 p. 626).
p. 626).Basic physiology of the bladder and urethra
Renal physiology: glomerular filtration and regulation of renal blood flow
Renal physiology: regulation of water balance
Renal physiology: regulation of sodium and potassium excretion
The bladder has an endothelial lining (urothelium) on a connective tissue base (lamina propria), surrounded by smooth muscle (detrusor), with outer connective tissue (adventitia). The urothelium consists of multilayered transitional epithelium, with numerous tight junctions that render it impermeable to water and solutes. Detrusor muscle is a homogenous mass of smooth muscle bundles. c-kit antigen-positive ‘interstitial cells’ exist around detrusor bundles and in the suburothelium and play a role in modulating contractile behaviour of adjacent smooth muscle. The bladder base is known as the trigone—a triangular area with the two ureteric orifices and the internal urinary meatus forming the corners. Intravesical pressure during filling is low due to reciprocal relaxation in a bladder with normal compliance. The main excitatory motor input to the bladder is from the autonomic nervous system and is predominantly parasympathetic innervation (S2–4). Preganglionic nerve fibres are conveyed to the bladder in the pelvic nerves and then synapse with cholinergic post-ganglionic nerve cells in the pelvic plexus and on the bladder, which, when activated, cause muscle contraction. Sympathetic innervation (T10–L2) via the hypogastric plexus plays a role in urine storage (see  p. 626).
p. 626).
The bladder neck (and posterior urethra) is normally closed during filling. It is composed of circular smooth muscle (with sympathetic innervation) and is also referred to as the internal sphincter. High pressure is generated at the midpoint of the urethra in women and at the level of the membranous urethra in men where the urethral wall is composed of a longitudinal and a circular smooth muscle coat, surrounded by striated muscle (external urethral sphincter).
The striated part of the sphincter receives motor innervation from the somatic pudendal nerve derived from S2–4 in a region in the sacral spinal cord called ‘Onuf’s nucleus’. It has voluntary control, and ACh mediates contraction. The smooth muscle component of the sphincter has myogenic tone and receives excitatory and inhibitory innervation from the autonomic nervous system. Contraction is enhanced by sympathetic input (NA) and ACh. Inhibitory innervation is nitrergic (NO) (see  pp. 626–627).
pp. 626–627).
As the bladder fills, sensory afferent nerves (A-delta) respond to stretch in the bladder wall and send information about bladder filling via the spinothalamic tracts to the sacral micturition centre and up to the PMC. During urine storage, the PMC mediates enhanced external urethral sphincter activity (so causing constriction of the sphincter). There is also somatic outflow via the pudendal nerve to the external striated sphincter muscle to cause contraction, and sympathetic outflow to constrict the internal smooth muscle sphincter (bladder neck) and also to inhibit ganglia in the bladder wall. At a socially acceptable time, the voiding reflex is activated (Fig. 18.1). Neurones in the PAG in the pons trigger a switch to the PMC in the brainstem to activate the voiding reflex. Inhibition of somatic input relaxes the external striated sphincter muscle, and sympathetic inhibition causes coordinated bladder neck smooth muscle (internal sphincter) relaxation. Simultaneous stimulation of detrusor smooth muscle by parasympathetic cholinergic nerves causes the bladder to contract. Activation of nitrergic nerves reduces the intraurethral pressure, resulting in bladder emptying (also see  pp. 626–627).
pp. 626–627).
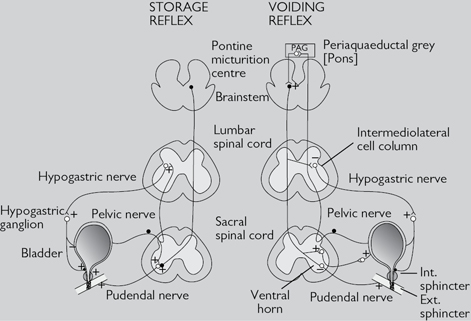
Fig. 18.1 Diagram representing the storage and voiding pathways of the micturition reflex. Micturition is stimulated by activity in the parasympathetic (pelvic) nerves and inhibited by activity in the sympathetic (hypogastric) nerves and pudendal nerves.
