1
Introduction
John S. Lucas
1.1 What is and What isn’t Aquaculture?
Give a person a fish and you feed them for a day;
Teach a person how to fish and you feed them for their life‐time;
Teach a person how to grow fish and you feed them and their neighbours for their life‐times.
(modified from a Chinese proverb)
Aquaculture continues to develop rapidly, especially through its growth in Asia. World aquaculture production is increasing much more rapidly than animal husbandry and capture fisheries, the other two sources of animal protein for the world’s population. There is widespread recognition that seafood production from capture fisheries is at its peak, and that aquaculture will become increasingly important as a source of seafood production, and ultimately the main source. There is widespread public interest in aquaculture. This is the context in which this textbook is written and we trust that it will convey some of the excitement of the rapidly developing discipline of aquaculture.
The term ‘seafood’1 is used inclusively in this textbook, i.e., for all animal and plant products from aquatic environments, including freshwater, brackish, and marine and hypersaline environments. The term ‘shellfish’, according to common usage, is used to describe aquatic invertebrates with a ‘shell’. In this way, bivalve and gastropod molluscs, decapod crustaceans and sea urchins are combined, while recognising the extreme diversity of morphology and biology within this grouping. The two groups that overwhelmingly constitute shellfish are the bivalves (oysters, mussels, clams, etc.) and decapod crustaceans (shrimp1, crayfish, crabs, etc.). The other major group of aquatic animals that is cultured is the fishes, also known as finfish. ‘Fish farming’ is used in the sense of aquaculture of fishes, crustaceans, molluscs, etc., but not plants.
There are many different forms of aquaculture and, at the outset of this book, it is important to establish what aquaculture is, what it isn’t and what distinguishes it from capture fisheries.
‘The definition of aquaculture is understood to mean the farming of aquatic organisms, including fish, molluscs, crustaceans and aquatic plants. Farming implies some form of intervention in the rearing process to enhance production, such as regular stocking, feeding and protection from predators. Farming also implies individual or corporate ownership of stock being cultivated.’
For statistical purposes:
- aquatic organisms that are harvested by an individual or corporate body that has owned them throughout their rearing period are classed as aquaculture products.
- aquatic organisms that are exploitable by the public as a common property resource, with or without appropriate licences, are the harvest of fisheries.
According to the FAO definition2, the two essential factors that together distinguish aquaculture from capture fisheries are:
- Intervention to enhance the stock.
- Ownership of the stock.
Thus, a structure to which fish are attracted and caught, e.g., a fish‐aggregating device (FAD) floating in the open ocean may be owned, but this does not confer ownership of the stock of attracted fish. Furthermore, the FAD facilitates capture, but does not enhance the fish stock that is being captured. This is capture fisheries production. Hatchery production of juvenile fishes is aquaculture: they are owned by the hatchery and may be sold as fingerling fish. Their ultimate capture, after being released into rivers to which they eventually return to breed is fishing. The released fingerlings enhance the stock, but they become a common property resource. The same applies where hatchery‐reared fish fingerlings are sold to fishing clubs and local government bodies to stock lakes and dams to improve recreational fishing.
Hydroponics and aeroponics, the cultivation of terrestrial plants with their roots in dilute nutrient solutions aren’t aquaculture. These are alternative methods for growing terrestrial plants.
Activities constituting aquaculture production, according to FAO3 are:
- hatchery rearing of fry, spat, postlarvae, etc.;
- stocking of ponds, cages, tanks, raceways and temporary barrages (e.g., dams) with wild‐caught or hatchery‐produced juveniles to be reared to market size;
- culture in private tidal ponds (e.g., Indonesian ‘tambaks’);
- rearing molluscs to market size from hatchery‐produced spat, transferred natural spatfall or transferred part‐grown animals;
- stocked fish culture in paddy fields;
- harvesting planted or suspended seaweed;
- valliculture (culture in coastal lagoons).
1.2 Origins of Aquaculture and Agriculture
The New Stone Age (Neolithic Age ca. 8,000–4,000 BC) was distinguished by the invention of farming. There were at least seven independent origins of farming during this Age: in China, New Guinea, Mexico, West Africa, the Andes, the Amazon basin and the Middle East (Figure 1.1). Wheat, rice, maize, barley and millet, which are still the major cereal crops, and the major root crops, potatoes and cassava, were all domesticated at various of these geographical locations during this period. Similarly, the husbandry of pigs, cattle, chickens, sheep, goats and horses, which are still major farm animals, was developed during the Neolithic Age. The origins of the plants and live‐stock that we farm go back a long way.

Figure 1.1 The court bakery of Ramses III. From the tomb of Ramses III in the Valley of the Kings, twentieth dynasty. (The Oxford Encyclopaedia of Ancient Egypt.)
(Courtesy of Wikimedia Commons).
These changes from hunting–gathering to agriculture and animal husbandry caused profound changes in lifestyle, from a nomadic to a settled existence. They resulted in greatly increased productivity from the land for human consumption and increased human populations per unit land area as a consequence. There were, however, disadvantages. The heavy dependence on a crop could lead to nutritional deficiencies and, if the crop failed, to starvation. Close proximity to other humans, domestic animals and opportunistic vermin led to the transmission of diseases. The hunter‐gatherer probably tended to be healthier, wih a more varied diet and less exposure to diseases.
The origin of aquaculture came some thousands of years after the Neolithic Age when culture of common carps (Cyprinus carpio) was developed in China where the carp is a native species (Figure 1.2). There is a long history of aquaculture in China (section 1.6). Common carps may have been farmed as early as 2000–1000 BC.4 The first aquaculture text is attributed to a Chinese politician, Fan Lei, and is dated about 500 BC. Fan Lei attributed the source of his wealth to his fish ponds: so his fish culture was more than a hobby. However, on three continents, Africa, America and Australia, aquaculture was not practised until it was introduced in recent centuries.

Figure 1.2 The common carp (Cyprinus carpio).
Source: Photograph by Piet Spaans. Reproduced under the terms of the Creative Commons Attribution License, CC‐BY‐SA 4.0.
The late origin of aquaculture compared with agriculture and its failure to develop in some continents is partly because humans are terrestrial inhabitants. We cannot readily appreciate the parameters of aquatic environments. There are some environmental factors that may profoundly affect aquatic organisms, such as:
- the very low content of O2 in water (<1%) compared with air (21%);
- high solubility of CO2 in water;
- pH;
- salinity;
- buffering capacity;
- dissolved nutrients;
- toxic nitrogenous waste molecules;
- turbidity;
- heavy metals and other toxic molecules in solution;
- phyto‐ and zooplankton concentrations; and
- current velocity.
Many of the diseases that afflict aquatic organisms are quite unfamiliar to us. Furthermore, virtually all the animals used in aquaculture are poikilotherms (their body temperature is variable and strongly influenced by environmental temperature) (‘cold blooded’). Their metabolic rates, and all functions depending on metabolic rate, are profoundly influenced by environmental temperature in ways that we do not experience as ‘warm‐blooded’ mammals (Figure 1.3).
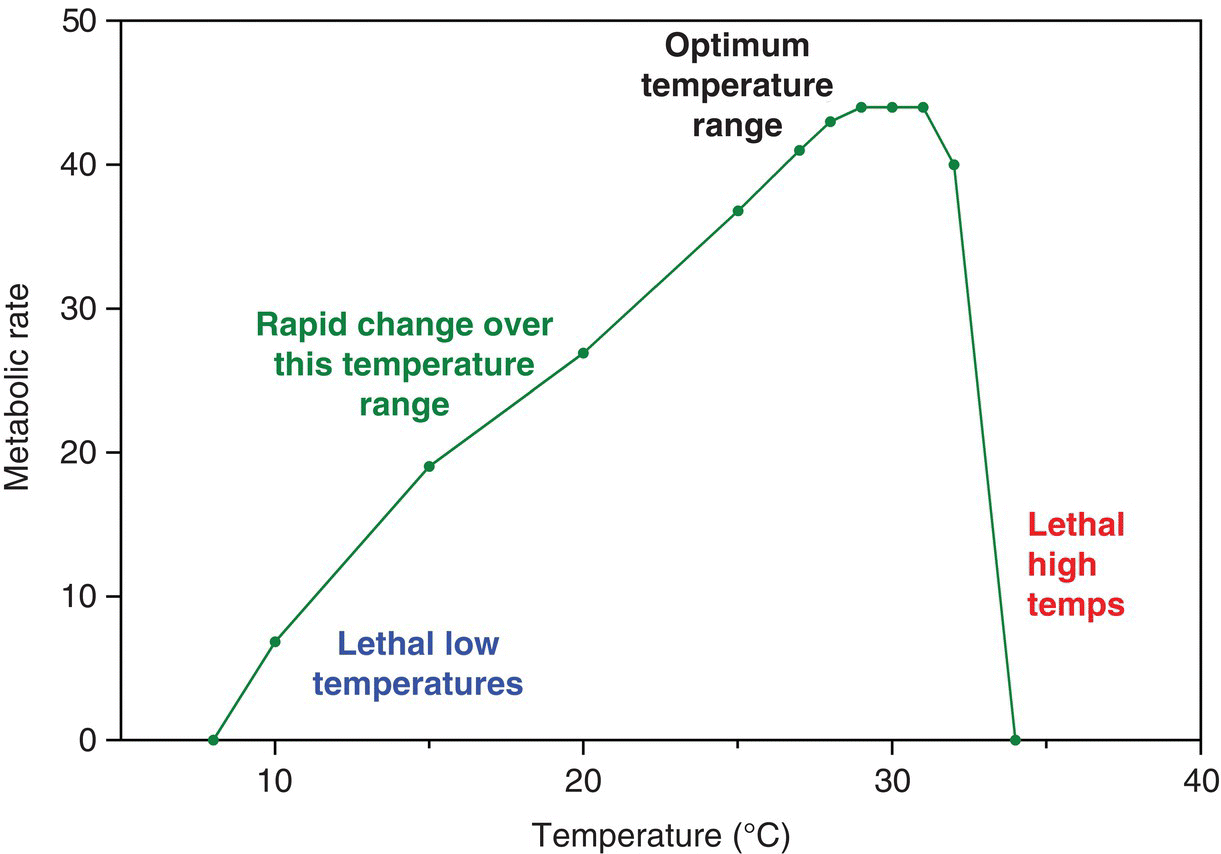
Figure 1.3 Metabolic rate over a temperature range in a poikilothem. Metabolic rate will be reflected in the rate of the animals’s oxygen consumption. Reproduced with permission from John Lucas.
