 |
|
Organic geochemistry is built on the application of organic chemical principles and analytical techniques to sedimentary geology. Carbon, nitrogen, oxygen, sulfur, hydrogen, calcium, and iron are the primary elements that living organisms utilize in their structural tissues, for energy harvesting, and for replication. Accumulation of organic matter in recent and ancient sediments is the most important link between the biosphere and geology. Not only are the organic materials in sedimentary rocks an economically important resource (coal and petroleum) but, as we shall see, they also provide a molecular record of life. The sedimentary burial of organic matter is also important to the global cycles of carbon, sulfur, and oxygen over geologic time.
In this chapter, we focus on understanding the production, degradation, and preservation of organic matter in sedimentary environments. We introduce the role of the carbon cycle in organic matter production, diagenesis, and preservation. Our focus will be on biochemically important compounds and the relationship of specific biomarkers to their precursor biota. We discuss mechanisms and factors that influence the accumulation of organic-rich sediments and evaluate biomarker distribution in recent sediments and ultimately in the sedimentary record. We close this chapter with a few examples illustrating how biomarkers are used to reconstruct paleoclimatic and paleoenvironmental conditions.
ORGANIC MATTER IN THE GLOBAL CARBON CYCLE
Organic geochemistry emerged in 1936, when Alfred Treibs discovered and described porphyrin pigments isolated from shale, oil, and coal. Treibs demonstrated that porphyrins originated from the degradation of chlorophyll, thereby linking the biochemicals in living organic matter to the ancient sedimentary record.
To understand the fate of organic matter in the biosphere and in sediments, it is useful to have an appreciation of how carbon is distributed among different geochemical environments and how it is transferred from one of them to another. We examine this topic at greater length in chapter 8, where we consider various ways to characterize environments and transfer processes for the purpose of creating analytical models of ocean chemistry. As you will see, there is no “best” way to model the global carbon cycle. Rather than begin that discussion now, let’s just consider the highly simplified model in figure 6.1, which is reminiscent of the party illustration we used in chapter 1. The inputs and outputs from the various reservoirs are roughly in balance, resulting in what can be considered a steady state system.
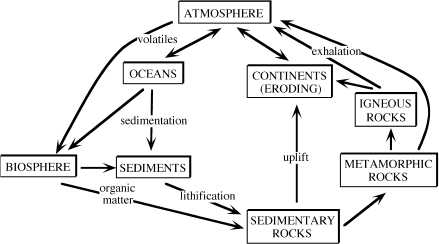
FIG. 6.1. Simplified version of the global carbon cycle indicating the principal reservoirs (boxes; see table 6.1 for amounts of carbon in most boxes) and pathways (arrows).
The Earth contains ∼1023 g of carbon, disseminated between sedimentary materials and active surficial reservoirs. Most of this carbon is sequestered in carbonate rocks (6.5 × 1022 g C) and organic materials, such as kerogen or coal (1.56 × 1022 g C) (Schlesinger 1997). The inventories show that in shales and other sedimentary rocks, essentially one out of every five carbon atoms is organic (table 6.1) and ∼90% of this preserved organic matter is amorphous kerogen, with the remaining 10% bitumen. Kerogen is insoluble, high molecular weight organic matter derived from algae and woody plant material that can yield petroleum products when heated. Bitumen is soluble in organic solvents and is formed from kerogen during petroleum generation.
Although a large fraction of the Earth’s organic and inorganic carbon is sequestered, only ∼0.1% (40 × 1018 g) of the carbon in the Earth’s upper crust is cycled throughout active surface reservoirs (table 6.1). The largest active reservoir is dissolved inorganic carbon (DIC) contained in the global ocean. Other inorganic reservoirs, such as soil carbonates and atmospheric carbon, and the organic reservoirs (soil humus, land plant tissue, dissolved organic carbon [DOC] in seawater, and carbon preserved in marine sediments) are one to two orders of magnitude smaller than the DIC reservoir.
Atmospheric carbon exists mainly as carbon dioxide, which is used by plants and other photosynthetic organisms, thereby linking the atmosphere with the biosphere and oceans (fig. 6.1). The ocean DIC reservoir moderates changes in atmospheric CO2 concentration and, at equilibrium, the oceans contain ∼56 times the carbon in the atmosphere.
TABLE 6.1. Major Reservoirs of Inorganic and Organic Carbon
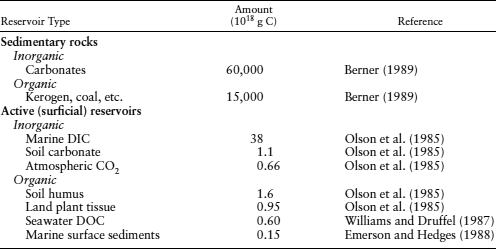
After Hedges and Keil (1995).
The organic reservoirs constitute only ∼8% of the total active surficial reservoirs and are typically composed of a complex mixture of heavily degraded substances (kerogen and bitumen), in which there are generally few readily identifiable biochemicals. The soil reservoir contains the largest concentration of organic and inorganic carbon in the terrestrial environment. Organic matter is almost totally recycled in terrestrial soils; therefore, terrestrial organic-rich deposits are typically confined to peat formations in bogs and low-lying swamps. Although the modern peat reservoir is small compared with organic-rich marine sediments, the presence of large coal deposits in the sedimentary record suggests that peat accumulation may have been an important process in the geologic past.
The amount of carbon in land plant tissue is similar to that contained in ocean waters and sediments. Together, these reservoirs contain approximately half as much carbon as is sequestered in soils. We have more to say about organic matter in soils in chapter 7, and we return to the topic later in this chapter, when we focus on diagenesis. For now, let’s turn our attention to the areas of the globe where most of the organic biomass is produced—the oceans.
ORGANIC MATTER PRODUCTION AND CYCLING IN THE OCEANS
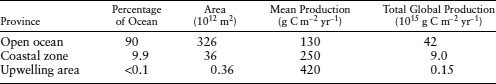
Organic matter is derived from the tissues of living organisms. Photosynthetic organisms (land and marine plants) capture sunlight energy, store it in organic compounds, and release the O2 that sustains other organisms. The organic matter produced by these organisms is almost completely utilized in the biosphere, but a small portion is preserved. Phytoplankton are the primary producers in the oceans, much as vegetation is in the terrestrial realm. Compared with the massive forests, marine phytoplankton are easily overlooked, owing to their small size and ephemeral nature. However, these organisms occupy almost 362 × 1012 m2 (Schlesinger 1997) of the Earth’s surface and consequently their presence in the open ocean accounts for almost half of the Earth’s photosynthesis (table 6.2). A simple version of the carbon cycle within the oceans is shown in figure 6.2.