The kidneys and ureters lie within the retroperitoneum (behind the peritoneal cavity). The hila of the kidneys lie on the transpyloric plane (vertebral level L1). Each kidney is composed of a cortex surrounding the medulla which forms projections (papillae) that drain into cup-shaped, epithelial-lined pouches called calyces. The calyx draining each papilla is known as a minor calyx, and several minor calyces coalesce to form a major calyx, several of which drain into the central renal pelvis (Fig. 18.2). The renal artery, which arises from the aorta at vertebral level L1/2, branches to form interlobar arteries which, in turn, form arcuate arteries and then cortical radial (interlobular) arteries, from which the afferent arterioles are derived. Venous drainage occurs into the renal vein. There are two capillary networks in each kidney—a glomerular capillary network (lying within Bowman’s capsule) which drains into a peritubular capillary network surrounding the tubules (PCT, LoH, DCT, and CDs). The capillaries drain into venous channels which drain into interlobular veins, then arcuate veins, eventually draining into the renal vein via interlobar veins.
•Anterior relations of the right kidney are, from top to bottom, the adrenal (suprarenal) gland, liver, and hepatic flexure of the colon. Medially and anterior to the right renal pelvis is the second part of the duodenum. The anterior relations of the left kidney are, from top to bottom, the adrenal gland, stomach, spleen, and splenic flexure of the colon. Medially lies the tail of the pancreas.
•Posterior relations of both kidneys are, superiorly, the diaphragm and lower ribs, and inferiorly (from lateral to medial), the transversus abdominis, quadratus lumborum, and psoas major muscles.
Each kidney has 1 million functional units, or nephrons (Fig. 18.3). These consist of a glomerular capillary network, surrounded by podocytes (epithelial cells) that project into Bowman’s capsule, which then drains into a tubular system. This includes the PCT, LoH, DCT, collecting tubule (CT), and CD. Blood is delivered to the glomerular capillaries by an afferent arteriole and drained by an efferent arteriole. An ultrafiltrate of plasma is formed within the lumen of Bowman’s capsule, driven by Starling forces across the glomerular capillaries. Reabsorption of salt and water occurs in the PCT, LoH, DCT, and CD, although the majority of glomerular filtrate is absorbed in the PCT (Table 18.1). The LoH generates hypertonicity; its descending limb is only permeable to water, whereas its ascending limb is only permeable to solutes. The role of the DCT is fine adjustment of the composition of urine by selective reabsorption or secretion of solutes.
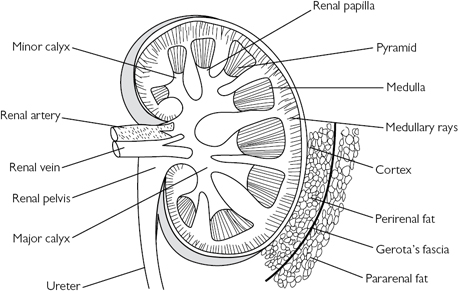
Fig. 18.2 Basic renal anatomy.
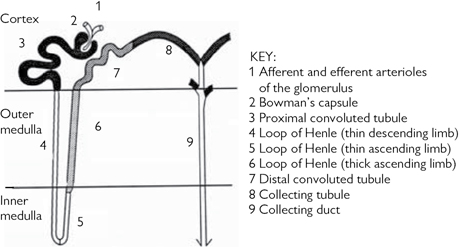
Fig. 18.3 The nephron.
Table 18.1 Solute and water reabsorption by different parts of the nephron
| Solute | PCT | LoH | DCT | CD |
| Sodium | 66% | 25% (in TAL) | ~5% | ~5% under control of aldosterone |
| Potassium | Majority | Reabsorption by intercalated cells; secretion by principal cells | ||
| Water | 66% (by osmosis) | ADH  permeability to water permeability to water |
||
| Magnesium | 80% | <5% | ||
| Phosphate | 90% | |||
| Calcium | 60% passive transport | 20% | 10% active transport | |
| Bicarbonate | Majority | |||
| Glucose | Majority | |||
| Amino acids | Majority |
CD, collection duct; DCT, distal convoluted tubule; LoH, Loop of Henle; PCT, proximal convoluted tubule; TAL, thick ascending limb (of the LoH).
Clearance is the volume of plasma that is completely cleared of solute by the kidney per minute. The clearance ratio for a substance indicates the amount of active reabsorption or excretion (i.e. ratio <1 = actively reabsorbed; >1 = actively excreted). Clearance of a substance from plasma can be expressed mathematically as:

where U is the concentration of a given substance in urine, P is its concentration in plasma, and V is the urine flow rate.
