“The explanation of how atoms combine to form molecules and why the resulting molecules have the unique shapes that identify them is one of the major triumphs of quantum mechanics.”
—Fine and Beall, Chemistry for Engineers and Scientists
(Reference E, p. 544)
With bonding we enter the realm of chemistry, the examination of how it is that elements combine. We are concerned about this phenomenon here because the types of bonds that may be formed are related to the basic electronic structure of atoms involved as explained by quantum mechanics.1 Bond shapes, molecular shapes, the polarization of molecules and intermolecular bonds, and the properties of the liquids and the crystal structures, and the mechanical properties of the solids that may be formed from atoms are all influenced, if not determined, by their electronic structures. And so I want to provide just a little bit of an illustration of bonding, simplified, just to give you a taste of what might lie ahead in chemistry.
I first present a very simple description of ionic, covalent, and metallic bonds, essentially definitions. (To better understand these bonds, you may wish to read the beginning of Appendix D through the second section, “The Propensity to Bond.”) I then go a bit further to describe a few examples. To do this I consider the atoms of mainly three elements: oxygen or carbon atoms in molecular combination with atoms of hydrogen.
BOND TYPES
An ionic bond forms when an atom loses one or more electrons to become a positively charged cation while another atom acquires the electrons from the first atom to become a negatively charged anion, and the cation and anion are then electrostatically attracted to each other to bond together to make a molecule. One example is sodium combining with chlorine to form sodium chloride, NaCl, common table salt.
As described in Chapter 14, what drives the bonding is the reduction in overall energy when the atoms acquire or lose electrons so that low-energy just-filled shells of electrons are produced in the resulting ions. (And, remember, things in nature tend to be in states of lowest energy.) Several cations and anions can be combined together, as long as the number of electrons lost by the atoms to form cations is the same as the number of electrons acquired by atoms to form anions. For example, two atoms of aluminum may each lose three electrons, forming cations in reaction with three oxygen atoms, each of which acquires two of the electrons, forming the compound aluminum oxide, Al2O3. (With trace amounts of different transition-metal impurities replacing some of the aluminum atoms, the compound may take on a blue color, forming a sapphire, or a red color, forming a ruby.)
Some atoms like to share electrons with other atoms to form covalent bonds, as occurs for example when two hydrogen atoms bond to form a hydrogen molecule, the molecule making up hydrogen gas. In this case, for example, each hydrogen atom “thinks of itself” as having two electrons to complete a heliumlike completed first electron shell.
All bonds are some combination of ionic and covalent. But there is much, much more to it and this is an oversimplification. For example, there is the metallic bond. The billions of atoms in a metallic solid (or liquid, as for example with mercury) are held together by a “sea” of outer electrons from all of the atoms in the liquid or solid. Although the number of electrons locally balances the positive charges of the protons in the nuclei of each of the atoms, the outer electrons of each atom are essentially free to move around within the solid or liquid, and it is these electrons that can carry electrical current.
And there are other bonding mechanisms that I haven't described. All of these many mechanisms can be spread with varying amounts of applicability across the whole spectrum of the electronic structures of the elements. This is the large palette that nature and the chemists have to work with in creating the multitude of compounds and substances that surround us. (And most of what surrounds us are compounds.) We present briefly now just a few examples of the bonding of atoms and the chemistry of molecules.
CARBON, THE CHAMELEON!
Carbon is perhaps the most versatile of elements. Shown darkly shaded with the nonmetals at the bottom of Group IVA in the periodic table (Table IV), carbon is nevertheless a border element, bonding with either metals or nonmetals.
The carbon atom consists of two 1s electrons, two 2s electrons, and two 2p electrons surrounding a nucleus of six protons and six neutrons, although some isotopes have more neutrons. The 2s and 2p states are so close in energy that they can collectively distort to form hybrid2 states, to accommodate particular bonding situations. And a carbon atom bonds readily with another carbon atom. Partly for this reason, carbon forms more compounds than any other element. It is the core element of organic chemistry and the key element of biology and all of life.
