3
Life before Birth I: The New Reproductive Technologies
Then if children make so much trouble, why do people have 'em?
From Jude the Obscure, Thomas Hardy (1895)
The human egg is a Mrs. Bennet, desperate to marry off her daughters…It is a truth universally acknowledged that a sperm must be in want of a matching strand of DNA.
From Afterparty, Daryl Gregory (2014)
Reproductive freedom is not just the ability not to have a child through birth control. It’s the ability to have one if and when you want.
Pamela Madsen, Founder and first Executive Director of The American Fertility Association
3.1 Introduction
Ethical debate about reproductive medicine and people’s reproductive rights – a notion to which we will return later – lags significantly behind what is technically possible (as we discussed in more a more general context in the previous chapter). This is thrown into very sharp focus by the case of Natalie Evans and her partner Howard Johnson in the early years of this century. Natalie was 30 when she had to have her ovaries removed as part of a successful treatment for cancer. In November 2001, before her cancer treatment, she and Howard undertook in vitro fertilisation (IVF), producing embryos that were frozen so that they could attempt to have a family at a later stage. Unfortunately, the couple split up. Natalie’s only hope of having a child that was genetically hers was to have the frozen embryos transferred to her womb to try to become pregnant. Howard however refused to give his consent to this procedure, as was his right under the UK Human Fertilisation and Embryology (HFE) Act (1990). Natalie embarked upon a series of appeals against the judgement that she should not be allowed to use the embryos in an attempt to have a child. In April 2007, the European Court of Human Rights finally ruled against her,and her fight to save the embryos was lost. The significance of this is that the principle of shared responsibility for the future of stored embryos takes precedence over one partner’s desire to try to create a pregnancy with a child that is genetically their own.
The ethical issues here arise from the fact that the two parents have an equal say in the fate of embryos created by artificial reproductive technologies. This chapter will analyse and discuss aspects of the complex arguments behind this apparently unproblematic statement. Before embarking on our discussion, we need to say that there is considerable overlap between this chapter and the next. This is deliberate because we wanted readers to be able to use them as ‘stand‐alone’ articles if they so wished.
3.2 Gametes Outside the Body
The United Kingdom was the first country in the world to pass legislation to regulate fertility treatment. The Human Fertilisation and Embryology Authority (HFEA) was set up to oversee the 1990 HFE Act by a system of inspection and licensing of clinics that offered any treatment involving the handling of gametes (eggs and sperm) outside of the body. This includes donor insemination (DI), IVF and more recently developed techniques such as intra‐cytoplasmic sperm injection (ICSI) and reception of oocytes from partner (ROPA). There are many variations and refinements of these techniques, but all are covered by the Act. There have been many challenges to its stipulations, some of which are exemplified in this chapter, and the public debate has intensified, often criticising the HFE Act and the work of the HFEA. It has been argued by the leading fertility specialist Lord Robert Winston that the HFEA should be abolished, claiming incompetence over its decisions in those cases where new ethical dilemmas are faced, such as debates about sex selection of embryos and creation of tissue‐matched embryos for sick siblings (so‐called saviour siblings). Lord Winston said that the HFEA should be replaced by
‘something a great deal less bureaucratic, which doesn’t inhibit research, which has a better consultation process with the public and which has a much more adequate inspection process’.
In response to this, Suzi Leather, then Chair of the HFEA, defended the authority, saying
‘…having a regulator has given the public confidence in the infertility sector and the system of regulation’.
She also supported the updating of the 1990 Act, as technologies had advanced so much since it came onto the Statute Book, that parts of it were out of date. It was the increase in medical and scientific knowledge, understanding and skill, coupled with the powerful needs of involuntarily childless couples that brought new and seemingly irreconcilable dilemmas regularly into conflict with the ageing legislation and thus into the news headlines. Concerned by the speed of medical advances, some religious leaders called for the instigation of a national body to debate ethical issues. Hard on the heels of criticism of the HFEA, the Christian leaders in England together with the Chief Rabbi expressed fears that controversial rulings on matters of reproductive medicine, for example, the granting of permission to clone human embryos for research into degenerative diseases, fuelled public disquiet and are being introduced without due discussion of the underlying moral principles.
In 2008 the HFE Act was updated to include a revised definition of parenthood, including removal of the phrase ‘need for a father’ and allowing unmarried heterosexual and same‐sex couples to have access to treatment. The European legislation also came to bear on the law surrounding the regulation of reproductive treatment. The European Union Tissues and Cells Directive (EUTCD) enables the safer and easier exchange of sperm and eggs between member states and an improvement of safety measures for this across the EU. This is perhaps just one example of the complicated legal processes that will have to be reviewed following the probable exit of the United Kingdom from the EU in 2019.
