In a video produced in 1996 by Robert Sapolsky for the Teaching Company entitled Biology and Human Behavior, Dr. Sapolsky stated
To the greater extent you’ve got all the neurons you’re ever going to have to deal with by the time we’re 4 or 5 years old and, unfortunately, all you do from there is lose them.
Until recently this belief—stated by Dr. Sapolsky such a short time ago—was shared by almost all clinicians and neuroscientists. We are not criticizing Dr. Sapolsky, whose outstanding contributions to the field were cited in the previous chapter. Rather, we wish to show that even astute Stanford professors once believed the brain was a static organ. The authors of this book were no more enlightened at that time.
Additionally, an abundance of new research has established that the brain is not the fixed structure we used to envision. Our brains have more plasticity (or ability to change) than we previously imagined. Although the rate of change is especially prominent early in life, altering the structure of the brain in response to environmental factors remains a feature across the entire life span.
The almost unbelievable prenatal development of the central nervous system (CNS) is a topic we briefly touched on in Chapter 2. The fundamental miracle of the brain is that a single fertilized egg ultimately develops into 100 billion neurons with 100 trillion connections. In utero, this process is largely genetically driven. After birth, interactions with the environment play a greater role in determining the direction of development.
PHASES OF DEVELOPMENT
The cellular events can be divided into four phases:
1. Neurogenesis: The production, migration, and development of distinct new cell types—nerve or glial—from undifferentiated stem cells.
2. Cell expansion: The branching of axons and dendrites to make synaptic connections.
3. Connection refinement: The elimination of excessive branching and synaptic connections.
4. Apoptosis: Programmed neuronal cell death.
Neurogenesis
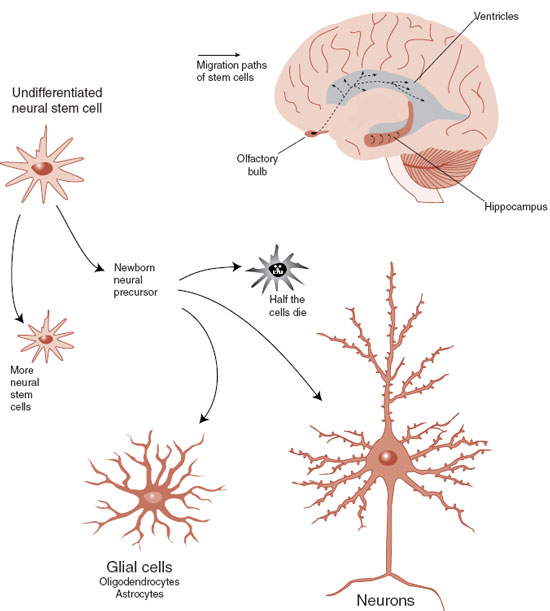
The cells of the intestinal epithelium are turned over every 2 weeks, whereas skin cells are replaced every 1 to 2 months. The adult brain is not nearly this prolific, but is not barren either. In 1998 unequivocal newborn neurons were identified in the hippocampus of elderly subjects whose brains were examined shortly after death. We now know that undifferentiated neural stem cells remain in the CNS and continue to divide throughout life (Figure 8.1). Neural stem cells can develop into neural precursors that grow into neurons or support cells. However, they must migrate away from the stem cell’s milieu before they can differentiate, and only about half the cells successfully move and transform.
The locations and potential for division of stem cells in the adult human brain is a hot, controversial topic. Clear evidence for adult neurogenesis has been established in two areas of the brain: the subgranular zone (SGZ) in the hippocampus and the subventricular zone (SVZ) of the lateral ventricles (Figures 8.2 and 8.3). Newborn cells are identified by tagging them with a molecule such as bromodeoxyuridine (BrdU), a thymidine analogue that can be incorporated into newly synthesized DNA. A florescent antibody specific for BrdU is then used to detect the incorporated molecule and thereby indicate recent DNA replication.
FIGURE 8.1  The process of neurogenesis starts with an undifferentiated neural stem cell that has the capacity to migrate and transform into functioning nerve cells. (Adapted from Gage FH. Brain, repair yourself. Sci Am. 2003;289(3):46-53.)
The process of neurogenesis starts with an undifferentiated neural stem cell that has the capacity to migrate and transform into functioning nerve cells. (Adapted from Gage FH. Brain, repair yourself. Sci Am. 2003;289(3):46-53.)
