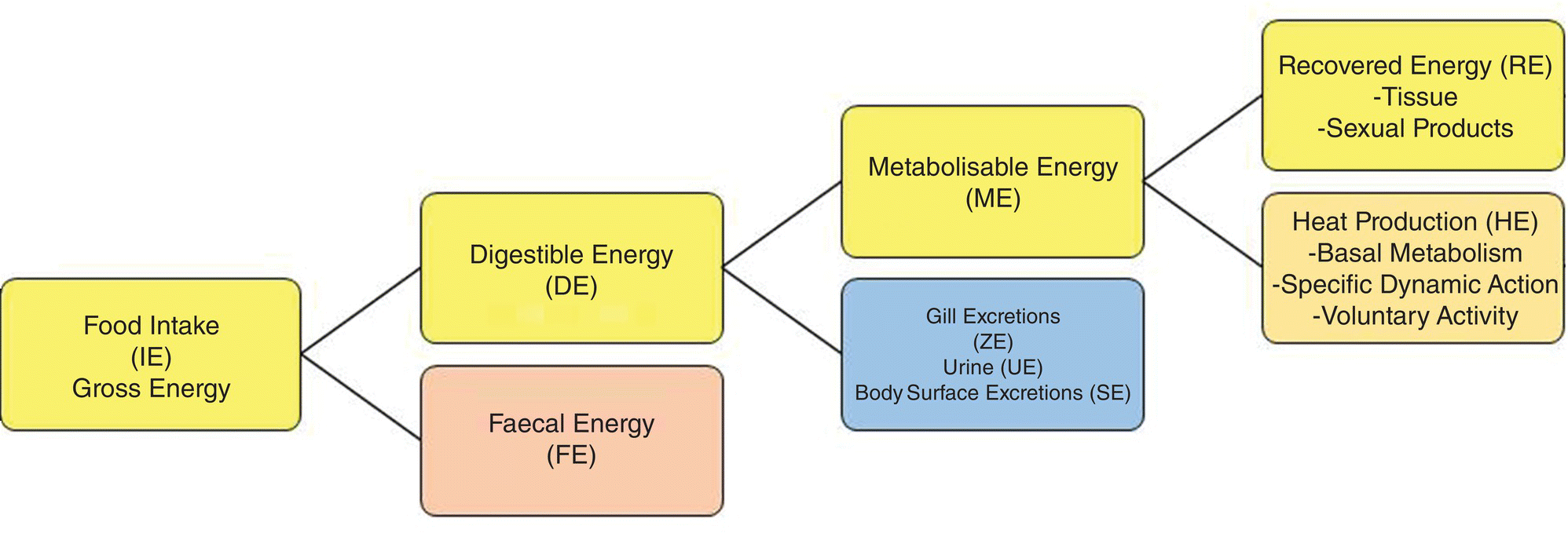
Figure 8.1 Bioenergetic diagram of the paths of the loss and utilization of energy derived from the consumption of dietary macronutrients.
Basal metabolism, calculated as basal metabolic rate (BMR), is the energy used for the maintenance of basic physiological activities. Heat increment (HE) is energy consumed in excess of BMR to fulfil needs for the digestion and molecular transformation of the macronutrients consumed. Specific dynamic action (SDA) is another term for HE that is used in diagrams of the partitioning of energy. Some of the energy consumed can ultimately be lost in the form of excretions from gills (ZE) the body surface (SE), such as the energy used to produce a protective coat of mucus, or urine (UE) that is produced. Any of the consumptive energy that remains can be used for voluntary activity such as swimming, water column migrations and predation.
Whatever amount of energy that remains after loss to voluntary activity is commonly termed recovered energy (RE) that is applied to the production of added tissue (growth) or the development of sexual organs and associated gametes. If the goal is growth, then, the RE would ideally be converted into tissue protein. Under this scenario, energy needs, other than those of RE would ideally be served through the metabolism of carbohydrates and lipids to serve as the sources of energy for BMR, SDA, and voluntary activity to maximise therefore, the channelling of protein energy to growth. This concept, based on the efficient use of protein consumed for growth rather than as an energy source, is often termed ‘protein sparing’. As an analogy, the ecological literature uses the term ‘ecological efficiency’ to define RE/IE, the amount of energy that is transferred from one trophic level to next higher trophic level divided by the amount of energy consumed × 100. Ecological efficiencies are generally around 10% but can range from 6% to 37%.
The total energy consumed by an aquatic organism per unit of time can vary based on its size, the energy content of the food and temperature of the water. The amount of this energy ultimately available for growth is, in turn, influenced by the partitioning of the energy consumed to meet the physiological needs of the organism as defined by many variables in the environment in which it lives. For example, at higher temperatures, BMR and SDA demands increase but rates of food (energy) consumption will correspondingly increase and sufficiently offset those needs. Hence, there is a temperature window for organisms wherein growth increases with temperature until metabolic needs do not leave any energy available for growth. Hence, optimal culture conditions for aquatic organisms are characterised by the most efficient use of energy coupled with the satisfaction of nutrient requirements to achieve ‘optimum’ growth under the existing culture conditions. The higher energy requirements for BMR and for the increase in feeding activity induced by an increase in environmental temperature will generally be offset by increases in IE. Oxygen is necessary for the oxidation and energy released from the macronutrients consumed (Figure 8.2). Thus, the aquatic organism’s ability to transform dietary macronutrients to energy is influenced by the availability of oxygen as influenced by the concentration in the water and the uptake ability of the aquatic organism.
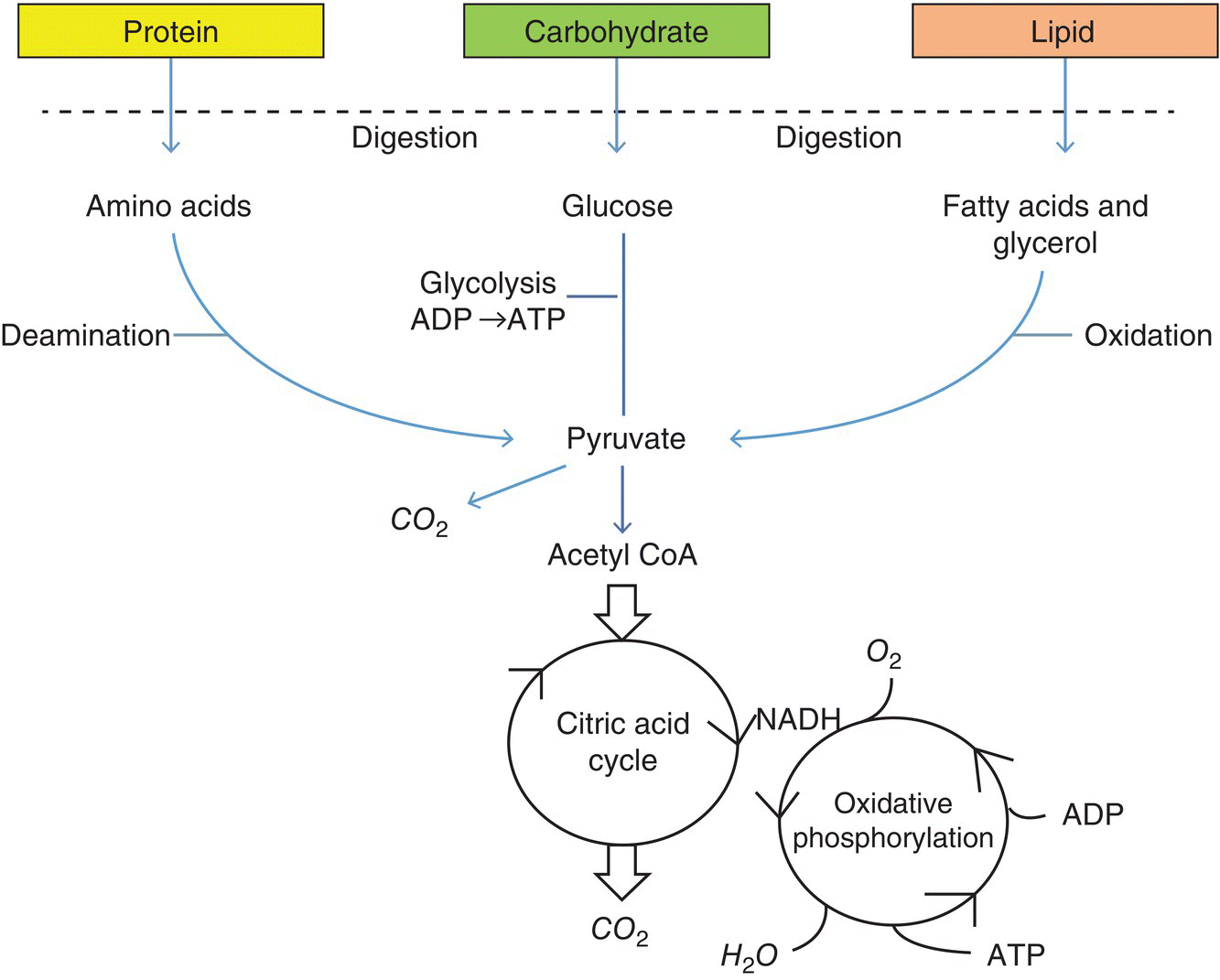
Figure 8.2 Paths to the production of energy derived from the digestion of protein, lipid and carbohydrate via the production of pyruvate from different processes.
8.3 The Relationship between Growth and Food Consumption
Three principal factors, temperature, organism size and ration influence the growth of fish. The ration, i.e., the amount of food consumed, is generally expressed as percent of body weight per unit of time and is the sole ‘driving force’. Therefore, growth is limited by any abiotic or biotic factor, or an array of these factors that limit the rate of consumption of food. Temperature is termed a rate‐controlling factor as the amount of food consumed generally increases with a concomitant increase in temperature. Weight, commonly related to size and/or age, is termed a ‘scaling’ factor because size, in association with temperature, influences the gross amount of food (energy) consumed. Growth/ration or G/R curves are often used to describe the relationship between growth and ration as influenced by both abiotic and biotic factors. Under an array of described conditions for culture and size/age of the organism, certain rations will result in different growth responses. Some rations will result in a negative growth rate (R0), no growth (Rmaint) when all energy derived from the ration is exclusively used to meet metabolic needs, optimal growth (Ropt), where the ratio, i.e., slope, of growth to ration is greatest, and maximum growth (Rmax) (Figure 8.3). Subtraction of Rmaint from Rmax yields what is termed the ‘scope for growth’ under the conditions provided for growth. The scope for growth is that amount of ration (energy) that can potentially be manifested in growth. The scope for growth varies according to level of ration and other factors such as temperature, age, aquaculture feeds, i.e., the quality of the feed being fed, salinity, light (photoperiod or intensity), oxygen concentration and uptake rates. The proportional division of the ration over more than one feeding per day and biomass density of the farmed organisms may also change the scope for growth. This array of physical, biological and behavioural factors influences both the amount of food consumed and the amount of energy required to address metabolic needs which determine the scope of growth that can be realised.
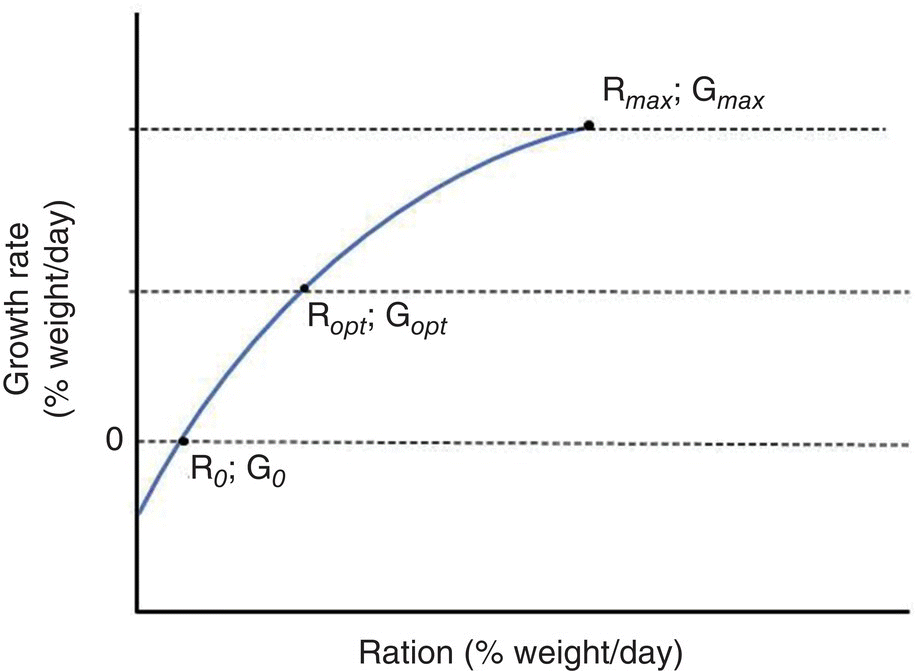
Figure 8.3 Typical growth‐ration curve illustrating no growth (G0), optimal growth (Gopt) and maximum growth (Gmax) and corresponding ration. Any ration that is below the zero‐growth line will result in a weight loss.
Thus, a major consideration for the feeding and nutrition of farmed aquatic organisms is not only the ration but also the composition (nutrient quality) of the feed. For example, insufficient protein levels, poor digestibility of the feed, etc., and a nutrient requirement being met marginally, can alter how much energy is ultimately available for growth. Most commercial farming is devoted to maximising the ‘scope for growth’, i.e., providing the ration that will maximise growth so that product turnaround is minimised. Efforts to feed a ration that will achieve optimal growth under existing conditions of culture, the level at which the feed will be used most efficiently, will not result in higher turnover to meet the desired market size. They will, however, reduce the cost per unit of product (protein) produced, an example of economic sustainability. Feed conversion efficiency (FCR) is a measure of the amount of feed fed divided by the amount of weight gain during a specified time of feeding, and is lowest when the feed efficiency, the inverse of FCR × 100, is highest. To measure the response to the efficacy of the feed and associated ration, both researchers in aquatic animal nutrition and farm managers often use FCR.
8.4 Requirements and Metabolic Functions of Nutrients
Knowledge of nutrient requirements for species of organisms that have become the focus of aquaculture enterprise is essential to achieving economic success. Most of the nutrient requirement knowledge is confined to only a small subset of fish and crustacean species. However, hundreds of species are farmed or have been the subject of evaluation as emerging species. For just marine species, 449 species have been the subject of some type of domestication strategy.