Glomerular filtration is driven by Starling forces—a hydrostatic pressure gradient between the capillary and Bowman’s capsule, which favours filtration, and colloid oncotic pressure which opposes filtration. The GFR is the clearance for any substance which is freely filtered and is neither reabsorbed, secreted, nor metabolized by the kidney. For such a substance, clearance is equivalent to the GFR. Where a substance is both filtered at the glomerulus and secreted by the renal tubules, its clearance will be greater than the GFR. Where a substance is filtered at the glomerulus but reabsorbed by the renal tubules, its clearance will be less than the GFR.
Clinically, the GFR is estimated using creatinine and is ~125mL/min. Of note, serum creatinine is an insensitive marker of early renal impairment, as the GFR needs to fall below 60–80mL/min before a rise in creatinine is seen.
The GFR is directly related to the renal plasma flow (RPF). Experimentally, the GFR can be accurately calculated by measuring the clearance of inulin (a substance which is freely filtered by the glomerulus and is neither secreted nor reabsorbed by the kidneys), using the equation:

Thus, the volume of plasma from which, in 1min, the kidneys remove all inulin is equivalent to the GFR. Factors affecting the GFR are:
•Rate of blood flow through the glomerulus.
•Permeability of the glomerular capillary wall (K).
•Surface area of the glomerular capillary bed (S).
•Differences in hydrostatic pressure between the glomerular capillary lumen (Pgc) and Bowman’s space (Pt).
•Differences in oncotic pressure between the glomerular capillary (πgc) and Bowman’s space (πt) (although autoregulation of blood flow tends to keep the GFR constant, despite a varying range of incoming perfusion pressures).
This can be represented by the equation:
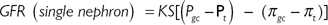
Normally about one-fifth (120mL/min) of plasma that flows through the glomerular capillaries (600mL/min) is filtered (filtration fraction = GFR/RPF).
The kidneys represent <0.5% of the body weight, but they receive 25% of cardiac output (~1300mL/min through both kidneys; 650mL/min per kidney). Combined blood flow in the two renal veins is about 1299mL/min, and the difference in flow rates represents the urine production rate (i.e. ~1mL/min).
RBF is defined as the pressure difference between the renal artery and the renal vein divided by the renal vascular resistance. The glomerular arterioles are the major determinants of vascular resistance. RBF remains essentially constant over a range of perfusion pressures (780–180mmHg, i.e. RBF is autoregulated). Autoregulation requires no innervation and probably occurs via:
•A myogenic mechanism:  pressure in the afferent arterioles causes them to contract, thereby preventing a change in RBF.
pressure in the afferent arterioles causes them to contract, thereby preventing a change in RBF.
•Tubuloglomerular feedback: the flow rate of tubular fluid is sensed at the macula densa of the juxtaglomerular apparatus (JGA), and, in some way, this controls flow through the glomerulus to which the JGA is opposed.
Sympathetic nerves innervate the glomerular arterioles. A reduction in circulating volume (such as blood loss) can stimulate sympathetic nerves, causing the release of NA (which acts on α1-adrenoceptors on the afferent arteriole) to cause vasoconstriction. This results in reduced RBF and GFR.
•Angiotensin II constricts the efferent and afferent arterioles and reduces RBF.
•ADH, ATP, and endothelin all cause vasoconstriction and reduce RBF and GFR.
•NO causes vasorelaxation and increases RBF.
•ANP causes afferent arteriole dilatation and increases RBF and GFR.
Total body water (TBW) is 42L. It is contained in two major compartments—the intracellular fluid (ICF or the water inside cells) which accounts for 28L and the extracellular fluid (ECF or water outside of cells) representing 14L. ECF is further divided into interstitial fluid (ISF; 11L), transcellular fluid (1L), and plasma (3L). Hydrostatic and osmotic pressures influence movement between the compartments. Water is taken in from fluids and food, and from oxidation of food. Water is lost from urine, faeces, and insensible losses. Intake and losses usually balance (72L/day), and TBW remains relatively constant. The role of the kidney is regulation of the volume and composition of ECF by constant adjustment of solutes and water to maintain a normal concentration.
