 p. 713) (Fig. 16.7).
p. 713) (Fig. 16.7).Aetiology and evaluation of male infertility
Investigation of male infertility
The hypothalamus secretes LHRH, also known as gonadotrophin-releasing hormone (GnRH). This causes the pulsatile release of anterior pituitary gonadotrophins called follicle-stimulating hormone (FSH) and LH, which act on the testis. FSH stimulates the seminiferous tubules to secrete inhibin and produce sperm; LH acts on Leydig cells to produce testosterone (Fig. 12.1).
Testosterone is secreted by the interstitial Leydig cells, which lie adjacent to the seminiferous tubules in the testis. It promotes the development of the ♂ reproductive system and secondary sexual characteristics. Steroidogenesis is stimulated by a cyclic adenosine monophosphate (cAMP)–protein kinase C mechanism which converts cholesterol to pregnenolone. Further steps in the biosynthesis pathway produce intermediary substances (dehydroepiandrosterone and androstenedione), prior to producing testosterone. In blood, 60% of testosterone is attached to sex hormone-binding globulin (SHBG), 38% is bound to albumin, and 2% is free. At androgen-responsive target tissues, testosterone is converted into a potent androgen dihydrotestosterone (DHT) by intracellular 5AR (see  p. 713) (Fig. 16.7).
p. 713) (Fig. 16.7).
Spermatogenesis: the seminiferous tubules are lined with Sertoli cells, which surround developing germ cells (spermatogonia) and provide nutrients and stimulating factors, as well as secreting androgen-binding factor and inhibin (Fig. 12.2). Primordial germ cells divide to form primary spermatocytes. These undergo a first meiotic division to create secondary spermatocytes (46 chromosomes), followed by a second meiotic division to form spermatids (23 chromosomes). Finally, these differentiate into spermatozoa. This process takes 72 days. The non-motile spermatozoa leave the seminiferous tubules and pass to the epididymis for storage and maturation (until ejaculation). Spermatozoa that are not released are reabsorbed by phagocytosis.
Mature sperm has a head, middle piece, and tail (Fig. 12.3). The head is composed of a nucleus covered by an acrosome cap containing vesicles filled with lytic enzymes. The middle piece contains mitochondria and contractile filaments which extend into the tail to aid motility. After deposition at the cervix, sperm penetrate cervical mucus and travel through the uterus to the site of fertilization in the Fallopian tube, during which time they undergo functional maturation (capacitation). Sperm start to penetrate the oocyte and bind to the zona pellucida. The activation phase is initiated (by ZP3), triggering hyperactivated motility and the acrosomal reaction which leads to enzyme release, penetration into the cytoplasm of the oocyte, fusion, and fertilization.
Of note, the seminal vesicles contribute 2mL, prostate 0.5mL, and Cowper’s glands 0.1mL to overall ejaculate/semen volume.
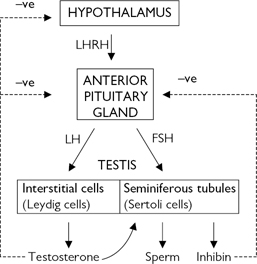
Fig. 12.1 Hypothalamic–pituitary–testicular axis.
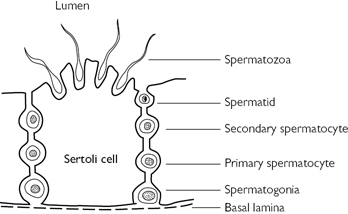
Fig. 12.2 Spermatogenesis in the seminiferous tubules of the testis.
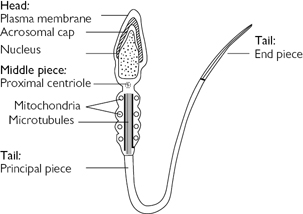
Fig. 12.3 Spermatozoa.
Failure of conception after at least 12 months of regular unprotected intercourse. The chance of a healthy couple conceiving is estimated at 20–25% per month, 75% by 6 months, and 90% at 1y.
Up to 50% of infertility is related to ♂ factors. An estimated 14–25% of couples may be affected at some point in their reproductive years. It can be primary (never conceived a child) or secondary (infertility after previous successful conception).
Due to failed fertilization of the normal ovum due to defective sperm development, function, or inadequate numbers. Abnormal epididymal function may also result in defective spermatozoa maturation transport or induce cell death. There may be abnormalities of:
•Morphology—teratozoospermia (<4% normal forms).
•Motility—asthenozoospermia (<40% motile sperm).
•Low sperm numbers—oligozoospermia (<15 × 106/mL).
•Anomalies of all three factors—oligoasthenoteratozoospermia or oligoasthenoteratospermia (OAT) syndrome.
•Absence of spermatozoa—azoospermia.