Research on nutrient requirements is commonly based on the use of purified diets, those that contain chemically defined ingredients. Response to graded levels of a nutrient under investigation, included by supplementing a control experimental diet, is commonly evaluated by growth, other physiological responses and possibly clinical manifestations as typically manifested in a dose‐response curve (Figure 8.4). This curve essentially represents an increasing proportion of the population responding to a dose (dietary level) as the dose increases. Therefore, each point generated on the curve is an observation for that proportion of the population that is responding to the dose. A number of methods estimating nutrient requirements, such as the broken‐stick regression and quadratic regression, have been proposed. The at times wide variability in reported requirements of nutrients is probably the result of shortcomings in experimental design or the choice of the model to estimate the requirement. The varied results arising from differences in approach were addressed by Shearer (2000) who provided a ‘set of protocols’ to achieve greater accuracy and therefore comparative value.

Figure 8.4 Typical dose‐response curve generated by measuring responses (weight gain, survival, enzyme activity, etc.) to graded levels of a dietary nutrient.
In contrast to fish, benthic organisms such as crustaceans, molluscs and echinoderms commonly do not consume experimental diets rapidly. Rather, these species tend to manipulate or break it up, given their characteristic feeding apparatuses. Thus, a difficulty is often encountered in arriving at a true estimate of the requirements of water‐soluble nutrients due to, in some cases, rapid loss caused by differential leaching (dissolution) into the water. As a result, requirements of water‐soluble nutrients such as some vitamins are often overestimates for these species. In contrast to terrestrial animals, nutrient requirements of aquaculture species are expressed as a percent dry weight of the diet, rather than as mg/g/day. Nutrient requirements can be expressed as g/kg of diet (macronutrients, such as protein, carbohydrate and lipid) or mg/kg diet (micronutrients such as vitamins and minerals). Knowledge of species‐specific nutrient requirements is ultimately applied to the formulation of practical diets consisting of feedstuffs that are readily available, highly digestible and collectively satisfy the requirements.
8.4.1 Protein
Although the literature contains descriptions of protein requirements of aquaculture organisms as a percentage of the diet, there is no true protein requirement. Rather, the protein requirement is actually based on the cumulative satisfaction of requirements for essential amino acids. There are 20 different amino acids (Table 8.1).
- Ten amino acids are termed essential or indispensable because animals cannot either synthesise them or synthesise them at a sufficient rate to meet metabolic demand, thereby invoking a dependence on dietary origins to meet the respective individual requirements of these amino acids.
- Another 10 dietary amino acids are termed non‐essential or dispensable because organisms have the ability to synthesise them, often from essential amino acids.
Table 8.1 Essential and non‐essential amino acids.
| Essential Amino Acids (EAAs) | Non‐essential Amino Acids (NEAAs) |
| Arginine (Arg) | Alanine (Ala) |
| Histidine (His) | Asparagine (Asn) |
| Isoleucine (Ile) | Aspartic Acid (Asp) |
| Leucine (Leu) | Cysteine (Cys) |
| Lysine (Lys) | Glutamic Acid (Glu) |
| Methionine (Met) | Glutamine (Gln) |
| Phenylalanine (Phe) | Glycine (Gly) |
| Threonine (Thr) | Proline (Pro) |
| Tryptophan (Trp) | Serine (Ser) |
| Valine (Val) | Tyrosine (Tyr) |
The requirement of an essential amino acid can be influenced by interactions with other essential amino acids, with non‐essential amino acids and even with non‐protein nutrients. For example, the possible dietary deficiency of a non‐essential amino acid such as cysteine can be compensated through its synthesis from methionine, an essential amino acid. Under these conditions, the methionine dietary requirement would be higher. A similar relationship exists between the essential amino acid phenylalanine and the non‐essential amino acid, tyrosine.
In the literature, taurine is often termed an amino acid, but is actually an organic acid, lacking the carboxyl group that is characteristic of all amino acids. Based on the results of several published research investigations, there is some evidence to suggest that taurine may be required by larval and early juvenile stages of fish and shrimp (NRC, 2011). For example, a dietary requirement of 1.09% taurine was found for juvenile yellow catfish, Pelteobagrus fulvidraco, fed a diet that contained only plant‐derived protein. Additional research is needed to determine whether differences in dietary taurine needs may be related to rate of synthesis from another amino acid, cysteine and/or to diets that contain protein that is derived exclusively from plant sources. These conditions may be further complicated by differential requirements as influenced by specific physiological needs.
Protein requirements, expressed as a percent of the diet, commonly range from 12–25% for mammals and birds in contrast to fish and crustaceans which reportedly require higher levels (35–55%). These data suggest that the efficiency of protein use, commonly termed Protein Efficiency Ratio (PER), which is expressed as the body weight increase per amount of protein consumed x 100, is seemingly much lower in mammals and birds than for aquatic organisms. However, these percent requirements that are used to formulate practical diets represent an absolute requirement. If a calculated relative requirement, expressed as protein intake (at maximum growth) per gram of body weight consumed per day, is calculated for an array of terrestrial and aquatic animal species, the median of values for each group of species (fish versus terrestrial vertebrate species) is very similar. The explanation for this similarity is that fish and other aquatic organisms are not consuming as much diet to satisfy the protein (array of essential amino acids) requirement as terrestrial vertebrates. The reduction in overall diet consumption is due to a lower need for energy which primarily originates from the dietary lipid and carbohydrate. Less dietary energy is needed to carry out a variety of physiological and behavioural activities. Aquatic aquaculture organisms are poikilotherms and energy is not needed to maintain body temperature. The principal excretory product from the breakdown of protein is ammonia which requires less energy to produce urea and uric acid (excretory products of terrestrial animals). The need for energy to perform locomotor activity is much less for aquatic organisms because of the natural buoyancy afforded by the aquatic environment. Hence, due to lower needs of dietary energy, less food needs to be consumed per body weight increase in aquaculture organisms, as illustrated by the feed conversion ratio (FCR) of fish being comparatively less than that for beef cattle, swine and poultry. Thus, aquaculture organisms are much more efficient in the conversion of diet consumed to protein deposition in the muscle where most of the growth is expressed. This efficiency of conversion is a defining aspect of highly sustainable farming, providing argument to focus on fish, crustacean, echinoderm and mollusc farming to meet future animal protein needs globally. Within a group of fish species, the existence of higher absolute protein requirements (% of total diet) and higher protein intake (relative requirement) is generally a characteristic of carnivores due to the need for protein to serve as a partial energy source. The carnivore’s comparatively low ability to digest dietary carbohydrate limits the efficient use of this macronutrient as a source of dietary energy.
For those amino acid requirement values that have been determined for certain species, it is clear that a good correlation exists between essential amino acid levels in the diet and those in the tissue where most of the dietary protein is deposited. This correlation has led to the Ideal Protein Concept, provision of the ideal dietary protein that would provide the correct proportional relationship (balance) of essential amino acids whereby protein in the diet would be used most efficiently. The high correlation of the proportional relationships among dietary and tissue essential amino acids can be used to help estimate the dietary requirements of other amino acids. If the requirement for lysine is determined and it is assigned a value of 100%, then the requirements for the other essential amino acids can be expressed as a percentage of lysine. Lysine is selected because it apparently has no other metabolic role than that of being included in protein deposition. In addition, lysine is generally recognised as the first or at least second limiting essential amino acid in practical feed formulations. Hence, formulating a feed based on satisfaction of the lysine requirement helps to ensure that required dietary levels of other essential amino acids will be provided. Unlike lysine, some essential amino acids are not used exclusively for protein deposition and have other metabolic roles. In these cases, the values obtained as a percent of lysine would be underestimates. A good example is the arginine requirement of crustaceans because arginine is part of the argino‐phosphate molecule associated with the contraction of crustacean musculature. When feeds are formulated to meet requirements of essential amino acids, digestibility of feedstuffs that contain essential amino acids is less than 100%. Therefore, data about digestibility will guide decisions as to how additional amounts to meet required levels will be provided in the diet. Despite all these considerations, the idea of an ideal protein concept based on the relationship of lysine to other essential amino acids in the tissue has practical merit toward the knowledge and selection of ingredients for the formulation of practical feeds.
8.4.2 Lipids
Lipids are water insoluble organic molecules that contain the highest amount of energy per gram among macronutrients. In addition to them being a source of energy, dietary lipids are used:
- as structural components of cellular membranes
- for transport as part of lipoprotein molecules
- to regulate metabolic processes as vitamin, hormone and prostaglandin molecules
- for reproduction.
Body lipids can also confer greater buoyancy and help to exclude pathogens by being part of a protective coating on the outer surface of many aquatic organisms.
There are two major classes of lipids, saponifiable (containing fatty acids) and non‐saponifiable (not containing fatty acids). Fatty acids are long chain carbon molecules that are composed of single or double bond carbon chains that have a carboxyl (COOH) end and a methyl (CH3) end. Due to the nature of the biosynthetic process, double bonds which confer the unsaturated nature to fatty acids are located between every other carbon atom. A certain nomenclature is used in the identification of fatty acids. For example, a 16:1n‐9 fatty acid is composed of 16 carbon atoms and one unsaturated bond that is located at the 9th carbon (n‐9) counting from the methyl end. Those fatty acids that have been determined as essential (EFAs) are composed of at least 18 carbon atoms and two unsaturated bonds. The most common essential fatty acids (common name in parenthesis) are either polyunsaturated fatty acids (PUFAs) such as 18:2n‐6 (linoleic acid) and 18:3n‐3 (linolenic acid), or long chain polyunsaturated fatty acids (LC‐PUFAs), also termed highly unsaturated fatty acids (HUFAs) such as 20:4n‐6 (arachidonic acid, ARA), 20:5n‐3 (eicosapentaenoic acid, EPA) and 22:6n‐3 (docosahexaenoic acid, DHA). Sources of the essential PUFAs and LC‐PUFA are confined to diet. Some aquaculture organisms can synthesise LC‐PUFAs from PUFAs, a metabolic ability that is confined to fatty acids that are common to either the n‐6 or n‐3 fatty acid family. LC‐PUFAs are very important as components of lipid molecules that compose part of the structure of cellular membranes, or are used in lipid transport, spawning, hatching, fertilisation and other physiological processes.
Triglyceride compounds, termed triacylglycerols (neutral lipids), are composed of a glycerol molecule with fatty acids attached to each of its three hydroxyl parts through an ester bond. Phospholipids (polar lipids) are of a similar structure but phosphate replaces one fatty acid, being similarly bound by an ester bond to one of the three hydroxyl parts of the molecule. Another organic molecule in turn is attached by an ester bond to the phosphate part which thereby confers a certain identity to the phospholipid molecule. For example, if the attached molecule is choline, ethanolamine or inositol, the respective phospholipid is called phosphatidylcholine, phosphatidylethanolamine and phosphatidylinositol. Phospholipids are important molecules composing the membrane of a cell and as part of lipoprotein molecules that are used in the circulatory transport of lipids.
Non‐saponifiable lipids are derived from isoprene and are termed isoprenoid lipids. These lipids include cholesterol, sex hormones such as testosterone and oestrogen, some vitamins (D, E, A and K) and pigments. Steroids such as cholesterol and sex hormones are cyclic compounds and pigments are isoprenoids that are responsible for the colour in aquatic organisms.
There is no true lipid requirement for an aquatic species as the dietary requirement can vary, in accordance with the meeting of a composite of several objectives, i.e., provision of sufficient lipid that will minimise the use of protein as an energy source while still meeting the dietary requirements of essential fatty acids (EFAs), polar lipids and some isoprenoids. Generally, in aquaculture the need to provide EFAs and other dietary lipids originates from an evolutionary loss of the inability to synthesise them at all or at a rate insufficient to meet requirements. The natural availability and comparatively high dietary levels of these compounds, by transfer up through the food chain, either directly or indirectly, is responsible for the loss of this biosynthetic ability. The greatest amount of dietary lipid naturally consumed by aquatic organisms is in the form of triglycerides that serve as a concentrated source of energy. Qualitative and quantitative fatty acid requirements derived from consumption of triglycerides or other lipid compounds vary based on trophic level/feeding habits, environmental temperature, and whether the aquatic organism lives in a freshwater, estuarine, or high salinity environment. An overview of fatty acid classes, nomenclature, metabolic use and biosynthetic pathways is shown in Figure 8.5. Based on published data of several species, some guidelines can emerge. For channel catfish (Ictalurus punctatus), a freshwater and warm water fish that is omnivorous, the requirement is 1% of PUFAs with either 18:3n‐3 (linolenic acid) or 18:2n‐6 being equally effective. However, at levels less than 1%, dietary LC‐PUFAs will produce the same response, indicating that LC‐PUFAs have a greater EFA activity than that of PUFAs. Rainbow trout have a 1 % dietary PUFA requirement, but in contrast to channel catfish, 18:6n‐2 provided at the same level does not exhibit an equal activity response, most probably attributable to its being a carnivorous, coldwater species. Generally, the dietary EFA requirement for marine species of fish and crustaceans is 0.5% to 2.0% of the diet contributed by 20:5n‐3 and 22:6n‐3, individually or in combination, with 20:4n‐6 sometimes manifesting equal activity. Juveniles require the higher 1% dietary level. In contrast, juveniles of the omnivorous freshwater prawn, Macrobrachium rosenbergii, require only 0.05% of dietary LC‐PUFAs to achieve significantly higher weight gain, and there is no difference in EFA activity relative to growth response among the 20:4n‐6, 20:5n‐3, and 22:6n‐3 fatty acids. This requirement of LC‐PUFAs from either the n‐3 or n‐6 family probably remains because of its omnivorous natural diet and phylogenetic ancestors that lived exclusively in saltwater.
