Chapter II.6.2
Overview of Tissue Engineering Concepts and Applications
General Introduction
History of Tissue Engineering
The term “tissue engineering” as recognized today was first introduced at a panel meeting of the National Science Foundation in 1987, which led to the first tissue engineering meeting in early 1988 (Nerem, 2006; Vacanti, 2006). However, tissue engineering strategies date back to the seventies and eighties for developing skin substitutes (Vacanti, 2006). Despite these early approaches for replacement, repair, and regeneration of failing organs, the true emergence of tissue engineering as a medical field started in the early nineties when tissue engineering was defined as an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain or improve tissue function (Langer and Vacanti, 1993). The field has since rapidly progressed worldwide, with nearly $4 billion invested in the field in the 1990s resulting in over 70 companies and several products on the market by the end of the millennium (Lysaght and Reyes, 2001). As of mid-2007, approximately 50 firms or business units with over 3000 employees offered commercial tissue-regenerative products or services with generally profitable annual sales in excess of $1.3 billion (Lysaght et al., 2008). Well over a million patients have been treated with these products. In addition, 110 development-stage companies with over 55 products in US Food and Drug Administration (FDA)-level clinical trials and other preclinical stages employed ~2500 scientists or support personnel, and spent $850 million development dollars in 2007. While early success has been challenging, technological advances alongside biological discoveries continue to propel the field of tissue engineering into exciting new frontiers.
Goals of Tissue Engineering and Classification
Goals of Tissue Engineering
Tissue engineering aims to restore tissue and organ function by employing biological and engineering strategies to clinical problems. The functional failure of tissues and organs is a severe and costly healthcare problem, as their replacement is limited by the availability of compatible donors (Langer and Vacanti, 1993, 1999).
Artificial prostheses and mechanical devices save and improve the lives of millions of patients, but are not ideal since they are subject to mechanical failure upon long-term implantation. Furthermore, mechanical devices rarely integrate with host tissues, and can trigger a host immune response damaging healthy tissue around the implant. In addition, surgical reconstruction of organs and tissues are attempted where the organs or tissues are moved from their original location to replace a damaged tissue, e.g., saphenous vein as bypass graft, patella tendon for anterior cruciate ligament (ACL) repair. However, often this strategy fails to replace all the functions of the original tissue. Additionally, development of malignant tumors, surgical complications, and morbidity at the donor sites are major problems in surgical reconstruction of tissues. Thus, tissue engineering has emerged as another alternative for tissue or organ transplantation. The primary goal of tissue engineering is to provide a biological substitute to treat tissue/organ loss or failure by integrating multiple aspects of engineering, biology, and medicine. By recapitulating the normal tissue development process, tissue engineering represents a strategy to restore, maintain, and improve tissue function, which ultimately aims toward complete organ replacement.
Classification of Tissue Engineering Approaches
Traditional Tissue Engineering Approaches
Tissue engineering includes two main strategies: (1) transplantation of a tissue grown in vitro consisting of an artificial matrix with cells and growth factors; and (2) in situ regeneration of tissue utilizing a combination of an artificial matrix and growth factors as a guiding template to induce host cell regeneration of the tissue in vivo (Figure II.6.2.1).

FIGURE II.6.2.1 Tissue engineering approaches may be classified into two categories: (A) transplantation of in vitro grown tissues; and (B) promotion of tissue regeneration in situ. In both approaches the scaffolds or artificial matrices, often biodegradable polymers, are integrated with microenvironmental factors (such as cytokines, growth factors, mechanical forces, physico-chemical factors, spatial and temporal signals, and extracellular matrix molecules).
In addition to traditional tissue engineering approaches, other methods used for tissue regeneration include local and systemic cell injection without a scaffold, and closed looped systems used as implantable or extracorporeal devices. While classically these methods are not regarded as tissue engineering, they have contributed significantly to tissue regeneration and are therefore briefly introduced here.
Cell Therapy
Cell therapy involves delivery of cells through systemic injection into the bloodstream or through direct transplantation into a local tissue (Mooney and Vandenburgh, 2008; Karp and Leng Teo, 2009). The major requirement for this strategy is to harvest the cells and grow them in large numbers for in vivo transplantation.
Direct injection of cells to a local site is a common strategy attempted to promote tissue regeneration. However, the survival of the delivered cells is typically low, often due to a lack of a rich nutrient and oxygen supply (Muschler et al., 2004). Alternatively, cells can be administrated via systemic injection, which relies on cells traveling through circulation to engraft in the target site. Cell transplants from bone marrow, peripheral blood or umbilical cord have been used to treat several blood-related diseases including leukemia, multiple myeloma, and immune deficiencies. The main goal of these strategies is to deliver hematopoietic (blood) stem cells to treat blood-related diseases (Thomas, 1987). Recently, mesenchymal stem cells, connective tissue progenitor cells, which repair or regenerate non-hematopoetic tissues, have been systemically injected to treat diseases including myocardial infarction (Barbash et al., 2003), bone diseases (Horwitz et al., 1999), and brain injury (Mahmood et al., 2003) in clinical trials. The main challenges for cell transplantation are: growing large number of cells without bacterial contamination; preservation of cell phenotype; and preventing accumulation of genetic mutations during culture expansion. Although cells have been successfully delivered to the heart to treat ischemic tissue following myocardial infarction, and into the joint to treat arthritis, irrespective of the delivery route, cell therapies face challenges due to widespread death of the transplanted cells, poor engraftment, and loss of control over the fate of the transplanted cells.
Closed Loop Methods
A variety of closed loop systems are used as extracorporeal or implantable devices which house the transplanted cells in a semipermeable membrane (Murua et al., 2008). The membrane permits diffusion of nutrients and excreted products, but prevents the movement of antibodies, pathogens or immunocompetent cells. There are several types of designs for such devices. Vascular type design uses a conduit structure around which the cells are transplanted in a chamber. As blood flows through the conduit it provides nutrients to the transplanted cells, while cell-secreted substances diffuse into the bloodstream (Langer and Vacanti, 1993; Patzer, 2001). Additionally, micro/macrocapsule-based systems have been used as closed loop systems (Aebischer et al., 1991; Winn et al., 1991) where cells are encapsulated within hydrogel droplets. The encapsulated cells can then be cultured in vitro or transplanted in vivo, either to repopulate a defect site or to produce growth factors or other molecules that will have an effect on the targeted cell population. Closed loop extracoporeal devices have been used for the treatment of liver, pancreas, and kidney pathologies. Major problems associated with these types of devices include fouling, fibrous tissue overgrowth, restricted and hindered diffusion, and immunogenic response (Wiegand et al., 1993).
Components of Tissue Engineering
Tissue engineering strategies typically involve multiple components including cells, a physical template, and a combination of growth cues that promote tissue regeneration and integration of the construct into a functional and organized tissue.
The Cell
Cell Source
Cells are the building blocks of tissues, and play a critical role in promoting tissue healing and regeneration. Within tissue engineering strategies, cells may be a component of the in vitro construct or may be recruited in vivo with the aid of immobilized or soluble signals. Cell types utilized for tissue engineering are selected from a variety of sources which include autologous cells from the patient, allogeneic cells from another human, and xenogeneic cells from a different species. However, allogeneic and xenogeneic cells often suffer from immune rejection.
Cell Type
Common cell types include stem cells (capable of self-renewal and differentiation into multiple lineages), differentiated mature cells, or a mixture of differentiated cells. Stem cells (see also Chapter II.1.7 “Stem Cells: Key Concepts” and II.6.4 “Cell Sources for Tissue Engineering: Mesenchymal Stem Cells”) may include embryonic stem cells and adult stem cells, such as mesenchymal stem cells and hematopoietic stem cells. Embryonic stem cells are an attractive cell source for tissue engineering as these cells can self-renew without differentiation, can be culture expanded, and most importantly can differentiate into any cell type. Since embryonic stem cells are isolated from an embryonic stage, they can develop into any of the three germ layers: endoderm (interior stomach lining, gastrointestinal tract, the lungs); mesoderm (muscle, bone, blood, urogential); or ectoderm (epidermal tissues and nervous system). Successful reprogramming of differentiated human somatic cells into pluripotent cells has led to the creation of induced pluripotent stem (iPS) cells (Takahashi and Yamanaka, 2006). These cells are functionally similar to embryonic stem cells, but do not require the destruction of an embryo and can be created from a patient’s own cells, eliminating the risk of host rejection (Takahashi et al., 2007). Adult stem cells include, for example, mesenchymal (Pittenger et al., 1999), hematopoietic (Baum et al., 1992), neural (Snyder et al., 1997), and hepatic (Petersen et al., 1999) stem cells. In particular, hematopoietic stem cells have been used in clinics for a few decades for treating blood diseases (i.e., bone marrow transplantation). Mesenchymal stem cells (MSCs), which can be transplanted as an allogenic cell source to another patient without immunosuppressive drugs, are capable of differentiation into multiple lineages that may produce tissues including bone, cartilage, and muscle, and have been approved for use in multiple systems to treat bone defects. Mesenchymal stem cells can also modulate the host immune response through paracrine or endocrine mechanisms, and are currently being applied in clinical trials for treatment of immune diseases. Nevertheless, adult stem cells are rare, challenging to isolate and expand without altering cell phenotype, and limited in their differentiation potential.
