A biological imperative, faced by all creatures, is to survive long enough to reproduce. Because of this, behavior related to survival and reproduction often appears to be built into the organism. That is, organisms come into the world with a range of behavior that aids survival and reproduction. Creatures that fly to avoid predators are likely born with the ability to fly. Thus, flying does not need to be learned; it results from the organism's species history. The complex array of motor movement and coordination involved in flying could be learned, but it is much more dependable when this behavior is based on genetic endowment.
For most animals, survival at birth depends on being able to breathe, digest food, and move about. When a worm is dangled over a young robin's head, this stimulus elicits opening the mouth and chirping. The behavior of the chick is the result of biological mechanisms and is elicited by the sight of the dangling worm. The relationship between the dangling worm (stimulus) and the open mouth (response) is a reflex. Presumably, in the evolutionary history of robins, chicks that presented a gaping mouth and chirped were fed and those that did not may have been ignored. That does not mean that there are no learned modifications of such initial behaviors (Walker, 1987, pp. 21–26). For example, Tinbergen and Kuenen (1957) observed that if feeding did not follow gaping to a realistic artificial stimulus thrush chicks stopped responding. In humans, reflexive crying to discomfort or hunger by an infant insures more effective care from the child's parents. Parents engage in a variety of caretaking behaviors in attempts to stop crying. Usually, parental responses such as changing a wet diaper, feeding, or burping the infant will stop the fussing.
Behavior relations that are based on the genetic endowment of an organism are called phylogenetic and are present on the basis of species history. Behavior that aids survival or procreation is often (but not always) unlearned. This is because past generations of organisms that engaged in such behavior survived and reproduced. These animals passed (to the next generation) the characteristics, in terms of genes, that allowed similar behavior. Thus, species history provides the organism with a basic repertoire of responses that are elicited by environmental conditions. Darwin said that these characteristics were naturally selected since they occurred through no action or intervention by man.
Fixed action patterns or FAPs are sequences of behavior (a series of connected movements) that are phylogenetic in origin. All members of a particular species (often all males or all females) engage in the FAP when the appropriate releasing stimuli are presented. Fixed action patterns have been observed and documented in a wide range of animals and over a large number of behaviors related to survival and reproduction. To illustrate, Tinbergen (1951) noted that the male stickleback fish responds with a stereotyped sequence of aggressive displays and movements when other male sticklebacks intrude on its territory during mating season. The female spider Cupiennius salei constructs a cocoon and deposits her eggs in it by engaging in a fixed sequence of responses (Eibl-Eibesfeldt, 1975). A greylag goose presented with an egg outside its nest will automatically roll the egg into the nest by reaching over the egg (with its bill) and pulling it carefully toward the nest. If the egg is removed, the bird continues with the fixed sequence of egg-retrieval actions. That is, the bird continues behaving as though the egg is present even though it has been removed. The following passage describes the FAP that the squirrel Sciurus vulgaris L. engages in while putting nuts away for the winter:
The squirrel … buries nuts in the ground each fall, employing a quite stereotyped sequence of movement. It picks a nut, climbs down to the ground, and searches for a place at the bottom of a tree trunk or a large boulder. At the base of such a conspicuous landmark it will scratch a hole by means of alternating movements of the forelimbs and place the nut in it. Then the nut is rammed into place with rapid thrusts of the snout, covered with dirt with sweeping motions and tamped down with the forepaws.
(Eibl-Eibesfeldt, 1975, p. 23)
Ethologists refer to such predictable and stereotypic behaviors as FAPs to imply that these behaviors are built-in and immutable. They are looking for heritable genetic-based factors with which to account for behavior. On the other hand the behavior science model prefers to consider all behaviors as flexible and adaptable, at least to some degree. So, given the adaptive ability of most animals, we refer to this behavior as flexible action patterns. Although the major topographic features of these types of reflex combinations may appear very similar across individuals and situations, there are numerous idiosyncratic differences that show flexibility. For example, all robins (Turdus americanis) build nests that appear very similar in construction. But, it is clear they do not all build in the same location, or use the same materials. There is great individual variation in all phases of nest construction, suggesting modification by the environment (ontogeny).
Reaction chains are similar to FAPs with one major difference—each response in a reaction chain requires an appropriate stimulus to set it off. Recall that once a FAP begins, the animal will continue the sequence even when the stimuli that set off the behavior are removed. In the previous squirrel example, if the nut is taken away from the squirrel, the animal will continue to dig a hole and bury a nonexistent nut. In contrast, a reaction chain requires the presence of a specific stimulus to evoke each link in a patterned sequence of behavior. An organism's performance produces stimuli that set off the next set of responses in the sequence; these behaviors produce the next set of stimuli followed by another set of responses. Presenting stimuli that prompt responses ordinarily occurring in the middle part of the sequence will start the chain at that point rather than at the beginning. Also, unlike FAPs, if the stimuli that evoke behavior are removed, the sequence is disrupted.
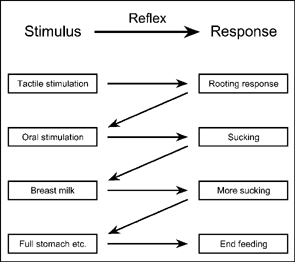
Reaction chains are like consecutive sets of reflexes where the stimulus that elicits the next response in the sequence is produced by the previous reflex. The nursing reaction chain of newborn babies is diagrammed in Figure 3.1. This sequence of reflexive responses may be initiated by tactile stimulation of the infant's cheek. This stimulation elicits the unconditioned rooting response, which involves turning the head towards the stimulation, opening the mouth, etc. Rooting results in mouth contact with the nipple; this oral stimulation in turn elicits sucking. Sucking produces breast milk in the infant's mouth, leading to further sucking. Eventually, internal stimuli arising from a full stomach, changes in blood chemistry, and endocrinology end the sequence and the baby stops feeding. (Note: When we discuss operant behavior and operant conditioning in a later chapter we also speak of response chains but in this case the sequences are learned and are described as interlocking three-term contingencies.)

FIG. 3.1 The nursing reaction chain of newborn babies is diagrammed. This sequence of reflexive responses is initiated by tactile stimulation of the infant's cheek. Stimulation of the cheek elicits the unconditioned rooting response that involves turning the head towards the nipple (rooting), opening the mouth, and sucking.
The principles that describe the reflex (and its conditioning) are similar for many different kinds of reflexes. For example, the laws that govern pupil contraction when a light is shined in the eye or principles describing the relationship between a sudden loud noise and a startle response also hold for the salivation produced when you eat a meal. Early work by Sherrington (1906) focused on the reflex, and the relationships that he discovered, almost a century ago, generalize to a remarkable variety of stimulus—response relationships. When food is placed in a dog's mouth, the salivary glands produce saliva. This relationship between food in the mouth and salivation is a reflex that is based on the genetic endowment of the organism and is not learned. Many reflexes serve defensive, protective or survival functions. Frequently such reflexes are not learned because they have to function before adequate experience is provided.
All organisms are born with a built-in set of reflexes, but many are particular to a species. Thus, humans are born with an array of responses that are elicited by specific stimuli. As illustrated above, tactile stimulation of the human infant's cheek evokes the rooting response—turning toward the stimulation with mouth open, which then receives the nipple. Also, as we have noted, in young robins, the so-called “begging” reflex (open mouth and chirping) serves a similar function—getting fed. Because these relationships are relatively invariant and biologically based, we refer to the eliciting event as the unconditioned stimulus (US). The related behavior following the stimulus is called the unconditioned response (UR). The term unconditioned is used because the reflex does not depend on an organism's experience or conditioning during its lifetime (i.e., learning).
When an unconditioned stimulus elicits an unconditioned response (US → UR), the relationship is called a reflex. Reflexive behavior is automatic in the sense that a physically healthy organism will always produce the unconditioned response when presented with an unconditioned stimulus. You do not choose to salivate or not when you have food in your mouth; the US (which is “food in the mouth”) draws out or elicits the UR of salivation. This is the way the animal (you) is built. However, there are times and conditions described below where the US does not elicit the UR. When repeated presentations of the US lead to a reduction of the UR we call that process habituation.
