22
Shrimps
Darryl Jory
22.1 Introduction
22.1.1 History of Shrimp1 Farming
In the last three decades, farming of various shrimp species has developed tremendously. In 2015, shrimp farms contributed ca. 60% of the world’s shrimp demand, continuing to replace traditional fisheries as market demand suppliers. In addition to generating 25 billion USD in trade, shrimp farming also provides employment for millions of people in developing nations, by:
- incorporating into production vast areas of previously unutilised coastal land unsuitable for other types of development;
- producing a valuable commodity that is increasingly marketed locally in many countries; and
- generating needed jobs and hard currency through significant exports.
The industry has also generated some environmental issues that have been acknowledged and properly addressed in most cases.
Shrimps have been grown in South‐East Asia for centuries by farmers who raised them as incidental crops in tidal fish ponds. Modern shrimp farming began in the 1930s, when Motosaku Fujinaga, a graduate of Tokyo University, succeeded in spawning the Kuruma shrimp2 (Marsupenaeus japonicus). He cultured larvae through to market size in the laboratory and successfully mass produced them commercially. Dr Fujinaga generously shared his findings and published papers on his work during the next 40 years. He was honoured by Emperor Hirohito with the title ‘Father of Inland Japonicus Farming’. In the early 1970s, researchers and entrepreneurs in various countries in Asia and Latin America became involved in promoting development of the industry, which grew steadily. Shrimp farming has come a long way in the last 25 years, and enormous progress has been made in developing technologies and methods to culture shrimps. The industry began a tremendous expansion in the early 1980s. Major references on global shrimp farming include Browdy and Jory (2001, 2009), and Alday‐Sanz (2010).
22.1.2 Current Status and Production
Shrimp farming has been practised worldwide in over 60 countries for at least the past four decades, but production is mostly concentrated in about 15 nations in Asia and Latin America (Figure 22.1). These regions account for about 95% of all farmed shrimps. Since 1992, about a dozen countries have contributed about 95% of farmed shrimp production and the top world producers have been, at various times, Ecuador, Taiwan, Indonesia, China and Thailand. The shrimp farming industry in China has really exploded in recent years, and the country has uninterruptedly been the world’s leading producer since at least 2003 (Figure 22.2).

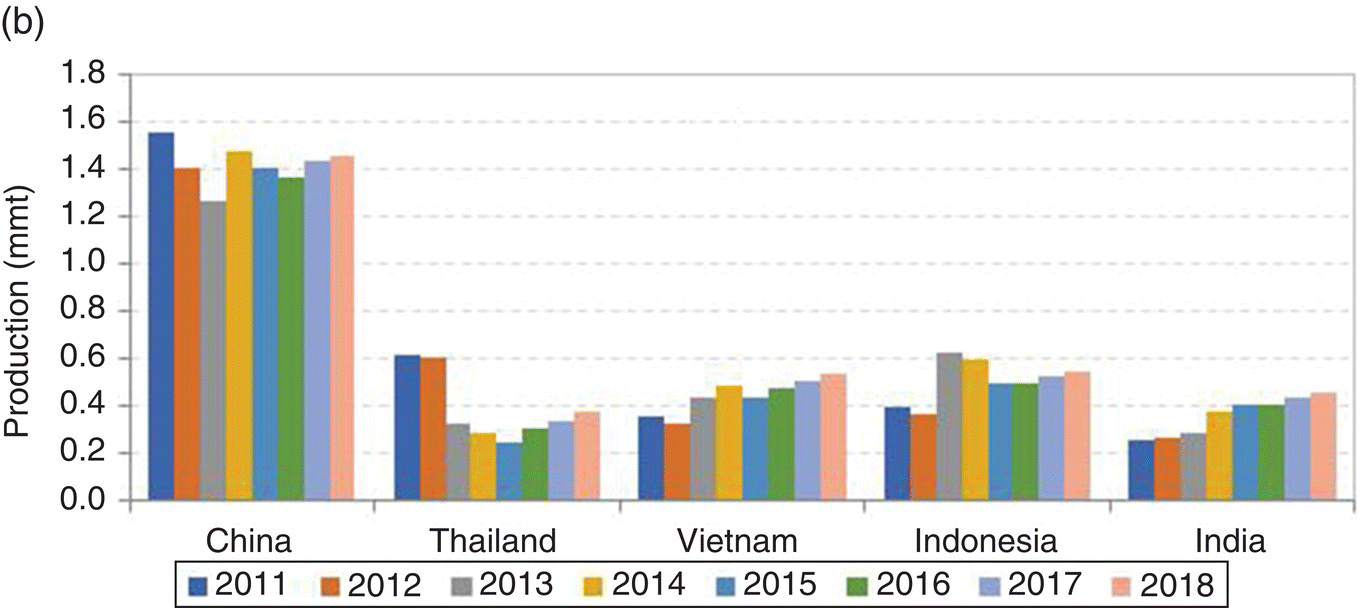
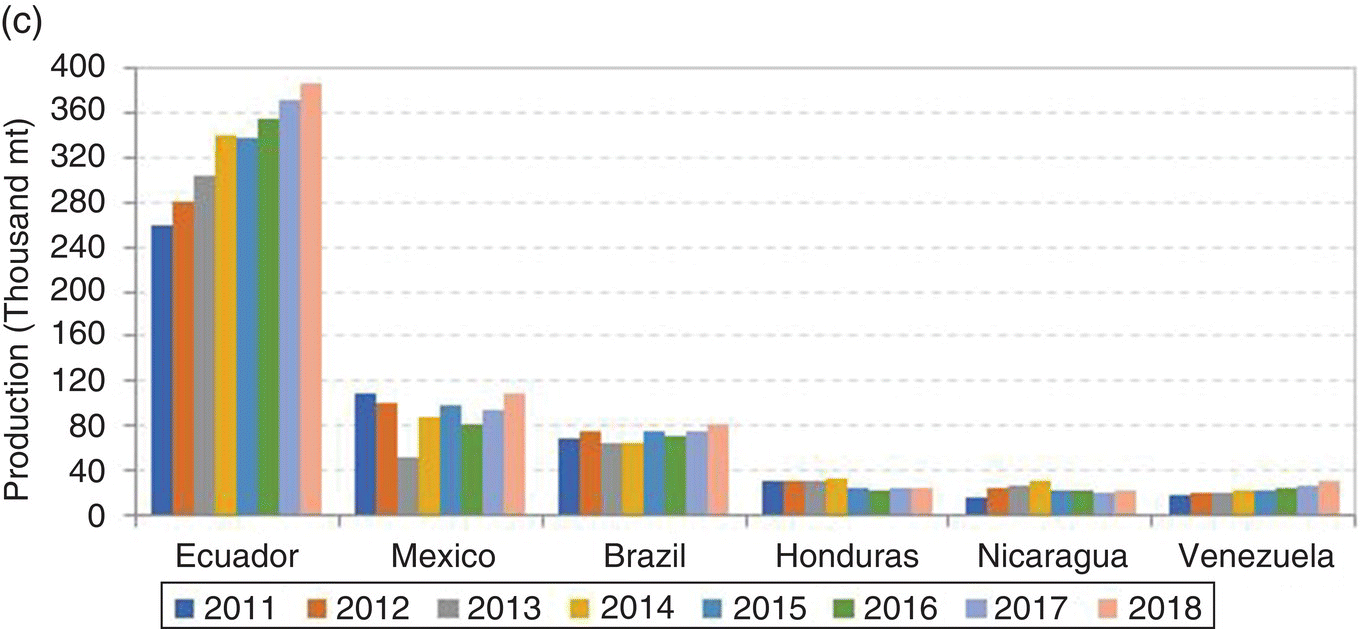
Figure 22.1 Total shrimp farming production (a) and production by the two major global regions: Southeast Asia (b) and the Americas (c).
Sources: FAO (2008−2011); FAO and GOAL3 2014 (2012−2013); FAO and GOAL 2015 (2014); GOAL 2016 (2015−2018). From Anderson et al. (2016). mmt = million t (or 1 x 106 t); thousand mt = thousand t (or 103 t). Source: Reproduced with permission from Darryl Jory.
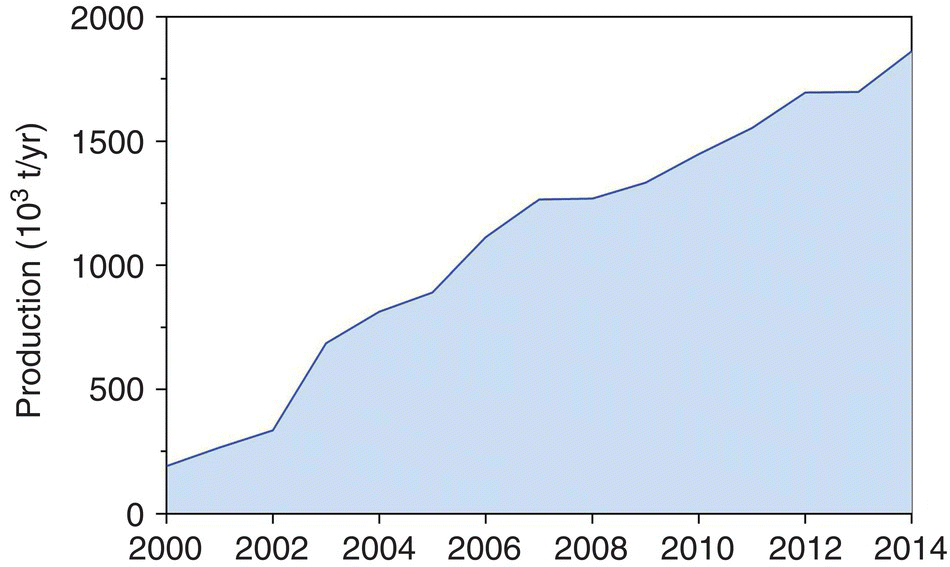
Figure 22.2 Recent growth of shrimp farming in China (FAO, 2016).
Source: Reproduced with permission from John Lucas.
Global production has been increasing steadily in the past two decades, from about one million t in 1995 to almost 5 million t: the latest global production estimates for 2014 (FAO) totals 4.875 million t.
Production is still increasing in various countries by responsibly intensifying culture methods, without having to develop large areas. Most of the best locations for farms have already been developed, but the industry can still expand in several countries, including various Latin American and African nations. There are shrimp farms in Algeria, Egypt, Nigeria, Ghana, Mozambique, Madagascar and probably several more African countries. Shrimp culture is a very minor activity in several European countries (Spain, Italy, France, Netherlands, Latvia and others), and Japan and the USA are relatively small producers owing to cool weather, high production costs, limited areas with suitable conditions, and the difficulty of competing cost‐efficiently with industry leaders. In the USA, at least 100 or more small producers, mostly using indoor recirculating systems, are growing shrimps for local niche markets.
Many developed countries are indirectly but nevertheless significantly involved in the shrimp farming industry as large consumers, as producers of supplies and materials for the industry, and in providing substantial technical expertise on production and processing techniques.
The industry generates at least 4 million direct jobs and many more indirectly through ancillary industries. It also generates USD 12−15 billion in international trade annually, ca. 10−15% of all seafood of all origins traded globally. It also creates much more additional economic activity centred on ancillary industries like aquafeeds, processing, various equipment, pharmaceutical chemicals, transportation, marketing, R&D and others. It is based mostly on one species with relatively few selectively‐bred, improved lines. The Pacific white or whiteleg shrimp (Litopenaeus vannamei) is the most important species in the world, with virtually all production coming from aquaculture. This species represents around 75% of all farmed shrimps globally, and over 40% of all shrimps produced in the world (FAO, 2016).
Between 1975 and 1985, global production of farmed shrimps increased by 300%, and between 1985 and 1995 by 250%. Since 1995, however, industry growth has been significantly slower due to various viral and bacterial diseases (Figure 22.1a). Costs have continued to increase and market prices have experienced substantial fluctuations over the past decade or so, with major variability and unpredictability in the costs of most inputs like energy and grains, with significant ingredient replacement in aquafeeds, and as the industry moves to comply with new international standards on product quality and the environment. Globally, industry growth in the past decade or so has fluctuated between ± 6%/yr and is currently expanding at around 4%.
