James Mooney, John W. Amburgy, D. Mitchell Self, Leah J. Schoel, and M.R. Chambers
Summary
Balloon kyphoplasty is a minimally invasive vertebral augmentation procedure used to treat painful vertebral compression fractures. The goal of any vertebral augmentation procedure is pain reduction, stabilization of the fracture, and improvement in patient function. Unique to balloon kyphoplasty is restoration of vertebral body height and reduction of kyphotic angulation through the use of inflatable bone tamps. In addition, the balloon tamps reduce the risk of cement leakage by facilitating an injection that follows the path of least resistance into and around the cavity created by the balloon. In this chapter, the diagnostic criteria, techniques, indications, and contraindications are discussed. Materials, equipment, imaging, and the procedure itself are described in detail. In addition, the importance of sagittal balance restoration and realignment is addressed as it relates to the risk of adjacent fractures. The risks and benefits of balloon kyphoplasty are summarized.
Keywords: balloon kyphoplasty, vertebral augmentation, vertebral compression fracture, bone cement, vertebral height, kyphotic angulation, sagittal balance, minimally invasive
Vertebral augmentation is a category of surgical procedures used to treat vertebral fractures and includes vertebroplasty, kyphoplasty, and implants. The goal of any vertebral augmentation procedure is the minimally invasive reduction and stabilization of a painful vertebral compression fracture (VCF). Unique to balloon kyphoplasty is restoration of vertebral body height and reduction of kyphotic angulation through the use of inflatable bone tamps. The balloon tamps reduce the risk of cement leakage by facilitating an injection that follows the path of least resistance into and around the cavity created by the balloon.
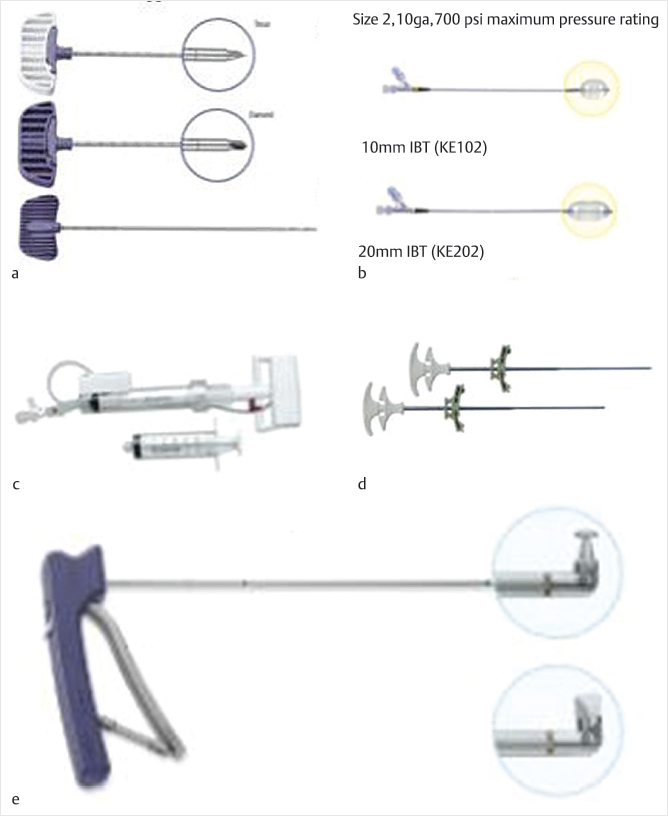
The surgical equipment is available through numerous sources. A comprehensive list of manufacturers, materials, and equipment for kyphoplasty can be found in ▶Table 11.1. Components available from Medtronic Kyphon are listed in the following sections and shown in ▶Fig. 11.1.
Table 11.1 Providers of materials and equipment for balloon kyphoplasty
Balloon kyphoplasty |
Ackermann |
Alphatec Spine |
BM Korea |
BPB Medico |
Biopsybell |
Depuy Synthes |
G-21 |
iMedicom |
KMC-Maxxspine |
Medtronic (Kyphon) |
Osseon |
Panmed US |
Rontis |
Taeyeon |
Synimed |
• Jamshidi-style needle: Typically, an 11- or 13-gauge cannulated needle used to gain access to the vertebral body via the pedicle or a peripedicular approach. The internal stylet may have a variety of bevel tips for increased penetration capability or for directional control. Once in position, the inner trocar is removed.
• Kirschner’s wire (K-wire): It is placed in the cannula, which is then removed for a Seldinger-technique establishment of the working channel.
• Osteointroducer: An 8- or 10-gauge cannulated introducer or working channel is placed over the K-wire, which is then removed. The osteointroducer may be beveled for directional control.
• Drill: It is used to cut and channel through cancellous bone for placement of the balloon.
• Curette: It is used to expand the cavity created by the drill to accommodate the expansion of the balloon.
• Inflatable bone tamps: These are available for use with 8- and 10-gauge introducers in three lengths (10, 15, and 20 mm) with volumes ranging from 3 to 6 mL and pressure ratings ranging from 300 to 700 psi.
• Inflation device: It has a manometer with a digital pressure gauge for controlled inflation.
• Cement delivery cannulas: It is an 8- or 10-gauge coaxial delivery system with an outer cannula and an inner rod or “pusher” for expelling cement.
Acrylic bone cement (ABC) is the most commonly used cement for vertebral augmentation. The main components of ABC are solid and liquid acrylic compounds that cure rapidly when mixed at room temperature and even faster when exposed to body temperature. A number of brands are commercially available. Disadvantages of using ABC include nonbiodegradability and significant mechanical mismatch with the osseous components of the vertebral body.1 Efforts have been made to improve the mechanical characteristics, porosity, and biodegradability of the products. Polymethyl methacrylate (PMMA) is the most popular bone cement. A modern version of PMMA was first used in the United Kingdom by Dr. John Charnley in total hip replacement surgery2 and was Food and Drug Administration (FDA) approved for treating VCFs in 2004.
In 2017, calcium phosphate cement (CPC) was redesigned by incorporating starch and BaSO4 to create a new cement. Biomechanical strengths measured by in vitro and in vivo models were not less than that of PMMA, while its biodegradability and osseointegrative capacities were significantly enhanced compared to PMMA.