The difficulties of appreciating and controlling the influences of these environmental factors still apply today, causing aquaculture programmes to have a relatively longer development period than other forms of food production. Even where there is well‐established technology for an organism there will still be site‐specific issues and progressive achievement of optimum husbandry. In agriculture we are much more readily able to appreciate the parameters influencing the success or otherwise of the output, and there is a very long history of attaining the skills needed.
A further major consequence of the late origin of aquaculture is that there has been relatively little genetic selection for many species and this is compared with the highly selected plants and animals of agriculture. Modern agriculture is based on organisms that are vastly different from their wild ancestors, and in many cases their wild ancestors no longer exist. This selection for desirable traits took place steadily and without any scientific basis over thousands of years of domestication. It was more intense, however, last century with scientific breeding programmes. Modern agriculture would be totally uneconomic, and the current world population would starve without these domesticated and genetically‐selected agricultural plants and animals. Much of aquaculture, by contrast, is based on plants and animals that are still ‘wild’. There are some species that have been subject to strong selection, hybridization, and molecular and genomic techniques (Chapter 7), such as:
- common carps;
- Atlantic salmon;
- rainbow trout;
- tilapias; and
- channel catfish.
Their breeding is based on broodstock that differ substantially from their ancestors in their genetics. Many other aquaculture species are based on wild broodstock obtained from natural populations. In some cases the life cycle has not yet been ‘closed’, i.e., the species has not been reared to sexual maturity and then spawned on a regular basis under culture conditions. Until the life cycle is closed, there is minimal potential for selective breeding.
1.3 Aquaculture and Capture Fisheries Production
Fishing activities, whether they are spearing individual fish, collecting shellfish from the shore, casting a net, fishing from a boat or the factory ships that ply the world’s oceans, are all tradional hunter–gatherer activities regardless of the degree of technology. Until recently, capture fisheries production exceeded aquaculture production: hunter–gathering was the major source of seafood. These fisheries suffer problems that are fundamental to hunter–gathering:
- variable recruitment and consequent unpredictability of stock size;
- difficulties in assessing stock size and its capacity for exploitation;
- difficulty in regulating exploitation to match the stock size; and
- relatively low productivity.
The natural productivity of the world’s water masses, fresh, brackish and marine, is huge, but finite; and a finite amount of plant and animal products can be harvested by fishing. For instance, the mean harvest from oceans that can be obtained for direct human consumption or consumption through use in fishmeal is ca. 2.5 kg/ha/yr of ocean surface. Furthermore, this huge but finite amount of harvest is within the scope of our current fishing capacity. Based on the FAO assessment of 600 marine fisheries, 77% are fully or over‐exploited, i.e., without capacity for increased harvest. This figure includes 17% of fisheries that are overexploited, 7% that are depleted and 1% recovering from depletion (Figure 1.4). This situation is reflected in the FAO data for annual capture fisheries production. Global capture fisheries production increased to 93 million t/yr (t = tonne)5 in about 1994 and since then has fluctuated between 89 and 97 million t/yr without any trend (Figure 1.5).6
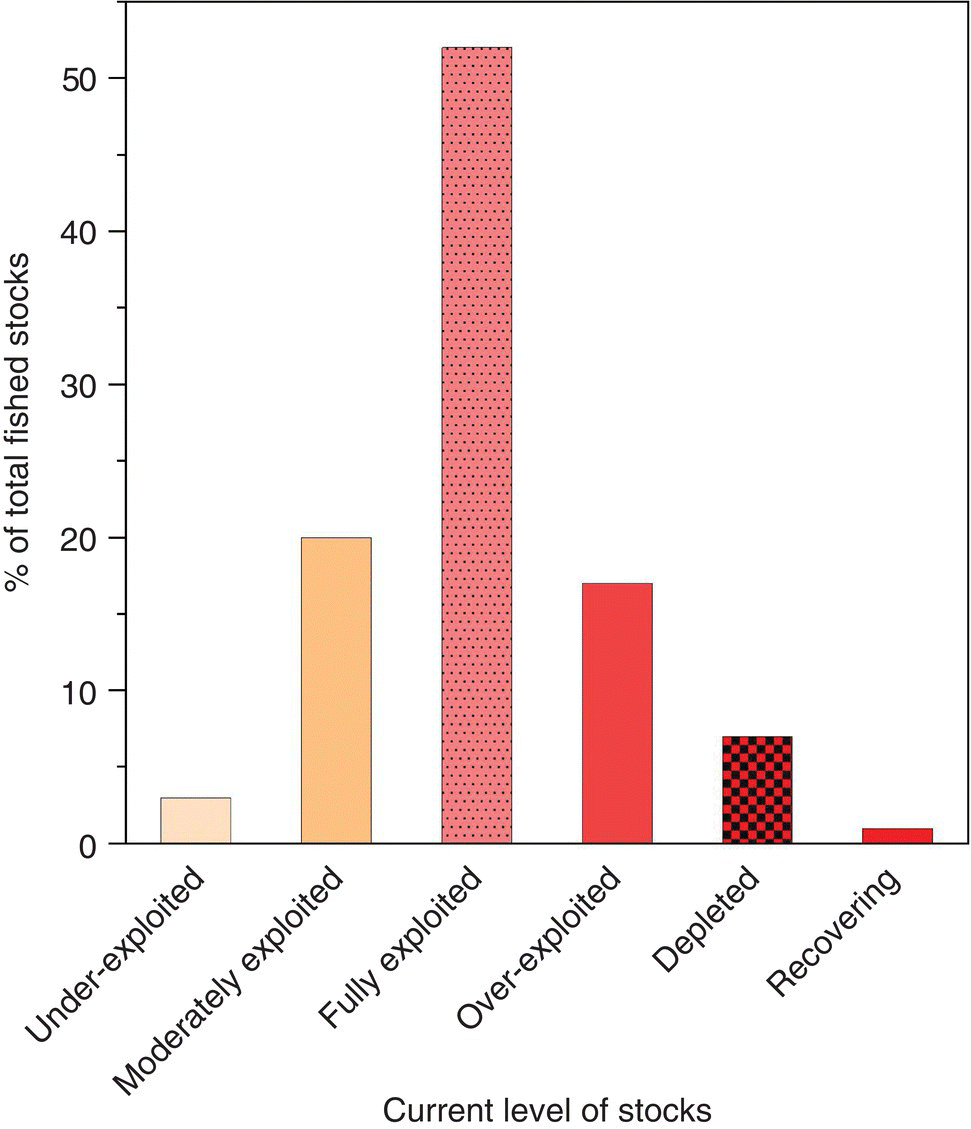
Figure 1.4 Percent status levels of 600 marine fisheries stocks based on the assessment of FAO. Data from http://www.fao.org/newsroom/common/ecg/1000505/en/stocks.pdf.
Source: Reproduced with permission from John Lucas.
