CHAPTER TWO
The Power of Long Telomeres
It’s 1987. Robin Huiras is twelve years old and standing on her school’s playing field, waiting to begin a timed mile-long run. The weather is good for running—it’s a chilly Minnesota morning—and Robin is fit and slender. Although she doesn’t enjoy being put through her paces by the gym teacher, she expects to do well.
She doesn’t. The gym teacher fires the starting pistol and almost immediately the other girls in the class are ahead of Robin. She tries to catch them, but the pack recedes along the red-dirt running path. Robin is no slacker—she gives everything she’s got, but as the race goes on she falls farther and farther behind. Her final time is one of the slowest in the class, almost as if she’d stopped partway through the course and taken a leisurely stroll across the finish line, but long after the race is finished Robin is still doubled over from the exertion, gulping for air.
The next year, when Robin is thirteen, she spots a gray strand threading its way through her brown hair. Then another gray hair appears, and another, until her hair takes on the light salt-and-pepper appearance that’s common among women in their forties or fifties. Her skin changes, too—there are days when normal activities leave deeply colored bruises on her arms and legs. Robin is only a teenager, but her energy is low, her hair is turning gray, and her skin is fragile. It’s as if she’s growing old before her time.
In a very real way, that was exactly what was happening. Robin has a rare telomere biology disorder, an inherited disorder that causes extremely short telomeres and, in turn, early cell aging. Well before people with telomere biology disorders are chronologically old, they can experience rapidly accelerated aging. Outwardly, it shows up in the skin. Melanocytes, for example, which are the skin’s color cells, lose their ability to keep the skin evenly toned. The result is age blotches and spots, along with gray or white hair, even at a young age. Fingernails and toenails look old, too. Because nails have cells that turn over quickly, they become ridged and split. Bones grow older, too: osteoblasts—cells that your bones need to stay solid and strong—can stop renewing themselves. Robin’s father, who had the same telomere biology disorder, had so much bone loss and muscle pain that he needed both of his hips replaced twice before the disorder took his life at age forty-three.
But an aged appearance and even bone loss are some of the milder effects of telomere biology disorder. The more devastating ones can include scarred lungs, unusually low blood counts, a weakened immune system, bone-marrow disorders, digestive problems, and certain cancers. People with telomere disorders do not tend to live full life spans, though the precise symptoms and the average length of life varies; one of the oldest known telomere disorder patients alive right now is in her sixties.
Severe inherited forms of telomere biology disorders such as Robin’s are an extreme form of much more common conditions, which we now collectively call “telomere syndromes.” We understand which genes accidentally go wrong to cause these inherited, severe forms, and what these genes do in cells. (Eleven such genes are known to date.) Thankfully, these extreme, inherited telomere syndromes are rare; they affect about one in a million people. And thankfully, Robin was eventually able to take advantage of medical advances and undergo a successful stem cell transplant (one which contained a donor’s blood-forming stem cells). One testimony to the transplant’s success is Robin’s platelet count. Because Robin’s blood stem cells could not effectively repair their telomeres, or make new cells, her platelets had plummeted to alarmingly low numbers, with counts as low as 3,000 or 4,000. (Low blood counts are a reason she couldn’t keep up during the mile run.) Six months after the transplant, Robin’s counts shot up to more normal levels of almost 200,000. Robin, who is now in her thirties and runs an advocacy organization for people with telomere biology disorders, has more wrinkles around her mouth and eyes than other people her age. Her hair is almost entirely gray, and she sometimes experiences severe joint and muscle pain. But habitual exercise helps keep the pain at bay, and the transplant has restored much of her energy.
