THE NEURONAL CELL
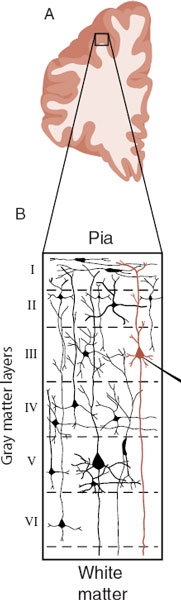
The human brain is the most complex organ known to exist in the universe. Its weight is just 3% of the body, but it consumes 17% of the body’s energy. The workhorse of the brain is the neuron. It is estimated that we have 100 billion neurons with 100 trillion connections. When we think of a neuron, we are typically thinking of a pyramidal neuron in the cerebral cortex. These neurons have a diamond-shaped cell body and usually reside in layers III or V of the gray matter (Figure 3.1).
Let us start with a brief review of cell biology. The cell body of the neuron is full of the usual assortment of organelles, although not in the same proportions as seen in non-neural cells. Structures such as the endoplasmic reticulum (ER) and mitochondria are found more frequently in neurons than in other brain cells, presumably because of the increased need for protein synthesis and energy production. The instructions for the functioning of the cell are contained in the DNA, which resides in the nucleus. The instructions are read when the DNA is transcribed into messenger ribonucleic acid (mRNA), which is translated into proteins in the cytoplasm (Figure 3.2). As mentioned in Chapter 1, this process is often called gene expression—two of our favorite words.
The ribosome is the organelle in which mRNA is translated into proteins (Figure 3.3). The ribosomes are usually attached to the rough ER, but can also be floating freely in the cytoplasm. The proteins, once they are refined, are used by the cell for structural (e.g., receptors), functional (e.g., enzymes), or communication (e.g., neuropeptides) purposes, to name a few.
The Golgi apparatus, which looks like ER without the ribosomes, is where much of the “posttranslation” refinement, sorting, and storage of proteins occurs. This structure enables proteins to be appropriately transported to distant sites within the cell.
The mitochondria are the remarkably abundant energy generators of the neuron. The brain requires considerable energy, even at rest, just to maintain an electrical gradient poised to respond at a moment’s notice. The mitochondria convert adenosine diphosphate into adenosine triphosphate (ATP) and it is ATP that the cell uses to perform its functions.
The dendrites are the part of the neuron that sprout off the cell body and look like tree branches. They are often called the ears of the neuron, because they receive input from other neurons and relay the signal to the cell body. Most dendrites have “little knobs” along their stalks that are called dendritic spines. Each spine is the postsynaptic receptor for an incoming signal from another neuron (Figure 3.4).
The morphology (structure and density) of the dendritic spines has been a source of considerable interest since Ramón y Cajal first identified them over 100 years ago. Spines can change in shape, volume, and number with remarkable speed and frequency. This plasticity is one of the great discoveries of modern neuroscience. We mention spines throughout this book as their morphology changes in a number of conditions, including substance abuse, mental retardation, schizophrenia, and learning (see Point of Interest).
The axon is perhaps the most unique structure of the neuron. Starting at the axon hillock and running anywhere from a few micrometers to the entire length of the spinal cord, the axon can transmit a signal quickly without degradation to other neurons or end organs. For this reason, the axon is often conceptualized as the telephone wire of the brain. Because the axon is devoid of ribosomes and incapable of protein synthesis, a process called axoplasmic transport enables the neuron to send material down the microtubules to the distal ends of the cell.


FIGURE 3.1  A. Cross section of the right prefrontal cortex (PFC). B. The six layers of neurons in the gray matter of the PFC. C. A stereotypical pyramidal neuron found in layer III of the cerebral cortex. ER, endoplasmic reticulum. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
A. Cross section of the right prefrontal cortex (PFC). B. The six layers of neurons in the gray matter of the PFC. C. A stereotypical pyramidal neuron found in layer III of the cerebral cortex. ER, endoplasmic reticulum. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
The terminal end of the axon forms the synapse (Figure 3.5). This is where one neuron talks to another—if the dendrites are the ears, the synapse is the mouth. Here the electrical signal streaming down the axon is converted into a chemical signal, so the impulse can pass from one cell to another. The neurotransmitters that form the basis of the chemical signal are stored in vesicles. When released, they diffuse across the synaptic cleft to receptors on the postsynaptic dendrite (more on this later in the chapter).