Other less commonly used bone cements include CPC, calcium sulfate cement (CSC), and magnesium phosphate cement (MPC). Chapter 7 provides a detailed discussion of the various cements and fill materials.
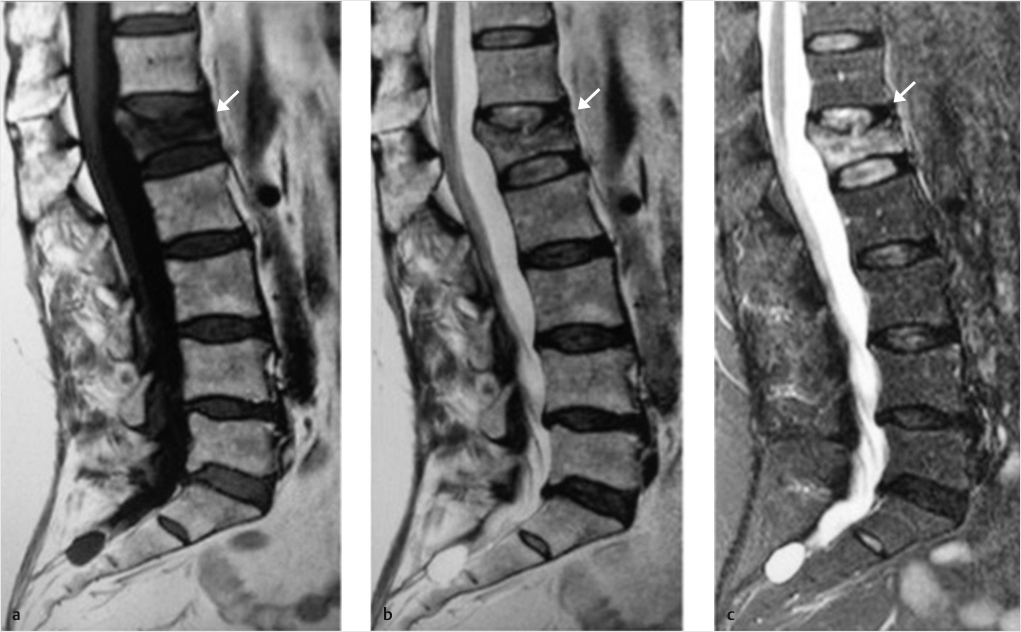
A patient with an acute or subacute vertebral body compression fracture will almost always complain of severe back pain. Physical examination will reveal tenderness to palpation and percussion over the corresponding spinous process. Subsequent radiographs, CT, MR, and/or nuclear bone scan imaging are used to confirm and characterize a fracture. Short tau inversion recovery (STIR) and T1-weighted sequences on MR imaging are considered the gold standard for evaluating VCFs. There is a high degree of correlation between increased STIR signal and a pain-generating fracture and the T1-weighted images well demonstrate fracture lines and marrow signal changes (▶Fig. 11.2). If MR imaging is not possible, nuclear bone scan imaging may be used to demonstrate radionuclide uptake in an acute or subacute fracture and can be used in combination with CT scanning for accurate anatomic characterization of the fracture.
Patients undergoing kyphoplasty are often elderly, are deconditioned, and have inherent vulnerability to perioperative stress. For example, inhalation agents are affected by the minimum alveolar anesthetic concentration, which decreases approximately 6% for every decade. Clearance and the volume of the central compartment decrease with age. Metabolism of medications and their durations of action depend on renal or hepatic excretion. It is important to titrate doses and prudent to use short-acting drugs. Preoperative assessment of organ function and reserve is essential to know how the patient might react with anesthesia.3 Although preoperative laboratory studies and testing will vary from patient to patient, we routinely include CBC with differential, chemistries, coagulation studies, anteroposterior (AP) chest radiograph, and an ECG.
Indications for kyphoplasty include intractable severe pain or moderate to severe persistent pain associated and correlating with a VCF. In 2017, a multidisciplinary expert panel of orthopaedic and neurosurgeons, interventional radiologists, and pain specialists, using the RAND/UCLA Appropriateness Method (RUAM), developed the Clinical Care Pathway (CCP), defining patient-specific recommendations for vertebral fragility fractures (VFF). The panel assessed the relative importance of signs and symptoms for the suspicion of VFF, the relevance of diagnostic procedures, and the appropriateness of vertebral augmentation versus nonsurgical management for a variety of clinical scenarios (▶Fig. 11.3). Their report included the following guidelines for relative and absolute contraindications (▶Table 11.2).
Absolute contraindications include active infection at the surgical site and untreated blood-borne infections. Strong contraindications include osteomyelitis, pregnancy, allergy to fill material, coagulopathy, spinal instability, myelopathy from the fracture, neurologic deficit, and neural impingement. Although dependent on degree, fracture repulsion and canal compromise are not generally a contraindication. Relative contraindications include cardiorespiratory compromise such that safe sedation or anesthesia cannot be achieved and in such cases the procedure may need to be done under local anesthesia. Breach of posterior vertebral cortex by tumor and tumor extension into the spinal canal are also relative contraindications for percutaneous vertebral augmentation techniques due to the potential for leakage of cement and/or displacement of tumor posteriorly.
Kyphoplasty may be performed under general anesthesia or monitored anesthesia care (MAC) with conscious sedation and local anesthetic. In our practice, with rare exceptions, we perform kyphoplasty under general endotracheal anesthesia. In patients who cannot tolerate general anesthesia due to severe cardiopulmonary disease, for example, sedation with MAC is used with caution to avoid oversedation and respiratory compromise.