ADH is secreted from the posterior pituitary in response to stimulus from changes in plasma osmolarity (detected by osmoreceptors in the hypothalamus) or changes in BP or volume (detected by baroreceptors in the left atrium, aortic arch, and carotid sinus). These changes also stimulate the thirst centre in the brain.
The action of ADH on the kidney:
•Increases CD permeability to water (via aquaporin water channel proteins) and urea.
•Increases LoH and CD reabsorption of sodium chloride (NaCl).
Body fluids become hypotonic, and ADH release and thirst are suppressed. In the absence of ADH, the CD is impermeable to water and a large volume of hypotonic urine is produced, so restoring normal plasma osmolarity.
Body fluids are hypertonic, and ADH secretion and thirst are stimulated. The CD becomes permeable; water is reabsorbed into the lumen, and a small volume of hypertonic urine is excreted.
The ability to concentrate or dilute urine depends on the countercurrent multiplication system in the LoH. Essentially, a medullary concentration gradient is generated (partly by the active transport of NaCl), which provides the osmotic driving force for the reabsorption of water from the lumen of the CD when ADH is present.
Children have a circadian rhythm in ADH secretion high at night and low during the day. Adults essentially have constant ADH secretion over a 24h period, with slight increases occurring around mealtimes. At these times,  ADH secretion probably acts to prevent sudden increases in plasma osmolarity that would otherwise occur due to ingestion of solutes in a meal.
ADH secretion probably acts to prevent sudden increases in plasma osmolarity that would otherwise occur due to ingestion of solutes in a meal.
NaCl is the main determinant of ECF osmolality* and volume. Two-thirds of sodium (Na+) is reabsorbed in the PCT by either primary active transport [Na+-K+-ATPase pump which transports Na+ in and potassium (K+) out] or by secondary active transport (specialized Na+ channels allow Na+ in and transfer of other solutes in and out of the cell).
Low-pressure receptors in the pulmonary vasculature and cardiac atria and high-pressure baroreceptors in the aortic arch and carotid sinus recognize changes in the circulating volume.  blood volume triggers
blood volume triggers  sympathetic nerve activity and stimulates ADH secretion, which results in reduced NaCl excretion. Conversely, when blood volumes are
sympathetic nerve activity and stimulates ADH secretion, which results in reduced NaCl excretion. Conversely, when blood volumes are  , sympathetic activity and ADH secretion are suppressed and NaCl excretion is enhanced (natriuresis). A variety of natriuretic peptides have been isolated which cause natriuresis. Under physiological conditions, renal natriuretic peptide (urodilatin) is the most important of these. ANP, released after atrial distension, may influence Na+ output under conditions of heart failure (acting to increase excretion of NaCl and water).
, sympathetic activity and ADH secretion are suppressed and NaCl excretion is enhanced (natriuresis). A variety of natriuretic peptides have been isolated which cause natriuresis. Under physiological conditions, renal natriuretic peptide (urodilatin) is the most important of these. ANP, released after atrial distension, may influence Na+ output under conditions of heart failure (acting to increase excretion of NaCl and water).
* Osmolality = mol/kg water; osmolarity = mol/L of solution.
Renin is an enzyme made and stored in the juxtaglomerular cells found in the walls of the afferent arteriole. Factors increasing renin secretion are:
•Reduced perfusion of the afferent arteriole.
•Reduced Na+ delivery to the macula densa.
Renin acts on angiotensin to create angiotensin I. This is converted to angiotensin II in the lungs by angiotensin-converting enzyme (ACE). Angiotensin II performs several functions, which result in the retention of salt and water:
•Stimulates aldosterone secretion (resulting in NaCl reabsorption and promoting the secretion of K+ and H+).
•Vasoconstriction of arterioles (efferent > afferent arterioles).
•Stimulates ADH secretion and thirst.
•Enhances NaCl reabsorption by the PCT.
K+ is critical for many cell functions. A large concentration gradient across cell membranes is maintained by Na+-K+-ATPase pump. Insulin and adrenaline also promote cellular uptake of K+.
The majority of K+ is reabsorbed in the PCT. The DCT can both reabsorb K+ in cases of K+ depletion (intercalated cells) and secrete it when there is K+ excess (principal cells). It is also secreted by the CD. Overall, the kidney excretes up to 95% of K+ ingested in the diet.
Factors promoting K+ secretion include:
• dietary K+ (driven by the electrochemical gradient).
dietary K+ (driven by the electrochemical gradient).