3.3 Techniques of Artificial Reproductive Medicine
3.3.1 Objections to Assisted Reproduction
As with many of the developments discussed in this book, there are individuals and groups who remain opposed to their use and it helps considerably in the ethical debate to understand the basis of such opposition. Thus it is useful, before describing what is physically involved in the basic techniques now routinely employed in the treatment of infertility, briefly to rehearse the religious objections to it as defined mainly by the Roman Catholic Church (many other Christian denominations and indeed other faiths are not opposed to assisted reproduction). Essentially the basic objection is the same as that to contraception and concerns the separation of acts of sexual intercourse and procreation. Any form of physical barrier between sperm and egg, such as a condom, or behaviour, such as coitus interruptus (withdrawal or ‘spilling the seed’), that prevents possible fertilisation is deemed to be morally wrong, as it is contrary to natural law. For many Roman Catholics their church’s teaching poses a great moral dilemma, because tradition also requires them not to act against their consciences. So if they believe that allowing repeated pregnancies and very large families is not what is right for them, what are they to do? Many other Christians believe that couples have a moral duty to limit the size of their families, and so the use of contraceptive methods is validated. Further, in order to produce a sperm sample for an assisted reproduction procedure involving gametes outside the body, masturbation is the most convenient method. The teaching of the Roman Catholic Church is that the male genital organs are for procreation and urination; therefore any other use of them (e.g. masturbation, which is a version of ‘spilling the seed’) is thus unethical. Roman Catholics are not the only people with this view. In some African cultures masturbation is believed to compromise potency, and men refrain from it. This has implications for DI, because men of this ethnic origin rarely volunteer to be sperm donors.
3.3.2 Donor Insemination
The first case of sperm donation in a medical setting that we know about was at the Jefferson Medical College in Philadelphia, United States, in 1884 but the practice was not used at all widely until well into the 20th century. The first modern scientific account of DI was published in 1945 by a British doctor, Dr Mary Barton, working in London. The paper, published in the British Medical Journal, attracted a huge amount of criticism from religious leaders (not just Roman Catholics), from politicians and from many members of the medical profession. The Archbishop of Canterbury called DI the work of the devil, which is a little ironic, considering that Dr Barton was a Christian. There were widespread calls to have the process made illegal but this did not happen. Nevertheless, for several years a good deal of secrecy surrounded this treatment both in the United Kingdom and in the United States. However, during the late 1960s and through the 1970s, societal attitudes to many issues became much more liberal and DI became more acceptable, albeit that couples who used it usually kept the fact secret; donor anonymity was also strictly preserved.
Treatment of a patient by DI is the culmination of a lot of commitment by many people. In the United Kingdom, the 1990 HFE Act states that in deciding to offer treatment, the clinical team should take into account the potential child’s right to have a father. DI is an appropriate treatment for couples in which the man has been shown by medical and scientific testing to be subfertile or infertile, perhaps as a result of chemotherapy for testicular cancer, or a naturally low sperm count. It is also an appropriate treatment for lesbian couples or single women wishing to have a family. However, in the latter cases, the ‘right to have a father’ was clearly an issue: the right of the child to a male parent came into conflict with the right of the women to become parents. This matter was addressed by the new version of the HFE Act (2008) as was mentioned earlier.
Over the last 40 or so years, there has been a significant change in Western attitudes concerning what is meant by marriage and ‘the family’. Although the majority of children are born to heterosexual couples in formally married unions, many are born to parents who are not formally married. Further, the passage of the Marriage (Same Sex Couples) Act in 2013 demonstrates the growing acceptance of other forms of procreation and family life in the United Kingdom. However, for some people this remains a controversial matter.
3.3.3 Gamete Donation
As was noted in the previous section, the history of sperm donation goes back many years and from 1953 the procedure has been facilitated by the relative ease of freezing sperm safely for long‐term storage. Eggs (ova) may also now be safely frozen (after many decades of research into cryopreservation techniques) and the first reported live human birth resulting from a frozen egg was in 1999.
There are three main steps in the donation of gametes: recruitment and screening of donors, testing, freezing and preparing the sperm and eggs and, finally, gamete transfer. Each stage is fraught with ethical problems. It may be difficult to recruit gamete donors, because they must undergo rigorous screening for infections such as HIV and hepatitis and adverse familial genetic traits such as Huntington’s disease and cystic fibrosis. Here, medical ethical issues arise for the donors themselves in particular if genetic tests reveal conditions of which the potential donor was unaware (see Chapter 6).
