3 Preprocedure Assessment Prior to Vertebral Augmentation
Scott Kreiner and Grace Maloney
Summary
Vertebral compression fractures (VCFs) are common and are increasing in number with the aging of the population. Patients with VCFs are optimally treated by accurate and early diagnosis and treatment. The patients with asymptomatic fractures must be recognized to be different than those with fractures and moderate to severe pain or pain that is increasing as the latter patients typically are not effectively treated with nonsurgical management (NSM). Correct identification of VCFs via cross-sectional imaging is important and the preprocedure assessment includes such factors as risks, cost, and the patient’s ability to tolerate NSM or percutaneous vertebral augmentation (PVA). If the patient is provisionally deemed to be a candidate for PVA, there should be an assessment of the indications and contraindications. The ideal candidate for vertebral augmentation is a patient with a symptomatic fracture seen on cross sectional imaging than cannot tolerate NSM and has positive physical examination signs and no absolute contraindication. The PVA procedure should be done with the appropriate equipment and personnel and in a facility designed to accommodate these procedures. After the augmentation procedure the patient should undergo the appropriate follow-up to ensure the optimal recovery from both the fracture and the procedure and they should also receive appropriate therapy for the underlying disorder that originally predisposed them to the vertebral fracture.
Keywords: vertebral compression fracture, percutaneous vertebral augmentation, contraindications, preprocedure assessment, sedation, postprocedure management
3.1 Introduction
Vertebral compression fractures are common and becoming more common as our population ages. Appropriate early diagnosis and management is key to obtaining optimal outcomes for these patients. While many patients have fractures that are only incidentally seen on imaging at some point after the fracture, the presence of a single compression fracture substantially increases the risk for future fractures. In addition to the commonly seen asymptomatic fracture, there is a prominent subset of patients that present with moderate to severe pain that does not resolve with NSM. For these reasons, early identification and optimal management of compression fractures improve outcomes in these patients.
3.2 Anatomy, Radiologic Imaging, Identification, and Interpretation of Vertebral Compression Fractures
Selection of appropriate patients for PVA is essential to obtaining optimal outcomes. Traditionally, the patient populations considered to benefit most from PVA generally fall into three categories as follows: elderly osteoporotic individuals with acute or subacute VCFs, individuals with compression fractures refractory to NSM, and individuals with compression fractures secondary to malignancy.
Chronicity of a VCF is an important factor to consider when considering PVA. While patients with older painful fractures commonly derive substantial benefit from PVA in terms of pain relief and improved functionality after failing conservative management, the best outcomes are seen in more acute fractures with evidence of bone marrow edema or fracture nonhealing.1
Identification of these fractures using current imaging technology is critical. The majority of osteoporotic VCFs occur at the thoracolumbar junction. Patient examination including palpation and percussion of the vertebral bodies can help identify the involved segment (see Chapter 4: Physical Examination Findings in Patients with Vertebral Compression Fractures). Imaging of the spine is ideally performed with magnetic resonance (MR) imaging. In cases of severe claustrophobia or MR imaging incompatibility (non-MRI compatible pacemaker, cochlear implant, etc.), imaging studies should include a computed tomography (CT) scan of the involved area. In order to distinguish between acute, subacute, and chronic fractures, the presence of edema on a sagittal T2 fat-saturated or short tau inversion recovery (STIR) sequence in combination with a sagittal T1-weighted sequence is the best indicator of an acute or subacute fracture. In cases of MR imaging incompatibility, CT scanning in combination with nuclear medicine bone scan or single-photon emission computed tomography (SPECT) imaging is usually required to distinguish between newer versus older fractures.2 In cases of complex fractures, the imaging evaluation with MRI and/or CT of the spine optimizes visualization of the fracture and may allow for more specific and directed treatment.
3.3 Preprocedure Assessment
While individuals with acute or subacute compression fractures will likely achieve good outcomes after PVA, other factors for consideration include procedural risks, procedure cost, and the patient’s functional ability and ability to tolerate NSM.