Phytoplankton production takes place in the upper 100 m of the ocean, where enough sunlight and nutrients delivered by rivers are available for phytoplankton to reduce carbon dioxide to form carbohydrate while splitting a mole of water and releasing a mole of oxygen:
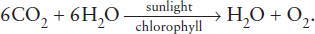
The most productive regions of the oceans are upwelling areas (table 6.2), where cold nutrient-rich bottom waters are brought to the surface and warm surface waters are pushed offshore by onshore winds. Coastal zones are also highly productive (table 6.2). Although the open ocean has the lowest mean production, its vast area is one to two orders of magnitude greater than the coastal and upwelling areas. Consequently, the open ocean constitutes 42% of the total global production, whereas coastal and upwelling zones make up only 9% and <1% of the global total, respectively. Oceanographers refer to the total mass of photosynthetic organisms at the ocean’s surface as primary production.
FATE OF PRIMARY PRODUCTION: DEGRADATION AND DIAGENESIS
Regardless of where the organic matter is produced—in large terrestrial forests, the soil reservoir, or lacustrine or marine environments—it is subject to degradation during deposition and to chemical changes after deposition (diagenesis), as we discussed in chapter 5. In the ocean water column, most marine primary production is consumed by animal plankton (zooplankton) and free-floating bacteria. The bacteria also decompose a large fraction of dissolved organic carbon and organic colloids produced by phytoplankton. Cole and coworkers (1988) found that net bacterial production is about twice that of zooplankton and accounts for the disappearance of 30% of primary production from the photic zone and, in some areas, as much as 70% of primary production may be degraded. Bacterial communities are important to the biogeochemical cycling of nutrients and organic matter in the oceans. The organic matter produced by phytoplankton may be consumed by zooplankton and eventually make its way up the food chain to higher trophic level organisms, such as fish. Alternatively, phytoplankton may be consumed by bacteria, which, in turn, may be consumed by bacteriovores. This process ultimately mineralizes nutrients and releases CO2 back to the surface waters. If the bacterial abundance is high, then a large fraction of the carbon fixed during photosynthesis is not passed to higher trophic levels. In chapter 8, we confirm that between 80 and 90% of the primary production is degraded to inorganic compounds (CO2, NO3, PO2, and the like) in surface waters and the remainder sinks below the euphotic zone (the depth to which light penetrates, where most primary production occurs) and into the deep ocean as particulate organic matter.
TABLE 6.2. Estimates of Marine Primary Production
After Hedges and Keil (1995).

FIG. 6.2. Schematic depicting the fate of organic matter in the oceans. Boxes represent organic reservoirs and arrows indicate pathways and processes.
Most of the organic matter sequestered in marine sediments is ultimately derived from organic matter synthesized by marine organisms inhabiting the surface waters of the oceans and transported to the seafloor (fig. 6.2). However, only a small fraction of the sinking organic matter survives transport to the seafloor to be preserved in the sediments. Extensive alteration of the organic matter in the water column can yield sedimentary organic matter having a chemical composition markedly different from that of the original material. Despite this alteration, a small fraction of particulate and dissolved organic material eventually reaches the sediment-water interface. There, the organic matter either undergoes further degradation by microbial communities or is preserved as kerogen and possibly petroleum if conditions are favorable.
FACTORS CONTROLLING ACCUMULATION AND PRESERVATION
In general, the preservation of organic materials depends on a complex interaction between the oxygen content of a system and the type of organic matter deposited within it (fig. 6.3). In the presence of oxygen, organic matter can be remineralized or converted to CO2. However, if this material is protected in some manner or is deposited in a suboxic or anoxic environment, it is likely to be preserved (fig. 6.3). Preservation is also enhanced in sediments underlying highly productive surface waters. In these environments, production of organic matter is much greater than its oxic degradation, so that much more organic matter reaches the sediments and is preserved. Although we have identified the key variables that influence organic matter preservation, you should realize that the mechanisms governing this process remain unclear. In this section, we address some of the mechanisms proposed to explain how organic matter is preserved in the sedimentary record.
Most organic matter in marine environments is concentrated in deltaic and continental shelf/slope sediments, whereas lower organic carbon (OC) concentrations are associated with high productivity areas and anoxic basins (table 6.3). Despite differences in OC concentration and key variables that influence organic matter preservation, a common mechanism appears to control organic matter preservation in these environments. More than 90% of the sedimentary OC from marine environments cannot be physically removed from its mineral matrix (Hedges and Keil 1995). Consequently, organic matter must be sorbed to mineral surfaces. The concentration of sorbed organic matter covaries with mineral surface area, such that more OC is associated with sediments that have highly irregular surfaces with small pores (Mayer 1994). Sorption of organic matter forms protective coatings that typically approach a single molecule-thick covering (often expressed as a monolayer equivalent, ∼0.5−1.0 mg OC m−2; Hedges and Keil 1995). These coatings contain both refractory (not easily degraded) and labile (easily degraded) organic matter that is preserved in the underlying anoxic sediments, because it is protected from mineralization during transport through the water column and oxygenated surface sediments. Formation of monolayer equivalents of organic matter on marine sediments implies that, at one time, the organic matter was in the dissolved phase, because it is unlikely that particulate organic matter would spread uniformly over mineral surfaces.
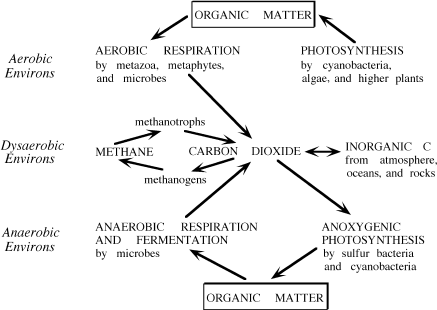
FIG. 6.3. The fate of organic matter in different redox environments. Arrows show pathways along which organic matter is produced or altered to various byproducts by different processes.
TABLE 6.3. Organic Carbon Preservation in Various Marine Environments
| Sediment Type | Organic Content (1012 g Cyr−1) |
| Deltaic sediments | 70 |
| Shelves and upper slopes | 68 |
| High-productivity slope and pelagic zone | 10 |
| Shallow-water shelf carbonates | 6 |
| Low-productivity pelagic sediments | 5 |
| Anoxic basins (e.g., Black Sea) | 1 |
From Hedges and Keil (1995).
Surface area is the primary control on organic preservation within continental shelf and slope environments, despite differences between these settings in primary production, bottom water oxygen concentration, sediment accumulation rate, and water depth (Keil et al. 1994; Mayer 1994). This control plays an important role in global organic matter preservation, because continental shelf and slope environments account for ∼45% of the Earth’s carbon burial (table 6.3).
Organic matter sorption is also the key mechanism in deltaic sediments, which account for another 45% of the total preserved OC, but the coatings on deltaic sediments are typically less than monolayer equivalents. Some of the sorbed OC to river sediments is removed when the sediments are deposited in a marine environment. The exact mechanism that causes this loss of coatings is unclear, but it may be related to desorption by seawater or direct oxidation (discussed later) on the mineral surface during sediment transport to the marine environment.