The SGZ is part of the hippocampus. The utility of creating new neurons in the hippocampus remains a mystery, but may facilitate the stabilization of new memories. The cells born in the SVZ, on the other hand, primarily migrate to the olfactory bulb. This might be helpful for a dog, but it is hard to imagine how this benefits the human brain. However, in 1999, Gould et al. identified newborn nerve cells in the neocortex (prefrontal, temporal, and parietal) of adult monkeys. They established that neural stem cells migrated through the white matter and differentiated into neurons and extended axons Figure 8.3). Unfortunately, the number of new cells is less than that found in the hippocampus or olfactory bulb and is inadequate for significant CNS repair. Future neurological treatments may come from finding methods (medications, exercise, magnetic stimulation, etc.) to invigorate the limited primate neurogenesis so as to replace damaged tissue.
FIGURE 8.2  The granule cell layer of just under the dentate gyrus (called the subgranular zone) is the most studied location of neurogenesis. (Adapted from Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433-1438.)
The granule cell layer of just under the dentate gyrus (called the subgranular zone) is the most studied location of neurogenesis. (Adapted from Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433-1438.)
A remarkable recent study seems to establish that adult neurogenesis does not generate new neurons that become working cells in the gray matter. Researchers utilized a remnant of the cold war to determine the age of neurons and nonneural brain cells in postmortem cortical biopsies.
Throughout the history of life, the amount of 14C carbon in the atmosphere remained almost constant until above-ground nuclear bomb testing during the 1950s and 1960s resulted in an explosive increase in 14C. The carbon isotope rapidly spread around the globe and was incorporated into any new organic molecules. Since the 1963 Test Ban Treaty, the amount of 14C in the atmosphere has steadily decreased.
The amount of 14C in a cell can be used to give an accurate date of the cell’s birth when plotted on the known curves of 14C available in the atmosphere over the last 50 years. For example, the 14C in rings in a tree will match the 14C in the atmosphere at the time that ring was formed. The neuroscientists in Sweden used this technique to determine the age of brain cells in seven individuals who died suddenly.
The researchers were able to separate nuclei of neurons and non-neuronal cells from various regions of the cortex. Then the DNA was extracted, analyzed for 14C, and plotted on the known levels of 14C in the atmosphere. The results are shown in Figure 8.4. These results seem to establish that all the adult human neurons in the neocortex were created perinatally and none since birth. Non-neuronal brain cells continued to form and were born, on average, 5 years after the birth of the individual. One limitation of this study is the exclusive use of healthy brains. We still do not know if new neurons replace damaged cells after trauma or ischemia.
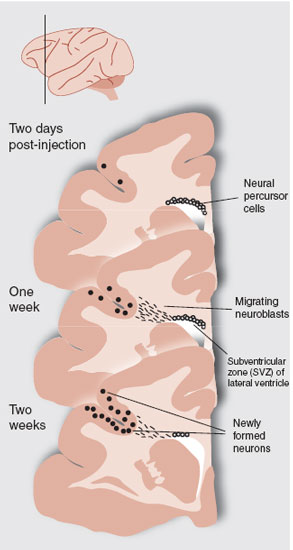
FIGURE 8.3  Adult macaque monkeys were injected with bromodeoxyuridine and sacrificed 2 hour, 1 week, and 2 weeks later. These sections from the prefrontal cortex show the development and migration of neural precursor cells from the subventricular zone (SVZ), through the white matter to the neocortex. Additional tests established that some of the cells were newly formed neurons. (Adapted from Gould E, Reeves AJ, Graziano MS, et al. Neurogenesis in the neocortex of adult primates. Science. 1999;286(5439):548-552.)
Adult macaque monkeys were injected with bromodeoxyuridine and sacrificed 2 hour, 1 week, and 2 weeks later. These sections from the prefrontal cortex show the development and migration of neural precursor cells from the subventricular zone (SVZ), through the white matter to the neocortex. Additional tests established that some of the cells were newly formed neurons. (Adapted from Gould E, Reeves AJ, Graziano MS, et al. Neurogenesis in the neocortex of adult primates. Science. 1999;286(5439):548-552.)
FIGURE 8.4  Postmortem analysis of 14C levels in DNA from neurons and non-neuronal brain cells shows that the neurons were created at the time of this individual’s birth. (Adapted from Bhardwaj RD, Curtis MA, Spalding KL, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564-12568.)
Postmortem analysis of 14C levels in DNA from neurons and non-neuronal brain cells shows that the neurons were created at the time of this individual’s birth. (Adapted from Bhardwaj RD, Curtis MA, Spalding KL, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564-12568.)
In summary, it appears that neurons are born in the SGZ and the SVZ throughout the life of mammals, but after birth they are not inserted as functional neurons into the cerebral cortex—at least in healthy brains. So why is it that the brain continues to make new neurons until we die?