The elderly osteoporotic patients with compression fractures are a group who derives a substantial benefit from PVA. Pain from a VCF is significantly limiting to patients of all ages, but the deconditioning associated with patient immobility is especially detrimental to the elderly population. Immobility places these patients at greater risk for pneumonia, pulmonary embolism, skin breakdown, and many other complications. Muscle atrophy from disuse is compounded by bracing and leads to decline in activities of daily life (ADLs), which is associated with increased mortality.3 Narcotic medications used for analgesia of the severe pain are more poorly tolerated by the elderly.4 When considering the risks of PVA as compared to the risks of immobility in this elderly osteoporotic population, the benefits of the procedure usually outweigh the risks and in elderly patients with moderate to severe pain the risks of NSM are usually greater than the risks of PVA.5
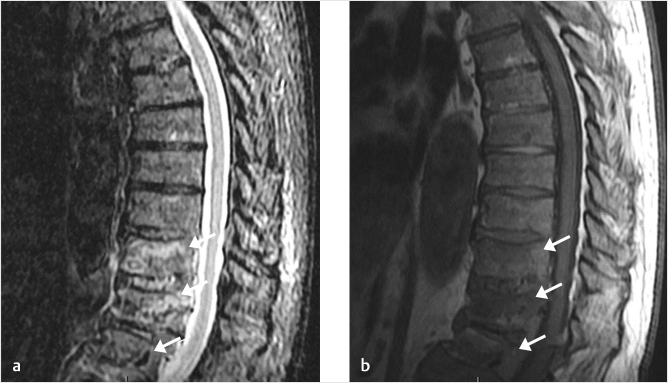
Fig. 3.1 Sagittal short tau inversion recovery (STIR; a) and T1-weighted (b) MR images show vertebral compression fractures with bone marrow edema (white arrows in a and b). The cause of these fractures was determined to be related to multiple myeloma following biopsy at the time of vertebral augmentation.
Fig. 3.2 Sagittal T2-weighted (a) and short tau inversion recovery (STIR; b) MR images show multiple vertebral compression fractures without significant bone marrow edema (white arrows in a and b) more typical of chronic vertebral fractures. The L2 vertebral body shows bone marrow edema (area within white circles) indicating an acute or subacute vertebral compression fracture. Intravertebral fluid-filled clefts are also seen in the L2 vertebral body (white arrowheads).
For the younger patient with a traumatic VCF, NSM is typically attempted first. Younger patients tend toward greater resilience in terms of functional decline, toleration of pain medications, and ability to heal bony injuries. However, in cases of severe or ongoing pain refractory to NSM, PVA may be reasonable provided that imaging findings correspond to the patient’s clinical presentation and physical examination findings.
The third group who benefits significantly from PVA are patients with pathologic fractures due to neoplasia.6 In cases of pathologic fractures (▶Fig. 3.1), if there is no known preexisting malignancy, bone biopsy performed at the time of the PVA can assist with diagnosis of the neoplasm.
3.4 General Indications, Contraindications, and Procedure Complications
As mentioned previously, compression fractures may be identified incidentally on imaging and are not causing pain (▶Fig. 3.2). However, in patients who present with substantial back pain and have a compression fracture, PVA may be appropriate. The major indications for vertebral augmentation are the presence of a symptomatic osteoporotic vertebral body fracture(s) that are refractory to medical therapy or that are too symptomatic for the patient to tolerate NSM and in vertebral bodies weakness or fracture due to neoplastic involvement.7 These fractures include symptomatic nondisplaced vertebral body fractures (seen on MR imaging or nuclear bone scan but has no obvious loss of vertebral body height) and patients with fracture progression and progressive loss of vertebral body height. In patients with mild pain, no functional impairment, or pain that is managed well with NSM such as oral medications and bracing, PVA may be performed later or not at all.
Absolute contraindications to PVA are infection at this site of the vertebral augmentation and an untreated blood-borne infection.8 A strong contraindication is osteomyelitis of the vertebral column.8 A contraindication that is usually contraindicated is for women who are pregnant.8 Relative contraindications that may or may not result in the discontinuation of the planned vertebral augmentation procedure include allergy to fill material, coagulopathy, spinal instability, myelopathy from the vertebral fracture, neurologic deficit, neural impingement, and fracture retropulsion with canal compromise.8
Overall, PVA is a very safe and well-tolerated procedure. Despite its optimal safety profile, there have been a few case reports of procedural complications. These include infections of the soft tissues, disk (diskitis), and/or bone9–14 (osteomyelitis), bleeding, and symptomatic extravasation of polymethyl methacrylate (PMMA).15–21 Intradiskal leakage of PMMA has also been shown to increase the risk of adjacent-level fractures.22
3.5 Equipment and Room Setup
Room setup and equipment used for the procedure will vary based on physician preference and availability (▶Fig. 3.3 and ▶Fig. 3.4). This section will provide minimum safe requirements for the performance of vertebral augmentation.
Fig. 3.3 Office-based procedure suite. Nurse (not shown) is to the left; note the monitor (black arrow), C-arm (black arrowhead), and oxygen (white arrow) available. Strict sterile technique is necessary for the performance of vertebral augmentation.
Fig. 3.4 The patient is placed in a prone position with towel rolls (black arrows) or gel pads under their shoulders and pelvis to put the spine in extension and facilitate vertebral fracture reduction.
