HISTORIC PERSPECTIVE
Over 100 years ago, Emil Kraepelin, a German psychiatrist, described the syndrome now called schizophrenia. Bleuler actually coined the term schizophrenia. Kraepelin called it dementia praecox.
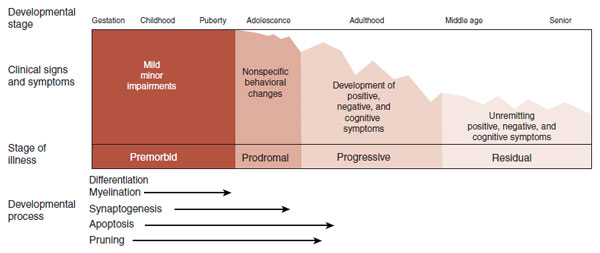
Kraepelin’s major contribution to psychiatry was recognizing that schizophrenia and manic depression are different disorders. The patient with schizophrenia has a persistent deteriorating course in mental functioning, whereas the patient with manic depression will experience periods of remission. Figure 23.1 shows a modern interpretation of the clinical course of schizophrenia.
Kraepelin was convinced that schizophrenia was an organic disease of the brain and spent considerable time and energy conducting postmortem studies on the brains of patients with schizophrenia. They had a good track record of identifying pathology, as one of his colleagues was the neuropathologist Alois Alzheimer. Unfortunately, Kraepelin was never able to discover a specific abnormality in the brains of schizophrenic patients. This pattern was to continue for a long time.
Numerous postmortem studies were conducted over the next 70 years comparing the brains of schizophrenic patients with healthy controls. Still no distinguishing pathology was isolated. The absence of gliosis in the tissue was of particular interest. Gliosis, sometimes called the glial scar, is considered the hallmark of neurodegenerative disorders and is found with such conditions as Huntington’s or Alzheimer’s diseases as well as with trauma and ischemia.
The absence of any significant neuropathology along with the burgeoning interest in psychoanalytic theory led to psychosocial explanations for schizophrenia. Terms such as the refrigerator mother and the double-bind were developed to explain the psychological turmoil that caused schizophrenia. Some clinicians even speculated that patients voluntarily chose to be psychotic to avoid conflict in their lives.

FIGURE 23.1  The typical clinical course of schizophrenia includes a relatively normal childhood interrupted in late adolescence or early adulthood by a dramatic deterioration from which few remit. (Adapted from Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28(2):325-334.)
The typical clinical course of schizophrenia includes a relatively normal childhood interrupted in late adolescence or early adulthood by a dramatic deterioration from which few remit. (Adapted from Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28(2):325-334.)
Although the development of chlorpromazine (Thorazine) in the early 1950s dramatically changed the treatment of schizophrenia, the identification of biologic abnormalities remained elusive. In 1972, Plum summarized the frustration when he called schizophrenia the graveyard of neuropathologists. Schizophrenia was actually dropped from the preeminent neuropathology textbook (Greenfield’s Neuropathology) for the next two editions and only added back in 1997. It was the emergence of significant findings on brain imaging studies that finally ended the debate about whether there are quantifiable (measurable) changes in the brain (more on this in the next section).
Although we follow convention and use the term “schizophrenia,” many researchers are struck by the variations between patients. For example, some patients have no hallucinations and mostly struggle with negative symptoms, while other patients have chronic hallucinations and few negative symptoms. To remind readers of this heterogeneity, some use the term “the schizophrenias.” In this book we will use the standard term schizophrenia.
MODERN EPIDEMIC?
E. Fuller Torrey calls schizophrenia an invisible plague. He believes that schizophrenia is a modern illness that has increased so gradually that the change is not perceptible during any single person’s lifetime. Additionally, the changes in diagnostic criteria that occur over the decades make comparisons between generations difficult. In spite of these difficulties, there is evidence to support Dr. Torrey’s belief.
The ancient Greeks and Romans were astute observers of human behavior. Reviews of the writings from ancient times provide the following descriptions of conditions that we easily recognize:
1. Epilepsy
2. Migraine headache
3. Melancholia
4. Anxiety
5. Chronic alcoholism
6. Delirium
The ancient writers did describe psychotic symptoms including hallucinations and delusions. However, in almost every case the psychosis cleared. There are no reports that describe an initial psychotic break in late adolescence or early adulthood with a chronic unremitting course. The absence of a condition that looks like schizophrenia stands in contrast to the good clinical descriptions of other neuropsychiatric syndromes. This softly suggests that the illness was not present in ancient Greece or Rome.
The 19th and 20th centuries saw an explosion in the institutionalization of patients with chronic mental illness. Many of these patients had schizophrenia. Figure 23.2 shows the growth in institutionalized patients as a percentage of the total population in four countries. There are many reasons for confining the seriously mentally ill: industrial revolution, changes in social norms, lack of effective treatments, and so on. An additional explanation is the emergence of a psychiatric epidemic.