Further investigation is needed to identify the best source of stem cells for each tissue engineering application. In the near future, it will be necessary to compare the performance of the different stem cell populations under the same testing conditions. Multiple variables should be addressed in the selection of the best stem cell population, including: (1) stem cell accessibility (e.g., the isolation of neural stem cells is invasive and relatively difficult compared to other stem cells); (2) number; (3) proliferation capacity; (4) differentiation profile; and (5) ethical issues.
In addition to stem cell populations, committed and/or differentiated cell types are also frequently used in tissue engineering approaches. For example, culture expanded chondrocytes have been used for over 15 years to treat cartilage defects (Brittberg et al., 1994). However, the potential of differentiated adult cells is often limited due to their low proliferation capability, loss of phenotype, and dedifferentiation in culture.
In addition to primary cells, the intrinsic biological potential and performance of a cell can be modified by transient or permanent alteration of specific genes (Hannallah et al., 2003). The introduction of new or altered genes is often accomplished with vectors created by modifying naturally-occurring viruses such as retrovirus, lentivirus, adenovirus or adeno-associated virus. There are several concerns for these approaches, such as transformation efficiency, safety of virus transfection, vector stability, and optimal function of the inserted genes. Non-viral transfection techniques have been developed to circumvent some of these issues; however, the long-term fate of these genetically modified cells still presents a potential risk.
The cell phenotype can also be regulated through manipulation of isolation and culture conditions. Although this may expand the available tools to manipulate cell characteristics, it can create more hurdles for consistently manufacturing high quality cells. In particular, care must be taken during the cell isolation process to maintain cell phenotype, purity, and differentiation state. It is critical to achieve a high purity of cells defined by rigorous characterization. In addition, it is important to consider that cell culture may “activate” cells, altering their phenotype from those found in situ. There are many decisions that must be made, often without suitable premise, such as the ideal number of cells to be transplanted, the maximum number of times cells can be passaged, the maximum length of time cells should be maintained in culture, the ideal differentiated state of the cells to produce a therapeutic effect, and the ideal storage conditions for the cells. These factors can significantly alter the outcome of a regenerative approach, and often need to be optimized on a case-by-case basis for specific animal models (Bueno et al., 2007).
Materials
Biomaterials are used to develop scaffolds, which provide a template for cells to organize and restore structure and function to damaged or dysfunctional tissues. Guidance can be achieved through biophysical and biochemical cues that direct cell behavior, morphology, adhesion, and motility (Hubbell, 1995; Langer and Tirrell, 2004). Biomaterials can, at the same time, be used to supply nutrients, drugs, and bioactive factors that direct specific tissue growth. Accordingly, the material should be non-toxic and degradable within a clinically useful range into fully biocompatible products. Ideally, the material should also possess physico-mechanical and engineering properties suitable for the intended application, and be compatible for further functionalization with bioactive molecules. Biomaterial scaffolds for tissue engineering are discussed in more detail in Chapter II.6.3 “Tissue Engineering Scaffolds.”
To accommodate these material requirements, the field of tissue engineering has witnessed tremendous development of new biomaterials over the past few decades. These materials are derived from both natural and synthetic sources, and possess a broad spectrum of structural and functional properties that make them suitable for many clinical applications.
Natural Materials
A wide range of natural-origin polymers, including proteins and polysaccharides, are used as carriers for cells and bioactive molecules (Malafaya et al., 2007). Natural materials are advantageous due to their inherent biological recognition through receptor–ligand interactions, cell-mediated proteolysis and remodeling, and low toxicity.
Protein-based natural polymers include collagen, gelatin, silk fibroin, fibrin, elastin, and soybean. Collagen is a major component of the extracellular matrix, the natural cell scaffold, which interacts with cells in all tissues, providing essential signals for the regulation of cell anchorage, migration, proliferation, differentiation, and survival (Yang et al., 2004). As a result, collagen has been studied for engineering artificial skin (collagen IV), bone (collagen I), and cartilage (collagen II), resulting in several tissue engineering products. Bilayered collagen gels seeded with human fibroblast and keratinocytes are used as a bioengineered artificial skin by Organogenesis, Inc. under the name of Apligraf®. Gelatin is a natural polymer derived from collagen that has significantly lower antigenicity in contrast to collagen and has been used for engineering bone, cartilage, and skin. Gelatin is also well-suited to serve as a carrier for bioactive molecules, including fibroblast growth factor and transforming growth factor (Ito et al., 2003). Silk fibroin has received significant attention as a versatile natural polymer due to its high strength-to-weight ratio and slow degradation (Sofia et al., 2001). Another commonly used natural material is fibrin, the structural component of blood clots that provides a transitory matrix for cell migration during wound healing. Fibrin has been used as a matrix for studying the regeneration of bone, cartilage, skin, nerve, and spinal cord (Ahmed et al., 2008).
Another class of natural polymers is polysaccharides that contain monomers (monosaccharides) linked together by O-glycosidic bonds to form linear and branched polymers (Malafaya et al., 2007). Several polysaccharides, including chitosan and alginates, have been studied for tissue engineering applications. Chitosan, the fully or partially deacetylated form of chitin found particularly in the shell of crustaceans, has attracted significant attention, due to its biocompatible properties, for regeneration of skin, bone, cartilage, and vascular grafts (Park et al., 2000; Mi et al., 2001; Kim et al., 2003). Alginates in the form of hydrogels, beads, and scaffolds are a widely utilized polysaccharide polymer used to promote angiogenesis and regenerate bone and cartilage (Marijnissen et al., 2002; Simmons et al., 2004; Tilakaratne et al., 2007). Other commonly employed polysaccharides include starch, hyaluronan, chrondoitin sulphate, cellulose, dextran, and polyhydroxyalkanoates.
Unfortunately, there are several limitations of natural materials which include purification, cost, immunogenic responses, and lack of mechanical properties and processability. Although some of these disadvantages have been avoided through recombinant protein expression technologies (Rodriguez-Cabello et al., 2005), synthetic biomaterials (see below) present a paradigm shift that overcomes many of these challenges while opening the door for custom-designed biomaterials.
Synthetic Materials
Many synthetic polymers have been designed and fabricated for tissue engineering purposes. Biodegradable synthetic polymers offer a number of advantages compared to natural materials, such as controlled mechanical properties and degradation kinetics, easy processability into custom shapes and structures, and easy modification of the material for specific applications.
The vast majority of synthetic biomaterial-based polymers belong to the polyester family which includes poly(glycolic acid), poly(lactic acid) and their copolymer poly(lactide-co-glycolide) (PLGA) (Shalaby, 1988). Polyesters have long been used in the clinic in the form of degradable sutures, and have more recently been studied to promote regeneration of bone, cartilage, bladder, skin, and vasculature. In particular, PLGA and its constituent polymers are used in a vast array of applications due to their ease of degradation by hydrolysis of ester bonds, controllable degradation rate, and minimal inflammatory response. Another class of polyester used in tissue engineering is polylactones, such as poly(caprolactone) (PCL), which are less crystalline than PLGA and degrade at a much slower rate. Interestingly, caprolactone-based materials have an added advantage given their shape memory properties which enables the polymer to change their shape after an increase in temperature (Lendlein et al., 2001). Other classes of polyesters that have been explored include poly(ortho esters) and poly(propylene fumarate) (Kharas et al., 1997). Recent development of elastomeric polyesters has shown significant promise as tissue engineering biomaterials that mimic the mechanical properties of soft tissues due to their elasticity compared to other polyesters, e.g., poly(glycerol sebacate) and poly(diol citrate) (Yang et al., 2006; Nijst et al., 2007).
Poly(anhydrides) are another class of synthetic polymers developed from the condensation of diacids or a mixture of diacids. These polymers are biocompatible and have well-defined degradation characteristics. Several poly (anhydride) homopolymers and copolymers have been synthesized to control their degradation rate and mechanical properties (Domb, 1987). Amino acid-based polymers, e.g., L-tyrosine based poly(carbonate), poly(imminocarbonate), and poly(phosphate) (Pulapura and Kohn, 1992; Gupta and Lopina, 2004) have been developed as a substitute for poly amino acids where the peptide linkages are partially replaced with non-peptide links. Biodegradable polyurethane elastomers have also been developed as synthetic biomaterials for regeneration of heart, cartilage, and skin (Guan et al., 2005). Polyurethanes with degradable linkages and biocompatible components have been developed from amino acids and peptides with a wide range of mechanical properties and degradation kinetics (Skarja and Woodhouse, 2001; Sarkar et al., 2009). Synthetic polymers with photopolymerizable groups are based on poly(ethylene glycol) and poly(acrylates). Upon exposure to ultraviolet radiation and cross-linking agents, these polymers can cross-link to form three-dimensional networks.