Rate of Neurogenesis
The rate of neurogenesis is modulated by various factors. It is known that enriched environments and exercise will increase neurogenesis (Figure 8.5). Additional research suggests that gonadal steroid hormones may also enhance new cell production. For example, testosterone will stimulate nerve cell production in songbirds (and possibly middle-aged men seeking to recapture the vigor of their youth). The point is that various factors may promote neurogenesis and could be utilized as a part of treatment for neurological disorders.
POINT OF INTEREST
The studies establishing an increase in neurogenesis with enriched environments for mice have also documented increased size of the dentate gyrus of the hippocampus. Additionally, the mice showed improved memory performance on hippocampal-dependent memory tasks (water maze). These findings validate the link between neurogenesis, neuronal growth, hippocampal size, and memory.
Stress, on the other hand, has an inhibitory effect on neurogenesis. Extended maternal separation is a well-characterized model of early life stress for a rodent. As adults, such rodents will show protracted elevations of corticotropin-releasing factor, adrenocorticotropic hormone, and corticosterone (cortisol equivalent in a rat), as well as behavioral inhibition in response to stress. Work from Gould’s laboratory has shown that rats exposed to prolonged maternal separation will have an enduring blunting of neurogenesis. When the corticosterone is experimentally lowered, the neurogenesis rebounds to normal levels—suggesting that hypersensitivity to corticosterone may be the mechanism of action that suppresses neurogenesis.
The relationship among early stress, the hypothalamic-pituitary-adrenal (HPA) axis, and neurogenesis is particularly interesting because of the role of the hippocampus in modulating the HPA axis. We can conceptualize that a healthy hippocampus is necessary to put the brakes on an activated HPA axis after the stressful event dissipates. The above research suggests that early stress (an unavailable mother) leads to a smaller hippocampus due to insufficient neurogenesis, which results in an overactive HPA axis and all those secondary problems discussed in the previous chapter.
Stem Cells
One potential way to rebuild a damaged brain is to implant human embryonic stem cells that have been isolated from a very immature embryo (only 100 to 200 cells). So far it has been difficult to get these immature cells to differentiate into functioning neurons outside the olfactory bulb and hippocampus. The problem may be the absence of biochemical signals that normally prompt the developing stem cell to migrate and differentiate.
FIGURE 8.5  A study by Brown et al. showed that an enriched environment and physical exercise stimulated neurogenesis in the hippocampus but not in the olfactory bulb in mice. (Graph adapted from Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042-2046.)
A study by Brown et al. showed that an enriched environment and physical exercise stimulated neurogenesis in the hippocampus but not in the olfactory bulb in mice. (Graph adapted from Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042-2046.)
Parkinson’s disease involves the loss of dopamine neurons. Stem cells offer a potential source for cell replacement therapy. A group in Japan reported a successful treatment in monkeys with chemically induced Parkinson’s disease. The important difference may have been a “cocktail” of several growth factors that coaxed the undifferentiated cells to develop into neurons and then into authentic dopamine neurons. These cells were injected into the bilateral putamen of the “Parkinsonian” monkeys. Figure 8.6 shows a diagram of the procedure and some results. Unfortunately, studies with humans have been disappointing and this potentially exciting intervention remains a dream.
FIGURE 8.6  A. Harvesting embryonic stem cells, stimulating the development of dopamine neurons in several cultures, and injecting them into a monkey with chemically induced Parkinson’s disease. B. Improved neurological scores over 14 weeks. (Adapted from Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115(1):102-109.)
A. Harvesting embryonic stem cells, stimulating the development of dopamine neurons in several cultures, and injecting them into a monkey with chemically induced Parkinson’s disease. B. Improved neurological scores over 14 weeks. (Adapted from Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115(1):102-109.)
Cell Expansion
The second phase of neuronal growth, often called synaptogenesis, describes the extensive growth of axons and dendrites to make synaptic connections—a process that primarily occurs early in life. The tips of axons and dendrites have growth cones that appear to reach out with fingerlike structures, filopodia, and literally pull the growth cone to its destination. The axons are guided in a specific direction by chemical signals that attract and repel the growth cone, that is, chemoattractants and chemorepellents.
In a comprehensive postmortem analysis, Huttenlocher determined synaptic density across the life span in two regions of the human brain from 14 individuals. Using an electron microscope and special stains, he counted synaptic connections from thin sections taken from the visual cortex and prefrontal cortex. The results, displayed graphically in Figure 8.7, show that the greatest synaptogenesis takes place shortly after birth and occurs sooner for the visual cortex. Maximum synaptic density is reached before the first year in the visual cortex, but not until 3.5 years in the prefrontal cortex. Synaptogenesis is accompanied by an increase in the size of the nerve cell, presumably to support the increased metabolic needs created by the expanded axons and dendrites.