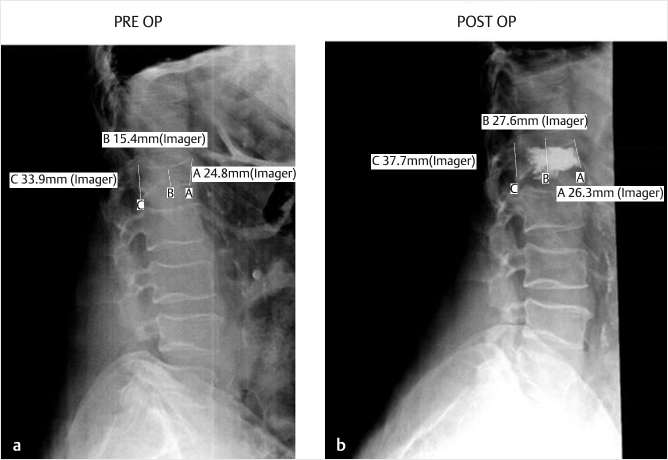
The patient is positioned prone on the operating table with shoulder and hip bolsters to aid in spinal extension and vertebral height restoration. All pressure points are padded and checked. With fluoroscopic guidance for localization, the area of planned surgery is prepped and draped in the usual sterile fashion. Appropriate prophylactic antibiotics are administered. Fluoroscopy is used to identify the pedicles and fractured vertebral body. Fluoroscopic views include true AP and lateral views. Another technique includes the en face view, directed down the longitudinal axis of the pedicle (▶Fig. 11.4).
Table 11.2 Absolute and relative contraindications for kyphoplasty
Condition |
Panel recommendation |
Active infection at surgical site |
Absolute contraindication for curent vertebral augmentation (VA). |
Untreated blood-borne infection |
Absolute contraindication. Preoperative antibiotic (parenteral) therapy is required. Once cultures are negative, following an appropriate period of antibiotic therapy, one can proceed with caution. |
Osteomyelitis |
Usually a strong contraindication for VA. In rare situations, VA may be considered, for example, if the patient is not stable for an open procedure and the infection is chronic and caused by a less virulent organism. The infection may then be controlled locally with antibiotic-loaded cement and long-term antibiotic suppression. |
Pregnancy |
Although VA is usually contraindicated in pregnant patients, there may be exceptional situations in which benefits could prevail over risks. Radiation exposure to the fetus should be minimized. |
Allergy to fill material |
Relative contraindication, depending on the severity of the allergy. If prior reactions were not associated with severe anaphylaxis, the allergy can be pretreated with steroids, Tylenol, and Benadryl. Alternatively, another fill material can be chosen. |
Coagulopathy |
Relative contraindication. Try to normalize/correct clotting function if possible (international normalized ratio [INR] < 1.7). The risk of bleeding should be balanced against the complications from bed rest. Caution in patients with thrombocytopenia (platelets less than 30,000/μL). |
Spinal instability |
Relative contraindication, depending on the degree of instability and level of fracture. If needed, plan an additional intervention to address instability, possibly but not necessarily in the same session. |
Myelopathy from the fracture |
Relative contraindication. Decompression and stabilization is the preferred option, but VA may be considered if the patient is unable to undergo surgery. Coordination with spine surgeon and neurologist is mandatory. |
Neurologic deficit |
Relative contraindication. Additional decompression with or without stabilization may be required. Patients should be informed about the risk of cement in the spinal canal. Coordination with spine surgeon and neurologist is mandatory. |
Neural impingement |
Relative contraindication, depending on the degree. Take extra care to avoid delivery of cement into canal or neural foramen. May need an additional open procedure. |
Fracture retropulsion/canal compromise |
Generally not a contraindication. Avoid hyperextension or aggravating stenosis. A CT scan may be used to determine integrity of posterior wall. |
Local anesthetic is injected and a small stab incision is made with a no. 15 or a no. 11 blade approximately 1 cm superior and 2 cm lateral to the superior pedicle border. For a standard transpedicular approach, the Jamshidi needle is inserted and guided under direct fluoroscopy through the pedicle into the vertebral body. Other approaches and access techniques may also be used (see Chapter 6). The inner cannula is removed and replaced with a K-wire. Using the Seldinger technique, the Jamshidi cannula is removed and the cannulated osteointroducer is passed over the K-wire and advanced through two-thirds of the AP vertebral body diameter. The K-wire and inner cannula are removed when the outer cannula position is confirmed. The drill is passed manually with fluoroscopic guidance to create a void for the bone tamp and to obtain biopsy material for pathology. Core bone biopsies may be obtained through the introducer or a biopsy cannula (▶Fig. 11.1).
Next, a 10- or 15-mm bone tamp connected to a syringe prefilled with iodinated contrast is inserted through the cannula and, under direct fluoroscopic guidance, guided into the tract created by the drill. The radiopaque markers on the balloon tamp are visualized distal to the cannula sheath in both AP and lateral fluoroscopic images. When a bilateral approach is used, this procedure is repeated identically on the contralateral side.
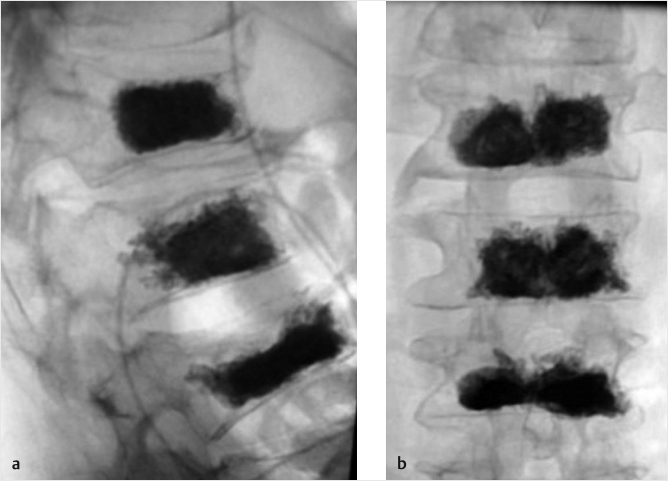
The use of balloon tamps allows for safe and gentle end plate reduction, displacement of trabecular bone, and the creation of a void. The balloon tamps are incrementally inflated while being monitored with AP and lateral imaging. Digital manometers incorporated into the inflation devices demonstrate increases in pressure with each increase in volume. The pressure then gradually diminishes as the trabecular bone is displaced. This process is repeated as safely tolerated. Fracture reduction is guided by the degree of end plate distraction, height restoration, and reduction of kyphotic angulation. Pressure, volume, and fluoroscopic images will all dictate an endpoint. There should be no breach of lateral wall or anterior cortex of the vertebral body. The final balloon volume is recorded and one or both tamps are deflated and removed. The cement-delivery cannula is inserted through the working channel and advanced until the tip of the cannula reaches the anterior extent of the void created by the tamp, preferably just posterior to the anterior cortical wall of the vertebral body. The rod is used to expel cement from the cannula. As cement is delivered, the cannula and rod are retracted gently to allow room for the cement to fill the void. As the cement extends posteriorly, the injection should be slow to watch for extravasation. The cement should extend from superior to inferior end plate and be located between the pedicles. The cement can extend up to the posterior vertebral body wall, but it is important to watch for and recognize extension of cement beyond this margin. This process is repeated on each side until an adequate fill is achieved. As the cement begins to harden, the cannulas are removed. The internal fixation and stabilization of the vertebrae is achieved through the hardening of the cement injected into the vertebral body. Final fluoroscopic images are taken to document the final cement position. Wounds are dressed with Dermabond or Steri-Strips (▶Fig. 11.5).
