21 Clinical Presentation and the Response to Vertebral Augmentation
Alexios Kelekis and Dimitrios K. Filippiadis
Summary
The presence of a vertebral compression fracture (VCF) can be defined anatomically as an objective loss in vertebral body height or by imaging criteria including increased signal on the fluid sensitive magnetic resonance (MR) imaging sequences. There are a number of clinical scenarios that may produce symptomatic VCFs including neoplasia, trauma, and osteoporosis. Vertebral fractures are common and have adverse effects on mobility and pulmonary function and can lead to prominently increased rates of morbidity and mortality. The kyphosis produced by the fracture also places increased strain on the adjacent vertebral bodies and increases the risk of an adjacent or additional vertebral fracture. Patients with VCFs complain of pain when transitioning from one position to another and the physical examination techniques used to identify patients with VCFs include closed fist percussion and the inability for the patient to assume a supine position comfortably. Patients with vertebral compression fractures can be asymptomatic but can also present with severe pain and prominently limited function. Patients with persistently painful fractures also tend to not improve with nonsurgical management (NSM) and should undergo vertebral augmentation to treat their symptomatic fractures. Treatment of these fractures can produce both short term and long term benefits regardless of the underlying cause of the fracture and vertebral augmentation has been shown in multiple studies to significantly decrease patients’ morbidity and mortality. The appropriate diagnosis and treatment of VCFs can produce better results and optimal outcomes for a large at risk patient population.
Keywords: clinical presentation, vertebral compression fracture, physical examination, morbidity, mortality
21.1 Clinical Presentation
VCF can be defined as the reduction in the height of the individual vertebral body by 20% over 10 to 20% of the vertebral body or a loss of vertebral height of at least 4 mm.1 There are numerous pathophysiologic processes that contribute to VCFs including osteoporosis, neoplasms (e.g., myeloma, metastasis, lymphoma, and hemangioma), osteonecrosis, and trauma.1 The burden of illness for VCFs results in a total annual hospitalization cost that is higher than the costs for myocardial infarction, cardiovascular arrest, and breast cancer.2 VCFs result in both direct and indirect effects on the patients’ quality of life and costs to the health care systems.1 The typical clinical appearance in a patient with an acute VCF is severe back pain lasting for weeks to months or more. Additionally, apart from intractable pain, vertebral fractures negatively affect health status in a variety of ways including but not limited to progressive deformity, impaired mobility, reduced pulmonary function, sleep difficulty, eating disorders, weight loss, clinical depression, anxiety, and an overall decrease in the patient’s quality of life. Additional symptoms may include sciatica or radiculopathy, numbness, tingling, muscle spasm, weakness, and bowel or bladder changes. Neglected fractures can evolve to vertebra plana, retropulsion, and may even cause paralysis due to compression of the spinal cord or cauda equina.3
When compared to age-matched controls, patients with VCFs have a 40% lower survival rate, which is typically attributed to all the aforementioned clinical symptoms and biomechanical changes.4 In these patients the resultant decreased mobility and bed rest are important predictors of adverse outcomes leading to complications such as functional decline and potentially deadly adverse events such as pneumonia or pulmonary embolism.5
Patients with VCFs suffering from severe pain will usually have mobility impairment which leads to increased morbidity and mortality rates.4,5 Pain in these patients results both from the fracture itself as well as the vertebral body’s instability with micro- or macro-movements (▶Video 21.1). The pain, in combination with the altered spinal biomechanics, the increased kyphotic angle, and the global spinal sagittal imbalance, creates a compensatory stance which in turn causes paraspinal muscular contraction that often results in chronic back pain. This imbalance plays an important role in increasing body sway, gait unsteadiness, and risk of falls in fragile patients who have already had an osteoporotic fracture.6 Prior to vertebral augmentation and restabilization of the fracture (▶Video 21.2), the incident fracture disrupts the ability of the adjacent intervertebral disk to pressurize, and the resulting force transmitted to the adjacent vertebral body cortex is doubled, which produces an increased risk of future VCFs.5 The change in the kyphotic angle also causes significant mechanical effects that result in decreased thoracic and abdominal space with subsequently decreased pulmonary function, decreased appetite, and a negative nutritional impact on an already frail patient.7,8
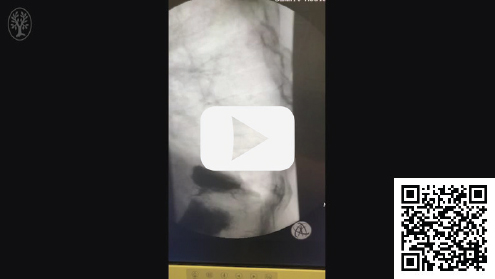
Video 21.1 Lateral thoracic fluoroscopic video of the thoracic spine showing a needle entering the posterior vertebral body of T9 and previous vertebral augmentation with PMMA at the T10 and T11 levels. The VCF at T9 is mobile and the superior end plate can be seen to move superoinferiorly with respiration.
