

Diabetes is so common in this country that it touches nearly everyone’s life—or will. The statistics on diabetes are staggering, and a diagnosis can be frightening: diabetes is the third leading cause of death in the United States. According to the most recent statistics compiled by the National Institutes of Health (NIH), as of 2007, a staggering 10.7 percent of the U.S. population over nineteen years old, or 23.5 million people, have diabetes, with about 350 million diabetics worldwide. About 25 percent of those people do not know they have diabetes. About 88 percent have blood sugars in excess of the very high levels recommended by professional diabetes associations. This number will no doubt increase. It is estimated that 66 million U.S. citizens already have “pre-diabetes,” which I would treat as diabetes. Most death certificates of diabetics do not list diabetes as the underlying cause of their heart attacks, strokes, kidney failure, hypoglycemia, ketoacidosis, or fatal infections. If it were included, it might well be the leading cause of death in the United States. On top of this, there is growing evidence that the incidence of many forms of cancer goes up considerably for people with elevated blood sugars. According to the American Diabetes Association, more than 1.6 million new diabetics will be diagnosed each year—more than double the number predicted in the 2007 edition of this book.
Even more alarming, the incidence of type 2—or what was once known as maturity-onset diabetes—among children eighteen years old and younger has skyrocketed. A Yale University study of obese children between ages four and eighteen appeared in the March 14, 2002, issue of the New England Journal of Medicine. The study found that nearly a quarter had a condition that’s often a precursor to diabetes. According to USA Today’s story on the report the same day, “The incidence of type 2 diabetes, the form that usually occurs in adults, has increased in young people, especially Hispanics, blacks, and Native Americans. Some studies suggest that in some regions, the incidence of type 2 in children has jumped from less than 5%, before 1994, to up to 50%.” That children are increasingly getting a disease that once targeted fifty- to sixty-year-olds presents a new and frightening potential public health disaster.
A huge portion of U.S. health care costs ($218 billion in 2007) is spent on the treatment of diabetes (mostly for long-term complications).
Each year, tens of thousands of Americans lose their eyesight because of diabetes, the leading cause of new blindness for people ages twenty to seventy-four. Ninety to ninety-five percent of diabetics have type 2 diabetes. Because 80 percent of type 2 diabetics are overweight, many inappropriately feel that the disease is their own fault, the result of some failure of character that causes them to overeat.
Since you are reading this book, you or a loved one may have been diagnosed recently with diabetes. Perhaps you have long-standing diabetes and are not satisfied with treatment that has left you plagued with complications such as encroaching blindness, foot pain, frozen shoulder, inability to achieve or maintain a penile erection, restrictive lung disease, hip and leg pain, or heart or kidney disease.
Although diabetes is still an incurable, chronic disease, it is very treatable, and the long-term “complications” are fully preventable. For sixty-five years, I’ve had type 1 diabetes, also called juvenile-onset or insulin-dependent diabetes mellitus (IDDM). This form of diabetes is generally far more serious than type 2, or non-insulin-dependent diabetes mellitus (NIDDM), although both have the potential to be fatal.* Most type 1 diabetics who were diagnosed back about the same time I was are now dead from one or more of the serious complications of the disease. Yet after living with diabetes for all these years, instead of being bedridden or out sick from work (or dead, the most likely scenario), I am more fit than most nondiabetics who are considerably younger than I. I regularly work 12-hour days, travel, sail, and pursue a vigorous exercise routine.
I am not special in this regard. If I can take control of my disease, you can take control of yours.
In the next several pages I’ll give you a general overview of diabetes, how the body’s system for controlling blood sugar (glucose) works in the nondiabetic, and how it works—and doesn’t work—for diabetics. In subsequent chapters we’ll discuss diet, exercise, and medication, and how you can use them to control your diabetes. If discussion of diet and exercise sounds like “the same old thing” you’ve heard again and again, read on, because you’ll find that what I’ve espoused is almost exactly the opposite of “the same old thing,” which is what you’ve probably been taught. The tricks you’ll learn can help you arrest the diabetic complications you may now be suffering, may reverse many of them, and should prevent the onset of new ones. We’ll also explore new medical treatments and new drugs that are now available to help manage blood sugar levels and curtail obesity and even overeating.