Through 2011, farmed shrimp production increased steadily in east Asia and averaged a 6% annual growth rate from 2008 to 2011. The GOAL (Global Outlook for Aquaculture Leadership) surveys indicate that production decreased from 3.45 million t to 3.25 million t in 2012 (down 5.8%) and 3.21 million t in 2013 (down 1.1%) due to the impact of the Early Mortality Syndrome (EMS; also known as Acute Hepatopancreatic Necrosis Syndrome, AHPND) in China, Vietnam, Thailand and Malaysia. In 2014, production increased significantly to 3.49 million t (up 8.5%) in large part due to larger harvests reported in China, India and Vietnam. Figure 22.1 shows production in Southeast Asia, China and the Americas, by the major Latin American producing nations, which include Ecuador, Mexico, and Brazil.
In Asia and the Americas the industry is expected to continue its consolidation into very large, vertically‐integrated companies that can maximise economies of scale and efficiency. And it is expected to expand significantly in the next two decades, particularly in India, Southeast Asia and Latin America, with current production expected to nearly double by 2030.
Currently, close to 75% of farmed shrimp production in the world is Pacific white shrimp, and this percentage will continue to increase (Figure 22.3). Improvements in selective breeding, nutrition, health management, disease diagnosis and prevention, and more efficient and intensive production systems have supported this industry expansion.
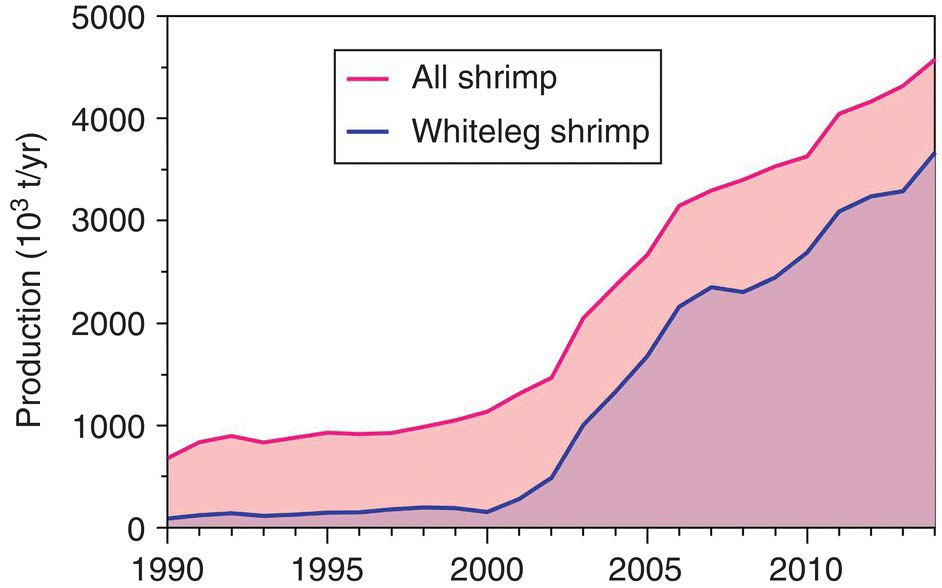
Figure 22.3 Recent output from farming Pacific white shrimp or whitteleg shrimp (Litopenaeus vannamei) versus output from farming the black tiger shrimp, Penaeus monodon.
Source: Reproduced with permission from John Lucas.
Asian farmers have demonstrated that Specific Pathogen Free (SPF) L. vannamei, often from selective breeding programs, can be efficiently grown at very high densities with relatively low environmental impacts. However, while there are farms in the Americas that have been able to successfully convert from semi‐intensive to more intensive production modes, these are relatively rare. There does not appear to be any real move towards the widespread adoption of this technology in the region, as land availability has not been a constraint to industry establishment and growth. Production models in the Americas remain largely based on:
- large ponds (5−10 ha) stocked at 10−30 animals/m2;
- using hatchery‐reared postlarvae (PLs) often selected for growth and survival against prevailing pathogens;
- with some mechanical aeration;
- reduced water exchange rates; and
- continued use of manufactured aquafeeds.
22.2 Cultured Species
There are about 2500 species of shrimps worldwide, but only seven species are farmed to some degree. All belong to the family Penaeidae, characterised by a rostrum with ventral and dorsal teeth, and last thoracic segment with gills. Out of the commercially farmed species, two account for around 95% of global production:
- the black tiger shrimp4 (Penaeus monodon) in Asia; and
- the Pacific white shrimp (Litopenaeus vannamei), native of the western coast of the Americas, but widely and often indiscriminately introduced throughout Asia, Africa, Europe and elsewhere.
Black tiger shrimp alone probably constituted 60–70% of the world’s production of farmed shrimps until the end of the century, when the Pacific white shrimp began to be introduced to Asia, where it has essentially replaced black tigers in production and world markets. Both species are popular in local and export markets, and although both are relatively easy to produce, L. vannamei has some very definite advantages regarding domestication, larval rearing, tolerance to high density culture and requirements for lower protein aquafeeds. The seedstock (PL) for both species can be produced in hatcheries using relatively simple technologies. Both can tolerate a wide range of salinity, from slightly greater than freshwater (1–2‰) to full‐strength ocean water (35–40‰; L. vannamei will grow well at up to 55‰). Both species readily eat formulated commercially manufactured feeds.
22.2.1 Western White Shrimp5 (Litopenaeus Vannamei)
This species is known as the Pacific white or whiteleg shrimp (Figure 22.4). Its original range was from the Gulf of California southward to northern Peru, but it is now a cosmopolitan species. Males reach a total length of 187 mm and females 230 mm. It is well suited for farming, because it:
- breeds well in captivity;
- can be stocked at small sizes;
- grows fast and at uniform rates;
- has comparatively low protein requirements (20–25%) and, together with the Indian white shrimp, Fenneropenaeus indicus, is considered among the most omnivorous of the cultured penaeid species; and
- adapts well to variable environmental conditions.

Figure 22.4 The Pacific white shrimp or whitteleg shrimp (Litopenaeus vannamei).
Source: Reproduced with permission from Darryl Jory.
Litopenaeus vannamei has advantages over P. monodon in that it breeds in captivity more readily. Overall survival in hatcheries is relatively high at 40–60%.
Many hatcheries in Latin America have captive stocks of L. vannamei broodstock, some pathogen free, some pathogen resistant, with some having been in captivity for 40 years. Established selective breeding programs in both Asia and the Americas have made significant advances in the genetic improvement of important traits like growth and disease resistance.
22.2.2 Black Tiger Shrimp (Penaeus Monodon)
Penaeus monodon is the largest (363 mm maximum length) and fastest growing (up to 5.5 g/week) of the farmed shrimps (Figure 22.5). The species is native to the Indian Ocean and the south‐western Pacific Ocean, from Japan to Australia, and dominates production everywhere in Asia except for Japan and China. This species can tolerate a wide range of salinities and is grown in inland low‐salinity ponds in some countries. It is, however, very susceptible to several shrimp pathogens, although it appears to be less affected by Early Mortality Syndrome EMS = AHPND than L. vannamei. Breeding in captivity is relatively more difficult to induce and hatchery survival is lower (30−40%).

Figure 22.5 The black tiger shrimp (Penaeus monodon).
Source: Reproduced with permission from Darryl Jory.
22.3 Grow‐Out Systems
Shrimp grow‐out operations span a wide continuum in terms of intensity, complexity and technology. Globally, four grow‐out production systems are generally recognised, which share some characteristics, but differ in other aspects (Table 22.1).
Table 22.1 Various shrimp farming systems, based on stocking density, production inputs and operational parameters. PL, postlarvae.
| Intensity | ||||
| Parameter | Extensive (low density) | Semi‐intensive (medium density) | Intensive (high density) | Intensive (very high density) |
| Stocking density (PL/m2) | 1–5 | 5–25 | 26–120 | 120–500 |
| Pond/tank area (ha) | Ponds (5–100) | Ponds (1–25) | Ponds/tanks (0.1–5.0) | Tanks (0.1–1.0) |
| Seedstock source | Wild | Some wild/mostly hatchery | Hatchery | Hatchery |
| Water exchange (% daily) | Tidal (ca. 5%) | Pumping (5–10%) | Pumping (up to 25%) | Pumping (25%+) |
| Management requirements | Minimal | Moderate | High | Very high |
| Feed | Natural productivity | Natural productivity and formulated feeds | Natural productivity / mostly formulated feeds | Natural productivity / mostly formulated feeds |
| Fertilisation | No | Generally yes | Sometimes | Sometimes |
| Mechanical aeration | No, minimal water exchange | Water exchange and some mechanical aeration | Mechanical aeration | Mechanical aeration |
| Aeration levels (hp/ha) | 0–2 | 2–5 | 6–20 | 20–60 |
| Production cycle (days) | 100–210 | 100–210 | 80–140 | 80–120 |
| Annual production (kg/ha) | 50–500 | 500–5000 | 5000–20 000 | 20 000–40 000 |
Moving from the lowest towards the highest stocking systems, there is:
- progressive reduction in the area of ponds used, eventually moving towards more intensive systems like tanks and raceways, and even indoors for more controlled conditions and biosecurity;
- progressive increase in capital, production costs and stocking densities, and risk;
- a trend for increased use of intermediate nursery or multiple phases; and
- an overall intensification in production practices, management and technology.
The last involves a more thorough preparation of ponds or tanks, the lining of ponds with high density polyethylene liners (HDPE, which allow faster restocking and the use of higher levels of mechanical aeration), total use of laboratory‐produced seed‐stock, use of better formulated and more nutrient‐dense feeds, improved pond management practices and others. Up to the mid‐ to late‐1990s, it also involved higher rates of water exchange, but for the last two decades there has been a strong global trend towards reducing exchange rates and the volume of water required to produce shrimps (i.e., m3 water/kg shrimps) (Figure 22.6). This has even developed to the point of reaching zero exchange (except for replacement of water lost to evaporation and other processes) and recirculation to improve biosecurity and operational cost‐efficiency. There are also trends towards increased use of technology, aeration, technical and professional labour, and a tendency to move away from the coastline to higher ground, and even towards ocean cages. Most farms produce at least two crops per year, although some farms can reach or surpass four or more annual crops, particularly those using nurseries or multi‐phase production systems.

Figure 22.6 Water pumping station for a shrimp farm. Farms in Latin America tend to operate with greater volumes of water exchange than their counterparts elsewhere.
Source: Reproduced with permission from Darryl Jory.
Early on, when the industry was young, farms were mostly extensive, and there are still many such farms in Thailand, Indonesia, India, Vietnam, Bangladesh, Central and South America, among other countries. Since around 1980, however, there has been a trend towards refitting farms to increase intensity of production. The more intensive farms are more typical in countries such as Taiwan, Japan and the USA (Jory et al., 2001).
22.3.1 Extensive Systems (Low Stocking Densities)
Shrimp farms with low stocking densities are typically located in tropical water impoundments ranging from ca. 2 ha to > 25 ha and are located adjacent to estuaries, bays, and coastal lagoons and rivers. They frequently involve polyculture with herbivorous fishes such as mullet, milkfish, tilapia and others. At some of these farms, liming materials are sometimes applied to ponds if soils are acidic or have accumulated considerable amounts of organic matter that is not removed. Some operations may sometimes use animal manures or other organic materials to stimulate production of natural food for their shrimps (Ponds are filled by tides and any water exchange (typically < 5−10% per day) is also by tidal action. Ponds are typically stocked with wild shrimp postlarvae (PL), when naturally available, by opening pond gates to incoming tides.
In all cases, these shrimp feed on natural phyto‐ and zooplankton, small plants and animals living in or on the pond substrate (particularly amphipods and polychaetes), and particulate organic matter suspended in the water or lying on the bottom. This natural production may be promoted with applications of organic or chemical fertilisers. In this extensive aquaculture the cultured shrimps are essentially part of a natural ecosystem that provides their nutritive and other requirements. Construction and operating costs are typically low, and production rarely surpasses 400–500 kg/ha in production cycles that last 100–160 days. Because it is illegal – and inefficient − in several countries to build new shrimp farms in tidal and mangrove areas, very few new extensive shrimp farms are being built anymore.