Severe inherited telomere syndromes carry a powerful message for all of us, because what is happening inside Robin’s cells is also happening inside your own. It’s just happening to her faster than it’s happening to you. In all of us, telomeres shrink with age. And premature cellular aging can happen—in a slower way—to basically healthy people. We can think of all of us as being susceptible, to some extent, to telomere syndromes of aging, although to much lesser degrees than Robin and her father. Patients with the inherited telomere syndromes are powerless to stop the premature aging process, because it takes place with overwhelming speed in their bodies, but the rest of us are luckier. We have much more control over premature cellular aging, because—to a surprising extent—we have some real control over our telomeres.
That control begins with knowledge—knowledge about telomeres and how their length corresponds to your daily habits and health. To understand the role telomeres play in your body, we need to turn to an unlikely source. We need to spend some time with pond scum.

POND SCUM SENDS A MESSAGE
Tetrahymena is a single-celled organism that swims valiantly through bodies of freshwater, searching for food or a mate. (There are seven sexes of Tetrahymena, a curious fact to ponder next time you are splashing around in a lake.) Tetrahymena is, literally, pond scum. Yet it’s almost adorable. Seen under a microscope, it boasts a plump little body and hairlike projections that make it look like a fuzzy cartoon creature. Look at it long enough, and you might notice a resemblance to Bip Bippadotta, the wild-haired Muppet who scats the famously infectious song “Mahna Mahna.”
Inside Tetrahymena’s cell is its nucleus, its central command center. Deep within that nucleus is a gift to molecular biologists: twenty thousand tiny chromosomes, all identical, linear, and very short. That gift makes it relatively easy to study Tetrahymena’s telomeres, those caps at the ends of chromosomes. That gift is the reason that in 1975, I (Liz) was standing in a laboratory at Yale, cultivating millions of tiny Tetrahymena in big glass lab jars. I wanted to collect enough of their telomeres to understand just what they were made of, at the genetic level.
For decades, scientists had theorized that telomeres protect chromosomes—not just in pond scum but in humans, too—but no one knew exactly what telomeres were or how they worked. I thought that if I could pinpoint the structure of the DNA in telomeres, I might be able to learn more about their function. I was driven by my desire to understand biology; at this point, no one knew that telomeres would prove to be one of the primary biological foundations of aging and health.
By using a mixture of what was essentially dish detergent and salts, I was able to release Tetrahymena’s DNA from its surrounding matter, out of the cell. Then I analyzed it, using a combination of the chemical and biochemical methods that I’d learned during my PhD graduate years in Cambridge, England. Under the dim, red, and warm safelight of the lab’s darkroom, I reached my goal. The darkroom was quiet; only a trickle of water sounded as it ran next to the old-fashioned developing tanks. I held a dripping X-ray film up to the safety light, and excitement surged through me as I understood what I was seeing. At the ends of chromosomes was a simple, repeated DNA sequence. The same sequence, over and over and over. I had discovered the structure of telomere DNA. And, in the ensuing months, as I toiled over pinpointing its details, an unexpected fact rose up: Remarkably, these tiny chromosomes were not as identical as they had seemed. Some had ends with more, and some had ends with fewer numbers of the repeats.
No other DNA behaves in this strangely variable, sequential, repeating way. The telomeres of pond scum were sending a message: There is something special here at the ends of chromosomes. Something that would turn out to be vital for the health of human cells. That variability in the lengths of the ends turns out to be one of the factors that explains why some of us live longer and healthier than others.

Figure 7: Tetrahymena. This tiny one-celled creature, which Liz studied to decode the DNA structure of telomeres and to discover telomerase, provided the first precious information about telomeres, telomerase, and a cell’s life span. This foreshadowed what was later learned in humans.
TELOMERES: THE PROTECTORS OF OUR CHROMOSOMES
It became clear from that dripping X-ray film that telomeres are composed of repeated patterns of DNA. Your DNA consists of two parallel, twisting strands that are made up of just four building blocks (“nucleotides”) that are represented by the letters A, T, C, and G. Remember grade school field trips, when you had to hold hands with a buddy as you walked through a museum? The letters of DNA operate on the buddy system, too. A always pairs with T, and C always pairs with G. The letters on the first strand of DNA pair up with their partners on the second strand. The two make up a “base pair,” which is the unit we measure telomeres in.