Aristotle about 350 BC developed principles of association that were rediscovered by psychologists and Pavlov (a physiologist) in the 1900s (Hothersall, 1990, p. 22). Sherrington (1906) studied many different types of reflexes, and formulated the laws of reflex action. Because reflexive behavior occurs across most or all animal species from protozoa (Wawrzyncyck, 1937) to humans (Watson & Rayner, 1920), and because associative or respondent conditioning builds on reflexive behavior, it is important to describe the laws of the reflex. The laws are general in that they hold for all eliciting or unconditioned stimuli (e.g., food in the mouth, a touch of a hot surface, a sharp blow just below the knee, a light shining in the eye) and the corresponding unconditioned responses (salivation, quick finger withdrawal, an outward kick of the leg, pupil contraction).
The unconditioned stimuli that elicit unconditioned responses may vary in intensity. For example, light that is shining in the eye may be bright enough to hurt or so faint that it is difficult to detect. A tap below the knee, causing a kick, may vary from a modest to a heavy blow, etc. The intensity of the eliciting US has direct effects on the elicited reflex. Three primary laws of the reflex describe these effects:
1. The law of the threshold is based on the observation that at very weak intensities a stimulus will not elicit a response, but as the intensity of the eliciting stimulus increases there is a point at which the response is elicited. That is, there is a point below which no response is elicited and above which a response always occurs. The uncertainty region, where roughly 50% of the stimuli that are presented produce a response, is called the threshold.
2. The law of intensity-magnitude describes the relationship between the intensity of the eliciting stimulus and the size or magnitude of the elicited response. As the intensity of the US increases so does the magnitude of the elicited UR. A light tap on the patella tendon (just below the kneecap) will evoke a slight jerk of the lower leg; a stronger tap will produce a more vigorous kick of the leg (the patella reflex). Of course, there are upper limits to the magnitude of the tap. If a hammer is used to smash into the knee, the result is a broken kneecap and no movement for a long time.
3. The law of latency concerns the time between the onset of the eliciting stimulus and the appearance of the reflexive response. Latency is a measure of the amount of time that passes between these two events. As the intensity of the US increases, the latency to the appearance of the elicited UR decreases. Thus, a strong puff of air will elicit a quick blink of the eye. A weaker puff will also elicit an eye blink, but the onset of the response will be delayed.
These three laws of the reflex are basic properties of all reflexes. They are called primary laws because, taken together, they define the relationship between the intensity of the eliciting stimulus (US) and the unconditioned response (UR). Reflexes, however, have other characteristics and one of these, habituation, has been shown in animals as simple as protozoa and as complex as humans.
One of the more documented secondary properties of the reflex is called habituation. Habituation is observed to occur when an unconditioned stimulus repeatedly elicits an unconditioned response and the response gradually declines in magnitude. When the UR is repeatedly elicited it may eventually fail to occur at all. For example, Wawrzyncyck (1937) repeatedly dropped a 4-g weight onto a slide that the protozoa Spirostomum ambiguum were mounted on. The weight drop initially elicited a contraction or startle response that steadily declined to near zero with repeated stimulation.
An interesting report of human habituation, in a dangerous setting, appeared in the July 1997 issue of National Geographic. The small island of Montserrat has been home to settlers since 1632. Unfortunately, the relatively silent volcano on the island reawakened in July 1995. Suddenly the quiet life that characterized living on Montserrat was rudely interrupted. Before the major eruption of the volcano, a large group of inhabitants refused to evacuate the island and these people suffered through several small volcanic explosions:
… Gerard Dyer and his wife, Judith, [have] been staying with friends in St. John's, about as far north of the volcano as you can get. … People could get passes to visit the unsafe zone, which is how Gerard came to be working on the flanks of Soufriere Hills that bright morning.
“If you have animals and crops, you can't just leave them” said Gerard as we walked back to his truck. “You have to come look after them and hope nothing happen.” As he spoke, the volcano made a crackling sound like distant thunder—blocks of solid lava rolling down the side of the dome. Gerard didn't even look up.
Montserratians have become so used to the volcano's huffing and puffing that the initial terror has gone. As one woman said, “At first when there was an ashfall, everybody run. Now when the ash falls, everybody look.”
(Williams, 1997, p. 66)
In this example, Gerard has been repeatedly exposed to the sound (US) of minor volcanic explosions. At first, this sound elicited a startle/panic response, accompanied by running, but these URs habituated to near zero with repeated eruptions of the volcano. A similar process is observed when people live under an airport flight path; initially the sound of a jet taking off or landing is bothersome, but after some time the sound is barely noticed.
There are a number of general properties that characterize habituation (Thompson & Spencer, 1966). Some of the more important principles of habituation are: (1) the decrease in the habituated response is large at first but this decrement gets progressively smaller as habituation is continued; (2) if the unconditioned stimulus is withheld for some time, the habituated response recovers; and (3) when habituation is repeatedly produced, each series of stimulus presentations generates progressively more rapid habituation. In other words, habituation occurs more quickly on a second series of unconditioned stimulus presentations than on the first, and then even faster on a third set. This may represent the simplest form of learning and also a rudimentary form of memory (Tighe & Leaton, 1976).
Habituation is a behavioral process that has come about because of phylogenetic history. Those animals that habituated were more likely to survive and produce offspring. A herbivore that runs away each time the grass rustles gets less to eat than one that stands its ground. A rustling grass sound may indicate the presence of a predator, or simply the wind blowing. Repeated activation of respondent mechanisms, when unnecessary, stresses the animal as well, which is not good in terms of health and physiology.
At the physiological level, habituation is possible because of the way the reflex arc is constructed. To explain, a sensory neuron with a sensory transducer enters the spinal cord and synapses onto a motor neuron. When activated by a touch, for example, the sensory neuron generates an action potential in an effector neuron across a synapse that in turn causes muscle or gland activity. Synapses have many inputs, some of which are excitatory and some that are inhibitory. The presence of the synapse between sensory and motor neurons allows for the presence of inhibitory input and habituation of the UR (Thompson & Glanzman, 1976).
In addition to phylogenetic history, the behavior of an organism is affected by environmental experience. Each organism has a unique ontogenetic history or lifetime of conditioning. Change in behavior as a result of such experience is called learning and consists of organism—environment interactions. Events in the physical and social world impact the behavior of organisims. Learning builds on species or phylogenetic history to determine when, where, and what kind of behavior will occur at a given moment.
For example, salivation is involved with the digestion of food. People do not learn to salivate to the taste of food; this is a phylogenetic characteristic of the species. After some experience where you learn that McDonald's goes with food, you may salivate to the sight of the golden arches of McDonald's, especially if you are hungry and like hamburgers. Salivating at the sight of McDonald's arches occurs because of respondent conditioning—you were not born that way. It is, however, important to note that respondent conditioning and other learning processes themselves evolved because they provided some sort of reproductive advantage. Those organisms whose behavior came under the control of arbitrary (but important) environmental events presumably gained an advantage over those that did not. Through Darwinian evolution and selection, respondent conditioning became a means of behavioral adaptation. In other words, organisms with a capacity for respondent learning were more likely to survive and reproduce—increasing their genes in the population.
Respondent conditioning involves the transfer of the control of behavior from one stimulus to another by S—S pairing. In Chapter 1, we saw that the sound of a bell could come to elicit salivation after the bell had been associated with food. This kind of conditioning occurs in all species, including humans, and is common in everyday life. Imagine you are out for an early morning walk and pass a bakery where you smell fresh doughnuts. When this happens, your mouth begins to water and your stomach starts to growl. These conditioned responses occur because, in the past, the smell has been associated (paired) with food in the mouth (doughnuts).
Figure 3.2 shows the classical conditioning of salivation described by Pavlov (1960). The upper panel indicates that an arbitrary stimulus such as a light (CS) is presented just before food (US) is placed in a dog's mouth. After several pairings of the light with the food, the light is presented alone. If the light now elicits salivation (test phase), it is called a conditioned stimulus (CS), and salivation to the light is called the conditioned response (CR).
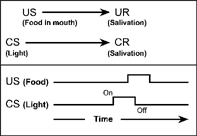
FIG. 3.2 Simple respondent conditioning. An arbitrary stimulus such as a light (CS) is presented just before food is placed in a dog's mouth (US). After several pairings of light and food, the light is presented alone. If the light now elicits salivation, it is called a conditioned stimulus (CS), and salivation to the light is a conditioned response (CR).