Sediments underlying highly productive O2-poor waters are typically enriched in organic matter, but these marine environments are rare and constitute only ∼5% of the global organic preservation. Monolayer equivalent sorption cannot explain the high organic matter concentrations (>5%) observed in these sediments. Less than 15% of the OC in these sediments can be separated from the mineral matrix, so these mineral surfaces must have coatings equivalent to several monolayers.
Many factors may lead to the formation of these thicker coatings. Among these are elevated DOC in sediment porewaters, condensation reactions, the presence of sulfides, or very brief O2 exposure times. Elevated DOC concentrations may lead to increased organic sorption to the mineral surfaces. DOC may also form organic-bearing sulfides that are resistant to degradation. Degraded biomolecules may combine to form complex high-molecular-weight substances (condensation reactions). The coating that these substances produce is most likely resistant to microbial degradation and strongly bound to the mineral surface. In addition, the coating may promote further condensation reactions, thereby protecting the organic matter sorbed to the mineral surface. Rapid sedimentation rates in highly productive areas may enhance preservation, as the O2 exposure time may be limited. This limited exposure may permit the preservation of labile organic matter and enhance the formation of resistant substances through condensation.
Degradation in Oxic Environments
Organic matter preservation in deep-sea sediments is typically poor (table 6.3), which suggests that some degradation mechanism may overcome surface area protection. Oceanic sediments have coatings with less mass than monolayer-equivalents, potentially formed from direct oxidation during transport. Slow accumulation rates and oxygenated waters in deep-sea environments give rise to long O2 exposure times and may inhibit organic preservation.
The most direct evidence of this oxygen degradation is seen in “oxidation fronts” in deep-sea turbidites, where slumping exposes sedimentary organic matter to O2-rich bottom waters for long periods of time. Molecular O2 reacts with OC and reduced minerals along sharp redox fronts, which can reduce the organic matter content by up to 75%. Oxidation continues until the deposit is covered by either another turbidite flow or by gradual pelagic sedimentation (Hedges and Keil 1995).
We have seen how organic matter is produced and where it is likely to accumulate and be preserved. Now we examine the transformations that organic matter undergo during diagenesis, as described in chapter 5. Microbial communities and other biologic agents primarily control diagenetic transformations, although some chemical transformations catalyzed by mineral surfaces do occur.
During diagenesis, sediments undergo compaction and consolidation, with a simultaneous decrease in water content and increase in temperature. Biologic alteration of OC eventually ceases as temperatures increase (>50°C) during the later stages of burial, a process called catagenesis. The boundary between diagenesis and catagenesis is not well defined but it essentially coincides with the onset of oil formation.
As we have seen, degradation of OC begins in the water column and continues after sedimentation (fig. 6.2). Different compounds in the organic matter degrade at different rates and only some compounds survive in a recognizable form. We examine this aspect of degradation more closely in the next section. During diagenesis, residual organic matter from microbial degradation undergoes condensation to yield macromolecules of insoluble brown organic material. The result of diagenesis is a condensed organic residue, or geopolymer, which contains varying amounts of largely unaltered refractory organic material. In soil environments, diagenesis yields humin, brown coal (or lignite) in coal-forming swamps, and kerogen in marine and deep lacustrine sediments.
Humic substances are found in soils, terrestrial and marine sediments, coal deposits, and all aquatic environments. Humics can account for almost all the organic carbon in freshwater environments, giving waters a brown color or possibly a green tint. Humin in soils is derived from terrestrial plants that decompose, forming OC that condenses during diagenesis to form the insoluble humic residue.
Coals may also form during diagenesis of organic matter, and the type of coal formed typically reflects its precursor plant material. For example, humic coals are formed mainly from the woody tissues of vascular plant remains. These coals are typically stratified, have a lustrous, black/dark brown appearance, and go through a peat-forming stage. Sapropelic coals, on the other hand, are not stratified and are dull in appearance. They form in quiet, anoxic shallow waters from fine-grained, organic-rich sediments that contain varying amounts of algal remains and degraded peat swamp plants. Unlike humic coals, formation of sapropelic coals does not typically involve a peat-forming stage. Instead, these coals follow a pathway similar to kerogens, which we discuss next.
Coals typically form as a result of two main processes: peatification, dominated largely by biologic activity, followed by coalification, in which heat and pressure are the most important agents of change. Peatification and early coalification are equivalent to diagenesis. Late-stage coalification, which is essentially catagenesis, parallels the onset of metamorphism. The boundary between the early and late stages of coalification is not sharp, because the actions of the biologic and physicochemical agents may overlap. The principal stages of coal formation begin with peat formation followed by the formation brown coal, bituminous coal, and eventually anthracite.
Kerogen is the highly complex organic material from which hydrocarbons are produced as the material is subject to increasing burial and heating. It is by far the most abundant form of organic carbon in the Earth’s crust and it occurs primarily in sedimentary rocks as finely disseminated organic macerals (chunks of organic matter, analogous to minerals in rocks). Until recently, kerogen was thought to form during the late stages of diagenesis from the alteration of huminlike material. This material was supposedly derived from the condensation of insoluble geopolymers, which formed from microbial degradation of organic matter. Kerogen is now thought to form during the early stages of diagenesis from mixtures of partially altered, refractory biomolecules (Tregelaar et al. 1989).
Kerogen is modified by temperature ultimately to yield petroleum hydrocarbons. The potential yield of petroleum products typically depends on the type of kerogen. There are four types of kerogens distinguished by their maceral groups: lignite, exinite, vitrinite, and inertinite. Type I kerogens (lignite), which are derived from algal or bacterial remains, are relatively rare but have a high oil potential. These materials formed in fine-grained, organic-rich muds deposited under anoxic conditions in quiet, shallow water environments, such as lagoons and lakes. Type II kerogens (exinite) are the most common and are usually formed in marine environments from mixtures of phytoplankton, zooplankton, and microbial organic matter under reducing conditions, but they can also be formed from higher plant debris. Type II kerogen has a lower yield of hydrocarbons than Type I but it has still produced oil shales of commercial value and sourced a large number of oil and gas fields (Killops and Killops 1993). Type III kerogen (vitrinite) is derived from vascular plants and may contain identifiable woody plant debris. Vitrinite is usually not extensively altered by microbial degradation, which may be a result of the material’s rapid sedimentation and burial. Vitrinite generally occurs as coals or coaly shales; therefore, it is similar to coal in terms of its composition and behavior with increasing burial. Type IV kerogen (inertinite) is composed of primarily black opaque debris potentially formed from the deposition of highly oxidized, higher plant material. It has no hydrocarbon generating potential and is sometimes not considered a true kerogen.
A CRASH COURSE IN ORGANIC NOMENCLATURE
You need some knowledge of organic chemistry to understand the principal aims, methods, and results of organic geochemical investigations, so let’s briefly examine some geochemically important structures and outline their nomenclature.