Due to complexities in the design and synthesis of biomaterials, recent techniques in combinatorial approaches to develop and screen synthetic biomaterials are rapidly gaining interest in this field (Anderson et al., 2004; Kohn, 2004; Yang et al., 2008). Such techniques can be used to rapidly synthesize a library of polymers by altering the structure and composition of polymers and to characterize their interactions with cells in a high-throughput manner.
Semi-Synthetic Materials
Combinations of natural and synthetic polymers have particular advantages compared to pure natural or synthetic counterparts for some applications. These biosynthetic hybrids are usually designed by incorporation of biologically active macromolecules onto the backbone of synthetic polymers to optimize both structure and function (Seliktar, 2005). From a tissue engineering perspective, the objective of using a biosynthetic hybrid material is to reproduce the intricate extracellular stimulation of the native cellular environment, yet exhibit enhanced control over the material properties (compared to purely natural materials). For example, semi-synthetic PEG(polyethylene glycol)-fibrinogen hydrogel has been developed to control cell migration and tissue regeneration where the synthetic PEG component controls density, stiffness, and biodegradability, and the fibrinogen component presents biofunctional domains for cell-mediated remodeling (Dikovsky et al., 2006).
Scaffold Design
Scaffolds act as the synthetic analog of the natural extracellular matrix. The role of scaffolds is to recapitulate the normal tissue development process by allowing cells to formulate their own microenvironment (Lee et al., 2008). The scaffold provides the necessary support for cells to attach, proliferate, and maintain their differentiated function and subsequent regeneration of new tissues. Ideally, a scaffold should have the following characteristics: (1) three-dimensional highly porous structure with an interconnected pore network to facilitate cell/tissue growth and diffusion of nutrients, metabolic waste, and paracrine factors; (2) biodegradable or bioresorbable with controllable degradation and resorption rates to match cell/tissue growth in vitro and in vivo; (3) suitable surface chemistry for cell attachment, proliferation, and differentiation; (4) mechanical properties to match those of the tissues at the site of implantation; and (5) be easily processed to form a variety of shapes and sizes.
Conventional Fabrication Methods
The formation of a porous structure is the main goal of scaffold fabrication. Most methods for fabricating porous scaffolds, including particulate leaching, freeze drying, gas infusion, and phase separation, create isotopically distributed interconnected pores. Porous structures are developed by introducing particles or bubbles when the scaffold is solidified, which are later removed leaving behind an interconnected network of pores. Although these techniques are relatively simple for developing a three-dimensional structure, they are limited by uncontrolled pore size and connectivity, poor mechanical strength, and residual solvent/porogens (Yang et al., 2001). Hydrocarbon templating is a combination of two distinct foam processes where water-insoluble hydrocarbon particulates are leached with the precipitation of the polymers. This process has several advantages, including control over scaffold thickness and pore structure, although use of organic solvents is typically a disadvantage for biological applications as it is difficult to remove completely.
Fiber-Based Scaffolds
Fibrous scaffold structures may be developed by electrospinning of polymers to generate continuous micro- or nanoscale diameter fibers. Additionally, the orientation of fibers can be controlled during electrospinning to develop random or aligned fibers. Electrospun scaffolds are advantageous due to their high surface-to-volume ratio and structural similarity to natural extracellular matrix. However, it is difficult to control the distance between fibers, an important factor that influences the migration of cells. Self-assembly of biopolymers, e.g., peptides and nucleic acids, using noncovalent interactions including H-bonding, hydrophobic, electrostatic interactions, and van der Waals forces into three-dimensional structures has also been used for scaffold development. The main advantage of such biopolymers is that their self-assembly relies on specific biorecognitions (e.g., DNA hybridization) which therefore make the formation of scaffold highly predictable and programmable.
Solid-Free-Form Methods
To enhance control over the three-dimensional organization of porous scaffolds, rapid prototyping techniques borrowed from electrical and mechanical engineering can be used. These techniques involve computer-assisted methods with solid-free-form (SFF) fabrication techniques (Hollister, 2005). Specifically, they involve acquiring a two-dimensional image of a target specimen by nondestructive imaging, then developing macroscale three-dimensional architecture with software, and finally fabrication of the three-dimensional matrix with highly precise and automated layer-by-layer SFF processes. There are several SFF processes, including laser-based, ink-jet type printing-based, and nozzle-based approaches. These methods offer precise control of the three-dimensional structure of the scaffolds; however, they are associated with higher costs and require more complex equipment compared to conventional salt leaching or gas foaming processes.
Hydrogel-Based Scaffold
Hydrogels are typically cross-linked networks swollen in water to suspend cells in three dimensions. Photocross-linkable hydrogels are frequently used to encapsulate cells (Ferreira et al., 2007a). Polymers with photocross-linkable moieties are polymerized into three-dimensional networks in the presence of cross-linkers under UV radiation. By controlling the structure with defined cross-linking density, mechanical properties, mass transport, and degradation characteristics, the gels can be tuned for a range of applications. Hydrogels are an appealing three-dimensional scaffold because they are structurally similar to the extracellular matrix of many tissues, can often be processed under relatively mild conditions, and may be delivered in a minimally invasive manner (Drury and Mooney, 2003). In addition, hydrogels are advantageous due to high water content, facile transport properties, and controlled degradation kinetics (Nuttelman et al., 2008). Furthermore, hydrogels can be chemically modified to improve the adhesion and proliferation of the cells on the gel matrices through inclusion of adhesion peptides, i.e., RGD(Arg-Gly-Asp tripeptide). In order to achieve more control over the cell placement within the hydrogels, three-dimensional patterned hydrogels are used. However, major problems with using hydrogels include structural instability and overall inferior mechanical properties for placement within dynamic environments.
Development of Tissue Engineering Constructs
Strategies for Tissue Engineering
As outlined above, there are two main strategies to develop a tissue engineering construct. To facilitate tissue growth, scaffolds can be transplanted with or without cells. Each strategy’s success largely depends on effective integration of different factors including cells, scaffolds, and environmental cues (e.g., growth factors, biological molecules).
Cells with Scaffold Transplantation
Scaffolds provide a substrate upon which cells attach, proliferate, and produce extracellular matrix within a predefined three-dimensional orientation. However, the main challenges for this strategy include: generating a complete functional tissue; preserving cell viability and function during transplantation; biological and mechanical integration with the surrounding tissue; and supplying oxygen and nutrients to the transplanted cells (Muschler et al., 2004). The transplanted cells within the core of large defects often die before contributing to the healing process. Moreover, the scaffolds are often filled with blood clots which present a harsh environment for the transplanted cells. Regardless, this strategy can be useful for transplantation of thin tissue grafts, such as corneal and skin grafts, where an immediate connection to the vascular system is not essential.
Scaffold-Only Transplantation
Another strategy involves in situ tissue regeneration by providing an instructive scaffold that can guide and control the regeneration process in vivo (Zhao and Karp, 2009). This process uses an artificial scaffold matrix that, once implanted, recruits host cells and relies on the body’s innate regenerative ability. The scaffold recruits such cells through signal presentation (Richardson et al., 2001; Simmons et al., 2004; Lee et al., 2008). For example, transplantation of PEG-collagen matrices with enzyme cleavable matrix metalloproteinase (MMP) links that release bone morphogenic protein-2 (BMP2) can promote regeneration of critical size bone defects effectively through invasion of the host cells (Lutolf et al., 2003a). The absence of MMP sensitive linkages and/or BMP-2 hindered the regeneration process, indicating that transplantation of scaffolds with appropriate soluble and insoluble signals is critical. Thus, the success of scaffold transplantation-based approaches largely depends on the appropriate presentation of signals to mediate host cell mobilization and coordination of the subsequent behavior of the cells, e.g., adherence, migration, and proliferation.
Biological Factors in Tissue Growth
Type of Biological Factors
Biological factors including hormones, cytokines, growth factors, extracellular matrix molecules, cell surface molecules, and nucleic acids can significantly influence the function and behavior of cells in the scaffold. The temporal and spatial coordination of cellular processes is orchestrated by these signals from the extracellular environment. A large number of biomolecules have been explored to induce tissue regeneration, but the majority of these molecules can be categorized as follows: (1) small molecules, e.g., corticosteroids, hormones, are used for intercellular and intracellular signaling by binding to specific protein receptors; (2) proteins and peptides act on the cells as mitogens, morphogens, growth factors, and cytokines; and (3) oligonucleotides, either DNA or RNA, can either affect gene transcription and/or translation or can be incorporated into the cell’s genome.