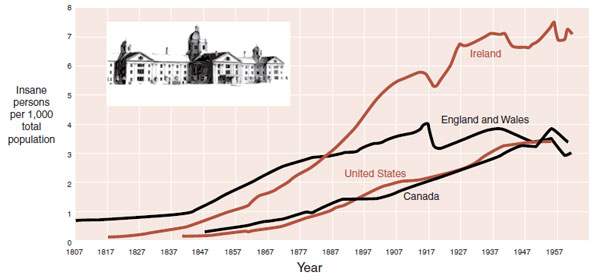
FIGURE 23.2  The epidemic of mental illness in the last two centuries. (Adapted from Liberman RP, Musgrave JG, Langlois J. Taunton State Hospital, Massachusetts. Am J Psychiatry. 2003;160(12):2098;
The epidemic of mental illness in the last two centuries. (Adapted from Liberman RP, Musgrave JG, Langlois J. Taunton State Hospital, Massachusetts. Am J Psychiatry. 2003;160(12):2098;
Torrey EF, Miller J. The Invisible Plague: The Rise of Mental Illness from 1750 to the Present. New Brunswick, NJ: Rutgers University Press; 2001.)
Although we may never know for sure whether schizophrenia is a modern epidemic or has been around for ages, the topic raises the issue of etiology. What causes schizophrenia? We will start with what is known about the brain of patients with schizophrenia.
GRAY MATTER
The development of brain imaging techniques provided a way to examine schizophrenic brains in live people. The first computed tomography scans in schizophrenia were published in 1976 and showed enlarged lateral ventricles in a group of patients with chronic schizophrenia. Others quickly replicated this study. However, it was magnetic resonance imaging (MRI), with its ability to differentiate gray and white matter, that finally provided irrefutable evidence of the biologic nature of schizophrenia.
The most famous MRI studies were the original studies on twins. E. Fuller Torrey and Daniel Weinberger et al. at the National Institute of Mental Health recruited monozygotic twins from the United States and Canada. They originally studied 15 sets of twins who were discordant for schizophrenia: one had the illness, whereas the other was unaffected. MRI was done in all 30 participants. The most remarkable finding was that in 12 of the 15 sets of twins the affected individual was easily identified by visual inspection of corresponding coronal scans (Figure 23.3).
The results of the twin study have been replicated and extended. The most common finding remains enlarged ventricles, but better technology has allowed more detailed analysis. Patients with schizophrenia show consistent but subtle decreases in total brain volume and total gray matter volume. These results provide an explanation for the increased ventricle size; that is, the ventricles expand to fill the void left by the loss of gray matter.
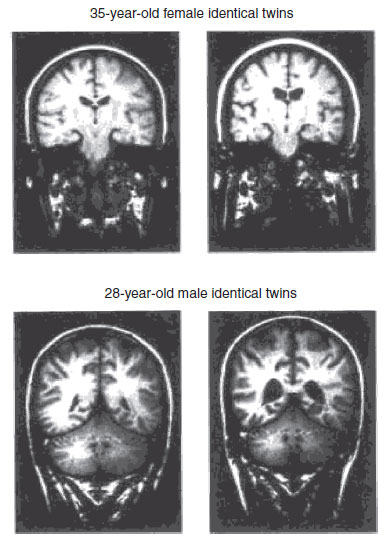
FIGURE 23.3  Coronal magnetic resonance imaging of two sets of twins discordant for schizophrenia. The enlarged lateral ventricles are readily apparent in the subjects on the right. (Courtesy of Drs. E. Fuller Torrey and Daniel Weinberger.)
Coronal magnetic resonance imaging of two sets of twins discordant for schizophrenia. The enlarged lateral ventricles are readily apparent in the subjects on the right. (Courtesy of Drs. E. Fuller Torrey and Daniel Weinberger.)
Judith Rapoport’s laboratory performed sequential MRI scans on children with childhood-onset schizophrenia and compared the findings with age-matched controls in an effort to follow up the changes in the brain that occur during adolescence. They found that the rate of change was greater for those children with schizophrenia. Figure 23.4 shows that the children with schizophrenia had striking loss of gray matter along with decreased brain size and increased ventricles.
Adolescence is a time of remodeling of the connections in the brain to create a more efficient organ. Processes such as pruning, apoptosis, and synaptogenesis are accepted features of the maturing brain (see Figures 8.8, 19.4, and 20.8). Studies such as the one described here suggest that schizophrenia may be the result of overly exuberant remodeling of the gray matter.
Certainly, the process of gray matter reduction occurs in the same time frame as the usual onset of schizophrenic symptoms. Although no definitive evidence exists to prove this theory, some genetic studies suggest that altered expression of genes that control synaptic plasticity contributes to the development of schizophrenia.
Reduced Neuropil Hypothesis
As stated before, traditional microscopic examination of the gray matter of schizophrenics will not identify anything unusual. So it has been difficult to explain the gray matter loss. Investigators at Yale utilized labor-intensive three-dimensional analytic tools to estimate cellular densities. They compared neuronal density in three regions of the brain from patients with schizophrenia and from healthy controls. The regions were Brodmann’s areas 9 and 46 in the prefrontal cortex (PFC) and area 17 in the visual cortex. They found increased density of neurons but not glial cells in the gray matter of schizophrenic patients (Figure 23.5).