• rate of flow of tubular fluid.
rate of flow of tubular fluid.
•Metabolic alkalosis (acidosis exerts the opposite effect).
The normal pH of ECF is 7.4 ([H+] = 40nmol/L). Several mechanisms are in place to eliminate acid produced by the body and maintain body pH within a narrow range.
Buffer bases that take up H+ ions in the body include:
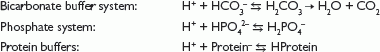
The Henderson–Hasselbalch equation describes the relationship between pH and the concentration of the conjugate acid and base.
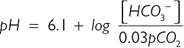
From this equation, it can be seen that alterations in bicarbonate (HCO3–) or carbon dioxide (CO2) will affect pH. Metabolic acid–base disturbances relate to a change in HCO3–, and respiratory acid–base disorders relate to alterations in CO2.
(See Fig. 18.4.)
HCO3– is the main buffer of ECF and is regulated by both the kidneys and lungs. Eighty-five per cent is reabsorbed in the PCT. Carbonic acid is first produced from CO2 and water (accelerated by carbonic anhydrase). The carbonic acid dissociates, and an active ion pump (Na+/H+ antiporter) extrudes intracellular H+ into the tubule lumen in exchange for Na+. Secretion of H+ ions favours a shift of the carbonic acid–bicarbonate equilibrium towards carbonic acid, which is rapidly converted into CO2 and water. CO2 diffuses into the tubular cells down its diffusion gradient and is re-formed into carbonic acid by intracellular carbonic anhydrase. HCO3– formed by this reaction is exchanged for chloride and passes into the circulation. Essentially, with each H+ ion that enters the kidney, a HCO3– ion enters blood, which bolsters the buffering capacity of the ECF.
The remaining HCO3– is absorbed in the DCT where cells actively secrete H+ into the lumen via an ATP-dependent pump. The DCT is the main site that pumps H+ into the urine to ensure complete removal of HCO3–. Once HCO3– has gone, phosphate ions and ammonia buffer any remaining H+ ions.

Fig. 18.4 Diagram showing bicarbonate reabsorption in the proximal convoluted tubule.
CA, carbonic anhydrase; Cl–, chloride ion; CO2, carbon dioxide; H+, hydrogen ion; HCO3–, bicarbonate; H2CO3, carbonic acid; H2O, water; HPO42–, phosphate ions; H2PO4–, phosphoric acid; Na+, sodium ion; pCO2, partial pressure of carbon dioxide.
•CKD stage 5 (eGFR 10–15mL/min).
•Acute renal failure due to persistent hyperkalaemia, metabolic acidosis, fluid overload, symptomatic uraemia, sepsis, and multiorgan failure, drug or toxin poisoning.
Arteriovenous fistula (requires 8wk to mature).
The dialysis machine pumps blood and dialysate through a dialyser. The dialysate is composed of water, Na+, K+, dextrose, calcium, chloride, magnesium, and HCO3– or acetate (as a buffer). The fluids are on opposite sides of a semi-permeable membrane and pumped in opposite directions (countercurrent flow). Waste products of metabolism diffuse from blood to the dialysate. Accumulated fluid is removed by convection of water and dissolved solutes (including proteins) down a pressure gradient (ultrafiltration). Heparin is used to prevent clotting.
4h sessions performed three times per week in a hospital-based dialysis unit or at home.
Anaemia, CVD [hypertension, arrhythmias, myocardial infarction, peripheral vascular disease (PVD), CVA], acquired renal cystic disease and renal tract cancers, renal osteodystrophy, neuropathy, ED. Fistula problems include thrombosis, stenosis, aneurysm, and infection.
Tenckhoff catheter which allows instillation of fluid into the peritoneum.
Fluid and solute exchange occurs between peritoneal capillary blood and the dialysis solution, using the peritoneum as the dialysis membrane. Small-molecular-weight solutes diffuse down a concentration gradient from blood into the dialysate. Water movement is achieved by osmosis from the ECF compartment to the hypertonic peritoneal dialysate.
Continuous ambulatory dialysis (CAPD) involves continuous dialysis using 3–5 exchanges per day of fluid, performed at home. Automated peritoneal dialysis (APD) allows fast instillation and drainage of large volumes of fluid if more intense dialysis is needed or for dialysis overnight.