•Primary vs secondary infertility.
•Duration of time of infertility.
•♀ partner age and fertility status (♀ fertility potential is reduced significantly after age 35).
•Varicocele (present in ~40%).
•Functional sperm disorders: immunological infertility (antisperm antibodies); head or tail defects; Kartagener’s syndrome (immotile cilia); dyskinetic cilia syndrome.
•Ejaculatory problems: retrograde ejaculation causes absent or low volume ejaculate.
•Testicular injury: testicular torsion; trauma; RT.
•Endocrine disorders: Kallmann’s syndrome (isolated gonadotrophin deficiency causing hypogonadism); Prader–Willi syndrome (hypogonadism, short stature, hyperphagia, obesity); pituitary gland adenoma, radiation, or infection.
•Hormone excess: excess prolactin (pituitary tumour); excess androgen (adrenal tumour, congenital adrenal hyperplasia, anabolic steroids); excess oestrogens.
•Genetic disorders: including Klinefelter’s syndrome (47,XXY) with azoospermia,  FSH/LH, and
FSH/LH, and  testosterone; Klinefelter’s mosaicism (46,XY/47,XXY); XX ♂ and XYY syndromes.
testosterone; Klinefelter’s mosaicism (46,XY/47,XXY); XX ♂ and XYY syndromes.
•Deletions in the azoospermic factor (AZF) gene on the Y chromosome are associated with abnormal spermatogenesis, which can be inherited by ♂ offspring. Microdeletions of region AZFa has associations with Sertoli cell-only syndrome; AZFb microdeletions with maturation arrest and AZFc microdeletions with azoospermia/severe oligozoospermia.
•Cystic fibrosis (CF) is an autosomal recessive disorder with abnormality of the CF transmembrane conductance regulator (CFTR) gene on chromosome 7p. CFTR gene mutations are associated with congenital bilateral absence of the vas deferens (CBAVD), causing obstructed azoospermia. The ♀ partner should also be tested for CFTR gene mutation, and if they are also a carrier, the couple should be counselled on the  risks of having a child with CF or CBAVD.
risks of having a child with CF or CBAVD.
•♂ genital tract obstruction: due to congenital absence of the vas deferens; agenesis of the seminal vesicles/Wolffian duct abnormalities; epididymal obstruction or infection; Müllerian prostatic cysts; inguinoscrotal or pelvic surgery.
•Systemic disease: renal failure; liver cirrhosis; CF.
•Drugs: chemotherapy; steroids; antiandrogens; alcohol; recreational drugs (marijuana); sulfasalazine; smoking.
•Environmental factors: pesticides; heavy metals; hot baths.
•Infection: genital tract infections are found in 10–20%. Chlamydia trachomatis can attach to, and penetrate, sperm; Ureaplasma urealyticum reduces sperm motility. HIV infection, previous prostatitis, and bilateral epididymitis reduce semen quality. Post-pubertal bilateral mumps orchitis can also contribute to reduced fertility.
•Malignancy: testicular tumours, lymphoma, leukaemia (and their adjuvant therapies and treatments).
•Sexual and reproductive: duration of problem; frequency and timing of intercourse; use of vaginal lubricants (adversely affects sperm function); previous successful conceptions; previous birth control; erectile or ejaculatory dysfunction.
•Partner’s history: age; previous pregnancies; previous investigation for subfertility; medical history.
•Developmental: age at puberty; history of undescended testes; gynaecomastia.
•Medical and surgical: detailed assessment for risk factors—recent febrile illness; post-pubertal mumps orchitis; varicocele; testicular torsion, orchidopexy, trauma, or tumour; STIs; UTI; genitourinary and pelvic surgery; RT; respiratory diseases associated with ciliary dysfunction; diabetes.
•Drug and environmental: previous chemotherapy; exposure to substances which impair spermatogenesis or erectile function; alcohol consumption; smoking habits; hot baths.
•Family history: hypogonadism; undescended testes; parent infertility issues.
Perform a full assessment of all systems, with attention to general appearance (evidence of secondary sexual development; signs of hypogonadism; gynaecomastia). Urogenital examination should include assessment of the penis (phimosis, hypospadias, chordee) and the presence of testes, and measurement of testicular consistency, tenderness, and volume with a Prader orchidometer (normal >20mL—varies with race); palpate the epididymides (assess for tenderness, swelling/fullness, and nodules) and spermatic cord (vas deferens present or absent, nodules, varicocele); DRE of the prostate. Absence of the vas is associated with CF.
Of note, the patient’s ♀ partner should also undergo full screening and assessment for infertility by a gynaecologist, either in a separate consultation or in a joint clinic.