Diabetes is the breakdown or partial breakdown of one of the more important of the body’s autonomic (self-regulating) mechanisms, and its breakdown throws many other self-regulating systems into imbalance. There is probably not a tissue in the body that escapes the effects of the high blood sugars of diabetes. People with high blood sugars tend to have osteoporosis, or fragile bones; they tend to have tight skin; they tend to have inflammation and tightness at their joints; they tend to have many other complications that affect every part of their body, including the brain, with impaired short-term memory and even depression.
At the center of diabetes is the pancreas, a large gland about the size of your hand, which is located toward the back of the abdominal cavity and is responsible for manufacturing, storing, and releasing the hormone insulin. The pancreas also makes several other hormones, as well as digestive enzymes. Even if you don’t know much about diabetes, in all likelihood you’ve heard of insulin and probably know that we all have to have insulin to survive. What you might not realize is that many diabetics may not need insulin shots.
Insulin is a hormone produced by the beta cells of the pancreas. Insulin’s major function is to regulate the level of glucose in the bloodstream, which it does primarily by facilitating the transport of blood glucose into most of the billions of cells that make up the body. The presence of insulin stimulates glucose transporters to move to the surface of cells to facilitate glucose entry into the cells. Insulin also stimulates centers in the hypothalamus of the brain responsible for hunger and satiety. Indeed, there is some insulin production even as one begins to eat, before glucose hits the bloodstream. Insulin also instructs fat cells to convert glucose and fatty acids from the blood into fat, which the fat cells then store until needed. Insulin is an anabolic hormone, which is to say that it is essential for the growth of many tissues and organs.* Too much and it can cause excessive growth—as, for example, of body fat and of cells that line blood vessels. Finally, insulin helps to regulate, or counterregulate, the balance of certain other hormones in the body. More about those later.
One of the ways insulin maintains the narrow range of normal levels of glucose in the blood is by regulation of the liver and muscles, directing them to manufacture and store glycogen, a starchy substance the body uses when blood sugar falls too low. If blood sugar does fall even slightly too low—as may occur after strenuous exercise or fasting—the alpha cells of the pancreas release glucagon, another hormone involved in the regulation of blood sugar levels. Glucagon signals the muscles and liver to convert their stored glycogen back into glucose (a process called glycogenolysis), which raises blood sugar. When the body’s stores of glucose and glycogen have been exhausted, the liver, and to a lesser extent the kidneys and small intestines, can transform some of the body’s protein stores—muscle mass and vital organs—into glucose.
As recently as ninety years ago, before the clinical availability of insulin, the diagnosis of type 1 diabetes—which involves a severely diminished or absent ability to produce insulin—was a death sentence. Most people died within a few months of diagnosis. Without insulin, glucose accumulates in the blood to extremely high toxic levels; yet since it cannot be utilized by the cells, many cell types will starve. Absent or lowered fasting (basal) levels of insulin also lead the liver, kidneys, and intestines to perform gluconeogenesis, turning the body’s protein store—the muscles and vital organs—into even more glucose that the body cannot utilize. Meanwhile, the kidneys, the filters of the blood, try to rid the body of inappropriately high levels of sugar. Frequent urination causes insatiable thirst and dehydration. Eventually, the starving body turns more and more protein to sugar.
The ancient Greeks described diabetes as a disease that causes the body to melt into sugar water. When tissues cannot utilize glucose, they will metabolize fat for energy, generating by-products called ketones, which are toxic at very high levels and cause further water loss as the kidneys try to eliminate them (see the discussion of ketoacidosis and hyperosmolar coma in Chapter 21, “How to Cope with Dehydration, Dehydrating Illness, and Infection”).
Today type 1 diabetes is still a very serious disease, and still eventually fatal if not properly treated with insulin. It can kill you rapidly when your blood glucose level is too low—through impaired judgment or loss of consciousness while driving, for example—or it can kill you slowly, by heart or kidney disease, which are commonly associated with long-term blood sugar elevation. Until I brought my blood sugars under control, I had numerous automobile accidents due to hypoglycemia, and it’s only through sheer luck that I’m here to relate this.
The causes of type 1 diabetes have not yet been fully unraveled. Research indicates that it’s an autoimmune disorder in which the body’s immune system attacks the pancreatic beta cells that produce insulin. Whatever causes type 1 diabetes, its deleterious effects can absolutely be prevented. The earlier it’s diagnosed, and the earlier blood sugars are normalized, the better off you will be.
