Woody Guthrie, the Depression-era folk singer (“This Land Is Your Land”), developed Huntington’s disease late in his thirties. The disease is an autosomal dominant neurodegenerative disorder, which often is not expressed until middle age. Woody’s son, Arlo Guthrie, who popularized the Vietnam era song about Alice’s Restaurant, has not developed the disorder as he did not inherit the deviant gene from his father. Huntington’s disease is an example of simple Mendelian genetics—one dominant gene leads to the disorder. With other simple Mendelian disorders, such as cystic fibrosis and sickle cell disease, the affected individual must inherit two recessive genes—one from each parent. (Single gene changes with large effects.)
Unfortunately, the genetic patterns of the common psychiatric disorders are not this simple. As a matter of fact, they remain incomprehensibly complex. We have yet to find a gene or a set of genes that can explain any major psychiatric disorder. Analysis of the human genome has revealed that the genetic mechanisms are more complex than we had imagined. Furthermore, events from the environment (toxins, abuse, isolation, etc.) can alter gene expression by changing the molecules around the DNA. The genetics of mental illness remains a mystery.
HERITABILITY OF MENTAL ILLNESS
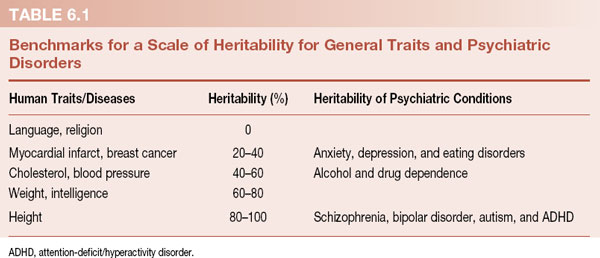
There is no question that psychiatric disorders are genetically linked. The twin and adoption studies (Figure 1.1 and Table 1.1) show that behavioral traits and mental illness run in families. The DNA that puts individuals at risk for mental disorders is passed from parent to child. Geneticists use the term heritability, which they define as the proportion of observed variance in a group of individuals that can be explained by genetic variance. What does that mean? Well, heritability (h2) of a trait is the total proportion of the total variance that is genetic (h2 = VG/VP). Clear as mud. A better way to understand heritability is shown in Table 6.1.
Kenneth Kendler created the scale in Table 6.1 to correlate heritability percentages with common traits. Language and religion have zero genetic influence. Asian babies raised by American families speak English without an accent and vice versa. At the other extreme of the scale is height. In the absence of malnutrition or a pituitary tumor, height is almost completely determined by the genes inherited from the parents. Monozygotic twins rarely differ in height by more than half an inch.
The heritability of psychiatric disorders is placed next to this scale for comparison. We cannot think of any psychiatric condition that does not have some genetic influence. Even traumatic brain injury, which should be the result of a random accident, is probably linked to impulsive and risk-taking genes. The common psychiatric disorders have moderate heritability, while the serious mental disorders have strong genetic components. The take-home message is clear—mental disorders are influenced by genes, although some more than others. But which genes are causing the problems? First, a word about the human genome.
JARGON
The comedian Steve Martin said, “Those French ... they have a different word for EVERYTHING!!!” At times, it seems like geneticists are speaking French, only without the passion. For example, gene is the term to describe a unit of heredity—a portion of DNA that codes for proteins or RNA. An allele is one of two or more forms of the DNA sequence for a gene. Such a gene is polymorphic. It gets worse. For example, the protein catechol-O-methyltransferase has a common polymorphism (Val158Met) at the chromosome 22q11.2. Ugh! We try to minimize the jargon and stick to simple terms. Sacré bleu!

HUMAN GENOME PROJECT
Genome is the term used to refer to the sum total of genetic information in a particular organism. The human genome is the entire DNA code contained in the chromosomes in each nucleated cell. The Human Genome Project was a large international scientific study focused on sequencing the nucleic acids (called base pairs) for the entire human genome. It was the Manhattan Project for biologists. They started in 1990 and completed the project in 2003. Important findings included the following:
– the human genome is made up of 3 billion base pairs,
– less than 2% of the DNA codes for proteins, and
– there are only approximately 23,000 protein-coding genes.
Although it is hard to find an example in which the Human Genome Project has advanced our medical treatment, it certainly has transformed our conceptualization of the storage of genetic information. The most shocking aspect is that we have so few protein-coding genes—just about 23,000. This is a bit unsettling especially when one considers that Caenorhabditis elegans (a flat worm ˜1 mm in length) has about 20,000 protein-coding genes. The governor of South Carolina seems so much more intelligent than a flat worm, yet they have about the same number of protein-coding genes. How can this be? Clearly, there is more to complexity than the total number of protein-coding genes.