•Semen analysis: two specimens should be taken at least 4wk apart, collected after 2–7 days of sexual abstinence. Avoid lubricants or spermicides. Place into a sterile container. Deliver the specimens to the laboratory within 1h (ideally keeping the specimen warm in a shirt or trouser pocket). Ejaculate volume, liquefaction time, and pH are noted (Table 12.1). The sample is centrifuged at 3000g for 15min to produce a pellet, which then undergoes microscopic examination by phase contrast optics at ×200 magnification. The pellet can then be stained and re-inspected. Microscopy techniques measure sperm concentration, total numbers, morphology, and motility (Table 12.2). The mixed agglutination reaction (MAR) test detects antisperm antibodies (useful for asthenozoospermia, which can be associated with immunological infertility), although this is not commonly performed in clinical practice now. The presence of leucocytes (>1 × 106/mL in semen) suggests infection, and cultures should be requested. Low or absent ejaculate volume may suggest absence or hypoplasia of the vas deferens or seminal vesicles, ejaculatory duct obstruction, hypogonadism, or retrograde ejaculation. If semen analysis has been abnormal on two or more tests, further andrological investigation is recommended.
•Hormone measurement: serum FSH, LH, and testosterone (Table 12.3). In cases of isolated low testosterone level, it is recommended to test early morning and free testosterone levels. If LH/FSH or testosterone levels are abnormal, consider checking prolactin, as a raised level is associated with sexual dysfunction, infertility, and pituitary disease.
Offer selective genetic testing (i.e. karyotype, Y deletions) to men with sperm counts of <10 million/mL.
•Karyotype: 5–10% of azoospermic patients have Klinefelter’s syndrome (47,XXY).
•Y chromosome (AZF) microdeletion assay:
•AZFa: microdeletion predicts no spermatogenesis (Sertoli cell only).
•AZFb: associated with maturational arrest (spermatogenic rest).
•AZFc: associated with severe oligozoospermia.
If a patient carries AZFa or AZFb microdeletions, no sperm will be identified either in the ejaculate or on testicular biopsy. This has implications when considering semen retrieval for in vitro fertilization (IVF). In men with AZFc deletion, sperm are detectable in around 50%; however, the ♂ offspring produced will also be infertile.
•Post-orgasmic urine analysis: consider for patients with low ejaculate volume. The presence of >10–15 sperm per HPF confirms the diagnosis of retrograde ejaculation.
•Antisperm antibodies: associated with testicular trauma, torsion, or surgery, infection, and ductal obstruction, and seen after vasectomy reversal. Can be associated with lower pregnancy rates. Not routinely tested; some would advocate testing for couples with unexplained infertility.
•Scrotal USS: is used to assess for testicular, epididymal, and vasal abnormalities, and detection of varicocele.
•TRUS: is indicated for low ejaculate volumes, to investigate seminal vesicle obstruction (>1.5cm width) or absence, and ejaculatory duct obstruction (>2.3mm).
•Abdominal USS: if unilateral or bilateral absence of the vas deferens, as this is associated with renal anomalies.
•Vasography: the vas deferens is punctured at the level of the scrotum and injected with contrast. A normal test shows the passage of contrast along the vas deferens, seminal vesicles, and ejaculatory duct, and into the bladder, which rules out obstruction.
•Venography: used to diagnose and guide embolization treatment of varicocele.
Performed for azoospermic patients to help differentiate between obstructive and non-obstructive causes. Simultaneous sperm retrieval can be carried out [testicular exploration and sperm extraction (TESE)] for use in intracytoplasmic sperm injection (ICSI) treatment, either at the time or at a later date (following freezing and storage). The degree of spermatogenesis can be histologically scored by the Johnsen score.1 The Johnsen score ranges from 1 (neither germ cells nor Sertoli cells present in the sample) to 10 (complete spermatogenesis, many spermatozoa). Only mature spermatozoa (score 8 or above) can be used for fertility treatment. Of note, the spermatozoa retrieved from infertile men have a high risk of chromosomal and genetic abnormalities which can be passed on to offspring.
Table 12.1 WHO semen analysis characteristics
| Semen analysis parameter | Lower reference limit (95% CI) |
| Serum volume | 1.5mL (1.4–1.7) |
| pH | ≥7.2 (normal range is 7.9–8.1) |
| Total sperm count | 39 × 106 per ejaculate (33–46) |
| Sperm concentration | 15 × 106 per mL (12–16) |
| Motility | 40% progressive + non-progressive (38–40) 32% progressive motility (31–34) Forward progression > grade 2 |
| Sperm morphology | 4% normal forms (3–4) |
| Vitality | 58% live spermatozoa (55–63) |
| Time to liquefy | 5–25min |
| WBC | <1 × 106 WBC per mL |
| MAR test (for antisperm antibody) | <50% motile spermatozoa with bound particles |
| Zinc | ≥2.4μmol per ejaculate |
| Semen fructose | ≥13μmol per ejaculate |
Adapted from the World Health Organization reference values for human semen characteristics.  http://www.who.int/reproductivehealth/topics/infertility/cooper_et_al_hrupdf Table II: page 37 in ‘Human Reproduction Update, Vol.16, No.3 pp. 231–245, 2010 doi:10.1093/humupd/dmp048’.