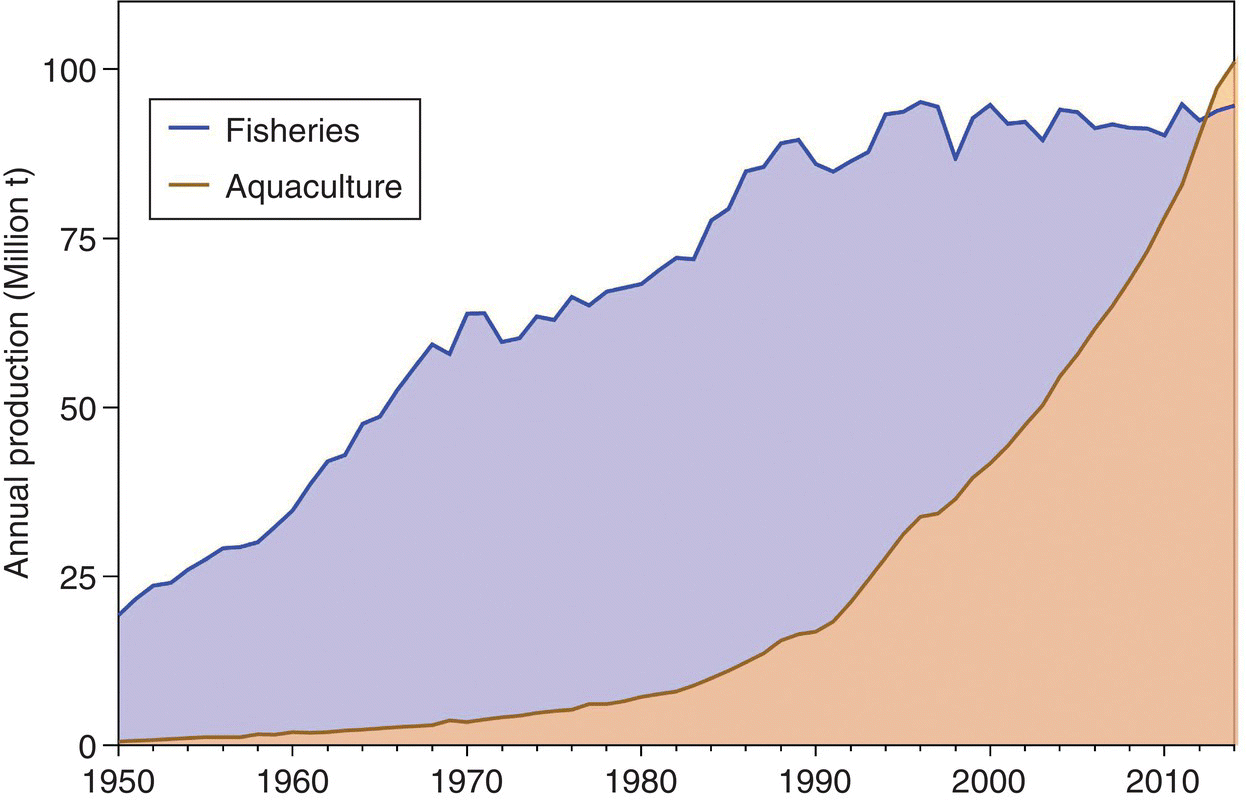
Figure 1.5 Global production of aquaculture and capture fisheries per year from 1995 to 2015. Data from http://www.fao.org/fishery/statistics/global‐aquaculture‐production/query/en.
Source: Reproduced with permission from John Lucas.
There are two further factors in capture fisheries production. About one‐quarter of capture fisheries production is used to make fishmeal, i.e., dried fish products, based on sardines and anchovies (Figures 5.10, 27.4), and fish wastes. Fishmeal is used as a source of n‐3 fatty acids in feeds for agricultural animal husbandry, e.g., pig feeds, but it is also extensively used in feeds for aquaculture. Thus, the effective annual production from global capture fisheries for direct human consumption is in the order of 70 million t/yr. The other 20–30 million t subsequently finds its way into the human diet by indirect processes involving substantial losses from the feed to incorporate it into a diet for human consumption (see later).
A further factor that doesn’t appear in FAO fisheries statistics is the substantial proportion of capture fisheries that consists of:
- bycatch = non‐target species; and
- discards = individuals of the target species that don’t fit the commercial criteria.
There are no precise data, but these amount to a substantial component of total fisheries’ catch and high proportions die through the handling. Bycatch and discards vary according to the nature of fishery and some trawl fisheries are among those with the highest levels of bycatch. Thus, it is not unreasonable to assume that this wastage amounts to at least 10% on top of the 90 million t/yr global fishery: about 10 million t/yr.
In contrast to capture fisheries, aquaculture production of animals and plants grew at a mean rate of 6.7% over the same period (Figure 1.5) (Table 1.1). All the increase in global seafood production in the past two decades has come from aquaculture.
Table 1.1 Global aquaculture production over 20 years from 1993 and 2013 by developing and developed countries. Data for China are also shown separately.*
Data from mailto:mailto:jorgen.bjorkli@balsfjord.kommune.no]
| Aquaculture production | |||
| 1994 | 2014 | Mean | |
| (106 t) | (106 t) | % increase/yr | |
| World | 27.8 | 101.1 | 6.7 |
| Least developed countries | 0.8 | 4.0 | 8.4 |
| % of world total | 2.9 | 4.0 | |
| Developing countries | 24.3 | 93.3 | 7.0 |
| % of world total | 87.4 | 92.2 | |
| Developed countries | 3.5 | 4.8 | 1.6% |
| % of world total | 12.6 | 4.7 | |
| China | 17.5 | 59.2 | 6.3% |
| % of world total | 62.9 | 58.1 | |
* http://www.fao.org/fishery/statistics/global‐aquaculture‐production/query/en
In the second edition of this textbook it was predicted on page 5 that:
‘…aquaculture production … will inevitably overtake global capture fisheries production. In view of the fact that the percent contribution of aquaculture to global seafood supply seems to be increasing exponentially this may happen sooner than later.’
This prediction was fulfilled in a surprisingly brief time when aquaculture contributed >50% of global seafood production in 2015 (Figure 1.5). Furthermore, it will continue to increase in relative importance and be the source of increased food supply from aquatic environments. Unlike capture fisheries, aquaculture is not limited by the natural productivity of the world’s water masses.
Aquatic plants (very predominantly seaweeds) contribute substantially to aquaculture production. Aquatic plant productions from aquaculture and capture fisheries were ca. 29.3 million t and 1.09 million t wet weight, respectively, in 2015 (Figure 1.6) (FAO).7 It may seem from these production numbers that aquaculture is still considerably behind fisheries as a source of seafood for human consumption and aquaculture still needs to increase substantially before it reaches capture fisheries as the major source of high‐protein seafood for human consumption. In fact:
- A substantial proportion of the seaweed is consumed in various forms (not just sushi) (Table 15.2). Some is consumed directly in a number of countries, especially South Korea, China and Japan. Korean consumption is 15.6 kg wet wt/person/yr (GAIN, 2015). However, not all consumption is in Asian countries. Laverbread (bara lafwr or bara lawr in Welsh) is a traditional Welsh delicacy made from the seaweed. It may be eaten fried with bacon.
- Not all seaweeds have low food protein levels. Red algae (Rhodophyta) in particular have high protein levels: in the order of 33–47% of dry wt, depending on the species and season (Fleurence, 1999).
- But, as described, a substantial portion of the capture from fisheries is used for fishmeal production and not direct human consumption.8 About 21 million t of the fishery production of fish, shrimp and molluscs in 2016 went for fishmeal, reducing the total for human consumption to 72.5 million t. Aquaculture produced 73.8 million t of fish, shrimp and molluscs in 2016, almost all for human consumption.
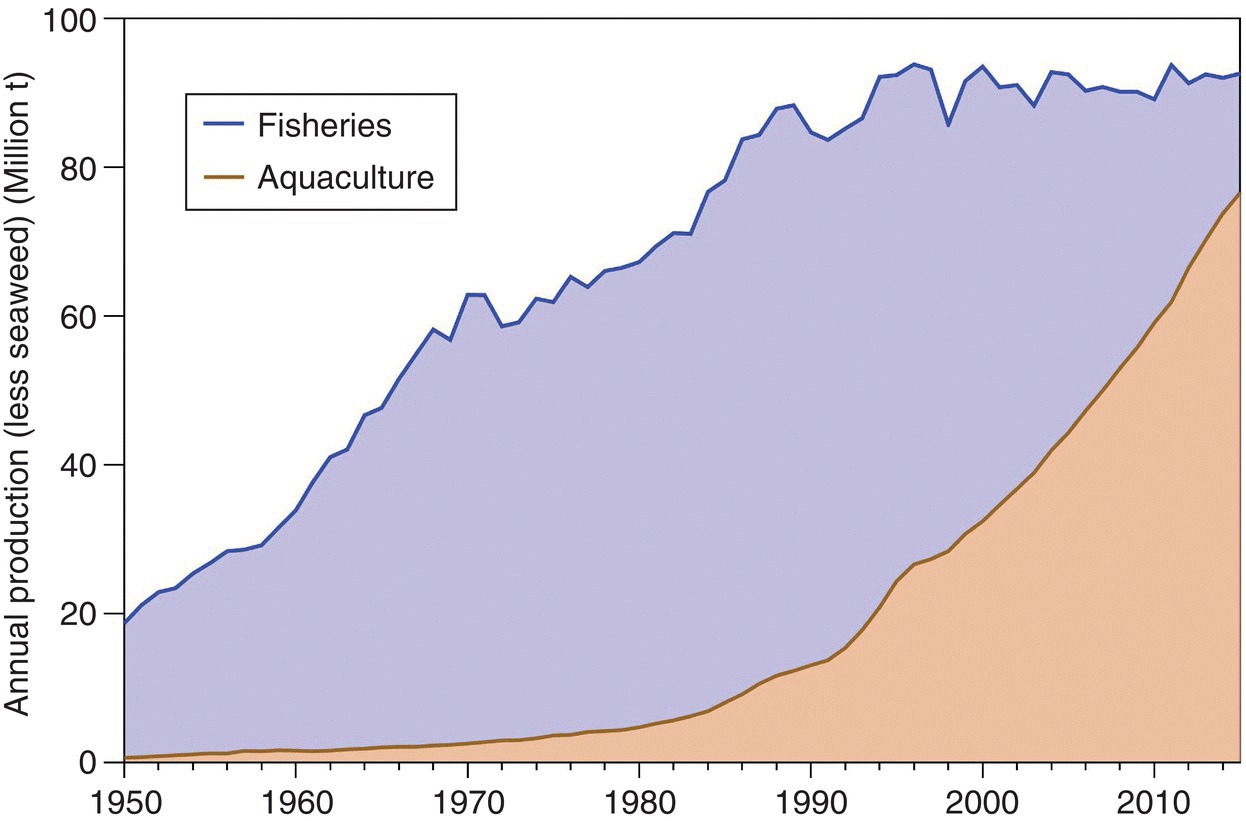
Figure 1.6 Global production of aquaculture and capture fisheries, excluding aquatic plants (mainly seaweeds) from 1995 to 2015. Data from http://www.fao.org/fishery/statistics/global‐aquaculture‐production/query/en Note that > 20 million t of capture fisheries products are used for fishmeal, etc., and not directly for human consumption.