Protein-Coding Genes
For decades, the central dogma of biology purported that DNA makes RNA, which in turn makes proteins. Here is the key point: it was presumed that proteins orchestrate all the important functions in a cell—regulatory, metabolic, structural, etc. So, how can the governor of South Carolina and a flat worm have roughly the same number of protein-coding genes? The answer seems to be hidden in the other 98% of the genome that is not coding for proteins. This portion of the genome, which has been called “junk DNA,” was presumed to be useless, inert DNA that was only there to connect the important sections together. In fact, the “junk DNA” is more active and more important than most people had imagined.
It’s an RNA World
A new respect for the “junk DNA” developed after the mouse genome was sequenced and compared with the human genome. Mice and people share many of the same protein-coding genes—this was expected. However, much to everyone’s amazement, the two species also share vast regions of “junk DNA.” This was remarkable because the mouse and human lineage diverged over 75 million years ago. If large sections of “junk” DNA were preserved over that time, then they must be important for the function of the cell. Equally revealing, it appears that the complexity of an organism is related to non–protein-coding DNA. Figure 6.1 shows how the percent of non–protein-coding DNA increases with the complexity of the organism.
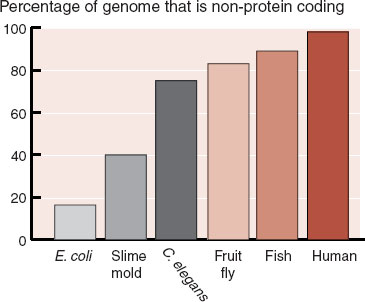
FIGURE 6.1  The percent of the genome that does not code for proteins increases as the complexity of the organism increases. This may explain why the governor of South Carolina is more complex than a C. elegans. (Adapted from Taft RJ, Pheasant M, Mattick JS. The relationship between non-protein-coding DNA and eukaryotic complexity. BioEssays. 2007;29:288-299.)
The percent of the genome that does not code for proteins increases as the complexity of the organism increases. This may explain why the governor of South Carolina is more complex than a C. elegans. (Adapted from Taft RJ, Pheasant M, Mattick JS. The relationship between non-protein-coding DNA and eukaryotic complexity. BioEssays. 2007;29:288-299.)
Further evidence of the importance of RNA comes from analysis of the transcriptional activity of the DNA. Even though less than 2% of the DNA codes for proteins, fully 85% of the genome is transcribed into RNA. The scientific literature has exploded with a dazzling array of various newly discovered non–protein-coding RNA molecules that differ from our old friend messenger RNA (see Figure 3.2). Molecules with such names as microRNA, long non-coding RNA, and small nucleolar RNA are examples of RNA that are transcribed throughout the genome. Even introns, the RNA spliced out of the protein-coding messenger RNA, seem to be functional. Figure 6.2 shows a schematic representation of the new conceptualization of the genome.
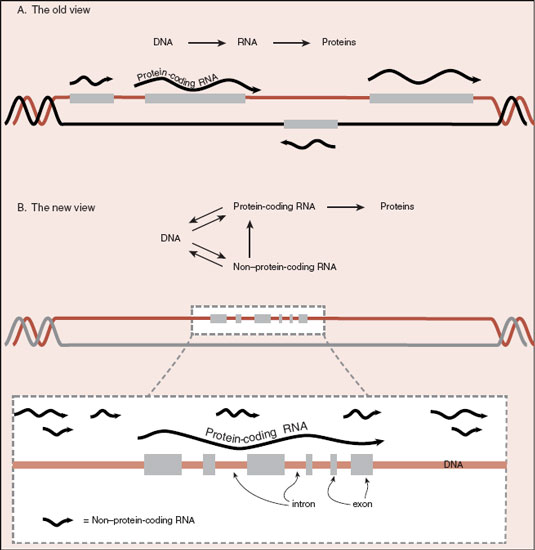
FIGURE 6.2  A. It used to be so simple. DNA made RNA, which made proteins. B. Now it appears that there are fewer protein-coding genes and much more non–protein-coding RNA.
A. It used to be so simple. DNA made RNA, which made proteins. B. Now it appears that there are fewer protein-coding genes and much more non–protein-coding RNA.
A New Level of Gene Regulation
We know the cell would not expend energy transcribing the DNA into RNA if it was not beneficial. But what are all the non–protein-coding RNA molecules doing? Some of the functions that have been identified are as follows:
– Maintain DNA integrity,
– Transcriptional regulation,
– Post-transcriptional modification,
– Viral defense, and
– Epigenetic modification.