As with any procedure, it is of utmost importance that the physician performing this procedure has been trained in the performance of vertebral augmentation and is prepared to manage any complications that may arise during the procedure. It is also important that appropriately trained personnel including nurse, and radiologic technology and other appropriate procedural or operating room personnel are present to assist the interventionalist/surgeon during this procedure.
Patients undergoing PVA should have intravenous access in place for the administration of fluids and medications as needed. Sedation may be beneficial in vertebral augmentation cases, as these patients typically have moderate to severe pain associated with their fractures, and the process of obtaining access to the vertebral body and reducing the fractured bone can be painful. The judicious use of local anesthetics is important and can help minimize the degree of sedation required to successfully perform this procedure. The decision to use sedation should be made on a case-by-case basis. If the physician performing the procedure decides to administer and supervise the sedation, they should be trained and qualified to do so. In these situations, a separate health care provider (registered nurse or other appropriately trained personnel) is required to assist with the administration of the medications and monitoring of the patient. Vital signs should be recorded at regular intervals and, if sedation is administered, pulse oximetry and cardiac monitoring must be used.
Because of the nature of the fracture and the frailty of patients who typically develop fractures that require treatment, antibiotic prophylaxis is recommended to decrease the risk of perioperative infection. Cephalosporins (cefazolin or cefuroxime) are the preferred drug due to their low toxicity, though Vancomycin and clindamycin are alternatives in patients with an allergy to cephalosporins or penicillin. These antibiotics cover primarily gram-positive organisms and may be combined with gentamycin, which covers primarily gram-negative organisms, and has been shown to penetrate into the intervertebral disk much more readily than other perioperative antibiotics.23
Strict sterile technique should be followed at all times as they pertain to the facilities, materials, patient preparation, physician preparation, and PVA materials preparation. Examples include, but are not limited to, the following:
• Skin overlying the target region should be prepared for an aseptic procedure, preferably using chlorhexidine-alcohol or povidone-iodine. The area should then be draped to create a sterile field.
• Barriers including sterile gloves, sterile gown, hat, and masks should be utilized during the procedure.
• Sterile equipment should be utilized, including a sterile C-arm cover.
• Single-use syringes/needles and single-dose vials should be employed.
Fig. 3.5 The patient is sterilely prepped and draped and gowns, gloves, and masks are worn for this sterile image-guided procedure. The needles are placed into the vertebral body with a mallet (white arrow) typically using fluoroscopic guidance.
3.6 Procedure
• The patient is typically placed in a prone position on the table. Pillows should be placed under the upper chest and pelvis to promote thoracic and lumbar extension, which will assist in the reduction of the compressed vertebrae.
• CT or fluoroscopic viewing should be utilized to guide the entry to the vertebral body (▶Fig. 3.5). See Chapter 6: Approaches to the Vertebral Body, for the various approaches to achieve optimal outcomes.
• It is desirable to achieve as much fracture reduction as possible. This reduces the risk of adjacent-level fracture and minimizes the kyphotic deformity.
• The cement, once prepared, may then be injected into the vertebral body. Volumes of cement ranging from 15 to 25% of the noncompressed vertebral body volume at the same level is necessary to adequately treat the fracture and to restore the vertebral body strength and stiffness as well as to relieve pain.24 Extravasation of cement from overfilling the vertebral cavity should be avoided.
• Incisions should receive some type of closure via a simple stitch, Dermabond, or Steri-strip-type closure bandage. In addition, if there is wound drainage, an appropriate dressing should be applied.
• The patient should be maintained in the prone immobile position while the cement sets. Setting time is temperature and cement specific though typically in the range of 10 to 20 minutes. Refer to product recommendations for the working time of the specific PMMA used.
3.7 Postprocedure Management
Most commonly, vertebral augmentation is performed as an outpatient procedure. It is appropriate to discharge the patient home following appropriate postanesthesia care. Neurological assessments should occur frequently during the immediate postprocedure recovery. Initiation of ambulation postprocedure must be carefully supervised.
As these patients are being treated for a fracture, at discharge, the patient should be instructed to avoid activities that may lead to additional or adjacent-level fracture. This includes activities that substantial increase the axial load on the spine such as lifting heavy objects, jumping from a height, or pulling objects against considerable resistance. Patients should also receive the usual postinterventional procedure instructions to minimize infection. These include keeping the incision clean and dry and no soaking in a bath, hot tub, pool, or lake for at least 48 hours after the procedure.
Finally, the cause of the fractures should be determined and treated. While not within the scope of this text, this includes diagnosis and management of osteoporosis and/or referral for malignancy workup.