Several large systematic reviews of randomized control trials have examined the difference between bilateral and unilateral approaches for kyphoplasty. Some differences were observed between the two approaches in terms of height restoration, correction of kyphotic angulation, and patient ratings of pain.4,5
In an analysis of 15 randomized control trials including 850 patients, Yang et al found no difference in quality-of-life or complications from surgery between bilateral and unilateral kyphoplasty.4 Chen et al found that the unilateral approach resulted in a shorter operative time, smaller amount of cement injected, and lower risk of cement leakage.5 There were no statistically significant differences in visual analog scale pain scores, height changes, or kyphotic angle changes between the groups.5 Papanastassiou et al examined the differences between unilateral and bilateral kyphoplasty in multiple myeloma patients and found no difference in clinical or radiological outcomes.6 Huang et al in a review of five studies including 253 patients found no clinically important differences but suggested that unilateral kyphoplasty is advantageous due to decreased operative time and cost.7 Similarly, in a systematic review and meta-analysis including 563 patients, Sun et al noted that unilateral approach led to decreased surgical time, cement consumption, and cement leakage; reduced radiation dose and hospitalization costs; and improved short-term general health.8
There are also substantial data supporting the bilateral approach for optimal outcomes in vertebral augmentation with balloon kyphoplasty. In a retrospective study of 296 patients with osteoporotic VCFs, Bozkurt et al showed that although there was no statistically significant difference between unilateral kyphoplasty and vertebroplasty regarding height restoration of the fractured vertebral body, there was a further advantage of significant height restoration of bilateral kyphoplasty compared to the other two techniques.9 The advantage of height restoration with a bilateral technique is also supported by a meta-analysis of five studies that reported a short-term follow-up that indicated bilateral balloon kyphoplasty had a significantly (p = 0.03) better degree of anterior vertebral height restoration than unilateral balloon kyphoplasty.10
In general, based on the above studies and analyses, a unilateral approach provides an advantage regarding procedure time, procedure and hospital costs, radiation dose, and improved short-term health and cement extravasation. Additionally, there appears to be no significant difference in the unilateral versus bilateral approach with respect to pain reduction, quality-of-life improvement, or procedural complications. Finally, balloon kyphoplasty using a bilateral approach has been shown to provide significantly better short-term height restoration than the same procedure performed via a unilateral approach.
As with any surgical procedure, possible adverse events include infection, hemorrhage, cardiac arrest, and stroke. Other risks specific to kyphoplasty include cement leakage and emboli, spinal cord compression, and nerve injury. Risks related to balloon failure are generally minimal. With failure or rupture of the balloon, pressure will rapidly drop to or near zero and a small amount of contrast medium and saline escape. The balloon may be gently removed after deflation. Fortunately, the complication rate for kyphoplasty is very low and this procedure has been shown to lead to significant and sustained reduction of pain, disability, and opioid analgesic usage, which results in a significantly improved quality of life.11–16
The physical consequences of vertebral deformity, sagittal imbalance, and kyphosis include reduced pulmonary function, early satiety and gastric distress, difficulty with balance, postural compensation with altered gait and reduced velocity, chronic back pain, reduced activity and function, increased fracture risk independent of bone mineral density, and increased mortality.9,10,17–28
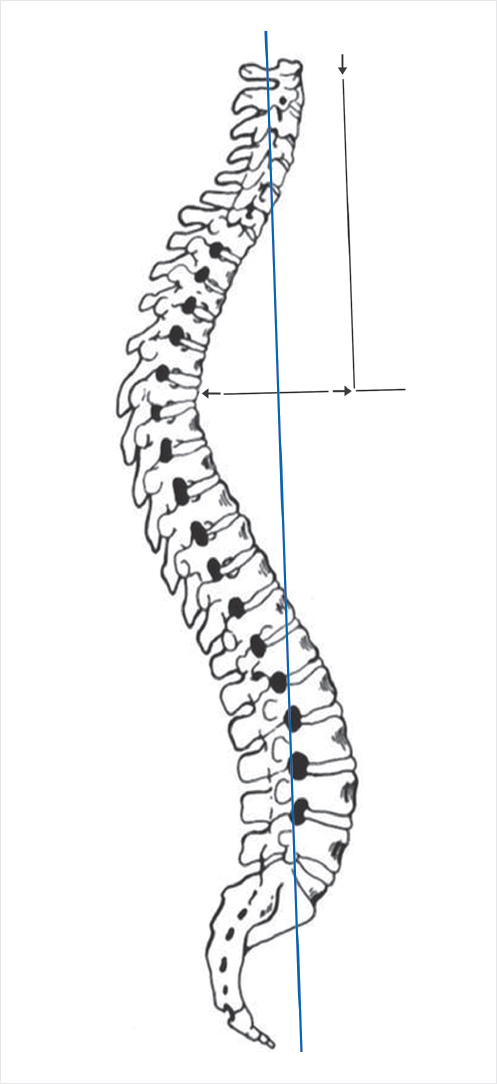
Sagittal balance is recognized as an important factor in determining outcomes following spine surgery and is included in the radiographic assessment of spinal deformity.29–32 Many patients with VCFs present with sagittal imbalance due to the kyphosis caused by the compression fracture itself. Sagittal balance is determined with respect to a plumb line drawn from the middle of the C7 vertebral body. In normal sagittal balance, the line will pass through a point at the posterosuperior aspect of the S1 vertebral body. A patient is in positive balance if the line passes greater than 2 cm anterior to this point and in negative balance if the line passes greater than 2cm posterior to the same point (▶Fig. 11.6).