Organic compounds consist principally of carbon atoms linked to nitrogen, oxygen, sulfur, and other carbon atoms. The basic carbon skeleton can be arranged in simple straight chains, branched chains, one or more rings, or combinations of these structures. The simplest organic compounds are hydrocarbons, which consist solely of carbon and hydrogen atoms. Carbon atoms have four valence electrons that must be satisfied by either single covalent bonds (see chapter 2) to four separate atoms or some combination of single and double bonds. A double bond is one that involves two valence electrons. Hydrocarbons that have only single bonds are saturated, whereas those that contain double bonds are referred to as unsaturated.
The carbon atoms can be linked together to form either straight-chain (aliphatic) or simple cyclical (alicyclic) structures. Saturated aliphatic hydrocarbons are alkanes, where the suffix -ane refers to the lack of any double bonds in the structure. An unsaturated aliphatic hydrocarbon is called an alkene, where the suffix -ene denotes a double bond. Alkanes (and alkenes) are named according to the number of carbon atoms in the chain: methane (1), ethane (2), propane (3), butane (4), pentane (5), hexane (6), heptane (7), octane (8), and so on. These compounds are typically represented by two-dimensional (“sawtooth”) drawings, in which the kink at each line segment represents a carbon atom. Leftover valence electrons (that is, ones not involved in bonding carbon atoms to each other) attach hydrogen atoms to the structure. For example, figure 6.4a shows dodecane, a straight chain, aliphatic hydrocarbon with 12 carbon atoms and no double bonds. It is understood that two hydrogen atoms attach to the carbon atom at each kink in the chain, and three attach at each end of the chain. The compound shown in figure 6.4b, dodecene, is essentially the same compound shown in figure 6.4a with one double bond. The carbon atoms at each end of the double bond would have only one attached hydrogen atom.
Stable configurations of carbon atoms also occur where double (C=C) bonds alternate with single (C—C) bonds to form a pattern like ∼C—C=C—C=C—C=C∼. This configuration, referred to as conjugated, is common in polyunsaturated compounds. If conjugation occurs in a ring structure with three double and three single bonds (fig. 6.4c), the configuration is aromatic. Aromatic compounds are unsaturated and are typically very stable.
The groups of elements bonded to the carbon backbone (whether chain or ring) are called functional groups, because they are usually reactive and thus influence the chemical behavior of the compound. Functional groups involve combinations of hydrogen atoms with one or more atoms of another element, most commonly oxygen, nitrogen, or sulfur. Some of these, like hydroxyl (–OH), are familiar from inorganic chemistry. Alcohols and phenols are based on combinations of −OH with aliphatic or aromatic groups, respectively (see the phenol in fig. 6.4d). Carbohydrates, such as sugars and starches, are a major class of aliphatic compounds with a large number of hydroxyl groups in place of hydrogen atoms. Organic compounds are often grouped under the name of the functional group that they contain. For example, carboxylic acids are compounds that bear a carboxyl group. Some geochemically important functional groups are listed in table 6.4.
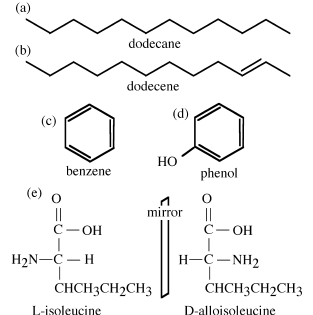
FIG. 6.4. Structural representations of some organic molecules. (a) Dodecane; (b) dodecene; (c) benzene; (d) phenol; (e) L- and D-enantiomers of isoleucine.
TABLE 6.4. Some Functional Groups in Organic Molecules
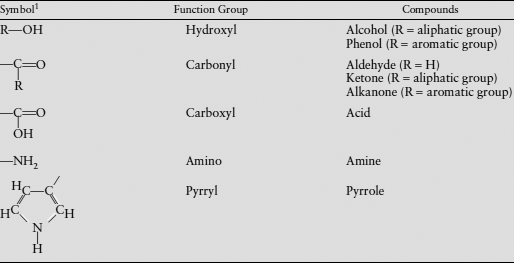
1R denotes groups of atoms that may be joined to the function group.
Although we typically represent organic compounds with two-dimensional drawings, these compounds actually have three-dimensional structures. Different spatial configurations of atoms attached to a central carbon atom can exist. This phenomenon is called stereoisomerism. Several compound classes exhibit this property, in which two structurally identical compounds exist as mirror images that cannot be superimposed. Your hands provide a straightforward illustration of this property: each hand has the same structure of four fingers and a thumb, but you cannot superimpose them (palms facing up). Similarly, certain amino acids and carbohydrates have identical structures but differ by the placement of their functional groups on one side of the central carbon, called the chiral carbon. These mirror images, called enantiomers, have similar physical properties but rotate light in different directions. Compounds that rotate light clockwise are called dextrorotatory and are denoted by the prefix D-, whereas counter-clockwise rotation is referred to as levorotatory and denoted by the prefix L-. For example, the amino acid isoleucine can exist in either L-or D-forms (fig. 6.4e). The only difference between these two compounds is the placement of an amino group, which is on opposite sides of the central carbon atoms. (As you can see, isoleucine has two chiral carbon atoms, which is a special case. When the L-form of this compound changes to its D-form, this process is called epimerization and the D-form is referred to as alloisoleucine. You will encounter an example of this in worked problem 6.3.)
CHEMICAL COMPOSITION OF BIOLOGIC PRECURSORS
Despite all the potential changes that may alter organic matter along various pathways from production to preservation, some organic compounds still reflect their original source materials, whether those are higher plants, marine or lacustrine algae, or bacteria. It is important to understand the chemical composition of these sources so that we can relate these compounds to those preserved in the sedimentary record.
All organisms are composed of compounds derived from carbohydrates, proteins, and lipids. In addition, significant amounts of lignin (structural tissues) are present in higher plants. Let’s now review the composition of the main chemical classes of compounds and their biochemical functions in living organisms. The structural representations and important functional groups for these compounds are discussed in an accompanying box.
The carbohydrates are aliphatic compounds containing only carbon, hydrogen, and oxygen, with a ratio of hydrogen to oxygen similar to water. Their general formula is represented by (Cn(H2O)n). Organisms use carbohydrates as food reserves and structural materials. Polysaccharides (many saccharide units) are major components of cell membranes in plants, bacteria, and fungi.
Cellulose, the main structural unit in higher plants, is the most common carbohydrate. Cellulose is composed of roughly 10,000 glucose (C6 monosaccharide) units that are, in essence, energy reserves. This energy is stored as starch, which is a component of the cellulose. Energy is derived from this reserve by breaking down the polysaccharides into individual glucose units.
Hemicellulose compounds are the next most abundant group of carbohydrates. These compounds are complex mixtures of polysaccharides containing between 50 and 2000 monosaccharide units that form a matrix around the cellulose fibers in plant cell walls. These compounds are present in nonwoody tissues and fruits but are only a minor component in woody tissues.