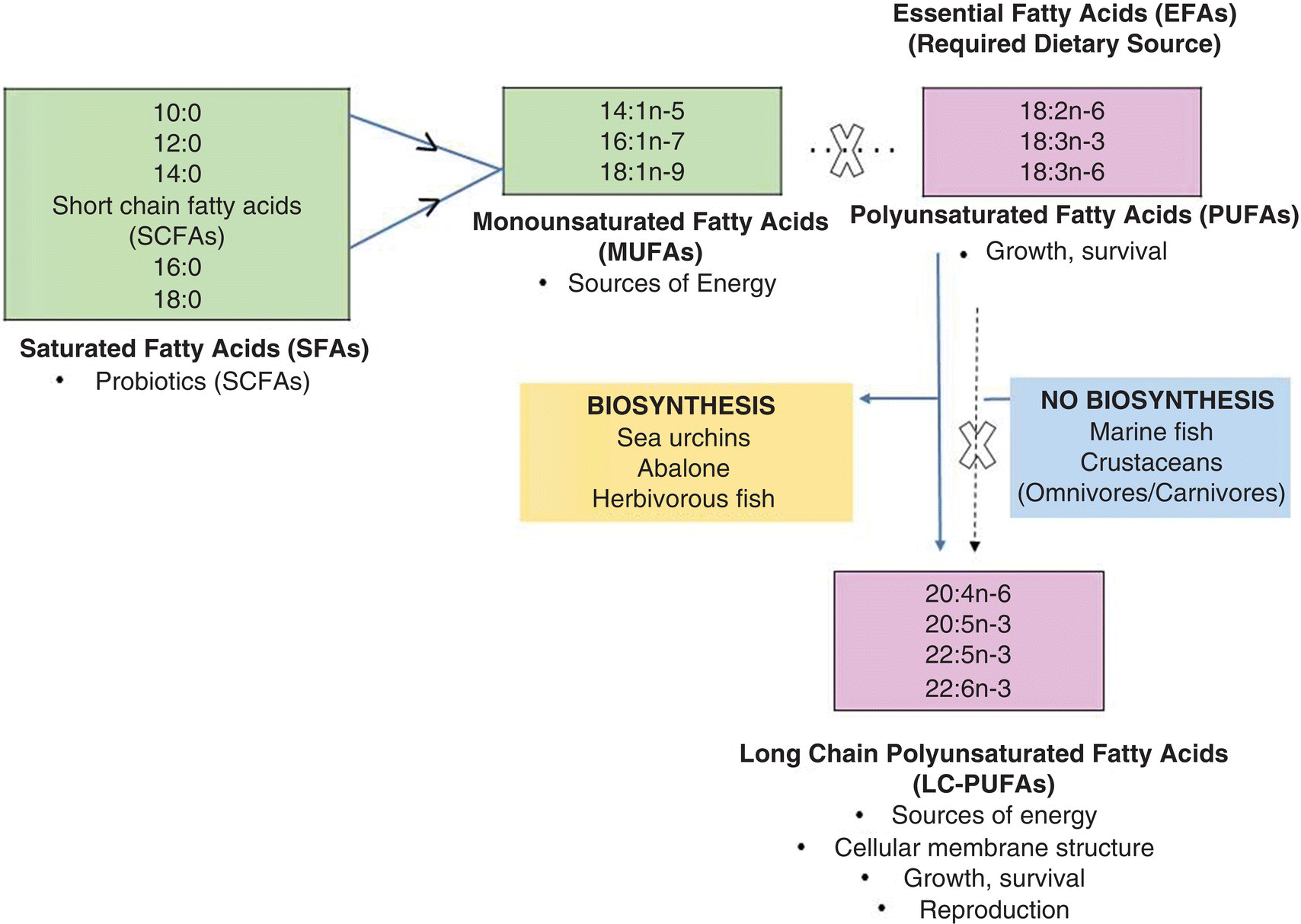
Figure 8.5 Classes, biosynthetic pathways, sources and physiological roles of fatty acids.
The ability of marine sea urchins and abalone (gastropod) to elongate and desaturate PUFA to LC‐PUFA starkly contrasts with the absence of this biosynthetic ability in marine fish and crustaceans. Juvenile sea urchins, Strongylocentrotus droebachiensis, that are characteristically herbivorous have the ability to elongate and desaturate n‐3 and n‐6 PUFA to n‐3 and n‐6 LC‐PUFA (20:5n‐3 and 20:4n‐6), respectively. The abalone Haliotis fulgens has the same intrinsic biosynthetic ability, affirmed by the lack of synthesis by gut microflora. Fatty acid composition differed among different tissues as well as within the different classes of lipid that composed the tissues. Growth of both Haliotis tuberculata and H. discus hannai is positively influenced by dietary LC‐PUFA, specifically 20:4n‐6, 20:5n‐3, and 22:6n‐3. Levels (mg of fatty acid/g dry weight) of LC‐PUFA (20:5n‐3, 22:6n‐3 and 20:4n‐6) within the polar lipids in the tissue of H. fulgens were selectively retained during starvation, suggesting the importance of these fatty acids as cellular components and essential nutrients. In contrast, LC‐PUFA in the neutral lipids of tissue decreased, probably due to a lack of dietary PUFAs as precursors for synthesis.
Takeuchi (1997) has addressed the wide EFA requirement patterns for aquatic species, particularly fish, as associated with life history stage, feeding, salinity and temperature. A detailed compilation of reported species‐specific fatty acid requirements of fish and shrimp can be found in NRC (2011). Glencross (2009) reviewed the requirements and metabolism of EFAs for fish, crustaceans and other species and addressed prospects for the use of grain, animal and algal derived oils as sources of EFAs in the formulation of aquafeeds.
8.4.3 Carbohydrates
Carbohydrates are synthesised by plants and consist of simple and complex molecules. Six simple sugars such as glucose and fructose are called monosaccharides, and when bonded together produce oligosaccharides consisting of three groups, disaccharides, trisaccharides and tetrasaccharides. Much larger ‘complex’ carbohydrate molecules consisting of chains of repeating monosaccharides are termed polysaccharides and are represented by such compounds as starch, glycogen, dextrin, mannan, fibre and cellulose. These polysaccharides are present in plants and plant‐derived feedstuffs, serving as comparatively inexpensive sources of energy in the diets formulated for fish, crustaceans, echinoids and gastropods. This energy is derived from the oxidation of the monosaccharide glucose, the primary building block of starches, that is ultimately derived from a sequence of enzymatically effected digestive processes. Glucose undergoes glycolysis to produce ATP from ADP with the concomitant formation of pyruvate. With the release of carbon dioxide, pyruvate is transformed into acetyl coenzyme A which enters the citric acid cycle to produce additional ATP.
Unlike many terrestrial homoeothermic species, fishes, being mostly carnivorous, do not naturally consume carbohydrates and accordingly a reduced ability to enzymatically digest carbohydrate has evolved. This lack of efficient use of dietary carbohydrate reduces its potential role as a source of energy in formulated diets for fish as well as crustaceans that have carnivorous feeding habits. For those aquatic species that are either omnivorous or herbivorous, most carbohydrates are readily digestible and can serve as a comparatively inexpensive source of energy in formulated feeds. However, for some species, dietary cellulose and fibre in aquafeeds do not serve as effective sources of energy due to the lack of enzymes to confer efficient digestion and they often cause reduced growth rates.
Due to these physiological differences imposed by feeding habits, there can be no true requirement for carbohydrate among aquatic species. However, some empirically‐based guidelines for inclusion of carbohydrates in feed formulations have been established. For carnivorous species, the total amount of dietary carbohydrate should not exceed approximately 20% of soluble carbohydrate. Feed formulations for herbivorous and omnivorous species can typically range from 25 to 45% (dry weight), somewhat imposed by the amount of lipid that can be included in the diet. As carbohydrate in the form of fibre is not digestible and therefore unavailable, efforts are typically directed at limiting the dietary level to 5–7%. The results of some investigations have suggested that the presence of fibre may enhance the digestibility of other nutrients by reducing the rate of gut passage. Some carbohydrates are added to formulated feeds to act as binders to preserve the stability in water or, if provided in sufficient quantity, to render a feed to float under particular conditions (temperature and pressure) of feed manufacture termed extrusion (see section 8.6.2).
8.4.4 Dietary Macronutrient Relationships (Protein: Energy Ratio)
A dietary protein‐energy ratio is expressed as the proportional amount of dietary protein relative to the total amount of macronutrient energy as calculated from the energy equivalents of protein, lipid and carbohydrates. Organisms do use some dietary protein as an energy source, but that amount can be ‘spared’ if sufficient quantities of lipid and carbohydrate are provided. Thus, protein requirements are not only determined by the composite satisfaction of the essential amino acids, but also some species‐specific need for protein as an energy source, despite other energy sources being provided in sufficient quantity. Providing highly digestible lipid and carbohydrates in sufficient quantities (combination) will therefore allow for the most efficient use of protein, i.e., its deposition as tissue (growth). The goal is the formulation of diets whereby energy derived from protein is minimised and all remaining energy needs are typically satisfied by dietary lipids and carbohydrates. Thus, for particular feed formulations, there are protein: energy ratios that will yield the highest growth. If the digestibility of the protein and energy sources are known, then these ratios can be expressed as the ratio of digestible protein to digestible energy. There have been numerous studies that have evaluated dietary protein to energy ratios for fish and crustaceans and they are generally expressed without reference to the digestibility of the protein and energy sources. Some ratios are inversely expressed as energy to protein ratios. Upon review, despite the variety of carbohydrate, protein and lipid sources used, the reported ratios are surprisingly consistent, generally ranging between 20–30 mg/kJ (33 to 50 kJ/g) for crustaceans and 23.8 to 30.0 mg/kJ (33 to 42 kJ/g) for fish.
As previously stated, carnivorous species are generally less capable of digesting carbohydrate so that feed formulation must compensate for this physiological characteristic. Carbohydrate containing, plant‐derived ingredients as sources of energy must be carefully evaluated relative to amount and digestibility. Lipid levels higher than those included in diets for herbivores and omnivores are commonly needed. In studies evaluating lipid to carbohydrate ratios in diets containing three levels of protein fed to the herbivorous pink abalone Haliotis corrugata protein and carbohydrate appear to be the principal sources of energy. Thus, for this abalone species, the focus would be on provision of highly digestible carbohydrate to provide energy to spare use of protein as an energy source. The amount of dietary lipid could therefore be comparatively low, but at a level sufficient to satisfy the essential fatty acid requirements. The P: E ratios that yielded the highest growth were 23.1 and 24 mg/kJ (41.7 to 43.5 kJ/g).
Age/life history‐specific changes in protein and/or energy demands would be expected as observed for larval forms (more protein for growth) and those individuals entering reproduction (more lipid for storage) and these changes must be accommodated to retain efficiency of protein metabolism.
8.4.5 Vitamins
Vitamins are organic molecules that are essential for growth, most serving as components of coenzymes for the catalysis of metabolic reactions. They cannot be synthesised and are termed essential micronutrients because they are required in small amounts (mg/kg or µg/kg). Vitamins are divided into two groups based on their solubility in either water or lipid. Four ‘lipid‐soluble’ vitamins, E, A, D and K are absorbed with lipid from the intestine into the circulatory system. A group of 11 vitamins, biotin, niacin, folic acid, thiamine, riboflavin, pantothenic acid, pyridoxine, cyanocobalamin, ascorbic acid, choline and myo‐inositol are water‐soluble vitamins. These vitamins are transferred from the intestine into the circulatory system via water. Choline, ascorbic acid and myo‐inositol are required at relatively higher concentrations. They are called megavitamins and are not components of coenzymes.
Dietary excesses of water‐soluble vitamins are not stored, but rapidly excreted from the body. Therefore, deficiencies will rapidly appear, often causing a variety of metabolic problems, some of which are manifested as clinical responses. For example, a dietary deficiency of ascorbic acid in fish is commonly manifested as ‘broken back syndrome’ (scoliosis/lordosis) which arises from a deficiency of collagen that serves as the organic matrix of bones. Specifically, the absence of sufficient collagen causes a decalcification of the bone which leads to the lack of ‘rigidity’ of the backbone, often manifested as scoliosis. Other water‐soluble vitamin deficiencies that are clinically exhibited by aquaculture species include poor appetite, haemorrhaging, fatty liver, hyperirritability, an array of aberrant haematological changes and others (NRC, 2011). Although each vitamin supports specific structural or metabolic functions, clinical manifestation of deficiencies may be the same for different vitamins. In contrast, lipid‐soluble vitamins are stored such that dietary requirements are difficult to determine. However, when tissue levels reach a threshold caused by excessive dietary levels, a condition termed hypervitaminosis occurs and clinical responses, such as reduced appetite and degeneration of the liver, are manifested.
Vitamins are commonly added as premixes in formulations to manufacture feeds for aquaculture species. Table 8.2 shows vitamin requirements of species of fish and shrimp reported from 1980 through 2010 and compiled in NRC (2011). Although published reports of vitamin requirements precede 1980, 1980 was arbitrarily selected as the cut‐off year for reporting requirements because by then studies had assumed a more controlled and rigorous methodology that presumably yielded more accurate results. When a range was reported, the mid‐point was used as an independent value in the calculation of the mean and the level of variation. If there were only two published estimates of a requirement, both values were listed rather than calculating a mean and a standard deviation. The measure of variation (standard deviation) is typically high and the range of values are most probably the result of a combination of factors that include species, the experimental diet, feeding behaviour, response criteria used to discriminate deficiency and the method used to estimate a requirement.