Few studies have directly examined the relationship between kyphoplasty and sagittal vertical axis (SVA) correction. Pradhan et al retrospectively reviewed 65 patients undergoing level 1 to 3 kyphoplasty procedures to examine the effects of single and multilevel kyphoplasty on local and overall sagittal alignment of the spine. The authors found that the majority of kyphosis correction by kyphoplasty was limited to the vertebral body treated and that correction over longer spans with multilevel kyphoplasties could achieve improved sagittal balance.33 In a study of 21 patients who underwent kyphoplasty, Yokoyama et al found that the preoperative SVA was 7 ± 3.9 cm, demonstrating a significant shift to anterior (positive) sagittal balance as compared to a healthy group that measured 1.45 ± 2.7 cm. After kyphoplasty, the SVA decreased to 5.02 ± 2.91 and this decrease correlated with the kyphotic reduction of the treated vertebrae.34
Reports examining the correlation between restoration of sagittal balance alignment and clinical outcome parameters have been mixed. Several studies have shown that improvements in sagittal balance after kyphoplasty are correlated with surgical outcomes involving pain and quality-of-life scores.35–37 Particularly in patients with adult degenerative spinal deformity, correlations between sagittal balance correction and surgical outcomes have been strong. However, other studies have demonstrated no relationship between sagittal balance alignment restoration and clinical outcome parameters.38–42 The FREE trial, a randomized, nonblinded trail comparing kyphoplasty with nonsurgical management of acute painful vertebral fractures, reported that patients with the greatest quality-of-life improvement, based on the SF-36 PCS (physical component summary) scores, had the better kyphosis correction (5.18 degrees) compared with the subgroup having the least quality-of-life improvement (1.98 degrees of angulation correction). Similarly, patients with the highest kyphotic angulation correction had higher SF-36 PCS quality-of-life improvement.35
Additional fractures have been reported following vertebral augmentation procedures, but the causal relationship between the procedures and subsequent adjacent vertebral fractures is doubtful as patients with osteoporosis who do not undergo surgery also develop additional fractures.43–53 A fracture rate above that of the natural history of osteoporosis has not been demonstrated. Additional fractures occur more quickly in patients who have undergone augmentation as compared to those who have not. This may explain the perception that augmented patients fracture more often (▶Fig. 11.7).20,54–56
Regardless of bone mineral density, age, and other clinical risk factors, vertebral fractures confirmed radiographically—even if asymptomatic—signal-impaired bone quality and strongly predict new vertebral and other nonvertebral fractures. The presence of a single vertebral fracture increases the risk of subsequent vertebral fractures fivefold and the risk of hip and other fractures twofold to threefold.57 Following an initial fracture, osteoporotic patients not treated with systemic antiosteoporosis therapy develop an additional fracture at twice the rate (20%) of those on antiresorptive medication and even fewer vertebral fractures are seen in patients receiving anabolic bone agents with dramatic absolute and relative risk reductions of 3.6 and 86%, respectively.52,55,58,59
Vertebral augmentation may serve to decrease additional or adjacent-level fractures. Numerous studies have demonstrated a lower incidence of adjacent fractures in patients treated with kyphoplasty (4.2%), compared to published rates in untreated patients with osteoporotic fractures (20%).15 In retrospectively analyzing 240 patients with painful VCFs, Baek et al found that risk for adjacent-level fractures after vertebral augmentation decreased significantly when the SVA was less than 6 cm and the segmental kyphotic angle was less than 11 degrees.60 In a matched prospective study, Palombaro reported a 6-month adjacent fracture rate of 37% in patients treated with balloon kyphoplasty and 65% in nonsurgically treated patients.61
Kyphosis resulting from a wedge-shaped fracture shifts the center of gravity of the upper body forward. This increases the flexion bending moment and leads to a compensatory stance with hamstrings foreshortened (knees bent) and paraspinal muscle activity increased in the effort to maintain balance. In a finite element study, Rohlmann et al measured intradiskal pressure (IDP) in the disks adjacent to a fractured vertebra before and after augmentation. The elastic modulus of PMMA varied between 1,000 and 3,000 MPa and the volume between 4 and 10 mL. The effects of volume and elastic modulus of bone cement on IDP were negligible. Augmentation of the fractured vertebral body with bone cement had a much smaller effect on IDP than did the vertebral fracture itself (with or without compensatory upper-body shift). In cadaveric studies completed in 2005, Berlemann et al described a “stress riser” effect weakening a functional spinal unit (two vertebral bodies and the intervening disk), whereby increased stiffness of the treated vertebra altered the load transfer to the noncemented adjacent level.55 Much more pronounced than any stiffness increase resulting from cement injection is the effect on spinal load resulting from the fracture and upper-body shift.62 Restoration of anterior vertebral body height and correction of kyphosis reduces the compressive forces by reducing the bending moment.63
Luo et al analyzed “stress profiles” of 28 cadaveric spine specimen comprising three thoracolumbar vertebrae and intervening disks and ligaments before and after compression injury to one of the three vertebrae, and again after vertebroplasty. Induction of the injury reduced IDP to an average of 47% of prefracture value at the affected level and 73% of baseline values at adjacent levels. Injury also transferred compressive load bearing from the nucleus to the annulus and from the disk to the neural arch. Vertebroplasty partially reversed these changes, increasing mean IDP to 76 and 81% of baseline values at fractured and adjacent levels, respectively. Following injury, a 14-fold increase in creep deformation of the vertebral body under load was noted. Vertebroplasty also reversed these changes, reducing deformation of the anterior vertebral body by 62% at the fractured level and 52% at the adjacent level, compared to postfracture values.64
Balloon kyphoplasty has been shown to significantly (p < 0.001) restore more than 80% of the original vertebral height following a wedge fracture and to correct vertebral wedge fracture deformity in up to 92% of patients65,66 with changes remaining stable for at least two years following surgery.65 Local kyphosis reduction continues to be one of the advantages of balloon kyphoplasty over vertebroplasty. Reduction of kyphosis with kyphoplasty has been shown to be greater than that for vertebroplasty (3.7–8 degrees vs. 0.5–3 degrees) and this kyphotic correction allows for a more effortless upright posture leading to relaxation of the paraspinal muscles, reduced pain, and fewer additional VCFs.12
Although there is limited evidence that postural reduction (preoperative positioning) is the most important factor for kyphosis correction,67 there is strong evidence that the balloon tamps used in kyphoplasty enhance reduction greater than 4.5-fold over positioning maneuvers alone and account for over 80% of the reduction.35–37,40–42,68–79
More information on additional or adjacent VCFs after vertebral augmentation can be found in Chapter 17.