http://www.who.int/reproductivehealth/topics/infertility/cooper_et_al_hrupdf Table II: page 37 in ‘Human Reproduction Update, Vol.16, No.3 pp. 231–245, 2010 doi:10.1093/humupd/dmp048’.
Table 12.2 Grading of sperm motility
| Grade | Type of sperm motility |
| 0 | No motility |
| 1 | Sluggish; no progressive movement |
| 2 | Slow, meandering forward progression |
| 3 | Moving in a straight line at moderate speed |
| 4 | Moving in a straight line at high speed |
Table 12.3 Clinical diagnosis on hormone assay
| FSH | LH | Testosterone | Diagnosis |
|
|
Normal | Normal | Seminiferous tubule damage (defective spermatogenesis) |
| Normal | Normal | Normal | Normal or bilateral genital tract obstruction |
|
|
|
Normal/
|
Primary hypogonadism (due to testicular failure) |
|
|
|
|
Secondary hypogonadism (hypogonadotropic hypogonadism due to defects at the pituitary or hypothalmus level) |
FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Reference
1Johnsen S (1970). Testicular biopsy score count—a method for registration of spermatogenesis in human testes. Normal values and results in 335 hypogonadal males. Hormones 1:2–25.
Defined as a sperm concentration of <15 million/mL of ejaculate. This will be identified in around 25% of patients presenting with infertility.
•Androgen deficiency/hypogonadism.
•Other: recreational drugs; medication (antiandrogens, anabolic steroids); smoking; alcohol; testicular trauma, infection, and maldescent; genetic defects on Y chromosome; chromosomal abnormalities (Klinefelter’s syndrome); neoplasia.
Often associated with abnormalities of morphology and motility. The combined disorder is called OAT syndrome. Common causes of OAT include varicocele, undescended testes, idiopathic, drug and toxin exposure, and febrile illness. Oligozoospermia is also associated with  risk of DNA fragmentation known to reduce the rate of natural conception and increase the risk of pregnancy loss.
risk of DNA fragmentation known to reduce the rate of natural conception and increase the risk of pregnancy loss.
•Hormone analysis: sperm counts of <5–10 million/mL require hormone investigation (FSH and testosterone). Severe oligozoospermia (<1 million/mL) is associated with seminiferous tubular failure, small soft testes, and  FSH. Include prolactin levels, particularly if the testosterone level is low, as hyperprolactinaemia can adversely affect spermatogenesis.
FSH. Include prolactin levels, particularly if the testosterone level is low, as hyperprolactinaemia can adversely affect spermatogenesis.
•Genetic analysis: indicated for sperm counts of <10 million/mL.
•Scrotal USS: to identify any varicocele.
Lifestyle advice and modification of risk factors. Correct the underlying cause (i.e. varicocele repair or embolization may improve testosterone levels, sperm production, and parameters). Idiopathic cases may respond to empirical medical therapy (clomiphene). There is limited evidence that antioxidants may be of benefit. Where these measures fail, couples will require assisted reproductive techniques (ART) (see  p. 575).
p. 575).
The 2y follow-up cumulative pregnancy rates are around 27% in couples where oligozoospermia is the cause of infertility.
Defined as an absence of sperm in the ejaculate fluid (and post-ejaculate urine). This is identified in around 10% of patients presenting with infertility.
•Epididymal obstruction: the commonest cause. Can be idiopathic or related to infection (i.e. Chlamydia, gonococcal), post-surgery, or due to agenesis.
•Vasal aplasia: CBAVD presents with a low ejaculate volume and impalpable vasa.
•Vas deferens obstruction: post-surgery or post-vasectomy.
•Ejaculatory duct obstruction: presents as low ejaculate volume (<1.5mL) in the presence of palpable vas deferens, with acidic pH (pH <7.0), low or absent semen fructose, and dilated seminal vesicles. Causes can be post-infective, post-surgery, or congenital, or due to a Müllerian duct cyst.
•Intratesticular obstruction: accounts for 15% of OA and presents with normal-sized testis, normal FSH/LH, and fullness of the epididymides. It can be congenital or acquired or secondary to trauma or infection/inflammation.
•Distal seminal duct obstruction: functional obstruction may be related to neuropathy or use of SSRI medication.