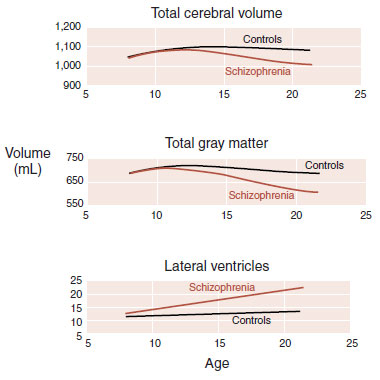
FIGURE 23.4  Children with schizophrenia have loss of brain volume and gray matter as well as enlargement of lateral ventricles through adolescence. (Adapted from Gogtay N, Sporn A, Rapoport J. Structural brain MRI studies in childhood-onset schizophrenia and childhood atypical psychosis. In: Lawrie S, Johnstone E, Weinberger D, eds. Schizophrenia: from Neuroimaging to Neuroscience. New York, NY: Oxford University Press; 2004.)
Children with schizophrenia have loss of brain volume and gray matter as well as enlargement of lateral ventricles through adolescence. (Adapted from Gogtay N, Sporn A, Rapoport J. Structural brain MRI studies in childhood-onset schizophrenia and childhood atypical psychosis. In: Lawrie S, Johnstone E, Weinberger D, eds. Schizophrenia: from Neuroimaging to Neuroscience. New York, NY: Oxford University Press; 2004.)
It appears that schizophrenic patients have the same number of neurons as healthy controls, but they are packed together in less space—called the reduced neuropil hypothesis. The tighter packaging of the schizophrenic neurons results from reduced cell size, less branching, and decreased spine formation. Figure 23.6 shows a drawing of this process. Figure 23.7 shows examples of actual spine formation from schizophrenic patients and controls. The key point is that it is not neuronal loss, but rather the loss of the richness of the dendritic connections that causes the reduced gray matter in schizophrenia. Presumably, this also results in deficient information processing.
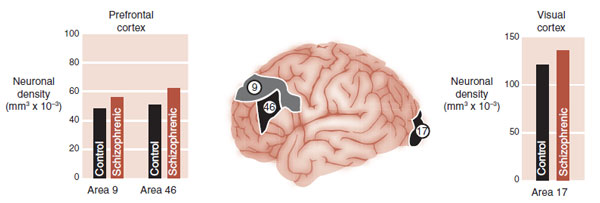
FIGURE 23.5  Patients with schizophrenia have increased neuronal density in all three areas of the brain tested. Glial density was no different. (Adapted from Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45(1):17-25.)
Patients with schizophrenia have increased neuronal density in all three areas of the brain tested. Glial density was no different. (Adapted from Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45(1):17-25.)
Although the underlying cause of neuronal atrophy remains unknown, recent evidence suggests a role for altered neuronal apoptosis. Apoptosis is usually associated with programmed cell death (see Figure 8.9). However, sublethal apoptotic activity may result in synaptic elimination without frank cell death. Apoptosis is controlled by pro- and antiapoptotic proteins, which may be disturbed in schizophrenia.
One further point worth mentioning is that these studies highlight the extensive whole brain involvement of schizophrenia. That is, it is not a disorder of just one region of the brain. Rather, schizophrenia seems to affect almost the entire cortex.
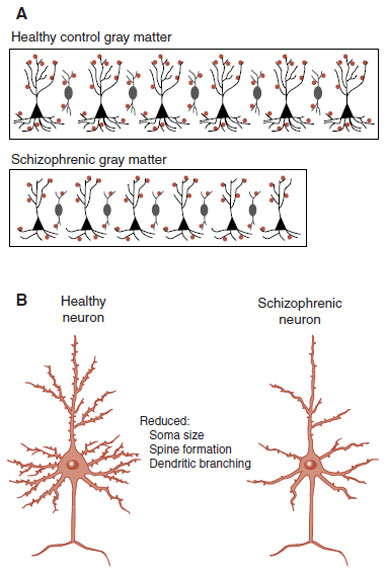
FIGURE 23.6  A. Schematic representation of the increased density but decreased size of schizophrenic gray matter. B. Suspected neuronal atrophy of schizophrenic pyramidal neurons, which results in defective connectivity. (A adapted from Selemon LD. Increased cortical neuronal density in schizophrenia. Am J Psychiatry. 2004;161(9):1564.)
A. Schematic representation of the increased density but decreased size of schizophrenic gray matter. B. Suspected neuronal atrophy of schizophrenic pyramidal neurons, which results in defective connectivity. (A adapted from Selemon LD. Increased cortical neuronal density in schizophrenia. Am J Psychiatry. 2004;161(9):1564.)
Functional Brain Imaging
Traditionally, the hallmark of schizophrenia has been hallucinations and delusions. In actuality, the symptoms of schizophrenia are made up of the following three categories of impairment.
1. Positive symptoms: hallucinations and delusions
2. Negative symptoms: lack of motivation, apathy, and so on
3. Cognitive impairment
The cognitive dysfunction, which includes problems with attention, memory, and executive function, may be the most detrimental aspect of the illness. They have a greater negative impact on the individual than the positive symptoms. Likewise, cognitive functioning is the best predictor of long-term outcome from the disorder.