At the time they are diagnosed, many type 1 diabetics still produce a small amount of insulin. It’s important to recognize that if they are treated early enough and treated properly, what’s left of their insulin-producing capability frequently can be preserved. Type 1 diabetes typically occurs before the age of forty-five and usually makes itself apparent quite suddenly, with such symptoms as dramatic weight loss and frequent thirst and urination. We now know, however, that as sudden as its appearance may be, its onset is actually quite slow. Routine commercial laboratory studies are available that can detect it earlier, and it may be possible to arrest it in these early stages by aggressive treatment. My own body no longer produces any detectable insulin at all. The high blood sugars I experienced during my first year with diabetes burned out, or exhausted, the ability of my pancreas to produce insulin. I must have insulin shots or I will rapidly die. I firmly believe—and know from experience with my patients—that if the kind of diet and medical regimen I prescribe for my patients had been utilized when I was diagnosed, the insulin-producing capability left to me at diagnosis would likely have been preserved. My requirements for injected insulin would have been lessened, and it would have been much easier for me to keep my blood sugars normal.
According to the NIH, approximately 233,600 people died in 2005 from diabetes, but it is likely that most deaths due to diabetes are underreported. (Is a diabetic’s death from heart disease, kidney disease, or stroke, for example, really a death from diabetes?) It is the NIH’s contention that “the risk for death among people with diabetes is about twice that of people without diabetes of a similar age.”
Certainly everyone has to die of something, but you needn’t die the slow, torturous death of diabetic complications, which often include blindness and amputations. My history and that of my patients support this.
The Diabetes Control and Complication Trial (DCCT), conducted by the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), began in 1983 as a ten-year study of type 1 diabetics to gauge the effects of improved control of blood sugar levels. Patients whose blood sugars were nearly “normalized” (my patients’ blood sugars are usually much closer to normal than were those in the intensive care arm of the trial because of our low-carbohydrate diet) had dramatic reductions of long-term complications. Researchers began the DCCT trying to see if they could, for example, lessen the frequency of diabetic retinopathy by at least 33.5 percent.
Instead of a one-third reduction in retinopathy, they found more than a 75 percent reduction in the progression of early retinopathy. They found similarly dramatic results in other diabetic complications and announced the results of the study early in order to make the good news immediately available to all. They found a 50 percent reduction of risk for kidney disease, a 60 percent reduction of risk for nerve damage, and a 35 percent reduction of risk for cardiovascular disease. This reduction continues to this day, many years after the study was terminated. I was present at the meeting where the results were announced and was congratulated by many of the physicians who had previously endured my long-term insistance that diabetics were entitled to the same blood sugars as nondiabetics.
I believe that with truly normal blood sugars, which many of my patients have, these reductions can be 100 percent.
The patients followed in the DCCT averaged twenty-seven years of age at the beginning of the trial, so reductions could easily have been greater in areas such as cardiovascular disease if they had been older or followed for a longer period of time. The implication is that full normalization of blood sugar could totally prevent these complications. In any case, the results of the DCCT are good reason to begin aggressively to monitor and normalize blood sugar levels. The effort and dollar cost of doing so do not have to be remotely as high as the DCCT’s findings suggested.
Different from type 1 diabetes is what is officially known as type 2. This is by far the more prevalent form of the disease. According to statistics from the American Diabetes Association, 90–95 percent of diabetics are type 2. Furthermore, as many as a quarter of Americans between the ages of sixty-five and seventy-four have type 2 diabetes. A study published by Yale University in 2002 found that 25 percent of obese teenagers had type 2 diabetes.
(A new category of “pre-diabetes” has been recently called latent autoimmune diabetes, or LADA. This category applies to mild diabetes with onset after the age of thirty-five, in which the patient has been found to produce an antibody to the pancreatic beta cell protein called GADA, just as in type 1 diabetes. Eventually these people may develop overt diabetes and require insulin. When the symptoms of diabetes finally occur, they are often more severe than at the “onset” of type 1 diabetes.)
Approximately 80 percent of those with type 2 diabetes are overweight and are affected by a particular form of obesity variously known as abdominal, truncal, or visceral obesity. It is quite possible that the 20 percent of the so-called type 2 diabetics who do not have visceral obesity actually suffer from a mild form of type 1 diabetes that causes only partial loss of the pancreatic beta cells that produce insulin.* If this proves to be the case, then fully all of those who have true type 2 diabetes may be overweight. (Obesity is usually defined as being at least 20 percent over the ideal body weight for one’s height, build, and sex.)