Bowel adhesions, obesity, inoperable hernias, stoma.
As for haemodialysis and also catheter infection, peritonitis, membrane (peritoneal) failure, and hernia.
Intravascular (tunnelled) catheters, placed in the femoral or internal jugular veins.
Removal of solutes with water is achieved by convection down a pressure gradient. It does not require dialysate. Large volumes of filtrate are removed and need to be replaced during this process. This is a controlled and slow process, avoiding large intravascular fluid shifts and minimizing electrolyte disturbances, arrhythmias, and hypotension. It is particularly useful for the haemodynamically compromised patients with acute renal failure. Anticoagulation is required.
Blood is filtered continuously across a highly permeable synthetic membrane.
Similar, but lower, incidence than for haemodialysis. Includes thrombosis of the catheter or access vein, bleeding or clotting, sepsis, fluid overload, alkalosis, and hypotension.
This describes the combination of dialysis and ultrafiltration (diffusion and convection removal of solutes).
End-stage renal disease.
•Active malignancy is a contraindication to receiving immunotherapy. Generally should remain cancer-free for 2y before transplantation can be considered. Higher-risk tumours, including melanoma, breast cancer, and colorectal carcinoma, must be disease-free for longer (5y). Lower-risk tumours of the skin (basal and squamous cell carcinomas) can undergo transplantation immediately after treatment.
•Active infection (bacterial and viral, including hepatitis B and C, HIV, CMV, and TB).
•Significant CVD (including recent myocardial infarction).
•Primary hyperoxaluria (requires combined renal and liver transplant).
•Untreated psychological disorders, IV drug use, or alcohol excess.
•USS of the kidneys, ureters, and bladder.
•Urodynamic studies for LUTS (if clinically indicated).
•History: including cause and length of time of renal failure, previous interventions for renal impairment, and previous transplants. Coexisting conditions such as diabetes mellitus (which might require pancreatic transplant). Symptoms of voiding dysfunction which will require treatment before transplant.
•Cardiology work-up: stress test and ECG.
•Bloods: FBC, U&E, LFTs, glucose, calcium, magnesium, clotting, viral serology [HIV, hepatitis, CMV, syphilis, Epstein–Barr virus (EBV), human T lymphotropic virus (HTLV)].
•Tissue typing for human leucocyte antigen (HLA)-A, B, and DR phenotypes.
•Blood cross-match to detect preformed antibodies. The recipient’s sera are mixed with donor lymphocytes. Recipient antibodies will bind to, and lyse, donor cells if the cross-match is positive.
•Cadaveric (heart-beating): brainstem-dead* donor with supported ventilation and circulation.
•Cadaveric (non-heart-beating): rapid retrieval is required to minimize ischaemia from these patients without active circulation.
•Live-unrelated: donation must comply with regulations set by the Unrelated Live Transplant Regulatory Authority (ULTRA).
* Brainstem death: requires two sets of test by two doctors (registered for >5y). The criteria include absence of: corneal reflex, cranial nerve motor function, vestibulo-ocular reflex, and cough and gag reflex. Pupils are fixed and unresponsive, no spontaneous movement, and apnoea off ventilator (PaCO2 >6.7kPa).
•History of metastatic cancer or cancer with a higher recurrence risk (i.e. lymphoma).
•Significant cardiac disease [previous myocardial infarction, coronary artery bypass graft (CABG), angina].
•Diabetes, hypertension, significant pulmonary or vascular disease.
•Active systemic renal disease, i.e. SLE.
•HIV or other active infection.
•History: including family history of disorders such as glomerulonephropathies, polycystic kidney disease, SLE). Clinical and psychological evaluation in living donors, BP check.
•Bloods: FBC, U&E, cholesterol, glucose, clotting.
•ABO and HLA compatibility: kidneys should be allocated to the recipient with the lowest number of HLA mismatches.
•Viral serology (hepatitis B and C, HIV, CMV, EBV, syphilis, TB): establish any family history of high-risk renal disease or prion disease (CJD).
•eGFR (24h urine collection or EDTA in living donors).
•Renal imaging to assess donor anatomy, choose the kidney (leave the donor with the better kidney), and plan the technique.
•Higher success rates than deceased donors and shortens waiting times.
•Long-term follow-up of donor is recommended for surveillance of hypertension or renal impairment.