FIGURE 3.2  Messenger ribonucleic acid (mRNA) carries the genetic instructions from the nucleus to the cytoplasm where translation into proteins occurs. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Messenger ribonucleic acid (mRNA) carries the genetic instructions from the nucleus to the cytoplasm where translation into proteins occurs. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
ELECTRICAL SIGNALING
All living cells maintain a negative internal electronic charge relative to the fluid outside of the cell—roughly –60 mV in a neuron. Nerve cells use the depolarization (rapid change in the electrical charge) to communicate with other nerves or end organs. There are two basic steps in this process. The neuron first receives signals through the dendrites, which are called postsynaptic potentials. Second, the cell sums the incoming impulses and if they are high enough then it sends an impulse down the axon, which is called an action potential.
POINT OF INTEREST
Enhanced branching and spine formation have been found consistently in rats raised in enriched environments when compared with rats raised in standard wire cages. The neurons in the figure below are from rats raised in different environments. Note the increased branching (also called arborization) of the neuron from the rat raised in the enriched environment. Abundant dendritic branching with multiple connections seems to be a microscopic sign of a healthy active brain.
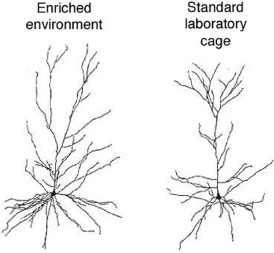
From Kolb B, Forgie M, Gibb R, et al. Age, experience and the changing brain. Neurosci Biobehav Rev. 1998;22(2):143-159.
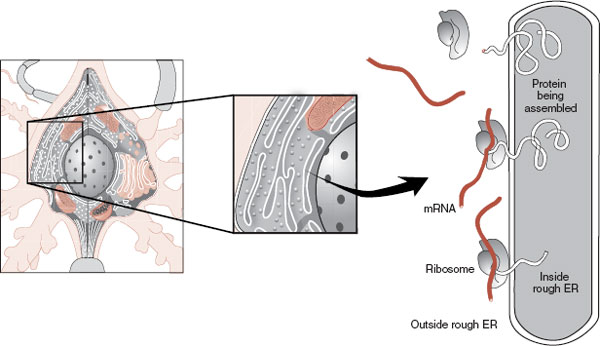
FIGURE 3.3  Messenger ribonucleic acid (mRNA) binds to a ribosome, initiating protein synthesis. Proteins synthesized on the rough endoplasmic reticulum (ER) as shown are eventually inserted into the membrane. Proteins synthesized on free ribosomes (not shown) are utilized in the cytosol. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Messenger ribonucleic acid (mRNA) binds to a ribosome, initiating protein synthesis. Proteins synthesized on the rough endoplasmic reticulum (ER) as shown are eventually inserted into the membrane. Proteins synthesized on free ribosomes (not shown) are utilized in the cytosol. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
FIGURE 3.4  Spines are the “little knobs” on the dendrites. They are on the receiving side of the electrochemical signal from other neurons—also called the postsynaptic membrane.
Spines are the “little knobs” on the dendrites. They are on the receiving side of the electrochemical signal from other neurons—also called the postsynaptic membrane.
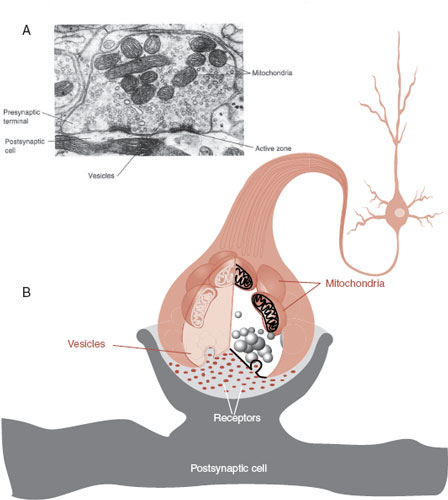
FIGURE 3.5  A synapse as seen with an electron microscope (A) and in a schematic drawing (B). Note the high concentration of vesicles filled with neurotransmitter and mitochondria to power the rapid processing. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
A synapse as seen with an electron microscope (A) and in a schematic drawing (B). Note the high concentration of vesicles filled with neurotransmitter and mitochondria to power the rapid processing. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
A single pyramidal cell will receive input from 1 to 100,000 neurons through the postsynaptic synapses (spines) on the dendrites and cell body. When the neurotransmitters bind with the receptor at the postsynaptic synapse, ions flow into the neuron and change the electrical potential making it more positive or more negative—or what is called depolarization and hyperpolarization.