Sperm donors must commit themselves to months of weekly donation, which involves producing samples in the clinic. Male donors are only paid expenses for this service, so there is no financial inducement. As has been mentioned, for many years, the identity of sperm donors was kept secret, under the laws and protocols surrounding the procedure. However, in the United Kingdom, pressure began to build in the 1990s to change the law and allow people conceived via gamete donation (i.e. sperm or eggs) to know the identity of the donor. For many people, not knowing the identity of a biological parent was very distressing or even traumatic and some have needed extensive counselling to help them deal with this. For such people, the change in UK law announced in 2004 was very welcome although it did not apply directly to those who were at that time experiencing distress. The change in law applies to any donor‐conceived person born after 31 March 2005, who, once they are 18 years old, may be informed of the donor’s name and identity. In order to obtain this information, application must be made to the HFEA, which holds all the relevant information on the donors, including their genetic history and name and address.
Egg (ovum) donation is a more invasive procedure than sperm donation. Women have to be under 36 years of age and must undergo the same rigorous genetic screening as male donors. The actual egg collection process involves firstly hormonal suppression of the natural ovarian cycle of egg maturation and release; this is followed by hormone injections for 10–14 days to stimulate the ovaries to produce an increased number of ova as well as scans and blood tests to monitor this process. A final hormonal ‘trigger’ injection is administered 36 hours ahead of egg collection. This is performed under either general anaesthetic, heavy sedation or local anaesthetic and involves ultrasound‐guided retrieval of the eggs from the ovaries through a needle either through the woman’s abdomen or trans‐vaginally. Because of the commitment, manipulation of the body’s natural processes and possible discomfort involved, egg donors are allowed to be compensated at a rate of up to £750 per donation cycle. Some women do this entirely altruistically, either for family members or for strangers. However a procedure known as ‘egg sharing’ in which women undergoing IVF treatment themselves are encouraged to donate ‘extra’ eggs usually involves some financial compensation (e.g. reduction in cost of IVF treatment, if being performed outside the National Health Service (NHS)). Even so, there is still a shortage of egg donors in the United Kingdom and this has led to ‘ovum tourism’ to countries where egg donors are more generously paid.
This shortage of egg donors in the United Kingdom and other countries has led to consideration of other sources of eggs, particularly aborted female foetuses. We discuss abortion in greater depth in the next chapter. Here we need to say that from 16 to 20 weeks of gestation, female foetuses accumulate around six to seven million oocytes (immature egg cells).In 1992, it was shown that in mice, foetal oocytes could be brought to maturity in the laboratory, and it was this that led to the proposal that we present as a case study here (repeated from Chapter 1).
In the United Kingdom, the proposal was put out to public consultation in 1994. There were suspicions that ‘pro‐life’ groups (see next chapter) had ‘hijacked’ the consultation, but there was sufficient opposition, based on a range of objections, including a widely expressed repugnance, for the HFEA not to approve the procedure. Rather strangely, that decision had been pre‐empted in Parliament in the adoption about two months earlier of an amendment to the Criminal Justice Act, which makes it illegal to use foetal tissues or cells in fertility treatments.
Nevertheless, research on the topic continued in other countries and in 2003, Dr Tal Biron‐Shental of the Meir hospital, Kfar Saba, Israel, announced that she and her team had succeeded in producing functional ova from oocytes of late‐aborted (22–33 weeks) human foetuses. This ignited the debate once more and it was conducted with the same intensity as in the 1990s. In the United Kingdom it was never likely that the HFEA would reverse its decision especially in view of the pressure to abolish donor anonymity (which, as mentioned above, resulted in a change in the law in 2005). For a person to be told that the egg that had led to their conception was donated by foetus that was never born was considered to be somewhat shocking. Although some research on the topic has been carried out since 2003, there is now very little interest in pursuing this route to egg donation.
We return now to our main theme. Having decided to accept a patient for treatment, the clinic chooses a gamete sample based on information kept about its donor, such as skin and eye colour, build and other physical features. Where possible, the match is made to the patient’s partner. With DI, the chosen sperm sample is thawed, and the best cells are chosen and then inserted directly into the woman’s uterus in a very small droplet at the most fertile time, that is, when she has ovulated. This process is known as intrauterine insemination (IUI). Clinics will often warn patients that they should look upon their DI as a course of at least six cycles rather than a one‐off treatment because the success rate for a single cycle of DI may be as low as 11%. Very little DI treatment is available through the NHS in the United Kingdom and depending upon the amount of other treatment necessary, patients can face considerable expense. Nevertheless, in the United Kingdom about 2500 children per year are born from donated gametes, the majority of which are inevitably sperm. All this information must be given to patients attending a fertility clinic, so that their consent to treatment is informed. Donated eggs on the other hand are used via IVF (see next section) with a similar success rate to ‘routine’ IVF of around 30% per cycle.