Figure 8: Telomere Strands Up Close. At the tips of the chromosome are the telomeres. The telomere strand is made up of repeating sequences of TTAGGG that sit across from their base pair partners, AATCCC. The more of these sequences we have, the longer our telomeres. In this diagram we depict just the DNA of telomeres, but it is not bare like this—it is covered by a protective sheath of proteins.
In human telomeres (as would later be discovered), the first strand consists of repeating sequences of TTAGGG, and they are coupled with their pairs, AATCCC, on the second strand, twisted into the helix shape that is DNA.
These are the base pairs of telomeres that, repeated thousands of times, offer a way of measuring their length. (Note some of our graphs measure telomere length in a unit called a t/s ratio, instead of base pairs, which is just another way to measure telomeres.) The repeating sequence highlights the differences between telomeres and other DNA. Genes, which are made of DNA, live within a chromosome. (Inside a cell we have twenty-three pairs of chromosomes, for a total of forty-six.) This genetic DNA is what forms your body’s blueprint, its instruction manual. Its paired letters create complicated “sentences” that send instructions for building the proteins that make up your body. Genetic DNA can help determine how quickly your heart beats, whether your eyes are brown or blue, and whether you’re going to have the long legs and arms of a distance runner. The DNA of telomeres is different. First of all, it doesn’t live inside any gene. It sits outside of all the genes, at the very edges of the chromosome that contains genes. And unlike genetic DNA, it doesn’t act like a blueprint or code. It’s more like a physical buffer; it protects the chromosome during the process of cell division. Like beefy football players who surround a quarterback, absorbing the hardest blows from the onrush of opposing players, telomeres take one for the team.
This protection is crucial. As cells divide and renew, they need their precious chromosome cargoes of genetic instruction manuals (the genes) to be delivered intact. Otherwise, how would a child’s body know to grow tall and strong? How would your cells know to produce the body traits that make you feel like you? Yet cell division is a potentially dangerous time for chromosomes and the genetic material inside. Without protection, chromosomes and the genetic material they carry could easily become unraveled. The chromosomes can break, can fuse with others, or can mutate. If your cell’s genetic instruction manuals were scrambled like this, the result would be disastrous. A mutation can lead to cell dysfunction, cell death, or even proliferation of a now-cancerous cell, and as a consequence you probably wouldn’t live very long.
Telomeres, which seal off the ends of the chromosomes, keep this unthinkable event from happening. That is the message sent to us by the special repeating sequences of telomere DNA. Jack Szostak and I (Liz) discovered this function in the early 1980s, when I isolated a telomere sequence from Tetrahymena and Jack put it into a yeast cell. The Tetrahymena telomeres protected the chromosomes of the yeast during cell division by donating some of their own base pairs.
Every time a cell divides, its precious “coding DNA” (which makes up the genes) is copied so it can stay safe and whole. Unfortunately, with each division, telomeres lose base pairs from the sequences at the two ends of each chromosome. Telomeres tend to shorten as we get older, as our cells experience more and more divisions. But the trend is not just a straight line. Take a look at the graph on the next page.
In the Kaiser Permanente Research Program on Genes, Environment, and Health study of one hundred thousand people’s salivary telomere length, telomeres on average grew shorter and shorter as people progressed from their twenties, hitting rock bottom at around age seventy-five.1 In an interesting coda, telomere length appears to stay the same or even go up as people live past seventy-five. This trend is probably not true lengthening happening; it just looks that way because the folks with shorter telomeres have passed away by this age (which is called survival bias—in any aging study, the oldest people are the healthy survivors). It’s the people with longer telomeres who are living into their eighties and nineties.