The pattern of cognitive impairment in schizophrenia implicates the frontal cortex. Hypofrontality is a term sometimes used to describe this problem. However, functional imaging studies have given inconsistent results. Weinberger et al. recognized that the function of the frontal lobe must be measured when it is engaged in a cognitive challenge.
To test this theory, patients and healthy controls underwent xenon XE 133 inhalation procedure for regional cerebral blood flow measurements while they were performing the Wisconsin Card Sorting test (Figure 23.8). The control subjects showed increased activation of their frontal lobes while performing the test, but the schizophrenic patients did not. Furthermore, there was a good correlation between the change in blood flow in the frontal cortex and percent errors on the test.
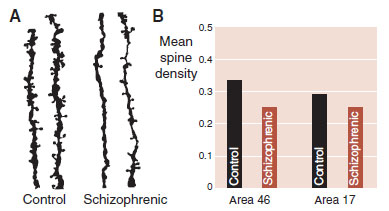
FIGURE 23.7  A. Drawings of actual dendrites and spines from pyramidal neurons in the dorsolateral prefrontal cortex of controls and schizophrenic patients. B. Mean spine density in frontal cortex and visual cortex from controls and schizophrenic patients. (Adapted from Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65-73.)
A. Drawings of actual dendrites and spines from pyramidal neurons in the dorsolateral prefrontal cortex of controls and schizophrenic patients. B. Mean spine density in frontal cortex and visual cortex from controls and schizophrenic patients. (Adapted from Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65-73.)
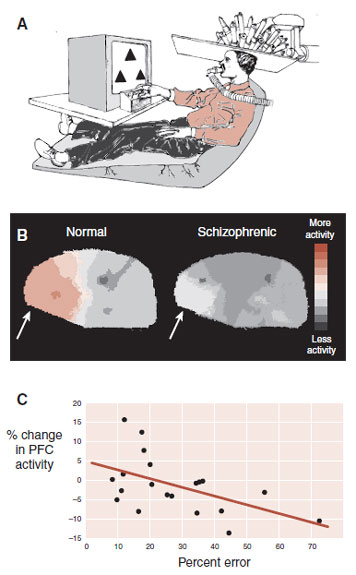
FIGURE 23.8  A. Subjects were scanned while performing the Wisconsin Card Sorting task. B. Healthy subjects displayed increased blood flow to the prefrontal cortex (PFC), whereas those with schizophrenia did not (arrow). C. Percent errors on the task correlated with change in PFC blood flow. (Adapted from Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2): 114-124.)
A. Subjects were scanned while performing the Wisconsin Card Sorting task. B. Healthy subjects displayed increased blood flow to the prefrontal cortex (PFC), whereas those with schizophrenia did not (arrow). C. Percent errors on the task correlated with change in PFC blood flow. (Adapted from Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2): 114-124.)
In summary, these results suggest that the cognitive impairment in patients with schizophrenia comes from impaired frontal lobes. Atrophic, disconnected neuronal cells presumably cause the PFC dysfunction.
Inhibitory Neurons
The activity of the large pyramidal neurons in the gray matter is modulated by smaller local interneurons (see Figure 2.3). Most of the interneurons are γ-aminobutyric acid (GABA) neurons and hence are inhibitory. There are several types of GABA neurons. Figure 23.9A shows two of them: parvalbumin and calretinin. The location of their inhibitory input seems to have different effects on the pyramidal neuron.
The GABA interneurons are important in the discussion of schizophrenia because there is evidence that the parvalbumin neurons are impaired in patients with the disorder. GABA is synthesized from a number of enzymes, one of which is called glutamic acid decarboxylase (GAD). One form of messenger RNA (mRNA) that encodes for GAD (GAD67) has been shown repeatedly to be decreased in patients with schizophrenia. It is one of the most consistent findings in postmortem studies. Figure 23.9B, C shows the results of a study comparing the expression of GAD67 in patients and controls.
Of particular interest, the deficiency in GAD67 expressing interneurons seems to be limited only to the parvalbumin neurons and is not found in other GABA neurons. Furthermore, the number of parvalbumin neurons is not reduced in patients with schizophrenia. So the GABA neurons that are implicated in schizophrenia are not limited in number, but have decreased expression of important genes that might impair the function of the cortex.
Working memory depends on the coordinated firing of pyramidal neurons in the PFC. The inhibitory interneurons are essential for the synchronization of the output from the pyramidal neurons. Tasks such as the delayed response task (see Figure 20.3) require inhibitory control to bridge the time between stimulus presentation and behavioral response. The cognitive impairment in patients with schizophrenia may be due to a failure to properly coordinate the pyramidal neurons.
WHITE MATTER
Schizophrenia also appears to be a disorder of disrupted connectivity. The white matter tracks that connect different regions of the cortex, as well as the cortex with the deeper brain structures, may also play an important role in the disruption of good connections. White matter is composed of the myelinated axons that transport the signals generated by the neurons. Figure 23.10 shows a drawing of some of the long and short white matter tracks. Disruption of the integrity of the white matter tracks leads to degradation of the neuronal signal.