Delivery and Presentation of Biological Factors
The challenges and strategies to deliver these factors vary due to the differences in their physical and chemical properties. Presentation of factors as soluble cues is an important strategy to control the interaction of cells and their microenvironment. Strategies to embed factors within scaffold matrices or encapsulate them within micro- and nanoparticles have been used to control the release of the agents. Presentation of a single or multiple factors has been achieved by release of factors from polymeric scaffolds with controlled degradation characteristics or diffusion of the factors out of the scaffold. This allows delivery of multiple soluble cues to cells at distinct rates. This strategy has been demonstrated to release vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF) in a PLGA scaffold to promote the growth of blood vessels in situ (Richardson et al., 2001). A similar strategy has been employed to regenerate bone with transplanted stromal cells in alginate scaffolds through dual delivery of BMP2 and transforming growth factor-β3 (TGF-β3) (Simmons et al., 2004). Additionally, presentation of factors in a spatio-temporally controlled manner is important for the development of spatially complex tissues. In particular, scaffolds developed by layer-by-layer deposition can control the releases of different growth factors and proteins. For example, by controlling spatial gradients of nerve growth factor (NGF) within the scaffold, axonal outgrowth can be achieved (Moore et al., 2006).
In addition to presenting growth factors in a soluble form, an alternative strategy is to bind factors to a surface, in either random or specific orientations. Non-covalent association of the matrix components, e.g., glycosaminoglycans (GAGs), can slowly release and potentiate binding to the cell membrane receptors (Sakiyama-Elbert and Hubbell, 2000). Alternatively, covalently bound growth factors can also influence cell behavior, i.e., covalently conjugated epidermal growth factor (EGF) enhances the survival of mesenchymal stem cells in a Matrigel™-based scaffold (Fan et al., 2007). The presence of protease sensitive peptide sequences within such growth factor proteins allows these molecules to be released on demand (Zisch et al., 2003). In addition, the adhesion of cells, which is a crucial step for cell survival and is mediated by cell-surface receptors with cell adhesion proteins, on the scaffold matrices can also be controlled by presentation of specific peptides and carbohydrates. In particular, a prototypical three amino acid sequence arginine-glycine-aspartic acid (RGD) is frequently found in many adhesion proteins and binds to many integrin receptors on cells. RGD peptide sequences have therefore been covalently immobilized on a synthetic material surface at a defined density and orientation to guide cell adhesion (Silva et al., 2008). To note, the graft density of RGD is essential to balance cell adhesion versus migration (Gobin and West, 2002). Finally, the covalent immobilization of protease sensitive cleavable linkages (i.e., MMP sensitive links) on synthetic matrices mimics the native extracellular matrix which can be used, in addition to soluble growth factors and other chemotactic cues, to guide migration of cells (Gobin and West, 2002; Lutolf et al., 2003b).
Mechano-Chemical Factors in Tissue Growth
In addition to soluble and immobilized biological factors, the physical and/or chemical nature of the scaffold is also known to play an important role in regulating cell fate. For example, recent studies suggest surface chemistries can regulate the differentiation of MSCs (Benoit et al., 2008). Similarly, the structure of scaffold can also influence the shape of stem cells, which impacts other cell functions such as differentiation. For instance, MSCs cultured in highly cross-linked hydrogels exhibit round morphology, but can be induced to spread by reducing the cross-linking density via photodegradation (Kloxin et al., 2009). Moreover, nanoscale geometry and matrix size can also influence cell adhesion, proliferation, and migration. By altering surface topography, cytoskeletal organization can be altered which directly influences molecular and biomechanical signals. Furthermore, mechanical forces exerted by scaffold matrix and the elasticity of the material also influence the cell fate. For instance, matrix stiffness can control the differentiation of MSCs (Engler et al., 2006; Winer et al., 2009). In conclusion, the physical environment, consisting of geometry, time-varying stress, strain, fluid flow, pressure, and potentially other biophysical parameters, e.g., osmotic pressure and electrical field, can regulate cell phenotype and tissue structure in a three-dimensional environment.
Integration of Multiple Factors
Successful integration of the appropriate cells, scaffolds, soluble cues, and mechano-chemical factors is a key in regulating cell fate and ultimately regenerating a functional tissue. Hence, combinatorial approaches in which the effect of different factors in combination is examined are useful (Flaim et al., 2005). Thus, an engineered “niche” or microenvironment is a useful approach for critical understanding of biology, as well as for engineering tissue regeneration (Lutolf and Blau, 2009). Static in vitro tissue engineering systems often fail to take into account the multiple factors that contribute to the healing and regeneration process. Thus, it is critical to understand the system as a whole through dynamic in vitro systems, such as bioreactors and relevant in vivo models.
Models for Tissue Engineering
Bioreactors
Importance of Bioreactors
The traditional tissue engineering approach of growing tissues on two-dimensional surfaces (i.e., petri dishes) or in three-dimensional scaffolds is limited by mass transfer, since the diffusion of metabolites, oxygen, and carbon dioxide under static conditions can only support a certain thickness of tissue (Lees et al., 1981). Consequently, cartilaginous tissue grown in petri dishes has been reported to reach only a maximum height of 0.5 mm (Martin et al., 1998). Multicellular spheroids also develop large, central necrotic regions with increasing diameter (Sutherland et al., 1986). Bioreactors can hence provide an enhanced environment for tissue growth. Their higher rates of mass transfer provide improved oxygen influx and waste disposal. Various cell seeding strategies (i.e., traditional static seeding through gravity, dynamic seeding which takes place in an agitated environment or seeding in a perfusion system) allows for desirable cell density and homogeneity, and mechanical conditioning (Martin and Vermette, 2005). By designing bioreactors it is possible to provide the desired conditions, i.e., mechanical signals, temperature, flow rate, oxygen, and carbon dioxide concentrations for simulating the in vivo environment required for regeneration of functional tissues (Burg et al., 2000; Freed et al., 2006). Bioreactors are discussed in more detail in Chapter II.6.6 “Bioreactors for Tissue Engineering.”
Types of Bioreactors
Traditional industrial reactors such as the continuous stirred-tank reactor (CSTR) have been adapted as bioreactors in tissue engineering. In particular, the stirred-flask reactor improves reactor performance by agitating the media used for seeding scaffolds (Vunjak-Novakovic et al., 1996). Another system is the rotating-wall vessel, which aims to minimize the shear stress and turbulence of previous approaches. This system was developed for cell culture experiments in space, and thus is often referred to as a microgravity reactor (Unsworth and Lelkes, 1998). Another interesting bioreactor is the hollow fiber reactor that permits the cultivation of highly sensitive cells such as hepatocytes. Cells can either be situated on the exterior or in the lumen of the fibers. These reactors can also introduce two different solutions to the tissue culture system that are optimized to either provide metabolites or remove waste products. Bioartificial liver systems based on this reactor type are in clinical trials to treat patients with hepatic failure (Gerlach et al., 2008).
Another method of seeding and culturing cells is the direct perfusion reactor that can perform continuous perfusion of a cell suspension through a scaffold. This system can increase the efficiency and uniformity of cell seeding (Wendt et al., 2003). There are several other bioreactors designed to grow tissues that primarily serve a biomechanical function. Mechanical constraints can be induced through bioreactors for the successful regeneration of functional tissue, as demonstrated by the in vitro generation of a tissue-engineered heart valve (Karim et al., 2006) and a trachea (Martin et al., 2004; Macchiarini et al., 2008).
Limitations and Challenges
While bioreactors have been shown to enhance uniformity of cell seeding and perfusion of three-dimensional cultures, reactor conditions such as mass transfer properties, cell seeding, mechanical environment, and hydrodynamics must be uniquely optimized for each tissue. Fortunately, mathematical modeling (Mehta and Linderman, 2006) and computational fluid dynamics are available to help understand the complex interactions of these factors (Hutmacher and Singh, 2008). However, even within the best designed bioreactor, one limitation remains, i.e., the transport of nutrients inside the tissue. To overcome this barrier, controlled vascularization of the tissue must be fully understood and applied.
Role of In Vivo Models
While in vitro development of tissues is the starting point of a tissue-engineered product, an appropriate in vivo model is crucial to validate the tissues’ function. Several factors cannot be assessed in vitro, for example the role of angiogenesis in a newly created tissue, host immune reactions to the graft, as well as functional considerations, such as rheological properties of engineered vessels and innervation of the graft (Fabian Schmidt, 2008). These factors must be tested to understand the efficacy of an engineered tissue or organ. Different animal models are used to investigate different tissue products, as all models have limitations that make them appropriate to assess some features but not others. For example, an immune-compromised mouse system is appropriate for initially evaluating graft integration with host tissue, but is not appropriate for evaluating host rejection. Thus, for any tissue-engineered product it is critical to analyze the outcomes of an in vivo study in the context of the model and its efficacy.
Applications of Tissue Engineering
The main goal of tissue engineering is to regenerate and replace human tissues and organs through a combination of biological, clinical, and engineering approaches.