Notice that a new feature of the environment (a light) has come to regulate the behavior (salivation) of the organism. Thus, classical (Pavlovian or respondent) conditioning involves the transfer of behavior control to new and often arbitrary aspects of the environment. To experience this sort of conditioning, try the following: Read the word lemon and consider the last time you ate a slice of lemon. Many people salivate at this CS because the word has been contiguously (near in time) paired with the sour taste of the fruit. This shift in controlling stimulus from food to word is possible because of the anatomy described above involving the critical synapse onto the final common neural pathway. In this case, input to the visual system ends up activating the neuron that innervates the salivary gland.
Because the CR is a response elicited by the CS, it is often called a respondent. The terms conditioned response and respondent are interchangeable throughout this text. The process of presenting stimuli together in time (pairing or associating stimuli) so that a CS comes to regulate the occurrence of the conditioned response is called respondent conditioning. Technically, respondent conditioning involves establishing a conditional probability between the CS and US (the occurrence of the US is conditional on the presence of the CS).
Note that the association is between the CS and US (i.e., the word lemon and the real fruit in the mouth) because they have been paired together at some time in the past—not because of some cognitive (internal mental) association of events. This is an important point: The word “association” is sometimes taken to mean an internal mental process that a person or other animal performs. We hear people say, “the dog salivates when the bell is sounded because it has associated the sound with the food.” In contrast, a behavior analyst points to the physical association of stimuli (CS and US) that occurred in the past. In other words, the association is between events—it does not refer to mental associations. The word lemon (CS) elicits salivation (CR) because the word has occurred at a time and place when the chemistry of a lemon (US) produced salivation (UR).
The usual measures of behavior for respondent conditioning are magnitude (amount of salivation) and latency (time to salivation) of response following presentation of the US or CS. Magnitude and latency make sense as behavioral measures because respondent conditioning often involves the actions of smooth muscles and glands or responses such as eye-blinks and skin resistance (the UR or CR) that vary on these two dimensions.
When a conditioned stimulus (CS) is repeatedly paired with an unconditioned stimulus (US), the CS comes to produce the conditioned response (CR). The increase in the CR to the presentation of the CS is called respondent acquisition. In one experiment, Anrep (1920) demonstrated the conditioning of the salivary reflex to a tone stimulus. The acquisition procedure involved turning on the tone for a brief period, and then placing food in a dog's mouth. Anrep measured the conditioned response as the number of drops of saliva during 30-s intervals wherein the tone occurred without food. Figure 3.3A (acquisition) shows that the amount of salivation to the tone increases rapidly during the first 25 trials and then levels off, or reaches its maximum called the asymptote. In other words, with repeated pairings of the CS and US, the magnitude of the conditioned response increases. Once the conditioned reflex reaches asymptote, however, further CS—US pairings have no additional effects.
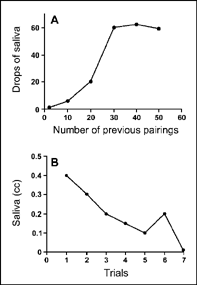
FIG. 3.3 The acquisition and extinction of salivation. The acquisition curve (A) is taken from an experiment by Anrep (1920), who paired a tone (CS) with food placed in a dog's mouth (US). The extinction curve (B) is from Pavlov (1960, p. 53), who presented the CS (sight of food) in the absence of the US (food in the mouth). Results are portrayed as a single experiment.
It is important to note that the asymptote for the conditioned response depends on the intensity of the unconditioned stimulus. As the intensity of the US increases, the magnitude of the UR also increases up to a point. The magnitude of the UR limits the maximum strength of the CR. Thus, the more food a dog is given, the greater the amount of salivation. If a dog is given 2 oz of meat, there will be more salivation than if it is presented with 1 oz. A tone that is associated with 2 oz of food will elicit salivation as a CR at a higher level (at asymptote) than a tone associated with 1 oz of food. It is clear that these relationships are limited by an organism's physiology. If a dog is given 1 lb of steak it will probably salivate at maximum strength, and a change to 2 lb will have no further effect. Similar limits are observed for reflexes such as variation in pupil size in response to light, magnitude of the knee jerk in response to a tap, and the degree of startle in response to noise.
Notice that the conditioned response of salivation appears to be identical to the unconditioned response. That is, when conditioning to the tone has occurred, turning it on will elicit salivation. This response to the tone seems the same as the salivation produced by food in the dog's mouth. In fact, early theories of learning held that the tone substituted for the food stimulus. This implies that the CS—CR relationship is the same as the US—UR relation. If the CS—CR and the US—UR relationships are the same, then both should follow similar laws and principles. And the laws of the reflex govern the US—UR relationship, as you have seen.
If the CS—CR and US—UR relationships are the same, then the law of intensity-magnitude should hold for conditioned stimuli and responses. That is, a rise in the intensity of the CS should increase the magnitude of the CR. In addition, the CS—CR relation should follow the law of latency. An increase in the intensity of the CS should decrease the latency between the CS onset and the conditioned response. Research has shown that these, and other laws of the reflex, typically do not hold for the CS-CR relation (Millenson, 1967). Generally, a change in the intensity of the conditioned stimulus decreases the strength of the conditioned response. In Anrep's (1920) experiment, the tone occurred at a particular intensity, and after conditioning it elicited a given magnitude and latency of salivation. If Anrep had increased the sound, there would have been less salivation and it would have taken longer to occur. Thus, the CS—CR relation is specific to the original conditioning and does not follow the laws of the reflex. One reason is that the CS—CR relationship involves processes such as respondent discrimination (see below).
Pavlov (1960) reported a very simple experimental procedure that he called respondent extinction. The procedure involves repeatedly presenting the CS and not presenting the US. Figure 3.3B (extinction) shows the decline in salivation when Pavlov's assistant, Dr. Babkin, repeatedly presented the CS but no longer fed the dog. As you can see, the amount of salivation declines and reaches a minimal value by the seventh trial. This minimum level of the CR is often similar to the value obtained during the first trial of acquisition and probably reflects the respondent level of this behavior. Respondent level, or baseline, refers to the strength of the target response (e.g., salivation) before any known conditioning has occurred.
A distinction should be made between extinction as a procedure and extinction as a behavioral process. The procedure involves presenting the CS but not the US after conditioning has occurred. As a behavioral process, extinction refers to the decline in the strength of the conditioned response when an extinction procedure is in effect. In both instances, the term extinction is used correctly. Extinction is the procedure of breaking the CS—US association, resulting in the decline of the CR.
The decline in the strength of the CR is often rapid. This statement is true for the conditioning of salivation, but other types of conditioned responses may vary in resistance to extinction. Even with salivation, Pavlov noted that as the time between trials increased, the CR declined more slowly. A test trial is any instance in which the CS is given in the absence of the unconditioned stimulus. Of course, repeated test trials are the same as extinction. The slower extinction of salivation with longer intervals between test trials may reflect what is called spontaneous recovery.
Spontaneous recovery is the observation of an increase in the conditioned response after respondent extinction has occurred. Recall that after repeated presentations of the CS without the US, the conditioned response declines to respondent level. Following extinction of the response to respondent level, after some time passes, the CS will again elicit the CR and the more time that passes between the first and second extinction sessions the more the spontaneous recovery (Brooks & Bouton, 1993).
The typical effect is seen in Figure 3.4, which shows the course of extinction and spontaneous recovery from another experiment by Pavlov (1960). In this experiment, the CS was the sight of meat powder, and the US was food in the dog's mouth. As you would expect, the sight of meat powder eventually elicited a conditioned response of salivation. When extinction began, the dog responded with 1 cc of salivation at the sight of the CS. By the fifth extinction trial, the animal showed almost no salivation to the sight of food powder, but after 20 minutes of rest without stimulus presentations, the CS again elicited a conditioned response. Note, however, that the amount of salivation on the spontaneous-recovery trial is much less than the amount elicited on the first extinction trial.