Source: Reproduced with permission from John Lucas.
Aquaculture is now greater or at least equivalent to capture fisheries as the major source of animal seafood for direct human consumption.
The relative proportions by quantity and value of fish, molluscs, crustaceans and aquatic plants from aquaculture in 2014 are shown in Figure 1.7. Fish constitute about half the weight and value of aquaculture production. Plants and shellfish each constitute about a quarter of the quantity. There are, however, major changes between relative quantities and relative values. Fish increase to 60% of the value of aquaculture products. Crustaceans (mainly shrimp) show a very large increase to be valued at about a quarter of total production. Molluscs decline in relative value and plants decline even more.
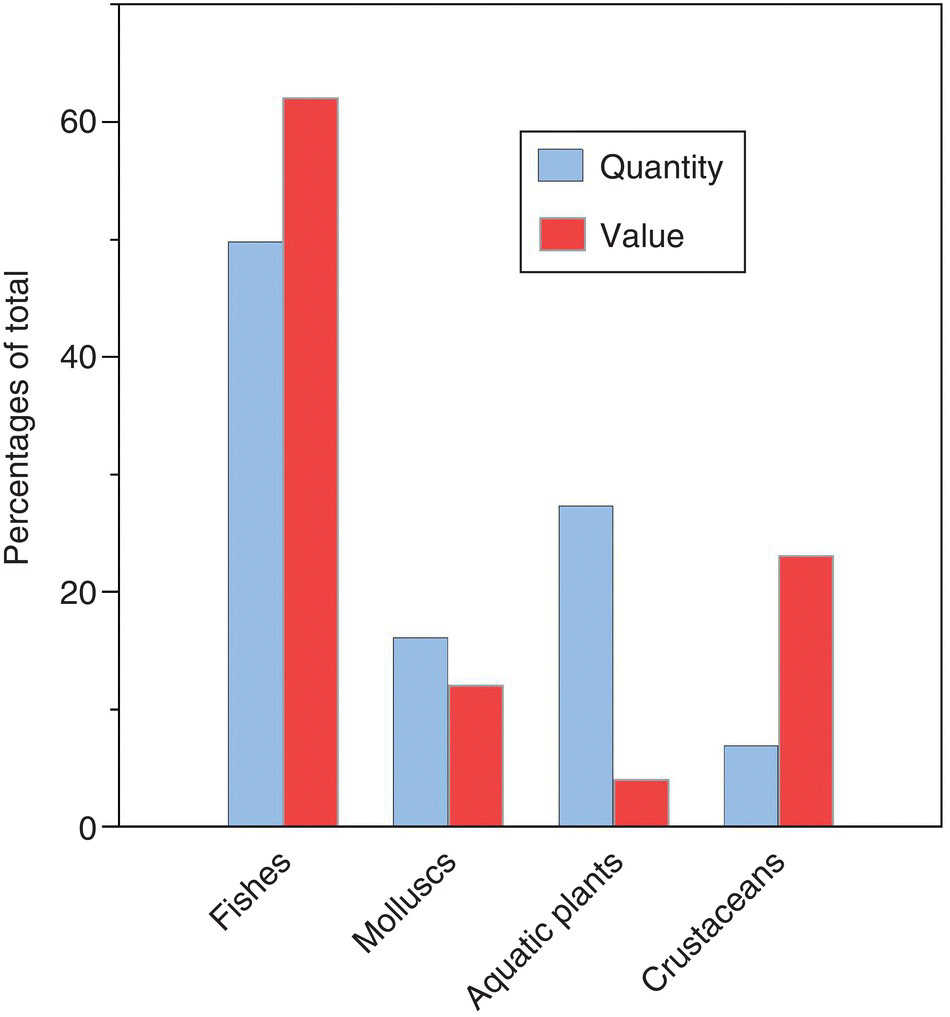
Figure 1.7 Relative proportions of fish, molluscs, crustaceans and aquatic plants in global aquaculture production in 2013. A. By quantity. B. By value. Data from http://www.fao.org/fishery/statistics/global‐aquaculture‐production/query/en.
Source: Reproduced with permission from John Lucas.
Food security for the world’s population, especially in poorer countries, is a major factor that pre‐occupies international organisations such as the FAO (Chapter 27). To put aquaculture and fisheries production in perspective of providing animal protein for the world’s current population: global production of slaughtered meat from livestock (pork, beef, chicken and lamb) is in the order of 300 million t/yr compared with about 70 million t/yr from capture fisheries (for direct human consumption) and 70 million t from aquaculture (excluding aquatic plants). This 140 million t from aquaculture and fisheries is pre‐slaughtered weight, and slaughtered weight (after removal of viscera, heads and shells) is probably around 50–60%. This value is not easy to estimate as it varies markedly with the kind of seafood and country of consumption. Consequently, seafood makes up 20–25% of all animal protein produced/yr for human consumption. Aquaculture is currently about 10% of all animal protein produced/yr based on these calculations. With fisheries production static and with livestock production increasing at about 1.5%/yr,9 aquaculture is increasing at 7%/yr. Thus, it seems inevitable that aquaculture will become increasingly important in terms of global food security.
1.4 The Efficiency of Aquaculture for Food Production
The value of aquaculture goes beyond replacing capture fisheries as the main source of seafood; it is one of the most efficient ways of producing protein for human consumption.
In bivalve culture, for instance, protein is generated without any feed input. There are many examples of high levels of production in the various forms of bivalve culture (Chapter 24). However, floating raft culture of the mussel, Mytilus galloprovincialis, in the Galician rias, is exceptional. Tradition raft culture in these rias, coastal inlets of NW Spain, has been practised for many decades and, although this production represents the pinnacle of this aquaculture technique, it none‐the‐less is indicative of what may be achieved. There are > 3,000 rafts each of ca. 500 m2 surface area in the region. They each have ca. 500 ropes hanging down from them with about 12 m available below the water surface for mussel attachment (see Figure 24.15). The mussels attach to the ropes by their byssal threads at densities of > 400/m. Factors such as whether the mussels are thinned out during early development affect the yield, but a typical yield for commercial‐sized mussels after a year of growth is ca. 200 kg/rope gross wt (Pérez‐Camacho et al., 2013). The yield is >60 t/raft/yr gross wt. Gross weight includes the shell and some mantle cavity water and the flesh wt is about 15% of the gross weight, ca 0.9 t/raft/yr. Edible protein content of the flesh is about 60 % of the wet flesh wt. This represents about 0.5 t/raft/yr of edible protein. There are multiple rafts in a hectare of ria ocean surface and dense rafts will produce 16 t /ha/yr or more of meat (Duarte et al., 2009). This meat yield is together with the usual healthy, nutritious substances of sea‐foods, including n‐3 fatty acids.
This is considerably more productive than from the equivalent husbandry of terrestrial livestock. The mussels suspended from the raft have no supplementary feeding, only the particulates that they filter from the water. The equivalent on land is free‐range husbandry on unimproved pastures where the free‐range livestock are stocked at low densities without fodder or grain to supplement the natural vegetation, e.g., free‐range cattle, sheep and goats. The stocking rates will vary markedly, depending on the quality and quantity of the natural vegetation. For beef cattle the stocking rate may be several animals/ha. Furthermore, there is dependence on weather conditions. During droughts the stocking density must be severely reduced, and stock may die from starvation or dehydration. In periods of flooding rain, the live‐stock may drown. Both situations occur regularly in regions of the world. Bivalve farming is essentially independent of weather conditions as long as the chosen culture site is protected from heavy waves and has water parameters that are comfortably within the animals’ tolerances.
Fish culture is also efficient in producing edible protein compared with other kinds of animal husbandry. In the data of Table 1.2 the carp are only exceeded in protein conversion and protein content in one box: the % protein content of edible weight. The outcome is that carp have the highest protein conversion efficiency, followed by chicken, pork and beef. This is a typical pattern of food conversion with beef cattle showing consistently poor values. Beef meat is highly regarded and expensive, but it wouldn’t be produced on the basis of return for input.
Table 1.2 Feed conversion rates and other parameters for carp compared with livestock.*
| Carp | Chicken | Pig | Beef | |
| FCR (kg feed/kg live wt) | 1.5 | 2.3 | 5.9 | 12.7 |
| Feed conversion (kg feed/kg of edible wt) | 2.3 | 4.2 | 10.7 | 31.7 |
| Protein content (% of edible wt) | 18 | 20 | 14 | 15 |
| % protein conversion efficiency | 30 | 26 | 13 | 5 |
*http://www.vaclavsmil.com/wp‐content/uploads/docs/smil‐article‐2002‐nitrogen‐and‐food‐production.pdf (June 2016)
Efficiency with fish aquaculture extends beyond carp to top predators such as Atlantic salmon. The data for Atlantic salmon, chickens and pigs are for animals in intensive culture on highly developed diets (Table 1.3). All have excellent food conversion ratios, but Atlantic salmon has the highest value. Its advantage is compounded by having the highest percent retention of energy and protein from the feed. On top of this the Atlantic salmon have the highest return of edible protein/body weight.
Table 1.3 Feed conversion rates and other parameters for Atlantic salmon compared with livestock.