Table 8.2 Mean ± SD vitamin (water‐soluble and lipid‐soluble) requirements (mg/kg) of species of fish and shrimp published from 1980 to 2011 (as reported in NRC, 2011). (n) = number of reported values. If estimates were limited to one or two values, no mean was calculated. If the estimated value was presented as a range, the mid‐point was used in the calculation. B12 and D values are presented as µg/kg.
| Water Soluble | |||
| Vitamin | Fish | Shrimp | Shrimp/Fish Ratio |
| Thiamin | 1; 11.2 (2) | 68.0 ± 47.0 (3) | x |
| Riboflavin | 6.0 ± 2.4 (9) | 22.5; 80 | x |
| Pyridoxine | 6.6 ± 4.4 (10) | 110.1 ± 31.5 | 16.7 |
| Pantothenic acid | 20.8 ± 8.3 (7) | 323.3 ± 369.6 (3) | 15.5 |
| Niacin | 19.1 ± 9.6 (7) | 219.1 ± 198.2 (3) | 11.5 |
| Biotin | 0.77 ± 1.1 (8) | 2.2; 0.4 | x |
| B12 (µg) | 54.2 ± 39.3 (3) | 200; 10 | x |
| Folic acid | 1.6 ± 1.1 (7) | 1.9; 21 | x |
| Choline | 1260.5 ± 1059.6 (8) | 6200; 600 | x |
| Myo‐inositol | 396.0 ± 153.4 (5) | 1351.0 ± 1659.2 (3) | 3.4 |
| Ascorbic Acid | 101.4 ± 133.1 (75) | 159.4 ± 131.8 (20) | 1.6 |
| Mean = | 9.7 | ||
| Lipid Soluble | |||
| A | 7.8 ± 13.0 (5) | 16.3 ± 24.9 (3) | |
| E | 72.8 ± 46.3 (18) | 87, 99 | |
| D (µg) | 22.7 ± 20.0 (4) | 10 | |
| K | 1.9, 0.2, <10 | 35, 185 | |
Despite the variation, the levels of water‐soluble vitamins reported among an array of freshwater species are comparable. This observation is not unexpected because of the commonality of the metabolic roles of these vitamins among species. For marine fish, little information is known about requirements of water‐soluble vitamins except for ascorbic acid and the reported requirements for this vitamin are very similar among several species. There is little information about requirements for lipid‐soluble vitamins; nonetheless reported estimates for both freshwater and marine fish are similar. The means of reported water‐soluble vitamin requirements of shrimp species are commonly greater (1.6 to 16.7 X) than those reported for fish. This difference most probably does not reside in phylogeny, but rather loss due to leaching during a protracted period of food consumption that is characteristic of shrimp species. Loss would naturally lead to an overestimation of the true requirement with greater departures corresponding to those vitamins with higher solubility. Vitamin requirements of other potential aquaculture species are becoming the subject of investigation. The inclusion of a vitamin pre‐mix in a diet for the sea urchin, Lytechinus variegatus, was found to be a necessary ingredient for weight gain and organ production.
8.4.6 Minerals
There are approximately 20 inorganic elements (minerals) that are considered to be essential nutrients. They are divided into two groups, macro‐elements, those required at levels of g/kg of diet, and micro‐elements or trace elements, those required at levels of mg/kg of diet. Macro‐elements and micro‐elements considered essential for aquatic animals are listed in Table 8.3. These nutrients are required for the successful function of a variety of physiological processes such a muscle contraction (Mg, P), production of skeletal structures (Ca, P), osmoregulation (Na, Cl) and as components of respiratory pigments (Fe, Cu), that include haemoglobin (fish) and haemocyanin (crustaceans). Minerals are also components of vitamins and hormones, and serve as cofactors that are essential for the enzyme catalysis of specific chemical reactions. Although minerals are naturally present in feedstuff ingredients that are part of formulations of manufactured feeds for aquaculture organisms, mineral mixes need to be included to compensate for anticipated deficiencies or reduced bioavailability of some minerals. Various sources of phosphorous such as fishmeal, soybean meal and calcium phosphate are differentially available to cultured aquatic organisms. For example, phosphorous derived from monobasic calcium phosphate, Ca (H2PO4)2, is much more available to fish than the dibasic form, CaHO4P.
Table 8.3 Essential macro‐elements and micro‐elements for animal aquaculture species.
| Macro‐elements | Micro‐elements (Trace elements) |
| Cations | |
| Calcium (Ca) | Cobalt (Co) |
| Magnesium (Mg) | Copper (Cu) |
| Potassium (K) | Chromium (Cr) |
| Sodium (Na) | Fluorine (Fl) |
| Iodine (I) | |
| Anions | Iron (Fe) |
| Chlorine (Cl) | Manganese (Mn) |
| Phosphorous (P) | Molybdenum (Mo) |
| Sulphur (S) | Nickel (Ni) |
| Selenium (Se) | |
| Silicon (Si) | |
| Tin (Sn) | |
| Vanadium (V) | |
| Zinc (Zn) |
Controlled experiments have achieved good estimations of some mineral requirements. These experiments have generally been designed with recognition of uniquely external factors that may influence estimates. Dietary supplementation of macro‐ and micro‐elements is based on a variety of factors. In some cases, rearing condition, i.e., fresh, marine or estuarine water may serve as a significant source to meet a requirement and must be considered in the overall estimate. Sources of dietary protein that have notable mineral components can also influence requirements. In addition, caution needs to be exercised so that experimental diets do not include feedstuffs that contain compounds that render dietary minerals unavailable to the organism. For example, feedstuffs derived from cereal grains and oil seed meals contain phytate which binds with (sequesters) calcium, thereby reducing bioavailability for use in meeting the requirement of the organism. Reported requirements of fish and shrimp are generally within the same order of magnitude (NRC, 2011).
8.4.7 Life History and Reproductive Stage Dependent Nutrient Requirements
8.4.7.1 Larvae
Larval fish and crustaceans are typically fed cultured live food such as algae, rotifers, copepods, and Artemia nauplii (section 9.2). In some cases, enrichment of the nutritional quality of these live organisms, commonly LC‐PUFAs, is frequently accomplished through different approaches, affording the ability to identify qualitative dietary requirements through observations of growth and survival responses. Larvae of the northern rock sole Lepidopsetta polyxystra have been fed rotifers that have consumed liposomes enriched with taurine.
The use of formulated diets as substitutes for live food in larval nutrition offers the advantages of control of nutrient composition, convenience of storage and use, and a reduction of cost of production associated with the labour‐intensive effort required to successfully maintain mass culture of live organisms. Moreover, the use of formulated diets eliminates the potential introduction of pathogenic organisms originating from live foods.
Despite noteworthy advances over the past 30 years, challenges remain in achieving an understanding of the nutrition of larval forms of fish and crustaceans and the development of successful feed formulations and feeding practices for them (Hamre et al., 2013), As a result, considerable constraints on their production and availability for intensive production practices exist. With the advantage of nutrient control afforded by formulated diets, a corresponding opportunity to determine qualitative and quantitative nutritional requirements becomes possible. Although promising, knowledge of nutrient requirements of larvae is substantially lacking, mostly qualitative in nature. Lack of specific quantitative knowledge remains plagued by poor consumption, particularly early larval stages and/or digestion. Only when a water‐stable, formulated diet that is readily consumed, digested and assimilated with an equivalency to live feeds will it be possible to investigate specific nutrient requirements with a high level of accuracy and confidence. In particular, estimation of requirements of water‐soluble nutrients is plagued by leaching from diets that lack physical integrity and have a characteristically high surface area to volume ratio.
A chronological progression toward the successful use of microparticulate diets for the culture of larval forms of aquaculture species has occurred over the recent two decades. In some cases, the success of complete replacement of live food is limited to certain stages of larval development. Use of commercially available larval feeds is commonly recommended as supplements rather than full substitutes for live food (see section 9.5).
The successful production of larvae through use of formulated diets has rarely translated to their modification and use for the determination of nutrient requirements. However, some progress has revealed that larvae do exhibit some unique differences in nutrient requirements. These distinctions are probably due to a greater demand associated with early growth and development combined with a reduced ability to digest and absorb particular macronutrients. It is commonly acknowledged that both fish and crustacean (penaeid shrimp) larvae have greater requirements for dietary EPA (at least % LC‐PUFA) and also exhibit a requirement for phospholipids, specifically phosphatidylcholine. As these species are known to synthesise PC, the existing rate of synthesis probably falls short of satisfying this early growth stage requirement. A dietary need of at least 2 % of dietary phospholipids for good growth and survival of larval fish and crustaceans has been often reported with phosphatidylcholine (PC) and phosphatidylinositol (PI) having the greatest nutritional value (NRC, 2011). This requirement is not related to the possible need for additional choline or inositol, two required water‐soluble vitamins that are part of the phospholipid molecules. Quantity and quality of dietary fatty acids affect food intake and absorption efficiency of nutrients in larvae of gilthead seabream, Sparus auratus and the Senegalese sole, Solea senegalensis.
Both larval and juvenile crustacean species exhibit a unique requirement for cholesterol. Attempts to completely or partially substitute dietary cholesterol with plant‐derived sterols (phytosterols) have not been successful. Cholesterol is synthesised from dietary phytosterols, as indicated by the exclusive presence of cholesterol in body tissue of those larvae/juveniles fed diets containing phytosterols. Apparently, the rate of synthesis is insufficient to satisfy a specific cholesterol requirement that cannot be ‘spared’. The deficiency response is commonly manifested in reductions in growth rates.
8.4.7.2 Broodstock
A reliable supply of larvae to produce juveniles (postlarvae) for stocking into production systems is integral to the success of an aquaculture enterprise. Therefore, providing essential nutrients to broodstock to reach reproductive maturity and for transfer to gametes (egg and sperm) to produce viable eggs and larvae is very important. However, broodstock nutrition is the least studied area of aquatic animal nutrition and along with larval nutrition definitely represent an impediment to conferring greater efficiency (sustainability) in aquaculture enterprises. For example, the nutritional status of females influences the time to reproductive maturity, the number of eggs produced per spawn or per unit of body weight (fecundity) and egg size, egg hatchability and survival of larvae through the early developmental stages.
The evident lack of widespread research focusing on broodstock nutrition appears to be limited by the length of time and corresponding resources, such as holding units and number of broodstock needed to conduct rigorous and meaningful research. This lack of information has imposed a dependency on fresh food as feed, exclusively or in association with formulated diets as part of broodstock husbandry. A detailed review of the role of the nutrition of broodstock on reproductive performance is found in Izquierdo et al. (2001) for fish and in Wouters et al. (2001) for species of penaeid shrimp. Salient details are presented in the following generalised summary, but reported species‐specific requirements are not addressed.
An increased demand for energy is needed for not only the production of viable gametes but also expression of secondary sexual characteristics and activities associated with reproductive behaviour. If more dietary energy is needed to meet the demands of the reproductive state, then less net energy is available to channel to growth. For macronutrients, the dietary protein level for broodstock is recommended to be aligned with those levels fed to grow‐out fish, but higher levels of dietary protein have been studied and used. Different dietary protein levels can exert different responses in broodstock. Intermediate levels of protein yielded higher relative fecundity (number of eggs per 100 g of female) in a species of tilapia, Oreochromis niloticus. For tilapia, the total eggs and the number of eggs per kg female were higher from fish fed with medium dietary protein (27.6 and 35%) than those fed with higher protein levels (42.6 and 50.1%). The higher protein diet yields heavier and larger eggs that are spawned between longer time intervals. Dietary lipid levels in formulated diets are commonly increased to meet the increased metabolic demand for energy and a commonly used level is 18%. Certain nutrients have been repeatedly shown to be required at dietary levels higher than those for juveniles and subadults and specifically include n‐3 EFAs, alpha‐tocopherol (vitamin E), ascorbic acid (vitamin C) and vitamin B6 (pyridoxine).
The EFA content of gonads corresponds well to the dietary EFA content and n‐3 LC‐PUFAs are essential for normal development of eggs and embryos, and improve the overall quality of eggs. These fatty acids may also serve as a source of energy during embryonic development. Dietary eicosapentaenoic acid (EPA) and arachidonic acid (AA) are correlated with higher rates of fertilization, possibly due to their observed role in ensuring good sperm motility. The quantitative LC‐PUFA content of formulated broodstock diets generally falls within the range of 1–2% with qualitative needs demonstrating some species‐specificity. Docosahexaenoic acid (22:6n‐3) is an important dietary component especially for transfer to larvae where the importance of this essential fatty acid for larval development has been documented. Higher dietary levels of vitamins E and C are important in ensuring high fecundity and high rates of fertilization. Vitamin C also manifests a role in vitellogenesis (egg quantity and quality) and steroidogenesis. Both vitamins C and E are antioxidants and presumably serve in a protective role for sperm cells during steroidogenesis and in ensuring good motility of sperm. Insufficient levels of dietary vitamin E have been associated with immature gonads and reductions of both hatching rates and survival of offspring. Viable eggs, fertilised eggs and larval survival were used as indicators to evaluate the combined roles of LC‐PUFA and vitamin E in affecting broodstock performance of gilthead seabream, Sparus aurata. Based on the available research results, specific nutrient recommendations for broodstock diets include 250 mg/kg of vitamin E and 100‐250 mg/kg of vitamin C. The observed positive effects of dietary phospholipids (egg quality), the carotenoid astaxanthin (buoyant and hatched eggs, increased percentage of normal larvae) and pyridoxine (synthesis of steroid hormones) have led to recommended inclusion. The required qualitative and quantitative dietary nutrient enhancements needed by broodstock will increase feed cost; however, the higher cost should be compensated by healthy broodstock yielding higher egg production and survival of larvae, particularly for marine fish. Reliable availability of juveniles for stocking into farming systems continues to be a major bottleneck in increasing marine fish production and contribution to animal protein food security globally.
During the maturation of the reproductive organs of crustaceans, nutrients are primarily transported from the hepatopancreas (mid‐gut gland). Therefore, those nutrients have been identified as important because they characteristically accumulate in the ovary during reproductive maturation and are notably found in natural food organisms that serve as successful broodstock feeds. As gonadogenesis is induced by the cutting (ablation) of the eyestalk (see section 6.2.2), a demand on good nutrition arises immediately. There is a demand for lipid, both as a source of energy and as EFAs, particularly LC‐PUFAs such as 20:5n‐3, 22:6n‐3 and 20:4n‐6 which characteristically accumulate in comparatively high concentrations in ovarian tissue. During maturation of the ovary, triglyceride content increases and this lipid is transferred to the eggs that are ultimately produced. The ovarian triglycerides principally serve as energy sources, but certain dietary fatty acids, particularly 20:5n‐3 and 22:6n‐3, are important in maturation of ovaries, fecundity and egg quality. In addition, arachidonic acid (20:4n‐6) and other n‐6 fatty acids are known to be precursors of prostaglandins which are assumed to play an important role in reproductive processes such as vitellogenesis. Based on presence and concentration in the ovary, phospholipids, particularly phosphatidylcholine and phosphatidylethanolamine, and cholesterol are considered to be important in the processes of reproductive maturation. Carotenoids, particularly the xanthophyll astaxanthin, accumulate in the ovaries as part of a lipovitellin protein. These lipid compounds have substantial antioxidant properties and confer colour to the ovaries which becomes a pigment source for transfer to eggs and larvae. In general, the recommended levels for inclusion in broodstock diets are 2% phospholipids, 50 mg/kg astaxanthin and a total lipid level of 10%, higher than that found in grow‐out feeds (~7%). Crustaceans are unable to synthesise LC‐PUFAs and carotenoids de novo, so diet must be the source. Cholesterol can be synthesised from phytosterols, but the rate of synthesis is insufficient to support the dietary requirement.