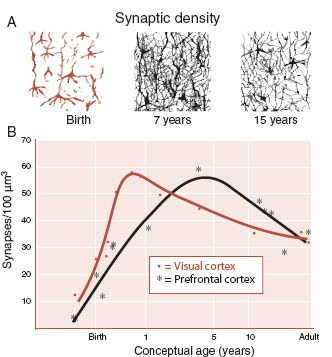
FIGURE 8.7  A. Schematic representation of changes in synaptic density. B. Measured synaptic densities in visual and prefrontal cortex from 14 individuals who died of non-neurological diseases. (Adapted from Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167-178.)
A. Schematic representation of changes in synaptic density. B. Measured synaptic densities in visual and prefrontal cortex from 14 individuals who died of non-neurological diseases. (Adapted from Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167-178.)
Connection Refinement
The third phase of neuronal cell development often referred to as pruning, or synaptic elimination, entails retraction and elimination of excessive connections. The brain makes many more connections than are needed and the weak ones (or those not used) regress. As with synaptogenesis, the pruning proceeds at different rates for different regions of the brain, based on need. For example, sensory and motor areas go through refinement before the regions devoted to executive functions. Clearly, moving and seeing are more essential than writing a poem.
The late and extensive reduction of the frontal cortex correlates with psychological tests measuring improved executive function. It is paradoxical that the maturing process that our adolescents struggle through is actually a refinement and reduction of the gray matter and not growth. We (the authors) have teenage children. Studies suggest they have more gray matter than we do, but we dominate when it comes to wisdom and smarts. The point is this: less is more. This is not the usual pattern in nature. Typically bigger is better. But then elephants and whales, with the largest brains, do not rule the planet like humans.
Disordered pruning may be the pathological basis for such conditions as autism and schizophrenia. A consistent finding in patients with autism is greater total brain volume, which is not present at birth but develops during the first few years of life. It has been suggested that this is due to abnormal connectivity and a lack of pruning. Schizophrenia, which typically develops in late adolescence and is associated with a loss of gray matter, may be the result of excessive pruning.
POINT OF INTEREST
Mark Twain said, “When I was a boy of 14, my father was so ignorant I could hardly stand to have the old man around. But when I got to be 21, I was astonished at how much he had learned in 7 years.” With this clever statement, Twain captures the refinement of the adolescent prefrontal cortex that a teen experiences but does not recognize.
Apoptosis
Programmed cell death, apoptosis, is the final phase of the sculpting of the brain. It is called programmed, which reflects the fact that the cells actually carry genetic instructions to self-destruct. Neurotrophins, discussed next, save the neuron by turning off the genetic program. When the intracellular “self-destruct button” has been activated, apoptosis proceeds in a characteristic process: cell shrinkage, fragmentation, and phagocytosis of the cellular remnants. This is distinguished from necrotic cell death, which results from trauma and is characterized by rapid cell membrane lysis.
Modifications of any of these four phases—for example, enhancing neurogenesis or retarding apoptosis—are potential targets for treatment interventions for such conditions as depression and Alzheimer’s disease. Active research for such treatments is going on.
Brain Imaging Studies
The postmortem studies by Huttenlocher provided an in-depth analysis of synaptic connections, but were limited by the total number of brains that he could analyze. Brain imaging technology, on the other hand, allows the noninvasive examination of a larger number of subjects, in real time, with the potential to be repeated as the subjects age. Unfortunately, brain imaging does not have the resolution to the level of the synapse, so studies follow the trajectory of larger structures, such as gray matter thickness or volume.
The noninvasive magnetic resonance imaging technology has allowed extensive studies of brain development beginning in childhood and progressing into adulthood. One study, shown in Figure 8.8A, scanned 145 healthy subjects, 99 of whom had at least two scans. Gray matter development proceeded on a trajectory of growth until 10 to 12 years of age followed by a decrease in adolescence—consistent with the pattern of synaptogenesis followed by connection refinement. The age of maximal volume (an indirect measurement of development) varies by brain region.
NEUROTROPHIC GROWTH FACTORS
Neurotrophic factors—literally growth factors for nerve cells—are best defined as any molecule that affects the nervous system by influencing the growth or differentiation of neurons or glia. Neurotrophic factors, affectionately called “brain fertilizers,” are the stimulus behind neurogenesis and synaptogenesis (Figure 8.9). Rita Levi-Montalcini et al. accidentally discovered the first growth factor 40 years ago (see Figure 1.9). They were able to isolate a protein from a mouse sarcoma that stimulated the growth of nerve cells as well as increased the size and branching of the cells. Although called nerve growth factor (NGF)—implying that it stimulates all nerve cells—this particular neurotrophin only affects sympathetic neurons and some sensory ganglion cells. Several decades of research have established four principles regarding the cells affected by NGF.