22.3.2 Semi‐Intensive Systems (Medium Stocking Densities)
Shrimp farms that operate at medium stocking densities (Table 22.1) are built above the high‐tide line and include a pumping station, water distribution canals. reservoirs and use of formulated feeds. In general, farm layout is relatively more symmetrical than extensive farms and ponds are harvested by draining through a net or by using a harvest pump. Pond preparation may be elaborate, with dry‐out once or twice a year, tilling and liming with various liming materials, and fertilisation with N, P, Si and other compounds to promote natural production. Producers may also apply various extracellular enzyme preparations and bacterial inocula to improve water quality, but the benefits of these treatments remain to be conclusively established. Organic fertilisers (mostly manure and agricultural by‐products) are sometimes used. In recent years, many farmers have adopted exchange practices of 2–5%. These lower exchange rates reduce pumping costs, and minimise fertiliser needs and the possibility of pathogen introduction. Formulated and pelleted feeds with 15–35% crude protein are usually applied 1–3 times per day, typically by manual broadcasting over pond surfaces from boats and levees. Feed quantity applied is calculated and adjusted based on the shrimp biomass estimated from results of cast net sampling and feeding charts typically provided by feed manufacturers, and also from historical data collected at each farm. Natural productivity in the ponds is important for juvenile shrimp growth during the early weeks at all intensities of shrimp culture.
Subsequently, there is variation in the degree of dependence on formulated feeds. Dependence on formulated feeds is not as great at this moderate density as it is at higher culture densities; hence, in the continuum of culture practices, this is semi‐intensive culture.
22.3.3 Intensive Systems (High Stocking Densities)
Shrimp farms using intensive culture and high stocking densities (Table 22.1) typically have ponds of 0.1–2 ha, although various designs of raceways and above‐ground tanks are also used, sometimes in greenhouses or hard buildings. Ponds are frequently lined with (high‐density polyethylene) HDPE or other commercial, plastic, inert liners. Preparation before stocking is more meticulous, and management is often more elaborate, with feed applied 6–24 times a day. Mechanical aeration is absolutely necessary to support the large shrimp biomass (and in some cases, liquid oxygen – LOX − is required) and heavy feeding rates needed. Aerators and/or air‐diffuser systems are placed in ponds and operated throughout the cycle, usually with increasing number of units and longer hours of operation as the cycle progresses. Generally, 4–12 hp/ha is used, with the amount increasing as the biomass of shrimp increases (Table 22.1). These systems are often stocked with juveniles from nurseries, rather than with postlarvae.
22.3.4 Intensive Systems (Very High Stocking Densities)
At the upper end of the continuum, systems with very high stocking densities (Table 22.1) include the highest level of environmental control, to the point of some being located indoors in greenhouses and other structures. Annual production can reach 20–100 t/ha and higher, but there are currently only a few of these farms, mostly in various Asian countries. Examples of these advanced farms and technology include the pioneer operations by (still ongoing) Belize Aquaculture Ltd (BAL) in Belize and (the now closed) OceanBoy Farms in Florida, USA. Pond management at these farms was designed to be based on zero water exchange, heavy aeration (up to 60 or more hp/ha) and the promotion of a bacteria‐dominated and stable ecological system. This compares with the highly unstable and traditional phytoplankton‐dominated system typical of semi‐intensive systems. The feeding regime used promotes the growth of heterotrophic bacteria and essentially makes the pond into a large outdoor bioreactor, akin to a sewage oxidation pond. In these high‐density, zero‐water‐exchange systems, the pond ecology shifts (at weeks 9 to 10 after stocking) during the production cycle from an autotrophic phytoplankton‐based community to a heterotrophic bacteria‐based community. This shift improves water quality through fast digestion (oxidation) of organic waste without production of toxic metabolites; and also recycles wastes into nutritious bacterial flocs, the basis for ‘natural production’ in this system.
22.4 Preparation of Ponds6
General details of ponds, site requirements, layout, design and water turnover are given in Chapter 4. For a review of pond preparation and management, see Boyd et al. (2010)
Adequate pond preparation provides young shrimps with an environment that is relatively free of predators and competitors, with an ample supply of adequate natural food organisms, and environmental conditions that minimise stress and promote growth and survival. Pond preparation involves several sequential procedures, including:
- pond draining and drying;
- pH mapping;
- soil tilling;
- disinfection and liming;
- fertilisation; and
- weir gate preparation and maintenance.
The first four steps above are not necessary in the case of fully‐lined ponds.
22.4.1 Pond Draining and Sludge Disposal
Effective removal of sludge that has built up on the pond bottom over a prior crop cycle is a very important step. If not removed, it will become an anaerobic, reduced sediment. This will generate toxic metabolites such as methane, hydrogen sulphide, ammonia, nitrite, ferrous iron, etc., affecting pond water quality and production during the following crop cycle. Organic sludge is a very important factor for the onset of EMS, as a substrate for the pathogenic Vibrio parahaemolyticus that causes this most important, devastating and recent disease. With the advent of a relatively new shrimp disease, EHP (acronym for a disease named after Enterocytozoon hepatopenaei, a tiny microsporidian parasite that affects shrimps by disrupting their digestive systems), it is very important to properly disinfect earthen ponds with calcium oxide (CaO, quick lime), applied at 7 t or more per ha over completely dry bottoms. The quick lime should be ploughed into the dried sediments to a depth of 10 to 12 centimetres and then the sediments moistened to activate the lime. Once the soil is moistened, the pH will rise to 12 or more within days and then gradually return to normal as the quick lime transforms to calcium carbonate.
After a production pond is harvested, all water inlet/outlet gates are opened to generate a strong water flow through the pond, to re‐suspend and flush out as much accumulated sludge as possible. The use in the ponds of probiotics that help digest organic matter in situ during the culture cycle can help alleviate the amount of organic matter that remains after a production cycle. And careful management of aquafeeds, with more feed applications per day, typically also helps with organic matter management.
22.4.2 Pond Drying and pH Mapping
Earthen ponds must be allowed to dry out for 2–4 weeks to promote decomposition of organic matter by bacteria; eliminate pathogens and eggs, larvae and adults of predator and competitors; and dry out undesirable filamentous algae.
The rate of organic matter decomposition is greatest at a soil pH of 7.5–8.5 and, for ponds built on acidic soils, farmers add various lime products to improve soil pH and promote decomposition of organic matter.
Optimal microbial action for decomposition of organic matter occurs at about 20% soil humidity. Pond dry‐out and disinfection may be the most effective methods for controlling epidemics of various shrimp pathogens, as well as various predators and competitors. UV radiation (via sunlight) and temperatures above 55°C will destroy several pathogens. If diseases have been detected within the last production cycle, a longer dry‐out (2–3 months) may be helpful. The pond surface must be cracked to a minimum of 5 cm, to oxidise the soil and eliminate anaerobic conditions. In acidic soils with pH ca. 7, pond bottom pH must be mapped promptly by sampling soil pH at various stations within the pond while soil humidity is about 30%, to calculate lime requirements. Once the pond surface is hard enough to walk on, soil pH is measured using standardised procedures.
Ploughing or turning the bottom soil of ponds (top 10–20 cm) is another optional step, which will depend on soil condition. Tilling the bottom soil of ponds can significantly promote oxidation of the lower layers of anaerobic sediments. Probiotics are sometimes also added to help digest organic matter.
22.4.3 Disinfection
Disinfection is important to eliminate eggs, larvae, juveniles and adults of species of fish, crustaceans, insects, and other predatory and competitor species. Several commercial products have been used to disinfect ponds before stocking shrimps. The use of pesticides (particularly chlorinated hydrocarbons and organophosphates) is not recommended.
Applying calcium oxide or calcium hydroxide at 5000 kg/ha will raise the pH to greater than 10 and will destroy pathogens. Only muddy areas must be treated like this, not the entire pond bottom, as this process will destroy desirable bacteria needed to promote the development of productive benthos. Piscicides (fish‐killer) such as rotenone and teaseed cake are routinely used to eliminate finfish, including mudfish.
22.4.4 Liming
Liming pond bottoms is a critical step in preparing earthen ponds. When calculating liming requirements, the type of lime product to use must be considered, as this will influence the amounts required (Table 22.2). One lime application is generally added 2 days after tilling with lime spreaders, applying lime more heavily to wet low areas than to higher dry areas. One week is allowed for the lime to react with the soil before applying fertiliser. The calculated neutralising values of various compounds used in pond liming range from 59% (sodium bicarbonate) to 208% (calcium magnesium oxide) (Table 22.2).
Table 22.2 Neutralising values of various compounds used in pond liming (Boyd et al., 2010).
| Compound | Chemical name | Neutralising value (%) |
| Agricultural limestone | Calcium carbonate | 100 |
| Agricultural limestone | Dolomite | 109 |
| Burnt lime | Calcium oxide | 179 |
| Burnt lime | Calcium magnesium oxide | 208 |
| Hydrated lime | Calcium hydroxide | 135 |
| Hydrated lime | Calcium magnesium hydroxide | 151 |
| Soda ash | Sodium carbonate | 94 |
| Baking soda | Sodium bicarbonate | 59 |
| Other compounds | Calcium silicate | 86 |
| Other compounds | Calcium phosphate | 65 |
When liming requirements cannot be calculated, the following liming rates, using calcium carbonate, CaCO3, can be used (see also Chapter 4):
- for pH 6.5–7.5 (500 kg/ha);
- for pH 6.0–6.5 (1000 kg/ha);
- for pH 5.5–6.0 (2000 kg/ha);
- for pH 5.5–5.0 (3000 kg/ha); and
- for pH < 5.0 (4000 kg/ha).
22.4.5 Weir Gate Preparation and Entrance Screening
Configuration and placement of weir gate restriction boards and screens to prevent escapes and entry of predators, while at the same time allowing water to enter and exit continuously, is vital for cost‐effective production.
Very fine (200–250 µm) screens are installed in the inlet structure to initially fill ponds. When pond filling is complete, these filters are replaced with 300‐ to 500 µm screens. Bag net filters are used in the inlet structures to augment effective filtering surface area, and reduce screen clogging. Bag net filters of 2–5 m in length are the best, but there is no limit to the length or size of the bag net filter and, in general, the longer the better. Multiple bag configurations (where a filter bag is placed inside another) are a cost‐effective means of decreasing the mesh aperture size without installing a finer screen. Ponds are inspected for proper completion of necessary preparation steps before filling with water.
22.4.6 Natural Productivity
Proper management of natural productivity in shrimp ponds is critical to promote and sustain plankton blooms, and microbial and benthic community productivity. A vigorous phytoplankton bloom will support a healthy benthic community and will contribute significantly to stabilising and maintaining adequate water quality in shrimp ponds. This happens:
- by promoting oxygen production through photo‐synthesis;
- by decreasing levels of various metabolites and toxic substances;
- by improving pond water and bottom pH (critical in in ponds with acid‐sulphate soils);
- by limiting, through shading, growth of filamentous bottom algae; and
- by increasing turbidity which reduces bird predation.
Natural productivity is also important because it supports the generation of detritus, the particulate organic material produced from the dead bodies, non‐living fragments and excretions of living organisms. In nature, organic detritus is an important food source for many estuarine organisms, and in shrimp ponds it can have an important role. Shrimp feed on detritus, and derive nourishment by stripping the micro‐organisms from the detritus as it passes through their gut. In addition, their faecal pellets may be recolonised and the process repeated until all the organic material has been utilised. By maximising the recycling capability of organic detritus within the culture environment, nutritionists and pond managers have an opportunity to reduce feed and production costs, improve FCR, and reduce environmental impacts.
22.4.7 Initial Fertilisation
Fertilisation of shrimp ponds is an effective means of stimulating natural food production that can help reduce feed costs. Through fertilisation, managers can promote those pond ecosystem components that are beneficial to shrimp production and discourage those that are detrimental. Several organic and inorganic nutrient sources can be used to fertilise shrimp ponds (section 4.4.2). They vary in their effectiveness because of differing nutrient density, solubility in water, potential toxicity, C/N ratios and other factors. Appropriate fertilisation rates vary depending on fertiliser type and the ambient nutrient concentration in the water. Various dynamic processes in ponds can also affect fertilisers, producing dissolved nutrient concentrations after fertilisation that may substantially differ from calculated concentrations.
Fertilisation involves the application of fertilisers over the entire pond bottom before filling with water. The objective of inorganic fertilisation during preparation and during the grow‐out cycle is to promote and maintain population densities of diatoms and green microalgae of at least 80 000–100 000 cells/mL
There is no universally optimal N/P ratio, and each farm must determine by trial and error the most suitable inorganic fertilisation regime for its prevailing conditions and needs. In areas that have markedly different dry and wet seasons, there are different optimal fertilisation ratios for each season, as well as different optimal stocking densities and other significant management differences.