Although it is customary to perform kyphoplasty as an outpatient procedure, many affected patients are elderly and have multiple medical comorbidities; thus, an overnight admission may be indicated. Elderly patients have an inherent progressive loss of functional reserve in all organ systems. Common causes of postoperative morbidity include atelectasis, bronchitis, pneumonia, delirium, heart failure, and myocardial infarction.3
Increased vulnerability to perioperative stress favors minimally invasive surgery with shorter operative times and a shorter hospital stay. Meticulous intraoperative management of coexisting disorders and postoperative pain control will help mitigate patient stress. When treating elderly patients, the clinician should always keep in mind that changes in pharmacokinetics and pharmacodynamics render some medications more potent in geriatric patients. Morphine clearance, for example, is decreased in the elderly, leading to a decreased narcotic requirement for pain relief. There is an increase in brain sensitivity to opioids with age.3
Patients are encouraged at discharge to resume all of their typical daily activities as soon as feasible with few restrictions. Patients are examined at 2 weeks postoperatively to evaluate their response to the procedure, their progress in healing, and to determine the need for additional care including physical therapy. Back-strengthening programs are often useful in these patients after kyphoplasty. All patients presenting with VFF should be referred for bone mineral density monitoring and osteoporosis education with treatment as indicated. The treatment of their underlying disorder of osteoporosis will be discussed in greater detail in Chapter 34. If their pain is not resolved or significantly diminished following kyphoplasty, re-evaluation with imaging is indicated. There are countless nonsurgical causes of back pain as well as frequent additional vertebral fractures after the initial vertebral augmentation procedure.
Kyphoplasty is a minimally invasive procedure designed to relieve pain and improve function in patients with pain and disability associated with VCFs. Results of numerous studies have demonstrated significant and durable pain relief, reduced disability, improved function, and enhanced quality of life after kyphoplasty in patients with VCFs resulting from a wide range of etiologies. In addition, restoration of vertebral height, correction of kyphotic angulation, and improved sagittal balance may decrease the risk of future vertebral fractures.
Performed under general anesthesia or MAC with sedation and local anesthetic, kyphoplasty has an extremely low complication rate in experienced hands and has been shown to significantly improve the rates of patient morbidity and mortality. Kyphoplasty remains a first-line treatment option for patients with painful VCFs unresponsive to medical therapy.
[1] He Z, Zhai Q, Hu M, et al. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J Orthop Translat 2014;3(1):1–11
[2] Charnley J. Anchorage of the femoral head prosthesis to the shaft of the femur. J Bone Joint Surg Br 1960;42-B:28–30
[3] Kanonidou Z, Karystianou G. Anesthesia for the elderly. Hippokratia 2007;11(4):175–177
[4] Yang S, Chen C, Wang H, Wu Z, Liu L. A systematic review of unilateral versus bilateral percutaneous vertebroplasty/percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Acta Orthop Traumatol Turc 2017;51(4):290–297
[5] Chen H, Tang P, Zhao Y, Gao Y, Wang Y. Unilateral versus bilateral balloon kyphoplasty in the treatment of osteoporotic vertebral compression fractures. Orthopedics 2014;37(9):e828–e835
[6] Papanastassiou ID, Eleraky M, Murtagh R, Kokkalis ZT, Gerochristou M, Vrionis FD. Comparison of unilateral versus bilateral kyphoplasty in multiple myeloma patients and the importance of preoperative planning. Asian Spine J 2014;8(3):244–252
[7] Huang Z, Wan S, Ning L, Han S. Is unilateral kyphoplasty as effective and safe as bilateral kyphoplasties for osteoporotic vertebral compression fractures? A meta-analysis. Clin Orthop Relat Res 2014;472(9):2833–2842
[8] Sun H, Lu PP, Liu YJ, et al. Can Unilateral Kyphoplasty Replace Bilateral Kyphoplasty in Treatment of Osteoporotic Vertebral Compression Fractures? A Systematic Review and Meta-analysis. Pain Physician 2016;19(8):551–563
[9] Bozkurt M, Kahilogullari G, Ozdemir M, et al. Comparative analysis of vertebroplasty and kyphoplasty for osteoporotic vertebral compression fractures. Asian Spine J 2014;8(1):27–34
[10] Feng H, Huang P, Zhang X, Zheng G, Wang Y. Unilateral versus bilateral percutaneous kyphoplasty for osteoporotic vertebral compression fractures: A systematic review and meta-analysis of RCTs. J Orthop Res 2015;33(11):1713–1723
[11] Eidt-Koch D, Greiner W. Quality of life results of balloon kyphoplasty versus non surgical management for osteoporotic vertebral fractures in Germany. Health Econ Rev 2011;1(1):7
[12] Papanastassiou ID, Phillips FM, Van Meirhaeghe J, et al. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur Spine J 2012;21(9):1826–1843
[13] Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009;373(9668):1016–1024
[14] Van Meirhaeghe J, Bastian L, Boonen S, Ranstam J, Tillman JB, Wardlaw D; FREE investigators. A randomized trial of balloon kyphoplasty and nonsurgical management for treating acute vertebral compression fractures: vertebral body kyphosis correction and surgical parameters. Spine 2013;38(12):971–983
[15] Kicielinski KP, Pritchard Patrick R, Ruiz H, et al. Patient Experience Following Kyphoplasty: Safety, Efficacy and Patient Satisfaction. J Adv Med Med Res 2015;10(1):1–11