MicroRNA provides a good example of what the non–protein-coding RNA may be doing in the cell. MicroRNAs have the capacity to silence gene expression by blocking the messenger RNA. MicroRNAs have complementary nucleic acids that bind to the nucleic acids on the messenger RNA, effectively shutting down any translation of the mRNA into protein—like putting gum in a typewriter. Mechanisms such as this and others that are beyond the scope of this book enable non–protein-coding RNA to affect and influence gene expression.
This new view of the genome is relevant for mental illness because non–protein-coding RNA is highly expressed in the brain. Small changes in these RNA molecules can affect the expression of multiple genes and may underlie neurodevelopmental, neurodegenerative, and psychiatric disorders. Recent studies have shown that some microRNAs are downregulated while others are upregulated in schizophrenia.
AN RNA WORLD
The origins of life in the primordial world remain a mystery. The development of a mechanism to pass along the instructions for building, maintaining, and replicating the cell was an essential step for sustaining any life form. It has been assumed that life started with DNA. Yet, DNA requires proteins to build RNA, which in turn makes the required proteins. It is a chicken-and-egg dilemma. New thinking postulates that RNA was the original hereditary molecule. Lincoln and Joyce from the Scripps Research Institute recently created a self-replicating RNA that can, by itself, reproduce indefinitely. It is possible DNA was developed later in evolution as a more stable form of storage and proteins evolved as molecules more adapted to control the functions of the cell.
FINDING THE OFFENDING GENES
Our DNA is subjected to a constant cycle of damage and repair. Radiation, toxins, and the natural decay of molecules take a toll on the DNA. Most damage is irrelevant as it is quickly repaired or results in cell death. Changes to the DNA that endure can have an effect on the cell that occurs along a spectrum from benign to pathogenic. Finding the changes that affect the behavior of the organism is the Holy Grail of psychiatric genetics.
It is easier to find the offending genes if the pathophysiology of a disease is known. For example, an abnormal type of hemoglobin is known to be the cause of sickle cell anemia, and the errant gene has been identified down to the single altered nucleotide. The pathophysiologies of psychiatric disorders, however, remain obscure. For many years, the prevailing theories about psychiatric pathology developed from the effects of psychiatric medications—the monoamine hypothesis or dopamine hypothesis—what we call the “chemical imbalance” theories. However, analyses of genes that control for serotonin and dopamine or their receptors have failed to locate abnormalities that correlate with the frequency of the disorder. When such genes are identified, they are either too common in the unaffected population or fail to replicate in further studies.
Single Genes
Linkage Studies
Prior to the era of genome-wide studies, we used to read reports linking a specific gene or chromosomal region with a particular disease. This was done by analyzing families with an illness and searching for the genes. Figure 6.3 shows a pedigree for an Amish family with bipolar disorder. The heritability of the disorder is readily apparent. The location of the gene or genes causing the problem is much harder to find.
Researchers used linkage studies to narrow down the location of the problematic gene. Linkage studies are based on the understanding that genes are located on the arms of chromosomes and that during meiosis some swapping of genes occurs between the arms. The closer two genes are to each other on the chromosome arm, the more “linked” they are. Consequently, using known locations for various genes, and the co-occurrence with the disorder, researchers can estimate where the gene might be. In this situation, the gene was narrowed down to the tip of the short arm of chromosome 11. Unfortunately, and this is the problem with most single gene studies, subsequent research failed to replicate the initial finding.
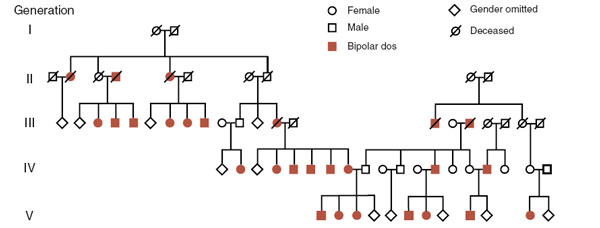
FIGURE 6.3  The high prevalence of bipolar disorder in this Amish family is shown in this pedigree. Linkage studies suggested a single gene located on chromosome 11. (Adapted from Law A, Richard CW 3rd, Cottingham RW Jr, Lathrop GM, Cox DR, Myers RM. Genetic linkage analysis of bipolar affective disorder in an Old Order Amish pedigree. Hum Genet. 1992;88:562-568.)
The high prevalence of bipolar disorder in this Amish family is shown in this pedigree. Linkage studies suggested a single gene located on chromosome 11. (Adapted from Law A, Richard CW 3rd, Cottingham RW Jr, Lathrop GM, Cox DR, Myers RM. Genetic linkage analysis of bipolar affective disorder in an Old Order Amish pedigree. Hum Genet. 1992;88:562-568.)