Pavlov (1960) argued that spontaneous recovery shows little weakening of the CS—CR relation-ship during extinction. He went on to suggest that “internal inhibition” came to block the connection between stimuli and responses. Pavlov viewed conditioning phenomena as an index of brain processes, and in this regard saw behavior as a reflection of central nervous system functioning. In this sense, spontaneous recovery reflected underlying physiological processes, and one of these was an active but temporary “dampening” of associative connections between the CS and the conditioned response. Pavlov called this apparent physiological blocking of the CS—CR relationship “internal inhibition.”
In contrast to Pavlov's hypothetical physiological account (he did not actually observe any neural activity), a behavioral analysis of spontaneous recovery suggests that the CS—CR relation is weakened by extinction, but the context or features of the situation in general maintain some level of control over the conditioned response. During respondent conditioning, many stimuli not specified by the researcher as the CS, but present in the experimental situation, come to regulate behavior. For example, background odors, general illumination of the room, the presence of particular researchers, the passage of time, and all the events that signal the start of a conditioning series come to exert some control over the conditioned response. Each time a recovery test is made, some part of the situation that has not yet been extinguished evokes the CR. This gradual decline in contextual stimulus control through repeated extinction also accounts for progressively less recovery of the conditioned response.
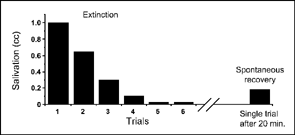
FIG. 3.4 Extinction and spontaneous recovery of salivation elicited by the sight of meat powder (Pavlov, 1960), with data replotted from Bower and Hilgard (1981, p. 51).
Pavlov conducted a large number of conditioning experiments and discovered many principles that remain useful today. One of his important findings concerned the principle of respondent generalization. Respondent generalization occurs when an organism shows a conditioned response to values of the CS that were not trained during acquisition. For example, respondent acquisition will occur when a specific stimulus, such as a 60-dB tone at a known frequency (e.g., 375 Hz), is associated with a US (e.g., food). After several pairings, the CS elicits a conditioned response, in this case salivation. If a 60-dB tone of 375 Hz is now presented without the US (a test trial), the animal will salivate at maximum level. To show generalization, the researcher varies some property of the conditioned stimulus. For example, a 60-dB tone of 75, 150, 225, 300, 375, 450, 525, 600, and 675 Hz is presented on test trials, and the magnitude of the conditioned response is measured. Figure 3.5 shows possible results of such an experiment. As you can see, the amount of salivation declines as the test stimulus departs in both directions from the value used in training. This graph, which plots stimulus value against magnitude of response, is called a generalization gradient.
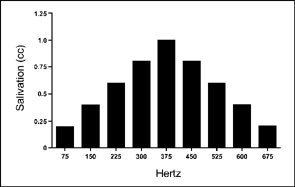
FIG. 3.5 A hypothetical generalization gradient for the salivary response. In this idealized experiment, training would occur at 375 Hz and then CSs ranging from 75 to 675 Hz would be presented.
Interestingly, a similar generalization gradient may not occur if the intensity rather than the tonal quality of the CS is varied. That is, if decibels rather than cycles per second (Hertz) are varied in the generalization test, a different result might occur. A few studies have shown that as the intensity of the CS increases, so does the magnitude of the conditioned response (Heinemann & Chase, 1970; Razran, 1949). Heinemann and Chase (1970) found that proportionally more conditioned responses were elicited as the sound intensity of the CS increased. Based on this finding, Heinemann and Chase suggest that there may be consistent increases in the strength of the CR as the intensity of the CS increases, although not all research has supported this finding (Ernst, Engberg, & Thomas, 1971). A conservative conclusion is that as the CS greatly departs from the value that was originally established, the conditioned response becomes weaker (see also Thomas & Setzer, 1972).
Generalization is an adaptive process that allows the organism to respond similarly even when conditions do not remain exactly the same from trial to trial. Consider a situation in which a predator's approach (US) is associated with the sound of snapping twigs, rustling grass, and waving shrubs (CS). An organism that runs away (CR) only in the presence of these exact stimulus conditions would probably not last long. This is because the events that occurred during conditioning are never precisely repeated—each approach of a predator produces variations in sounds, sights, and smells. Even in the laboratory where many features of the environment are controlled, there is some variation in stimuli from one trial to the next. When a bell is paired with food, the dog may change its orientation to the bell and thereby alter the sound; room humidity and other factors may also produce slight variations in tonal quality. Because of generalization, a CS—CR relationship can be strengthened even though the stimulus conditions are never exactly the same from trial to trial. Thus, generalization was likely an adaptive process, allowing organisms to respond to the vagaries of life.
Another conditioning principle that Pavlov discovered is called differentiation or discrimination. Respondent discrimination occurs when an organism shows a conditioned response to one stimulus but not to other similar events. This is a process at the other end of the continuum from generalization. A discrimination-training procedure involves presenting both positive and negative conditioning trials. For example, a positive trial occurs when a CS+ such as a 60-dB tone is associated with an unconditioned stimulus like food. On negative trials, a 40-dB tone is presented (CS−) but never paired with food. Because of stimulus generalization, the dog may salivate to both the 60-dB (CS+) and 40-dB (CS−) tones on the early trials. However, if the procedure is continued, the animal will no longer salivate to the CS− (40-dB tone), but will show a response to the CS+ (60-dB tone). Once such a differential response occurs, we may say that the dog discriminates between the tones.
Respondent discrimination is another adaptive process. It would be a chaotic world if an animal spent its day running away from most sounds, sights, and smells, generalizing to everything. Such an animal would not survive and reproduce because there would be no time for other essential activities, like eating, drinking, and procreating. Discrimination allows an organism to budget its time and responses in accord with the requirements of the environment. In the predator example, noises that are reliably associated with an animal that considers you a main course should become CS+ for flight or fight. Similar noises made by the wind or harmless animals are CS− for such behavior. Notice, however, that there is a fine line between discrimination and generalization in terms of survival.
There are several ways of arranging the temporal relationship between the presentation of a CS and the unconditioned stimulus (US). So far we have described a procedure in which the CS is presented a few seconds before the US occurs. This procedure is called delayed conditioning (the presentation of the US is slightly delayed relative to the CS) and is shown in Figure 3.6A).
Delayed conditioning is considered the most effective way to condition simple autonomic reflexes like salivation. In the diagram, the CS is turned on, and 3 s later the US is presented. The interval between the onset of the CS and the onset of the US (called the CS—US interval) determines the effectiveness of conditioning. For autonomic responses like salivation, blood pressure, skin temperature, hormone levels, and sweat secretion, a CS—US interval of 5–30 s appears to be most effective. A brief CS—US interval of about 0.5 s seems to be optimal for the conditioning of quick skeletal responses such as a knee jerk, eye blinks, and retraction of a limb from a hot surface. In human eyeblink conditioning, a delay of 0.4 s between CS and US produces the fastest conditioning in young adults but a longer delay of about 1 s is more effective with older people (Solomon, Blanchard, Levine, Velazquez, & Groccia-Ellison, 1991).
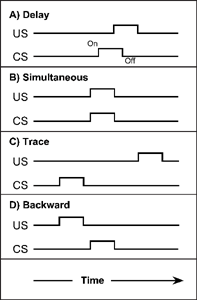
FIG. 3.6 Several temporal arrangements between CS and US commonly used for simple respondent conditioning. Time is shown in the bottom panel of the figure and moves from left to right. The other panels depict the temporal arrangement between US and CS for four basic respondent conditioning arrangements. For example, delayed conditioning is shown in panel A, where the CS is turned on and, a few seconds later, the US is presented.
Another temporal arrangement is called simultaneous conditioning, where the CS and US are presented at the same time. This procedure is shown in Figure 3.6B, where the CS and US are presented at the same moment. For example, at the same time that the bell rings (CS), food is placed in the dog's mouth (US). Compared with delayed conditioning, where the CS precedes the US briefly, simultaneous conditioning produces a weaker conditioned response (Smith & Gorme-zano, 1965; White & Schlosberg, 1952). One way to understand this weaker effect is to note that the CS does not signal the impending occurrence of the US in simultaneous conditioning. Based on this observation, many researchers have emphasized the predictiveness of the CS as a central feature of classical conditioning (see Rescorla, 1966). That is, the CS works because it provides information that “tells” the organism a US will follow. In simultaneous conditioning, however, there can be no predictive information given by the CS and yet some conditioning occurs. This suggests that predictiveness may facilitate conditioning, but is not necessary for it (Papini & Bitterman, 1990).