When selecting inorganic fertilisers, the type (nutrient class, composition and solubility), the N/P ratio, daily dose rates and frequency of application must be considered. The main nutrients needed in shrimp ponds to promote phytoplankton blooms are N, P and Si. Si compounds stimulate production of the very desirable diatoms, the cells of which are enclosed in an Si compound. However, Si fertilisation is rarely needed at water salinities over 25‰ or so. The most common silicate fertiliser used is sodium metasilicate, which is expensive and not widely available. Commonly used inorganic fertilisers are urea and sodium nitrate as N sources, and monoammonium phosphate (MAP), diammonium phosphate (DAP) and triple superphosphate (TSP) as P sources. Urea is the most commonly used source of N because it is widely available, inexpensive and effective. Nitrate‐based fertilisers are very effective, but more expensive and less available, and purchasing and possessing these fertilisers can be problematic in some places due to its other potential uses as explosives. An N/P ratio of 15–20:1 promotes the development of diatom blooms; however, the N/P ratios used in many shrimp farming areas worldwide can vary from 1:1 up to 45:1.
22.5 Reproduction and Maturation
22.5.1 Hatchery Production and the Life Cycle
The typical life cycle of a shrimp in nature begins with adult animals migrating up to several kilometres offshore, maturing, mating and spawning. The eggs initially sink, but after a few hours they hatch and the nauplii (the first larval stage) float to the surface. Shrimps usually spawn in areas where favourable currents will eventually bring the developing larval stages inshore into nursery areas such as estuaries, large bays and coastal lagoons. These areas provide abundant natural food and adequate conditions for survival and growth. Developing shrimps remain in nursery areas for several months, and then begin maturing and moving offshore to spawn and complete their life cycle (Figure 22.7).
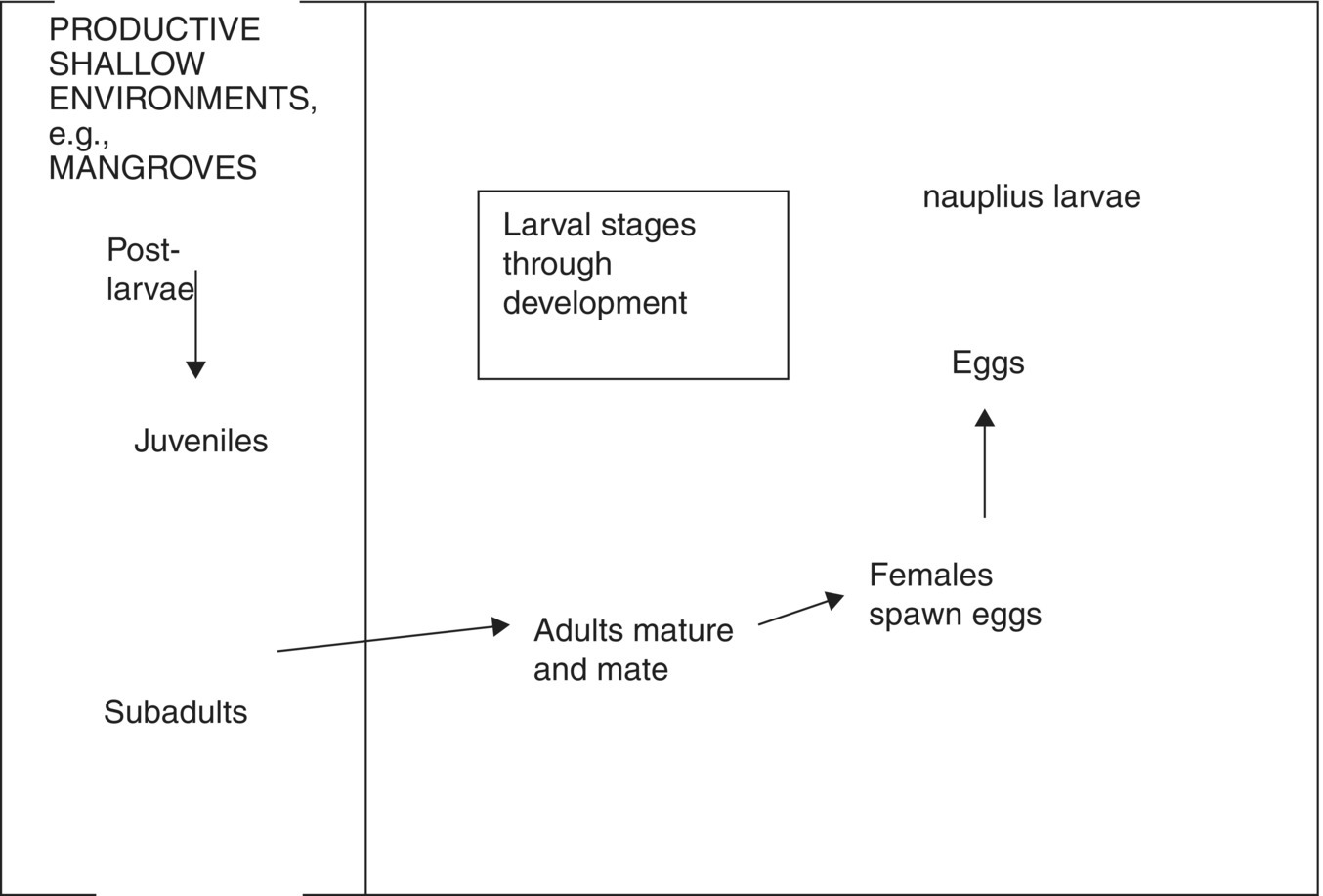
Figure 22.7 A broad outline of a shrimp life cycle.
Source: Reproduced with permission from Darryl Jory.
Hatcheries in the western hemisphere (for L. vannamei) are typically large (Figure 22.8), often belong to a vertically integrated operation that includes a grow‐out farm and a processing plant, and they frequently produce excess nauplii that are sold to smaller hatcheries, or postlarvae that are sold to other farms. Many of these operate on two larval culture phases, stocking 100−250 nauplii/L, transferring from first to second phase at PL7(instar 7), and harvesting at PL12‐14. This western hemisphere hatchery model has been transferred and adapted to Asia with the introduction of L. vannamei there. In the eastern hemisphere, many backyard and medium‐scale hatcheries are used to produce most of the seedstock used by farmers. However, in the past 12 years or so, very large hatcheries have also started operating in Asia, often as part of integrated operations. Some have very sophisticated genetic improvement programs.

Figure 22.8 Large tanks for rearing shrimp larvae in a commercial hatchery in Latin America.
Source: Reproduced with permission from Darryl Jory.
Larval development from egg to PL is complex and involves three stages: nauplius, zoea and mysis. The nauplius has five or six sub‐stages, and the zoea and mysis generally have three sub‐stages each, with each sub‐stage lasting few to many hours. The larval development process takes ca. 15 days. As larvae develop and consume the yolk sac, their diet switches to phytoplankton and then to zooplankton. After the mysis stage, they consume a variety of organisms, including brine shrimp (Artemia species). All these live organisms are produced in the hatchery (see Chapter 9), but major advances have been made recently to develop alternative inert diets (section 9.4), including synthetic Artemia. After they become PL, the animals look like small adult shrimps and are able to feed on zooplankton, detritus and commercial feeds. After several days as PL, they are ready to be stocked into nursery or grow‐out systems. With the increasing importance of genetic improvement programs to address biosecurity concerns for various shrimp diseases, hatcheries will play an increasingly important role in the support and expansion of the industry. For detailed information on hatchery procedures refer to Juarez et al. (2010).
22.5.2 Broodstock Maturation
It is very important to be able to produce seedstock on demand, consistently and in sufficient numbers to support the industry. Maturation of shrimp broodstock is undertaken routinely in many countries, and at least 26 penaeid shrimp species have been matured and spawned in captivity to produce viable eggs. However, the life cycle has only been closed consistently and dependably for a few shrimp species.
Large hatcheries typically have separate infrastructure to carry out broodstock maturation. The maturation section of a hatchery is normally isolated from other sections, to reduce noise levels and stress caused by human activity. Maturation tanks are typically round, about 3–5 m in diameter and 60–100 cm in height, and they are gently sloped towards a central drain to facilitate removal and siphoning of uneaten food and other undesirable debris.
Environmental conditions in maturation facilities duplicate or even intensify conditions known to stimulate reproduction. Each species has optimum ranges at which maturation will be facilitated. The value of these parameters and their rate of variation over time are critical to stimulate the reproductive process. The water supply system, closed or open, must continually provide maturation tanks with clear unpolluted water with oceanic characteristics and a daily exchange capacity of 200–300%. Many hatcheries now use a recirculation water system for broodstock operations. Optimum water temperature for maturation is typically around 28–29°C. An oceanic salinity of 30‰ is considered optimum, although maturation may occur between 28‰ and 36‰, and pH must be maintained between 8.0 and 8.2.
22.5.3 Open vs. Closed Thelycum Species
Penaeid shrimps belong to two groups based on the structure of the female thelycum, a receptacle structure on the ventral thorax of females where the spermatophore is deposited by the male at mating. The management of maturation procedures is different for open‐ and closed‐thelycum species
22.5.4 Maturation Procedures
The maturation process is relatively simple and includes selecting or sourcing good prospective broodstock (screened for absence of viruses) and holding them under stable, optimal environmental conditions, minimal stress and adequate nutrition using both natural and artificial diets. Exclusion and control of opportunistic shrimp pathogens, such as various bacteria, fungi and protozoea, is critical. It is accomplished by maintaining the best water quality possible and by periodic prophylactic treatments.
Adequate nutrition is another factor critical for shrimp maturation, promoting sexual maturation and mating, and improving fertility and offspring quality. Maturation diets typically include combinations of commercial dry and semi‐moist pelleted feed supplements, together with natural organisms such as various molluscs (clams and other bivalves, squid), crustaceans, fish and ‘bloodworms’. Bloodworms are marine polychaetes rich in the polyunsaturated long‐chain fatty acids (PUFAs) that are essential for shrimps to mature, and the worms are commercially available. Using natural, live or fresh feeds can create serious biosecurity concerns, and it is likely that some major shrimp diseases have been widely spread due to careless practices.
22.5.5 Mating and Spawning
Mating in shrimps is characterised by particular courtship behaviour, and various pheromones are involved in sex attraction. Females with one or both eyestalks ablated (removing glands that affect ovary development) will normally spawn multiple times over a period of several weeks. Nauplii can be collected directly from maturation tanks, but in most commercial operations mated females are usually removed and placed in individual spawning tanks of 100–500 L. This is particularly important in operations implementing selective breeding programs.
22.6 Hatchery Design and Larval Culture
22.6.1 Hatchery Design
Shrimp hatcheries may be set up in a warehouse structure or building shell and have indoor and outdoor infrastructure and facilities. Hatchery design follows the typical pattern with various areas for microalgae culture, laboratory, brine shrimp production, seawater treatment and holding, spawning, larval and PL rearing. Support infrastructure includes storage space, offices, and aeration and electrical power generating capability. There are small, medium and large shrimp hatcheries, and all have the same basic infrastructure:
- Small hatcheries are usually a family operation, with very low set‐up and operating costs, using simple techniques and untreated water, low culture densities, and with larval culture tank capacity under 50 000 L. These generally produce either nauplii or PL, and often suffer disease outbreaks and water quality problems.
- Small‐ and medium‐scale hatcheries are usually based on the Taiwanese design; with large culture tanks between 5000 and 50 000 L.
- Large hatcheries use elaborate techniques to operate controlled culture environments (Figure 22.9). They target annual production of 100 million PL or more and are typically based on the ‘Galveston’ system. When large hatcheries have water quality and disease problems, they can require several months to get back on line.

Figure 22.9 Large outside tanks for rearing shrimp postlarvae (PL), Brazil.
Source: Reproduced with permission from Darryl Jory.
22.6.2 Larval Culture Methods
Worldwide, various methods are used to produce shrimp larvae in hatcheries. These are all modifications of the two basic methods, the Taiwanese and the Galveston methods. The latter is a modification of the former and differs from it in that microalgae are cultured outside the larval tank and are added as required. Over the years, many researchers have helped modify and refine these techniques.