Protein levels in the ovary also increase during maturation, probably arising from the transfer of vitellogenin from the hepatopancreas where it is synthesised. It appears, therefore, that higher protein levels should be included in a formulated diet for broodstock and this need is also supported by the high levels found in fresh food that are fed as ‘maturation diets’. Evidence for the essentiality of a specific quantity or quality of carbohydrate in formulated diets for crustacean broodstock has yet to be observed.
Similar to fish, vitamins, A, C and E have been found to be required dietary ingredients to achieve desired reproductive performance in broodstock. Vitamin E deficiency has resulted in abnormalities in sperm and a reduction in the hatching rate of eggs. High hatching rates have also been observed in response to increases in dietary ascorbic acid (vitamin C) a compound with antioxidant properties.
8.5 Digestion and Assimilation of Food
An understanding of the digestion and assimilation of food is important as choice of highly digestible feedstuffs as sources of nutrients for aquaculture species is essential in achieving low food conversion ratios or high feed efficiencies. Maximizing nutrient availability and assimilation for growth and other essential metabolic processes achieves high growth efficiency, a critical component of sustainability. Hence, a summary of the knowledge of the processing of food consumed by fish, crustaceans and echinoderms/molluscs will serve as an important foundation of the understanding of nutrient availability and metabolism. The following descriptions of digestion and assimilation of nutrients are derived from more extensive descriptions found in Ceccaldi (1997) for crustaceans and Horn (1998) for fish. The processes of digestion and assimilation of nutrients by fish and crustaceans are collectively shared with monogastric (one stomach) terrestrial animals.
8.5.1 Fish
Fish initially evolved as carnivores followed by the evolution of herbivory, omnivory and detrivory in some species. However, 85% of all species of fish remain carnivores. Carnivores do not have the ability to digest carbohydrates effectively, so dietary protein and lipid collectively serve as the source of energy. In contrast, carbohydrates can serve as an excellent source of energy for herbivores, omnivores and detritivores and therefore formulated diets can contain less proportional protein and lipid, commonly resulting in feed formulations that are less expensive.
The morphology of the guts of species differs according to feeding strategy and length is generally a defining characteristic. For example, detritivores need to efficiently process large quantities of nutrient poor food to derive the required amounts of nutrients. Accordingly, they characteristically have long intestines where detrital material is moved through quickly. The intestines have a thin mucosal lining to facilitate rapid uptake of nutrients. Representative species of aquaculture interest are carp, tilapia and milkfish. Carnivorous species generally have short (absolute length) intestines with thick mucosal linings and the rate of transport through is comparatively longer. However, along the proximal portion of the intestine of some of these fish there are pyloric caeca which are characterised by having a large surface area to assist in the efficient uptake of available nutrients. Food passes through the caeca at the same rate as that of the intestine. Representative species include trout, tuna and hybrid striped bass. Many herbivores have a thick‐walled modified stomach call a gizzard that is primarily used to physically break down food in conjunction with chemical digestion effected by enzymes. The intestinal length of herbivores commonly falls between those of detritivores and carnivores.
Most of the digestion of foods consumed by fish occurs in the intestine followed by absorption through columnar epithelial cells that have a ‘brush border’ composed of microvilli. Based on their morphology they contribute about 90% of the total absorptive surface area of a fish’s gastrointestinal tract.
Prior to entrance into the stomach (if one exists), some physical breakdown of food may be accomplished by teeth, if present, in the mouth or the pharynx. The food, reduced to smaller particles, is combined with mucous originating from goblet cells which are specialised epithelial cells of the oesophagus. For those species that have stomachs there is both physical digestion, effected by the muscular tissue and enzymatic digestion. The initial stages of chemical digestion focus on protein that is broken into smaller chains of amino acids by the action of the enzyme pepsin in association with HCl, both originating from the oxyntopeptic cells of the mucosa of the stomach. Pepsin is an endopeptidase that hydrolyses peptide bonds (bonds between amino acids) in the interior of protein molecules. With the secretion of HCl, the pH of the stomach contents decreases to 1–2 and returns to between 4 and 7 when this partially digested food mixture moves out of the stomach into the intestine where the remaining digestion of protein and other macronutrients (lipid, carbohydrate) occurs. Bicarbonate from the pancreas neutralises the acidic mix from the stomach whereby the pH of the intestine falls within a range of 7 to 9. Most of the digestive enzymes present within the intestine are secreted from the exocrine cells of the pancreas and the brush border cells of the intestine itself.
The pancreas secretes two major endopeptidases, trypsin and chymotrypsin, which operate at a pH optimum of 7–7.5 and continue the digestion of proteins by specifically hydrolysing peptide bonds that remain in the interior of protein molecules. These endopeptidases are highly specific, executing the hydrolysis of peptide bonds where the carboxyl group of arginine and lysine is combined with the amino group of another amino acid. Trypsin activates chymotrypsin through proteolysis (breakdown) of its inactive form (chymotrypsinogen) and chymotrypsin A specifically hydrolyses peptide bonds at the carboxyl end of amino acids with an aromatic side chain such as tyrosine and phenyalanine. Carboxypeptidases A and B continue the hydrolysis of peptide bonds of the polypeptide molecules created by the endopeptidases, focusing on the peptide bonds that have free carboxyl and amino groups located at the end of the molecules. The peptide bonds of the resulting tripeptides and dipeptides are further hydrolysed by the action of aminopeptidases that originate from the brush border cells of the mucosal layer of the intestine, reducing them to their constituent amino acids.
Other enzymes produced by and released from the pancreas are amylases which hydrolyse the glycolytic bonds in polysaccharides to produce oligosaccharides and then disaccharides. Disaccharides are further hydrolysed to monosaccharides by disaccharidases which are released from the brush border of the mucosal cells. In preparation for digestion, lipid in the intestine is physically reduced in size to droplets that are emulsified by a mixture of phospholipids and bile salts produced by the liver and delivered via the gall bladder. The ester bonds of the emulsified lipid are then hydrolysed by lipases delivered from the pancreas and triglycerides are reduced to diglycerides and then monoglycerides, fatty acids and glycerol. Phospholipases are also present to hydrolyse the phosphorous and glycerol ester bond found in phospholipid molecules.
The absorption of nutrients in the form of the building blocks of macronutrients, i.e., amino acids, fatty acids and monosaccharides, occurs in the intestine, either actively (via carrier molecules) or passively (diffusion). The cellular sites of the absorption of the nutrients are called enterocytes. Fatty acids, monoglycerides, fat soluble vitamins, cholesterol and other lipophilic nutrients combine with bile salts and phospholipid to form a micelle whereby these nutrients are transported to the brush border of the enterocyte into which they are released via passive diffusion. Glucose and other monosaccharides, amino acids, small peptides and even some intact proteins are engulfed at the brush border of the enterocytes through a process called pinocytosis. Amino acids, glucose, di‐ and tripeptides and water‐soluble vitamins, aligned with specific proteinaceous carrier or transporter molecules, are actively absorbed across the membranes of the brush border cells into the circulatory system. These nutrients are then transported to the liver to be either stored or oxidised to produce energy.
8.5.2 Crustaceans
As monogastric (single stomach) animals, the digestive morphology and enzyme controlled digestive processes of crustaceans are similar to those of fish. Nonetheless, some noteworthy differences exist. Prior to transfer to the mouth, crustaceans physically manipulate and reduce the size of food obtained through external mouthparts, specifically mandibles and maxillipeds. Joining the mouth to the stomach is the oesophagus which is short, straight and vertically oriented. The inner surface of the stomach is coated by a chitin‐protein complex. The anterior region of the stomach has a thin epithelium whereas the posterior region that includes the cardiac and pyloric regions is also reinforced with calcified ‘teeth’ that are used to break down the entering food into smaller particles. The particles are then separated by size, either transported out of the stomach or physically retained by filters in the stomach for additional breakdown. The very fine food particles released from the stomach are ultimately passed to an important organ, the hepatopancreas, which is also termed the mid‐gut gland. This tissue is composed of two sections and each section, consisting of 2–3 lobes, opens into a digestive tract that contains secondary and tertiary tubules. These tubules are the glandular part of the mid‐gut and produce an array of enzymes to facilitate chemical digestion. Trypsin is the principal proteolytic enzyme and chymotrypsin is either not present or present in very small amounts. Carboxypeptidases, aminopeptidases and dipeptidases complete the protein digestion. Enzymes to affect the chemical digestion of carbohydrates and lipids are also present. Amino acids and molecules that are the products of lipid and carbohydrate digestion are absorbed at the brush border cells of the intestine. The mid‐gut gland manifests liver‐like functions, both storing and metabolizing organic compounds and storing minerals.
8.6 Formulation, Manufacture and Digestibility of Feeds
A variety of ingredients, feedstuffs, premixes and supplements generally compose an aquafeed either research or practical use (Table 8.4). The qualitative and quantitative ingredient composition of a feed is termed a formulation. Ingredients used in formulations to produce feed for aquatic organisms are often the by‐products of the processing of food for humans. The choice of feedstuff ingredients must ultimately be governed by production practices that are sustainable and not used for human consumption. A primary goal in the development of a formulation is to minimise the number of ingredients while ensuring that they are highly digestible to achieve efficient satisfaction of nutrient requirements. Glencross et al. (2007) compiled a group of strategies designed to evaluate ingredients for use in aquafeeds and this work is an excellent foundation to guide the development of international standards for choice and use of feedstuffs in aquafeeds. Ideally, manufacturers will recognise the benefits of these standards/practices and accordingly accept and implement them.
Table 8.4 Examples of ingredients typically found in arrays of laboratory/research diets, farm‐made feeds and commercial feeds for aquatic animals. Proportional amounts vary dependent on choice of other ingredients, production systems used, type of manufacture and species.
| Laboratory Research | Farm‐made/Supplemental | Commercial Extruded/Pelleted |
| Vitamin‐free casein | Fishmeal | Fishmeal |
| Isolated soy protein | Rice bran | Soybean meal |
| Gelatine | Coconut meal/Coconut oil cake | Poultry by‐product meal |
| Refined soy lecithin | Lupine seed | Poultry feather meal |
| Dextrin | Cassava leaves flour | Wheat grain/ |
| Wheat middlings | ||
| Phosphatidylcholine | Peanut meal | Corn gluten meal |
| Wheat starch | Soybean meal | Blood meal |
| Alpha‐cellulose | Trash fish | Ground wheat |
| Egg albumin | Coconut/ground nut oil | Wheat flour |
| Fish protein concentrate | Chicken feed | Squid meal |
| Fish/vegetable oil | Fish/vegetable oil | Fish/vegetable oil |
| Vitamin/mineral premixes | Vitamin/mineral premixes | Vitamin/mineral premixes |
| Canthaxanthin | Soybean hulls | Astaxanthin |
8.6.1 Feed Manufacture (Farm‐Made Feeds)
Farm‐made feeds are still produced for use in small‐scale culture operations in many countries due to either the scarce availability of commercially produced pelleted feed or the prohibitive cost of these feeds for use in such rudimentary, sometimes subsistence farming, endeavours. Examples of ingredients in farm‐made feeds include shrimp waste, fresh leaves, corn silage and beef liver. Choice of ingredients is governed by availability and cost. Generally, the formulations of farm‐made feeds are species‐specific, but recommended ingredient compositions are lacking. The use of unprocessed animal ingredients, the extensive variety of conventional and raw materials that may be used, and the lack of consistent quality and nutrient composition of locally available ingredients cause concern about the ongoing value of farm‐made feeds. Quality assurance is not a component of production practices. Use of some locally available feedstuffs as ingredients in formulations of commercially‐manufactured diets are appealing. However, the common lack of sufficient amounts as well as inconsistent nutrient composition caused by differences in collection time or vagaries in preparation practices are foundation for reservations about use.
The use of farm‐made feeds for small farms continues and production methods that will improve consistency and efficiency are the ongoing subject of investigation. For example, the desire to use floating feeds that do not require the costly use of the feed manufacturing technology of extrusion (see following section) has led to the testing of different arrays of ingredients to produce a pelleted dough that has positive buoyancy characteristics. The use of farm‐made feeds will continue. However, with anticipated increases in scales of production throughout the world to meet future per capita protein demand, commercially‐manufactured aquafeeds will replace farm‐made feeds in those instances where the economic advantages of such a feeding practice is revealed. The goal of environmentally and economically sustainable production practices will ultimately be realised through adoption of such practices that will dramatically reduce waste and disease transmission and limit vagaries in annual production.
8.6.2 Feed Manufacture (Dry Pelleting)
The manufacture of pelleted feed consists of a sequence of steps. Held in storage facilities, the feedstuffs intended for use as ingredients of a formulation may be subject to a preliminary grinding process. The goal of this process is to enhance digestibility as well as establish a homogeneous ingredient mixture. Subsequent to the grinding process, the ingredients are batched together and micronutrients such a mineral and vitamin premixes are added separately. Mixing and additional grinding of the entire mixture follows. Pelleted feeds are produced with the aid of steam (15–18 %) to moisten the mixture and a simultaneous heating between 65 and 85 °C before passage through a pellet die to provide the desired size of a compressed sinking pellet. Pelleted feeds are also produced by subjecting the final mixture to a slightly different procedure whereby a greater amount of moisture (~25%) is added to the mixture followed by exposure to a higher range of temperature (90–150 °C) in an extruder barrel. This mixture is then forced through a pellet die (extrusion pelleting). As the pellet passes through the die, some of the moisture is vaporised, causing the pellet to expand. Pellets are dried to contain between 8 to 10% moisture and can be either sinking or floating, as determined by the ingredient composition of the mixture.
The type of manufacturing process itself can be a positive factor through enhancement of the nutritive value of the feed. Through the addition of comparatively higher amounts of water combined with the use of a higher manufacture temperature prior to extrusion, the starch contained within the feedstuff ingredients is gelatinised. Gelatinised starch has been shown to be more digestible than other forms of starch for trout and carp, thereby conferring a greater amount of available dietary energy. The pellet expansion that occurs during extrusion also produces a surface on which a lipid coating can be applied. The ability to introduce lipids in this manner offers the opportunity to increase the energy content of feed, enhance palatability, and substantially reduce the quantity of fines (dust) that can accumulate after manufacture and during storage/transport, thereby minimizing the amount of feed that is unavailable for use by the farmer.