Whereas cellulose is the dominant structural material in plants, the polysaccharide chitin is the principal structural compounds in most fungi, some algae, mollusks, and arthropods (insects and crustaceans). This compound is composed of chains of glucose units with an attached amino (NH2) functional group. Eubacterial cell walls contain murein, another polysaccharide. It is possible to distinguish some eubacteria based on the compounds that are contained on the exterior of their cell walls.
Similarly, other carbohydrates (specifically enantiomers; see accompanying box) may be characteristic of, but not necessarily exclusive to, different organisms. Fungal polysaccharides are predominantly D-glucose, D-galactose, and D-mannose, whereas marine algae such as Chlorophyta contain a large proportion of L-rhamanose, and Phaeophyta are rich in D-ribose. Freshwater algae and higher aquatic plants, however, contain significant amounts of L-arabinose and D-xylose (Killops and Killops 1993).
Proteins, which consist of linked amino acids, are the principal storage sites for nitrogen in organisms. The amino acids contain an amino group (NH2) and a carboxyl group (COOH) that can bond through the elimination of water to form peptide linkages. Structurally, all amino acids are chiral molecules with the exception of glycine, which has two hydrogen atoms bonded to the chiral carbon atom. There are only 20 amino acids involved in protein synthesis and the L-configuration of these compounds is utilized exclusively, owing to the stereospecificity of enzymes in all organisms except bacteria. The predominant use of D-amino acids by bacteria may help identify the source of these compounds in complex mixtures, provided that diagenesis has not occurred.
Plants are able to synthesize all the amino acids necessary for biomass production, and most amino acids are derived from glutamic acid. Animals, on the other hand, are not able to synthesize all the amino acids required for protein synthesis; therefore, their essential amino acids are supplied by eating plants.
Proteins typically constitute a substantial portion of the bulk OC in an organism. Owing to their ability to form fibers, proteins provide supportive tissues such as skin and bone (collagen) or hooves and claws (keratin) in animals. The function of these protein-derived structural materials is analogous to that of cellulose and lignin materials in plants.
Lipids are those compounds that are insoluble in water but are extractable in solvents known to dissolve fats (such as chloroform, hexane, toluene, and acetone). Most organic geochemists use this broad definition of lipids, because it encompasses all the compound classes of geologic importance. The most abundant and geochemically important lipids (such as those that are principal components of oil source rocks) include the following groups.
Glycerides are esters of the alcohol glycerol that can bond with one, two, or three carboxylic acids (-COOH) to form mono-, di-, and triglycerides, respectively. Among the triglycerides, fats are important compounds.
Fats are composed of fatty acids, which are straight-chain carboxylic acids (RCOOH) that typically contain between 12 and 36 carbon atoms. Saturated fatty acids are found in animals, whereas unsaturated and polyunsaturated fatty acids are common in plants. Unsaturated acids have a lower melting point for a given chain length, which is why some unsaturated plant-derived fats are oils whereas saturated animal fats are solids. The C16 and C18 saturated fatty acids are most abundant in animals, whereas the C18 mono-, di-, and triunsaturated fatty acids are the dominant forms in plant tissues. Polyunsaturated fatty acids are more common in algae than in higher plants. Fatty acids are predominantly even-numbered carbon compounds, because their biosynthetic pathway incorporates two carbon atoms (acetyl units) that are derived from glucose. Similar to carbohydrates, fats are used as an energy reserve by animals and plants, but the principal difference is that fats provide twice the energy as carbohydrates during oxidation. This is particularly useful during processes that require a substantial amount of energy, such as seed and fruit generation.
Waxes serve mostly to protect organism membranes, as in, for example, the waxes that coat plant leaves. These biochemicals are mixtures of fatty acid esters and fatty alcohols, whose carbon chains range from C24 to C28. Waxes are mostly even-carbon–numbered owing to their synthesis from fatty acids (also even-carbon numbered). Hydrocarbons, principally long-chain n-alkanes, are also a major constituent of waxes, but these compounds contain predominantly odd numbers of carbon atoms in the range of C23 to C33 with the majority of these being C27, C29 and C31. The odd-number preference arises because n-alkanes are formed from an aliphatic acid (CH3(CH2)nCH2COOH) by the elimination of the carboxyl group (COOH), which contains one carbon atom. Higher plants and fungi contain a similar distribution of n-alkanes, whereas these compounds are almost absent in most bacteria.
Terpenoids are a class of lipids whose structures and functions vary markedly from the basic building block of chlorophyll and gums of higher plants to volatile sex pheromones. All the different terpenoids, however, have a similar structural component made of five carbon atoms called an isoprene unit. Terpenoids are classified by the number of constituent isoprene units.
Monoterpenoids consist of two isoprene units (hence, they are C10 compounds) and some are highly volatile. They are usually found in algae and constitute the essential oils of higher plants. These oils, like citronella or menthol in peppermint, give the plant a specific odor. Due to their volatility, some of these compounds, such as insect pheromones, act as attractants. Others, such as chrysanthemic acid in pyrethrum flower heads, are natural insecticides.
Sesquiterpenoids (C15 compounds), formed from three isoprene units, function not only as the essential oils of higher plants but also as fungal antibiotics. Farnesol is a common acyclic compound found in many plants and in the chlorophyll of some bacteria.
Diterpenoids (C20 compounds) contain four isoprene units and form resins, which seal damaged tissues with protective coatings and inhibit insect and animal attack. Other compounds in this class cause some bitter taste in plants, which can also act as a protective mechanism.
Triterpenoids (C30) contain six isoprene units and are believed to develop from squalene, which is a ubiquitous 30-carbon compound in many organisms. Most of the triterpenoids are either tetracyclic (four rings) or pentacyclic (five rings). The pentacyclic triterpenoids typically constitute the resins of higher plants, but another group of these compounds (hopanoids) is found in bacteria. In addition, other triterpenoids have been identified as the precursors to certain petroleum hydrocarbons. Although you may be not familiar with the majority of these compounds, you probably recognize the tetracyclic triterpenoids known as steroids (or sterols). These compounds contain four rings in their structure, which result from the oxidation of squalene followed by cyclization (ring formation). This reaction produces two different compounds that are the precursors to all plant (cycloartenol) and animal (lanosterol) sterols. Oxidation of lanosterol produces cholesterol (C27 compound), which is the precursor to all other animal steroids. The majority of the sterols that are found in the geologic record range between C27 and C30. Sterols are rare in bacteria, but hopanoids are abundant, which may provide a means to distinguish the source of organic matter in sedimentary environments.
Tetraterpenoids (C40) consist of eight isoprene units. The most important group of these compounds is the carotenoid pigments. Carotenoids are essential for the production of vitamin A and are found most organisms. Carotenoid pigments are responsible for the red coloration in “red tide” blooms of dinoflagellates. Carotenes and xanthophylls (from the Greek for contain oxygen) are highly unsaturated and can absorb relatively short wavelengths. These compounds are major photosynthetic pigments that aid in the capture of sunlight energy and transfer this energy to chlorophyll. Certain carotenoids are characteristic of different photosynthetic organisms.