Few single gene linkage studies have yielded enduring results for mental illness. The apolipoprotein E gene is one exception. The E4 allele (there are three common alleles) is associated with late-onset Alzheimer’s disease. People with two copies of the gene have 15 times the risk of developing the disease. People with any copies of the gene who sustain head trauma suffer more residual cognitive problems. Unfortunately, there are few other single gene linkage studies with such significant findings in our field. Furthermore, the Human Genome Project has transformed the search to identify the pathogenic genes.
Many Small Changes
Single Nucleotide Polymorphism
If single problematic genes do not cause the common psychiatric disorders, then maybe the disorders arise from many minor changes—not one big bang but the cumulative effect of many small hits. Small changes in the DNA are one-letter variations called single nucleotide polymorphisms (SNPs—pronounced “snips”) (Figure 6.4). Genome-wide studies have identified that humans have numerous SNPs that seem to have little effect on observable characteristics (phenotype) of the person. For example, James Watson (of Watson and Crick fame) had his genome sequenced. They discovered he had 3.3 million SNPs. Of interest, 10,000 of the SNPs occurred in protein-coding genes, which might have caused a malfunction in building an important protein—yet he won a Nobel Prize and is alive in his eighties.
Many of the common SNPs co-occur with various diseases. However, the majority of people who have a particular SNP do not develop the disease—that is, a particular SNP by itself has a low predictive value of developing the disease. But maybe common diseases are caused when enough of the common SNPs show up in the same unlucky person. So, the natural next step has been huge studies with large numbers of subjects attempting to correlate numerous SNPs with the disease states. Unfortunately, the studies thus far have been a big disappointment. The results continue to have low predictive value.

FIGURE 6.4  A hypothetical example of a single nucleotide polymorphism. In this single strand of DNA, a “C” has been replaced with a “G.” This is roughly one nucleotide change in a strand of 1,000 nucleotides, which is about the difference between any two humans.
A hypothetical example of a single nucleotide polymorphism. In this single strand of DNA, a “C” has been replaced with a “G.” This is roughly one nucleotide change in a strand of 1,000 nucleotides, which is about the difference between any two humans.
Structural Variation
Copy Number Variation
It was not until 1956 that geneticists could identify and count the number of human chromosomes—called karyotyping. They were surprised to find 46 chromosomes instead of 48 as they had expected. Subsequently, curious clinicians looked at the chromosomes of children with inherited disorders. In 1959, Jérôme Lejeune, a French pediatrician and geneticist, karyotyped the chromosomes in children with Down syndrome—what was called mongolism at the time. Lejeune discovered that these children had an extra chromosome, which was later identified as chromosome 21 (Figure 6.5 A).
The presence of the extra chromosome in Down syndrome, or in some cases just an extra part of the chromosome, has profound effects on the cognition and physical development of the child. While it is not clear what molecular problems result from the additional DNA, we know something goes awry. It may be that the extra DNA results in excessive gene expression, which overwhelms the precise mechanisms of normal development—like having too many cooks in the kitchen.
Henry Turner, an endocrinologist in Illinois, first reported in 1938 a syndrome in girls of short stature and undeveloped secondary sexual characteristics. It was not until 1959 that the syndrome, which has since taken his name, was recognized as resulting from the absence of an X chromosome. Affected women have 45 chromosomes—only one X. Down syndrome and Turner syndrome are examples of genetic abnormalities at the macro level—large additions or deletions in the total quantity of DNA. SNPs, on the other hand, are at the other end of the spectrum—single-letter changes. There is a spectrum of alterations between these two extremes (whole chromosome additions/deletions and SNPs) and these are called copy number variations (CNVs).
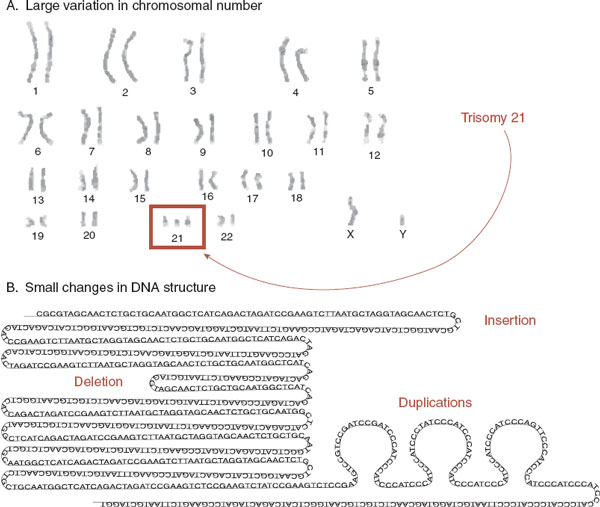
FIGURE 6.5  Structural variation—macro and micro. A. The karyotype of a male child with Down syndrome showing the addition of an extra chromosome. B. Copy number variations are smaller insertions, deletions, or duplications within the DNA strand.