FIGURE 8.8  A. MRI analyses from 145 healthy subjects showing the changing gray matter volumes for different regions of the brain for males. B. Myelination of the frontal lobes correlates with motor speed and peaks in at 39 years of age. (A Adapted from Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2(10): 861-863. B Adapted from Bartzokis G, Lu PH, Tingus K, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554-1562.)
A. MRI analyses from 145 healthy subjects showing the changing gray matter volumes for different regions of the brain for males. B. Myelination of the frontal lobes correlates with motor speed and peaks in at 39 years of age. (A Adapted from Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2(10): 861-863. B Adapted from Bartzokis G, Lu PH, Tingus K, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554-1562.)
1. NGF mediates cell survival.
2. Cells that do not receive enough NGF die.
3. Target organs produce NGF.
4. Specific NGF receptors are present on innervating nerve terminals.
Because NGF is specific for a limited subset of peripheral nerves, it was assumed for many years that there were a host of other neurotrophic factors following similar rules waiting to be discovered. Unfortunately, the fortuitous discovery of NGF has not been repeated with other factors. Identifying and purifying factors that are excreted in such minute quantities has turned into an arduous task. Several factors have been discovered and are being studied, such as neurotrophin-3, glial cell line–derived neurotrophic factor, and insulinlike growth factor, to name a few, but the one that is of most interest to the mental health community is brain-derived neurotrophic factor (BDNF).
The actions of neurotrophins such as BDNF are mediated primarily through Trk (for tyrosine kinase) receptors on the nerve cell membrane. The extracellular portion of the receptor binds with the neurotrophic factor, which in turn initiates an intracellular cascade that leads to gene expression. As yet, there are no small molecule agonists or antagonists for these receptors, which hinders a better understanding of the action of the neurotrophic factors.
Signaling the Trk receptor can lead to changes in three aspects of the cell state:
1. Cell survival or death.
2. Synaptic stabilization or elimination.
3. Process growth or retraction.
The effect is determined by the specific neurotrophic factor, the combination of receptors signaled, and the intracellular pathways expressed in that cell. Disruption of the neurotrophic factor signaling is presumably an explanation (and possible treatment) for some neurodegenerative diseases, for example, Huntington’s, Parkinson’s, and Alzheimer’s diseases. Likewise, the enduring symptoms resulting from substance abuse may be the result of inappropriate neurotrophic factor synthesis.
FIGURE 8.9  Neurotrophic factors mediate cell proliferation and elimination by promoting cell growth, stimulating synaptogenesis, and preventing apoptosis.
Neurotrophic factors mediate cell proliferation and elimination by promoting cell growth, stimulating synaptogenesis, and preventing apoptosis.
TREATMENT
NEUROTROPHINS
Medicating with neurotrophins or stimulating their increased production is an exciting prospect for future neuropsychiatric treatment. Some studies suggest that this may be the mechanism by which lithium, electroconvulsive therapy, and antidepressants resolve depression (see Chapter 21). If this is true, future treatments for depression will focus on more effective ways to stimulate growth factors. Other researchers have examined the therapeutic benefits of growth hormones for such disorders as amyotrophic lateral sclerosis and multiple sclerosis. The biggest difficulty is finding effective yet unobtrusive methods to deliver the neurotrophins to the CNS.
CRITICAL PERIODS
As we have said before, genes control development in utero, but the environment influences the direction after birth. Early interactions during the time of great cellular expansion mold the brain’s anatomy with an ease and permanence that can never be repeated or completely undone. These stages in development when the environmental input is so crucial to determining the structure of the brain are called critical periods.
Language acquisition is a good example of a critical period. Early exposure to native sounds has a lasting impact on speech, which is hard to alter later in life. Johnson and Newport demonstrated this by examining English proficiency in Korean and Chinese speakers who arrived in the United States between the ages of 3 and 39. They found that subjects who arrived in the United States before the age of 7 had equivalent fluency to native speakers, whereas those who arrived after the age of 7 had a linear decline in performance until puberty (Figure 8.10).
The development of the visual cortex provides another example of a critical period. David Hubel and Torstern Wiesel (who later received the Nobel Prize for this work) established the importance of normal visual experience early in life for proper development of the visual cortex in cats and monkeys. In a series of experiments, they sutured one eyelid shut in kittens and later monkeys for short lengths of time during various weeks after birth. They established, down to the level of the neuron, the profound effect of visual deprivation at critical periods of development.