Imaging
MRI studies on patients and controls have found a small but nonsignificant trend toward reduced white matter in schizophrenia. Other studies using the more recently developed diffusion tensor imaging (DTI) technology (see Figure 3.13) have found abnormalities in patients with schizophrenia compared with healthy controls. Some insight into the significance of these findings can be gleaned from comparing the result of DTI studies in other demyelinating diseases. For example, multiple sclerosis and human immunodeficiency virus (both known to induce cognitive impairment and hallucinations with some patients) also produce changes in the DTI analysis. These results suggest that all three diseases may share some similar white matter degradation.
FIGURE 23.9  A. γ-Aminobutyric acid (GABA) interneurons have inhibitory input on the pyramidal neurons in the prefrontal cortex. B. Glutamic acid decarboxylase (GAD)67 expression cells from the prefrontal cortex of a control and a patient with schizophrenia. C. Mean number of GAD67 messenger RNA (mRNA) expression neurons by gray matter layer. PFC, prefrontal cortex. (A Adapted from Tamminga C, Hashimoto T, Volk DW, et al. GABA neurons in the human prefrontal cortex. Am J Psychiatry. 2004;161(10):1764. B, C Adapted from Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258-266.)
A. γ-Aminobutyric acid (GABA) interneurons have inhibitory input on the pyramidal neurons in the prefrontal cortex. B. Glutamic acid decarboxylase (GAD)67 expression cells from the prefrontal cortex of a control and a patient with schizophrenia. C. Mean number of GAD67 messenger RNA (mRNA) expression neurons by gray matter layer. PFC, prefrontal cortex. (A Adapted from Tamminga C, Hashimoto T, Volk DW, et al. GABA neurons in the human prefrontal cortex. Am J Psychiatry. 2004;161(10):1764. B, C Adapted from Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258-266.)
Myelin
Oligodendrocytes
Oligodendrocytes are one of the glial cells that support the neurons. Specifically, the oligodendrocytes provide layers of myelin that insulate the axons and enhance the speed of transmission of neural impulses (see Figure 3.10). Diseases that affect the integrity of the myelin sheath impair the function of the brain and can cause psychotic symptoms in some cases. One particular disease—metachromatic leukodystrophy—usually begins with demyelination of the frontal lobes.
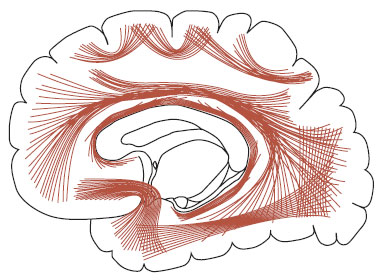
FIGURE 23.10  A drawing of the white matter tracks connecting various regions in the brain. (Adapted from Gray’s Anatomy of the Human Body as displayed at Bartleby.com.)
A drawing of the white matter tracks connecting various regions in the brain. (Adapted from Gray’s Anatomy of the Human Body as displayed at Bartleby.com.)
The rare late-onset form of metachromatic leukodystrophy occurs in about the same time frame as schizophrenia—from adolescence to young adulthood. Reviews of such cases have noted that over half the individuals had psychotic symptoms including auditory hallucinations and bizarre delusions.
Others have looked at the oligodendrocyte population in patients with schizophrenia. Hof et al. counted the number of oligodendrocytes in the white matter of Brodmann’s area 9. They found that there was a 27% decrease in the number of oligodendrocytes in patients with schizophrenia compared with the controls (see Figure 23.11).
The application of microarray analysis (see Figure 1.11) has further implicated the involvement of myelin in schizophrenia. Hakak et al. applied postmortem tissue from patients with schizophrenia and controls to microarray chips to identify gene expression. In other words, they wanted to see which genes were active in which subjects.
More than 6,000 genes were compared between the schizophrenic and control subjects. Only 17 genes were significantly downregulated in the schizophrenic patients. Of these, six were myelin related. The other 11 showed no particular pattern. The authors concluded that the results gave a clear indication that deficient oligodendrocytes and myelination are involved in schizophrenia.
FIGURE 23.11  A. One oligodendrocyte can provide the myelin covering over many axons. B. Patients with schizophrenia have less oligodendrocytes in their white matter. (Graph adapted from Hof PR, Haroutunian V, Friedrich VL Jr, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53(12):1075-1085.)
A. One oligodendrocyte can provide the myelin covering over many axons. B. Patients with schizophrenia have less oligodendrocytes in their white matter. (Graph adapted from Hof PR, Haroutunian V, Friedrich VL Jr, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53(12):1075-1085.)
AUDITORY HALLUCINATIONS
In 1863, Broca described lesions of the left frontal cortex in patients with language expression deficits (see Figure 1.3). Roughly 10 years later, Wernicke described a different language deficit associated with lesions of the superior temporal lobe. The region Wernicke described was a part of what is now called the auditory cortex. The perception of sound starts in the ear, then proceeds through the brain stem and thalamus before reaching the auditory cortex on the superior aspect of the temporal lobe (see Figure 23.12). White matter tracts called the arcuate fasciculus connect the auditory cortex with the frontal cortex.
Wernicke and later Kraepelin both postulated that auditory hallucinations were due to temporal lobe abnormalities since neurologic causes of auditory hallucinations pointed in that direction. Indeed, an auditory aura preceding a seizure suggests the temporal lobe as the nidus of the electric activity. Likewise, hallucinations can result from strokes that involve the temporal lobes. However, until recently the neuronal correlates of auditory hallucinations for schizophrenia were unknown.