Tetrapyrrole pigments consist of four pyrrole units (a five-member ring structure, in which four of the atoms are carbon and the fifth member is a NH group linked by a double-bonded CH group). These structures can either be a ring or large open chain. The pigments are involved in photosynthesis as either primary or accessory pigments. Chlorophylls are members of this group that have a large ring structure called a porphyrin. Chlorophyll is found in all algae, higher plants, and cyanobacteria.
Lignin, characterized by phenolic structures (aromatic six-member carbon rings bonded to OH groups, illustrated in fig. 6.4d), is a major, unique component of the structural tissues of higher plants. These compounds are derived from monosaccharides. Lignin forms around the fibrous woody core (xylem) of terrestrial plants and acts as a support structure as the plant grows. Cellulose constitutes up to 40–60% of wood and lignin essentially makes up the remainder. This high-molecular-weight material has a highly complex structure consisting of many different types of alcohols that undergo condensation reactions to ultimately form lignin.
Now that we are familiar with some of the biochemically important compounds, we can use these as biomarkers to gain insight into the types of organisms that contribute to the total organic matter in sedimentary environments. Biomarkers survive diagenesis almost intact or their diagenetic pathways can be traced, whereas other organic compounds are altered and may not necessarily reflect their original source. Here we investigate the role of biomarkers as source indicators in sedimentary environments, using lignin and certain lipids as examples.
There are many differences in the biochemical compositions of the principal groups of organisms. For example, the presence of lignin in sediments may indicate a higher plant contribution to the total organic matter of a sedimentary environment, because, as we have seen, lignin is present only in higher plants. We have also seen that higher plants contain protective waxes, in which even-carbon-number fatty acids predominate, on their leaves. The predominance of even-over odd-carbon-number fatty acids in organic residue, then, is a further biomarker for higher plants. Hydrocarbons (n-alkanes) are also a major constituent of the waxes, but these compounds contain predominantly odd numbers of carbon atoms, so an odd-over-even predominance of n-alkanes also suggests input from higher plants.
Compounds derived from the steroid class can also be indicative of certain groups of organisms. Phytoplankton primarily consist of abundant C28 sterols, whereas zooplankton typically contain C27 sterols, particularly cholesterol. In contrast, major plants are dominated by the C29 sterols, and C27–C29 sterols are typically associated with fungi.
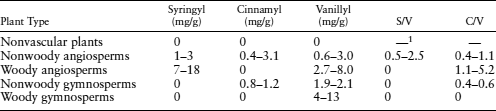
Lignin and its associated phenolic units can be used to differentiate among the types of vascular plants that contribute organic matter in complex sedimentary environments. To do this in the laboratory, a geochemist liberates the phenolic units from the lignin structure through an oxidation process. Oxidation produces three groups of structurally related compound groups, vanillyl (V), syringyl (S), and cinnamyl (C), which are diagnostic of lignin. These compound groups are absent from nonvascular plants and vary according to vascular plant type (table 6.5), which makes source determination possible. For example, nonwoody angiosperms can be distinguished from the other three groups of vascular plants based on their S/V ratios, and woody angiosperms have a distinct C/V ratio. Another approach is to plot the S/V ratios versus C/V ratios, which can also provide a means to differentiate these sources. Let’s see how this works with a real example.
TABLE 6.5. Distribution of Lignin Oxidation Products in Various Plant Tissues
1—, not applicable.
Worked Problem 6.1
The syringyl, cinnamyl, and vanillyl concentrations have been measured at different depths within a lacustrine sediment core. The calculated S/V and C/V ratios are shown below. How can we determine the source of organic matter that has accumulated in this lake basin over time?
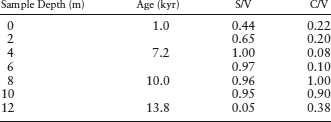
We can compare the S/V and C/V values with table 6.5 to determine planet sources for each core sample and investigate any vegetation changes. The basal portion of the core has low S/V and C/V ratios, which suggests input from nonwoody gymnosperms, such as ferns. Between 8 and 10 m depth, both the S/V and C/V ratios are high, reflecting a change to nonwoody angiosperms (marsh or grassland plants) and possibly a tundra landscape. High S/V and low C/V ratios between 4 and 6 m indicate input from woody angiosperms, such as deciduous trees, making another vegetation change to a boreal biome. A decrease in S/V ratios from 4 m to the top of the core with relatively constant C/V ratios suggests a progressive enrichment in woody gymnosperms (conifers), mostly likely at the expense of the woody angiosperms (deciduous trees). Changes in the lignin parameters (S/V and C/V) thus indicate that climate changed from a fern-dominated landscape 13 kyr ago to tundra by 10 kyr. Climate change forced the tundra to give way to boreal forest by 7 kyr, which eventually gave way to a pine forest.
APPLICATION OF BIOMARKERS TO PALEOENVIRONMENTAL RECONSTRUCTIONS
Biomarkers can also be used to quantify specific environmental conditions. For example, the distributions of some biomarkers are sensitive to temperature (because of the metabolic pathways involved in their synthesis) and time (because of the kinetics of their reactions).
One family of temperature-sensitive lipids is the (C37−C39) unsaturated ketones or alkenones. These compounds have a structure similar to long chain alkenes (remember, the -ene suffix denotes double bonds) and can have between two and four double bonds. The number of double bonds is a function of temperature. A higher proportion of unsaturated compounds is associated with colder temperatures. For C37:3 and C37:4, 37 indicates the number of carbon atoms and 3 or 4 is the number of double bonds, respectively. These unsaturated alkenones are produced by the coccolithophore algae Emiliani huxleyii, which first appeared in the geologic record during the late Pleistocene approximately 250,000 years ago (Killops and Killops 1993). By culturing these organisms at various temperatures, Simon Brassell and coworkers (1986) established a linear relationship between the degree of unsaturation and growth temperature. This relationship is known as the 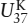 index:
index:
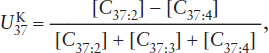
where the square brackets are the concentrations of the di-, tri-, and tetraunsaturated ketones. The di- and tri-unsaturated compounds are dominant in most sediments, so this index can be simplified to:
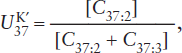
Values of 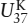 can be related to the growth temperature and hence sea surface temperature (SST) in the case of marine sediments through the following relationship (Prahl et al. 1988):
can be related to the growth temperature and hence sea surface temperature (SST) in the case of marine sediments through the following relationship (Prahl et al. 1988):
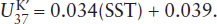
Equation 6.3 holds well for most oceans, but there are some, such as the Southern Ocean, for which a slightly different temperature calibration has been developed. Regardless of the calibration, the 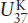 index appears to provide reasonable temperatures in the modern oceans and consequently has been used to determine SST on glacial/interglacial time scales.
index appears to provide reasonable temperatures in the modern oceans and consequently has been used to determine SST on glacial/interglacial time scales.