https://www.thieme.de/de/q.htm?p=opn/cs/19/11/10618073-85b5d5e3
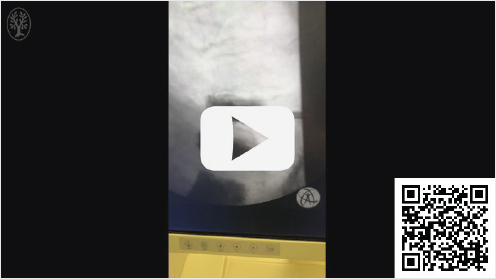
Video 21.2 Lateral thoracic fluoroscopic video of the thoracic spine taken after vertebral augmentation with PMMA at the T9 level shows two cannulas and bone fillers within the T9 vertebral body that is now stable. No movement of the superior end plate is seen with respiration. https://www.thieme.de/de/q.htm?p=opn/cs/19/11/10618072-eaf53de7
During clinical examination, the entire spinal length should be examined for physical signs and symptoms that are reliable for diagnosing the presence of a vertebral fracture. On using a firm, closed-fist percussion, the patient will complain of a sharp, sudden, fracture pain.9 This is typically an effective physical exam maneuver in patients with VCFs as it has been shown to have a sensitivity and specificity for detecting symptomatic VCFs of 87.5 and 90%, respectively. Patients with vertebral fractures also complain of pain during rotation and positional changes (turning while lying or standing) and Postacchini et al found that pain-related behaviors such as grimacing, sighing, and requesting help with position changes were correlated with the presence of a VCF as confirmed by MR imaging and were not present in the control patients without fractures.19 A useful physical examination sign is the presence of back pain when lying supine.9 The patient is asked to lie supine on the examination couch with only one pillow. The clinical sign is positive when a patient is unable to lie supine due to severe pain in their spine.9 Despite the presence of some reliable signs of a painful VCF, clinical examination cannot stand alone for the diagnosis of a vertebral fracture as preoperative imaging will provide valuable information to confirm the diagnosis as well as to assess the fracture anatomy including the posterior vertebral body wall integrity and can exclude other causes of back pain that can mimic a VCF.1 Radiographs of the spine in anteroposterior and lateral projections still remain the simplest and most direct approach and can provide basic information. The absence of a fracture on a radiograph, however, does not exclude the presence of a fracture, especially in the osteoporotic patient population. MR imaging with Short TI Inversion Recovery (STIR) and T1-weighted sequences are the most sensitive for fracture detection and should always be used in case of clinical suspicion. It can be useful to assess the fracture’s age and healing status (acute vs. chronic, incompletely healed vs. consolidated).1 Tanigawa et al reported that the improvement postvertebral augmentation is closely related to the bone edema pattern illustrated in the pretherapeutic MR imaging.10 The authors showed that patients with an extensive bone marrow edema pattern involving more than 50% of the vertebral body reported significantly greater clinical improvement than either patients with fractures that had no bone marrow edema.10
Apart from the clinical signs and symptoms directly caused by a VCF, there is a long list of indirect effects. Immobility of patients with VCFs results in loss of bone density and muscle strength as well as muscular contractures and pressure sores.11 In the acute phase the bone mineral density loss can be as high as 2% per week, which not only predisposes the patient to additional VCFs but the loss can only be reversed by either high impact exercise or anabolic bone agents.11 The muscle strength loss is also rapid, declining approximately 10 to 15% per week and roughly half of the patient’s strength is lost within four to five weeks after the fracture.11 VCFs are also associated with decreased cardiac performance (including increased heart rate, shorter diastolic times, reduced coronary blood flow, decreased stroke volume, and left ventricular function with lower cardiac output), deep venous thrombosis, and pulmonary compromise (with average of 9% decrease in forced vital capacity, 25–50% decrease in respiratory capacity, deconditioning of respiratory muscles, and increased risk of pneumonia).11 Indirect effects of VCFs are also reported in the gastrointestinal system (loss of appetite, constipation, fecal impaction, and glucose intolerance), in the urinary tract (infection, sepsis, and calculus formation), and in the central nervous system (imbalance, increase sensitivity, and intolerance to pain, anxiety, depression, and insomnia).11,12
21.2 Response to Vertebral Augmentation
One of the confusing aspects of patients with VCFs is the dichotomy of how these patients present. On one hand VCFs are common in the general population with a population-based study by Sanfélix-Genovés et al confirming that one in three women over the age of 50 has osteoporosis and one in five will have a VCF.20 Additionally, one in ten fractures will be classified as moderate to severe. Out of all the patients that had the fractures, only 1.5% were aware of their fractures. This situation exemplifies the dichotomous situation that asymptomatic VCFs are quite common and don’t need treatment but symptomatic fractures are less common and when the symptoms from the fractures are severe the patients need treatment.