Carbon, in its most abundant naturally occurring form (allotrope), is graphite or graphitelike, where each carbon atom is bonded to three others in sheets of connected hexagons, with carbon atoms at each “corner” of the hexagons. Isolated sheets are the allotrope of carbon known as graphene, which is described along with related “fullerenes,” buckyballs, and nanotubes in Chapter 22. The tight bonding of carbon atom to carbon atom within the sheets results because one 2s and two 2p electrons hybridize to provide connections equally to the three neighboring carbon atoms. Graphite is thus soft and slippery because the sheets can slide over each other. Meanwhile, each carbon atom's fourth n = 2 level electron is free to wander around to carry an electrical current when driven to do so by a battery or other voltage source. So, graphite is electrically conducting.
In contrast, at high temperature and pressure, the carbon atom's two 2s and two 2p electrons hybridize into a lobed tetrahedral arrangement that allows each carbon atom to bond with four others to make diamond, the hardest substance known to us, and also a perfect electrical insulator (there are no free conduction electrons). This same tetrahedral bonding is evident in the compound methane, CH4, the major constituent of natural gas, where each of four hydrogen atoms bonds by sharing its electron into one of the four equally spaced tetrahedral lobes of the hybridized carbon states.
CROOKED CONNECTIONS
While the H2O molecule in liquid water or ice tends to be drawn into a tetragonal arrangement with the help of noncovalent intermolecular hydrogen bonds with its neighbors, the isolated H2O molecule is bent to nearly a right angle: that is, the two hydrogen atoms attach to the oxygen atom at a relative angle of 104.5 degrees, as shown in Figure 15.1(c). (Many other molecules show a similar behavior of connections at odd angles.)
This bent structure was first explained over seventy years ago by Nobel Prize–winning chemist Linus Pauling in his work on the nature of the chemical bond.3 The perpendicular orientation of dumbbell-shaped oxygen 2p spatial states (such as the 2p spatial state for the hydrogen atom shown at the bottom of the second “column” of Figure 3.8) is the root cause for this bent shape. Figure 15.1 shows the oxygen atom, starting at the bottom left, as a more-simply-drawn, “unfuzzy” depiction of two oxygen 2p states, one with its lobes along the x axis and one with its lobes along the vertical y axis. The (a), (b), and (c) steps described in the caption show the formation of the bent molecule. (More recent calculations show that this picture of the bonding isn't quite correct,4 as the oxygen states involved in the bonding are somewhat hybridized5 with the 2s state, but the influence of the 2p states in providing a directionality for the bonds is still there.)
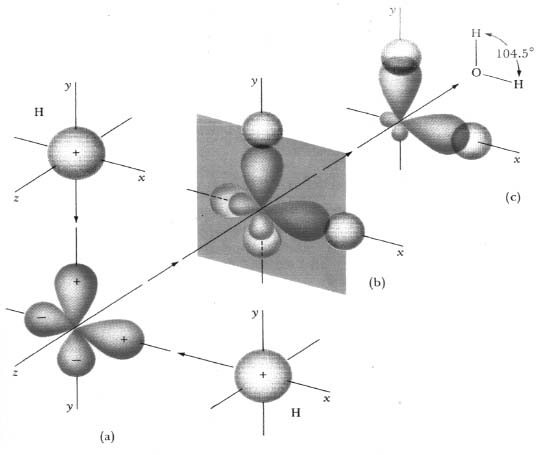
Fig. 15.1. The isolated H2O water molecule's bent shape. (a) The hydrogen atoms with their 1s spherically symmetric spatial states approach the oxygen atom with its two perpendicular dumbbell-shaped 2p spatial states along the x and y axes. In (b) the states draw together and in (c) their spatial states begin to overlap. Also shown in (c): the mutual repulsion of the clouds of electrons that are drawn toward the hydrogen atoms spreads the angle from 90 degrees to 104.5 degrees. (Figure 18-2 from Fine and Beall, Reference E, with permission from Dr. Leonard W. Fine.)
Odd angles in the structure of the molecules of many compounds are explained by the strange shapes of the various atoms’ spatial states that result from the quantum mechanical solutions of Schrödinger's equation. There is simply no classical explanation for these angles.