Certain feed ingredients may interfere with or be partially subject to loss in conjunction with the feed manufacturing process. For example, rice bran is not a preferred feed ingredient because it can be abrasive on equipment used in the manufacturing process. Extrusion manufacture of feed causes a loss (elimination of activity) of approximately 50% of ascorbic acid. To provide for the desired dietary level, some type of compensation such as the addition of higher amounts or the presence of a stabiliser needs to be addressed.
Physical quality of the feed will vary according to ingredient composition and processing. The differences can affect growth by negatively affecting consumption and the digestibility of the feed. Physical (hardness, integrity, density, oil absorption) and nutritional (availability and digestibility of nutrients) characteristics have been defined as qualitative measures of high energy extruded feed. Differences in the water stability of feeds can confer different digestibility and feed intake characteristics relative to the species under study. For example, harder pellets have been found to both reduce and increase feed intake and are also characterised by higher retention times in the gastrointestinal tract.
The principal feedstuff components of a feed are added to serve as protein and energy sources. Examples of common sources of protein used in feed formulations include fishmeal, soybean meal and poultry by‐product meal. A variety of fishmeal sources such as menhaden, anchovy and herring are used. The best plant protein source is soybean meal, but other plant oil seed sources that are used include cottonseed meal and canola meal. The content of protein in animal‐derived feedstuffs ranges from 50 to 85 %, whereas protein content of plant‐derived sources ranges from 20 to 50 %. Mixtures of different protein sources are commonly used in an effort to provide sufficient and balanced levels of available amino acids. Sources of lipid to provide both energy and a source of EFAs are generally marine‐derived oils or combinations of marine‐derived and plant‐derived oils to provide a mixture of n‐3 LC‐PUFA and PUFA and n‐6 LC‐PUFA and PUFA, respectively. A greater total proportion of plant‐derived feedstuffs can be used in feed formulation for herbivorous and omnivorous species because of their ability to digest carbohydrates more efficiently. These carbohydrates serve as complementary sources of energy.
During the past 30 years, considerable effort has been directed toward the reduction or elimination of marine‐derived meals and oils from diets because both these feedstuffs are not sustainable. In addition, unpredictable availability of these feedstuffs accompanied by fluctuating costs is not conducive to sustaining a successful aquaculture enterprise that requires feed. Therefore, feedstuffs selected to compose formulations must be continuously and readily available while contributing to the lowest possible cost of production expressed as per unit of protein. To attend to this goal, feed manufacture may involve the use of computer software to achieve ‘least cost’ formulations which are based on the selection of a combination of feedstuffs guided by an array of restrictions in meeting the fulfilment of nutrient requirements. These restrictions include maximum and minimum limits that are imposed to avoid problems arising in manufacture or in the introduction of properties/characteristics that reduce the availability of the nutrients to the aquatic species. Thus, the availability of phosphorous, often present in plant‐derived ingredients, may be reduced by up to 33 % if sufficient phytate, which binds with phosphorous, is present in the combination of plant‐derived feedstuffs used in a specific formulation. Provisions to avoid this extant lack of variability can be part of the least cost formulation.
8.6.3 Digestibility of Feedstuffs and Nutrients
Digestibility of a feed, different feedstuffs and specific nutrients by most species of farmed fish and shrimp is principally determined by an indirect method that involves the addition of an indigestible marker to an experimental diet. The most common marker used is chromic oxide, but others include ash, silica, crude fibre, polythene, stable isotopes and titanium oxide. To yield accurate determinations of digestibility, the marker must be non‐toxic, inert (not metabolised) and not alter the process of digestion and rate of flow through the digestive tract. The relative increase of the marker in the faeces versus the amount in the diet is used to calculate a percentage which is called the apparent digestibility co‐efficient. The term ‘apparent’ is used because faeces contain organic material such as enzymes and epithelial cells of the intestine that are the respective products of the digestion process and the movement through the intestinal tract. These minor additions contribute to a slight reduction in calculated digestibility coefficients., Among nine different feedstuffs of marine origin (fish, crab, scallop, shrimp head, and squid meals) apparent digestibility coefficients for dry matter ranged from 46 to 102% and protein digestibility coefficients ranged from 64 to 89% for the marine shrimp, Litopenaeus vannamei.
8.6.4 Anti‐nutrients and Contaminants
Choices of feedstuffs for inclusion in formulated feeds must also consider the presence of naturally occurring compounds that prevent/inhibit the efficiency of digestion or the uptake of particular nutrients. Compounds that can adversely affect the activity of digestive enzymes are generally proteins, both simple and complex, and this undesirable characteristic can be neutralised through treatments such as heat, fermentation or alcohol extraction. A good example of a current effort to circumvent the introduction of potential anti‐nutrients into feeds is manifested by the evaluation of fermented soybean meal as a partial replacement for fishmeal.
One group of ‘anti‐nutritional’ factors inhibit the activity of proteinases during the digestive process. Some of these factors are broadly active, affecting an array of proteinase enzymes, whereas others specifically focus on trypsin and carboxypeptidases. The activity of amylases, needed for carbohydrate digestion, is restricted by the presence of saponins that commonly have their source in feedstuffs that originate from beans, grains and tubers. A 0.2% addition of saponins from soybean to diets containing plant meals and fed to Atlantic salmon caused inflammation in the distal intestine and reduced digestibility of lipid, fatty acids and minerals. In some cases, minimum levels of these digestion inhibitors have been recommended for inclusion in diets. Thus, the level of plant‐derived feedstuffs that can be used as alternatives to fishmeal may be influenced by their respective dietary levels of saponins as well as provision of the proper amounts and balance of essential amino acids.
Inhibitors of lipase digestion are commonly found in feedstuffs derived from seeds that have characteristically high levels of lipid (soybeans, sunflower and peanut/groundnut). Also included in this group of feedstuffs that contain lipase inhibitors are cereals such as wheat, barley and sorghum. These inhibitors interfere with the digestion process by binding to emulsified lipid droplets or they can actually form a complex with the enzyme itself. Generally, lipid digestion in fish is very high, but exceeding particular levels of cereals or seed meals in formulated diets will probably exert a deleterious effect unless some neutralizing treatment can be introduced or applied.
Phytic acids or salts of phytic acid (phytate) are naturally found in plant feedstuffs often used in feed formulations. These compounds characteristically reduce the availability of minerals for absorption. Phytic acid mainly binds with K+ and Mg2+ and can precipitate out as a salt with other cations such as Mn2+ and Ca2+. Soybean meal and rapeseed meal contain 10–15 g/kg and 50–75 g/kg of phytate, respectively. A diet containing 0.5% (5 g/kg) of phytic acid and fed to rainbow trout reduced growth by 10%. Feeding a diet containing 0.21% (2.1 g/kg) of phytic acid adversely affected the activity of trypsin in the intestinal tract of Atlantic salmon. Elimination of the adverse effects of phytic acid and phytate can be effectively addressed through the inclusion of the enzyme phytase in formulated pelleted diets for fish and crustaceans (see the following section).
Gossypol is a yellow lipid‐soluble compound that is found in seeds of cotton plants of the genus Gossypium, except for a ‘glandless’ variety, and has been connected to a variety of toxic effects in several species of fish. The amount of gossypol present in cottonseed meal varies, depending on species and respective growth under different climatic conditions. Toxicity is defined through a variety of responses that include reductions in growth, feed consumption, reproduction and the percentage of red blood cells in the plasma. Histological indicators of gossypol toxicity are lesions on the liver, kidney spleen and reproductive organs. Toxic levels of gossypol appear to vary with species. Age and size of the organisms as well as other ingredients of manufactured feeds can influence the magnitude of response to the same level of dietary gossypol. Potential toxicity of gossypol can be avoided through use of either proportionately low levels of cottonseed meal or glandless varieties containing no gossypol.
The development of accurate methods to identify contaminants in feeds is an important area of future research that can positively impact efficiency of production. The ultimate goal is the definition of limits of their content whereby such information is effectively delivered to feed manufacturers. This information would hopefully serve as the foundation of universal (global) acceptance of regulations for quality control of manufacture of aquafeeds.
8.6.5 Feedstuff Alternatives and Additives
The feed industry and animal food producers continually evaluate alternative feedstuffs and additives that appear to have potential to increase efficiency of production through reduction of cost per unit of animal protein. Glencross et al. (2007) provide a review of strategies to evaluate ingredient suitability for aquaculture feeds based on the foremost considerations of digestibility, palatability and nutrient utilization and interference. The utility of an alternative feedstuff also lies in characteristics of consistent availability and easy storage, handling and shipping. Finally, use of any feedstuff alternative and/or additive must be judged by whether the environmental footprint associated with its manufacture or procurement is comparatively low. These considerations contribute to goals of economic and environmental sustainability. Comprehensive assessments of possible changes in the protein source on the health of the animal, the consumer and the environment relative to inclusion of the alternative feedstuff must be conducted. Another guiding criterion that governs the use of alternatives would be lack of competition for use as sources of human food. An array of aquafeed additives, such as enzymes, carotenoids, prebiotics and probiotics, has become the subject of increased interest by aquafeed manufacturers and commercial growers to increase efficiency of production. Some are already being added into commercially produced feeds, whereas others lack a conclusive demonstration of value or common application.
8.6.5.1 Alternative Feedstuffs – The Search for Fishmeal and Fish Oil Replacements
Despite successful efforts to reduce levels of fishmeal and fish oil in aquafeeds, demand may, at least temporarily, increase due to the need to adopt intensive production systems to address resource conservation, combined with the dramatic increase in the farming of fed carnivorous marine species.
Both feedstuffs are excellent sources of required nutrients, specifically essential amino acids and fatty acids. However, fishmeal and fish oil are ingredients that inflict dramatic pressure on the sustainability of forage (pelagic) fisheries, such as menhaden and anchovy to meet the demand. Progress toward the use of both plant and animal‐derived alternative feedstuffs, in concert with appropriate economic and regulatory incentives, will substantially improve the perception of aquaculture as being a highly favourable complement to capture fisheries to meet global protein demand through the use of feeds containing sustainable ingredients.
A number of potential sources of alternatives to the use of fishmeal have been identified. Plant‐based protein sources include canola, soybeans, peas, lupines and wheat. Soy protein concentrate and wheat are potentially good options, but their use is not cost‐effective. During the past 20 years, an abundance of published investigations using different fed aquatic species has focused on the use of different soybean meals to serve as a complete or partial replacement of fishmeal. These studies have revealed that generally soybean meal protein can effectively substitute for up to 50% of the fishmeal protein. Plant‐derived protein sources are not as digestible as equivalent amounts of animal‐derived sources because they contain fibre and insoluble starches that are not easily digested by carnivores and some omnivores, and therefore cannot be used as a source of energy. Exclusive use of protein from one plant source results in a deficiency in one or more essential amino acids to meet the dietary requirements. Therefore, successful use of plant‐derived sources of dietary protein will most probably be realised through the inclusion of other‐sources of plant protein in combination with dietary supplements of individual amino acids to ultimately meet species‐specific requirements.
Use of plant‐derived protein sources in aquafeeds is also associated with other detrimental consequences such as the absence or reduced levels of LC‐PUFAs, the reduction of palatability and corresponding reduction in feed consumption, and nutrient interaction whereby, for example, dietary phosphorous is nutritionally unavailable to the farmed organisms and consequently released into the environment. Inclusion of the widely used soybean meal as a substitute could also be limited by lack of availability due to demands for its use in the manufacture of feeds for terrestrial animal production species and foods for human consumption.
Rendered meals derived from waste products of land animal processing as well as defatted meal prepared from insects fed fish processing wastes establish them as strong replacements for fishmeal. They are attractive options because they share important characteristics of high protein content, the desired balance of essential amino acids, and high digestibility. Fishmeal derived from the wastes of the processing of both cultured and captured fish also represents at least a partial substitute of fishmeal derived from the processing of pelagic fish like menhaden or sardines. Recycling of wastes to produce these feedstuffs contribute to both environmental and economical sustainability.
The use of plant‐derived oils such as soybean oil as a 1:1 substitute for fish oil is not possible due to the absence of essential LC‐PUFAs that are characteristic of marine fish oils, i.e., marine food chains. A combination of plant and fish oils, thereby reducing the use of fish oils, has been the feed formulation strategy in the production of effective salmonid feeds. The fish oil portion of the total oil content of the feed proportionately increases during the few months prior to the intended harvest. This feed management practice is designed to enhance the content of LC‐PUFAs in the muscle tissue and thereby enhance nutritional value of the fillets sought by the consumer. Finishing diets containing a fish oil have been successfully used to ‘restore’ the fatty acid composition of fish tissue after feeding a diet containing plant‐based oil for most of the grow‐out period. Fish oil derived from the waste left from the processing of fish from the culture and capture fisheries also appears to be an attractive, cost effective source of required LC‐PUFAs to include in diets.
Sources of protein and oil derived from single cell culture, provided as the entire organism or as an extract (lipid), are receiving considerable attention. Promising results have been realised under small‐scale laboratory production; extrapolation to large scale production for cost‐effective use in aquafeeds will likely become a reality. Future provision of dietary LC‐PUFAs could ultimately reside in plants that have been genetically modified to produce LC‐PUFAs.
The use of a meal derived from the culture of insects has become the subject of interest as an alternative ingredient in feeds for aquatic organisms (see section 27.3.3.1; Figure 27.9). Deficiencies in essential amino acid or LC‐PUFA content can be rectified by the type of food fed to larval forms of insects. When fish offal was provided as food for soldier fly larvae in different proportions of the total diet for 21 days, tissue levels of LC‐PUFA expressed as weight percent of the total lipid, increased by 2.3 to 2.7 %. This notable level of change was also achieved when larvae were fed for only 24 hours after being removed from a control diet consisting of no fish offal. This specific insect meal effectively replaced 15 % of the fishmeal protein and 38 % of the fish oil ingredient in a diet fed to rainbow trout Oncorhynchus mykiss. Total lipid in insect meal characteristically exceeds that of fishmeal (~30% vs. ~ 10 %) and may need to be reduced by an extraction procedure if an economically and environmentally acceptable product for use in aquafeeds is to be developed.
8.6.5.2 Feed Additives
The aquafeed industry uses certain feed additives with the principal intent to increase production efficiency through improvement of growth and production, and enhancement of resistance to pathogens. Additives are also used to achieve a colouration that will be appealing to the consumer. This section will focus on carotenoids, enzymes, prebiotics and probiotics.