This proceeds in two ways:
Depolarize (excitatory) with an influx of positive ions such as Na+.
Hyperpolarize (inhibitory) with an influx of negative ions such as Cl–.
These are appropriately called excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) (Figures 3.6 and 3.7). The EPSP and IPSP are, respectively, the accelerator and brake for the brain. An EPSP is more likely to generate an action potential; an IPSP inhibits the generation of an action potential. The goal for a healthy brain is to maintain the correct balance—if there is too much excitation, one can have a seizure; if there is too much inhibition, the brain is sluggish, even comatose. Actually, most of us want our brains to be activated during the day and inhibited at night—something our hunter–gatherer brains are not always wired to accommodate.
The Action Potential
The decision to fire an action potential is made at the axon hillock. Moment to moment, the sum of all the incoming EPSPs and IPSPs at the axon hillock determines whether the neuron sends an impulse down the axon. If the potential has depolarized to the threshold, then an action potential is generated. Figure 3.8 shows how the neuron requires enough depolarization (excitation) but not too much hyperpolarization (inhibition) to generate an action potential.
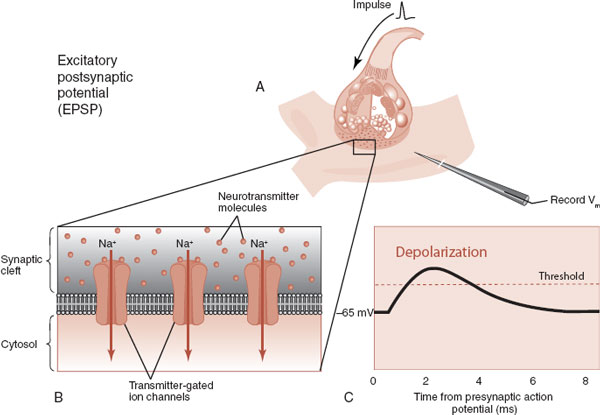
FIGURE 3.6  Neurotransmission from an excitatory neuron (A) promotes the entry of positively charged sodium ions into the dendrite (B). The resulting depolarization generates an EPSP (C). (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Neurotransmission from an excitatory neuron (A) promotes the entry of positively charged sodium ions into the dendrite (B). The resulting depolarization generates an EPSP (C). (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
FIGURE 3.7  Neurotransmission from an inhibitory neuron (A) promotes the entry of negatively charged chloride ions into the dendrite (B). The resulting hyperpolarization generates an IPSP (C). (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Neurotransmission from an inhibitory neuron (A) promotes the entry of negatively charged chloride ions into the dendrite (B). The resulting hyperpolarization generates an IPSP (C). (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Once the threshold (–40 mV) has been reached at the axon hillock, the neuron “pulls the trigger” and shoots an action potential down the axon. Because of the unique design of the voltage-gated sodium channels, the action potential maintains its integrity as it proceeds along the axon—there is no diminution in the signal. This occurs because the voltage-gated sodium channels facilitate a rapid influx of positive ions. Like other ion channels, the voltage-gated sodium channel is a protein embedded in the lipid member of the cell. The “gated” nature of the channel allows a large and rapid influx of the ions once the threshold has been crossed. The voltage-gated channel is like a trap waiting to be sprung. When the switch is tripped, the ions pour into the axon.
Electrochemical Signaling
As Otto Loewi demonstrated in 1921, the communication within the nervous system is both electrical and chemical. Figure 3.9 shows a representation of the arrival of the action potential at the synaptic terminal, the release of the neurotransmitters, and the generation of an excitatory or inhibitor postsynaptic potential in the dendrite of the neighboring neuron. This example is of a dopamine neuron that is an excitatory neuron, but the same principles apply to all the neurons and neurotransmitters.