Structural variation—macro and micro. A. The karyotype of a male child with Down syndrome showing the addition of an extra chromosome. B. Copy number variations are smaller insertions, deletions, or duplications within the DNA strand.
CNV is a term that describes small insertions, deletions, or duplications within the DNA molecule (Figure 6.5B). CNVs range in size from hundreds up to millions of base pairs of DNA. Remarkably, they are harder to detect than SNPs because CNV detection requires microarray technology, which is different from the method used to sequence the genome. Recently, attention has turned to the role CNVs may play in mental illness. Many labs are comparing CNVs in normal populations with those in patients with disorders. Studies suggest that CNVs play a role in schizophrenia, autism, and attention-deficit/hyperactivity disorder (ADHD). The recent study in ADHD highlights some of the problems. Four hundred and ten children with ADHD were compared with 1,156 healthy, matched controls. Fourteen percent of the ADHD children had large rare CNVs, while only 7% of the controls had similar genetic deficits. While this is a significant difference, it is also disappointing for it shows that most children with ADHD do not have CNVs and many healthy controls do.
Missing Heritability
In spite of the technological advances in analyzing the genome, the genetic causes of the common medical and psychiatric disorders remain elusive. At this time, a good family history remains more clinically useful than expensive genetic sequencing. Where is the missing heritability? One answer is that the genetic mechanisms are more complicated than previously expected. Another answer may be that events from the environment can alter the genome, further complicating the picture. This is the topic of the next section.
EPIGENETICS
Every nucleated cell in the body contains a complete copy of the organism’s genetic code. Cardiac cells, liver cells, and neurons all contain the same DNA. Yet, only a fraction of the genes in any particular cell are expressed. Most are literally switched “off,” so that only the appropriate DNA is transcribed for any given cell line. During embryonic development, different cell lines emerge by turning on some genes (at the appropriate time) and turning off all the rest.
Understandably, transcription is under tight control in any cell. Having the correct “key” to a particular gene is one mechanism the cell uses to limit transcription. Each gene has a sequence of DNA called the promoter region that signals the starting point for RNA synthesis. Transcription factor proteins specific for that promoter, along with RNA polymerase, bind with the DNA and synthesize RNA (Figure 6.6). (Less is known about the control of transcription of non–protein-coding RNA.) Another mechanism to control gene expression is for a cell to limit access to the promoter region. Remarkably, events during the course of one’s life can affect these mechanisms.
Unfolding the DNA
The DNA in each of our 46 chromosomes is one long strand of a double-helix fiber, which would measure approximately a meter if laid end to end. The cell must package these strands into the nucleus (Figure 6.7). This is accomplished by using dense proteins called histones—vacuum storage bags at the cellular level. The DNA, which is negatively charged, wraps around a series of positively charged histones. These “beads on a string” are then folded into a compact structure called chromatin. The chromatin is further coiled and folded to form the chromosome.
The histones do more than just package the DNA. They also regulate gene expression. DNA is inaccessible to regulatory signals when it is folded in its chromatin structure. Transcription factors cannot initiate gene expression when they cannot physically reach the promoter region of the DNA. Transcription of the gene requires the unfolding of the chromatin, the unwrapping of the histone, and the exposure of the promoter region.
Epigenetics
The attachment of other molecules to the DNA or histones can have a tremendous impact on gene expression. Epigenetics (literally meaning “above the genetics”) has come to mean changes in gene expression that take place without a change in the sequence of A, T, C, and G nucleotides. In other words, the specific genetic code is not changed, but the accessory molecules hanging on the nucleotides and/or histones may alter gene expression. In particular, the addition (or subsequent removal) of methyl or acetyl groups to the DNA or the histone proteins can alter access to the promoter region of the gene. Typically methyl groups decrease gene expression, while acetyl groups increase gene expression.
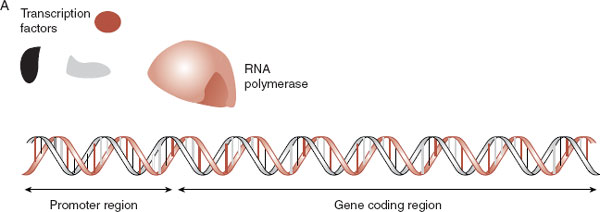
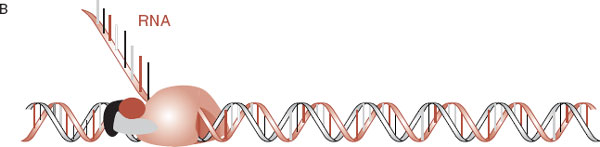
FIGURE 6.6  Transcription factors and RNA polymerase (A) must combine and lock onto the promoter region of the gene before the DNA can be transcribed into mRNA (B).