Replacing/Regenerating Target Organs
Investigators have attempted to engineer almost every mammalian tissue. The following section describes some key developments in the field for several tissues:
• Skin: Tissue-engineered skin aims to restore barrier function to patients for whom this has been severely compromised, e.g., burn patients. Skin is a widely explored engineered tissue, and several commercial skin products are available, e.g., Epicel® by Genzyme is a product based on autologous cells grown to cover a wound; Apligraf® by Organogenesis is a dual layer skin equivalent with keratinocytes and fibroblasts on collagen gel; Dermagraft® by Advanced Tissue Science uses a similar approach with dermal fibroblast on resorbable polymer, among many others (MacNeil, 2007). These products treat burns, ulcers, deep wounds, and other injuries. Collagen, fibrin, hyaluronic acid, and poly(lactic glycolic acid) are mainly used in skin substitute matrices. Keratinocytes, melanocytes, and fibroblasts compose the majority of cells in skin tissue, thus expanding and transplanting these cells within biocompatible matrices is key to successful skin regeneration (Priya et al., 2008). Additionally, to achieve effective healing, tissue-engineered skin must attach to the wound bed and avoid rejection by the immune system. Despite great success in engineering skin tissue, pitfalls include scar tissue formation, wound contraction, incomplete healing of deep wounds, lack of full recovery of skin function, and imperfect regeneration of skin components such as glands and hair follicles (MacNeil, 2007). Thus, incorporating our understanding of the requirements of skin regeneration into new three-dimensional model systems is important to develop a fully functional skin replacement.
• Liver: Liver transplantation is end-stage treatment for many liver diseases. Several factors including drug use, alcohol abuse, and viruses like Hepatitis can cause acute liver failure. It is important to completely replace the damaged liver or support the patients that wait for donor organs or suffer from chronic liver diseases with tissue-engineered livers (Kulig and Vacanti, 2004). Intense efforts exist to develop a bridging device that can support a patient’s liver function until a donor is available, e.g., dialysis, charcoal hemoperfusion, immobilized enzymes or exchange transfusion. Several extracorporeal systems use patients’ own cells in a hollow-fiber, spouted-bed or flat-bed device, which reduce the chance of immune rejection. Tissue engineering approaches to transplant hepatocytes consist of several critical steps: culture and expansion of hepatocytes in a three-dimensional polymer substrate, maintaining the viability and differentiated state of the cells, engrafting a sufficient number of hepatocytes with vascularization for survival of the graft, and attaining the complex structural geometry of liver. Several bioartificial livers (BAL) have been developed and designed to flow patient’s plasma through a bioreactor that houses/maintains hepatocytes sandwiched between artificial plates or capillaries. These BAL devices have different hepatocyte sources, treatment, and perfusate (i.e., blood or plasma). The liver tissue engineering field has developed two main strategies: (1) transplantation of suspended hepatocytes with extracellular matrix components; (2) use of biodegradable scaffolds to provide a platform for hepatocyte attachment. Recent advancement of microfabrication techniques has improved the three-dimensional design of artificial livers with microcapillary beds to mimic physiological conditions (Powers et al., 2002). However, challenges remain in liver tissue engineering due to the complex vascularized architecture of the liver, relatively high volumetric oxygen consumption rate of the tissue, and need for long-term culture for applications such as the evaluation of toxicity, efficacy, and infection (Griffith and Swartz, 2006).
• Pancreas: Diabetes is the fifth highest cause of death in the US, and diabetes-related expenses were estimated at more than $100 billion in 2007 (American Diabetes Association, 2008). One type of diabetes is type 1 diabetes mellitus (T1DM), an autoimmune disease which destroys the insulin-secreting cells of the pancreas. T1DM is relevant to tissue engineering, since it can be treated by replacing the destroyed pancreatic islet cells. Techniques for pancreatic tissue engineering aim to release insulin from transplanted islets into the blood to restore normal blood glucose levels. Three main approaches are used: a tubular membrane that encapsulates islets and connects to blood vessels; hollow fibers containing islets embedded in a polymer matrix; and encapsulation of islets in microcapsules (Lacy et al., 1991; Sullivan et al., 1991; Lanza et al., 1995). The membranes used in the perfusion devices and coatings in microcapsules are developed from biocompatible polymers that allow insulin to diffuse into the bloodstream, while protecting the cells from destruction by immune cells. An insufficient source of islet cells presents a challenge, but recent advances with stem cells may overcome this.
• Heart: Cardiovascular disease is the leading cause of death in the US. Many patients are left with damaged or malfunctioning cardiac tissue that lead to arrythmias and diminished cardiac output. Tissue engineering is actively pursing treatments for myocardial infarction, congenital heart defects, and stenotic valves through regenerating cardiac tissues. Heart valves are developed by transplanting autologous cells onto a scaffold, growing and maturing the cell-seeded scaffold, and finally transplanting the valves into the patient. Scaffolds can be made from biomaterials, but decellularized heart valves from donors or animal and biomaterials are also used (Wilson et al., 1995). Decellularized heart valves consist of extracellular matrix which is repopulated with host cells, but they can potentially produce severe immune response (Simon et al., 2003). Alternatively, biomaterial-based heart valves are designed from various natural materials, e.g., collagen, fibrin, and synthetic polymers, e.g., PLGA, poly(hydroxy butyrate) (Hoerstrup et al., 2000). Biomaterial-based heart valves have advantageous characteristics, including malleability and improved mechanical strength. Cells for cardiovascular applications are usually obtained from donor tissues, e.g, peripheral arteries with mixed populations of myofibroblasts and endothelial cells, as well as established cell lines of myofibroblasts and endothelial cells. There are several successful applications of tissue-engineered heart valves for both in vitro and in vivo models. Additionally, efforts have been made to regenerate myocardium by scaffold-based approaches with natural and synthetic materials (Kofidis et al., 2002; Shimizu et al., 2003). Furthermore, recent devlopments have shown stem cells to be promising tools for the field of cardiovascular tissue engineering (Caspi et al., 2007). However, the complex metabolic, electrical, and mechanical nature of heart tissues continues to present a significant challenge to engineering myocardial tissues (Parker and Ingber, 2007).
• Blood vessels: Poly(tetrafluoro ethylene) PTFE and Dacron® grafts have traditionally been used as vascular grafts, particularly for large diameter vessels. However, these grafts are largely unsuccessful for small diameter blood vessels, due to thrombogenicity and compliance mismatch (Edelman, 1999). Tissue engineering strategies therefore provide great opportunities for development of blood vessels. Decellularized arteries with well-preserved extra cellular proteins have been repopulated with cells both in vitro and in vivo to generate blood vessels (Bader et al., 2000; Kaushal et al., 2001). Polymer-based scaffolds are often used as a template to guide the regeneration of blood vessels. By generating sufficient extracellular matrix and adequate mechanical responsiveness, scaffold guided blood vessels have shown significant promise (Niklason et al., 1999). Also, recently developed microfluidic and microfabrication techniques allow the complex architecture of a blood vessel with defined microstructures to be realized (Fidkowski et al., 2005). In an alternative approach, sheets of smooth muscle cells and extracellular matrix are generated under flow conditions in the absence of scaffold, and are subsequently rolled over a support to mature into a vessel structure (L’Heureux et al., 1998). An emerging approach focuses on combining endothelial cells with mesenchymal stem cells or smooth muscle cells to help stabilize the blood vessel and help aid in the vessel pruning process (Ferreira et al., 2007b). It is important to note that formation of new blood vessels is critical for engineering any tissue to supply nutrients and oxygen to cells. Unfortunately, vascularization of large organs is still a challenge for tissue engineering. Several approaches, including delivery of one or more growth factors from scaffold, have been used to stimulate angiogenesis in engineered tissues (Richardson et al., 2001).
• Nervous system: Generation of nerve tissues is a major focus in tissue engineering (Schmidt and Leach, 2003; Zhang et al., 2005). Injuries in the central nervous system (CNS) are often accompanied by permanent functional impairment, unlike peripheral nervous system (PNS) damage where the axons are able to re-extend and re-innervate, leading to functional recovery (Belkas et al., 2004). This is because the environment of the damaged site in CNS blocks the regeneration of neurons. Peripheral nerve grafts that include synthetic or biological substrates have been developed to act as a bridge to guide the nerve regeneration process. To restore the structures lost in the disorganization of axons during injury it is critical to build a bridge that spans the lesion gap with all the morphological, chemical, and biological cues that mimic normal tissue. Both natural and synthetic polymer bridges and conduits have been shown to aid nerve regeneration, and these graft materials may be seeded with Schwann cells, for example, which aid the regeneration process (Bellamkonda and Aebischer, 1994; Zhang et al., 2005). Studies have also shown that controlled release of neurotrophic factors, neuronal adhesion molecules, and growth factors induces a local sprouting response, stimulates reinnervation, and remodels the growing axons (Schnell et al., 1994; Kapur and Shoichet, 2004; Slack et al., 2004). These methods of delivering cells and factors might also treat neurodegenerative disorders in vivo (i.e., encapsulated dopamine producing cells for treating Parkinson’s disease) (Lindvall et al., 1990).