TREATMENT
CALM DOWN
We often treat patients who have too much cerebral activity. Anxiety, attention-deficit/hyperactivity disorder (ADHD), insomnia, and mania are four conditions in which the brain is going too fast. Such patients have too much excitation and not enough inhibition. It is encouraging that more research is being directed toward treatments that increase inhibitory potentials (e.g., γ-aminobutyric acid). The challenge is to selectively inhibit the annoying trait without slowing down the whole brain: calmer but not stupid.
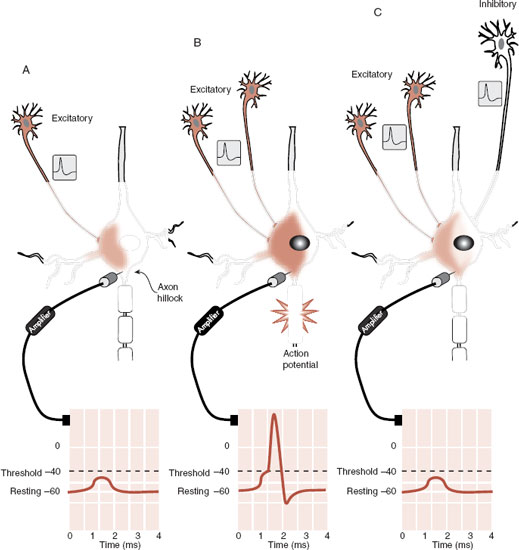
FIGURE 3.8  A signal from one excitatory neuron (A) increases the EPSP but is not sufficient to reach the threshold. Two excitatory impulses reaching the neuron at the same time (B) generate an EPSP that reaches the threshold at the axon hillock and an action potential is fired. However, with an inhibitory input from a third neuron (C), the membrane potential again fails to reach the threshold.
A signal from one excitatory neuron (A) increases the EPSP but is not sufficient to reach the threshold. Two excitatory impulses reaching the neuron at the same time (B) generate an EPSP that reaches the threshold at the axon hillock and an action potential is fired. However, with an inhibitory input from a third neuron (C), the membrane potential again fails to reach the threshold.
The arrival of the action potential at the terminal depolarizes the membrane, which opens the voltage-gated calcium channels. The voltage-gated calcium channels are similar to the voltage-gated sodium channels except that they are permeable to Ca2+. Consequently, there is a large and rapid influx of Ca2+, which is required for exocytosis and the release of the neurotransmitter.
NON-NEURONAL CELLS
The glial cells that make up the rest of the cells in the central nervous system (CNS) actually outnumber the neurons by 9:1. Traditionally seen as supportive cells with no roll in communication, recent research has shown that glial cells modulate the synaptic activity. There are three kinds of glial cells: astrocyte, oligodendrocyte, and microglia. The microglia are similar to macrophages found in the peripheral tissue. They respond to injury with a dramatic increase in their numbers and remove cellular debris from the damaged area. (For more information on microglia, refer Chapter 9.)

FIGURE 3.9  This is an example of the electrochemical signaling from a dopamine neuron. If this were an inhibitory neuron, γ-aminobutyric acid or glycine, for example, the postsynaptic potential would be an inhibitory postsynaptic potential (IPSP) and not an excitatory postsynaptic potential (EPSP).
This is an example of the electrochemical signaling from a dopamine neuron. If this were an inhibitory neuron, γ-aminobutyric acid or glycine, for example, the postsynaptic potential would be an inhibitory postsynaptic potential (IPSP) and not an excitatory postsynaptic potential (EPSP).
The oligodendrocyte is considered the CNS equivalent of the Schwann cell in the peripheral nervous system (Figure 3.10; see also Figure 20.12). Schwann cells are the cells that wrap myelin around the axons of the neurons and by acting as an electrical insulator they greatly increase the speed of the transmission of the action potential. This process of myelinization is not complete at birth and proceeds rapidly in the first years of life, which has a dramatic effect on behavior. In children, this process results in improved motor skills as they mature. Complete myelinization of the prefrontal cortex (PFC) is delayed until the second and even third decade of life. Hence why we worry about our teens—the bodies of adults without the brakes of matured frontal lobes.