The procedure for trace conditioning is shown in Figure 3.6C. The CS is presented for a brief period, on and off, and after some time the US occurs. For example, a light is flashed for 2 s and 20 s later food is placed in a dog's mouth. The term trace conditioning comes from the idea of a “memory trace” and refers to the fact that the organism must remember the presentation of the CS. Generally, as the time between the CS and US increases, the conditioned response becomes weaker (Ellison, 1964; Lucas, Deich, & Wasserman, 1981). For eyeblink conditioning (a puff of air in the eye US —→ an eye blink UR), the response to the CS does not occur when the CS and US are separated by as little as 2 s (Schneiderman, 1966). When compared to delay conditioning with the same interval, between the onset of the CS followed by the US, trace conditioning is not as effective—producing a weaker conditioned response. Recent research has extended trace conditioning to taste aversion learning (see Chapter 7) and to biochemical changes that help to bridge stimulus associations over the trace interval (Misanin, Goodhart, Anderson, & Hinderliter, 2002).
As shown in Figure 3.6D, backward conditioning stipulates that the US comes on and goes off before the CS comes on. The general consensus has been that backward conditioning is unreliable, and many researchers question whether it occurs at all (but see Barnet & Miller, 1976, and Heth, 1976, for supportive views). It is true that backward conditioning usually does not produce a conditioned response. That is, if you place food in a dog's mouth and then ring a bell, the bell will not elicit the response of salivation when presented later. Most conditioning experiments have used arbitrary stimuli such as lights, tones, and shapes as the conditioned stimulus. However, Keith-Lucas and Guttman (1975) found backward conditioning when they used a biologically significant CS.
These researchers reasoned that following an unsuccessful attack by a predator, the sights, sounds, and smells of the attacker would be associated with pain from the attack. Consider a situation in which a grazing animal is unaware of the approach of a leopard. The attack (US) comes swiftly and without warning (no CS). The animal survives the onslaught and manages to run away. In this case, the pain inflicted by the attack is a US for flight that precedes the sight of the predator (CS). For such a situation, backward conditioning would have adaptive value since the animal would learn to avoid leopards.
Keith-Lucas and Guttman (1975) designed an experiment to test this adaptive-value hypothesis. Rats were placed in an experimental chamber and fed a sugar pellet in a particular location. While eating the pellet, the rats were given a one-trial presentation of electric shock (US). After the shock, the chamber was made completely dark for 1, 5, 10, or 40 s. When the light in the chamber came back on, a toy hedgehog (CS) was presented to the rat. To make this experiment clear, eating sugar pellets was viewed as the laboratory equivalent of grazing, the shock represented an attack, and the appearance of the toy hedgehog substituted for the predator. Two control groups were run under identical conditions, except that one group saw the hedgehog but did not get shocked and the other group got the shock but did not see a hedgehog.
On the next day, each animal was returned to the situation and a number of responses were measured. Compared with the control groups, backward conditioning was found after a delay of 1, 5, and 10 s but not after 40 s. Relative to control animals, experimental subjects showed greater avoidance (fear) of the hedgehog, spent less time in the presence of the hedgehog, and ate less food. Presumably, the shock (US) elicited a fear-flight reaction (UR), and backward conditioning transferred this reaction to the toy hedgehog (CS). The fear induced by the hedgehog (CR) interfered with eating and produced avoidance of the toy animal. This experiment shows that with a biologically relevant CS, backward conditioning is possible. Despite this outcome, most researchers suggest that the backward arrangement of US and then CS does not result in reliable conditioning (but see Cole & Miller, 1999; Siegel & Domjan, 1971; Tait & Saladin, 1986, for backward inhibitory conditioning; also Arcediano & Miller, 2002, for timing and backward conditioning).
So far we have considered only first-order conditioning. To briefly review, in first-order conditioning, an apparently neutral stimulus is paired with an unconditioned stimulus. After several such pairings, the control of the response to the US is transferred to the neutral stimulus, which is now called a conditioned stimulus (CS). Second-order conditioning extends this transfer of control to events that have not been directly associated with the unconditioned stimulus. These events gain control over the response because of their pairing with an established conditioned stimulus. Thus, second-order conditioning involves pairing a second CS2 with an already functional CS1, rather than pairing a CS and US (Rizley & Rescorla, 1972). Such higher order conditioning is important because it extends the range of behavioral effects produced by respondent conditioning, especially with regard to learning word meanings (Staats, 1975) and evaluative conditioning in humans (De Houwer, Thomas, & Baeyens, 2001, for a review).
Some phobic reactions (i.e., an intense and seemingly irrational fear) that people have may be caused by higher order conditioning. Consider a person who refuses to sit with friends in the backyard on a nice summer day. The sight of flowers greatly upsets her and she says that “with so many flowers there are probably bees.” A possible interpretation is that the person has been stung (US) by a bee (CS1), and she has noticed that bees hover around flowers (CS2). The “phobic” fear of flowers occurs because of the pairing of bees (CS1) with flowers (CS2). Thus, phobic reactions and other emotional responses may sometimes involve higher order respondent conditioning (see Martin & Pear, 2006, on systematic desensitization and the fear hierachy).
Basic research on simple and complex (i.e., including contextual effects) respondent conditioning has applied importance. Recently, the US government has declared a war on the import and use of illegal drugs. One result of this is that more money is being spent on research to identify the factors that affect drug use and abuse. Several experiments have shown that conditioned stimuli can produce drug-like effects in both humans and other animals, disrupting behavior and producing physiological changes. In addition, stimuli that have been paired with drugs sometimes produce internal conditioned responses that are opposite to the unconditioned effects of the drug. For example, when animals are injected with insulin (US), the unconditioned response is a reduction in blood sugar (UR). The response to a stimulus (CS) that has been paired with insulin is exactly the opposite; blood sugar levels increase (Siegel, 1972, 1975).
Similar counteractive effects have been found with drugs other than insulin. For example, amphetamine reduces appetite, but a CS that has been paired with it increases food intake (Poulos, Wilkinson, & Cappell, 1981). Pentobarbital is a sedative, but the response to a conditioned stimulus associated with pentobarbital counteracts the drowsiness ordinarily associated with the drug (Hinson, Poulos, & Cappell, 1982).
Effects such as these suggest that respondent conditioning plays a major role in drug tolerance. Here is how it works. With repeated pairings of a drug (US) and a CS (e.g., injection process), the conditioned response gains in strength and increasingly opposes the unconditioned effects of the drug. This means it will take larger and larger amounts for the user to experience the same degree of effect. In everyday life, conditioned stimuli arise from the time of day that a drug is taken, the way it is administered (e.g., using a needle), the location such as a tavern or home, and social events like a party or dance.
Notice that, in the case of tolerance, the reduction in the effects of the drugs (UR) is not due to habituation; rather, it is the result of the counteractive effects (CR) to the injection process and setting (CS). When more of a drug (US) is needed to obtain the same drug effects (UR), we talk about drug tolerance (Baker & Tiffany, 1985). Thus, the counteractive effects of CSs are major components of drug tolerance.
The concept of homeostasis helps to clarify the control by the CS over conditioned responses opposite to those induced by the US. Homeostasis is the tendency for a system to remain stable and to resist change. In terms of a biological system, homeostasis refers to the regulation of the system by negative feedback loops. For example, the body maintains a temperature within a very fine tolerance. If the environment warms up or cools down, physiological mechanisms (sweating or shivering) involving the sympathetic and parasympathetic nervous systems are activated to reduce the drift from normal body temperature. In terms of drug exposure, when a drug (US) is administered it upsets the stability of the system, i.e., it may increase heart rate or reduce respiration (UR). If some aspect of the environment is consistently present when the drug is delivered (e.g., drug paraphenalia, a person, or the room), then that stimulus becomes a conditioned stimulus (CS) capable of eliciting a conditioned response that is often preparatory and compensatory (CR). If the US drug causes heart rate to increase, the conditioned compensatory (homeostatic) response (CR) will be a heart rate decrease; that is, the learned component (CSneedle →CRheart rate decrease) counteracts the unlearned response to the drug (USdrug →URheart rate increase). This counteracting homeostatic effect may be so great that it nullifies the responses to the drug and the user no longer experiences the typical high, a process called tolerance. The onset of tolerance can be dangerous for the drug user. If a larger dose of the drug is taken to overcome tolerance and the compensatory counteracting response is not produced, an overdose can occur. Further, if the preparatory stimuli (CSs) elicit their responses and the drug (US) is not delivered, a condition that we call craving or withdrawal occurs.