• Bone: There are numerous clinical applications that can benefit from bone regeneration therapies including spinal fusion to alleviate back pain, temporomandibular joint reconstruction to alleviate jaw pain, and restoration of contour and shape within reconstructed craniofacial bone. Significant progress in the development of bone regeneration therapies has been achieved, yet large critical-sized defects which do not heal remain a significant clinical challenge. Although several inorganic-, polymeric-, and hybrid-based scaffolds have been examined to engineer bone, it has been difficult to develop a material that displays optimal mechanical properties and degradation kinetics for bone repair (Muschler et al., 2004). It is clear that the scaffolds for bone regeneration should have an interconnected macroporosity to allow three-dimensional bone growth throughout the scaffold (Gao et al., 2001; Karp et al., 2003, 2004). Implantation of empty macroporous scaffolds that are devoid of cells typically do not improve the healing response (Grande et al., 1999; Louisia et al., 1999; Petite et al., 2000). Although combining biodegradable scaffolds with cells is a commonly explored strategy (Goshima et al., 1991; Holy et al., 2000; Ma et al., 2001; Hutmacher and Sittinger, 2003) these strategies have low success rates, as many of the cells may die due to a lack of vascularization (Petite et al., 2000). Since most tissue engineering scaffolds have heterogeneous pore sizes, and since the amount of blood that fills a scaffold depends on the geometry of the scaffold pores (Whang et al., 1999), it is likely that there is a high variability in the amount of blood that fills these scaffolds; a critical phenomena that is often neglected. It is important to consider that the diverse compositions of scaffolds with respect to materials, porosity, surface chemistry, morphology, degradation rate, pore sizes, and mechanical properties, employed in the field of bone engineering makes it challenging to compare results between studies, and this limits what can be learned and applied by others. Controlled systematic studies and increased understanding of the osteogenic microenvironment is required to provide rational design criteria for the next generation of bone engineering approaches.
In addition to the tissues described above, there are many other tissues which have been targeted for regeneration by tissue engineering strategies. These include cartilage, muscle, kidney, blood, cornea, gastrointestinal tissues, vocal chord, and many others.
Other Applications of Tissue Engineering
There are several non-traditional, yet useful and important applications of tissue engineering strategies (Griffith and Naughton 2002; Ingber et al., 2006). In particular, the field of drug screening is in need of advanced in vitro methods for the assessment of the activity and toxicity of drugs before clinical studies are initiated (Saltzman and Olbricht, 2002). Since most drugs are metabolized in the liver, microscale hepatocyte systems may allow high-throughput screening for liver toxicity (Bhadriraju and Chen, 2002; Khetani and Bhatia, 2008). Furthermore, humans or animals “on a chip” are in development. These chips are essentially several reactors with different cell types connected in series which can simulate the pathway of a chemical substance through several parts of the body, such as the liver, brain, and fat (Viravaidya et al., 2004; Khamsi, 2005). In the future, tissue engineering-based drug testing and toxicity assays can provide an alternate strategy to reduce the use of in vivo animal testing. Moreover, an artificially engineered tissue-engineered system can provide enhanced control of tissue microenvironments, both in physiological and pathological conditions (e.g., cancer tumor), contributing to the understanding of complex tissues and organs (Ali et al., 2009).
Current Challenges and Future Directions
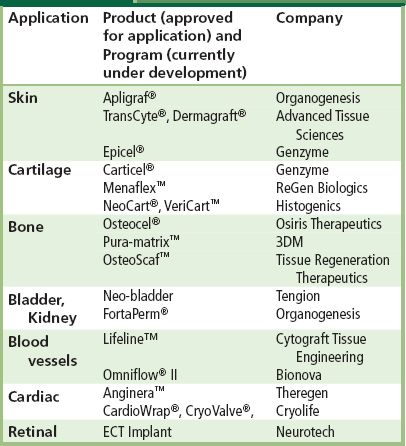
The past two decades have seen a dramatic increase in the exploration of tissue engineering as a promising approach to restore, maintain, and enhance tissues and organs. Tissue engineering concepts based on the application of a scaffold/cell construct have significant potential in the healthcare industry. Indeed, a number of engineered tissues have been approved by the FDA, and are used in the clinic for treatment of patients worldwide. Some examples of tissue engineering products and programs are included in Table II.6.2.1.
TABLE II.6.2.1 Tissue Engineering Products and Programs for Replacement or Restoration of Human Tissue Function
Challenges
Despite the excitement and early success, there are many hurdles to be addressed before tissue engineering reaches its eventual goal to treat millions of patients (Griffith and Naughton, 2002). In addition to efficacy, potential for facile scale-up, reliability, established regulatory routes, and societal acceptance issues, there are many technical challenges to overcome. Some of the major challenges are discussed below.
Cell Source
A cell source is one of the key aspects for an effective tissue engineering strategy. It is critical to access reliable cell sources that are adequate to repair the damaged tissue, and to understand at the molecular level how cells function. Specifically, it is important to comprehend how cells respond to molecular signals and integrate multiple inputs to generate a predictable response. Additionally, although local transplantation or injection of cells represents a potential approach, locally administered cells often die before significantly contributing to the healing response, due to diffusion limitations of nutrients and oxygen (Muschler et al., 2004). Cells need to be within ~200 µm of the nearest blood vessel, and it may take many weeks or months for vascularization to reach the cells, leading to cell and tissue death. This significantly reduces the capacity for an exogenous cell source to contribute to the regenerative process. In addition to issues with cell delivery, embryonic stem cells are fraught with several ethical concerns, but recent federal approval to expand research with these stem cells has invigorated the field of tissue engineering. More importantly, the recent development of iPS cells may replace embryo-derived embryonic stem cells, as iPS cells avoid the destruction of embryos, providing a cell source for drug screening, in vitro models, and future clinical applications. However, currently many existing techniques for creating iPS cells utilize viral transfection techniques that produce a very low yield. Consequently, there is currently a plethora of research ongoing to develop non-viral and highly efficient iPS cell techniques.
Vascularization
Vascularization, the growth of blood vessels, is a major engineering hurdle to overcome in creating artificial organs, particularly large-scale three-dimensional tissues (Soker et al., 2000). It is critical to have effective transport of oxygen, nutrients, and removal of cell-secreted waste for the survival of cells. This is the major reason why most successful engineered organs are restricted to tissues, such as skin, cartilage, and ligament. For these tissues, thin layers of cells (e.g., 1–2 cell layers in skin tissue) that are well-accessible to the blood vessels are sufficient and a proximal blood supply is not essential for survival (Rouwkema et al., 2008). For other tissues and organs, several potential strategies exist to address the issue of vascularization. Recent tissue studies have focused on prevascularizing the tissue constructs prior to implantation or delivering angiogenic growth factors. Although these methods have shown promising results, more critical issues remain as to methods of developing the complex vascular network using scalable technology and integrating the systems with host vasculature.
Material Design
It is a challenge to develop an ideal instructive biomaterial that can effectively induce the growth of tissues. In spite of significant progress in biomaterial development, it is not fully understood how the scaffold should be used to control cell–matrix interactions (Place et al., 2009). The type of signals and the mode of their presentation (e.g., density and organization of adhesion ligands, delivery of growth factors and cytokines) as soluble or insoluble cues can dramatically impact the functionality of the scaffold. Thus, it is critical to develop large-scale screening systems that can thoroughly and systematically analyze these effects. Specifically, new materials that control and manipulate transcription factors for regulating development and morphogen expression can be useful to control the formation of new tissues. It is important to consider how a cell acts at the molecular level, i.e., the cell’s biochemical pathways and how these pathways are affected. Thus, the design of such materials is extremely important to understand the concerted effect of all the factors on the cell functions which are guided by their supporting scaffold, growth factor and cytokine profiles, and biomechanical forces. Additionally, immune rejection of engineered tissues and organs presents a serious problem (Chan and Mooney, 2008). Strategies to evade the host immune system are clearly needed; particularly technologies that can alter or reduce the inflammatory response to increase tolerance will help to overcome this problem.
Future Perspectives
Multiple challenges remain for translation of tissue-engineered products to the clinic. Cell type, source, and manipulation are critical parameters that need to be further studied and defined, in order to achieve the best clinical outcomes. Many approaches are too complex for scale-up to industrial level manufacture. Ideally, tissue-engineered products involving cells should be amenable to cryopreservation (Griffith and Naughton, 2002; Pancrazio et al., 2007). It is also critical to consider that in vivo animal models may not adequately represent the human condition. Furthermore, it is still not clear how the FDA will regulate combination products. This significantly increases the risk for new companies to develop multi-component systems.
Recent developments in the field of gene microarray analysis and imaging (e.g., magnetic resonance imaging (MRI)) provide valuable tools and strategies for advancing the field (Yamada et al., 2006; Pancrazio et al., 2007). More quantitative approaches such as computational modeling and systems biology will be useful to understand the mechanism of tissue development and regenerative processes. Although significant advances have been accomplished, most regenerative therapies are still in the developmental phase. Understanding the fundamental biology associated with normal tissue development is critical for the creation of highly integrated approaches to achieve controlled cell differentiation and tissue formation.