•Hormonal abnormality: hypogonadotrophism (Kallmann’s syndrome, pituitary tumour).
•Abnormalities of spermatogenesis: commonest cause is idiopathic (60% of NOA). Others are secondary to testicular torsion or trauma, viral orchitis, chromosomal anomalies (i.e. Klinefelter’s syndrome), or testicular failure (seen as raised FSH/LH and both vasa present).
•Hormone assay: raised FSH is suggestive of a non-obstructive cause (i.e. reduced spermatogenesis presents with  FSH associated with
FSH associated with  inhibin). Normal FSH with normal testes indicates
inhibin). Normal FSH with normal testes indicates  likelihood of obstruction.
likelihood of obstruction.
•Semen analysis: the volume and pH of the semen can be indicative of the underlying pathology. Seminal vesicle fluid is alkaline, so its absence (due to obstruction) will result in an acidic pH of the ejaculate (and reduced volume), whereas prostatic fluid is acidic, so its absence will result in a raised pH.
•Genetic testing/chromosomal analysis: chromosomal anomalies tend to be associated with lower sperm counts. Karyotyping is used to identify Klinefelter’s syndrome in patients presenting with azoospermia, small soft testes, gynaecomastia,  FSH/LH, and
FSH/LH, and  testosterone. Assessment should also be made for Y chromosome (AZF) microdeletions. CF gene analysis is indicated for ♂ patients with unilateral or bilateral non-palpable vas deferens. Remember to include the ♀ partner to exclude carrier status in both.
testosterone. Assessment should also be made for Y chromosome (AZF) microdeletions. CF gene analysis is indicated for ♂ patients with unilateral or bilateral non-palpable vas deferens. Remember to include the ♀ partner to exclude carrier status in both.
•TRUS: assesses for absence or blockage of vas deferens and ejaculatory duct obstruction. Exclude CF in patients with vas deferens defects.
•Renal tract USS: CBAVD as it is associated with unilateral renal agenesis.
•Vasogram: to assess for vas deferens obstruction.
•Testicular biopsy: to help distinguish between obstructed and non-obstructed cases where the aetiology is not clear clinically. Can be combined with sperm retrieval for later therapeutic use.
•Bilateral absence or agenesis of vas deferens (CBAVD): this is associated with mutations in the CFTR gene. Most of these patients are not candidates for reconstruction, as they have a defect in sperm transport from mid epididymis to the seminal vesicles, and therefore ART are required in the form of microsurgical epididymal sperm aspiration (MESA). Alternative options include percutaneous epididymal sperm aspiration (PESA) and TESE.
•Obstructive cause with normal testis: if an isolated obstruction of the epididymis is identified, vasoepididymostomy can be performed. A microsurgical reversal of vasectomy (vasovasostomy) can be performed in the case of previous vasectomy, with good results. There is a trend to offer simultaneous TESE (for semen retrieval and storage), particularly if there has been a long interval since the vasectomy and if the ♀ partner is older (>35y). If reconstruction is not possible, TESE alone may be needed.
•Ejaculatory duct obstruction: transurethral resection of ejaculatory duct; deroofing or incision of any obstructing cysts. Alternatives include using MESA or TESE instead.
•Intratesticular obstruction: requires TESE.
•Primary testicular failure with testicular atrophy: microsurgical TESE with ICSI and IVF. Consider artificial insemination by donor (AID) if this fails.
•Primary testicular failure with normal testis: TESE with ICSI and IVF, or AID.
The chances of retrieving sperm by TESE in NOA are around 50%.
The results of ICSI are better with ejaculated (vs retrieved) semen, and from semen extracted from men with OA (vs NOA). Higher birth rates are seen in OA. Live birth and pregnancy rates for NOA with successful spermatozoa extraction is around 30–50%.
Dilatation of veins in the pampiniform plexus of the spermatic cord. For grading, see Table 12.4.
Found in 15% of men in the general population, with 20–40% of ♂ presenting with primary infertility and 45–80% of men with secondary infertility. Rare prior to puberty; present in ~10% of adolescents. Bilateral or unilateral (left side affected in 90%).
Incompetent valves in the internal spermatic veins lead to retrograde blood flow, vessel dilatation, and tortuosity of the pampiniform plexus. The left internal spermatic (testicular) vein enters the left renal vein at right angles and is under a higher pressure than the right vein, which enters the vena cava obliquely at a lower level. As a consequence, the left side is more likely to develop a varicocele. Most are idiopathic; rarely are caused by an underlying renal or retroperitoneal malignancy.
Testicular venous drainage is via the pampiniform plexus, a meshwork of veins encircling the testicular arteries. This arrangement normally provides a countercurrent heat exchange mechanism which cools arterial blood as it reaches the testis. Varicoceles adversely affect this mechanism, resulting in elevated scrotal temperatures and consequent deleterious effects on spermatogenesis (± loss of testicular volume over time).