While the cause of type 1 diabetes may still be somewhat mysterious, the cause of type 2 is less so. As noted previously, another designation for type 2 diabetes is insulin-resistant diabetes. Obesity, particularly visceral obesity, and insulin resistance—the inability to fully utilize the glucose-transporting effects of insulin—are interlinked. For reasons related to genetics (see Chapter 12, “Weight Loss—If You’re Overweight”), a substantial portion of the population has the potential when overweight to become sufficiently insulin-resistant that the increased demands on the pancreas burn out the beta cells that produce insulin. These people enter the vicious circle depicted in Figure 1-1. Note in the figure the crucial role of dietary carbohydrate in the development and progression of this disease. This is discussed in detail in Chapter 12.
Insulin resistance appears to be caused at least in part by inheritance and in part by high levels of fat—in the form of triglycerides released from abdominal fat—in the branch of the bloodstream that feeds the liver. (Transient insulin resistance can be created in laboratory animals by injecting triglycerides—fat—directly into their liver’s blood supply.)† Abdominal fat is associated with systemic inflammation, another cause of insulin resistance, as are infections. Insulin resistance by its very nature increases the body’s need for insulin, which therefore causes the pancreas to work harder to produce elevated insulin levels (hyperinsulinemia), which can indirectly cause high blood pressure and damage the circulatory system. Excessive levels of insulin in the blood down-regulate the affinity for insulin that insulin receptors all over the body have naturally. This “tolerance” to insulin causes even greater insulin resistance.
So, to simplify somewhat, inheritance plus inflammation plus fat in the blood feeding the liver causes insulin resistance, which causes elevated serum insulin levels, which cause the fat cells to build even more abdominal fat, which raises triglycerides in the liver’s blood supply and enhances inflammation, which causes insulin levels to increase because of increased resistance to insulin.
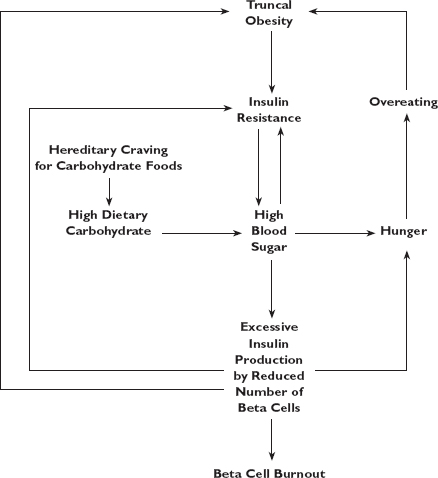
Fig. 1-1. The vicious circle of insulin resistance.
If that sounds circular, it is. But note that the fat that is the culprit here is not dietary fat.
Triglycerides are in circulation at some level in the bloodstream at all times. High triglyceride levels are not so much the result of intake of dietary fat as they are of carbohydrate consumption and existing body fat. (We will discuss carbohydrates, fat, and insulin resistance more in Chapter 9, “The Basic Food Groups.”) The culprit is actually a particular kind of body fat. Visceral obesity is a type of obesity in which a special kind of fat is concentrated around the middle of the body, particularly surrounding the intestines (the viscera). A man who is viscerally obese has a waist of greater circumference than his hips. A woman who is viscerally obese has a waist at least 80 percent as big around as her hips. All obese individuals and especially those with visceral obesity are insulin-resistant. The ones who eventually become diabetic are those who cannot make enough extra insulin to keep their blood sugars normal.
Though treatment has many similar elements—and many of the adverse effects of elevated blood sugar are the same—type 2 diabetes differs from type 1 in several important ways.
The onset of type 2 diabetes is slower and more stealthy, but even in its earliest stages abnormal blood sugar levels, though not sky-high, can cause damage to nerves, blood vessels, heart, eyes, and more. Type 2 diabetes is often called the silent killer, and it is quite frequently discovered through one of its complications, such as hypertension, visual changes, or recurrent infections.*
Type 2 diabetes is, at the beginning, a less serious disease—patients don’t melt away into sugar water and die in a few months’ time. Type 2, however, can through chronically but less dramatically elevated blood sugars be much more insidious. Because so many more people are affected, it probably causes more heart attacks, strokes, and amputations than the more serious type 1 disease. Type 2 is a major cause of hypertension, heart disease, kidney failure, blindness, and erectile dysfunction. That these serious complications of type 2 diabetes can progress is no doubt because it is initially milder and is often left untreated or treated more poorly.