Transcription factors and RNA polymerase (A) must combine and lock onto the promoter region of the gene before the DNA can be transcribed into mRNA (B).
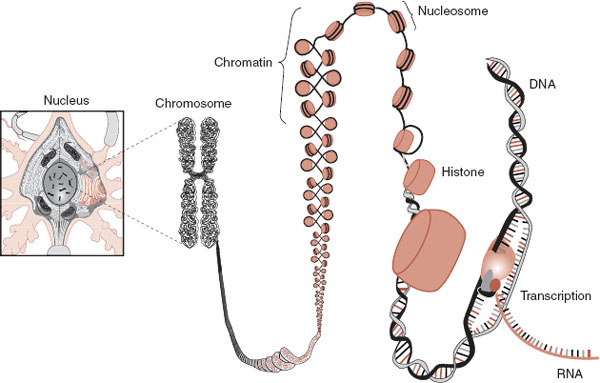
FIGURE 6.7  The DNA must be properly packaged to fit into the nucleus. For gene expression (transcription) to occur, the appropriate section must be unfolded and exposed.
The DNA must be properly packaged to fit into the nucleus. For gene expression (transcription) to occur, the appropriate section must be unfolded and exposed.
FIGURE 6.8  A. Addition of a methyl to the cytosine nucleotide. B. Methylation of the promoter region of the gene prevents transcription factors from locking onto the DNA and prevents gene expression, which silences the gene.
A. Addition of a methyl to the cytosine nucleotide. B. Methylation of the promoter region of the gene prevents transcription factors from locking onto the DNA and prevents gene expression, which silences the gene.
DNA Methylation
DNA methylation is the best understood of the epigenetic alterations. DNA methylation involves the addition of a methyl group (CH3) to one of the nucleotides—in this case cytosine (Figure 6.8). The methyl group is only a problem when it is added to cytosines found in the promoter region of genes. DNA methylation of the cytosine nucleotide in the promoter region effectively silences that gene by preventing the transcription factors from binding with the DNA. If transcription cannot get started, gene expression does not occur.
Chromatin Remodeling
Chromatin remodeling is another epigenetic mechanism regulating gene expression. The addition or deletion of various molecules to the histone proteins can affect the structure of the chromatin. The chromatin structure can be more tightly wrapped or more loosely exposed depending on which molecules are attached to the histone. Open chromatin structure allows greater access to the promoter region of a gene and enhances gene expression.
There are many molecules that can be attached to the histone, but the two most widely reported in behavioral studies are methyl and acetyl groups. The methyl groups tend to compress the chromatin structure and silence the gene. Acetylation, on the other hand, opens up the chromatin structure and allows the transcription factors greater access to the DNA and hence greater gene expression (Figure 6.9).
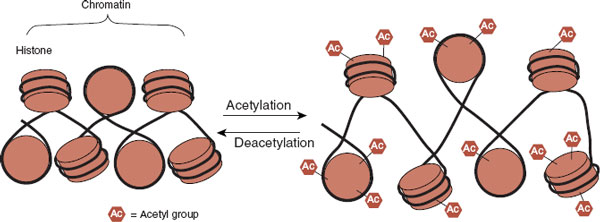
FIGURE 6.9  The addition of acetyl groups to the histones opens up the chromatin and allows greater access to the genes. The result is generally increased gene expression.
The addition of acetyl groups to the histones opens up the chromatin and allows greater access to the genes. The result is generally increased gene expression.
Environmental Events and Epigenetics
Now, this is where it gets really interesting! It turns out stimuli from the environment (toxins, radiation, trauma, etc.) can alter gene expression through epigenetic mechanisms. Physical or emotional events during one’s life can literally alter gene expression through epigenetic mechanisms—without changing the genetic code. For example, smoking can increase the risk of cancer through epigenetic mechanisms. Smoking can turn off tumor suppressor genes. Without the tumor suppressor genes producing suppressor proteins, tumors can grow unimpeded. The individual’s behavior has negative health effects through epigenetic mechanisms.
Epigenetics may also explain why identical twins do not always develop the same diseases—an issue broached in Chapter 1. Although genetically identical, the twins do not live identical lives and grow increasingly discordant for epigenetic markers as they age. Figure 6.10 shows chromosome 17 stained for methylation from two sets of twins—one 3 years old and the other 50 years old. The methylation pattern is practically identical in the young twins, but much more out of synch in the older twins. The events of one’s life are recorded on the epigenome.
Agouti Gene
The Agouti gene in mice is a wonderful example of how environmental factors—in this case the mother’s diet—can affect health through epigenetic changes. The Agouti gene is a problem. It not only turns the mouse’s hair yellow but also predisposes it to diabetes, obesity, and cancer. Fortunately, the Agouti gene can be turned off if the promoter region in front of the gene is methylated—transcription factors cannot attach to the gene and it is silenced. This is good for the mouse. Figure 6.11 shows the results of a study by Waterland and Jirtle analyzing the methylation of the Agouti gene and the coat of the mouse. More methylation results in a browner, and healthier, mouse.