Worked Problem 6.2
How can we determine changes in SST in the Pacific Ocean over glacial/interglacial time scales at midlatitudes and in the tropics? Oceanographers have recovered ocean sediment cores that span the past 50,000 years, and we have determined the 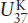 values from the concentrations of the di- and tri-unsaturated C37 alkenones for several radiocarbon dated samples. The
values from the concentrations of the di- and tri-unsaturated C37 alkenones for several radiocarbon dated samples. The 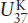 values for the tropical core for the last glacial maximum and the Holocene are 0.925 and 0.99, respectively. In the midlatitudes, the last glacial maximum and Holocene
values for the tropical core for the last glacial maximum and the Holocene are 0.925 and 0.99, respectively. In the midlatitudes, the last glacial maximum and Holocene 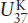 values are significantly smaller at 0.53 and 0.65, respectively.
values are significantly smaller at 0.53 and 0.65, respectively.
Using equation 6.3, the SST during the last glacial maximum in the tropical Pacific Ocean was:
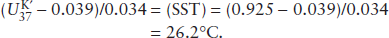
and the Holocene temperature was:
(SST) = (0.99 − 0.039)/0.034 = 28.0°C.
This corresponds to a 1.8°C temperature increase from the last glacial maximum, which is consistent with other ways of estimating SST. If we examine the temperature changes in the midlatitudes, we see that during the last glacial period,
(SST) = (0.53 − 0.039)/0.034 = 14.4°C,
and Holocene temperatures were on the order of:
(SST) = (0.65 − 0.039)/0.034 = 18.0°C,
which is ∼4°C warmer. This is also consistent with other proxy records that suggest that midlatitude glacial oceans were much colder than tropical oceans and that the glacial/Holocene temperature rise was more pronounced in the midlatitudes.
We noted earlier that all living organisms (except bacteria) manufacture L-amino acids, rather than D-amino acids. The chiral (L- versus D-) configuration of amino acids is sensitive to both time and temperature. L-amino acids convert or racemize (for one chiral carbon atom and epimerize for two chiral carbon atoms) to their D-amino acid equivalents over time after the proteins are formed and effectively protected from the biologic processes of the organism. The change from L- to D-amino acids continues until an equal mixture of the two forms exists.
Bacteria preferentially produce D-amino acids. These compounds are nearly ubiquitous in the biosphere and can come into contact with a potential sample of preserved nonbacterial material, thereby introducing amino acids not indigenous to the sample. To reduce the risk of contamination, most organic geochemists prefer to sample materials that are least likely to have been accessible to bacteria. They sample shells of marine organisms (mollusks and foraminifera), land snails, and ostrich eggs, because the amino acids in these materials are protected from the surrounding environment owing to a layer of calcium carbonate deposited over protein layers in the shell.
Reliable ages and temperatures can be calculated from the D/L values of a sample provided that a reasonable kinetic model is established to quantify the relationships among time, D/L ratios, and temperature. This is typically accomplished through artificial heating experiments of age-dated fossil shells. The relationship between the age of a sample and measured D/L value is expressed as (McCoy 1987):
t = ln([1 + D/L]/[1−K′D/L]) − ln([1 + D0/L0]/[1−K′D0/L0])/(1+K′)Ae−Ea/RT,
where t is the age of the sample, D/L is the ratio of D to L enantiomers, K′ is 1/Keq (equilibrium constant ∼0.77 for alloisoleucine/isoleucine [A/I]), D0/L0 is the D/L ratio of the sample at t = 0 (∼0.01, owing to a small amount of racemization during sample preparation), Ea (the activation energy in J mol−1), and A (the entropy factor) are the Arrhenius parameters of the racemization reaction, which are determined experimentally. R is the gas constant (1.9872 cal K−1 mol−1), and T is the effective diagenetic temperature in K.
Application of this equation to generate numerical age estimates is limited by the temperature dependence of the racemization reaction and the variable temperature history of many sample sites. This variability leads to imprecise and potentially inaccurate age dates. However, if samples are collected from different environments with similar postdepositional temperature histories, then differences in D/L ratios can be used to identify relative age differences between samples. This approach is known as aminostratigraphy.
Age estimates may also be made from an empirical relationship between the measured D/L ratios and the sample age. The D/L ratios of independently dated samples can be used to calibrate ages of other samples on the basis of their D/L ratios, provided that all the samples have had similar postdepositional temperature histories.
By solving equation 6.4 for temperature (T), we can estimate the effective diagenetic temperature of a sample terms of its age, measured D/L values, and the Arrhenius parameters (McCoy 1987):
T = −Ea/R ln(ln{([1 + D/L]/[1 − K′(D/L]) − ([1 + D/L]/[1 − K′D/L])}/At[1 + k′]).
This equation can be simplified for an interval of time bracketed by two independently dated samples to:
T(t2−t1) = Ea/R ln(A/k),
where
| k | = (k2t2 − k1t1)/(t2 − t1) |
| = ln(c/b)/[(1 − K′)(t2 − t1)], (6.7) |
in which b = (1 + D1/L1)/(1 − K′D1/L1) and c = (1 + D2/L2)/(1 − K’D2/L2). D1/L1 and D2/L2 refer to the D/L ratios for the younger and older samples, respectively. The numerical ages of the younger and older samples are represented by t1 and t2, respectively.
Worked Problem 6.3
How can we determine the effective diagenetic temperature of fossil shells deposited at different times following the last glacial maximum? Eric Oches and coworkers (1996) collected fossil gastropod shells from silt in the Mississippi Valley and measured their D/L ratios. They also determined the Arrhenius parameters (A = 1.658 × 1017, K′ for alle/ile, and Ea = 29,235 cal mol−1), measured the equilibrium constant for isoleucine epimerization (K′ = 0.77), and obtained radiocarbon dates for each sample.
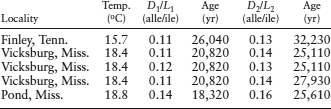
The values of b and c in equation 6.7, for the Finley, Tenn., samples, are therefore:
b = (1 + 0.11)/(1 − [0.77 × 0.11]) = 1.21,
c = (1 + 0.13)/(1 − [0.77 × 0.13]) = 1.25.
Substituting these values into equation 6.7, we can determine the value of k:
| k | = ln(1.25/1.21)/[(1 + 0.77)(32,320 − 26,040)] |
| = 3.18 × 10−6. |
Using this value in equation 6.6, we can find T:
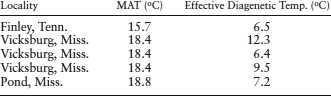
T = (29,235 cal mol−1)/(1.9872 cal K−1 mol−1)ln(1.658 × 1017/3.18 × 10−6) = 279.7 K = 6.5°C.
Determining the temperatures for all sample sites using this procedure (see below), we find that temperatures between 20,000 and 30,000 years ago were much cooler than the mean annual temperature (MAT) for all locations. These results agree with the trend in midlatitude sea-surface temperatures that we calculated in worked problem 6.2. These temperatures were much cooler during the last glacial maximum than at present.