Individuals with type 2 still make insulin, and many will never require injected insulin to survive, though if the disease is treated poorly, they can eventually burn out their pancreatic beta cells and require insulin shots. Because of their resistance to the blood sugar–lowering effects of insulin (though not its fat-building effects), many overweight type 2 diabetics actually make more insulin than slim nondiabetics.

Since high blood sugar is the hallmark of diabetes, and the cause of every long-term complication of the disease, it makes sense to discuss where blood sugar comes from and how it is used and not used.
Our dietary sources of blood sugar are carbohydrates and proteins. One reason the taste of sugar—a simple form of carbohydrate—delights us is that it fosters the production of neurotransmitters (principally serotonin) in the brain that relieve anxiety and can create a sense of well-being or even euphoria. This makes carbohydrate quite addictive to certain people whose brains may have inadequate levels of or sensitivity to these neurotransmitters, the chemical messengers with which the brain communicates with itself and the rest of the body. When blood sugar levels are low, the liver, kidneys, and intestines can, through a process we will discuss shortly, convert proteins into glucose, but very slowly and inefficiently. The body cannot convert glucose back into protein, nor can it convert fat into sugar. Fat cells, however, with the help of insulin, do transform glucose into saturated fat.
The taste of protein doesn’t excite us as much as that of carbohydrate—it would be the very unusual child who’d jump up and down in the grocery store and beg his mother for steak or fish instead of cookies. Dietary protein gives us a much slower and smaller blood sugar effect, which, as you will see, we diabetics can use to our advantage in normalizing blood sugars.
In the fasting nondiabetic, and even in most type 2 diabetics, the pancreas constantly releases a steady, low level of insulin. This baseline, or basal, insulin level prevents the liver, kidneys, and intestines from inappropriately converting bodily proteins (muscle, vital organs) into glucose and thereby raising blood sugar, a process known as gluconeogenesis. The nondiabetic ordinarily maintains blood sugar immaculately within a narrow range—usually between 70 and 95 mg/dl (milligrams per deciliter),* with most people hovering near 83 mg/dl. There are times when that range can briefly stretch up or down—as high as 160 mg/dl and as low as 65—but generally, for the nondiabetic, such swings are rare.
You will note that in some literature on diabetes, “normal” may be defined as 60–120 mg/dl, or even as high as 140 mg/dl. This “normal” is entirely relative. No nondiabetic will have blood sugar levels as high as 140 mg/dl except after consuming a lot of carbohydrate. “Normal” in this case has more to do with what is considered “cost-effective” for the average physician to treat. Since a postmeal (postprandial) blood sugar under 140 mg/dl is not classified as diabetes, and since the individual who experiences such a value will usually still have adequate insulin production eventually to bring it down to reasonable levels, many physicians would see no reason for spending their valuable time on treatment. Such an individual may be sent off with the admonition to watch his weight or her sugar intake. Despite the designation “normal,” an individual frequently displaying a blood sugar of 140 mg/dl is a good candidate for full-blown type 2 diabetes. I have seen “nondiabetics” with sustained blood sugars averaging 120 mg/dl develop diabetic complications.
Let’s take a look at how the average nondiabetic body makes and uses insulin. Suppose that Alice, a nondiabetic, arises in the morning and has a mixed breakfast, that is, one that contains both carbohydrate and protein. On the carbohydrate side, she has toast with jelly and a glass of orange juice; on the protein side, she has a boiled egg. Her basal (i.e., before-meals) insulin secretion has kept her blood sugar steady during the night, inhibiting gluconeogenesis. Shortly after the sugar in the juice or jelly hits her mouth, or the starchy carbohydrates in the toast reach certain enzymes in her saliva and intestines, glucose begins to enter her bloodstream. The mere presence of food in her gut as well as the rise in her blood sugar signal her pancreas to release the granules of insulin it has stored in order to offset a jump in blood sugar (see Figure 1-2). This rapid release of stored insulin is called phase I insulin response. It quickly corrects the initial blood sugar increase and can prevent further increase from the ingested carbohydrate. As the pancreas runs out of stored insulin, it manufactures more, but it has to do so from scratch. The insulin released now is known as the phase II insulin response, and it’s secreted much more slowly. As she eats her boiled egg, the small amount of insulin of phase II can cover the glucose that, over a period of hours, is slowly produced from the protein of the egg.
Fig. 1-2. Phase I and phase II insulin response in a normal, nondiabetic person.