•Full informed consent should be taken. Risks of nephrectomy include: hypertension, CRF, and mortality (<0.03%).
The kidney, ureter, vena cava, aorta, and renal vessels may be taken en bloc from a cadaveric donor. Transport media include University of Wisconsin preservation solution (with glutathione and adenosine) with the graft, then packed in ice. Living donor kidney and ureter may be harvested via a laparoscopic or open approach.
The renal graft is most commonly placed into the iliac fossa, and the vessels anastomosed to the external iliac vein and artery (alternatives include common iliac vessels). An extravesical ureteric reimplantation is performed, and a ureteric stent inserted.
Immunosuppression is used as prophylaxis against graft rejection. Agents utilized include:
•Calcineurin inhibitors (CNIs)—ciclosporin or tacrolimus, which inhibit IL-2 production. Blood level monitoring is required.
•Corticosteroid (prednisolone or methylprednisolone).
•Azathioprine, an antimetabolite producing bone marrow suppression.
•Antithymocyte globulin (ATG), a polyclonal antibody which can lyse human leucocytes.
•OKT3, a monoclonal antibody directed against T lymphocytes.
•Rapamycin, an mTOR inhibitor, involved in IL-2 downregulation.
Common regimens include CNI, MPA, and corticosteroid. Steroids can be stopped at 3–12 months post-surgery for patients on a combination of CNI and MPA. Azathioprine is an alternative to MPA in low-risk patients. The consent process should include the need for long-term immunosuppression and explanation of the related side effects ( risk of malignancy, infection, diabetes, hypertension, tremor, renal and liver problems, osteoporosis, etc.).
risk of malignancy, infection, diabetes, hypertension, tremor, renal and liver problems, osteoporosis, etc.).
•Monitor fluid balance with input and output charts and daily weights.
•Radionuclide renal scan to assess graft blood flow and exclude extravasation.
•Removal of the catheter at 7 days if no extravasation.
•Antiplatelet inhibitors (aspirin, dipyridamole).
•Antibacterial and antifungal prophylaxis.
•Avoid NSAIDs and ACE inhibitors.
•Be wary of drug interaction, and alter doses of drugs accordingly.
Graft dysfunction can occur early or late and may be due to medical or surgical causes.
This manifests as renal function deterioration (oliguria, rising creatinine, hypertension, and proteinuria). Patients may be asymptomatic or have systemic symptoms (fever) or local symptoms, including graft tenderness or swelling. Investigate with percutaneous renal biopsy.
•Hyperacute rejection (intraoperatively or within days): due to preformed antibodies to allograft major histocompatibility complex (MHC). Treatment is graft removal.
•Accelerated rejection (within first week): due to cellular presensitization. Treatment is intensive antirejection treatment, with temporary dialysis support.
•Acute allograft rejection (occurs in the first 3 months): affects around 20–30% (90% respond to steroids). Classified as acute cellular rejection (ACR) which is T-cell-mediated or acute humoral rejection (AHR) which is antibody-mediated. ACR: treat with a steroid bolus. If this fails, employ intensified immunosuppression, conversion to tacrolimus, and T-cell-depleting agents. AHR: steroid bolus, conversion to tacrolimus, and IV immunoglobulin treatment.
•Chronic allograft rejection (months to years): is multifactorial and affects around 30%. Treatment options include conversion from CNI to mTOR inhibitor, or CNI reduction with MPA ± steroid cover.
•Post-transplant malignancy. Most affect the skin (40%) or lymphatic system (11%).
•Graft loss due to recurrence of original renal disease.
•Urinary tract: urinary leak, ureteric stricture/obstruction, fistula, stones, perinephric collections (lymphocele, haematoma, urinoma, abscess).
•Vascular complications: renal artery stenosis or thrombosis, renal vein thrombosis, pseudoaneurysm, arteriovenous fistula, atheromatous vascular disease.
•Diabetes (thought to be due to use of corticosteroids and tacrolimus).
Recipient death rate at 12 months is 5%. Graft survival rate is >85% at 1y, 60–70% at 5y, and 40–50% at 10y. The best results are with living donors.
Review every 6–12 months. Monitor renal function, immunosuppression, and side effects. Monitor cholesterol (may be raised due to ciclosporin or rapamycin). Diabetes control may be difficult due to steroids. Perform surveillance for the development of malignancy.