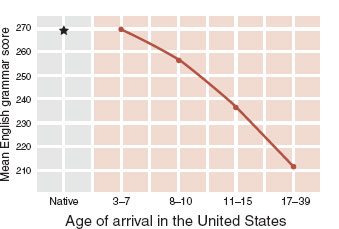
FIGURE 8.10  Language acquisition as measured by English grammar tests for native speakers compared with Korean and Chinese immigrants. (Adapted from Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cognit Psychol. 1989;21(1):60-99.)
Language acquisition as measured by English grammar tests for native speakers compared with Korean and Chinese immigrants. (Adapted from Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cognit Psychol. 1989;21(1):60-99.)
FIGURE 8.11  Injecting radioactive proline into an eye allows visualization of the cortical innervation in layer IV from that eye when the animal is sacrificed several weeks later. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007; Purves D, Augustine GJ, Fitzpatrick D, et al. Neuroscience. 5th ed. Sunderland, MA: Sinauer; 2011.)
Injecting radioactive proline into an eye allows visualization of the cortical innervation in layer IV from that eye when the animal is sacrificed several weeks later. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007; Purves D, Augustine GJ, Fitzpatrick D, et al. Neuroscience. 5th ed. Sunderland, MA: Sinauer; 2011.)
Figure 8.11 shows how the experiments by Hubel and Wiesel were conducted. Radioactive proline was injected into an eye where it was absorbed and transported down the axons to the lateral geniculate nucleus (LGN) of the thalamus. Some of the labeled proline that spilled out of the terminal would be taken up by the LGN neurons and transported to the visual cortex. The location of the radioactive molecules can be visualized using autoradiography.
Figure 8.12 shows the results—both real and schematic—of suturing one eye shut for differing periods of time early in life. The autoradiographs in the B row show a normal cat compared with a cat whose eye was closed from 2 weeks until 18 months. This demonstrates—as do the drawings in the C row—how the neurons from the occluded eye regress and the neurons from the good eye expand. The D row shows the terminal branching, arborization, of the LGN neurons in the visual cortex for a normal eye and one that was occluded for just 1 week at 30 days of age. Hubel and Wiese have estimated that the critical period for ocular deprivation in a cat is 3 to 4 months.
In humans, the critical period for vision extends out to 5 or 6 years of age. For example, children with congenital cataracts can have substantial and permanent visual deficits if the occluded lens is not corrected early enough. However, adults with cataracts regain their preexisting visual acuity when the lens is replaced because occlusion past the critical period does not damage the cortex.
Strabismus, often called lazy eye, is a misalignment of the eyes due to improper control by the eye muscles. Children with this condition experience double vision. The response of the brain to receiving two images is to suppress the input from one eye. This can result in low acuity or even blindness in the suppressed eye due to developmental impediments during the critical period. One form of treatment is to patch the good eye and force development in the cortical regions connected to the lazy eye.
ADULT NEUROPLASTICITY
Plasticity is defined as the capability to be formed or molded. Neuroplasticity describes the brain’s ability to adapt to environmental factors that cannot be anticipated by genetic programming. The description of critical periods described above suggests a bleak picture for plasticity in the adult brain, but there is more hope for your old cortex than one might think. For example, the organization of the representation of the sensory input in the cortex (called cortical maps) is continually reshaped by experience.
DISORDER
PERSONALITY
Extrapolating these studies to behavior, one wonders about critical periods and personality development. For example, what is the effect of early exposure to TV? Recent studies suggest that too much time in front of the TV leads to attentional problems for children, poorer academic achievement, and increased aggression. What neuronal connections fail to develop when children spend too much time watching TV?
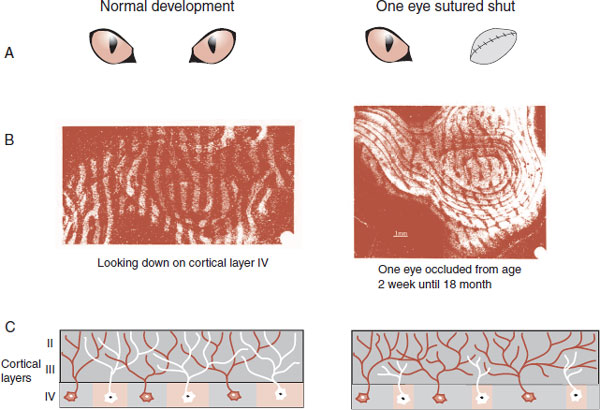
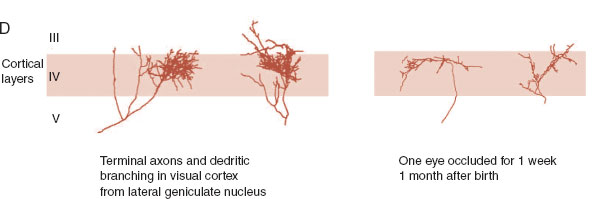
FIGURE 8.12  A–E. Comparing the normal development of the visual cortex on the left with development on the right when one eye has been sutured shut for varying lengths of time. E. Electrodes inserted horizontal to the cortex show distinctive patterns of ocular dominance depending on which eyes developed properly. (B. From Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299(5884):583-591. D. From Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260(5115):1819-1821. Reprinted with permission from the AAAS.)