Insulin acts in the nondiabetic as the means to admit glucose—fuel—into the cells. It does this by activating the movement of glucose “transporters” within the cells. These specialized protein molecules protrude from the cytoplasm of the cells and their outer surfaces to grab glucose from the blood and bring it to the interiors of the cells. Once inside the cells, glucose can be utilized to power energy-requiring functions. Without insulin, the cells can absorb only a very small amount of glucose, not enough to sustain the body.
As glucose continues to enter Alice’s blood, and the beta cells in her pancreas continue to release insulin, some of her blood sugar is transformed to glycogen, a starchy substance stored in the muscles and liver. Once glycogen storage sites in the muscles and liver are filled, excess glucose remaining in the bloodstream is converted to and stored as saturated fat. Later, as lunchtime nears but before Alice eats, if her blood sugar drops slightly low, the alpha cells of her pancreas will release another pancreatic hormone, glucagon, which will “instruct” her liver and muscles to begin converting glycogen to glucose, to raise blood sugar. When she eats again, her store of glycogen will be replenished.
This pattern of basal, phase I, then phase II insulin secretion is perfect for keeping Alice’s blood glucose levels in a safe range. Her body is nourished, and things work according to design. Her mixed meal is handled beautifully. This is not, however, how things work for either the type 1 or type 2 diabetic.
Let’s look at what would happen to me, a type 1 diabetic, if I had the same breakfast as Alice, our nondiabetic.
Unlike Alice, because of a condition peculiar to diabetics, if I take a long-acting insulin at bedtime, I might awaken with a normal blood sugar, but if I spend some time awake before breakfast, my blood sugar may rise, even if I haven’t had anything to eat. Ordinarily, the liver is constantly removing some insulin from the bloodstream, but during the first few hours after waking from a full night’s sleep, it clears insulin out of the blood at an accelerated rate. This dip in the level of my previously injected insulin is called the dawn phenomenon (see here). Because of it, my blood glucose can rise even though I haven’t eaten. A nondiabetic just makes more insulin to offset the increased insulin clearance. Those of us who are severely diabetic have to track the dawn phenomenon carefully by monitoring blood glucose levels, and can learn how to use injected insulin to prevent its effect upon blood sugar.
As with Alice, the minute the meal hits my mouth, the enzymes in my saliva begin to break down the sugars in the toast and juice, and almost immediately my blood sugar would begin to rise. Even if the toast had no jelly, the enzymes in my saliva and intestines and acid in my stomach would begin to transform the toast rapidly into glucose shortly after ingestion.
Since my beta cells no longer produce detectable amounts of insulin, there is no stored insulin to be released by my pancreas, so I have no phase I insulin response. My blood sugar (in the absence of injected insulin) will rise while I digest my meal. None of the glucose will be converted to fat, nor will any be converted to glycogen. Eventually much will be filtered out by my kidneys and passed out through the urine, but not before my body has endured damagingly high blood sugar levels—which won’t kill me on the spot but will do so over a period of days if I don’t inject insulin. The natural question is, wouldn’t injected insulin “cover” the carbohydrate in such a breakfast? Not adequately! This is a common misconception—even by those in the health care professions. Injected insulin—even with an insulin pump—doesn’t work the same as insulin created naturally in the body. Conventional insulin/diet therapy resulting in high blood sugar after meals is a guaranteed slow, incremental, “silent” death from the ravages of diabetic complications.
Normal phase I insulin is almost instantly in the bloodstream. Rapidly it begins to hustle blood sugar off to where it’s needed. Injected insulin, on the other hand, is injected either into fat or muscle (not usually into a vein) and absorbed slowly. The fastest insulin we have starts to work in about 20 minutes, but its full effect is drawn out over a number of hours, not nearly fast enough to prevent a damaging upswing in blood sugars if fast-acting carbohydrate, like bread, is consumed.
This is the central problem for type 1 diabetics—the carbohydrate and the drastic surge it causes in blood sugar. Because I know my body produces essentially no insulin, I have a shot of insulin before every meal. But I no longer eat meals with fast-acting or large amounts of carbohydrate, because the blood sugar swings they caused were what brought about my long-term complications. Even injection by means of an insulin pump (see the discussion near the end of Chapter 19, “Intensive Insulin Regimens”) cannot automatically fine-tune the level of glucose in my blood the way a nondiabetic’s body does naturally.