A group in Switzerland has done extensive imaging studies of schizophrenic patients when they were hallucinating. In the past, the time it took to scan a person was so long it obscured the difference between the hallucinating state and the nonhallucinating state. Now with rapid functional MRI scans, the differences can be detected. Patients were asked to press a button with the onset of hallucinations and keep it pressed for as long as they lasted. Images during hallucinations were compared with images when the voices were silent. Figure 23.13A shows the activity in the gray matter of the auditory cortex during hallucinations for one patient.
POINT OF INTEREST
Numerous researchers have documented a correlation between auditory hallucinations and the size of the temporal lobe; that is, a smaller temporal lobe predicts more hallucinations. This seems counterintuitive. Why does less brain tissue produce positive symptoms?
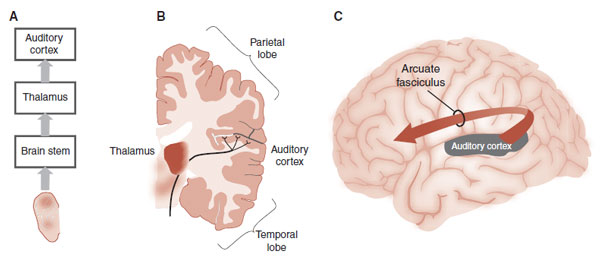
FIGURE 23.12  A. Pathways from the ear to the cortex. B. Auditory signals synapse in the thalamus before reaching the auditory cortex. C. The Arcuate fasciculus is composed of white matter tracts that connect the auditory cortex with the frontal cortex.
A. Pathways from the ear to the cortex. B. Auditory signals synapse in the thalamus before reaching the auditory cortex. C. The Arcuate fasciculus is composed of white matter tracts that connect the auditory cortex with the frontal cortex.
FIGURE 23.13  A. Functional magnetic resonance imaging (fMRI) showing the gray matter regions activated when schizophrenic patients are experiencing auditory hallucinations (arrows). B. Diffusion tensor imaging showing the areas of altered white matter tracts for patients who hear auditory hallucinations compared to healthy controls. (A from Dierks T, Linden DE, Jandl M, et al. Activation of Heschls gyrus during auditory hallucinations. Neuron. 1999;22(3):615-621. B from Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61(7):658-668.)
A. Functional magnetic resonance imaging (fMRI) showing the gray matter regions activated when schizophrenic patients are experiencing auditory hallucinations (arrows). B. Diffusion tensor imaging showing the areas of altered white matter tracts for patients who hear auditory hallucinations compared to healthy controls. (A from Dierks T, Linden DE, Jandl M, et al. Activation of Heschls gyrus during auditory hallucinations. Neuron. 1999;22(3):615-621. B from Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61(7):658-668.)
Recently, this same research group used DTI to look at white matter tracts in schizophrenic patients with hallucinations compared with patients without hallucinations and healthy controls. Remarkably, they found that patients with hallucinations have significantly more alterations of the white matter tracts of the arcuate fasciculus (Figure 23.13B).
Taken together, these studies suggest that auditory hallucinations are derived from abnormalities in the regions that register external sounds. The patient with auditory hallucinations may misidentify inner speech as coming from an external source due to lack of integrity of the system. It is reminiscent of a phone or television picking up other signals and playing more than one sound track at a time.
These studies highlight the complexity of schizophrenia (or the schizophrenias). For just one symptom, abnormalities have been identified in both the gray matter and white matter for patients with schizophrenia. Clearly, schizophrenia is a confusing disorder with diverse and numerous effects on multiple areas of the brain.
ETIOLOGY
We have tried to establish that schizophrenia appears to be a disorder of disconnectedness. There is no specific brain region affected, but rather a dysfunction of circuits within and between regions. Additionally, the onset of the full disorder suggests a neurodevelopmental disruption. It is plausible to envision that schizophrenia results from the abnormal expression of genes that govern maturational processes. However, what goes wrong and how does all this occur?
Genetics
One of the most consistent findings in schizophrenia research is the heritable nature of the illness and related illnesses. Figure 1.1 shows the striking power of the genes with this disorder. The closer one is related to someone with schizophrenia, the more likely that person is to get the illness. However, even the monozygotic twin of a person with schizophrenia only has approximately a 50% chance of getting the illness. Furthermore, patients with schizophrenia are less likely to procreate. If this is a genetic disorder with Mendelian properties, we would expect it to decline in frequency over many generations. Clearly, there is more involved than just genes in the traditional sense.
Environment
Prenatal Complications
Adverse environmental events are known to be potential triggers for developing schizophrenia. Maternal infection is one well-known risk factor for schizophrenia. Obstetric complications are also associated with schizophrenia. A large prospective study that followed up children from birth through adulthood found that the odds of schizophrenia increased linearly with increasing number of hypoxia-associated obstetric complications.