Bibliography
1. Aebischer P, Tresco PA, Winn SR, Greene LA, Jaeger CB. Long-term cross-species brain transplantation of a polymer-encapsulated dopamine-secreting cell line. Exp Neurol. 1991;111(3):269–275.
2. Ahmed TA, Dare EV, Hincke M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):199–215.
3. Ali OA, Huebsch N, Cao L, Granoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8(2):151–158.
4. American Diabetes Association. Economic costs of diabetes in the U.S in 2007. Diabetes Care. 2008;31(3):596–615.
5. Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22(7):863–866.
6. Bader A, Steinhoff G, Strobl K, Schilling T, Brandes G, et al. Engineering of human vascular aortic tissue based on a xenogeneic starter matrix. Transplantation. 2000;70(1):7–14.
7. Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868.
8. Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89(7):2804–2808.
9. Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26(2):151–160.
10. Bellamkonda R, Aebischer P. Review: Tissue engineering in the nervous system. Biotechnol Bioeng. 1994;43(7):543–554.
11. Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7(10):816–823.
12. Bhadriraju K, Chen CS. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov Today. 2002;7(11):612–620.
13. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895.
14. Bueno EM, Laevsky G, Barabino GA. Enhancing cell seeding of scaffolds in tissue engineering through manipulation of hydrodynamic parameters. J Biotechnol. 2007;129(3):516–531.
15. Burg KJ, Holder Jr WD, Culberson CR, Beiler RJ, Greene KG, et al. Comparative study of seeding methods for three-dimensional polymeric scaffolds. J Biomed Mater Res. 2000;52(3):576.
16. Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100(2):263–272.
17. Chan G, Mooney DJ. New materials for tissue engineering: Towards greater control over the biological response. Trends Biotechnol. 2008;26(7):382–392.
18. Dikovsky D, Bianco-Peled H, Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27(8):1496–1506.
19. Domb LR. Polyanhydrides I: Preparation of high molecular weight polyanhydrides J. Polym. Sci., Part A. Pol Chem. 1987;25:3373–3386.
20. Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351.
21. Edelman ER. Vascular tissue engineering: Designer arteries. Circ Res. 1999;85(12):1115–1117.
22. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689.
23. Fabian Schmidt JH. In Vivo Animal Models. In: Meyer U, Handschel J, Wiesmann HP, Meyer T, eds. Tissue Engineering Fundamentals of Tissue Engineering and Regenerative Medicine. Berlin Heidelberg: Springer; 2008;773–779.
24. Fan VH, Tamama K, Au A, Littrell R, Richardson LB, et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25(5):1241–1251.
25. Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28(17):2706–2717.
26. Ferreira LS, Gerecht S, Shieh HF, Watson N, Rupnick MA, et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101(3):286–294.
27. Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, et al. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11(1–2):302–309.
28. Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2(2):119–125.
29. Freed LE, Guilak F, Guo XE, Gray ML, Tranquillo R, et al. Advanced tools for tissue engineering: Scaffolds, bioreactors, and signaling. Tissue Eng. 2006;12(12):3285–3305.
30. Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, et al. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7(4):363–371.
31. Gerlach JC, Zellinger K, Patzer Li JF. Bioartificial Liver systems: Why? what? whither?. Regen Med. 2008;3(4):575–595.
32. Gobin AS, West JL. Cell migration through defined, synthetic ECM analogs. Faseb J. 2002;16(7):751–753.
33. Goshima J, Goldberg VM, Kaplan AI. The origin of bone formed in composite grafts of porous calcium phosphate ceramic loaded with marrow cells. Clin Orthop. 1991;269:274–283.
34. Grande DA, Breitbart AS, Mason J, Paulino C, Laser J, et al. Cartilage tissue engineering: Current limitations and solutions. Clin Orthop. 1999;(Suppl. 367):S176–S185.
35. Griffith LG, Naughton G. Tissue engineering: Current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014.
36. Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7(3):211–224.
37. Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26(18):3961–3971.
38. Gupta AS, Lopina ST. Synthesis and characterization of -tyrosine based novel polyphosphates for potential biomaterial applications. Polymer. 2004;45(14):4653–4662.
39. Hannallah D, Peterson B, Leiberman JR, Fu FH, Huard. Gene therapy in orthopaedic surgery. Instr Course Lect. 2003;52:753–768.
40. Hoerstrup SP, Sodian R, Daebritz S, Wang J, Bacha EA, et al. Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102(19 Suppl. 3):III44–III49.
41. Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518–524.
42. Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell-seeding density and culture period. J Biomed Mater Res. 2000;51(3):376–382.
43. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–313.
44. Hubbell JA. Biomaterials in tissue engineering. Biotechnology (NY). 1995;13(6):565–576.
45. Hutmacher DW, Singh H. Computational fluid dynamics for improved bioreactor design and 3D culture. Trends Biotechnol. 2008;26(4):166–172.
46. Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(Suppl. 1):S45–S64.
47. Ingber DE, Mow VC, Butler D, Niklason L, Huard J, et al. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006;12(12):3265–3283.
48. Ito A, Mase A, Takizawa Y, Shinkai M, Honda H, et al. Transglutaminase-mediated gelatin matrices incorporating cell adhesion factors as a biomaterial for tissue engineering. J Biosci Bioeng. 2003;95(2):196–199.
49. Kapur TA, Shoichet MS. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J Biomed Mater Res A. 2004;68A(2):235–243.
50. Karim N, Golz K, Bader A. The cardiovascular tissue-reactor: A novel device for the engineering of heart valves. Artif Organs. 2006;30(10):809–814.
51. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4(3):206–216.
52. Karp JM, Shoichet MS, Davies JE. Bone formation on two-dimensional poly(DL-lactide-co-glycolide) (PLGA) films and three-dimensional PLGA tissue engineering scaffolds in vitro. J Biomed Mater Res A. 2003;64(2):388–396.
53. Karp JM, Sarraf F, Shoichet MS, Davies JE. Fibrin-filled scaffolds for bone-tissue engineering: An in vivo study. J Biomed Mater Res A. 2004;71(1):162–171.
54. Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7(9):1035–1040.
55. Khamsi R. Labs on a chip: Meet the stripped down rat. Nature. 2005;435(7038):12–13.
56. Kharas GB, Kamenetsky M, Simantirakis J, Beinlich KC, Rizzo AT, et al. Synthesis and characterization of fumarate-based polyesters for use in bioresorbable bone cement composites. J Appl Polym Sci. 1997;66(6):1123–1137.
57. Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26(1):120–126.
58. Kim SE, Park JH, Cho YW, Chung H, Jeong SY, et al. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: Implications for cartilage tissue engineering. J Control Release. 2003;91(3):365–374.
59. Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63.
60. Kofidis T, Akhyari P, Boublik J, Theodorou P, Martin U, et al. In vitro engineering of heart muscle: Artificial myocardial tissue. J Thorac Cardiovasc Surg. 2002;124(1):63–69.
61. Kohn J. New approaches to biomaterials design. Nat Mater. 2004;3(11):745–747.
62. Kulig KM, Vacanti JP. Hepatic tissue engineering. Transpl Immunol. 2004;12(3–4):303–310.
63. L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. Faseb J. 1998;12(1):47–56.
64. Lacy PE, Hegre OD, Gerasimidi-Vazeou A, Gentile FT, Dionne KE. Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets. Science. 1991;254(5039):1782–1784.
65. Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492.
66. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926.
67. Langer RS, Vacanti JP. Tissue engineering: The challenges ahead. Sci Am. 1999;280(4):86–89.
68. Lanza RP, Kuhtreiber WM, Ecker D, Staruk JE, Chick WL. Xenotransplantation of porcine and bovine islets without immunosuppression using uncoated alginate microspheres. Transplantation. 1995;59(10):1377–1384.
69. Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: State of the art. Tissue Eng Part B Rev. 2008;14(1):61–86.
70. Lee SJ, Van Dyke M, Atala A, Yoo JJ. Host cell mobilization for in situ tissue regeneration. Rejuvenation Res. 2008;11(4):747–756.
71. Lees RK, Sordat B, MacDonald HR. Multicellular tumor spheroids of human colon carcinoma origin Kinetic analysis of infiltration and in situ destruction in a xenogeneic (murine) host. Exp Cell Biol. 1981;49(4):207–219.
72. Lendlein A, Schmidt AM, Langer R. AB-polymer networks based on oligo(epsilon-caprolactone) segments showing shape-memory properties. Proc Natl Acad Sci USA. 2001;98(3):842–847.
73. Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, et al. Neural transplantation in Parkinson’s disease: The Swedish experience. Prog Brain Res. 1990;82:729–734.
74. Louisia S, Stromboni M, Meunier A, Sedel L, Petite H. Coral grafting supplemented with bone marrow. J Bone Joint Surg Br. 1999;81(4):719–724.