Carotenoids are compounds that are responsible for the pigmentation found in farmed species. Lacking the biochemical ability to synthesise these compounds de novo, animals are dependent on an exogenous source (food) of them to achieve colouration. Astaxanthin, a xanthophyll, is the primary carotenoid found in the tissue of fish and crustaceans. Therefore, to achieve the appropriate level of pigmentation in meeting consumer acceptance of farmed aquatic species, the feed must contain a sufficient concentration of carotenoids. The combined dietary level of carotenoids derived from feedstuffs is commonly insufficient to produce the desired colour and intensity of pigmentation. Therefore, a carotenoid is generally added to the feed. Different astaxanthin‐containing animal‐derived products are used in aquafeeds (NRC 2011) and copepod, shrimp, krill or crab oil have the highest concentrations, ranging from 50–150 mg/kg.
The reddish orange colouration of the flesh of salmon is due to the presence of two oxygenated carotenoids, astaxanthin and canthaxanthin (xanthophylls) which are commonly added as synthetic forms in the feed fed to salmon. Recommended dietary levels of these specific xanthophylls range from 50 to 150 mg/kg to maintain tissue levels that yield consumer acceptance. Natural sources of astaxanthin which may ultimately become cost‐effective additives include the red yeast, Phaffia rhodozyma and Hematococcus species of algae. The different carotenoids found in plants such as lutein and zeaxanthin are metabolic precursors in the synthesis of astaxanthin and canthaxanthin but use of these xanthophylls as dietary additions does not yield pigmentation that is commensurate with animal‐derived carotenoids. Therefore, the most efficient method to attain the desired pigmentation in a farmed species is to provide the carotenoid that is principally found in the tissue. When singular sources of different carotenoids were included in diets fed to juvenile lobsters, Homarus sp. which contain astaxanthin as the tissue carotenoid, the intensity of pigmentation in the shell and tissue was found to be directly related to the proximity of the specific dietary carotenoid to astaxanthin in the biosynthetic pathway. This carotenoid‐specific graded response would most probably be manifested in other aquaculture species in other phyla such as molluscs and echinoderms. The ability of organisms to retain the desired pigmentation is influenced by an array of factors which include rates of absorption, transport, metabolism and excretion. Maintaining particular intensity and colour is critical to achieving a marketable product.
The use of enzymes in aquafeeds has gained interest in an effort to eliminate the adverse effects on production of certain compounds that exist in plant feedstuffs. The primary limitation to use of exogenous enzymes in aquafeeds is cost and the loss of activity that generally ceases around 95 °C. Thus, the enzymes cannot withstand the much higher temperatures used in the preparation of mixtures of feed ingredients prior to compressed or extruded pelletisation. Capitalizing on the potential benefit of enzyme additives lies in technology that will successfully protect them from high temperatures or surface application subsequent to the manufacture of the feed.
Phytase, a microbial‐derived enzyme, has been used to improve the availability of phosphorous that composes part of the phytate compound found in plants. It eliminates the ability of phytate to bind micro‐elements (see section 8.4.6) and cause them to be unavailable for uptake. As the phosphate is enzymatically removed from the phytate molecule, the capacity to bind to micro‐elements is eliminated. The release of phosphorous confers an improved availability, as manifested by increased amounts of phosphorous retained in bone and less phosphorous appearing in faeces. This combined result has been repeatedly demonstrated for several species of fish fed diets containing phytase. The ideal phytase would be heat stable, effective at low pH and low phytate concentrations, and demonstrate a sustained activity within the gastric region. There is some evidence of the beneficial effect of the addition of carboxyhydrases in diets containing carbohydrates whereby increased digestibility has been determined, but convincing evidence as that found from studies with pigs and poultry is lacking. A mix of directly fed microbials (DFM) and enzymes, i.e., spores of three Bacillus strains, and xylanase, amylase and protease improved the gut health, performance (body weight gain associated with improved feed efficiency) and welfare of poultry. Synergistic activities between the DFM and the enzymes increased the digestion of nutrients, thereby decreasing the FCR. With the additional of this microbial and enzyme mix, energy utilization from high fibre diets was found to be as good as that from a diet containing a lower amount of fibre. These results may have applicability to aquatic organisms.
Use of probiotics and prebiotics in aquaculture has continued to engender interest and widespread application in aquaculture enterprise may eventually be realised. Probiotics are live microbials that are generally added to the diet of an organism to modify the microbial composition (microbiome) of the gastrointestinal tract. Recognised probiotics include bacteria of the genus Bacillus and different lactic acid bacteria such as Lactobacillus sp. and Streptococcus sp. Dietary probiotics have been found to confer disease resistance in shrimp farming. Establishment and maintenance of these changes in the gut microbiome are founded on yielding enhanced growth and increased efficiency of digestion, and increased immunocompetence/disease resistance. Successful use of probiotics is dependent on the ability to preserve the viability of the live organisms under the conditions of feed manufacture. Nonetheless, some evidence suggests that dead cells, freeze dried cells or extracts can be added to diets to serve as a vehicle for the control of pathogenesis. Some probiotics have been produced for introduction into the culture environment, but the claims of success lack documentation of consistency and reliability.
In contrast to probiotics, prebiotics are compounds that, when added to an organism’s diet, promote the growth of bacterial species and/or enhance the metabolic activity of resident intestinal bacteria to improve feed efficiency. Oligofructose and inulin are two inert/indigestible compounds that have been shown to alter the intestinal microbiome and enhance nutrient transport in the small intestine of mice fed diets containing them. This observation established an early foundation for the potential benefit of their use as additives to diets for monogastric aquatic animals. Other potentially effective prebiotics include oligosaccharides such as mannanoligosaccharides, fructooligosaccharides and galatooligosaccharides (see section 8.4.3). Even certain dietary fatty acids and carbohydrates have altered the composition of microbial populations in the gastrointestinal tract of fish. These demonstrated effects are good evidence to expect that dietary prebiotics will continue to elicit interest and continued testing in aquafeeds whereby the altered intestinal microbiome confers a higher resistance to disease. Such an achievement will serve as a positive contribution to the elimination of use of sub‐therapeutic levels of antibiotics as part of the management of production systems and thereby hinder the occurrence of unwanted antibiotic resistant strains of pathogens.
Currently, documentation of the beneficial effects of probiotic and prebiotic additives to feed to improve growth rate, digestion, immunity and resistance to pathogenic infections is encouraging. However, before these additives become routine additions to aquafeed, extensive standarised evaluation of response and cost effectiveness will be required for application to the diverse array of aquaculture species.
Recent investigations have demonstrated the effectiveness of the addition of short chain fatty acids (SCFAs), often termed organic acids, as dietary additives that confer antibiotic activity. Low concentrations of formic acid have been effective in inhibiting the growth of five different species of Vibrio grown in liquid media. Enhanced immune responses to Steptococcus galactiae in red hybrid tilapia and to Vibrio harveyi in Penaeus monodon have been observed in response to specific dietary SCFAs.
8.7 Nutrition Management Strategies
Applying knowledge of nutritional requirements to the formulation and manufacture of feeds is complemented by an effective provision of these nutrients combined with control of anti‐nutrient compounds, the management of feedstuffs and the use of additives. In addition, natural food whose growth can be promoted and sustained in ponds can also play a noteworthy role in satisfaction of nutrient requirements and reductions in costs of production. The common goal of these strategies is to achieve the highest feed efficiency (lowest FCR).
The expected increase in the global demand for animal protein, particularly demand for farmed seafood, coupled with restricted resources (water, land, energy and capital) and global environmental change will undoubtedly impact how aquatic animals will be farmed. The characteristics of different production strategies and their comparative relationships to meet the challenges are presented in Figure 8.6. Under most scenarios, that movement will require a transition to highly intensive culture systems (NRC, 2011) that preserve sustainability with the goal of achieving the highest production per unit of area (space) and other resources utilised. In most cases, a large proportion of the variable costs associated with intensive systems will lie in the purchase, storage and provision of a nutritionally complete and balanced feed. Development of feeding practices for formulated feeds and, in some cases, capitalizing on natural food organisms as a complementary resource within the production unit itself will be essential for ensuring economic and environmental sustainability. The potential interest in production systems that address the trophic feeding behaviour of two or more species simultaneously has continued to gain interest, evaluation and application. These systems are termed Integrated Multi‐Trophic Aquaculture (IMTA) and they serve to meet the important goal of funnelling nutrient excess that can be characteristic of intensive monoculture systems into production of another species.
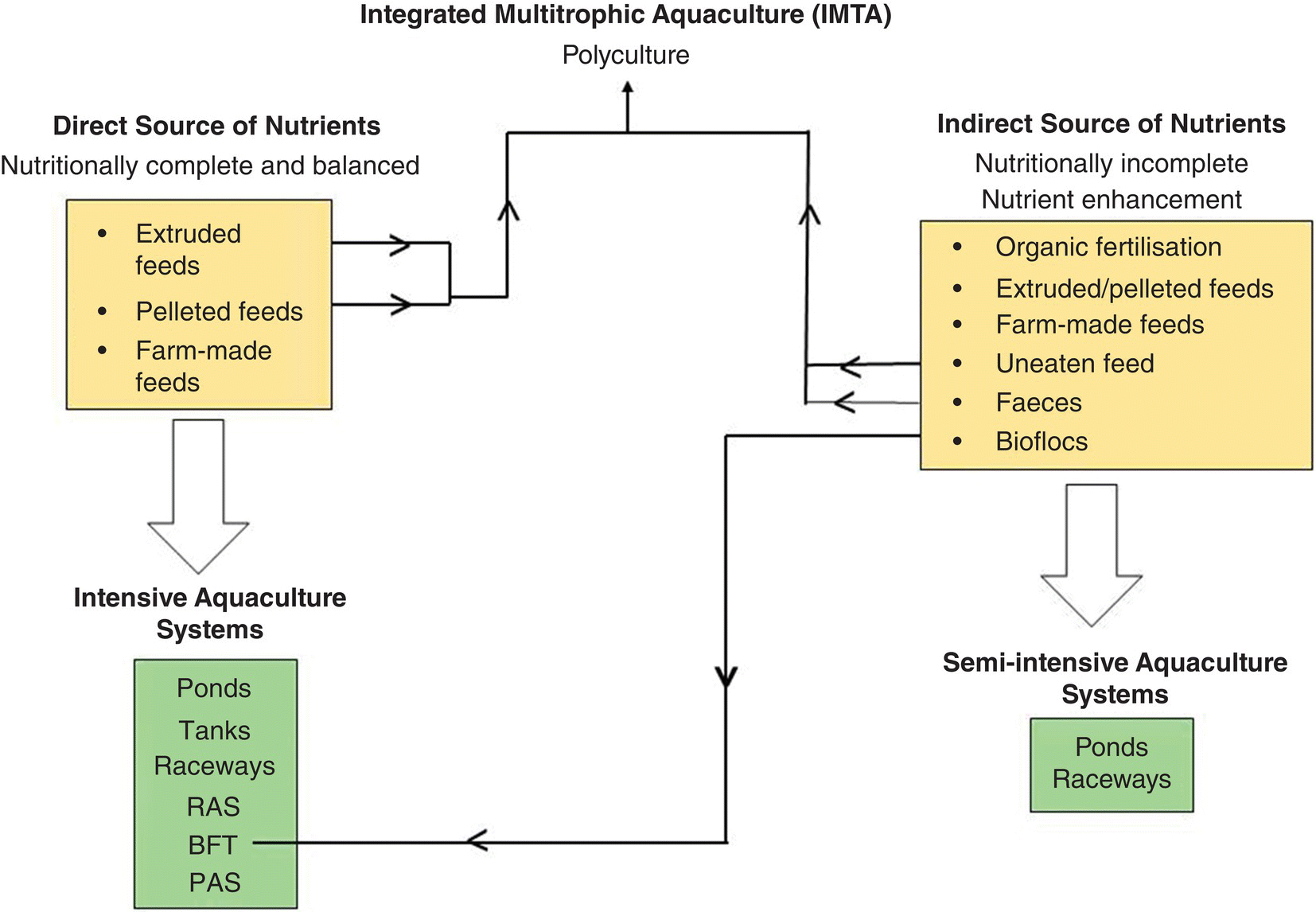
Figure 8.6 Sources of nutrients and associated aquaculture production systems; RAS‐recirculating aquaculture systems; BFT‐biofloc technology; PAS‐partitioned aquaculture systems.
8.7.1 Use of Natural Food as Complementary Nutrient Sources
Other practices that together use formulated feed, organic fertilisers and live food resources derived from a pond production unit itself have evolved to reduce feed costs and ensure good water quality for culture. A noteworthy advance is founded in biofloc technology (BFT) which has improved farming in traditional freshwater and saltwater ponds by conservation of water and land resources in concert with biosecurity and higher feed efficiency. The technology promotes the production of bioflocs which are aggregates of algae, protozoans, bacteria and particulate organic matter that help to maintain good quality water for culture through uptake of nitrogen and phosphorous. In addition, animal consumption of bioflocs is a good source of nutrients (nitrogen) and will usually permit a reduction in the protein content of the feed that translates into a corresponding cost reduction of the feed. BFT systems are a good example of addressing the special challenges of intensive aquaculture through management techniques designed to establish ecological stability. These systems are impressive and proven, having been in operation at the commercial level for shrimp and tilapia species since the beginning of the 21st century. Variations of this biotechnology exist and warrant the need to understand and establish common principles whereby a successful transition for application to other species can be realised.
The biology of some farmed species dictates a different approach whereby the best approach lies in semi‐intensive production systems where use of nutritionally complete and balanced feeds is not needed. Two good examples are crayfish and freshwater prawns. Crayfish, Procambarus clarkii and P. acutus, are often grown in the USA in shallow ponds where a crop grown in rows (section 23.4.5.2), often certain strains of rice, has been previously planted and then filled with water to a shallow depth. Upon filling of the pond, crayfish emerge from burrows where they had previously retreated when the water was drained. The plant, which may or may not be harvested, does not serve as a principal source of nutrition per se, but rather as an indirect source of nutrition by serving as a substrate (detrital food chain) whereby secondary productivity is enhanced and is consumed by the crayfish. The crayfish are not fed any commercially‐produced feed.