In a study by Suzuki et al the authors treated patients with NSM in patients who had a 7 out of 10 level of pain for one year.21 After a full year of NSM, only 10% of the patients reported little or no pain whereas 76% of the patients still had pain that they regarded as severe and averaged a 6 out of 10 level of pain. In another study by Bornemann et al, the authors treated patient with painful VCFs with NSM for 6 weeks then offered the patients an additional 6 weeks of NSM or vertebral augmentation.22 They defined clinical success as a decreased amount of pain on the Visual Analog Scale (VAS) and no functional worsening on the Oswestry Disability Index (ODI) scale. After the initial 6 weeks of NSM only one patient met the criteria for clinical success and only five patients met the clinical criteria for success after an additional 6 weeks of NSM. The authors concluded that for the majority of patients with a pain level of a 5 out of 10 or more, NSM provides no clinical improvement but nearly all patients undergoing vertebral augmentation had rapid and substantial improvement. The authors went on to say that surgery was clearly much more effective than NSM and should be offered to patients much sooner.22
The dichotomy for VCFs is that while most fractures are asymptomatic and do not need treatment there are a subset that are painful and cause prominent disability that tend not to improve with NSM and should be treated promptly. The clinical care pathway for treating VCFs, discussed in detail in Chapter 14, reflects the current recommendation which focuses more on the patients’ clinical symptoms to determine the appropriate treatment rather than on an arbitrary requirement for NSM.
After vertebral augmentation there is significant and immediate pain reduction and functional improvement. In patients with acute or subacute osteoporotic fractures, the pain reduction amount after vertebral augmentation is 90% as opposed to approximately 80% for chronic osteoporotic fractures and hemangiomas and 60 to 85% in fractures due to neoplastic involvement.1 Tsoumakidou et al has reported the reduction in the requirement for analgesics to be 91% after vertebral augmentation and improved mobility is 84 to 93% and 50 to 88% after treatment for acute and chronic osteoporotic fractures, respectively.1 After vertebral augmentation there are short- and long-term benefits. The improvement in pain is the greatest potential short-term benefit, whereas prevention of recurrent pain due to fracture at the treated levels, limitation or reversal of height loss, and spinal deformity as well as improved functional capability are included in the potential long-term benefits.12
It has been reported by Mailli et al that vertebral augmentation is efficient and safe for symptomatic vertebral fractures independently of the number of vertebrae treated per session. Additionally the underlying cause of the vertebral fracture (benign or malignant) does not affect the technique’s safety and efficacy when treating a single VCF or multiple levels during a single session.13 Kelekis et al in a comparative prospective study among patients with VCFs treated with percutaneous vertebroplasty and a control group of healthy volunteers reported that patients prior to their vertebroplasty procedure had a statistically significant difference in load distribution variation as compared to the control group.14 After treatment this difference normalized in a statistically significant way showing the efficacy of vertebral augmentation in restoring equilibrium in load distribution.14 There is also a very significant reduction in mortality and morbidity after vertebral augmentation (see Chapter 22), with rates of mortality reduction after vertebral augmentation ranging from 11 to 55% and statistically significant reductions in morbidities.15,23
During follow-up, patients should be examined for pain that is both related and unrelated to the previous vertebral augmentation procedure. Immediate pain after treatment usually relates to soft tissue discomfort or a hematoma at the puncture site(s). Bracing after treatment is not mandatory although it can be helpful to provide additional support and increase patient comfort and should be considered in especially frail patients. The usual follow-up includes a patient visit and clinical examination within 7 to 14 days. This visit can include radiographs or cross-sectional imaging to assess the prior vertebral augmentation and/or look for additional fractures if the patient’s symptoms have returned. In case of pain or aches that seem unrelated to the previous vertebral augmentation procedure or an additional VCF, clinical examination and laboratory exams may be necessary to verify the origin of the pain. Most of the time the pain is of a benign origin and due to the changes in sagittal balance and altered loading of the intervertebral disks and facets and changes in muscle function.16 In those cases targeted treatment by image-guided injections should be explored.17 If there is recalcitrant pain and/or neurological symptoms during follow-up the patient should be examined thoroughly, including new MR imaging, as there is always the risk of infection, tumor growth, or additional fracture, either at the treated level or at another level.18
In conclusion, when patients are treated after a spinal fracture, whether it is due to benign or malignant disease they should be examined and the vertebral augmentation treatment planned for optimal patient outcomes. Vertebral augmentation is just one treatment among others for back pain and in many occasions the VCF will be just one of many pain generators especially in the patient population where VCFs commonly occur. Finding and attributing the pain to the appropriate pathology and treating the culprit that is producing the patient’s pain and discomfort are our responsibilities as physicians.