[16] Feltes C, Fountas KN, Machinis T, et al. Immediate and early postoperative pain relief after kyphoplasty without significant restoration of vertebral body height in acute osteoporotic vertebral fractures. Neurosurg Focus 2005;18(3):e5
[17] Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 2004;15(2):108–112
[18] Johnell O, Kanis JA, Odén A, et al. Mortality after osteoporotic fractures. Osteoporos Int 2004;15(1):38–42
[19] Hillier TA, Lui LY, Kado DM, et al. Height loss in older women: risk of hip fracture and mortality independent of vertebral fractures. J Bone Miner Res 2012;27(1):153–159
[20] Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285(3):320–323
[21] Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med 1998;128(10):793–800
[22] van Schoor NM, Smit JH, Twisk JW, Lips P. Impact of vertebral deformities, osteoarthritis, and other chronic diseases on quality of life: a population-based study. Osteoporos Int 2005;16(7):749–756
[23] Hallberg I, Rosenqvist AM, Kartous L, Löfman O, Wahlström O, Toss G. Health-related quality of life after osteoporotic fractures. Osteoporos Int 2004;15(10):834–841
[24] Silverman SL. The clinical consequences of vertebral compression fracture. Bone 1992;13(Suppl 2):S27–S31
[25] Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Balance disorder and increased risk of falls in osteoporosis and kyphosis: significance of kyphotic posture and muscle strength. Osteoporos Int 2005;16(8): 1004–1010
[26] Schlaich C, Minne HW, Bruckner T, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int 1998;8(3): 261–267
[27] Culham EG, Jimenez HA, King CE. Thoracic kyphosis, rib mobility, and lung volumes in normal women and women with osteoporosis. Spine 1994;19(11):1250–1255
[28] Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis 1990;141(1):68–71
[29] Booth KC, Bridwell KH, Lenke LG, Baldus CR, Blanke KM. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance). Spine 1999;24(16):1712–1720
[30] Berven SH, Deviren V, Smith JA, Hu SH, Bradford DS. Management of fixed sagittal plane deformity: outcome of combined anterior and posterior surgery. Spine 2003;28(15):1710–1715, discussion 1716
[31] Park SJ, Lee CS, Chung SS, Kang KC, Shin SK. Postoperative changes in pelvic parameters and sagittal balance in adult isthmic spondylolisthesis. Neurosurgery 2011;68(2, Suppl Operative):355–363, discussion 362–363
[32] Endo K, Suzuki H, Tanaka H, Kang Y, Yamamoto K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J 2010;19(3):435–438
[33] Pradhan BB, Bae HW, Kropf MA, Patel VV, Delamarter RB. Kyphoplasty reduction of osteoporotic vertebral compression fractures: correction of local kyphosis versus overall sagittal alignment. Spine 2006;31(4):435–441
[34] Yokoyama K, Kawanishi M, Yamada M, et al. Postoperative change in sagittal balance after Kyphoplasty for the treatment of osteoporotic vertebral compression fracture. Eur Spine J 2015;24(4):744–749
[35] Ranstam J, Turkiewicz A, Boonen S, Van Meirhaeghe J, Bastian L, Wardlaw D. Alternative analyses for handling incomplete follow-up in the intention-to-treat analysis: the randomized controlled trial of balloon kyphoplasty versus non-surgical care for vertebral compression fracture (FREE). BMC Med Res Methodol 2012;12:35
[36] Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech 2005;18(3):238–242
[37] Dong R, Chen L, Gu Y, et al. Improvement in respiratory function after vertebroplasty and kyphoplasty. Int Orthop 2009;33(6):1689–1694
[38] Dong R, Chen L, Tang T, et al. Pain reduction following vertebroplasty and kyphoplasty. Int Orthop 2013;37(1):83–87
[39] Berlemann U, Franz T, Orler R, Heini PF. Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective non-randomized study. Eur Spine J 2004;13(6):496–501
[40] Rollinghoff M, Siewe J, Zarghooni K, et al. Effectiveness, security and height restoration on fresh compression fractures: a comparative prospective study of vertebroplasty and kyphoplasty Minim Invasive Neurosurg 2009;52(5–6): 233–237
[41] Lovi A, Teli M, Ortolina A, Costa F, Fornari M, Brayda-Bruno M. Vertebroplasty and kyphoplasty: complementary techniques for the treatment of painful osteoporotic vertebral compression fractures. A prospective non-randomised study on 154 patients. Eur Spine J 2009;18(Suppl 1):95–101
[42] Kasperk C, Hillmeier J, Nöldge G, et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res 2005;20(4):604–612
[43] Ettinger B, Black DM, Mitlak BH, et al; Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 1999;282(7):637–645
[44] Zoarski GH, Snow P, Olan WJ, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol 2002;13(2, Pt 1):139–148
[45] Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology 2003;226(1):119–124
[46] Syed MI, Patel NA, Jan S, Harron MS, Morar K, Shaikh A. New symptomatic vertebral compression fractures within a year following vertebroplasty in osteoporotic women. AJNR Am J Neuroradiol 2005;26(6):1601–1604
[47] Legroux-Gérot I, Lormeau C, Boutry N, Cotten A, Duquesnoy B, Cortet B. Long-term follow-up of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Clin Rheumatol 2004;23(4):310–317
[48] Kobayashi K, Shimoyama K, Nakamura K, Murata K. Percutaneous vertebroplasty immediately relieves pain of osteoporotic vertebral compression fractures and prevents prolonged immobilization of patients. Eur Radiol 2005;15(2):360–367
[49] Jensen ME, Dion JE. Percutaneous vertebroplasty in the treatment of osteoporotic compression fractures. Neuroimaging Clin N Am 2000;10(3):547–568