Now, if I ate only the protein portion of the meal, my blood sugar wouldn’t have the huge, and potentially toxic, surge that carbohydrates cause. It would rise less rapidly, and a small dose of insulin could act quickly enough to cover the glucose that’s slowly derived from the protein. My body would not have to endure wide swings in blood sugar levels. (Dietary fat, by the way, has no direct effect on blood sugar levels, except that it can slightly slow the digestion of carbohydrate.)
In a sense, you could look at my insulin shot before eating only the protein portion of the meal as mimicking the nondiabetic’s phase II response. This is much easier to accomplish than trying to mimic phase I, because of the much lower levels of dietary carbohydrate (only the slow-acting kind) and injected insulin that I use.
Let’s say Bob, a type 2 diabetic, is 6 feet tall and weighs 300 pounds, much of which is centered around his midsection. Remember, at least 80 percent of type 2 diabetics are overweight. If Bob weighed only 170 pounds, he might well be nondiabetic, but because he’s insulin-resistant, Bob’s body no longer produces enough excess insulin to keep his blood sugar levels normal.
The overweight tend to be insulin-resistant as a group, a condition that’s not only hereditary but also directly related to the ratio of visceral and total body fat to lean body mass (muscle). The higher this ratio, the more insulin-resistant a person will be. Whether or not an overweight individual is diabetic, his weight, intake of carbohydrates, and insulin resistance all tend to make him produce considerably more insulin than a slender person of similar age and height (see Figure 1-3).
Many athletes, because of their low fat mass and high percentage of muscle, tend as a group to require and make less insulin than nonathletes. An overweight type 2 diabetic like Bob, on the other hand, typically makes two to three times as much insulin as the slender nondiabetic. In Bob’s case, from many years of having to overcompensate, his pancreas has partially burned out, his ability to store insulin is diminished or gone, and his phase I insulin response is attenuated. Despite his huge output of insulin, he no longer can keep his blood sugars within normal ranges. (In my medical practice, a number of patients come to me for treatment of their obesity, not diabetes. On examination, however, most of these very obese “nondiabetics” have slight elevations of their hemoglobin A1C (HgbA1C) test for average blood sugar.)
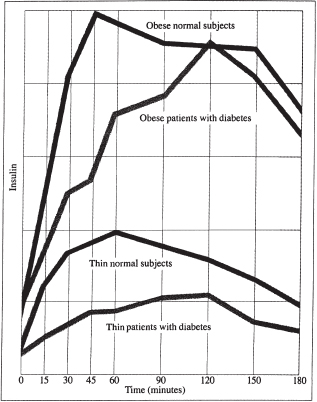
Fig. 1-3. Serum insulin response to glucose consumption of individuals with and without type 2 diabetes.
Let’s take another look at that mixed breakfast and see how it affects a type 2 diabetic. Bob has the same toast and jelly and juice and boiled egg that Alice, our nondiabetic, and I had. Bob’s blood sugar levels at waking may be normal.* Since he has a bigger appetite than either Alice or I, he has two glasses of juice, four pieces of toast, and two eggs. Almost as soon as the toast and juice hit his mouth, his blood sugar begins to rise. Unlike mine, Bob’s pancreas eventually releases insulin, but he has very little or no stored insulin (his pancreas works hard just to keep up his basal insulin level), so he has impaired phase I secretion. His phase II insulin response, however, may be partially intact. So, very slowly, his pancreas will struggle to produce enough insulin to bring his blood sugar down toward the normal range. Eventually it may get there, but not until hours after his meal, and hours after his body has been exposed to high blood sugars. Insulin is not only the major fat-building hormone; it also serves to stimulate the centers in the brain responsible for feeding behavior. Thus, in all likelihood, Bob will grow even more overweight, as demonstrated by the cycle illustrated in Figure 1-1.
Since he’s resistant to insulin, his pancreas has to work that much harder to produce insulin to enable him to utilize the carbohydrate he consumes. Because of insulin’s fat-building properties, his body stores away some of his blood sugar as fat and glycogen; but his blood sugar continues to rise, since his cells are unable to utilize all of the glucose derived from his meal. Bob, therefore, still feels hungry. As he eats more, his beta cells work harder to produce more insulin. The excess insulin and the “hungry” cells in his brain prompt him to want yet more food. He has just one more piece of toast with a little more jelly on it, hoping that it will be enough to get him through until lunch. Meanwhile, his blood sugar goes even higher, his beta cells work harder, and perhaps a few burn out.† Even after all this food, he still may feel many of the symptoms of hunger. His blood sugar, however, will probably not go anywhere near as high as mine would if I took no insulin. In addition, his phase II insulin response could even bring his blood sugar down to normal after many hours without more food.