To consider drug tolerance as a result of a conditioned response helps to explain instances of drug overdose. Heroin addicts are known to survive a drug dose that would kill a person who did not regularly use the drug. In spite of this high level of tolerance, approximately 1% of heroin addicts die from drug overdose each year. These victims typically die from drug-induced respiratory depression. Surprisingly, many of these addicts die from a dose that is similar to the amount of heroin they usually took each day. Siegel, Hinson, Krank, and McCully (1982) proposed that these deaths resulted from “a failure of tolerance. That is, the opiate addict, who can usually tolerate extraordinarily high doses, is not tolerant on the occasion of the overdose” (p. 436). They suggested that when a drug is administered in the usual context (CS+), the CRs that counteract the drug allow for a large dose. When the situation in which the drug is taken is changed, the CSs are not present, the opposing conditioned response does not occur, and the drug is sufficient to kill the user. Siegel and associates designed an animal experiment to test these ideas.
In one study rats were injected with heroin every other day for 30 days. The amount of heroin was gradually increased to a dose level that would produce tolerance to the drug. On nonheroin days, these rats were injected with dextrose solution (i.e., sugar and water). Both heroin and dextrose injections were given in one of two distinctive contexts—the ordinary colony room that the rats lived in, or a different room with constant white noise. A control group of rats was injected only with the dextrose solution in the two situations. The researchers predicted that experimental animals would develop a tolerance to the drug; this tolerance would occur if aspects of the room in which heroin injections were given became CSs that elicited opposing responses (CRs) to the drug.
To test this assumption, Siegel and colleagues (1982) on the test day doubled the amount of heroin given to experimental animals. The same high dose of heroin was given to the control group, who had no history of tolerance. Half of the experimental animals received this larger dose in the room where the drug was usually administered. The other addicted rats were injected with the higher dose in the room where they usually received a dextrose injection.
Figure 3.7 shows the results of this experiment. As you can see, the large dose of heroin killed almost all of the animals in the control group. For the two groups of animals with a history of heroin exposure, one group (same room) received the higher dose in the room where they usually were injected with heroin. Only 32% of the rats died in this condition, presumably because the CSs set off the opposing conditioned responses. This inference is supported by the mortality rate of rats in the different room group. These rats were injected with the double dose of heroin in a room that had never been associated with heroin administration. Twice as many animals in this condition died from the larger dose (64%) when compared to the same room group. It seems the effects of context during this kind of respondent conditioning can be a matter of life or death—tolerance to heroin (and perhaps other drugs) is relative to the situation in which the conditioning has occurred (Siegel, 2001).
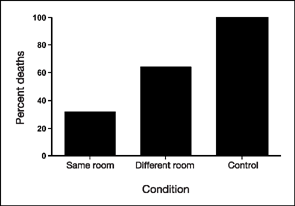
FIG. 3.7 Results of the experiment by Siegel, Hinson, Krank, and McCully (1982). The same room group of rats received the higher dose in the room where they usually were injected with heroin, and only 32% died. Twice as many animals in the different room condition died from the larger dose, presumably because they were injected in a room where heroin had not been given. Heroin killed almost all of the animals in the control group. Adapted from Siegel et al. (1982), Science, 216, 436–437.
What happens when the drug-related CS is presented without the drug US, as in the classical extinction procedure? In this case the elicited respondents are often called “cravings” and the process is known as conditioned withdrawal. The CS elicits reactions that are ordinarily countered by the US. However, when the US is not delivered and if those CR reactions occur, the subject experiences what is called withdrawal. A heroin addict can have their withdrawal symptoms immediately terminated by a heroin injection. If you are accustomed to having a cigarette after a meal, the craving you experience can be alleviated with a smoke.
Conditioned immunosuppression is another example of environmental influences altering what is generally considered to be internal and autonomously controlled processes. In this procedure, a CS (for example a novel flavor) is paired with a US drug that suppresses immune system function, such as the production of antibodies. (Note that drugs like cyclophosphamide are commonly administered to suppress rejection of a transplanted organ.) After several pairings the CS is presented alone and the immune system reaction is measured. Ader and Cohen (1981, 1985, 1993) were the first to systematically investigate and support this phenomenon. Clearly the next question is, can the immune system also be conditioned to increase immune reaction? It appears that it can. In a human study Buske-Kirschbaum, Kirschbaum, Stierle, Jabaij, and Helhammer (1994), after pairing a flavor CS and adrenaline injection US, subsequently raised NK (natural killer) cell production by presentation of the flavor alone.
The issue of conditioned enhancement of the immune system also speaks to the findings of placebo effects. How can a neutral substance, a placebo, have any effect on a person's physiological well-being? Many studies have shown that groups receiving a sugar pill do as well as those in the legitimate treatment group (Brody, 2000). How can this be possible when the placebo, by definition, cannot directly cause any change? The obvious conclusion is that there is respondent conditioning occurring. A CS, say the patient's “belief” (resulting from experience with doctors and medication) that they are receiving treatment, is presented and this verbal stimulus acts as a placebo to elicit the CR mechanisms of improvement. Even sham (fake) arthroscopic surgery for arthritis is as functional as actual surgery, and of course with fewer side effects and less cost (Moseley, O'Malley, Petersen, Menke, Brody, Kuykendall, et al., 2002). One thing that these types of studies indicate is that there is much greater two-way interaction between the environment and physiological mechanisms than has been suspected. Organisms are adaptive and they learn. It appears that organs (e.g., salivary glands) and organ systems (e.g., immune system) can also alter their functions as a result of experience. Obviously we need more research to expand and validate these topics.
We have so far examined CS and US relationships in isolation, ignoring for the most part the context or background in which these events occur. To investigate the effects of context on respondent behavior, researchers have arranged situations involving compound stimuli. In these cases, and to keep things somewhat simple, two conditioned stimuli (e.g., tone and light) are presented together before (delayed) or during (simultaneous) a US. This arrangement of two controllable stimuli (compound CSs) presented together can be shown to acquire the capacity to elicit a single conditioned response.
In an everyday example, the odor of food at a bakery or restaurant probably becomes a CS for salivation, having been paired with donuts or burgers and fries (US). But other related stimuli like the name, the order clerk, the location of the store, and the outdoor signs are also paired with eating. These additional features of the fast-food experience become conditioned stimuli that function as the context (compound CS) that evokes salivation. Differences in conditioning procedures related to compound stimuli result in the behavioral processes called sensory preconditioning, blocking, and overshadowing.
Pavlov (1960) first described overshadowing. A compound stimulus consisting of two or more simple stimuli are presented at the same time. For example, a faint light and loud tone (compound CS) may be turned on at the same time and paired with an unconditioned stimulus such as food. Pavlov found that the most salient element of the compound stimulus came to regulate exclusively the conditioned response. In this case the loud tone and not the faint light will become a CS and elicit salivation. The tone is said to overshadow conditioning to the light. This happens even though the weak light could function as a CS if it were originally presented by itself and was paired with a US.
Kamin (1969) reported an effect related to overshadowing that also involved compound stimuli. This effect is called blocking and describes a situation in which one CS paired with a US blocks a subsequent CS—US association. In blocking, a CS is paired with a US until the conditioned response reaches maximum strength. Following this conditioning, a second stimulus is presented at the same time as the original CS, and both are paired with the unconditioned stimulus. On test trials, the original CS evokes the CR but the second stimulus does not. For example, a tone (CS) may be associated with food (US) until the tone reliably evokes salivation. Next, the tone plus a light are presented together as a compound CS and both are associated with food (US). On test trials, the tone will elicit salivation but the light will not. The previously conditioned tone blocks conditioning of the light stimulus.