Alternatively, demyelinating disorders such as multiple sclerosis and Guillain-Barré have devastating effects on patients. Clearly, a neuron without its myelin is not as effective. Regarding mental illness, recent research suggests that some failure in myelinization may play a role in schizophrenia (see Chapter 23).
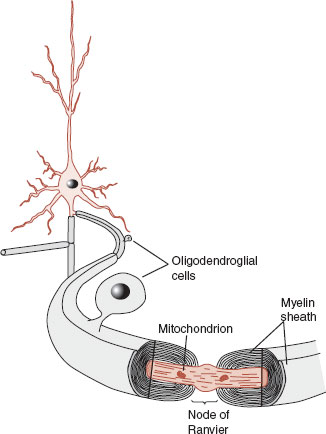
FIGURE 3.10  Oligodendroglial cells wrap a myelin sheath around the axon, providing electrical insulation. This can improve the speed of the transmission of an action potential up to 15 times. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Oligodendroglial cells wrap a myelin sheath around the axon, providing electrical insulation. This can improve the speed of the transmission of an action potential up to 15 times. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
The astrocyte is the star-shaped cell that fills the spaces between the neurons (Figure 3.11). We have already seen that the astrocyte plays a role in maintaining the blood–brain barrier, but other functions include regulating the chemistry of the extracellular fluid, providing structural support, and bringing nutrients to the neurons. Even more interesting is the role the astrocyte plays in modulating the electrical activity at the synapse. Research has shown that astrocytes encircle the synapse and have receptors that respond to the neurotransmitters released by the neuron. The astrocyte may in turn release its own neurotransmitter, which enhances the transmission of the signal. This may facilitate learning and memory. Additionally, there is evidence that the presence of astrocytes or their proteins increases the number of synapses a neuron will form. Clearly, the glial cells are more involved in the communication within the brain than previously thought.
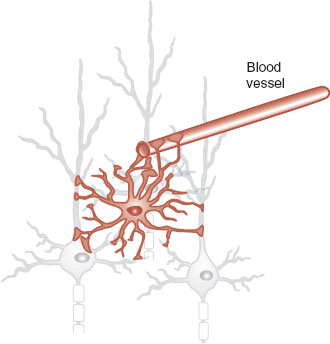
FIGURE 3.11  The astrocyte not only supports the neurons and blood vessels but also has some role in modulating the transmission of information.
The astrocyte not only supports the neurons and blood vessels but also has some role in modulating the transmission of information.
CIRCUITS
The capacity to communicate quickly over long distances (relative to the size of a cell) is the most unique feature of the nerve cell. Sending a signal, receiving feedback, and adjusting further responses is the essence of communication—cellular or social. The white matter tracts—bundles of axons just underneath the gray matter—connect the nerve cells with other regions of the cortex, subcortical nuclei, and end organs such as muscles and glands (Figure 3.12).
DISORDER
EPILEPSY
Increasing evidence is pointing to the astrocyte as playing an instigating role in epilepsy—a problem historically assigned to dysfunctional neurons. Analysis of specimens after surgical resection for epilepsy often shows prominent gliosis. Glutamate released from astrocytes can trigger experimental models of seizures. Finally, several effective antiepileptic drugs (valproate, gabapentin, and phenytoin) potently reduce astrocytic Ca2+ signaling—an event believed to precede seizure activity. If aberrant astrocytes are the nidus for seizures, then new treatments might be developed that can calm the astrocytes without dulling the neurons.
FIGURE 3.12  Beneath the gray matter are white matter tracts that allow communication between widely separated regions of the brain or between the brain and end organs. (Adapted from Rosenzweig MR, Breedlove SM, Watson NV. Biological Psychology. 4th ed. Sunderland, MA: Sinauer; 2005.)
Beneath the gray matter are white matter tracts that allow communication between widely separated regions of the brain or between the brain and end organs. (Adapted from Rosenzweig MR, Breedlove SM, Watson NV. Biological Psychology. 4th ed. Sunderland, MA: Sinauer; 2005.)