A–E. Comparing the normal development of the visual cortex on the left with development on the right when one eye has been sutured shut for varying lengths of time. E. Electrodes inserted horizontal to the cortex show distinctive patterns of ocular dominance depending on which eyes developed properly. (B. From Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299(5884):583-591. D. From Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260(5115):1819-1821. Reprinted with permission from the AAAS.)
FIGURE 8.13  The expansion of cortical representation of the dorsal fingers of a monkey before and after several weeks of controlled hand use. (Adapted from Jenkins WM, Merzenich MM, Ochs MT, et al. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63(1):82-104.)
The expansion of cortical representation of the dorsal fingers of a monkey before and after several weeks of controlled hand use. (Adapted from Jenkins WM, Merzenich MM, Ochs MT, et al. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63(1):82-104.)
Studies with monkeys have shown a startling capacity of the cortical maps to change in response to sensory input. In one classic study, animals were taught to rotate a disk using only digits 2, 3, and sometimes 4 to receive banana-flavored pellets. After several weeks of practice, the cortical regions (the cortical map) used by fingers 2, 3, and 4 were enlarged (Figure 8.13). Practice literally changed the brain.
In another experiment by the same group, the researchers measured the representation of the fingers on the cortex before and after amputation of the middle finger. Figure 8.14 shows how the cortex adapted to the change. Neurons deprived of their normal sensory input from the middle finger switch to responding to stimulation from the closest finger. Similar changes have been observed with humans after medical amputation. For some patients, when their face is scratched, they “feel” a sensation as though it was coming from their amputated lower arm—a phantom sensation. Functional imaging scans performed during such stimulation have shown activation of the somatosensory region for both the face and where the lower arm was. It appears that the sensory neurons in the brain do not die when input ceases. Rather, they shift their focus.
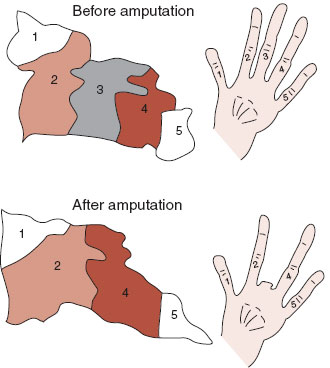
FIGURE 8.14  After amputation of a monkey’s finger, the cortical neurons reorganize. The neurons formally responding to stimulation of the third finger now respond to stimulation from the second and fourth fingers. Similar “re-mapping” also occurs in the thalamus. (Adapted from Merzenich MM, Nelson RJ, Stryker MP, et al. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224(4):591-605.)
After amputation of a monkey’s finger, the cortical neurons reorganize. The neurons formally responding to stimulation of the third finger now respond to stimulation from the second and fourth fingers. Similar “re-mapping” also occurs in the thalamus. (Adapted from Merzenich MM, Nelson RJ, Stryker MP, et al. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224(4):591-605.)
The Musician’s Brain
Learning to play a musical instrument is a complex task requiring practice, practice, practice. Musicians’ brains serve as a model of neuroplasticity. Figure 8.15 shows the enhanced signals from the somatosensory and auditory cortex for highly skilled musicians compared with controls. Figure 8.15A shows the representation of the cortical signal from the little finger on the left hand for string musicians and controls when the fingers were stimulated. This difference did not exist for the thumb or the fingers on the right hand. In Figure 8.15B, the musicians had a 25% greater neuronal excitation for the piano tones than the controls. In both cases, the musicians who had started playing at the youngest age displayed the largest signal.
FIGURE 8.15  Musicians (black arrow) and controls (white arrow) show differences in the somatosensory (A) and auditory (B) cortex. (Adapted from Pantev C, Engelien A, Candia V, et al. Representational cortex in musicians. Plastic alterations in response to musical practice. Ann N Y Acad Sci. 2001;930:300-314.)
Musicians (black arrow) and controls (white arrow) show differences in the somatosensory (A) and auditory (B) cortex. (Adapted from Pantev C, Engelien A, Candia V, et al. Representational cortex in musicians. Plastic alterations in response to musical practice. Ann N Y Acad Sci. 2001;930:300-314.)