22.6.3 Larval Nutrition
The larval feeding regime of shrimp hatcheries is typically based on microalgae and brine shrimp or on live feeds combined with formulated/prepared diets. The latter can be produced at the hatchery, although in recent years several commercial brands have become available. Most are ‘dry diets’ packaged under vacuum or in nitrogen‐filled cans or pouches, and others are ‘liquid diets’ and are produced in a range of particle sizes suited to the feeding habits of the larval stages for which they are intended. Crude protein levels typically range from 42% to 48%, whereas lipids are 12–16% and fibre is less than 5%. Artificial diets are not a complete replacement for natural food, but when used supplementary to natural food they can increase both survival rate and growth when compared with the feeding of natural diets (algae and brine shrimp) alone. It is likely that in the near future these dry and liquid inert diets will totally replace live feeds in hatcheries, with major savings in costs and resources.
Several species of microalgae are commonly produced in shrimp hatcheries, including species of the genera Tetraselmis, Isochrysis, Chaetoceros, Skeletonema and others. Isochrysis galbana and Chaetoceros gracilis are among the best, because of their relatively small size and significant content of highly unsaturated fatty acids (HUFAs). It is common to enrich brine shrimp nauplii with HUFA before feeding these to larval shrimp.
See also Chapter 9.
22.6.4 Probiotics, Vaccines and Immunostimulants
The ability to control pathogenic organisms, particularly bacteria, has been very important to the success of commercial‐scale hatcheries. The general method to control pathogenic and undesirable bacteria is through various water filtration and disinfection techniques (e.g., UV, chlorination). Other common procedures include hygienic procedures (personnel and equipment disinfection) and the use of non‐infected cultures of microalgae and brine shrimp. Use of antibiotics can result in development of resistance, chemical traces in shrimp tissues, and environment impacts.
Probiotics are widely used to promote survival and growth of larval shrimps, and can be an effective hatchery tool by:
- competitively excluding pathogenic bacteria;
- producing substances that inhibit growth in opportunistic pathogen species;
- providing essential nutrients;
- promoting digestion by supplying essential enzymes; and
- direct uptake of dissolved organic material mediated by bacteria.
There is much potential for improvement of probiotics, but more research is needed to identify suitable species and how to promote their population growth in larval culture tanks.
22.7 Seedstock Quality and Stocking
22.7.1 Seedstock Packing, Transportation and Reception
It is important that the delivery of seedstock (PL) is well co‐ordinated between the hatchery and the farm. Good planning and communication are required to ensure a smooth transition from the protected conditions of a hatchery to the conditions of an outdoor pond, tank or raceway (Fig. 22.10). Timing is critical to ensure that the PL are going into a grow‐out environment that has been adequately prepared and has the proper conditions to promote acceptable survival and production. Of particular importance, in the case of outdoor ponds, is that there is sufficient natural productivity to provide adequate nutrition to the animals being stocked. There is a window of about 10 days, starting about 10 days after pond filling begins, when pond conditions should be adequate for stocking. In general, ponds should not be stocked beyond 20 days after filling has begun.

Figure 22.10 Shrimp postlarvae held up for inspection.
Source: Reproduced with permission from Darryl Jory.
Seedstock of great quality may be severely stressed by inadequate water quality or high packing densities during the move from hatchery to farm. Inadequate packing and transportation of shrimp seedstock will significantly affect survival and health. Care must be taken to minimise stress and to provide the best handling and conditions possible.
Methods of packing and transportation of shrimp PL can vary significantly, depending on origin, species, distance between hatchery and farm, resources available and other factors. PL are typically transported to farms from hatcheries in plastic bags with water and added oxygen, within polystyrene foam or cardboard boxes and cooled to 18–22°C (down from the typical 28–30°C in hatchery larval culture tanks); or in 2−20 t tankers set up to provide water aeration / oxygenation.
Feed (e.g., frozen brine shrimp or inert commercial diets) is added to prevent cannibalism. Packing densities vary between 500 PL/L and 2000 PL/L, depending on species, age and estimated time in transit. When possible, PL are transported early in the morning or in the evening, to avoid high temperatures. Transport time should be as short as possible, ideally not exceeding 6–8 h. Reduced water temperature and use of various compounds to shipping water (including ammonia suppressants, buffers and activated carbon) increase PL survival over extended shipping.
Upon arrival, parameters such as water temperature, salinity, dissolved oxygen, pH and alkalinity must be determined to serve as the baseline for acclimation adjustments to match pond water characteristics.
22.7.2 Counting and Quality Control
There are various methods used to count shrimp PL and there is controversy regarding the accuracy of each. Volumetric subsampling is the most common technique: it involves concentrating all or most of the PL in a known volume of water and drawing a fixed number of subsamples (around five) using a beaker or similar container of known volume. The subsamples are counted and their average is extrapolated to the larger volume. Nowadays, there are also commercial, video‐based counters available that use computer image recognition and can count PL in real time.
Stocking only the best‐quality shrimp PL is critical to the success of a shrimp farm. Although many hatcheries will provide health certificates for their PL, it should be compulsory for the farm to carry out its own testing.
The strength or ‘hardiness’ of PL from different hatcheries or batches can vary significantly and the acclimation schedule must be tailored to the PL ‘fitness’. Stronger animals can be acclimated at a faster rate than weaker PL. Various stress tests are used to challenge PL and determine a suitable acclimation schedule.
22.7.3 Acclimation and Stocking
Most shrimp farmers spend substantial resources and effort during pond preparation to enable them to stock their PL into a grow‐out environment with the best possible environmental conditions. These are as free of predators, competitors and stress as possible, and with ample supply of adequate food organisms. Still, the transition from relatively benign conditions in hatcheries to those prevailing in open grow‐out systems, such as tanks and ponds, where water conditions continually or unpredictably change (day/night, dry/rainy seasons over the production cycle) can be a traumatic experience for PL unless the transition is gradual and stress is minimised.
The acclimation station, including all tanks and other water reservoirs and equipment (nets, siphons, buckets, tubing, others), is thoroughly cleaned and disinfected by scrubbing with chlorine or other disinfecting agent. Well‐functioning and calibrated equipment to monitor water parameters (temperature, salinity, pH and DO) before the PL arrive at the acclimation station is critical.
The typical acclimation process involves holding the PL for a period in tanks and slowly adding water from the pond to be stocked to equalise various parameters (mainly salinity and temperature). General acclimation recommendations that have been used for many years include:
- increase/decrease salinity by no more than 3‰/hr;
- avoid sudden temperature changes (>3–4°C);
- maintain DO levels at 6–7 ppm; and
- acclimation densities should not exceed 300–500 PL/L depending on animal size and duration of acclimation.
Salinity is probably the most critical parameter to manipulate during PL acclimation. Acclimated PL or nursed PL may be released into the pond using buckets or other containers at points at least 50 cm in depth, at 50 minute intervals and on the upwind side of the pond to maximise PL distribution throughout the pond. Excessive turbulence at release must be avoided to prevent damage to animals.
22.8 Production Management and Harvest
22.8.1 Water and Sediment Quality
Some water quality parameters must be routinely monitored to effectively manage shrimp production systems during grow‐out. Controlling various parameters can be difficult, but, if not managed properly, pond carrying capacity can be rapidly exceeded and ponds can crash within a few hours or days. Monitoring the quality and properties of intake water, pond water/soil conditions and organics, and other parameters in effluent are essential for good animal husbandry. It is also important for farms to be environmentally aware and maintain water management programmes to minimise any potential ‘downstream’ impacts. Table 22.3 shows a general range and monitoring frequency for various water quality parameters that are important in shrimp production systems. They are measured in the field with probes and meters or by taking water samples that are analysed in a laboratory on site. Measurements and samples are taken at several places within a pond, including the water intake, the central region, drain, and surface and bottom layers, which provides a more representative assessment of the parameter. Detailed information on pond water parameters, their monitoring and management are found in Chapter 4.
Table 22.3 General range and recommended monitoring frequency for various water quality parameters in shrimp production units (ponds, tanks and raceways).
| Parameter | Minimum value | Maximum value | Monitoring frequency* |
| Water temperature (°C) | 24 | 30 | 2–4×/day |
| Salinity (‰) | 15 | 45 | 1×/day |
| DO (ppm) | 3 | 12 | 2–12×/day |
| pH | 8.1 | 9.0 | 2×/day |
| Secchi disc (cm) | 30 | 50 | 1×/day |
| Alkalinity (mequiv.) | 100 | 200 | 1–7×/week |
| Total ammonia‐N (ppm) | 0.1 | 1.0 | 2–7×/week |
| Non‐ionised ammonia‐N (ppm) | – | 0.2 | 2–7×/week |
| NO3 (ppm) | 0.6 | 1.2 | 2–7×/week |
| NO2 (ppm) | – | 0.5 | 1–3×/week |
| Total N (ppm) | 0.6 | 2.5 | 2–7×/week |
| Phosphate (ppm) | 0.2 | 0.5 | 2×/week |
| Silicate (ppm) | 1.0 | 4.0 | 1×/week |
| H2S (ppm) | – | 0.1 | 1–7×/week |
* depending on production system used and standing shrimp biomass.
DO is one of the most critical physical parameters of a pond culture. Low DO is one of the most common causes of mortality and poor growth in high‐density shrimp ponds. Lethal levels seem to vary from about 0.5 to 1.2 ppm, depending on the species and hardiness of a particular population. DO levels can be relatively unstable as a result of wide fluctuations in photosynthetic oxygen production and the bacterial population affecting BOD. DO levels in the water column fluctuates over a day/night cycle, from a low at dawn to a high in mid‐afternoon, particularly due to phytoplankton photosynthesis during the day and then respiration at night. Hence it is appropriate to sample DO at dawn and mid‐afternoon. DO readings in the water column immediately adjacent to the bottom are essential, because shrimp spend most of their time feeding and resting in or on the sediment (Figure 22.11).

Figure 22.11 Standing on a plank above the pond with oxygen meter in hand and DO probe down in the water column.
Source: Reproduced with permission from Darryl Jory.
Salinity and temperature are extremely important parameters that affect several biotic and abiotic processes in the production system environment, but there is usually relatively little that can be done to modify them. Water temperature, however, may be controlled in indoor systems of high stocking density. Farms with free access to both good‐quality seawater and freshwater can mix these to desired salinities.
The pH does not usually reach levels that affect the shrimps. Values seldom exceed the range of 6–9 in sediments or the water column (except in acid‐sulphate soils). High pH (>9) is a lesser risk than low pH (<6). Low pH may affect the mineral deposition of the shrimp’s exoskeleton after moulting, resulting in ‘soft’ shrimps, and can destabilise some phytoplankton species that prefer alkaline conditions. pH in the water column fluctuates with phytoplankton photosynthesis and respiration in proportion to dissolved CO2, which is the reciprocal of DO. pH is lowest at dawn when dissolved CO2 is highest and highest in mid‐afternoon when dissolved CO2 is lowest.
Nitrogenous wastes resulting from protein digestion can accumulate and even reach dangerous concentrations, particularly at high stocking densities. Nitrite and unionised ammonia can accumulate to toxic levels periodically, particularly during massive die‐offs of phytoplankton. Nitrate is usually not toxic at levels typically found in ponds. Total ammonia and nitrate are managed through water exchange.
Water transparency is an index of plankton biomass present and is measured with a Secchi disc, typically once per day at mid‐morning. Acceptable values are between 30 cm and 50 cm. Readings of < 30 cm indicate high phytoplankton biomass or suspended sediment or both in the water column. Pond water colour usually reflects the predominant phytoplankton species, i.e., golden brown = diatoms; green = green flagellates; blue‐green = blue‐green algae; and red = dinoflagellates.
Pond sediments tend to deteriorate with successive cycles and within production cycles due to accumulation of organic matter and sludge deposition. Sediment quality can be determined from hydrogen sulphide level and redox potential (Eh), and by visual examination of sediment cores. Smelling a scoop of surface mud from various areas of the pond bottom is a quick check for hydrogen sulphide sediment. Redox potential of sediment is another quantitative indicator of sediment conditions. It is a measure of the proportion of oxidised to reduced substances, and is an indicator of the relative activity of aerobic and anaerobic bacteria in the sediment profile.
22.8.2 Water Management
Water exchange is an effective management tool to flush out wastes and, in some cases, to improve DO levels. At many large farms, water exchange often follows a fixed schedule rather than being a flexible alternative used when pond conditions require it. High exchange rates can be wasteful and have a negative effect on fertilisation and natural productivity by flushing out nutrients. As much as 35–40% of pond volume used to be exchanged daily at many farms some years ago, particularly ponds stocked at high and very high densities. More typical exchange rates for medium and high stocking densities currently are 2–10%. With the continuing incidence of, and increase in the number of shrimp pathogens, a significant reduction in water exchange can maximise biosecurity through the exclusion of pathogens in incoming water.