A new imaging technique, called diffusion tensor imaging (DTI), has been developed to assess the quality of the white matter tracks. Using a magnetic resonance imaging scanner, the technique involves following the movement of water (diffusion) in the brain. In most tissues, the water molecules move in every direction. In the white matter tracts, the water molecules tend to move along the length of the axons. Thus, with some Herculean number crunching on the computer, images showing remarkable detail of the white matter tracks can be produced (Figure 3.13). Although still a research tool, DTI is being used to identify white matter abnormalities in patients with psychiatric disorders (see Chapter 23).
The networks formed to coordinate a task are called circuits. We can imagine that catching a Frisbee requires such a circuit. The visual cortex registers the trajectory of the Frisbee, the frontal cortex acknowledges the emotional importance of making the catch, and the motor cortex signals the legs and arms to get in a position to grasp the spinning disc. The interplay between these and other regions of the brain enables the individual to move into the appropriate location and make the grab. We can imagine that the efficiency and accuracy of such a circuit for different people falls along a spectrum from poor to superior—whether due to genes or practice. It is the coordination of the circuit—not one particular region—that masters that catch.
Increasing evidence suggests that understanding circuits may help us understand the variants in behavior. The elusive nature of the biological causes of mental disorders has been the bane of psychiatry. While Broca could pinpoint the damaged region to explain his aphasic patients, conspicuous lesions corresponding to psychiatric conditions have not been easy to locate. Perhaps this is because it is not one specific region that is at fault, but rather the dysfunction of the circuit.

FIGURE 3.13  Visualization of white matter tracks in the brain using diffusion tensor imaging (DTI) technology. (Adapted from Brun A, Park HJ, Knutsson H, et al. Coloring of DT-MRI Fiber Traces Using Laplacian Eigenmaps. Available at http://lmi.bwh.harvard.edu/papers/papers/brunEUROCAST03.html. 2003.)
Visualization of white matter tracks in the brain using diffusion tensor imaging (DTI) technology. (Adapted from Brun A, Park HJ, Knutsson H, et al. Coloring of DT-MRI Fiber Traces Using Laplacian Eigenmaps. Available at http://lmi.bwh.harvard.edu/papers/papers/brunEUROCAST03.html. 2003.)
Figure 3.14 shows a schematic representation of a hypothetical circuit between the PFC, temporal cortex, and subcortical regions. Imaging studies suggest that dysfunction of emotional circuits may explain some psychiatric disorders—particularly depression, anxiety, and substance abuse. One common example involves the PFC. Insufficient activity from the PFC allows the expression of impulses from lower regions of the brain—a problem we will address in regard to anger, ADHD, and anxiety.
FIGURE 3.14  Dysfunctional circuits may explain the behavioral and cognitive symptoms in psychiatric disorders.
Dysfunctional circuits may explain the behavioral and cognitive symptoms in psychiatric disorders.
QUESTIONS
1. The pyramidal cells in the gray matter reside predominantly in which layers?
a. I and III.
b. II and IV.
c. III and V.
d. IV and VI.
2. Protein synthesis requires all of the following except
a. Rough ER.
b. Gene expression.
c. Transcription and translation.
d. mRNA.
3. Enhanced arborization of the dendrites is found with
a. Mental retardation.
b. Stimulating environments.
c. Usual laboratory environments.
d. Schizophrenia.
4. An IPSP
a. Results from the influx of sodium ions.
b. Depolarizes the cell.
c. Can induce seizures.
d. Hyperpolarizes the cell.
5. The neuron generates an action potential based on the postsynaptic potential at the
a. Axon hillock.
b. Synapse.
c. Nucleus.
d. Node of Ranvier.
6. Exocytosis of the neurotransmitters at the synapse requires opening of the
a. Voltage-gated sodium channels.
b. Voltage-gated calcium channels.
c. Excitatory postsynaptic channels.
d. Inhibitory postsynaptic channels.
7. The cell responsible for myelin in the CNS is
a. Astrocyte.
b. Microglia.
c. Schwann cell.
d. Oligodendrocyte.
8. Modulates electrochemical activity at the synapse:
a. Astrocyte.
b. Microglia.
c. Schwann cell.
d. Oligodendrocyte.
See Answers section at the end of the book.