Taken in total, these studies suggest that starting early is best, but even the late bloomers can change their brains and develop new skills. Clearly, as anyone who has tried to learn a new language, instrument, or sport knows, it requires work and diligence to master the task—or literally to change the brain. Fortunately, it appears that even an old cortex can adapt. Hubel and Wiesel described a monkey who had one eye occluded between days 21 and 30. Initially the monkey appeared blind in the deprived eye. Remarkably, 4 years later he had an estimated acuity of 20/80 in the bad eye and 20/40 in the normal eye.
Maladaptive Neuroplasticity
Prolonged practice has its dark side. Approximately 1% of professional musicians will develop a condition called focal dystonia: loss of control of skilled movements in the performing hand. It is usually a career-ending disorder and is believed to result from maladaptive neuroplasticity stimulated by long hours of repetitive movements. Neuroimaging studies show a fusion of the digital representations in the somatosensory cortex (Figure 8.16). It is almost as though the neurons are attempting to move two fingers when they should be only moving one. Consequently, individual finger movement is no longer possible.
Cellular Changes
The cellular mechanisms that accompany the cortical changes seen with monkeys rotating disks and musicians playing instruments (and hopefully readers of this book) are poorly understood. Some similar processes that may play a role in the dynamics of the neocortex were discussed in earlier chapters of this book. A good model is long-term potentiation (LTP). Figure 5.8 shows an example of new spine development induced with LTP. Such spine development along with branching of the dendrites is seen with enriched environments (Point of Interest Chapter 3 ). It is tempting to speculate that successful learning involves production of growth factor proteins that stimulate arborization of dendrites in the cortex, resulting in bigger, bushier neurons.
TREATMENT
STROKE
After a stroke, many patients will avoid using the affected limb and preferentially use the intact limb. Functional imaging studies of stroke patients have shown contraction of the cortical representation of the affected limb, which is how the brain responds when it is not used. A new kind of rehabilitation for stroke patients called constraint-induced movement therapy corrects this problem and improves function even in chronic conditions. Patients have their good limb restrained with a mitt or harness and are forced to use their impaired limb. Controlled trials have shown this treatment to improve motor skills as well as increase the cortical activity in the affected area. The brain has the capacity to change, but it must be exercised.
FIGURE 8.16  The circles are functional representations of the fingers from the right and left hands of a musician with focal dystonia of the left hand. The response from the somatosensory cortex is superimposed on a MRI. Note the fusion of the representations for the fingers from the affected left fingers that is not seen with the separated fingers from the right hand. The thumb—a digit not requiring as much movement when playing—is unaffected. (Adapted from Elbert T, Candia V, Altenmuller E, et al. Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport. 1998;9(16):3571-3575.)
The circles are functional representations of the fingers from the right and left hands of a musician with focal dystonia of the left hand. The response from the somatosensory cortex is superimposed on a MRI. Note the fusion of the representations for the fingers from the affected left fingers that is not seen with the separated fingers from the right hand. The thumb—a digit not requiring as much movement when playing—is unaffected. (Adapted from Elbert T, Candia V, Altenmuller E, et al. Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport. 1998;9(16):3571-3575.)
Adult Nerve Regeneration
Why is it that the nerves in the peripheral nervous system (PNS) can regenerate while those in the CNS cannot? Likewise, why can amphibians regrow long nerve connections, but mammals cannot? How come the most complex organ in the universe is less capable of repair than more primitive organs? It appears that the brain responds to injuries with actions that are intended to preserve the complex connections, but this ultimately also prevents regeneration.
Oligodendrocytes and astrocytes respond to CNS injury by forming what is called a glial scar. This scar is part of the inflammatory response and limits cellular damage by isolating and protecting the injured area. Unfortunately, the glial scar is full of molecules (not found in the PNS) that inhibit the growth cone and prevent regeneration. But more on this in the next chapter when we discuss immunity and inflammation.
1. Brain fertilizer
a. Apoptosis.
b. Critical period.
c. Focal dystonia.
d. Neurotrophins.
2. Programmed cell death
a. Apoptosis.
b. Critical period.
c. Focal dystonia.
d. Neurotrophins.
3. Maximum synaptogenesis
a. Apoptosis.
b. Critical period.
c. Focal dystonia.
d. Neurotrophins.
4. Inappropriate neurogenesis
a. Apoptosis.
b. Critical period.
c. Focal dystonia.
d. Neurotrophins.
5. Not one of the phases of development
a. Neurogenesis.
b. Synaptogenesis.
c. Neuroplasticity.
d. Pruning.
6. Factors that do not enhance CNS neurogenesis
a. Amputation.
b. Antidepressants.
c. Enriched environments.
d. Exercise.
See Answers section at the end of the book.