Kamin (1969) used a procedure called conditioned suppression (see Estes & Skinner, 1941). In conditioned suppression, a previously neutral stimulus (e.g., tone, light, etc.) is paired with an aversive US such as an electric shock. After several pairings, the originally neutral stimulus becomes a conditioned aversive stimulus (CSave). The CSave is said to elicit a conditioned emotional response (CER) that is commonly called anxiety or fear. Once the CSave has been conditioned, its effects may be observed by changes in an organism's operant behavior. For example, a rat may be trained to press a lever for food. After a stable rate of response is established, the CSave is introduced. When this occurs, the animal's lever pressing is disrupted, presumably because of the CER elicited by the CSave. Basically we could say that the CSave frightens the animal so it stops pressing the bar. Conditioned suppression is a widely used procedure in respondent conditioning, and as you will see later it is important in the study of human emotions.
Using a conditioned-suppression procedure, Kamin (1969) discovered the phenomenon of blocking. Two groups of rats were used: a blocking group and a control group. In the blocking group, rats were presented with a tone (CSave) that was associated with electric shocks for 16 trials. Following this, the rats received 8 trials during which the compound stimulus of tone and light was followed by shock. The control group did not receive the 16 light-shock conditioning trials but did have the 8 trials of tone and light paired with shock. Both groups were tested for conditioned suppression of lever pressing in the presence of the light. That is, the light was presented alone and suppression of bar pressing for food indicated the occurrence of the CER. Kamin found that the light suppressed bar pressing in the control group but did not affect lever pressing in the blocking group. In other words, prior conditioning with the tone blocked or prevented conditioning to the light. Functionally, the light was a CSave in the control group but not in the blocking group.
Blocking and overshadowing may also be interpreted as cases of redundant stimuli. If two or more stimuli have been paired with the same US then only one CS element of the compound is required for eliciting the CR. We intentionally generate compound stimuli (actually we can hardly avoid it) so that some aspect or other of the environment will gain eliciting properties. All stimulus manipulations are conducted in some place, be it the lab or elsewhere, and elements of that environment will be paired with the stimuli of interest. It is the repeated, consistent, and predictable nature of the specific CS—US pairing that tends to restrict the connection to only some stimuli.
Sensory preconditioning is another example of stimulus control by compound events. In this case, two stimuli such as light and tone are repeatedly presented together without the occurrence of a known Us—this is called preconditioning. Later, one of these stimuli is paired with an unconditioned stimulus for several trials and then the other stimulus is tested for conditioning. Even though the second stimulus was never directly associated with the US, it comes to elicit a conditioned response (Brogden, 1939; Pfautz, Donegan, & Wagner, 1978; Prewitt, 1967).
For example, a rat may be repeatedly exposed to 10 s of light with an accompanying 10-s tone. Following this preconditioning phase, the tone is paired with an electric shock. Subsequently, using a conditioned-suppression procedure, it is possible to show that the light will also suppress the animal's operant behavior. Notice that the light has never been paired with the shock but comes to have a CSave function based on previous association with the tone. Preconditioning is a way that stimuli may acquire corresponding or equivalent functions. It might be said that the light “stands for” the tone and the tone for the light.
The occurrence of overshadowing, blocking, and sensory preconditioning has led many researchers to the conclusion that cognitive processes underlie conditioning. This is because these effects (and others) seem to imply that an animal learns to expect certain events on the basis of predictive cues. That is, the sight of a predator becomes a predictive cue because the animal expects an attack. The CS is said to provide information about the occurrence of the US, and redundant information, as in blocking, is not processed by the organism.
Although this may be an intuitively satisfying account, inferring cognitive processes is not necessary to describe most of the research in respondent conditioning. Bolles (1979) has commented as follows:
Are we now in a position to conclude that conditioning is really a cognitive process, that it involves the expectancy of a … [US], and that the expectancies reflect predictive relationships the animal perceives between cues and consequences? Some psychologists have come to this conclusion. But others have shown restraint. Indeed, it turns out to be possible to account… [for many conditioning effects], all without recourse to any cognitive concepts. It can all be done with the clever application of [temporal pairing of stimuli] and other S-R principles. This remarkable development is the work of Wagner, and surprisingly, Rescorla himself. They have produced what is widely known as the Rescorla-Wagner model.
(p. 158)
As Bolles (1979) notes, the Rescorla—Wagner model (Rescorla & Wagner, 1972; Wagner & Rescorla, 1972) is an S-R pairing theory of respondent conditioning. That is, the Rescorla—Wagner model is a behavioral theory that does not make inferences about underlying cognitive/informational processing.
The basic idea of the Rescorla—Wagner model is that a conditioned stimulus acquires a limited amount of associative strength on any trial. We use the term associative strength to describe the relation between the CS and the magnitude of the conditioned response (CR). In general, associative strength increases over conditioning trials and reaches some maximum level. It is apparent that a given CS can acquire only so much control over a conditioned response. This is the maximum associative strength for the CS. Thus, a tone (CS) that is paired with 1 g of food will have maximum associative strength when salivation (CR) to the tone is about the same as salivation (UR) to the 1 g of food (US). That is, an unconditioned stimulus elicits a given magnitude of the unconditioned response. This magnitude sets the upper limit for the conditioned response. The CS cannot elicit a greater response than the one produced by the unconditioned stimulus.
A conditioned stimulus gains a certain amount of associative strength on any one trial. The amount of gain or increment depends on several factors. One obvious factor is the maximum associative strength that may accrue to the conditioned stimulus. As noted, this maximum is set by the magnitude of the US—UR relationship. An intense US will set a higher maximum value than a weaker one.
Another factor that affects the increment in associative strength on any trial is the change in associative strength or the difference between the present strength of the CS and its maximum possible value. As conditioning trials proceed, the CS gains associative strength, and this means that the difference between present and maximum strength decreases; there is less and less to gain on each trial. For example, assume a 10-trial experiment in which 1 g of meat evokes 2 cc of saliva and the meat is paired with a tone. In terms of change in associative strength, the most gain will occur on the 1 st trial, there will be less gain by the 5th, and there will be almost no gain in associative strength by the 10th trial.
The change in associative strength of a conditioned stimulus (CS1) is also affected by the strength of other conditioned stimuli (CS2, CS3, etc.) that elicit the conditioned response in that situation. Because there is a maximum associative strength set by the US, it follows that the associative strength of each CS will add together and reduce the difference between the present associative strength and the maximum possible value. Thus, if a tone has been frequently paired with meat, it will evoke almost maximum salivation. If a light is now introduced and presented along with the tone, it will show little control over salivation since most of the possible associative strength has accrued to the tone (blocking).
The Rescorla—Wagner model of respondent conditioning describes a large number of findings and has stimulated a good deal of research. The model makes counterintuitive predictions that have been confirmed in a variety of experimental settings. Since the early 1970s, scores of experiments have been conducted to test some of the implications of the model.
The three limiting conditions of maximum associative strength, difference between the current strength and maximum, and the number of additional CSs in the situation are represented by an equation suggested by Rescorla and Wagner (1972; see also Wagner & Rescorla, 1972) but simplified here for presentation:

The symbol ΔV stands for the amount of change in associative strength (or change in value of the stimulus, V) of any CS that occurs on any one trial. The symbol S is a constant that varies between 0 and 1, and may be interpreted as the salience (e.g., dim light versus bright light) of the CS based on the sensory capacities of the organism. The constant S (salience) is estimated after conditioning and determines how quickly the associative strength of the CS rises to a maximum. That is, a larger salience coefficient makes the associative strength of the CS rise more quickly to its maximum. The value VMAX represents the maximum associative strength as measured by the magnitude of the unconditioned response (UR). The symbol V represents the associative strength already accrued to the CS1 and VSUM is any associative strength gained by any other stimuli in the situation (VSUM = CS2 + CS3 + …CSN).
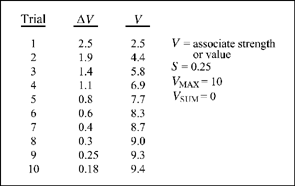
FIG. 3.8 A table of values for a 10-trial acquistion experiment based on solving the Rescorla—Wagner equation (our Equation 3.1). The symbols Vand ΔV refer to associative strength and change in associative strength for a given trial. The values of VMAX, VSUM and S are also given in the table. See text for details.