From Bjørkli, J. 2002. Protein og energiregnskap hos laks, kylling, gris og lam [Protein and energy account in salmon, chicken, pig and lamb], Norway: Norwegian University of Life Sciences (UMB). M.Sc. Thesis. (Reproduced with permission of J. Bjørkli, 2006.)
| Atlantic salmon | Chicken | Pig | Lamb | |
| FCRa | 1.15 | 1.79 | 2.63 | 6.3 |
| % Harvest yieldb | 86.0 | 65.6 | 72.5 | 46.9 |
| % edible portionc | 68.3 | 46.1 | 52.1 | 38 |
| % energy retentiond | 23 | 10 | 14 | 5 |
| % protein retentione | 31 | 21 | 18 | 5 |
a FCR = (kg feed fed)/(kg live wt gain).
b Harvest yield is yield of gutted and bled animals.
c Edible portion is ratio of total body weight that is normally eaten, muscle, body adipose tissue (+ liver, lung, and heart for pig). Skin is excluded for all animals.
d Energy retention = (energy in edible parts)/(gross energy fed).
e Protein retention = (kg protein in edible parts)/(kg protein fed).
There are several factors in energy efficiency that relate to most fishes. Energy saving comes from:
- poikilothermy (being ‘cold‐blooded’) in particular, whereby they don’t spend energy maintaining body temperature;
- neutral buoyancy, whereby less energy is spent producing a skeleton and on posturing against gravity. The skeleton consists of relatively fine bones, which result in the high % of edible flesh yield (but fine bones may show up in fish fillets);
- excretion of some ammonium, which saves the cost of detoxifying N waste molecules to urea; and
- relatively less expenditure on reproduction compared with birds and mammals.
It is notable in Table 1.3 that chickens, which typically are most near fishes in terms of good values for these parameters, have poor percent energy retention: birds tend to have relatively high metabolic rates.
Not all marine fishes currently have FCR values comparable to Atlantic salmon for which a very efficient feed has been developed over a number of years of research. Bluefin tuna are a very valuable fish, especially for the Japanese fresh fish market to be used as sashimi and sushi. Development of the aquaculture of tuna species has been slow and much of the cultured fish production is from captured small fish that are reared to a larger and more valuable size for marketing. Tuna prey on small fishes, such as sardines, herring and mackerel, and on squid. Since it hasn’t proved possible yet to produce a satisfactory artificial diet that is acceptable to the tuna, frozen sardines and other small fishes are used as feed. There are very high FCRs for Pacific Bluefin tuna on these feeds: 18:1 with a sardines feed and 23:1 with a mixed feed of sardines + squid meat + gelatine‐vitamins (Estess et al., 2014).
1.5 Has There Been a ‘Blue’ Revolution?
The rapid increase in aquaculture production in the 1990s led to suggestions that aquaculture was undergoing a ‘Blue’ Revolution that would transform the productivity of marine and other aquatic environments with new technology (e.g., Figure 1.8). This envisaged a revolution in productivity similar to the ‘Green Revolution’ in agriculture during the decades following World War II. The Green Revolution occurred where focused research developed modern agricultural practices, e.g., mechanisation, heavy fertilisation, heavy pesticide use, irrigation, genetically improved stocks and advanced feed formulations’. The Green Revolution, however, wasn’t an unmitigated success. There were adverse environmental effects which, Blue Revolution or not, aquaculture must avoid in its long‐term growth (Diana et al., 2013).

Figure 1.8 Sea Station 3000 was a fully submersible 3,000 m3 seacage (24 m diam x 16.5 m tall). In operation the seacage was submerged to below 10 m depth, ca. 800 m off the Kona Coast of the Big Island of Hawaii. It was stocked with up to 70 000 Seriola rivoliana (Kona Kampachi®). The Offshore Site Manager is standing on the spar of the semi‐submerged structure.
Source: Reproduced with permission from Kona Blue Water Farms, Inc., 2017.
The near exponential increase in quantity of animal and plant aquaculture production in the two decades from 1994 to 2014 came from increases in freshwater and marine aquaculture. There were similar increases in these two environments (Figure 1.9a and b). In terms of animal products production, however, and hence protein production directly for human consumption, the increase was substantially greater from freshwater aquaculture. Production of aquatic animals in freshwater environments increased by 34.4 million t/yr over these two decades compared with an increase of 13.7 million t/yr in the marine environment, in which much of the increased production was from seaweeds. This clearly establishes freshwater as the major environment of aquatic animal production (Figure 1.9). In view of the fact that the growth in aquaculture production of aquatic animals in particular occurred predominately in freshwater systems, which are often anything but blue, it might be appropriate to call the huge increase in aquaculture the ‘Brown’ Revolution.
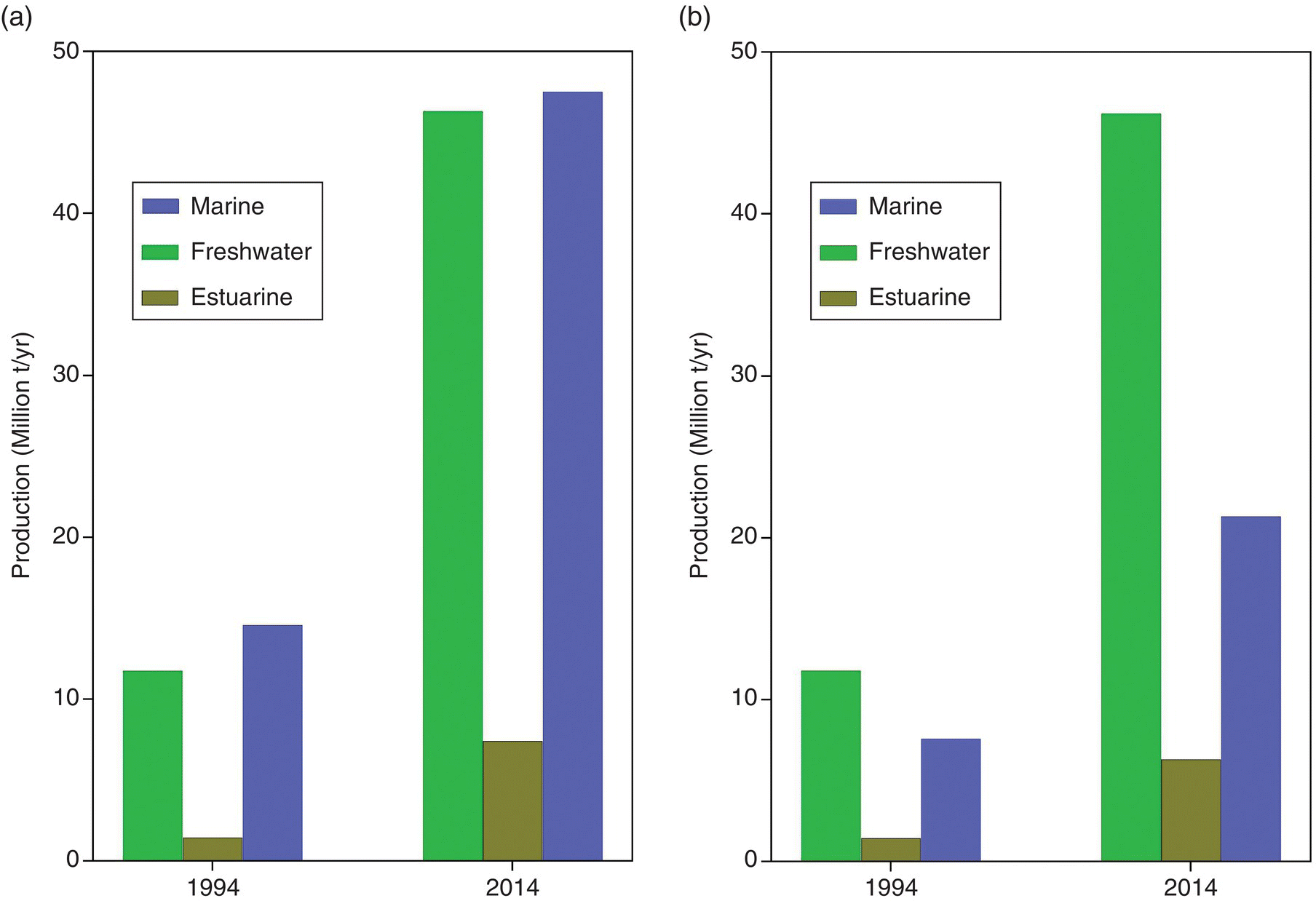
Figure 1.9 Increases in global aquaculture production of aquatic animals by environment between 1984 and 2014 by environment. (a) Date for all aquacultuare products. (b) Data only for animal products. Data from http://www.fao.org/fishery/statistics/global‐aquaculture‐production/query/en.
Source: Reproduced with permission from John Lucas.
It would be misleading, however, to see aquaculture developing purely as an expansion of pond culture. For example, there is a number of sophisticated developments in genetics, nutrition and disease control.
Furthermore, the open ocean is huge, but challenging, and it is possible that there will be a Blue Revolution, as technological developments enable increasing movement offshore into blue oceanic conditions (Simpson, 2011).
1.6 The Big Producers
There is a further very important statistic about the growth in aquaculture production: the marked division between least developed/developing countries and the developed countries (FAO classification10). Of the increase in global aquaculture production of 73.3 million t/yr over two decades, 1994 to 2014, 72.2 million t/yr came from the least developed/developing countries (Table 1.1). Aquaculture production in these countries increased at the remarkable rates of 8.4 and 7.0%/yr, respectively, over these two decades (Table 1.1). Compared with this, the 1.9%/yr growth of aquaculture production in developed countries was modest.11 The ‘Brown Revolution’ in aquaculture, like the ‘Green Revolution’ in agriculture, occurred primarily in the least developed and developing countries. In fact, there was an inverse relationship between the development category and aquaculture development rate (lowest = fastest, etc.).