Fertilisation of ponds with inorganic and organic nutrients is an effective means of stimulating the production of natural foods and reducing feed use. In the first few weeks, it may be necessary to fertilise a pond every day, then every other day, and eventually biweekly. Routine fertilisation may be enough to maintain adequate water transparency (30–50 cm), provide a continuous natural food supplement and improve water quality. Once feed applications reach about 25–30 kg/ha/day, no additional N or P fertilisation is usually needed, because uneaten feed and shrimp faeces supply enough. Liming compounds are added to ponds in some farms (ca. 20–100 kg/ha/week) during the cycle to increase alkalinity, particularly during the rainy season or in areas where low‐salinity waters are prevalent. Liming compounds are also added under the presumption that water quality will be improved and pathogens in the water reduced (Figure 22.12).

Figure 22.12 Liming shrimp ponds on a regular basis to improve water quality is a common management technique.
Source: Reproduced with permission from Darryl Jory.
22.8.3 Water Aeration and Circulation
Pond aeration is the primary life support system of many aquaculture ponds, including shrimp ponds. Management of DO aims at maintaining DO levels over 4.0 ppm or higher, if possible, throughout the pond, using the least equipment and with minimum cost. Efficient use of aeration and circulation equipment can take advantage of the oxygen supplied by photosynthesis during the day and by diffusion across the pond surface at night. Aerators are the mechanical devices that act as the heart and lungs of an aquaculture pond. They provide distinctive circulation and aeration effects, increasing the rate at which oxygen enters water. There are two main techniques for aerating pond water: one is to splash water into the air and the other is for air bubbles to be released into the water, so there are ‘splasher’ and ‘bubbler’ aerators.
Shrimp ponds require mechanical aeration when production biomass exceeds 2 t/ha and additional aeration at a rate of around 2 kW for each additional t. The moderate circulation effect provided by one or two aerators is desired from the time of stocking. Surface circulation must be ca. 4 cm/sec during feed applications. This speed will not disturb fresh pellets, but will keep finer particles and organics in suspension. Excessive water circulation may be managed by arranging aerators in uniform configurations or by other means. In a typical configuration, aerators generally create a central area of sediment deposition, and sometimes the sludge deposited may be removed by using suction hoses. There are central drains in some ponds with high stocking densities. Proper positioning of aerators and circulators is very important, because their operating efficiency is dependent on achieving adequate water circulation.
One of the needs for circulation and aeration is thermal stratification. Thermal stratification can occur in ponds when surface waters warm faster than deeper waters during the day. This may lead to DO depletion in the bottom water, because most of the DO in pond water originates from photosynthesis in the upper stratum of water or by diffusion from the air through the water surface. Paddlewheel and propeller, i.e., aspirator pump aerators, are especially efficient in circulating pond water. There are also circulators that supply little aeration but can prevent stratification. These are generally large, slowly revolving (50–150 rpm) propellers installed in ponds to quietly move large volumes of water.
22.8.4 Population Sampling and Health Assessment
Periodic sampling of shrimp populations is an important management tool during the production cycle. Cast netting is about the only effective sampling tool currently available and it is widely used. The process of sampling has the objective of generating information about a large group of individuals, a shrimp population in a pond, by looking at a small number of individuals. Most shrimp farms routinely carry out sampling programmes to:
- monitor population size;
- monitor individual and average size/weight of animals;
- evaluate the animals’ physical condition, appearance and product quality;
- assess the animals’ overall health; and
- test for the possible presence of known pathogens or diseases.
In the last few years, and as their use becomes widespread, it has been realised that properly managed feed trays or lift nets can provide adequate population estimates by combining daily feed consumption rates with percentage body weight curves.
Generally, to improve the validity of a shrimp population sampling programme, sample collection must be carried out after lowering the pond water level by experienced personnel using a large and heavy cast net, and the number of sampling stations and frequency of sampling must be as large as possible. It is important for a farm to establish adequate in‐house sampling methods that adequately reflect its needs and capabilities. Dugger and Jory (2016) recently reviewed shrimp sampling.
Health assessment and management on shrimp farms has become an important issue in the last 20 years or so, because of the increased importance of various diseases, particularly of viral origin. Shrimp hatcheries, nurseries and grow‐out facilities can be affected by many diseases of viral, bacterial, fungal or parasitic origin. Some diseases are so far only reported from some areas (Eastern or Western Hemisphere). The shrimp farming industry has been characterised through its relatively short history by a series of periodic, serious and financially devastating diseases, mostly of viral origin but lately also bacterial and fungal. These have probably caused many USD billions in financial losses to the industry.
There are now over 30 distinct viruses (or groups of viruses) known to infect shrimps. Viral diseases, particularly the white spot virus (WSSV) and Taura virus (TSV) have severely impacted on the shrimp farming industry worldwide, causing very important production and economic losses.
The latest major shrimp diseases affecting the industry are Early Mortality Syndrome (EMS)(=Acute Hepatopancreatic Necrosis Syndrome (AHPND), and Hepatopancreatic Microsporidiosis, known as EHP from the small microsporidian parasite, Enterocytozoon hepatopenaei, that causes it.
EMS/AHPND is a serious global disease caused by the pathogenic bacterium Vibrio parahaemolyticus, which causes hepatopancreas dysfunction and secondary Vibrio infections, and can result in up to 100% mortality in juvenile cultured shrimps. AHPND was first reported in China in 2010 and has since spread to other Asian countries including Thailand, Vietnam, India, Malaysia and the Philippines, and to Mexico and various Latin American countries. Estimated losses due to EMS exceed USD 1 billion annually. The best management for EMS is to stock clean animals into clean ponds, and to keep organic matter levels as low as possible in the grow‐out ponds.
EHP disrupts the digestive system of affected shrimps. It does not typically result in significant mortalities but reduces growth and significantly increases size variability. It has seriously affected farmed shrimp production in, and has been reported from Thailand, China, Malaysia, Indonesia, Vietnam and India, and more recently it was also reported from a Latin American country. Because EHP reproduces through very resistant spores, it is very difficult to eradicate and the most effective management practice is strict biosecurity and disinfection of affected facilities.
Several strategies have been tried to control viral diseases in shrimp farming, ranging from improved husbandry practices to stocking ‘specific pathogen‐free’ (SPF) or ‘specific pathogen‐resistant’ (SPR) species or stocks. Further information on shrimp diseases is provided by Cuéllar‐Anjel et al. (2010).
A cost‐effective health management and biosecurity programme requires reliable diagnostic tools that shrimp farmers can use to make adequate and timely decisions on management procedures to control or exclude pathogens. Virulent pathogens can produce catastrophic mortalities very rapidly, and shrimp farmers need this fast diagnostic capacity in order to respond effectively. Practical diagnostic methods that are accurate, sensitive, rapid and economical to conduct are already available, including molecular methods like polymerase chain reaction (PCR), dot‐blot gene probes and various methods for rapid fixation and staining.
Early signs of many health problems can be promptly observed by examining shrimps during regular feed tray monitoring or from weekly sampling. Some of these signs include:
- loss of appetite (empty guts);
- changes in colour (blueish or reddish);
- persistent soft shells or shell fouling;
- red discolouration (particularly in appendages and uropods);
- lethargic or disoriented behaviour;
- fouled or discoloured or black gills;
- blackened lesions on shell;
- opaque or white tail muscle; and
- various morphological deformities, such as cramped tail.
22.8.5 Harvest and Transport to Processing Plant
Shrimps are highly perishable and delicate, and no amount of manipulation can restore product quality once it is lost. Therefore, proper preparation, harvesting and preservation are critical for the product to be the best quality and command the best price. This preparation can be quite elaborate, because a pond ready to be harvested may have 5−30 t or more of shrimps, which must be properly collected, handled and packed. The most important objectives at harvest are to:
- minimise the quantity of shrimps left on the pond bottom;
- immediately chill shrimps to near freezing to prevent deterioration due to bacterial activity; and
- pack the shrimps in a manner that avoids physical damage.
The most important considerations of when to harvest a pond are shrimp size, price and maximising the economic return of the production cycle. Sometimes ponds need to be emergency‐harvested because of a disease outbreak. Many shrimp farmers use the moon phase to program their harvests, targeting periods of full and new moon. About 3–5 days before the harvest date, the texture (representative of the stage in the moulting cycle) of shrimps to be harvested is monitored by daily collection of a sample (100–300 animals/pond) to determine the percentage that are moulting. The particular requirements of the processing plant and the intended market determine what percentages of hard, soft and semi‐hard (post‐moulting) shrimps, are acceptable to proceed with a planned harvest. For example, for shrimps to be marketed whole, typically less than 5–8% should be soft and less than 15–20% should be semi‐hard. Some farmers also stop feeding a few days before the harvest, sometimes believing this will prevent the condition known as black or ‘exploded’ hepatopancreas.
Most shrimps are harvested by draining the production ponds, tanks and raceways. In preparing ponds for harvest, water levels are typically lowered to ca. 70% of their operational levels, beginning 24–48 h before the harvest. In larger ponds, it is often necessary to reduce water levels to 50% or so before beginning the harvest, so that the harvest does not last excessively long, which would stress the shrimps and reduce their quality.
Harvested shrimps are immediately separated into size classes and layered with ice or immersed in an ice slurry for transport to the processing plant. Within minutes after death, shrimps begin to deteriorate at normal pond temperatures (25–30°C) that promote bacterial decomposition. Initial spoilage, however, is due to digestive enzymes from the hepatopancreas. These break down proteins and reduce product weight, and quickly break down tissue at the junction of the head and tail, making the head appear loose and sagging, which is unacceptable for some markets. Bacterial and enzyme activity can be stopped by immediately reducing temperature to near freezing. It is very important that shrimps are killed by thermal shock when immersed in chilled water (4°C is maintained from the time the animals are harvested to the time they reach the plant). Often additional processing takes place at the pond bank. This includes dipping the shrimps in a solution of sodium metabisulphite to prevent melanosis or black spots, and reducing blackening of the head for head‐on shrimp product, removing crabs, fish and plant material. Shrimps must be handled carefully during packaging to minimise damage to the product and thus reducing its acceptability in markets.
Mechanical harvesting systems are also used to harvest shrimp ponds and assure product quality. These systems can be used to harvest ponds anytime without any tidal effects or needs.
Further details of post‐harvest technology for shrimps, including safety and health, live transport, post‐mortem processing and chilled storage life, are provided in Chapter 13. A processing plant that operates on a QA/QC (quality assurance/quality control) is shown in Figure 22.13.

Figure 22.13 Shrimp processing plant.
Source: Reproduced with permission from Darryl Jory.
Shrimps are marketed in a variety of forms: heads‐on shrimps (including live), shell‐on tails, peeled tails (including canned) (Figure 22.14), breaded tails and other value‐added products. These are transported to various markets in a variety of forms and packaging. Lucien‐Brun (2016) recently discussed in detail shrimp harvesting and processing, and the critical decisions and steps to maintain product quality.

Figure 22.14 Peeled and cooked is a common value‐added market form.
Source: Reproduced with permission from Darryl Jory.
22.9 Nutrition, Formulated Diets and Feed Management
22.9.1 Nutritional Requirements and Formulated Diets
With industry expansion, there has been an intensification of production and an increased dependence on the use of manufactured dry feed, which often represents the highest production cost. Protein is typically the most expensive macronutrient in shrimp feeds, and dietary protein levels from 18% to 50% have been recommended for various species and sizes; possibly because of their wide range of natural feeding habits. PL shrimps require a higher dietary protein level than older shrimps. Formulated shrimp feeds are complex products and the main components typically are wheat flour (20–35%), soybean meal (15–45%) and fishmeal (10−20%).
In recent years, there has been a strong effort to reduce the inclusion of fishmeal and fish oil, and replace these with alternative ingredients, including various meals and oils from agriculture products as well as rendered ingredients from the processing of other terrestrial meat industries (section 8.6.5.1). The remaining ingredients used in typical formulas include various lipids and micro‐ingredients that provide essential fatty acids, vitamins, minerals, attractants, binders, preservatives, pigments and health additives. At least 150 additives are currently used as ingredients in shrimp feeds.