Table 12.4 Varicocele grading system
| Grade | Size | Definition |
| 0 | Subclinical | Detected only on USS |
| 1 | Small | Palpable only with Valsalva manoeuvre |
| 2 | Moderate | Palpable without Valsalva |
| 3 | Large | Visible through the scrotal skin |
The majority are asymptomatic, although large varicoceles may cause pain or a heavy feeling in the scrotal area. Examine, both lying and standing, and ask the patient to perform the Valsalva manoeuvre (strain down). A varicocele is identified as a mass of dilated and tortuous veins above the testicle (‘bag of worms’), which decompress on lying supine. Examine also for testicular atrophy.
Urgently assess and investigate if there is a solitary, right-sided varicocele, a recent-onset symptomatic varicocele, and varicoceles which remain tense when the patient lies down, to exclude renal carcinoma causing vena caval obstruction or compression from other retroperitoneal masses.
•Scrotal Doppler USS: is diagnostic (venous diameter >3.5mm with patient supine) and allows for assessment of testicular health.
•Venography: is the ‘gold standard’ but is reserved for patients considering embolization or for varicocele recurring after treatment.
•Semen analysis: varicoceles are associated with low or absent sperm counts, reduced sperm motility, and abnormal morphology, either alone or in combination (OAT syndrome). The higher the grade of the varicocele, the lower the semen count.
•Urinary tract USS: reserve for a solitary, right-sided varicocele or suspicious presentations of an acute-onset, symptomatic varicocele that does not decompress on lying.
•Adolescents: pain, bilateral large varicoceles, varicocele in a solitary testis, small testicular volume/persistent delayed testicular growth by >20% (as compared with non-affected side), and for impaired semen quality.
•Adults: repair is indicated for symptomatic varicoceles and pain.
It is also performed for subfertility to improve semen parameters,1 with some studies showing improved pregnancy rates.1 It is recommended in the USA for a palpable varicocele associated with an abnormal semen analysis.2 The EAU recommends repair in men with a clinical varicocele, oligozoospermia, and otherwise unexplained couple infertility. Varicoceles are thought to be associated with a risk of  sperm DNA damage/fragmentation.3 Intact sperm DNA integrity is important for normal fertilization and growth of the embryo, and therefore, offering varicocele repair can potentially improve the success rates of pregnancy, including those in couples needing artificial reproductive techniques.4
sperm DNA damage/fragmentation.3 Intact sperm DNA integrity is important for normal fertilization and growth of the embryo, and therefore, offering varicocele repair can potentially improve the success rates of pregnancy, including those in couples needing artificial reproductive techniques.4
However, this remains controversial, and in the UK, the Cochrane review5 failed to identify significant benefit to pregnancy rates. NICE does not recommend routine varicocele repair for infertility reasons. Patients should be fully counselled on the limitations of varicocele repair for infertility before being booked for treatment.
This is the first-line management. It is an interventional radiological technique where the femoral vein is used to access the spermatic veins for venography and embolization (with coils or other sclerosing agents), with success rates of >90%.
•Microsurgical varicocelectomy ± Doppler USS guidance: reports the best surgical success rates and the fewest complications. Deliver the spermatic cord via a subinguinal approach, and use an operating microscope to isolate the veins and tie them off; 1–2% risk of testicular artery injury.
•Subinguinal (Marmar) approach: the external spermatic veins are accessed and ligated via a small transverse incision below the external ring.
•Inguinal (Ivanissevich) approach: the inguinal canal is incised to access the spermatic cord, and the external spermatic veins are tied off as they exit the internal ring.
•High retroperitoneal (Palomo) approach: a muscle-splitting incision is made near the anterior superior iliac spine, and the internal spermatic veins are ligated at that level.
•Laparoscopic: the internal spermatic veins are occluded high in the retroperitoneum.
Varicocele recurrence; hydrocele formation; testicular atrophy, haematoma; ilioinguinal nerve damage, and wound infection.
Overall 95% surgical success rate. Semen analysis should be repeated 3 months post-operatively for men undertaking the procedure for infertility reasons. Semen parameters can improve by up to 50% after varicocele repair, and overall, 70% of men have improvement of semen parameters. Patients with lower counts gain most from repair; the best results are with clinically apparent varicoceles.
When offering varicocele surgery for fertility reasons, check the ♀ partner’s age. If there is an older partner, and a significant (grade 3) varicocele, consider recommending that the couple proceed straight to IVF.
References
1Abdel-Meguid TA, Al-Sayyad A, Tayib A, et al. (2011). Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol 59:455–61.