The 10 major countries in terms of quantity of aquaculture production in 2014 are shown in Table 1.4. All except the lower‐ranked Norway and Egypt are Asian countries; and only two, Norway and Japan, are developed countries (FAO classification). The huge gap between China’s production and the next countries is notable. The increase in quantity of aquaculture by Indonesia in recent years to bring it to second in this list is notable. The increase is largely based on the expansion of seaweed production.
Table 1.4 The top 10 countries by percentage of aquaculture production in 2014.
| Order | Country | % World production |
| 1 | China | 58.1 |
| 2 | Indonesia | 14.2 |
| 3 | India | 4.8 |
| 4 | Vietnam | 3.4 |
| 5 | Philippines | 2.3 |
| 6 | Bangladesh | 1.9 |
| 7 | South Korea | 1.5 |
| 8 | Egypt | 1.4 |
| 9 | Norway | 1.3 |
| 10 | Japan | 1.0 |
Although the developing Asian countries have aquaculture industries of high‐value products, such as shrimp and scallops, for lucrative export markets, a high proportion of aquaculture in these countries continues to be from traditional pond culture of freshwater fish, especially carps and other cyprinids (Chapter 16). Figure 1.10 shows that carps together with other cyprinids are by far the most important fish by quantity in global production. They are almost a magnitude greater than the next three major groups of fish in aquaculture: catfishes, tilapia, and salmonids (salmon and trout). Carps are the most valuable fish in total global production, but they are substantially lower in value/unit weight than salmonids, which represent the high‐priced end of the market. There is ten times more culture production of carps than there is of salmonids, but their total value is not much more than two times the value of salmonids.
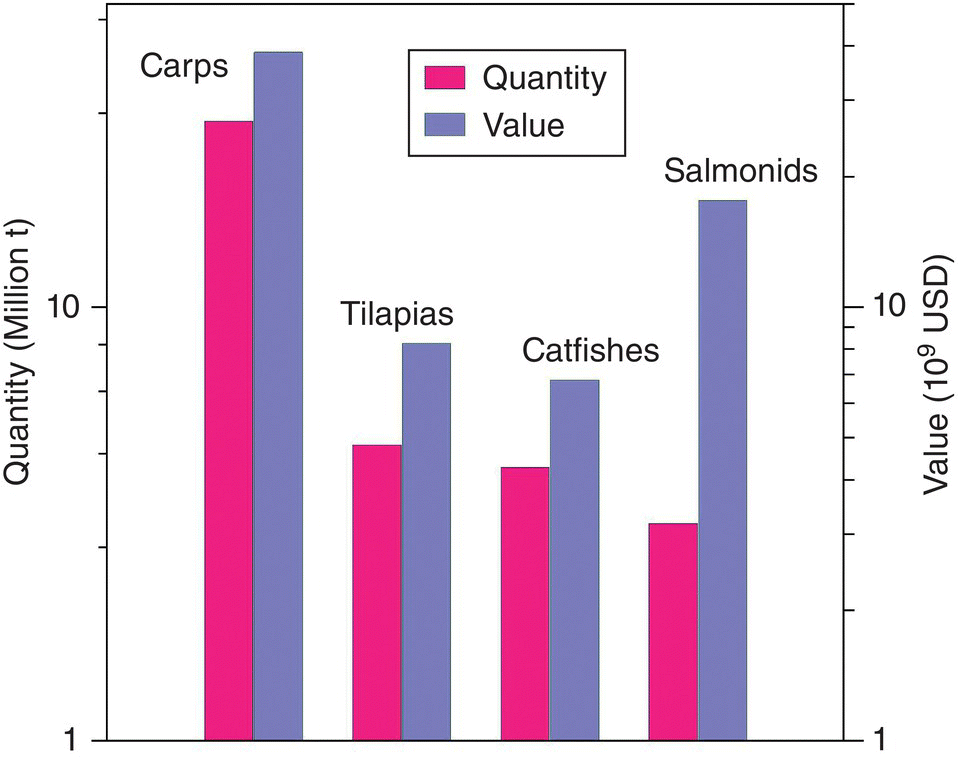
Figure 1.10 Annual production of major groups of fish in 2014. Note that both Y axes are logarithmic and there are very large differences. Data from http://www.fao.org/fishery/statistics/global‐aquaculture‐production/query/en.
Source: Reproduced with permission from John Lucas.
Freshwater fish are often cultured together as complementary species (polyculture; section 2.3). These fish species are herbivores, omnivores and detritivores, feeding low in the food chain and requiring little supplementary input of feeds. This aquaculture often involves simple ponds, basic technology and low stocking densities (Figure 2.4). The production is often enhanced by using inexpensive organic fertilisers, such as vegetable, farm animal and human wastes, to feed the cultured fish and to promote primary production in the ponds (integrated culture; section 2.4). In rural communities where animal protein is scarce and is prohibitively expensive from other sources, cultured fishes may form the major if not the exclusive source of animal protein.
In considering production versus consumption, there is a further complication when the data for total production of edible sea food (‘food fish’) from aquaculture together with capture fisheries are considered (Table 1.3). As the Asian region includes the major aquaculture producers, it has by far the greatest production, but the European and North American regions are relatively greater importers. Despite the very high production of seafood in Asia, when the total available supplies of edible seafood are related to population sizes, the consumption in kg/capita/yr is greatest in European and North America regions (Table 1.5). These affluent regions also consume the greatest amounts of animal products from animal husbandry. Affluence supports high consumption of animal protein from both land and sea. The African countries, which on average have a low intake of animal products/capita/yr from animal husbandry, also have a low consumption of seafood.
Table 1.5 Annual production of food fish* (million t) from aquaculture and capture fisheries in the major regions of the world. Food fish consumption/capita/yr is also included. The value of Total food supply is the summation of the positive (production and imports) and negative (non‐food use and exports) values.
Data from FAO 2007.
| Africa | North America | Central America | Asia | Europe | Oceania | World | |
| Total food fish production | 8.0 | 6.8 | 10.9 | 82.3 | 15.9 | 1.6 | 134.6 |
| Non‐food use | –1.2 | –0.9 | –12.6 | –10.1 | –3.5 | –0.4 | –28.8 |
| Imports | 2.5 | 5.0 | 1.3 | 12.5 | 15.4 | 0.5 | 37.3 |
| Exports | –1.8 | –3.1 | –3.8 | –14.6 | –12.8 | –0.9 | –38.8 |
| Total food supply | 7.6 | 8.0 | 4.0 | 70.2 | 15.2 | 0.8 | 106.7 |
| Consumption kg/capita/yr | 8.3 | 24.1 | 8.7 | 17.8 | 20.7 | 24.6 | 16.4 |
* Food fish in this Table refers to fish, crustaceans and molluscs destined for human consumption (cf. Chapter 26).
The relationship between animal protein consumption per capita not only applies to countries, but also to people within countries. As middle classes increase in developing countries the mean level of seafood consumption per capita will increase. Extrapolations from the current level of global aquaculture production to the future global populations levels need to take account of not only population growth, but also burgeoning middle classes in some countries.
There is another contrast between aquaculture in the developing Asian countries and in developed countries. Over 60% of fish production in developed countries is based on high market value species of carnivorous fish (Figure 2.7). These fish are reared in monoculture at high stocking densities and need inputs of high‐protein feeds. High‐protein feeds are expensive and usually require fishmeal as sources of animal protein and lipids for the carnivorous diet. Thus, this form of aquaculture uses low market value products from fisheries as feed to increase their value in the final products of culture. This is inefficient.
1.7 China
China had a flying start, as it were, in developing modern aquaculture. There is a long history of aquaculture in China.12 Some records suggest that common carp (Cyprinus carpio) were being farmed in earthen ponds as early as 1100 BC. Later, during the Tang dynasty (618–917 AD), culture was extended to five further carp species: silver carp (Hypophthalmichthys molitrix), big‐head carp (Aristichthys nobilis), grass carp (Ctenopharyngodon idellus), black carp (Mylopharyngodon piceus) and mud carp (Cirrhina molitorella). Fingerlings for all these species were collected from the wild in the Yangtze and Pearl Rivers.
Oyster‐farming was recorded as early as the Han dynasty (206 BC to 220 AD), while culture of marine finfish and shellfish developed during the Ming dynasty. Marine aquaculture and milkfish culture became popular aquaculture activities during the Ming dynasty (1368–1644 AD). It reached a level such that Xu Guangqi (1639 AD) recorded the methods for freshwater fish farming and prevention of fish diseases, and a treatise on mullet farming was produced.
Today, even if most countries were like developed countries and had modest levels of aquaculture growth over the past two decades, aquaculture would still have flourished on a global scale. China alone increased aquaculture production by 40 million t/yr over the period 1994 to 2014 (Table 1.1). It was a driving force of aquaculture expansion in the world. China accounts for about 60% of global aquaculture production (Tables 1.1 and 1.4).
A number of factors are responsible for the outstanding increase in aquaculture production in China. The major factor has been a deliberate policy of promoting aquaculture since 1978.13 There have been a series of five‐year plans promoting new developments and objective. These have included aquaculture which is treated as an important component of China’s food and commodity production. There is an integrated, if complex, system of components at three levels:
- National and local government bodies together with the Ministry of Science and Technology set out objectives for the five‐year plans.