Famine
Two large epidemiologic studies of in utero exposure to maternal starvation have shown an increased risk of schizophrenia among the offspring. In October 1944, a Nazi blockade of the western Netherlands precipitated a famine that did not remit until liberation in May 1945. During the blockade, daily food rations fell to <500 calories per person per day. In follow-up studies, the risk of developing schizophrenia in exposed children had doubled. Additionally, there was also a significant increase in births with neural tube defects.
The second study looked at the effects of famine caused by the Great Leap Forward in China during 1960 to 1961. Similar results were found (Figure 23.14). In both situations, the birth rate dropped during the famine. Some speculate that the lack of folate in the diet had a detrimental effect on the developing fetal brain. Folate is needed for DNA synthesis and repair. Its absence can lead to chromosomal instability. (Is this why the Irish, with their interminable famines, experienced a greater epidemic of mental illness? See Figure 23.2.)
Finnish Adoption Study
Many people conceptualize schizophrenia as resulting from some interaction between genes and the environment, but it has been hard to tease out the relation between these factors. The Finnish Adoption Study provides some interesting data to help understand this interaction. Researchers collected the names of all women who were hospitalized in Finland from 1960 to 1979 and diagnosed with schizophrenia. Then they identified the children from these women who were adopted away. They collected a similar number of adopted children as matched controls.
Detailed and blinded assessments were made of the adoptive families by experienced psychiatrists. Using specific scales, they divided families into those that were more healthy and those that were more dysfunctional. Additionally, each child was assessed for a schizophrenia spectrum disorder based on the Diagnostic and Statistical Manual of Mental Disorders-Third Edition-Revised (DSM-III-R) criteria, for example, schizophrenia, delusional disorder, depressive disorder with psychotic features, and schizotypal personality disorder.
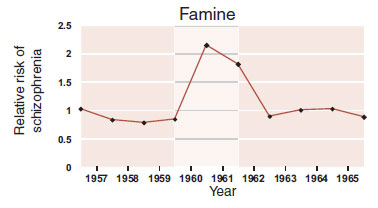
FIGURE 23.14  The relative rate of developing schizophrenia doubled for those born during the famine during 1960 to 1961 in one region of China.
The relative rate of developing schizophrenia doubled for those born during the famine during 1960 to 1961 in one region of China.
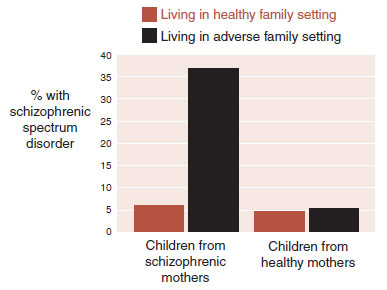
FIGURE 23.15  Children born to schizophrenic mothers and raised in more dysfunctional family environments were at increased risk for developing schizophrenic spectrum disorders.
Children born to schizophrenic mothers and raised in more dysfunctional family environments were at increased risk for developing schizophrenic spectrum disorders.
The remarkable results of this study are shown in Figure 23.15. Only the children born to mothers with schizophrenia and raised in an adverse family household showed an increased risk of developing schizophrenic spectrum disorders. This is an example of a genetic–environment interaction. Children at genetic risk for schizophrenia appear to be more sensitive to problems in the environment.
Gene Expression
In several chapters of this book, we have discussed the enduring effects that life events can have on the DNA. Environmental events change the genes—a cardinal feature of the neuroscience model (see Figure 6.13). Enhancing or silencing specific genes changes behavior. This enables animals to adapt their behavior to their particular environment. However, some events seem to silence important genes and have devastating effects on behavior. Schizophrenia may be such a condition.
Researchers are starting to look at changes in gene expression in patients with schizophrenia as a way to better understand the disorder. To proceed with such an examination, an important protein must first be identified and then the DNA that encodes for that protein must be analyzed. Although we imagine there are numerous such proteins, one that has been identified is reelin, a protein expressed by GABA interneurons. Reelin is recognized as crucial for neuronal migration, axonal branching, and synaptogenesis throughout brain development. Additionally, reelin and its mRNA have been found to be reduced in postmortem brains of schizophrenic patients.
We have previously discussed that the addition of methyl groups to the DNA limits gene expression— the methyl groups prevent the transcription factors from “zipping” off some mRNA. Tsuang et al. at the University of California in San Diego recently looked at the methylation of the reelin DNA from gray matter from postmortem brains of patients with schizophrenia. Not surprisingly, they found a distinct methylated signal in 73% of the schizophrenic samples but only in 24% of the control samples. To put it another way, the reelin DNA of the schizophrenic patients was three times more likely to be methylated.
Not only does this give a possible mechanism to explain the failure of gene expression in patients with schizophrenia but also fits with known environmental events that increase the risk of developing the disorder. For example, transient ischemia is known to increase DNA methylation. Likewise, folate is necessary for normal DNA methylation. This may explain why fetal hypoxia and maternal famine predispose some individuals to develop schizophrenia.
This is not to say that DNA methylation is the only mechanism to explain schizophrenia. Spontaneous mutation during spermatogenesis is another possible cause. It is known that schizophrenia is associated with increased paternal age, and older fathers are more likely to have increased de novo germline mutations. A recent analysis of genome-wide sequencing of DNA from 78 Icelandic families (219 individuals) found that it is the age of the father that determines the number of de novo mutations in the child (Figure 23.16). The important point is that changes to DNA— particularly vulnerable DNA—can be a way for us to understand the genetic/environmental etiology of schizophrenia.