75. Lutolf MP, Blau HM. Artificial stem cell niches. Adv Mater. 2009;21(32):3255–3268.
76. Lutolf MP, Lauer-Fields JL, Smoekel HG, Metters AT, Weber FE, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100(9):5413–5418.
77. Lutolf MP, Weber FE, Smoekel HG, Schense JC, Kohler T, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–518.
78. Lysaght MJ, Reyes J. The growth of tissue engineering. Tissue Eng. 2001;7(5):485–493.
79. Lysaght MJ, Jaklenec A, Deweerd E. Great expectations: Private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng Part A. 2008;14(2):305–315.
80. Ma PX, Zhang R, Xiao G, Franchesci R. Engineering new bone tissue in vitro on highly porous poly(alpha-hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res. 2001;54(2):284–293.
81. Macchiarini P, Jungebluth P, Go T, Asnagi MA, Rees LE, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030.
82. MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445(7130):874–880.
83. Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53(3):697–702 discussion 702–703.
84. Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59(4–5):207–233.
85. Marijnissen WJ, van Osch GJ, Aigner J, van der Veen SW, Hollander AP, et al. Alginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineering. Biomaterials. 2002;23(6):1511–1517.
86. Martin I, Padera RF, Vunjak-Novakovic G, Freed LE. In vitro differentiation of chick embryo bone marrow stromal cells into cartilaginous and bone-like tissues. J Orthop Res. 1998;16(2):181–189.
87. Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22(2):80–86.
88. Martin Y, Vermette P. Bioreactors for tissue mass culture: Design, characterization, and recent advances. Biomaterials. 2005;26(35):7481–7503.
89. Mehta K, Linderman JJ. Model-based analysis and design of a microchannel reactor for tissue engineering. Biotechnol Bioeng. 2006;94(3):596–609.
90. Mi FL, Shyu SS, Wu YB, Lee ST, Shyong JY, et al. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials. 2001;22(2):165–173.
91. Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2(3):205–213.
92. Moore K, MacSween M, Soichet M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006;12(2):267–278.
93. Murua A, Portero A, Orive G, Hernández RM, de Castro M, et al. Cell microencapsulation technology: Towards clinical application. J Control Release. 2008;132(2):76–83.
94. Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am., 86-A(7) 2004;1541–1558.
95. Nerem RM. Tissue engineering: The hope, the hype, and the future. Tissue Eng. 2006;12(5):1143–1150.
96. Nijst CL, Bruggeman JP, Karp JM, Ferreira L, Zumbuehl A, et al. Synthesis and characterization of photocurable elastomers from poly(glycerol-co-sebacate). Biomacromolecules. 2007;8(10):3067–3073.
97. Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, et al. Functional arteries grown in vitro. Science. 1999;284(5413):489–493.
98. Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, et al. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog Polym Sci. 2008;33(2):167–179.
99. Pancrazio JJ, Wang F, Kelley CA. Enabling tools for tissue engineering. Biosens Bioelectron. 2007;22(12):2803–2811.
100. Park YJ, Lee YM, Park SN, Sheen SY, Chung CP, et al. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials. 2000;21(2):153–159.
101. Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1267–1279.
102. Patzer 2nd JF. Advances in bioartificial liver assist devices. Ann N Y Acad Sci. 2001;944:320–333.
103. Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284(5417):1168–1170.
104. Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18(9):959–963.
105. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147.
106. Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8(6):457–470.
107. Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng. 2002;78(3):257–269.
108. Priya SG, Jungvid H, Kumar A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng Part B Rev. 2008;14(1):105–118.
109. Pulapura S, Kohn J. Tyrosine-derived polycarbonates: Backbone-modified pseudo-poly (amino acids) designed for biomedical applications. Biopolymers. 1992;32(4):411–417.
110. Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034.
111. Rodriguez-Cabello JC, Reguera J, Girotti A, Alonso M, Testera AM. Developing functionality in elastin-like polymers by increasing their molecular complexity: The power of the genetic engineering approach. Prog Polym Sci. 2005;30:1119–1145.
112. Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26(8):434–441.
113. Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65(3):389–402.
114. Saltzman WM, Olbricht WL. Building drug delivery into tissue engineering. Nat Rev Drug Discov. 2002;1(3):177–186.
115. Sarkar D, Yang JC, Gupta AS, Lopina ST. Synthesis and characterization of L-tyrosine based polyurethanes for biomaterial applications. J Biomed Mater Res A. 2009;90(1):263–271.
116. Schmidt CE, Leach JB. Neural tissue engineering: Strategies for repair and regeneration. Ann Rev Biomed Eng. 2003;5:293–347.
117. Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367(6459):170–173.
118. Seliktar D. Extracellular stimulation in tissue engineering. Ann NY Acad Sci. 2005;1047:386–394.
119. Shalaby S. Bioabsorbable Polymers. In: Swarbrick J, ed. Encyclopedia of Pharmaceutical Technology. Marcel Deker 1988;465–476.
120. Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24(13):2309–2316.
121. Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA. 2008;105(38):14347–14352.
122. Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35(2):562–569.
123. Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg. 2003;23(6):1002–1006 discussion 1006.
124. Skarja GA, Woodhouse KA. In vitro degradation and erosion of degradable, segmented polyurethanes containing an amino acid-based chain extender. J Biomat Sci.Polym Ed. 2001;12:851–873.
125. Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci. 2004;20(7):1769–1778.
126. Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci USA. 1997;94(21):11663–11668.
127. Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54(1):139–148.
128. Soker S, Machado M, Atala A. Systems for therapeutic angiogenesis in tissue engineering. World J Urol. 2000;18(1):10–18.
129. Sullivan SJ, Maki T, Borland KM, Mahoney MD, Solomon BA, et al. Biohybrid artificial pancreas: Long-term implantation studies in diabetic, pancreatectomized dogs. Science. 1991;252(5006):718–721.
130. Sutherland RM, Sordat B, Bamat J, Gabbert H, Bourrat B, et al. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986;46(10):5320–5329.
131. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676.
132. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872.
133. Thomas ED. Bone marrow transplantation. CA Cancer J Clin. 1987;37(5):291–301.
134. Tilakaratne HK, Hunter SK, Andracki, Me E, Benda JA, Rodgers VG. Characterizing short-term release and neovascularization potential of multi-protein growth supplement delivered via alginate hollow fiber devices. Biomaterials. 2007;28(1):89–98.
135. Unsworth BR, Lelkes PI. Growing tissues in microgravity. Nat Med. 1998;4(8):901–907.
136. Vacanti CA. History of tissue engineering and a glimpse into its future. Tissue Eng. 2006;12(5):1137–1142.
137. Viravaidya K, Sin A, Shuler ML. Development of a microscale cell culture analog to probe naphthalene toxicity. Biotechnol Prog. 2004;20(1):316–323.
138. Vunjak-Novakovic G, Freed LE, Biron RJ, Langer R. Effects of mixing on the composition and morphology of tissue-engineered cartilage. AIChE Journal. 1996;42(3):850–860.
139. Wendt D, Marsano A, Jakob M, Heberer M, Martin I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnol Bioeng. 2003;84(2):205–214.
140. Whang K, Healy KE, Elenz DR, Nam EK, Tsai DC, et al. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999;5(1):35–51.
141. Wiegand F, Kroncke KD, Kolb-BacHofen V. Macrophage-generated nitric oxide as cytotoxic factor in destruction of alginate-encapsulated islets Protection by arginine analogs and/or coencapsulated erythrocytes. Transplantation. 1993;56(5):1206–1212.
142. Wilson GJ, Courtman DW, Klement P, Lee JM, Yeger H. Acellular matrix: A biomaterials approach for coronary artery bypass and heart valve replacement. Ann Thorac Surg. 1995;60(Suppl. 2):S353–S358.
143. Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15(1):147–154.
144. Winn SR, Tresco PA, Zielinski B, Greene LA, Jaeger CB, et al. Behavioral recovery following intrastriatal implantation of microencapsulated PC12 cells. Exp Neurol. 1991;113(3):322–329.
145. Yamada Y, Fujimoto A, Ito A, Yoshimi R, Ueda M. Cluster analysis and gene expression profiles: A cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials. 2006;27(20):3766–3781.
146. Yang C, Hillas PJ, Baez JA, Nokelainen M, Balan J, et al. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18(2):103–119.
147. Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27(9):1889–1898.
148. Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering Part I Traditional factors. Tissue Eng. 2001;7(6):679–689.
149. Yang Y, Bolikal D, Becker ML, Kohn J, Zeiger DN, et al. Combinatorial polymer scaffold libraries for screening cell-biomaterial interactions in 3D. Adv Mater. 2008;20(11):2037–2043.
150. Zhang N, Yan H, Wen X. Tissue-engineering approaches for axonal guidance. Brain Res Brain Res Rev. 2005;49(1):48–64.
151. Zhao W, Karp JM. Controlling cell fate in vivo. Chembiochem. 2009;10(14):2308–2310.
152. Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. Faseb J. 2003;17(15):2260–2262.