Although the field of organic geochemistry has its origins in the study of petroleum, here we have concentrated on the application of organic geochemistry to paleoenvironmental and paleoclimatic reconstructions. We have examined the origin of organic matter and how it is cycled within the geosphere. The organic carbon reservoir is only a small fraction of the total global carbon content and represents only 8% of the active surface reservoirs. Despite its relatively small size, this reservoir is intricately linked to the biosphere and geosphere.
We showed that the oceans are the primary source of organic carbon in the sedimentary record and discussed how organic matter is transformed and altered during deposition and diagenesis. Most of the organic matter produced in surface waters is completely recycled within the water column and only a very small fraction gets preserved in sediments. Further alteration of this material occurs during diagenesis, owing to microbial degradation of labile organic matter. Degradation is typically followed by condensation to form kerogen, which is ultimately preserved in the sedimentary record.
We examined several mechanisms that may influence the accumulation and preservation of organic matter in marine environments. Preservation of organic matter is controlled by sorption to mineral surfaces. Organic matter preservation may actually be a competition between sorptive preservation and oxic degradation. We highlighted the geochemically relevant biologic compounds to give you an idea of which compounds can be used to identify inputs of organic matter from specific organisms. The lipid and lignin compounds are potentially the most useful biomarkers. Using alkenones, we were able to reconstruct sea surface temperatures from midlatitude and tropical regions of the Pacific over the past 35,000 years. We also examined how amino acid racemization can be used as a paleoenvironmental tool.
There are many more questions that organic geochemists try to answer, not only in the petroleum and paleoclimate fields but also from the modern environmental/contamination point of view. To gain insight into these fields, you may consider investigating some of the sources listed in the suggested readings for this chapter.
Only a few books dedicated to organic geochemistry have been written, and these are already dated. Most findings, especially on the biomarker front, are disseminated in articles in the following technical journals: Organic Geochemistry, Geochimica et Cosmochimica Acta, Marine Geochemistry, and Paleoceanography. For general background, you may wish to consult the following texts.
Eglinton, G., and M. T. J. Murphy. 1969. Organic Geochemistry: Methods and Results. New York: Springer-Verlag. (This book was the first definitive text in organic geochemistry and still provides background information on the occurrence and evolution of organic matter in sediments.)
Engel, M. H., and S. A. Macko, eds. 1993. Organic Geochemistry: Principles and Applications. New York: Plenum. (This text nicely shows applications of organic geochemistry to the formation of petroleum, reconstructing paleoenvironments, and understanding the fate of organic compounds.)
Killops, S. D., and V. J. Killops. 1993. An Introduction to Organic Geochemistry. New York: Wiley. (Top-of-the-line text that introduces basic concepts of organic geochemistry in an easily digestible manner.)
The following articles were referenced in this chapter.
Berner, R. A. 1989. Biogeochemical cycles of carbon and sulfur and their effect on atmospheric oxygen over Phanerozoic time. Palaeogeography, Palaeoclimatology, Palaecology 73: 97−122.
Brassell, S. C., G. Eglinton, I. T. Marlowe, U. Pflaumann, and M. Sarnthein. 1986. Molecular stratigraphy: A new tool for climatic assessment. Nature 320:129−133.
Cole, J. J., S. Findlay, and M. L. Pace. 1988. Bacterial production in fresh and saltwater ecosystems: A cross-system overview. Marine Ecology-Progress Series 43:1−10.
Emerson, S., and J. Hedges. 1988. Processes controlling the organic carbon content of open ocean sediments. Paleoceanography 3:621−634.
Hedges, J. I., and R. G. Keil. 1995. Sedimentary organic-matter preservation—an assessment and speculative synthesis. Marine Chemistry 492−493:81−115.
Keil, R. G., E. Tsamakis, C. B. Fuh, C. Giddings, and J. I. Hedges. 1994. Mineralogical and textural controls on the organic composition of coastal marine sediments—hydrodynamic separation using SPLITT-fractionation. Geochimica et Cosmochimica Acta 582:879−893.
Mayer, L. M. 1994. Surface area control of organic carbon accumulation in continental shelf sediments. Geochimica et Cosmochimica Acta 114:347−363.
McCoy, W. D. 1987. The precision of amino acid geochronology and paleothermometry. Quantitative Science Review 6: 43−54.
Oches, E. A., W. D. McCoy, and P. U. Clark. 1996. Amino acid estimates of latitudinal temperature gradients and geochronology of loess deposition during the last glaciation, Mississippi Valley, United States. Geological Society of America Bulletin 1087:892−903.
Olson, J. S., R. M. Garrels, et al. 1985. The Natural Carbon Cycle. Atmospheric Carbon Dioxide and the Global Carbon Cycle. J. R. Trabalka, ed. Washington, D.C.: U.S. Department of Energy, pp. 175−213.
Prahl, F. G., L. A. Muehlhausen, and D. L. Zahnle. 1988. Further evaluation of long-chain alkenones as indicators of paleoceanographic conditions. Geochemica et Cosmochemica Acta 52:2303−2310.
Schlesinger, W. H. 1997. Biochemistry: An Analysis of Global Change. San Diego: Academic.
Tregelaar, E. W., J. de Leeuw, S. Derenne, and C. Largeau. 1989. A reappraisal of kerogen formation. Geochimica et Cosmochimica Acta 53:3103−3106.
Williams, P. M., and E. R. M. Druffel. 1987. Radiocarbon in dissolved organic matter in the central North Pacific Ocean. Nature 330(6145):246−248.
(6.1) Briefly discuss how the chemical compositions of higher plants differ from those of bacteria and determine which biochemical compounds you would expect to survive diagenesis and why.
(6.2) You recover a sediment core from a Miocene age lake deposit. Knowing that Miocene woody forests were replaced by grasslands, discuss: (a) which organic compounds you would analyze to determine the organic matter sources to sinkhole sediments; and (b) what you would expect to find if indeed forests were replaced by grasslands.
(6.3) Identify three areas of the oceans where monolayer equivalents of various masses would potentially form, and discuss the key factors that control their formation in each environment.
(6.4) Draw the structures of the following organic compounds: octane, pentene, ethanol (ethyl alcohol), a phenol, and an alkenone with 37 carbon atoms and 3 double bonds (an unsaturated straight-chain ketone).
(6.5) Using the relationship 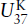 0.040(SST) − 0.110, recalculate the values in worked problem 6.2. What difference does this calibration make in the resulting values, and is this significant?
0.040(SST) − 0.110, recalculate the values in worked problem 6.2. What difference does this calibration make in the resulting values, and is this significant?
(6.6) Using the information in worked problem 6.3, calculate the age of fossil snail shells that have alle/ile ratios of: 0.09, 0.13, 0.19, 0.23, 0.35. Is this a linear relationship?