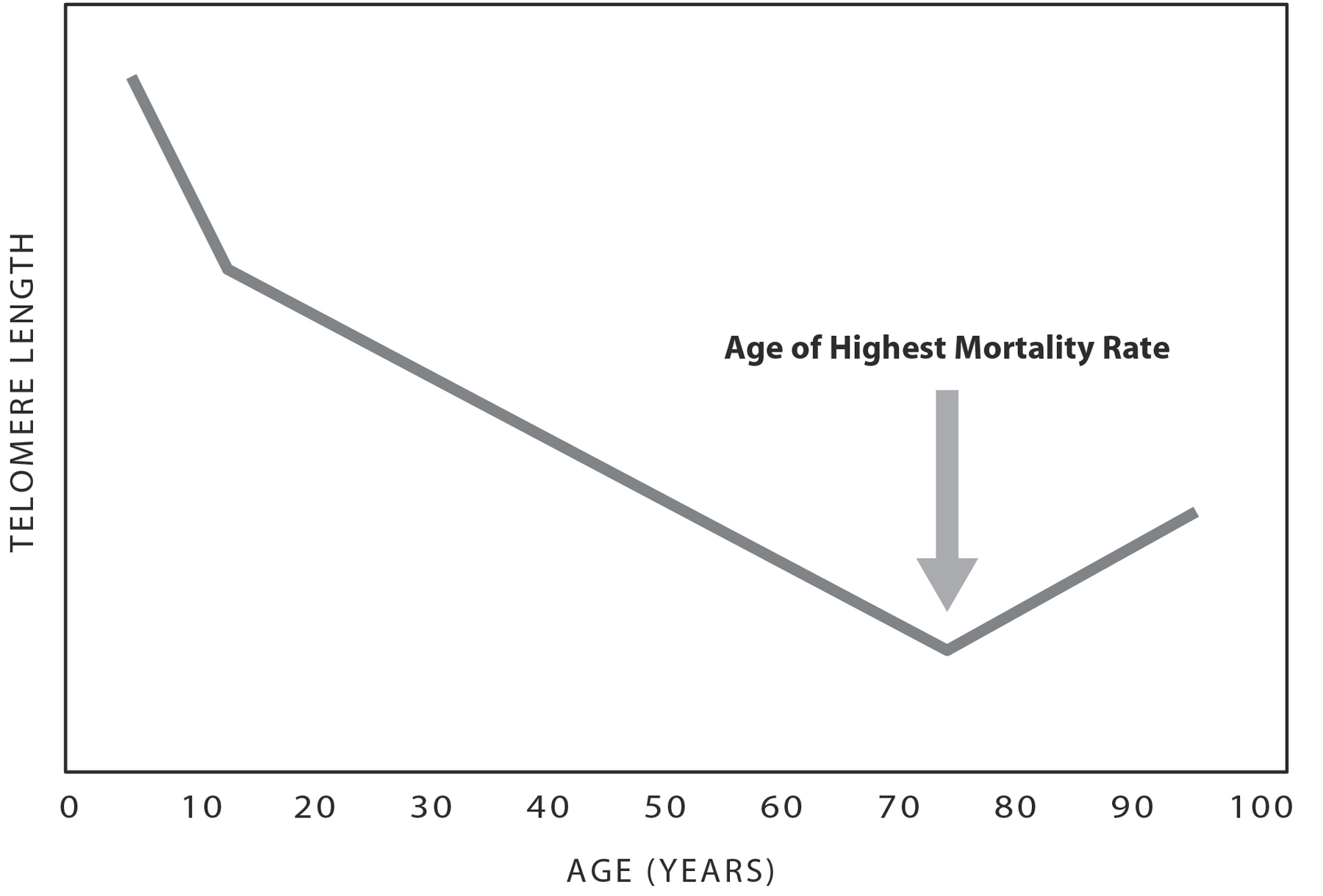
Figure 9: Telomeres Shorten with Age. Telomere length declines with age, on average. It declines fastest during early childhood and then has a slower average rate of decline with age. Interestingly, many studies find telomere length is not shorter in those who live to be a lot older than seventy years. This is thought to be due to “survival bias,” meaning that those still alive at this age tended to have been those people with longer telomeres. Their telomeres probably had been longer all along, starting from birth.