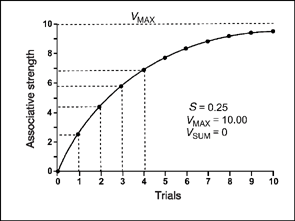
FIG. 3.9 The acquisition curve predicted by the Rescorla—Wagner equation (our Equation 3.1). Gain in associative strength, from trial to trial, declines as the CR comes closer to the asymptote. The asymptote or upper-flat portion of the curve is set in the equation by the value VMAX. The curve is based on the data in Figure 3.8.
Figure 3.8 is a table of values for an idealized experiment on the acquisition of a conditioned response based on Equation (3.1). Figure 3.9 is the graph of the associative strength V based on the data in the table. In this hypothetical experiment, a tone CS is repeatedly paired with an unconditioned stimulus such as food. In the figure, S is set at 0.25 and the asymptote (or maximum possible strength) is 10 arbitrary units of the conditioned response (e.g., salivation). The value of VSUM is assumed to be zero, so that all associative strength accrues to the CS. The value of ΔV is given by the equation when we substitute S = 0.25, VMAX= 10, and the value of Vis zero (V= 0) before conditioning begins. Based on Equation (3.1), the increase in associative strength from no conditioning to the first trial is
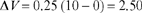
Notice that the value of V has changed from 0 to 2.50 (check this with the tabled values of Figure 3.8).
On each subsequent trial, the associative strength of the CS is 0.25 (salience) of the remaining distance to the asymptote or maximum. Thus for trial 2 we substitute the value 2.50 for V and obtain an increase of 1.88 for ΔV:
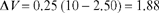
The associative strength of the CS (V), after the second trial is 2.50 + 1.88, or 4.38. This means that roughly half of the maximum associative strength (VMAX =10) of the CS has been acquired by trial 2.
The change in associative strength for trial 3 uses V = 4.38 from the second trial and obtains the value:
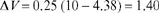
And the new estimate of V is 4.38 + 1.40, or 5.78 (used to obtain ΔV on the 4th trial). Estimates of ΔV and V for all 10 trials of the experiment are obtained in the same way, using Equation (3.1).
As you can see in Figure 3.9, the equation yields a negatively accelerating curve for the associative strength V, which approaches but never quite reaches maximum associative strength. You can see from the horizontal and perpendicular lines that the largest increase in associative strength is on the first trial, and this change corresponds to the difference in associative strength between trial 0 and trial 1 (2.5-unit increase). The change in associative strength (ΔV) becomes smaller and smaller over trials (check this out in the table of Figure 3.8). Notice how the values of ΔV and V depend on the salience, S, of the CS (tone). If the salience of the tone were different, say S = 0.50 rather than S = 0.25, a new set of estimates would be given by Equation (3.1) for ΔV and V.
As Bolles (1979) noted, the Rescorla—Wagner equation accounts for many respondent conditioning effects without making assumptions about cognitive processes. One important effect that we have already discussed is blocking. Equation (3.1) provides a behavioral account of this phenomenon. Consider what will happen when Vis almost equivalent to the value VMAX, and a second conditioned stimulus (CS2) is introduced. For example, a tone (CS1) is paired with shock until the tone evokes close to maximum response suppression. At this point, a light (CS2) is presented at the same time as the tone and conditioning continues. In Equation (3.1), the light is represented as VSUM and the tone as V. After the tone acquires close to maximum strength, little is left over for the light (VSUM) and the light has almost no suppressive effect on bar pressing. That is, the previous conditioning to the tone blocks conditioning to the light. Notice that it makes a big difference when the CS2 is introduced. If CS1 and CS2 are paired from the start, then (all things being equal) both stimuli will gain half of the increase in associative strength (ΔV).
Equation (3.1) can also be used to account for respondent extinction. In this case, the decline in associative strength (ΔV) is determined by the values of S, VMAX, V, and VSUM. As before, assume that a tone is paired with food until the tone (CS) elicits a conditioned response that is close to maximum; there are no other relevant stimuli, so VSUM = 0 and cancels out of the equation. Since the procedure is respondent extinction, the curve must decline toward no associative strength, which means that VMAX must be zero. If S = 0.25 and VMAX = 0 then the decline in associative strength on the first extinction trial is:
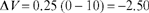
Thus, the value of the tone (CS) after the first extinction trial is 10.00−2.50, or 7.50 (V=7.50). Other values of the CS during extinction are determined in a similar fashion (compare with respondent acquisition). Figure 3.10 shows that the predicted extinction curve is the exact opposite of the acquisition curve of Figure 3.9. It is important to note that the actual associative strength of the tone before extinction is never exactly equal to the VMAX, but for simplicity we have assumed that it is in Figure 3.10.
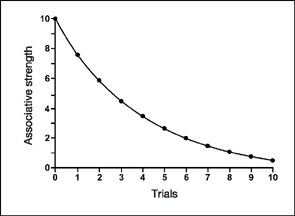
FIG. 3.10 The extinction curve predicted by the Rescorla—Wagner model. Notice that VMAX, or the asymptote, is zero because extinction is in effect.
As you can see, the Rescorla—Wagner equation describes many of the basic aspects of respondent conditioning (acquistion, extinction, and blocking) as well as others not discussed here (e.g., latent inhibition). The equation is usually said to describe processes of associative learning but, as Pear (2001, p. 427) notes, it is also possible to derive equations for operant choice (Chapter 9) from the Rescorla—Wagner model, and vice versa. Thus, both respondent and operant behavior could be related at the most fundamental levels. Advances in neurophysiology may help to show how this is possible in terms of the reward circuitry of the brain (e.g., Zink, Pagnoni, Martin-Skursky, Chappelow & Berns, 2004).
This chapter has introduced reflexive behavior, which is based on species history or phylogeny. It has explored reflexive sequences or fixed action patterns set off by a releasing stimulus and reaction chains where each response requires an appropriate stimulus to keep the sequence going. Reflexive behavior obeys the three laws of the reflex (threshold, magnitude, and latency) and secondary principles such as reflex habituation. Next, the discussion turned to the ontogeny of behavior and respondent conditioning, which involved pairing a CS (tone) with a US (food in mouth). Respondent behavior is elicited by the US and this function is established for the CS through conditioning. Two major processes of respondent acquisition and extinction were described and research examples were provided. It was also pointed out that respondent behavior shows spontaneous recovery even after an extinction procedure has occurred.
Organisms show generalization of respondent behavior over a stimulus gradient but also show discrimination (a differential response) when the US is presented with one stimulus and withheld to other values of the stimulus array. In addition to simple conditioning effects, temporal relationships between the CS and US are important, as in delayed, simultaneous, trace, and backward conditioning—and second-order conditioning. The implications of respondent conditioning were extended to an analysis of drug use and abuse, with some attention to context and drug tolerance. Finally, more advanced issues of complex conditioning and compound stimulus effects such as overshadowing, blocking, and sensory preconditioning were introduced. The Rescorla—Wagner model of conditioning was described and how the model could be expressed as an equation. This equation predicts the increase in the respondent over time (acquisition) and the decrease during extinction. The equation can also describe the process known as blocking.
Associative strength
Backward conditioning
Blocking
Change in associative strength
Compound stimuli
Conditioned response
Conditioned stimulus
Conditioned suppression
Conditioned withdrawal
Delayed conditioning
Elicited
Fixed action pattern
Generalization gradient
Habituation
Homeostasis
Law of intensity—magnitude
Law of latency
Law of the threshold
Maximum associative strength
Ontogenetic
Overshadowing
Phylogenetic
Placebo effect
Primary laws of the reflex
Reaction chain
Reflex
Rescorla—Wagner model
Respondent
Respondent acquisition
Respondent conditioning
Respondent discrimination
Respondent extinction
Respondent generalization
Respondent level
Salience
Second-order conditioning
Sensory preconditioning
Simultaneous conditioning
Spontaneous recovery
Tolerance
Trace conditioning
Unconditioned response