Highly efficient, i.e., sustainable, freshwater prawn, Macrobrachium rosenbergii, farming is realised within semi‐intensive systems to manage as much as possible the density dependent growth rates and the existence of intraspecific aggressive and agonistic behaviour. Under the semi‐intensive production system, consumption of benthic populations of natural pond organisms (macroinvertebrates) contributes to the satisfaction of nutrient requirements. With the use of distiller’s dried grains and solubles (DDGS) either applied as a fertiliser or provided in the form of a pelleted feed, standing populations of macroinvertebrates in prawn production ponds were significantly higher than those in the ponds where neither feed nor fertiliser was used. Consumption of benthic macroinvertebrate populations occurred, and certain taxonomic groups were preferentially consumed. The results suggest that at semi‐intensive stocking densities, less expensive nutritionally incomplete feeds could be used to satisfy nutrient requirements throughout the duration of the grow‐out period in ponds as long as populations of natural food organisms were sustained and consumed by prawns as part of their diet. As a result, it has been observed that commercially‐prepared pelletised feeds of a crude protein content of no less than 18%, such as corn gluten pellets, range cubes, or wheat middlings, are sufficient to achieve the same growth as that achieved with nutritionally complete, commercially‐manufactured prawn feed that commonly contains approximately 30% crude protein. Levels of macro‐ or micro‐nutrients within the pellets were insufficient to satisfy nutritional requirements, but were fulfilled through the consumption of the secondary productivity in the pond. Comparison of nutrient (fatty acid) composition of the pellet versus the tissue supported this assumption. Tissue analysis revealed that EFAs were completely absent in the pelleted feed, but requirements could be satisfied through consumption of natural biota. This management strategy also allowed use of a feed that contained no fishmeal or fish oil.
The semi‐intensive systems described for crayfish and freshwater prawn culture are highly sustainable, representing a trade‐off of intensive production for a reduction in the risk of the incidence of economic loss. Based on different feeding strategies that would be characteristic of intensive and these semi‐intensive systems, the cost of production per unit of protein would probably be comparable.
8.7.2 Integrated Multi‐TrophicAquaculture (IMTA)
IMTA systems are designed to improve the efficiency of production by combining a species receiving a manufactured feed with an extractive species that derives nutrients from faeces, excess (uneaten) feed or other nutrient overload originating from the fed species. For example, fish or shrimp would be the fed species and extractive species could be other fish or shrimp, echinoderms (sea cucumbers, sea urchins) or molluscs (mussels, clams and oysters). This approach helps to meet important goals of environmental sustainability through efficient nutrient cycling, as well as economic sustainability.
For example, intensive cage culture of species of fish in freshwater ponds or in the ocean offers the opportunity to increase yield by incorporating a species that is an inhabitant of the benthos as part of a polyculture system. Uneaten feed and faecal material fall through the cage and essentially become substrates for the colonization of nutrient rich microorganisms. Under the cages, benthic communities proliferate and become a source of food for a farmed benthic species. Preliminary evaluations of the potential for culture of the sea cucumber, Apostichopus japonicus, in the water column below fish cages in Japan, have been conducted. Such systems offer an opportunity to achieve both environmental and economic sustainability. Technological advances in IMTA may also find application to RAS systems whereby extractive species may assist in the improvement of water quality for culture of the fed species.
8.8 Feed Management
As already discussed, feed performance is founded in its formulation, manufacture, transport and storage. Growth/Ration (G/R) curves addressed in section 8.3 attest to the fact that maximum growth and high feed efficiency do not coincide at the same feeding rate. The amount of formulated feed consumed per aquatic organism per day is dependent on an array of factors that are both abiotic, i.e., water temperature, level of dissolved oxygen, salinity, photoperiod, barometric pressure and biotic, i.e., weight, intraspecific behaviour, health, age, life history, location in moult cycle (in crustaceans), natural food availability and whether a hybrid is the choice for culture. The time of day that feed is provided may also affect consumption, growth and feed efficiency. However, a plan to feed at a certain time of the day may not be logistically possible due to the sheer size of commercial operations, thereby resulting in feeding occuring over an extended interval of time during the day.
Because the amount of feed consumed is influenced by many factors, many operations offer feed to satiation. This method of feed management offers the opportunity to address the combined effect of all variables and presumably minimises the loss of uneaten feed which contributes to adverse environmental conditions, which include low oxygen concentration and increased phosphorous levels. For the culture of certain species of fish, this ability can be accomplished through the use of floating or slow sinking feeds whereby feeding behaviour can be observed. In order to feed to satiation, the act of feeding and the point of satiation must be observed, and this method of feeding can actually increase FCRs because determining satiation can be highly subjective. Higher amounts of feed are accordingly provided, weight gain is highest but FCRs are also higher. For those aquatic species that are benthic in nature, for example, shrimp, sea urchins and abalone, the amount of uneaten feed must be monitored to adjust feeding rates, particularly as biomass and age increases. For intensive culture of marine shrimp, feeding trays, positioned at different locations in a pond are used to monitor feed consumption (Figure 22.17). A certain amount of feed is placed in a feeding tray based on temperature and size of shrimp. Adjustments to the feeding rate, based on a percent of body weight, estimated mean weight and survival are made based on whether no feed or excess feed is found in the feeding tray after a certain period of feeding. The weight, generally age, of a species of aquatic organism has a specific feeding rate (% of body weight per day) that will achieve the lowest FCR and that feeding rate may be divided among several feedings. Feeding rates based on temperature and individual body weight of the Pacific white shrimp, Litopenaeus vannamei have been developed and applied in grow‐out systems.
Feed consumption, growth and accordingly feed conversion efficiency may be affected by feeding frequency or time of day. Generally, the feeding of larvae involves several feedings per day, due to a high energy demand. For feeding of juvenile, sub‐adult or adult aquatic organisms, the effect of feeding once or twice per day, or within certain intervals, i.e., every two days has been studied. An increase in daily consumption is commonly associated with an increase in frequency of feeding. If these feed management schemes do have a positive effect on feed efficiency, this effect must be weighed against the economic cost. No significant differences in amount of feed fed, weight gain or estimated survival were found when juvenile channel catfish were fed to satiation once daily, either in the morning or the afternoon or twice daily, morning and afternoon. Feeding to satiation every other day reduced weight gain but improved the FCR and this same result was observed with hybrid catfish. Restricted feeding rates (feeding to a maximum/pond area/day) also reduced weight gain and net yield while FCR correspondingly decreased. When more feed is fed under satiation feeding, weight gain and yield is higher but FCR also increases. The advantage of optimum weight gain rather than maximum weight gain is a concept that many farmers fail to acknowledge despite optimum weight gain being the obvious choice to achieve economic sustainability. A comprehensive review of feed management strategies used globally in aquaculture is available (Shipton and Hasan, 2013).
The use of demand feeders has gained popularity for feeding rainbow trout. The demand feeder is cone shaped and attached to it is a rod that extends into the water column. Inside the cone is a disc that has a diameter that is slightly smaller than the diameter of the cone at that location. The disc holds the feed from falling into the water. However, when the rod is ‘hit’ by a fish the disc moves and permits feed to fall into the water. The location of the disc can be changed to increase the amount of food that is released when the rod is triggered by the fish. This method of feeding minimises incidence of poor water quality and diminishes the detrimental effects on growth of fish that would result from aggressive encounters among fish of different size or some other behavioural hierarchy. Methods for distributing feed to cultured fish and shrimp are described in later chapters (see Figures 17.14, 17.15, 22.15 and 22.16).
8.9 Emerging Research Areas
Nutrigenomics, the study of the effects of foods and food constituents on gene expression, and metabolomics, the study of the production and identification of chemical biomarkers influenced by genetic and environmental factors, are areas of research with high potential for application to the development of feeds with both environmentally and economically sustainable properties. Metabolomics represents the molecular phenotype and may focus on the metabolic products of the whole organism, or tissue, cell or cell organelle level. The objective is to understand how alternative feedstuffs or different nutrients can impact and regulate gene expression and ultimately influence specific products of metabolism. With knowledge of the full gene sequence maps of aquaculture organisms, control of gene expression through diet and breeding of organisms can be used to optimise responses to changes in the environment and available resources. The capacity assumes particular significance in how changes in nutrition and feed management can effectively address regional changes in environments and their available resources as imposed by the phenomenon of global warming.
Some recent research results are based on an approach, geometric framework (GF) that contrasts with the traditional nutrient requirement evaluation responses to graded dietary levels. GF methodology investigates whether animals, if provided with an array of dietary choices, will self‐control the intake of specific nutrients to ultimately satisfy requirements. For example, consumption of dietary protein by adult sea urchins, Lytechinus variegatus, has been observed to be regulated and not affected by either ratios or levels of other dietary macronutrients, whereas consumption of dietary carbohydrate is not regulated. Knowledge of whether such selectivity is a strategy exhibited by other farmed aquatic animals has the potential to be applied to species and age‐specific formulation of aquafeeds.
8.10 Summary
- A significant amount of knowledge of nutrition, feed production and feed management for farming of animal aquatic species has been generated over the past 50 years and has had an important impact on the extraordinary increases in the volume and value of global aquaculture production.
- Due to reduced needs of aquatic organisms for energy, aquaculture organisms are more efficient because less food needs to be consumed per body weight increase (protein deposition) relative to terrestrial animals. Thus, this aspect of aquatic animal production is a defining characteristic of sustainable farming to meet the future global needs of animal protein.
- Additional noteworthy advances in the nutrition of farmed aquatic animals can be more readily achieved by embracing a systems‐based holistic approach founded on a continued focus on the determination of nutrient requirements and a complementary understanding of the metabolism and utilization of nutrients and their possible interactions.
- The sheer number of aquatic species being farmed warrants a goal of the establishment of feeds and feeding practices that will have common application based on similar phylogeny, feeding habits, life history and natural environment (temperature, salinity, etc.).
- Selection of new and alternative feedstuffs for use in aquafeeds, particularly sources of protein and fishmeal and fish oil substitutes, must be consistent with meeting the standards of environmental as well as economic sustainability. Choice of ingredients should also focus on alternatives that will achieve equivalent or greater benefits to animal health (disease prevention) and increase feed efficiency and thereby contribute to global food security.
- Application of environmentally and economically sustainable probiotic and prebiotic feed additives whereby the intestinal microbiomes are altered to achieve greater production efficiency and pathogenic resistance must be a future focus of aquaculture nutrition.
- The provision of nutrients to meet requirements in association with sustainable aquaculture should be composed of several species‐specific strategies that include not only direct provision of feeds but also indirect consumption of nutrients through the enhancement of primary and secondary productivity of pond production systems as well as the implementation of successful IMTA.
- With the development of full gene sequence maps for species of aquaculture importance, an increase in the knowledge of the effects of nutrients on gene expression (nutrigenomics), is possible. This information offers the ability to establish an understanding of the interaction of diet with such responses as susceptibility to disease and feed efficiency. In addition, identification of biomarkers, metabolic products that reflect changes in physiological response (metabolomics) can lead to a successful program of breeding that would promote efficient response under specific culture conditions. This knowledge will be useful in making adjustments at the molecular level to regional changes in environments and their available resources arising from the phenomenon of global warming.
References
- Ceccaldi, H. J. (1997). Anatomy and physiology of the digestive system. In: D’Abramo, L. R., Conklin, D. E., and Akiyama, D. M. (Eds.) Crustacean Nutrition, pp. 261–291. The World Aquaculture Society, Baton Rouge, LA, USA.
- D’Abramo, L. R., Conklin, D. E. and Akiyama, D. M. (Eds.) (1997) Crustacean Nutrition. Advances in World Aquaculture Volume 6. The World Aquaculture Society Baton Rouge, LA, USA.
- Duarte, C. M., Holmer, M, Olsen, Y., Soto, D., Marbá, N., Guiu J., Black, K. and Karakassis, I. (2009). Will the oceans help feed humanity? BioScience, 59(11), 967–976.
- FAO, (2014). The State of World Fisheries and Aquaculture: Opportunities and Challenges. Rome: FAO.
- FAO. (2009). How to Feed the World in 2050. FAO: Rome http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf [Website accessed January 2017]
- Glencross, B. D. (2009). Exploring the nutritional demand for essential fatty acids by aquaculture species. Reviews in Aquaculture, 1, 71–124.
- Glencross, B. D., Booth, M. and Allan, G. L. (2007). A feed is only as good as its ingredients – a review of ingredient evaluation strategies for aquaculture feeds. Aquaculture Nutrition, 13, 17–34.
- Hamre, K., Yufera, M., Ronnerstad, I., Boglione, C., Conceicao, L. E. C. and Izquierdo, M. (2013). Fish larval nutrition and feed formulation: knowledge gaps and bottlenecks for advances in larval rearing. Reviews in Aquaculture, 5 (Suppl 1.), 26–58.
- Horn, M. H. (1998). Feeding and digestion. In: D. H. Evans (Ed.) The Physiology of Fishes, 2nd edition. pp. 43–64. CRC Press LLC
- Izquierdo, M. S., Fernandez‐Palacios, H. and Tacon, A. G. J. (2001). Effect of broodstock nutrition on reproductive performance of fish. Aquaculture, 197, 25–42.
- NRC (National Research Council of the National Academies) (2015). Critical Role of Animal Science Research in Food Security and Sustainability. The National Academies Press, Washington, D.C.
- NRC (2011). Nutrient Requirements of Fish and Shrimp. The National Academies Press, Washington, D.C.
- NRC. (1993). Nutrient Requirements of Fish and Shrimp. The National Academies Press, Washington, D.C.
- Shearer, K. D. (2000). Experimental design, statistical analysis and modelling of dietary nutrient requirement studies for fish: a critical review. Aquaculture Nutrition, 6, 91–102.
- Shipton, T. A. and Hasan, M.R. (2013). An overview of the current status of feed management practices. In Hasan, M. R. and New, M. B. (Eds.) On‐farm Feeding and Feed Management in Aquaculture. pp. 3–20. FAO Fisheries and Aquaculture Technical Paper No. 583. Rome, FAO. http://www.fao.org/3/a‐i3481e.pdf [accessed January, 2017].
- Takeuchi, T. (1997). Essential fatty acid requirements of aquatic animals with emphasis on fish larvae and fingerlings. Reviews in Fisheries Science, 5, 1–25.
- Webster, C. D. and Lim, C. E. (Eds.) (2002). Nutrient Requirements and Feeding of Finfish for Aquaculture. CABI Publishing, New York, NY.
- Wouters, R., Lavens, P., Nieto, J. and Sorgeloos, P. (2001). Penaeid shrimp broodstock nutrition: an updated review on research and development. Aquaculture, 202, 1–21.