[50] Heini PF, Wälchli B, Berlemann U. Percutaneous transpedicular vertebroplasty with PMMA: operative technique and early results. A prospective study for the treatment of osteoporotic compression fractures. Eur Spine J 2000;9(5):445–450
[51] Diamond TH, Bryant C, Browne L, Clark WA. Clinical outcomes after acute osteoporotic vertebral fractures: a 2-year non-randomised trial comparing percutaneous vertebroplasty with conservative therapy. Med J Aust 2006;184(3):113–117
[52] Frankel BM, Monroe T, Wang C. Percutaneous vertebral augmentation: an elevation in adjacent-level fracture risk in kyphoplasty as compared with vertebroplasty. Spine J 2007;7(5):575–582
[53] Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000;39(12):1410–1414
[54] Lindsay R, Burge RT, Strauss DM. One year outcomes and costs following a vertebral fracture. Osteoporos Int 2005;16(1):78–85
[55] Berlemann U, Ferguson SJ, Nolte LP, Heini PF. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br 2002;84(5):748–752
[56] Yi X, Lu H, Tian F, et al. Recompression in new levels after percutaneous vertebroplasty and kyphoplasty compared with conservative treatment. Arch Orthop Trauma Surg 2014;134(1):21–30
[57] Gold DT. The nonskeletal consequences of osteoporotic fractures. Psychologic and social outcomes. Rheum Dis Clin North Am 2001;27(1):255–262
[58] Hoenig HM, Rubenstein LZ. Hospital-associated deconditioning and dysfunction. J Am Geriatr Soc 1991;39(2):220–222
[59] Miller PD, Hattersley G, Riis BJ, et al; ACTIVE Study Investigators. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA 2016;316(7):722–733
[60] Baek SW, Kim C, Chang H. The relationship between the spinopelvic balance and the incidence of adjacent vertebral fractures following percutaneous vertebroplasty. Osteoporos Int 2015;26(5):1507–1513
[61] Palombaro KM. Effects of walking-only interventions on bone mineral density at various skeletal sites: a meta-analysis. J Geriatr Phys Ther 2005;28(3):102–107
[62] Rohlmann A, Zander T Jony, Weber U, Bergmann G. [Effect of vertebral body stiffness before and after vertebroplasty on intradiscal pressure] Biomed Tech (Berl) 2005;50(5):148–152
[63] Disch AC, Schmoelz W. Cement augmentation in a thoracolumbar fracture model: reduction and stability after balloon kyphoplasty versus vertebral body stenting. Spine 2014;39(19):E1147–E1153
[64] Luo J, Annesley-Williams DJ, Adams MA, Dolan P. How are adjacent spinal levels affected by vertebral fracture and by vertebroplasty? A biomechanical study on cadaveric spines. Spine J 2017;17(6):863–874
[65] Ledlie JT, Renfro MB. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine 2006;31(1):57–64
[66] Gaitanis IN, Hadjipavlou AG, Katonis PG, Tzermiadianos MN, Pasku DS, Patwardhan AG. Balloon kyphoplasty for the treatment of pathological vertebral compressive fractures. Eur Spine J 2005;14(3):250–260
[67] Pflugmacher R, Bornemann R, Koch EM, et al. [Comparison of clinical and radiological data in the treatment of patients with osteoporotic vertebral compression fractures using radiofrequency kyphoplasty or balloon kyphoplasty] Z Orthop Unfall 2012;150(1):56–61
[68] Movrin I. Adjacent level fracture after osteoporotic vertebral compression fracture: a nonrandomized prospective study comparing balloon kyphoplasty with conservative therapy. Wien Klin Wochenschr 2012;124(9–10):304–311
[69] Kasperk C, Grafe IA, Schmitt S, et al. Three-year outcomes after kyphoplasty in patients with osteoporosis with painful vertebral fractures. J Vasc Interv Radiol 2010;21(5):701–709
[70] Grafe IA, Da Fonseca K, Hillmeier J, et al. Reduction of pain and fracture incidence after kyphoplasty: 1-year outcomes of a prospective controlled trial of patients with primary osteoporosis. Osteoporos Int 2005;16(12):2005–2012
[71] Grafe IA, Baier M, Nöldge G, et al. Calcium-phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine 2008;33(11):1284–1290
[72] Xing D, Ma JX, Ma XL, et al. A meta-analysis of balloon kyphoplasty compared to percutaneous vertebroplasty for treating osteoporotic vertebral compression fractures. J Clin Neurosci 2013;20(6):795–803
[73] Liu JT, Liao WJ, Tan WC, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int 2010;21(2): 359–364
[74] Kumar K, Nguyen R, Bishop S. A comparative analysis of the results of vertebroplasty and kyphoplasty in osteoporotic vertebral compression fractures. Neurosurgery 2010;67(3, Suppl Operative):ons171–ons188, discussion ons188
[75] Li X, Yang H, Tang T, Qian Z, Chen L, Zhang Z. Comparison of kyphoplasty and vertebroplasty for treatment of painful osteoporotic vertebral compression fractures: twelve-month follow-up in a prospective nonrandomized comparative study. J Spinal Disord Tech 2012;25(3):142–149
[76] Schofer MD, Efe T, Timmesfeld N, Kortmann HR, Quante M. Comparison of kyphoplasty and vertebroplasty in the treatment of fresh vertebral compression fractures. Arch Orthop Trauma Surg 2009;129(10):1391–1399
[77] Pflugmacher R, Kandziora F, Schröder R, et al. [Vertebroplasty and kyphoplasty in osteoporotic fractures of vertebral bodies -- a prospective 1-year follow-up analysis] RoFo Fortschr Geb Rontgenstr Nuklearmed 2005;177(12):1670–1676
[78] Movrin I, Vengust R, Komadina R. Adjacent vertebral fractures after percutaneous vertebral augmentation of osteoporotic vertebral compression fracture: a comparison of balloon kyphoplasty and vertebroplasty. Arch Orthop Trauma Surg 2010;130(9):1157–1166
[79] Shindle MK, Gardner MJ, Koob J, Bukata S, Cabin JA, Lane JM. Vertebral height restoration in osteoporotic compression fractures: kyphoplasty balloon tamp is superior to postural correction alone. Osteoporos Int 2006;17(12):1815–1819