References
[1] Tanigawa N, Komemushi A, Kariya S, et al. Percutaneous vertebroplasty: relationship between vertebral body bone marrow edema pattern on MR images and initial clinical response. Radiology 2006;239(1):195–200
[2] Maynard AS, Jensen ME, Schweickert PA, Marx WF, Short JG, Kallmes DF. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol 2000;21(10):1807–1812
[3] Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc 2004;52(8):1263–1270
[4] Cherasse A, Muller G, Ornetti P, Piroth C, Tavernier C, Maillefert JF. Tolerability of opioids in patients with acute pain due to nonmalignant musculoskeletal disease. A hospital-based observational study. Joint Bone Spine 2004;71(6):572–576
[5] Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009;373(9668): 1016–1024
[6] Berenson J, Pflugmacher R, Jarzem P, et al; Cancer Patient Fracture Evaluation (CAFE) Investigators. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011;12(3):225–235
[7] ACR. ACR–ASNR–ASSR–SIR–SNIS Practice parameter for the performance of vertebral augmentation. 2017
[8] Hirsch JA, Beall DP, Chambers MR, et al. Management of vertebral fragility fractures: a clinical care pathway developed by a multispecialty panel using the RAND/UCLA Appropriateness Method. Spine J 2018;18(11):2152–2161
[9] Abdelrahman H, Siam AE, Shawky A, Ezzati A, Boehm H. Infection after vertebroplasty or kyphoplasty. A series of nine cases and review of literature. Spine J 2013;13(12):1809–1817
[10] Alfonso Olmos M, Silva González A, Duart Clemente J, Villas Tomé C. Infected vertebroplasty due to uncommon bacteria solved surgically: a rare and threatening life complication of a common procedure: report of a case and a review of the literature. Spine 2006;31(20):E770–E773
[11] Mummaneni PV, Walker DH, Mizuno J, Rodts GE. Infected vertebroplasty requiring 360 degrees spinal reconstruction: long-term follow-up review. Report of two cases. J Neurosurg Spine 2006;5(1):86–89
[12] Park JW, Park SM, Lee HJ, Lee CK, Chang BS, Kim H. Infection following percutaneous vertebral augmentation with polymethylmethacrylate. Arch Osteoporos 2018;13(1):47
[13] Syed MI, Avutu B, Shaikh A, Sparks H, Mohammed MI, Morar K. Vertebral osteomyelitis following vertebroplasty: is acne a potential contraindication and are prophylactic antibiotics mandatory prior to vertebroplasty? Pain Physician 2009;12(4):E285–E290
[14] Walker DH, Mummaneni P, Rodts GE Jr. Infected vertebroplasty. Report of two cases and review of the literature. Neurosurg Focus 2004;17(6):E6
[15] Chen JK, Lee HM, Shih JT, Hung ST. Combined extraforaminal and intradiscal cement leakage following percutaneous vertebroplasty. Spine 2007;32(12):E358–E362
[16] Chen YJ, Tan TS, Chen WH, Chen CC, Lee TS. Intradural cement leakage: a devastatingly rare complication of vertebroplasty. Spine 2006;31(12): E379–E382
[17] Esmende SM, Daniels AH, Palumbo MA. Spinal cord compression after percutaneous kyphoplasty for metastatic compression fracture. Spine J 2013;13(7):831–832
[18] Grelat M, Le Van T, Fahed E, Beaurain J, Madkouri R. Rare complication of percutaneous technique: intradural cement leakage and its surgical treatment. World Neurosurg 2018;118:97
[19] Kulkarni AG, Shah SP, Deopujari CE. Epidural and intradural cement leakage following percutaneous vertebroplasty: a case report. J Orthop Surg (Hong Kong) 2013;21(3):365–368
[20] Teng MM, Cheng H, Ho DM, Chang CY. Intraspinal leakage of bone cement after vertebroplasty: a report of 3 cases. AJNR Am J Neuroradiol 2006;27(1):224–229
[21] Wu CC, Lin MH, Yang SH, Chen PQ, Shih TT. Surgical removal of extravasated epidural and neuroforaminal polymethylmethacrylate after percutaneous vertebroplasty in the thoracic spine. Eur Spine J 2007;16 (Suppl 3):326–331
[22] Jesse MK, Petersen B, Glueck D, Kriedler S. Effect of the location of endplate cement extravasation on adjacent level fracture in osteoporotic patients undergoing vertebroplasty and kyphoplasty. Pain Physician 2015;18(5): E805–E814
[23] Jackson AR, Eismont A, Yu L, et al. Diffusion of antibiotics in intervertebral disc. J Biomech 2018;76:259–262
[24] Martinčič D, Brojan M, Kosel F, et al. Minimum cement volume for vertebroplasty. Int Orthop 2015;39(4):727–733