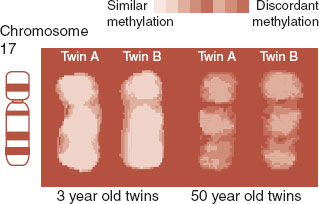
FIGURE 6.10  Color is used to show similar or discordant methylation patterns for each set of twins. The study suggests that epigenetic markers, similar when young, change over the course of one’s life. (Adapted from Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604-10609.)
Color is used to show similar or discordant methylation patterns for each set of twins. The study suggests that epigenetic markers, similar when young, change over the course of one’s life. (Adapted from Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604-10609.)
What is more remarkable is that a mother’s diet when she’s pregnant can affect the methylation of the Agouti gene in her pups. Mother mice fed a supplemental diet high in methyl groups (folic acid, vitamin B12, choline, and betaine) will produce a greater percentage of brown, healthy pups (Figure 6.12). This study shows a clear connection between an environmental event (in this case diet) and subsequent epigenetic effects on physical appearance and health.
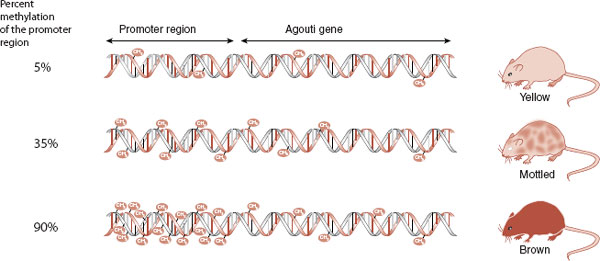
FIGURE 6.11  The Agouti gene causes the mouse to have a yellow coat and predisposes it to obesity, diabetes, and cancer. With increased methylation of the promoter region of the gene, the gene is not expressed and the mouse is brown and healthy.
The Agouti gene causes the mouse to have a yellow coat and predisposes it to obesity, diabetes, and cancer. With increased methylation of the promoter region of the gene, the gene is not expressed and the mouse is brown and healthy.

FIGURE 6.12  Pregnant mice fed a supplemental diet high in methyl groups will produce more pups with a silenced Agouti gene due to increased methylation of the gene.
Pregnant mice fed a supplemental diet high in methyl groups will produce more pups with a silenced Agouti gene due to increased methylation of the gene.
A fascinating study conducted in Sweden suggests that the diet of our grandparents has enduring epigenetic effects on our health. Researchers studied a small, isolated community in northern Sweden, which was subjected to periods of feast and famine in the 19th and early 20th centuries. People who enjoyed bountiful harvests during their preadolescent years had grandchildren who died younger. On the other hand, people who were underfed when they were between the ages of 8 and 12 sired grandchildren who lived longer. In other words, the diet of our grandparents during their preadolescent years may have epigenetic effects that are passed on to future generations. If this is true, what will the current epidemic of obesity in children have on future generations?
Epigenetics is of particular relevance in understanding mental illness. We know that environmental events (trauma, relationships, drugs, poverty, etc.) play a large role in the development of mental disorders. Epigenetic mechanisms may explain the link between events in the environment and gene expression. It is a topic that appears frequently in the coming chapters.
Perhaps one reason it is so hard to find the genes of mental illness is that epigenetic factors obscure the real culprits. If the genome is the hardware of inheritance, then epigenetics describes the software. A problem with both the hardware (the actual genetic code) and the software (the environment and how life events affect gene expression) would be especially difficult to identify.
Multifactorial
All mental disorders result from some problem with gene expression: too much or too little—either starting during embryonic development or beginning later in life. Yet, finding the genes involved remains hidden at this point. The genome has proved to be more complex than we imagined. Disorders are likely caused by hundreds of additions, substitutions, or deletions, each having a small effect. Epigenetic effects compound the difficulty of finding the offending genes. Furthermore, what appears to be one illness such as anxiety or schizophrenia could be many different conditions with a similar effect on a final common pathway behavior. This has been observed with the genetics of the different epilepsy syndromes. Clearly, the predisposition to a major mental illness is to some degree inherited, but the sources of the errors are too numerous and mysterious to identify with our current technology. The search continues.
Telomeres are the molecular caps on the end of each strand of DNA. Like a knot at the end of a rope, they identify the terminal portion of the chromosome. This ensures that the enzymes that repair the DNA will not mistake the end for a break in the strand and attempt to connect it to some other free end—a process that normally preserves the DNA, but under these circumstances scrambles the genetic code.