The take-home message is this: There are likely to be multiple genetic vulnerabilities to schizophrenia, which are rarely expressed but not uncommon. Insults from the environment, such as diet, infection, and ischemia, have detrimental and lasting effects on the DNA. Those individuals having both genetic vulnerability and environmental insult are the ones who develop schizophrenia.
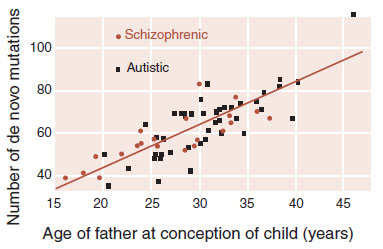
FIGURE 23.16  As the father’s age at conception of the child increases, the number of single nucleotide polymorphisms (SNPs) increases. A father at 20 years passes along on average 25 mutations, while a 40-year-old father passes on about 65. (Adapted from Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488:471-475.)
As the father’s age at conception of the child increases, the number of single nucleotide polymorphisms (SNPs) increases. A father at 20 years passes along on average 25 mutations, while a 40-year-old father passes on about 65. (Adapted from Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488:471-475.)
Dopamine Hypothesis
It does not seem proper to write an entire chapter on schizophrenia without mentioning dopamine. The dopamine hypothesis is an old and enduring theory purporting that overactivity of the dopamine system is part of the pathogenesis of schizophrenia. It was first proposed in 1966 on the basis of pharmacologic studies. Dopamine blocking agents provided the first effective treatment for the positive symptoms of schizophrenia. Furthermore, amphetamines, which increase dopamine at the synaptic cleft, can induce psychosis.
Although the popularity of the dopamine blocking agents is at an all-time high, the belief that dopamine overactivity causes schizophrenia has dwindled. Modulation of dopamine activity may be effective in diminishing psychotic symptoms, but there is minimal evidence to implicate the dopamine neurons in the pathogenesis of the disorder. Howes and Kapur put it nicely in their review of the dopamine hypothesis when they proposed that dopamine dysregulation is the final common pathway of psychosis that is manipulated by “upstream factors.” Most likely it is the disconnections among the glutamate and GABA neurons that are the etiopathogenesis of schizophrenia.
ANTIPSYCHOTICS AND THE SHRINKING BRAIN
One of the foremost concerns in the treatment of psychosis is the long-term effect of medication. Medication changes gene expression, which can, in some instances, propagate irreversible adverse outcomes, for example, tardive dyskinesia. While medications can be beneficial in the short run, we do not know the effects on the brain in the long run. A recent decadelong study out of Nancy Andreasen’s lab in Iowa produced unsettling results on this issue.
MRI scans were performed every three years on 211 first-episode patients treated with antipsychotic medication. On average, patients had three scans over 7.2 years. The investigators found that gray matter volumes decreased over time in all brain regions except the cerebellum. Patients who received the higher lifetime doses of medication had less gray matter. These results are consistent with controlled antipsychotic treatment studies in animals.
The potential neurotoxicity of antipsychotic medications reminds us to avoid unnecessary treatment and use the lowest dose possible, until more information is available.
QUESTIONS
1. Neurodevelopmental causes that could explain schizophrenia include excessive amounts of all of the following, except
a. Pruning.
b. Synaptogenesis.
c. Apoptosis.
d. Myelination.
2. Evidence that schizophrenia is a biologic disorder includes all of the following, except
a. The difference in lateral ventricles in the twin study.
b. Gliosis in the PFC.
c. Gray matter reduction in childhood-onset schizophrenia.
d. Hypofrontality.
3. All of the following support the reduced neuropil hypothesis, except
a. Oligodendrocyte dysfunction.
b. Reduced neural cell size.
c. Limited spine formation on the dendrites.
d. Increased density of gray matter.
4. Evidence that GABA interneurons are impaired in schizophrenia
a. Increased methylation of calretinin DNA.
b. Reduced parvalbumin neurons.
c. Reduced GAD67.
d. Increased reelin.
5. All of the following suggest white matter impairment in schizophrenia, except
a. Microarray analysis.
b. DTI.
c. Oligodendrocyte cell counts.
d. Significantly reduced white matter volume.
6. Auditory hallucinations have been shown to activate which region on fMRI?
a. Superior temporal lobe.
b. Broca’s area.
c. Wernicke’s area.
d. Arcuate fasciculus.
7. All are plausible theories on the etiology of schizophrenia, except
a. Methylation of DNA.
b. Spontaneous mutations.
c. Dopamine hypothesis.
d. Genetic/environmental interactions.
8. Match the following:
1. Bleuler |
A. Psychoanalytic theory |
2. E. Fuller Torrey |
B. Distinguished schizophrenia from bipolar disorder |
3. Kraepelin |
C. Hypofrontality |
4. Plum |
D. Invisible epidemic |
5. Refrigerator mother |
E. Coined the term schizophrenia |
6. Wisconsin Card Sorting |
F. Graveyard of neuropathologists |
See Answers section at the end of the book.