2American Urological Association and the American Society for Reproductive Medicine (2001). Report on varicocele and infertility. Available from:  https://www.auanet.org/guidelines/archived-documents.
https://www.auanet.org/guidelines/archived-documents.
3Wang YJ, Zhang RQ, Lin YJ, et al. (2012). Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online 25:307–14.
4Esteves SC, Roque M, Agarwal A (2016). Outcome of assisted reproductive technology in men with treated and untreated varicocele: systematic review and meta-analysis. Asian J Androl 18:254–8.
5Kroese AC, de Lange NM, Collins J, et al. (2012). Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev 10:CD000479.
Aim to identify and treat reversible causes of infertility and improve semen quality. Advice on modification of lifestyle factors (i.e. reduce alcohol consumption, avoid hot baths), and review medications.
Treat any positive semen, urine, or urethral cultures with the appropriate antibiotics.
•Secondary hypogonadism (pituitary intact): may respond to administration of hCG, which stimulates an increase in testosterone and testicular size. If the patient remains azoospermic after 6 months of treatment, FSH is added (human recombinant FSH) or human menopausal gonadotrophin (HMG). Pulsatile LHRH can be administered SC via a mini-pump and is used for treating secondary hypogonadism due to a hypothalamic cause (Kallman’s syndrome).
•Hyperprolactinaemia: is treated with dopamine agonists. Arrange an MRI to rule out a pituitary tumour.
•Antioestrogens (clomifene citrate 25mg od): are used empirically to increase LHRH, which stimulates endogenous gonadotrophin secretion. Used selectively for idiopathic oligozoospermia and OAT.
Vitamin E supplements have been shown to improve sperm function and IVF success rates; zinc and folic acid may help increase sperm concentrations.
ED may be treated conventionally (oral, intraurethral, intracavernosal drugs; vacuum devices or prostheses). Ejaculatory failure may respond to sympathomimetic drugs (desipramine), or electroejaculation or vibro-ejaculation (used in SCI) where an electrical stimulus is used to produce ejaculation. It is delivered via a rectal probe to the post-ganglionic sympathetic nerves that innervate the prostate and seminal vesicles.
•Epididymal obstruction: can be overcome by microsurgical anastomosis between the epididymal tubule and vas (vasoepididymostomy).
•Vas deferens obstruction: is treated by microsurgical re-anastomosis of the ends of the vas (vasovasotomy) and is used for vasectomy reversal. Highest success rates for finding viable sperm occur in the first 8y post-vasectomy (80–90%); overall pregnancy rates are ~50%.
•Ejaculatory duct obstruction: requires transurethral resection of the ejaculatory ducts (TURED).
•CBAVD: consider MESA or TESE.
•Varicocele: consider repair for men with a clinical (palpable) varicocele and abnormal semen analysis (oligozoospermia). First-line treatment is embolization. Optimal open repair is microsurgical subinguinal varicocelectomy.
(See Table 12.5.)
Sperm are removed directly from the epididymis by PESA or MESA. If these methods fail, TESE by conventional biopsy or microsurgical techniques, or testicular exploration and sperm aspiration (TESA) may be tried. Sperm undergo cryo-preservation until required. Later, they are separated from seminal fluid by dilution and centrifugation methods, with further selection of motile sperm and normal forms using Percoll gradient techniques. In men with complete AZFa and AZFb microdeletions, sperm extraction procedures are contraindicated as the likelihood of successful sperm retrieval is extremely low.
Prior to cryo-preservation, semen is initially quarantined and all men must undergo HIV and hepatitis B and C testing. If positive, semen can undergo washing techniques but should be stored separately. Additional analysis is performed for non-partner semen donations (selected genetic testing, syphilis and C. trachomatis testing). Semen is frozen, and samples are immersed in liquid nitrogen for storage (–196°C). Cryo-preservation inevitably does cause deterioration of semen quality, which is affected particularly by the freezing and thawing processes, but paternity success rates overall are comparable to fresh semen for ICSI.
•Following semen extraction (i.e. TESE) to facilitate future attempts at ART.
•Can be offered at the time of testicular biopsy (performed for diagnosis of infertility).
•Offered to men prior to undergoing chemo- or radiotherapy for cancer (i.e. prior to radical orchidectomy).
•For medical conditions associated with a risk of  semen quality.
semen quality.
•Following semen harvest after electroejaculation (SCI patients; psychogenic anejaculation).
•Intrauterine insemination (IUI): following ovarian stimulation, sperm are placed directly into the uterus.
•IVF: controlled ovarian stimulation with gonadotrophins produces oocytes which are then retrieved by transvaginal USS-guided needle aspiration. Oocytes and sperm are placed in a Petri dish for fertilization to occur. Embryos are incubated and cultured for 2–3 days and then transferred to the uterine cavity. Pregnancy rates are 20–30% per cycle.