References
[1] Tsoumakidou G, Too CW, Koch G, et al. CIRSE guidelines on percutaneous vertebral augmentation. Cardiovasc Intervent Radiol 2017;40(3):331–342
[2] Singer A, Exuzides A, Spangler L, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc 2015;90(1):53–62
[3] Clark W, Bird P, Gonski P, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2016;388(10052):1408–1416
[4] Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am 2008;90(7):1479–1486
[5] Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc 2004;52(8):1263–1270
[6] Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Balance disorder and increased risk of falls in osteoporosis and kyphosis: significance of kyphotic posture and muscle strength. Osteoporos Int 2005;16(8):1004–1010
[7] Yuan HA, Brown CW, Phillips FM. Osteoporotic spinal deformity: a biomechanical rationale for the clinical consequences and treatment of vertebral body compression fractures. J Spinal Disord Tech 2004;17(3):236–242
[8] Kado DM, Lui LY, Ensrud KE, Fink HA, Karlamangla AS, Cummings SR; Study of Osteoporotic Fractures. Hyperkyphosis predicts mortality independent of vertebral osteoporosis in older women. Ann Intern Med 2009;150(10): 681–687
[9] Langdon J, Way A, Heaton S, Bernard J, Molloy S. Vertebral compression fractures: new clinical signs to aid diagnosis. Ann R Coll Surg Engl 2010;92(2): 163–166
[10] Tanigawa N, Komemushi A, Kariya S, et al. Percutaneous vertebroplasty: relationship between vertebral body bone marrow edema pattern on MR images and initial clinical response. Radiology 2006;239(1):195–200
[11] Babayev M, Lachmann E, Nagler W. The controversy surrounding sacral insufficiency fractures: to ambulate or not to ambulate? Am J Phys Med Rehabil 2000;79(4):404–409
[12] Beall DP, McRoberts WP, Berven SH, Ledlie JT, Tutton SM, Parsons BP. Critique of the analysis of UpToDate.com on the treatment of painful vertebral compression fractures: time to update UpToDate. AJNR Am J Neuroradiol 2015;36(4):631–636
[13] Mailli L, Filippiadis DK, Brountzos EN, Alexopoulou E, Kelekis N, Kelekis A. Clinical outcome and safety of multilevel vertebroplasty: clinical experience and results. Cardiovasc Intervent Radiol 2013;36(1):183–191
[14] Kelekis A, Filippiadis DK, Vergadis C, et al. Comparative prospective study of load distribution projection among patients with vertebral fractures treated with percutaneous vertebroplasty and a control group of healthy volunteers. Cardiovasc Intervent Radiol 2014;37(1):186–192
[15] Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab 2010;95(3): 1174–1181
[16] Capozzi A, Scambia G, Pedicelli A, Evangelista M, Sorge R, Lello S. Clinical management of osteoporotic vertebral fracture treated with percutaneous vertebroplasty. Clin Cases Miner Bone Metab 2017;14(2):161–166
[17] Hofmann UK, Keller RL, Walter C, Mittag F. Predictability of the effects of facet joint infiltration in the degenerate lumbar spine when assessing MRI scans. J Orthop Surg Res 2017;12(1):180
[18] Beall DP, Coe JD, McIIduff M, et al. Serious adverse events associated with readmission through one year after vertebral augmentation with either a polyetheretherketone implant or balloon kyphoplasty. Pain Physician 2017;20(6):521–528
[19] Postacchini R, Paolino M, Faraglia S, Cinotti G, Postacchini F. Assessment of patient’s pain-related behavior at physical examination may allow diagnosis of recent osteoporotic vertebral fracture. Spine J 2013;13(9):1126–1133
[20] Sanfélix-Genovés J, Reig-Molla B, Sanfélix-Gimeno G, et al. The population-based prevalence of osteoporotic vertebral fracture and densitometric osteoporosis in postmenopausal women over 50 in Valencia, Spain (the FRAVO study). Bone 2010;47(3):610–616
[21] Suzuki N, Ogikubo O, Hansson T. The course of the acute vertebral body fragility fracture: its effect on pain, disability and quality of life during 12 months. Eur Spine J 2008;17(10):1380–1390
[22] Bornemann R, Hanna M, Kabir K, Goost H, Wirtz DC, Pflugmacher R. Continuing conservative care versus crossover to radiofrequency kyphoplasty: a comparative effectiveness study on the treatment of vertebral body fractures. Eur Spine J 2012;21(5):930–936
[23] Ong KL, Beall DP, Frohbergh M, Lau E, Hirsch JA. Reply to “At what price decreased mortality risk?” Osteoporos Int 2018;29(8):1929–1930