- There are the research and development, and tertiary training institutes. They are represented by the Chinese Academy of Fisheries Sciences and three Institutions.
- The National and Provincial Technology and Extension Centres assist in implementing the new technologies that are being developed by the R&D organisations into local farms and companies.
Aquaculture would grow much more rapidly in many countries with this level of government commitment.
Listed below is a series of important factors in China’s aquaculture growth. Some developments may not be considered appropriate in some countries, but they have been:
- identifying huge areas of potential sites for aquaculture, i.e., 2.6 million ha of suitable coastal sites and 17.5 million ha of inland freshwater sites;
- extending offshore areas used for mariculture from 10 m to 50 m depth;
- increasing utilisation of inland waters for aquaculture, e.g., it was 25.5% in 1995 compared with 4.3% in 1978 (this 4.3% already represented 100,500 ha);
- increasing productivity/unit area, through research, extension and better technology;
- increasing the number of species in culture including high‐value species such as shrimp, scallops and abalone for international markets;
- introducing about 60 species from abroad;
- domesticating about 20 new species from wild populations and improving other species through hybridisation and stock selection programmes; and
- producing huge numbers of fingerlings and juveniles from many large dedicated hatcheries.
Unlike the Green Revolution, where technological developments played a major role in increased productivity, the outstanding growth of aquaculture in China has not depended on major technological developments. While R&D and extension programs are now having a significant impact, earlier growth came from upscalation.
The result of this deliberate programme to promote aquaculture is that China leads the world in being one of the few, if only major country, in which aquaculture production substantially exceeds fisheries production. In 2014, the quantity of aquaculture production was three‐fold fisheries production.
It may not seem surprising that China, as the country with the largest population, is the greatest aquaculture producer. Some production versus population size data are presented in Table 1.1. China’s aquaculture production is ca. 36 kg/capita/yr. Mean production for all developing countries is ca. 17 kg/capita/yr and for developed countries it is <2 kg/capita/yr. Taken at face value these figures are misleading. For instance, China produces a lot of seaweed (see above), which is less used for human consumption, and, like other Asian countries, it exports high‐value aquaculture products to developed countries.
1.8 Issues for Developed Countries
‘No land‐based food production industries have undergone such intense and focused scrutiny as has aquaculture.’
Jose R. Villalon14
Some aquaculture developments over past decades during the period of rapid growth were environmentally‐destructive and not sustainable. These were especially in south east Asia and they were ‘get rich quick’ enterprises without any consideration of long‐term environmental impacts. This has led to a level of poor image and suspicion of aquaculture developments and products in some developed countries.
It is intrinsic to farming that it involves environmental modifications to various degrees to enhance production. For instance, grain farming such as wheat farming has irreversibly destroyed the environment in countless millions of hectares around the globe, with:
- native vegetation clear‐felled;
- soil structure destroyed;
- soil eroded;
- extinctions or near extinctions of native species dependent on the original environment; and
- introductions of exotic and pest species.
On the basis of contemporary environmental awareness it is very unlikely that grain farming would be permitted or even considered on this scale. However, the environmental damage has occurred over hundreds to thousands of years, is irreversible, and the alternative to grain farming is that the world population starves.
There have been capture fisheries for tens of thousands of years. However, since global fisheries have reached the point that it is no longer possible to extract more products (Figure 1.3), it is inconceivable that capture fisheries are not having widespread impacts not just on fished populations but on the adjacent aquatic ecosystems. The recent onset of ‘industrialised fishing’ has been a particular factor in reducing predatory fish populations in coastal and some oceanic regions to 80% or less of their previous levels (Meyers and Worm, 2013) These profound impacts are difficult to determine, but the Sea Around Us project is attempting this. “Broadly, the work of the project is aimed at a reappraisal of fisheries, from the benign activity that many interested people still perceive them to be, to a realization that they have become the driver for massive loss of biodiversity in the ocean” (my italics) (Pauly, 2007). There have been studies showing the flow‐down effects to lower trophic levels of over‐fishing predatory fish, so that the community structure is changed. An example is the effect on the ecosystem following the collapse from over‐fishing of the Atlantic cod fishery on the eastern Scotian Shelf (Bundy, 2005). The system switched from one dominated by bottom and near‐bottom feeders to one dominated by pelagic feeders, presumably because of the new high abundance of pelagic fishes.
Environmentally‐sound and sustainable aquaculture is a better alternative to fisheries for more than just increased seafood production.
Agriculture and fisheries are part of our heritage, but aquaculture, especially in the developed countries, is a late‐comer and has to make its way in regulatory circumstances that are much more rigorous. Agriculture and capture fisheries are accepted despite their environmental damage because we depend on them as necessary sources of food.
Aquaculture in developed countries must develop within the constraints of comprehensive environmental regulations that are sometimes unreasonable, e.g., the hydrological parameters of discharge water from ponds must be of higher quality than those of the intake water. It also has to develop within the constraints of existing stakeholders who may lodge objections to the proposed aquaculture development, e.g., for interfering with recreational boating, for spoiling a scenic location. If the aquaculture farm is a long‐standing fixture before the marina is proposed or the coastal community begins to develop in the region, these objections are more difficult to sustain, but long‐standing aquaculture operations are often uncommon in developed countries. Many proposed aquaculture projects founder on the number of permits and approvals required, legal and administrative costs, and the duration of processes in obtaining the final approval to go ahead with the project.
Despite the heavy scrutiny there is wide‐spread recognition in developed countries that aquaculture is the future if we are to meet the growing demand for seafood. It seems, however, that the governments of some developed countries are prepared to import increasing amounts of cultured seafood rather than facilitate growth of industries within their countries. The rates of aquaculture growth in developed countries over the next decades will strongly depend on government policies:
- to what extent they are content to accept increasing seafood imports; and
- to what extent they deliberately plan to support within‐country production.
If it is the latter, they will need to do more to ‘take the brakes off’ and facilitate new aquaculture ventures. Major growth of aquaculture will not occur in the face of government indifference and over‐regulation. In developing countries, the aquaculture industry growth has involved governments making decisions to facilitate aquaculture in general and some industries in particular.
1.9 An Allegory
Short‐tail lums are moderate‐sized herbivores that weigh up to 50 kg. Some of their meat was sold on the local market, but most was snap‐frozen and exported to Asian markets where it was highly valued. Harvesting was based on shooting lums at night when they were active. The lums were fixed in a spotlight and shot by professional shooters riding on the backs of 4WD vehicles. However, as the populations were harvested at unsustainable levels it was then necessary to drive further and longer to find lums. Furthermore, the remaining short‐tail lums were vehicle‐shy and the vehicles had to drive faster and travel further into the bush, smashing down swathes of bushes and small trees. Some similar‐sized carnivorous mammals were also shot because of the limited time for careful identification. In fact, as the lums became increasingly rare, the strategy became to shoot first and check later when appropriate‐sized mammals were spot‐lighted. Increasing numbers of the non‐target mammals were shot in this way, but they were of little commercial value. The short‐tail lum industry became marginally profitable and numbers of shooters left for other employment.
Amateur lum shooters were affected by the diminishing populations and sent a delegation to raise the issue with the appropriate government politician. It was proposed that reserves for short‐tail lum protection be established. Some biologists, however, pointed out that the rates of recovery of short‐tail lum populations, and the other diminished mammal populations, would not be limited by their rates of reproduction. Population recoveries would be limited by the long period required for recovery of their environment. Furthermore, lums travelled in small herds which merged into large breeding herds during the spring season. Most lums were now living singly and it was likely that breeding behaviour involving large merged herds, would no longer occur.
Independently, a farm to raise short‐tail lums was proposed in the region. Careful planning was made for the lum farm to minimise environmental impacts and to meet all the requirements for the new agriculture venture. The lums would be farmed at relatively high density and fed a comprehensive diet of grain, pelleted feeds and chaff, instead of grazing over a large area. The economic viability of the proposal was demonstrated. The short‐tail lum breeding stock would be obtained from other farms with semi‐domesticated stock. The proposal went to the Department of Primary Industries and New Enterprise (DPINE) after two years of planning and discussion with local, state and national government bodies to meet the criteria for a new agriculture development. The Department published the proposal and invited submissions from the various stakeholders.
When asked, most people recognized that farming lums was the only solution to maintain or obtain more lum products. However, they did not want a nearby farm (NIMBY – Not In My Backyard). Protest meetings were organised, including the remaining professional shooters and an environmental activist. Deputations were made to the Department and local politicians.
The bases for oppositions were:
- short‐tail lums are noisy animals and active at night;
- the smell of short‐tail lum faeces would pervade the air;
- the local abattoir would be resurrected with the return of its unpleasant odour;
- hectares of pristine countryside would be destroyed if the mode of farming changed with a new permit;
- semi‐domesticated lums could get out through the fences and mate with the remaining wild lums, weakening the genetic pool of the latter; and
- the remaining professional shooters would suffer further financial hardship through being in competition with lum farmers.
The application was refused by the Department of Primary Industries and New Enterprise (DPINE) in the face of this vigorous opposition.
Meanwhile, two species of flies and three species of beetles, which lay their eggs specifically in short‐tail lum faeces or in the ground beneath them, were dying out in this region due to scarcity of lum faeces. Two bird species and a lizard that depend on these insects and their larvae for food faced extinction. Their declines to extinction in the region were passing unnoticed. What didn’t pass unnoticed resulted from the reduced numbers of one of the non‐target mammals. Substantial numbers of this mammal, which preys on rodents, were incidentally shot and consequently populations of rats began to expand rapidly due to reduced predator pressure. The rats’ food became scare in their normal environment and they began searching new environments. Adjacent residential areas were inexplicably invaded by plagues of rats.