TELOMERES, THE DISEASESPAN, AND DEATH
Telomeres shorten with age. But can our telomeres really help determine how long we’ll live or how soon we enter the diseasespan?
Science says yes.
Short telomeres don’t predict death in every study, since there are many other factors that predict when we die. They do predict time of death in around half of studies, including the largest study yet. A 2015 Copenhagen study of more than sixty-four thousand people shows that short telomeres predict earlier mortality.2 The shorter your telomeres, the higher your risk of dying from cancer, cardiovascular disease, and of dying at younger ages generally, known as all-cause mortality. Look at figure 10, and you’ll see that telomere length is broken out by percentiles in ten groups. People in the 90th percentile of telomere length (with the longest telomeres) are at the left; people in the 80th percentile are just next to them; and so on all the way to the right side, where the people in the lowest percentile are represented. There is a graded response: people with the longest telomeres are the healthiest, and as telomeres get shorter, people get sicker and are more likely to die.

Figure 10: Telomeres and Death. Telomere length predicts mortality overall, and from different diseases. Those with the longest telomeres (90th percentile) have the lowest rate of death from cancers, heart disease, and all causes added up. (Figure is from the data in Rode et al., 2015.3)
The Kaiser Permanente study previously mentioned measured telomere length in one hundred thousand research volunteers who happened to be members of Kaiser’s health coverage plan. In the three years after their telomeres were measured, the people with shorter telomeres were more likely to die when all causes of death were combined.4 The study was controlled for differences among the subjects that might likely lead to differences in health and longevity, including age, gender, race and ethnicity, education, smoking, physical activity, drinking, and body mass index (BMI). Why did the scientists control for so many variables? Because any one, some, or all of these factors might in theory have been the real reasons contributing to the increased mortality, not the shortened telomeres. For example, a clear relationship exists between tobacco smoking histories and all-cause mortality rates. And many studies have found a relationship between more smoking and more telomere shortening. Yet even after correcting for all those potential explanations, the relationship between telomere shortness and all-cause mortality still held true. It does indeed look as though telomere shortness itself is a real contributor to our overall risks for mortality.
Over and over and over, telomere shortness has also been linked to the major diseases of aging. Many large studies have shown that people with shorter telomeres are more likely to have a chronic disease, such as diabetes, cardiovascular disease, lung diseases, impaired immune function, and certain types of cancers, or to develop one of these diseases over time.5 Many of these associations have now been reinforced by large reviews (called meta-analyses) that give us confidence that the relationships are accurate and reliable. Flip these findings, and the optimistic opposite is true: one study of a healthy elderly U.S. sample (the Health ABC study) showed that in the general population, people with longer telomeres in their white blood cells had more years of healthy life without any major diseases—a longer healthspan.6
TURN THE TIDE IN HEALTH
People like Robin Huiras, whose rare inherited disorder leads to telomeres that are drastically short, show us the power of telomeres. Sometimes, as in Robin’s case, it’s a kind of dark, corroding power that speeds up the cellular aging process. The good news is that we have learned a great deal about the nature of telomeres. By donating blood and tissue samples, for example, Robin and her family have helped researchers pinpoint one of the gene mutations that caused her disorder. That knowledge is a first step to better diagnoses, treatments, and, one day, a cure.
And you can use our knowledge about telomeres to turn the tide in health—in your health, the health of people in your community, and the health of generations to come. Because as you’re about to see, telomeres can change. You have the power to influence whether your telomeres are going to shorten early, or whether they are going to stay supported and healthy. To show you what we mean, we need to take you back to Liz’s lab. There, Tetrahymena telomeres began to behave in a strange, unexpected way.