Telomeres shorten with every cell division. Telomere shortening is an inevitable and unfortunate consequence of aging. As the telomeres grow shorter, the cells eventually reach the limit of their replicative capacity and progress to senescence or death (apoptosis). Consequently, telomere length can be considered a rough measure of cell age (see figure below).
Patients with mental disorders have been found to have accelerated shortening of their telomeres. Patients with mood disorders, schizophrenia, Alzheimer’s disease, and a history of childhood abuse have shorter telomeres when compared with healthy controls. Exercise appears to preserve telomere length.
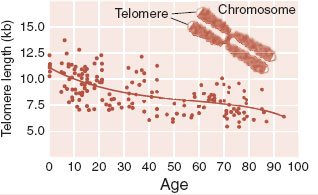
Adapted from Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353-2365.
Cancer is the result of cells that replicate continuously and without constraint. The telomeres—although small—are preserved. In many cancers, an enzyme called telomerase is produced that adds nucleotides back on to the end of the DNA and preserves the telomeres—and the cell. Actually every cell has the capacity to synthesize telomerase, but few do. Some people speculate that harnessing telomerase may be the fountain of youth.
THE NEUROSCIENCE MODEL
Up to this point in this book, we have been reviewing the basic parts of the brain. We started with the big organ and got increasingly small: from cortical structures, to cells, down to molecules. In this chapter, we have gone to the deepest level, the DNA—where it all begins. If you view this sequence of chapter in the opposite order, genes to molecules to cells to networks to behaviors and then the effects of the behaviors on the DNA, this is what we call the neuroscience model (Figure 6.13). It is the most up-to-date model for understanding normal behavior and mental illness.
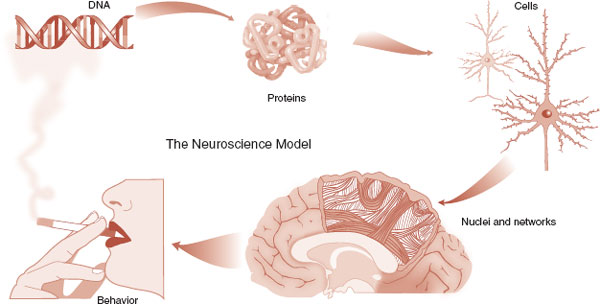
FIGURE 6.13  The neuroscience model. Although the term is not used, this is the prevailing model of understanding the physiology of normal behavior and mental illness.
The neuroscience model. Although the term is not used, this is the prevailing model of understanding the physiology of normal behavior and mental illness.
1. Why does the governor of South Carolina have so few protein-coding genes?
a. The governor believes protein-coding genes are another example of too much government control.
b. The governor is closely related to C. elegans.
c. The governor sold off the vast majority of protein-coding genes in an attempt to balance the state budget.
d. Because much of the complexity of the human species is controlled by other parts of the DNA.
2. All of the following lead us to believe “junk DNA” is important except
a. Non–protein-coding sections are highly expressed in the brain.
b. RNA has more capacity to control functions in a cell than previously recognized.
c. Defects in “junk DNA” have been identified in autism.
d. Some microRNAs have been implicated in schizophrenia.
3. What has happened to the “chemical imbalance” theories of mental illness in the genomic era?
a. Genome-wide studies fail to implicate genes that are associated with neurotransmitters or their receptors.
b. Genes for the serotonin receptor have been identified in families with bipolar disorder in multiple linkage studies.
c. The dopamine transporter gene on chromosome 11 is altered in most patients with childhood-onset schizophrenia.
d. A significant number of patients with panic disorder lack the gene for the A section of the GABA receptor.
4. Understanding epigenetics helps us understand
a. Why non–protein-coding sections are so highly expressed in the brain.
b. How events in the environment can alter gene expression.
c. The cognitive effects of trisomy 21.
d. The increased potential for insertions, deletions, and duplications in the seriously mentally ill.
5. Which of the following is most accurate?
a. Methylation increases gene expression and acetylation increases it.
b. Methylation increases gene expression and acetylation reduces it.
c. Methylation reduces gene expression and acetylation increases it.
d. Methylation reduces gene expression and acetylation reduces it.
6. All of the following are true of the Agouti gene in mice except
a. Methylation of the promoter region produces more brown pups.
b. What the pregnant mothers eat can have enduring effects on the pups’ DNA.
c. Pups with less expression of the Agouti gene are healthier.
d. A pregnant mother’s diet high in polyunsaturated fats will silence the Agouti gene.
7. All of the following are true about telomeres except
a. Identify the terminal end of a chromosome.
b. They are altered by the diet of one’s grandparents.
c. Shorter with increasing replication.
d. Are believed to correlate with some mental disorders.
See Answers section at the end of the book.