22.9.2 Feed Management
Shrimp feed management is a critical aspect for cost‐efficient, environmentally responsible shrimp production. Appropriate practices produce maximum shrimp growth and survival concurrent with the lowest FCR, while reducing feed inputs and minimising impact of effluents. Efficient feed management is the summation of several sequential steps, including feed selection, storage and handling, application methods, and feeding regimes. Determining when to feed requires knowledge of diet activity patterns, feeding frequency and time (subject to change with geographical location, season, species, size, age, stocking density, unusual environmental conditions and other stimuli). Calculating feed rations involves estimating survival, population size and biomass, and size distribution. Monitoring and continuously adjusting the amount of feed applied, according to changes in consumption caused by various biotic (e.g., amount, quality and availability of natural food items) and abiotic (water quality and other environmental parameters) factors, is important for effective feed management. Evaluating and adjusting feed input involves regular population sampling and proper monitoring of various water quality parameters.
Shrimps are bottom feeders, and it is difficult to estimate their feed consumption, unless feed trays or lift nets are used (Figure 22.15). Inadequate feed management may promote the onset of various diseases and water quality‐related problems, and may adversely affect production. Ineffective practices often include:
- applying feed during times convenient for employees (during daylight hours), but not necessarily at the best times for shrimps;
- inadequate handling and storage practices during bulk feed storage and after feed distribution to pond side; and
- overfeeding.

Figure 22.15 Using a feed tray at set locations.
Source: Reproduced with permission from Darryl Jory.
The best shrimp feed in the world will yield poor results if it is not handled, stored and used properly.
22.9.3 Factors that Affect Feed Consumption
Several factors can affect shrimp feeding behaviour, and it is important to understand these factors to make proper and timely management adjustments (Jory et al. 2001). The major factors affecting feeding behaviour are:
- Species, age and size. There are marked differences between species. Some species are much more active and aggressive while foraging for food, and this has to be incorporated into a feeding strategy. Feeding rate is a physiological function dependent on the growth stage of the animal; it decreases as the animal grows and approaches maturity.
- Availability of natural food. When natural food is freely available, the demand for formulated feeds is reduced. This is typical when the biomass of stocked shrimps is low during the first few weeks after stocking and until the natural carrying capacity of the pond is reached.
- Water quality. The most important parameters are temperature, DO, pH and salinity, but other parameters also influence shrimps (Table 22.3). For each parameter, animals have a range of tolerance and a narrower optimum range that promotes optimum feeding, growth and overall well‐being.
- Moulting. Shrimps moult periodically (days–weeks) throughout their lives, and this is a stressing period during which their appetite diminishes markedly. It can take 2–5 days for normal feeding to resume after moulting. Thus, it is important to recognise when there is a significant reduction in feed consumption, indicating high incidence of moulting.
- Quality of commercial feeds. Shrimps eat to fulfil their nutritional needs, and if the feed does not have enough energy or appropriate nutritional profile, their feeding activity will increase. Feed attractability and palatability are also important factors.
22.9.4 Feed Handling and Storage
Feed management at a shrimp farm begins upon arrival of a feed shipment. Poor storage and handling of feeds will result in product deterioration, reduced feed attractability and palatability, possible nutritional deficiencies and disease outbreaks, reducing growth rates and overall production. Upon reception of a feed batch, a few randomly selected bags are examined for physical integrity. In addition, twice a year feed samples are collected from newly received shipments (or when using a new feed) and analysed for proximate composition, mycotoxins and selected pesticides if pertinent (Jory et al., 2001; Jory, 2016).
Feed is ideally used within the first 2–4 weeks after manufacture and must not be stored for more than 2–3 months. At farms that feed several times over 24 h, the total feed ration is often distributed from the farm warehouse to the ponds once, usually early in the morning. The feed bags must then be protected from sunlight and rain by storing them off the ground in simple pond‐side sheds.
22.9.5 Application and Distribution
Formulated feeds may be distributed manually in large ponds from boats or mechanically using blowers mounted on vehicles and boats (Figures 22.16 and 22.17). In smaller ponds, tanks and raceways, automatic feeders with timing mechanisms can be used or broadcasting from the pond banks. Feed is applied exclusively using feeding trays at some farms. Newer and very sophisticated auto‐feeding systems rely on the feeding sounds of shrimps – using underwater microphones connected to computers and using elaborate software programs to provide continuous feed applications, if desired.

Figure 22.16 A feed blower used to feed shrimps in large ponds.
Source: Reproduced with permission from Darryl Jory.

Figure 22.17 Manually broadcasting feed from a boat or pond bank.
Source: Reproduced with permission from Darryl Jory.
It is important to distribute the feed evenly early in the grow‐out cycle, but, as the cycle progresses, shrimps react to changing pond microhabitats. They avoid areas where anaerobic sediments accumulate and noxious compounds, such as H2S, are produced, including internal drainage canals and areas close to the drainage structures. In addition, many shrimps will move to the deeper areas of ponds during the day to avoid light. Therefore, it may not be appropriate to apply feed in very shallow areas during daylight hours, because it is unlikely that shrimps will consume it.
22.9.6 Frequency and Timetables
All formulated feeds have an ideal feeding rate range that optimises growth and feed efficiency. This range varies with species, age and weight, stocking density, water quality, availability of natural foods, stress and other factors. At many farms, feeding is based on tables that do not properly consider natural feeding habits of shrimps or their physiological state. Increasing the frequency of feeding generally produces immediate benefits, including reduced nutrient and feed loss, and increased growth and feed utilisation efficiency. In Asia, it is common practice to feed up to six to seven times over a 24‐hr period regardless of the species farmed.
How many times and when to feed is an important decision that each shrimp farm must determine, based on the factors described and available resources. Feeding during the night typically becomes more important as the production cycle progresses and the availability of natural feed diminishes. In general, two feed applications per day are needed at the beginning of the production cycle, increasing as the cycle progresses to at least three or four applications per day.
22.9.7 Feed Rations
Feed rations can be calculated using a set schedule that accounts for animal weight and estimated biomass/survival in the pond. Feeding based on tables is still widely practised. There are several problems with relying on feeding tables:
- There are problems in accurately estimating survival, particularly when dealing with small animals in large (ca. 5 ha) ponds.
- Various factors, as outlined, affect feeding rates.
Feed consumption changes can usually be detected by proper monitoring of feed trays. Feeding rates are adjusted periodically (usually weekly), based on sampling estimates for individual average body weight, population size distribution and pond shrimp biomass. As shrimps grow the feed amounts used decrease as a percentage of the total shrimp biomass, but the absolute amount of feed increases together with increasing shrimp biomass.
22.9.8 Use of Feed Trays
Feed trays are also called observation or lift nets, feed inspection trays or umbrella nets. They are simple devices usually consisting of a frame and fine mesh that is lowered to the floor of the pond (Figure 22.15) and can also collect a sample of the shrimps feeding in it. There are several ways feed trays can be used, including as indicators of feed consumption, population and health assessments and other observations.
In using the feed trays as indicators of feed consumption, about 4−8/ha are used in ponds < 5 ha, whereas 2−5/ha are used in larger ponds (10–20 ha). A small percentage of the ration is placed in the trays and the ration is distributed throughout the pond. Trays are checked after 1−3 hr and the data are used to adjust rations. In the Peruvian system, the entire ration is applied in trays, which requires many more trays per pond.
In general, observation of feed consumption from a small number of trays is not an adequate measure of actual feed consumption, especially in large ponds. This is because there are considerable day‐to‐day variations in feed consumption.
22.10 Emerging Production Technologies and Issues
22.10.1 Diseases and Biosecurity
Viral diseases have had a considerable impact on commercial shrimp farming during the past two decades, significantly affecting the operation, management and design of shrimp farms. Another resulting consequence is increased awareness of the need for better husbandry methods to reduce the risks of exposure to pathogens, and also of the need for improved management practices to enhance shrimp health. Shrimp farming is a relatively new industry and has lagged behind in the development of practices for standard health management. In recent years, tremendous progress has been made in shrimp health management.
Biosecurity in shrimp aquaculture involves those practices that will reduce the probability of introduction and dissemination of a pathogen. Shrimp producers often give only limited attention to routine biosecurity on their farms. This is because of the misconception that the potential costs of implementing biosecurity measures will outweigh the benefits or because they do not have appropriate knowledge. Effective implementation of biosecurity protocols requires awareness, discipline and a commitment by farm owners to implement them. We have improved our knowledge of shrimp viral diseases significantly during the past 30 years, mainly due to their negative impact on the industry, but more efficient biosecurity is still very new to shrimp farming (Fegan and Clifford, 2001).
A cost‐effective, biosecure shrimp‐farming protocol involves:
- aggressive methods for pathogen exclusion from production systems;
- effective screening of seedstock;
- appropriate environmental management;
- effective health management, integrating genetic selection;
- specific pathogen‐free and/or pathogen‐resistant stocks;
- limited or zero water exchange;
- stocking strategies;
- feed management and use of immune stimulants to increase host defences; and
- strict and proactive health monitoring and farm management strategies.
Also important are farm location (site selection) and design. Relatively few shrimp farms have been specifically designed to prevent diseases, although it is more cost‐effective to incorporate disease prevention and treatment aspects during the planning stage than to redesign or refit existing farms. Eliminating or reducing water exchange is important to prevent viral diseases, to exclude pathogens from production systems, and to minimise stressful variations in water quality, which may trigger disease outbreaks.
22.10.2 Probiotics and Microbial Management
As described, the use of probiotics is relatively common to limit pathogenic bacteria in the disease‐prone intensive systems used to produce shrimp PL in commercial hatcheries. For some time now, the use of bacterial supplements has been recommended for use in aquaculture ponds to obtain a number of benefits, including:
- reducing blue‐green algae populations;
- preventing off‐flavour;
- reducing N and P levels;
- increasing DO; and
- promoting decomposition of organic matter.
Several commercial probiotic products are available to shrimp producers and are widely used with the objectives of promoting shrimp health and shrimp yield; and to manage organic waste and sludge in ponds, tanks and raceways, as well as pond effluent quality.
The pond microbial community plays a major role in the natural food availability, mineral recycling rates and DO dynamics in shrimp ponds. Effectively managing the microbial community can help prevent or reduce the risk of a disease outbreak, but, if mismanaged, the microbial community can also promote disease by creating conditions that facilitate growth of pathogenic bacteria. Microbial management is still a relatively new but most promising field for the shrimp farming industry.
22.10.3 Nursery Systems
Many shrimp farms are now using nursery systems as an intermediate production step between the hatchery and the final grow‐out pond. Nurseries are often also used as acclimation stations. These systems can be as simple as mesh enclosures in smaller, ponds to sophisticated indoor, recirculating systems (lined tanks/ponds in greenhouses or other cover, with areas of 300−7500 m2).
Nurseries are used to grow PL at high densities, with stocking densities from 500 to 10 000 PL/m3, stocking size of around 2‐mg and harvest sizes of 0.3−3 g and harvest biomass of 1−3 kg/m3. Nurseries typically produce strong, healthy and uniform juveniles with significant potential for compensatory growth after their transfer for final grow‐out to market size. Nursery systems have several advantages, including more control and efficiency, improved biosecurity and health management, and they allow for early stocking in areas with extreme seasonality.
A two‐stage grow‐out system using a nursery phase as a quarantine area increases biosecurity and can increase turnover (number of grow‐out production cycles) by reducing culture time to market size in grow‐out ponds. Therefore, the grow‐out pond is being used more efficiently as a biological system, and with greater capital and operating efficiency. A nursery system also provides improved accuracy to estimate the juvenile population before actual stocking in grow‐out ponds. Thus, stocking juveniles allows for a more accurate estimate of the initial population and biomass, and improving feeding rate estimates when formulated feed becomes up to 60% of the production cost. Indoor nurseries can broaden the temperature stocking windows for seasonal hatchery outputs, allowing greater efficiency for the hatchery and farm. Shrimp farms in areas of lower salinities can use the nursery as an acclimation system. Nursery head‐start strategies may allow farms without hatcheries to purchase seedstock in advance of the peak demand periods, possibly at lower cost and with improved certainty of seedstock delivery.
Managing nursery systems in tanks and raceways is relatively more difficult than standard grow‐out ponds stocked directly, but the many benefits derived from a two‐phase grow‐out strategy, using first a nursery system (indoor) followed by final grow‐out to market size (outdoor pond) can significantly improve production and profitability. For a thorough review of shrimp nursery systems, see Samocha (2010).