Postprandial (after-eating) blood sugar levels that I would call unacceptably high—140 mg/dl, or even 200 mg/dl—may be considered by other physicians to be unworthy of treatment because the patient still produces adequate insulin to bring them periodically down to normal, or “acceptable,” ranges. If Bob, our type 2 diabetic, had received intensive medical intervention before the beta cells of his pancreas began to burn out, he would have slimmed down, brought his blood sugars into line, and eased the burden on his pancreas. He might even have “cured” his diabetes by slimming down, as I’ve seen in several patients. But many doctors might decide such “mildly” abnormal blood sugars are only impaired glucose tolerance (IGT) and do little more than “watch” them. Again, it’s my belief that aggressive treatment at an early stage can save most patients considerable lost time and personal agony by preventing complications that will occur if blood sugar levels are left unchecked. Such intervention can make subsequent treatment of what can remain—a mild disease—elegantly simple.

I include some hopeful forecasts of future treatments in this first chapter because as you’re learning how to control your diabetes, hope is a valuable asset. But your hope should be realistic. Your best hope for controlling your diabetes is normalizing your blood sugars now. That does not mean that the future will not bring great things. Diabetes research progresses on a daily basis, and I hope as much as you do for a cure, but it’s still on the horizon.
Researchers are currently trying to perfect methods for replicating insulin-producing pancreatic beta cells in the laboratory. Doing this in a fashion that’s comparatively easy and cost-effective should not be an insurmountable task, and indeed the preliminary results are quite encouraging. Once patients’ cells are replicated, they can be transplanted back into patients to actually cure their diabetes. After such treatment, unless you were to have another autoimmune event that would destroy these new beta cells, you would, at least in theory, remain nondiabetic for the rest of your life. If you had another autoimmune attack, you would simply have to receive more of your replicated cells. Another very hopeful approach currently undergoing clinical trials in humans is the transformation of the precursors of beta cells (the cells that line the ducts of the pancreas) into actual beta cells without even removing them from your body. This may be achieved by the simple intramuscular injection of a special protein and is now being tested for efficacy and possible adverse effects at several centers.
Another potential approach might be to insert the genes for insulin production into liver or kidney cells. These are potential opportunities for a cure, and have successfully cured diabetes in rats, but there are still obstacles to overcome.
Yet another approach to replacing lost beta cells has been used by two competing companies to cure diabetes in animals. The technique involves a series of ordinary injections of proteins that stimulate the remaining beta cells to replicate until the lost ones have been replaced.
One problem with all of these “solutions” is that the immune systems of diabetics are still capable of destroying the new beta cells. The following paragraph describes a way around this problem.
A very promising new approach relies on the fact that most diabetics, even most type 1s, have a few beta cells that still replicate. Their immune systems, however, make white blood cells that destroy the new beta cells as fast as they are made—or faster. If the culprit white cells can be isolated, they can be replicated and used to create vaccines that can be injected into diabetics, stimulating their immune systems to destroy all of their culprit cells. A diabetic’s few remaining beta cells would then be able to replicate, eventually curing the diabetes. It’s possible that these new “former diabetics” would require antibody injections every few years to prevent the appearance of more culprit cells. I have applied for a patent on a method for isolating these culprit cells.
With respect to the replication of beta cells, the catch for me and other diabetics who no longer have any insulin-producing capacity is that the cells from which new beta cells would be replicated ideally should be your own, and after more than six decades I may have none. Had my diabetes been diagnosed, say, a year earlier, and had my blood sugars been immaculately controlled immediately upon diagnosis, the injected insulin might have taken much of the strain off my remaining beta cells and allowed them to survive.
Many people (including the parents of diabetic children) view having to use insulin as a last straw, a final admission that they are (or their child is) diabetic and seriously ill. Therefore they will try anything else—including things that will burn out their remaining beta cells—before using insulin. Many people in our culture have the notion that you cannot be well if you are using medication. This is nonsense, but some patients are so convinced that they must do things the “natural” way that I practically have to beg them to use insulin, which is as “natural” as one can go. In reality, nothing could be more natural. Diabetics who still have beta cell function left may well be carrying their own cure around with them—provided they don’t burn it out with high blood sugars and the refusal to use insulin.
I will personally answer questions from readers for one hour every month. This free service is available by visiting www.askdrbernstein.net.