Here ends the Allegory!
1.10 Diversity of Aquaculture
Freshwater cyprinid fish and seaweeds dominate world aquaculture production. However, far beyond these two large groups and the modest number of aquaculture species treated in this textbook, there is a huge diversity of species that are cultured. FAO provides quantity and commercial value data on aquaculture production of some hundreds of species of fish, shellfish and algae that are cultured for human consumption.
Even these three very broad categories of organisms do not encompass the whole range of aquacultured species. In addition to aquaculture for human consumption, there are:
- international and local industries producing live feeds for hatcheries, e.g., dried brine shrimp cysts, live ‘blood worms’, micro‐algae (Chapter 9);
- a huge worldwide aquaculture industry for ornamental fish, especially tropical freshwater and marine fish (Chapter 26);
- aquarium‐related industries producing aquarium plants and freshwater and marine invertebrates, including coralline algae/coral‐encrusted substrates (‘living rock’) for tropical marine aquaria;
- pearls and sponges are cultured for their traditional uses;
- crocodiles are farmed to produce skins for use in expensive ladies’ handbags; and
- speciality products sucah as sea cucumbers, sea urchins, bullfrogs and soft‐shell turtles (Chapter 21) are highly prized in Asian cuisine.
In particular, the Asian countries with large and traditional aquaculture industries are involved in culturing a wide diversity of species. Cen and Zhang (1998) indicate that at least 110 species (including introduced species) are cultured in China. These include freshwater and marine fish, shrimps, prawns, crabs, bivalves, gastropods, a variety of seaweeds and speciality products (Chapter 20). Aquaculturists in Taiwan have developed techniques for larval culture of more than 90 species of freshwater and marine fish. In Japan in 1997, 284 hatcheries together produced fingerlings of 88 fish species (Fushimi, 2001). Fingerlings of 73 species, amounting to 168 million fingerlings, were released into the environment in stock enhancement programmes.
1.11 Fishery Stock Enhancement and Restoration
This raises another aspect of the diversity of aquaculture: links between aquaculture and fisheries.
There is a long history of stock enhancement of restricted freshwater environments for subsequent fishing (typically recreational). This is typically with fingerlings from dedicated hatcheries (Figure 1.11). The fingerlings are not necessarily native to the water mass in all cases and this is not stock enhancement, in fact, it has more in common with pond aquaculture.

Figure 1.11 Stocking fingerlings into the Colorado River, near Moabi Park, California, USA.
Source: Photograph by Stephen Friedt. Reproduced under the terms of the Creative Commons Attribution License, CC‐BY‐SA 4.0.
Fishery stock enhancement and restoration in marine environments is more complex, at the very least because the released animals are not confined.
The Science Consortium for Ocean Replenishment (SCORE)15 recognises three basic types of fisheries enhancement:
- Sea ranching is the release of cultured juveniles into unenclosed marine and estuarine environments, i.e., not sea‐cages, for harvest at a larger size. The animals are not released with the expectation that they will contribute to spawning biomass of the fishery stock, although it is inevitable that some won’t be captured and may reach reproductive maturity.
- Stock enhancement is the release of cultured juveniles into a fishery stock that is recruitment‐limited to augment the natural supply of juveniles and increase harvests (the fishery may be commercial or recreational).
- Restocking (or restoration) is the release of cultured juveniles into a fishery stock to restore severely depleted spawning biomass to a level where it can once again provide regular, substantial yields.
Countries that are involved in restocking and stock enhancement programs with various culture species include Australia, China, Denmark, France, Iceland, Iran, South Korea, Norway, Oceania islands, Spain, Thailand, UK and USA. The programs involve a wide range of species: predominantly fishes, but also shellfish such as abalone, scallops and marine lobsters. Japan alone restocks fisheries with about 80 species.
The results of stock‐enhancement programmes for heavily overfished abalone stocks in Japan illustrate the variable success of some restocking programmes (Hamasaki and Kitada, 2008). The recapture rates of released abalone ranged from 1.4 to 23.8% at 13 locations: the released abalone contributed 6.9 to 83.5% of total catches at these locations and the economic efficiency of the stock‐enhancement was estimated to range from 0.4 to 6.2 over the 13 locations.16 This variability in abalone restocking data was between locations in particular, but it also occurred between years of release. The abalone restocking programmes began in the late 1960s, but annual catches in Japan declined from ca 6500 t in 1970 to 2000 t in the mid‐90s (Hamasaki and Kitada, 2008).
Stock enhancement involves a more sophisticated approach than rearing large numbers of juveniles and releasing them into the vicinity of the fishery stock, other factors are very important in the success of the process. These factors include genetic diversity of broodstock, conditioning and selection during culture in the hatchery to maximise the physiological and behavioural fitness of juveniles after release.
Factors that are adversely affecting the wild stock may need to be rectified together with the restocking, e.g., habitat restoration, predator control measures and fishing limits. Furthermore, there must be sufficient habitat available for the new releases, and there is no point in stocking a fishery where there are inadequate habitats for new releases or where the fished stock is not recruitment‐limited.
There is a developing research field for discerning optimum conditions for release of the aquaculture‐based animals into the field (Lorenzen et al., 2013).
1.12 Summary
- Aquaculture developed thousands of years later than terrestrial farming due, among other reasons, to our difficulty in appreciating the crucial parameters of aquatic environments.
- Aquaculture continues to grow rapidly in importance for seafood production while fisheries production has plateaued with many fisheries in a poor state.
- Aquaculture’s increase as a percentage of total seafood production is currently exponential and it passed capture fisheries as the main source of seafood in 2014.
- There is, however, a substantially greater proportion of aquatic plants in aquaculture production and, while significant amounts of seaweeds are consumed in various forms, the greatest need in the world is for animal protein. At its current rate of exponential growth in animal production, aquaculture should surpass fisheries as the main source of animal seafood before 2020.
- The greatest increases in animal seafood production, have been in low‐value freshwater fishes and within developing countries. Aquaculture growth per year was an amazing 11.9% in least developed countries and 7.4% in developing countries over the period from1994 to 2014. The values result from strong promotion of the industry in these countries.
- Aquaculture is hardly promoted in many developed countries; in fact it is often hampered by over‐regulation and public suspicion. There was some very environmentally‐destructive aquaculture in the 1980s and 90s and the industry needs to gain public confidence. Despite this, aquaculture is still growing in developed countries at an annual rate, 1.8%, slightly more than most livestock.
- Research has shown that production of some bivalves and fishes is highly efficient. Fishes are superior to major live‐stock species in critical parameters such as feed conversion rate, protein conversion rate and body protein content. Thus, beyond being a seafood alternative to capture fisheries, aquaculture has great potential for efficient food production
References
- Bundy, A. (2005). Structure and functioning of the eastern Scotian Shelf ecosystem before and after the collapse of groundfish stocks in the early 1990s. Canadian Journal of Fisheries and Aquatic Sciences, 62, 1453–1473.
- Diana, J.S., Egna, H.S., Chopin, T. et al. (2013). Responsible aquaculture in 2050: valuing local conditions and human innovations will be key to success. BioScience, 63 (4), 255–262. [doi: 10.1525/bio.2013.63.4.5]
- Duarte, C.M., Holmer, M., Olsen, Y. et al. (2009). Will the oceans help feed humanity? BioScience, 59 (11), 967–976.
- Estess, E.E., Coffey, D.M., Shimose, T. et al. (2014). Bioenergetics of captive Pacific bluefin tuna (Thunnus orientalis). Aquaculture, 434, 137–144.
- FAO. (2003). Aquaculture development in China: the role of public sector policies. FAO Fisheries and Aquaculture Department http://www.fao.org/docrep/006/y4762e/y4762e04.htm. [confirmed June 2016]
- Fleurence, J. (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends in Food Science & Technology, 10, 25–28.
- Fushimi, H. (2001). Production of juvenile marine finfish for stock enhancement in Japan. Aquaculture, 200, 33–53.
- GAIN Report. (2015). Global Agriculture Information Network. Report Number KS1529. 7/24/2015. USDA Foreign Agriculture Service. http://gain.fas.usda.gov/Recent%20GAIN%20Publications/2015%20Seafood%20Products%20Market%20Brief_Seoul%20ATO_Korea%20‐%20Republic%20of_7‐24‐2015.pdf (confirmed June 2016)
- Hamasaki, K., and Kitada, S. (2008). The enhancement of abalone stocks: lessons from Japanese case studies. Fish and Fisheries, 9 (3), 243–260.
- Lorenzen, K., Agnalt, A‐L., Blankenship, L. et al. (2013). Evolving context and maturing science: aquaculture‐based enhancement and restoration enter the marine fisheries management toolbox. Reviews in Fisheries Science, 21(3–4), 213–221.
- Meyers, R.A. and Worm, B. (2003). Rapid worldwide depletion of predatory fish communities. Nature, 423 (May 15), 260–263.
- Pauly, D. (2007). The Sea Around Us Project: documenting and communicating global fisheries impacts on marine ecosystems. Ambio, 36 (4), 290–295.
- Pérez‐Camacho, A., Labarta, U., Vinseiro, V. et al. (2013). Mussel production management: Raft culture without thinning‐out. Aquaculture, 406–407, 172–179.
- Simpson, S. (2011). The blue food revolution: making aquaculture a sustainable food source. Scientific American, 304, 54–61.