22.10.4 Inland Shrimp Production
Shrimp farms have traditionally been built in tropical coastal areas, very close to the ocean or to an estuary or river. Since around 2000, many shrimp farms have been developed in inland areas and desert coastlines that, in principle, do not appear suitable for this activity. Some of these farms are located in inland deserts with available underground water having specific chemical characteristics. They could provide a new direction for the expansion of the industry, because deserts and other dry lands constitute over 40% of the global land area.
22.10.5 Recirculation, Biofloc Technology and Reduced Water Exchange Systems
Improved water management regimes can help address viral disease problems and issues raised by environmental groups that have targeted shrimp farming, pressuring the industry to adopt more sustainable production practices.
Large‐scale application of zero‐exchange and recirculation technologies on existing farms has already increased producer confidence in the potential for reducing or eliminating routine water exchange through most or all of the growing season. There are several successful examples of the implementation of zero‐exchange production systems.
Browdy et al. (2001) reviewed various aspects of intensive closed technologies for shrimp production. Nutrient‐rich effluents from intensive production systems can contribute to the eutrophication of receiving waters, potentially affecting both natural biota and local culture operations. Water exchange can be reduced or eliminated, and supplementary aeration can have an essential role in the successful operation of intensive closed systems. Paddlewheel aeration must be increased by 10% or more over levels traditionally applied in intensive culture to maintain appropriate DO levels. Better placement of mechanical aerators and use of back‐up aeration and alarm systems are also necessary. The design and management of production facilities to re‐use water, minimise exchange and eliminate discharge will improve the outlook for more profitable and sustainable production technologies.
Another technology, borrowed from the wastewater industry, that continues to gain strength in shrimp farming is biofloc technology. Bioflocs are masses or aggregates (flocs) of bacteria, algae, protozoans and various forms of particulate organic matter like uneaten aquafeed, faeces and others. They are held together in a mucous matrix secreted by bacteria or by electrostatic attraction or connected by filamentous microorganisms. Animals that consume flocs, like nematodes and zooplankton, are also considered part of the biofloc community. They provide improved biosecurity, disease prevention, in‐situ water treatment and nutrient recycling (typically with improved nutrition and FCR). However, these systems can be expensive to setup and operate, require a high degree of technical expertise, and reliable and continuous mechanical aeration.
In recent years, a number of shrimp farms in Asia and Latin America have adopted a strategy of internally recirculating water and only replenishing from the outside that water lost by evaporation, seepage and other natural processes. This approach typically relies heavily on frequent treatment with various bacterial amendments, and has numerous advantages:
- significantly increased biosecurity;
- minimization of effluents released to surrounding waters;
- improved pumping efficiency;
- significantly reduced need for fertilization;
- more stable water quality parameters, including improved levels of DO;
and
- reduced pond downtime and faster restocking.
They also has some drawbacks, including:
- a high initial cost to retro‐fit the ponds, e.g., build sedimentation ponds or convert some existing production ones into sedimentation ones;
- management requires a higher degree of technical competency;
and
- depending on the shrimp biomass, up to total dependency on 24‐hr mechanical aeration.
And in some countries, a number of small indoor facilities for shrimp grow‐out have been developed. These depend on buying PL and aquafeed, using small plastic swimming pools or small tanks, and they are generally managed as biofloc systems. They are often a side business to other agriculture activities. They produce small amounts of shrimps (50‐400 kg/month), and service nearby niche markets for fresh shrimps, fetching very high prices for them.
22.10.6 Effluents
General impacts of effluents from coastal aquaculture farms on marine environments are considered in Chapter 5. This is an issue that shrimp producers and processors need to address while they are discharging effluent.
Settling basins are especially efficient for treating shrimp farm effluents, because the high concentrations of cations in seawater and brackish water tend to neutralise the negative charges on suspended clay particles, which will flocculate and settle. Plankton cannot be removed efficiently by sedimentation, and products such as aluminium sulphate, lime and selected organic colloids, often used in wastewater treatment to promote sedimentation, are not needed in shrimp farm settling basins.
22.11 Responsible Shrimp Farming and the Challenge of Sustainability
22.11.1 Domestication and Genetic Improvement
During its first three decades, commercial shrimp farming depended significantly on wild seedstock and broodstock. Wild seed supply was often unreliable and limited, and PL shortages severely afflicted the industry. Many factors affected the sourcing of broodstock and wild larvae, from global weather phenomena, such as El Niño and annual monsoons, to localised pollution and environmental degradation, and over‐fishing or over‐regulation of fisheries.
Shrimps have proved excellent candidates for domestication and genetic improvement, because of their high fecundity, short generation interval and the presence of additive effect of genetic variance for growth rate. When compared with most livestock industries, however, shrimp farming is still a newcomer to domestication and selective breeding.
Achieving domestication of selected shrimp species, together with selection, genetic improvement and, possibly, hybridisation and ploidy manipulation, should be major research objectives. An industry with the global importance that shrimp farming has achieved cannot depend on nature to supply its seedstock reliably.
22.11.2 Nutritional Requirements and Formulated Feeds
The development and use of compound feeds have been major factors in the expansion of the industry and will continue gaining importance. Growing demand for formulated feeds will increase competition for component resources, particularly fishmeal. There is an enormous but still unrealised potential to reduce the production cost and improve the nutritional performance of formulated feeds for shrimps. Extensive research must continue to improve our knowledge of the nutritional requirements of shrimps and to develop new diets that are species, area and even season specific. Reducing the cost of feeds is an aspect critical to further expanding the industry and improving its competitiveness relative to other protein sources, such as fish, beef, pork and poultry.
22.11.3 Disease Prevention, Diagnosis and Control
As described earlier, there have been spectacular collapses of shrimp farming industries in a number of countries, including the top producing countries, China, Thailand, Indonesia and Ecuador. Standard denominators among the shrimp farming industries of these countries were very fast, unregulated development and an increased incidence of diseases, particularly viral diseases. There are still very few alternatives to deal with viral infections, and the best procedure for disease management is exclusion. There will undoubtedly be new pathogens that the industry will have to confront and manage. The application of effective pathogen detection and disease diagnostic methods, particularly those based on molecular biology and continuously improved by the industry, are essential to better understand and prevent losses due to disease.
22.11.4 Best Management Practices
Best management practices (BMPs) are a practical way to approach environment management for shrimp farming. They are practices considered the most effective methods of reducing environmental impacts, while being compatible with resource management goals. Shrimp producers are faced with a variety of major certification schemes and the uncertainty of which one to choose. Some certifications are more important than others, depending on the targeted export market. It is an urgent matter for the major certification schemes to come together and harmonise their standards, so that producers can more cost‐effectively opt for this important product quality and marketing tool.
22.12 Summary
- Shrimps have been farmed commercially for almost 40 years. Shrimp farming is now carried out in at least 60 countries worldwide, but production is significantly localised in two major areas, Asia and the Americas, which account for about 95% of all farmed shrimps.
- Production has been increasing steadily in the past two decades, from about 1 million t in 1995 to about 4 million t currently.
- It is based mostly on one species with relatively few selectively‐bred, improved lines. The Pacific white or whiteleg shrimp (L. vannamei) is the most important species in the world, with virtually all production coming from aquaculture. This species represents around 75% of all farmed shrimps globally, and over 40% of all shrimps produced in the world (fisheries and aquaculture combined).
- Grow‐out production technology is still mostly extensive to semi‐intensive. There is considerable potential to improve production efficiency through innovation, standardisation and automation at various levels.
- Knowledge of the nutritional requirements of farmed shrimps is adequate but there is much room for improvement. Higher inclusion of land‐based ingredients has significantly reduced the use of limited ingredients of marine origin, like fishmeal and fish oil, which were very important in shrimp feeds during the first two decades of the industry.
- The history of the industry is one of periodic, major disease outbreaks (mostly viral) and continuous health management issues that disrupt supply chains and markets. It is of major concern to potential investors with few proactive alternatives available, other than strict biosecurity measures. This is because shrimps, being invertebrates, do not have specific immunity and cannot be vaccinated in the traditional sense.
- Ongoing consolidation within the industry both in Asia and the Americas is creating very large, vertically‐integrated companies that can maximise the economies of scale and efficiency.
- The shrimp farming industry is expected to expand significantly in the next two decades, particularly in India, Southeast Asia and Latin America, and current production will nearly double by 2030.
References
- Alday‐Sanz, V. (Ed.). 2010. The Shrimp Book. Nottingham University Press. Manor Farms, Main Street, Thrumpton. Nottingham, NG11 0AX, United Kingdom. 920 p.
- Anderson, J.L., Valderrama, D. and Jory, D.E. 2016. Global shrimp survey: GOAL 2016. http://advocate.gaalliance.org/global‐shrimp‐survey‐goal‐2016/
- Boyd, C.E., C.A. Boyd and Suwanit Chainark. 2010. Shrimp pond soil and water quality management. Pp. 281–303. In: Alday‐Sanz, V. (Ed.). 2010. The Shrimp Book. Nottingham University Press. Manor Farms, Main Street, Thrumpton. Nottingham, NG11 0AX, United Kingdom. 920 p.
- Browdy, C. L. and Jory, D. E. (Eds). (2001). The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture. Aquaculture 2001, World Aquaculture Society, Baton Rouge, LA.
- Browdy, C.L., and Jory, D. E. (Eds). 2009 The Rising Tide, Proceedings of the Special Session on Sustainable Shrimp Farming, World Aquaculture 2009. The World Aquaculture Society, Baton Rouge Louisiana USA.
- Browdy, C. L., Bratvold, D., Stokes, A. D. et al. (2001). Perspectives on the application of closed shrimp culture systems. In: The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture (Ed. by C. L. Browdy and D. E. Jory), pp. 20–34. Aquaculture 2001, World Aquaculture Society, Baton Rouge, LA.
- Cuéllar‐Anjel, J., M. Corteel, L. Galli, V. et al. 2010. Principal shrimp diseases, diagnosis and management. Pp. 517–621. In: Alday‐Sanz, V. (Ed.). 2010. The Shrimp Book. Nottingham University Press. Manor Farms, Main Street, Thrumpton. Nottingham, NG11 0AX, United Kingdom. 920 p.
- Dugger, D.M. and Jory, D.E. 2016. Regular population sampling of shrimp in ponds, parts 1 and 2. http://advocate.gaalliance.org/regular‐population‐sampling‐of‐shrimp‐in‐ponds‐part‐1/ and http://advocate.gaalliance.org/regular‐population‐sampling‐of‐shrimp‐in‐ponds‐part‐2/
- FAO Fishstat Plus (2016). Universal software for fishery statistical time series. Version 2.30. Fisheries Department, Fishery Information, Data and Statistical Unit. FAO, Rome.
- Fast, A. W. and Menasveta, P. (2000). Some recent issues and innovations in shrimp pond culture. Reviews in Fisheries Science, 8, 151–233.
- Fegan, D. and Clifford, H. C., III (2001). Health management for viral diseases in shrimp farms. In: The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture (Ed. by C. L. Browdy and D. E. Jory), pp. 168–98. Aquaculture 2001, World Aquaculture Society, Baton Rouge, LA.
- Jory, D.E. 2016. The proper management of commercial shrimp feeds, parts 1 and 2. http://advocate.gaalliance.org/the‐proper‐management‐of‐commercial‐shrimp‐feeds‐part‐1/ and http://advocate.gaalliance.org/the‐proper‐management‐of‐commercial‐shrimp‐feeds‐part‐2/
- Jory, D. E., Cabrera, T. R., Dugger, D. M., Fegan, D., Lee, P. G., et al. (2001). A global overview of current shrimp feed management: status and perspectives. In: The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture (Ed. by C. L. Browdy and D. E. Jory), pp. 104–52. Aquaculture 2001, The World Aquaculture Society, Baton Rouge, LA.
- Juarez, L.M., Moss, S.M. and Figueras, E. 2010. Maturation and larval rearing of the Pacific white shrimp, Litopenaeus vannamei. Pp. 305–352. In: Alday‐Sanz, V. (Ed.). 2010. The Shrimp Book. Nottingham University Press. Manor Farms, Main Street, Thrumpton. Nottingham, NG11 0AX, United Kingdom.
- Samocha, T.M. 2010. Use of intensive and super‐intensive nursery systems. Pp. 247–280. In: Alday‐Sanz, V. (Ed.). 2010. The Shrimp Book. Nottingham University Press. Manor Farms, Main Street, Thrumpton. Nottingham, NG11 0AX, United Kingdom. 920 p.