•Gamete intra-Fallopian transfer (GIFT): oocytes and sperm are mixed and deposited into the Fallopian tubes via laparoscopy. Variations include zygote intra-Fallopian transfer (ZIFT) and tubal embryo transfer (TET).
•ICSI: a single spermatozoa is injected directly into the oocyte cytoplasm (through the intact zona pellucida). The advantage is that fewer sperm are needed. ICSI is always combined with IVF, and the clinical pregnancy rate is 28–40% per cycle. Good success is seen with TESE, TESA, and PESA in combination with ICSI (better results with OA patients). Most report similar success between fresh and frozen semen. Offspring conceived through ICSI have a 3-fold higher risk of sex chromosome anomalies, compared to natural conception.
Table 12.5 Summary of techniques used in assisted reproduction
| Technique | Acronym | Indications |
| Percutaneous epididymal sperm aspiration | PESA | Epididymal obstruction (OA); ED |
| Microsurgical epididymal sperm aspiration | MESA | Epididymal obstruction (OA) |
| Testicular exploration and sperm aspiration | TESA | NOA; testicular failure; SCI; ED (can also use for OA) |
| Testicular exploration and sperm extraction | TESE | NOA; testicular failure; OAT; SCI (can also use for OA) |
| Microsurgical TESE | Micro-TESE (gold standard) | NOA; testicular failure; OAT (can also use for OA) |
ED, erectile dysfunction; NOA, non-obstructive azoospermia; OA, obstructive azoospermia; OAT, oligoasthenoteratozoospermia; SCI, spinal cord injury.
Bilateral division of the vas deferens to achieve ‘permanent’ sterilization for contraceptive reasons (also see  pp. 790–791). It is performed most commonly under LA. The no-scalpel (or Li) technique is associated with the lowest risks. Excise 1–2cm length of the vas and occlude the lumen; this is done most effectively by cauterization of the lumen of the divided vas using a needle diathermy and suturing the fascia between the two divided vas ends (fascial interposition). Alternative occlusion techniques are to suture ligate the ends of the vas or to fold over the ends of the vas and then tie.
pp. 790–791). It is performed most commonly under LA. The no-scalpel (or Li) technique is associated with the lowest risks. Excise 1–2cm length of the vas and occlude the lumen; this is done most effectively by cauterization of the lumen of the divided vas using a needle diathermy and suturing the fascia between the two divided vas ends (fascial interposition). Alternative occlusion techniques are to suture ligate the ends of the vas or to fold over the ends of the vas and then tie.
•Vasectomy should be considered irreversible.
•Couples need to use alternative contraception until two negative semen analysis specimens are achieved.
•Infection (wound; epididymitis).
•Early failure (due to recanalization); risk is around 1 in 200.
•Late failure; risk of pregnancy of around 1 in 2000.
Post-vasectomy semen analysis should be performed at 16wk (preferably after the patient has produced 24 ejaculates),1 with a second sample at around 20wk, post-operatively. The patient has ‘clearance’ (and alternative forms of contraception can stop) when there is confirmation of no motile spermatozoa on both tests; ideally, there should be two azoospermic specimens.
If motile spermatozoa are identified post-vasectomy, generally, this is evidence of failure. Special clearance may be granted to indicate that it is safe to reply on the vasectomy for contraception if there are <10 000 non-motile spermatozoa per millilitre in semen samples at least 7 months after vasectomy.
The best outcomes are seen using microsurgical techniques to first identify and then dissect the obstructed ends of the vas to ensure they are healthy and patent, and then re-anastomose using very fine non-absorbable sutures in layers to achieve closure. It can take up to 18 months to get the maximal number of spermatozoa reappearing in the semen. The keys points are that success is directly related to the interval of time since the vasectomy was performed, with longer durations being associated with poorer outcomes for patency and paternity rates (Table 12.6).2 It is also important to consider the age of the ♀ partner. For women aged <35y, vasectomy reversal is a preferable option for conception, rather than going straight to ICSI.
Table 12.6 Patency and pregnancy rates following vasectomy reversal over time
| Post-vasectomy | Patency rate (%) | Pregnancy rate (%) |
| <3y | 97 | 76 |
| 3–8y | 88 | 53 |
| 9–14y | 79 | 44 |
| >15y | 71 | 30 |
Data sourced from Belker AM, Thomas AJ Jr, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol 1991;145:505–11.
References
1Hancock P, McLaughlin E (2002). British Andrology Society guidelines for the assessment of post vasectomy semen samples. J Clin Pathol 55:812–16.
2Belker AM, Thomas AJ Jr, Fuchs EF, et al. (1991). Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol 145:505–11.