Finally, we also need to note that there is considerable variation between countries in respect of the laws concerning gamete (and especially sperm) donation. In Sweden, for example, donor anonymity was abolished in 1984, while in many other countries, including the United States, donors still remain anonymous. Most countries limit the number of individuals that can be conceived via the sperm of each donor and within that overall number may limit the number of families that may benefit. For example, a limit of 20 conceptions may be imposed but these may only be dispersed through ten families. Again, the United States does not impose these restrictions and in that respect is rather unusual. Finally, there are countries in which the availability of DI is restricted. In France, for example, it is not available to lesbian couples, while in some Muslim countries it is totally illegal.
3.3.4 In Vitro Fertilisation and Variations
As a treatment for infertility, DI has in many cases been superseded by newer technologies, all of which have been made possible by IVF. Previously infertile men can now be helped to become biological fathers. However, as for egg donation, described briefly above, IVF is a far more invasive treatment, not only of the patients’ bodies but also of their lives, involving as it does:
- Down‐regulation of the ovaries (stopping the natural cycle)
- Stimulation of ovulation during the treatment cycle (sometimes called ‘superovulation’)
- Semen collection, analysis and preparation
- Egg collection
- Insemination in vitro
- Fertilisation and embryo culture
- Embryo transfer, back to the woman’s uterus.
At any of these stages, the treatment can, and frequently does, fail. The latest UK figures available give a success rate for IVF in women under 35 of 32.2% and for those aged between 36 and 37, 27.7% per treatment cycle. This figure falls considerably in women over 37. There are some variations on the basic procedure, such as in vitro maturation (IVM) of eggs: treating the egg in various ways to assist the passage of the fertilising sperm or transferring a collected egg and some specially prepared sperm back into the woman’s fallopian tube. As with DI, patients are advised to view IVF as a course of treatment, but because many of the natural barriers to fertilisation are removed, it is statistically the more successful approach. As well as potential failures, a range of ethical problems marks each stage of IVF. Many of these, particularly those of consent to treatment, are common to issues in other medical specialisms, but some are specific to reproductive medicine. Ethical problems may arise, at least for some people, in the following situations:
- If the patient has no long‐term partner.
- If the prospective parents are of the same sex (for males this obviously also raises the issue of surrogacy). Note that the law allows same‐sex couples to use IVF.
- If gametes are donated, there may be concern about their origin – who are the donors and what was their motivation?
- If gametes are donated, there may also be concerns about the genetic status of the child.
As in all human situations, if something can go wrong, it may well do so. When the consequences of fertility treatment produce an ethical dilemma, it frequently seems that the debate lags behind what has actually happened. Thus, over the years there have been instances of what are called, rather trivially, IVF mix‐ups.There have been cases of using the wrong sperm to fertilise eggs, giving rise in one case to a white woman giving birth to mixed‐race babies. There have also been several examples of women being implanted with the wrong embryos such that black women have given birth to white babies and vice versa. Instances involving babies of the same ethnicities are harder to spot but may come to light in routine checks at the IVF clinic or through DNA testing. Indeed, to avoid embryo mix‐up, some clinics offer a DNA test at the eight‐cell stage of embryo development, albeit at extra cost. Fortunately, in relation to the total numbers of successful IVF treatments (see below), these mistakes are very rare but that does not detract from the anxiety and anguish that they may cause.
Over the last two decades, the technique of injecting a single sperm directly into an egg has been developed with increasing success. This is known as ICSI and for the patients involves all the stages of IVF. The difference lies in the considerable expertise needed by the embryologists, who manipulate the individual gametes by hand under a microscope, passing a single sperm cell through a very fine glass tube into the inside of the egg, before incubating it to produce an embryo, which is then transferred to the woman as in conventional IVF. This treatment is suitable for couples in which the man has a low sperm count or a high proportion of abnormal sperm cells.
Since the first IVF baby, Louise Brown, was born in 1978, more than six million children have been born through IVF worldwide with over 250,000 of those being born in the United Kingdom. In the United Kingdom, the National Institute for Health and Clinical Excellence (NICE) recommended that women under 40 should be allowed to have three cycles of IVF and those over 40 one cycle funded by the NHS. However, the financial constraints upon many NHS trusts is currently such that their individual policies are often much stricter than this and indeed some trusts do not fund IVF at all. If patients opt for private treatment, a single cycle of IVF can cost up to £5000, with drugs amounting to an additional £2000.
The funding of IVF treatment raises further ethical questions. Essentially, infertility is a condition of otherwise healthy people, so some might argue that limited funds are better deployed in providing, for example, more kidney machines, heart transplants or neonatal nursing care for sick babies already born. However, it is estimated that in the United Kingdom about one in seven couples who wish to have a baby is unable to do so. In some cases, the problems are resolved over time but that still leaves many who want to have a child but cannot do so. For many of these, involuntary childlessness is a condition that causes huge misery and distress; it has been likened to being bereaved but with no specific person to mourn. For these reasons it is widely regarded that IVF and other reproductive technologies should be available within national funding organisations (such as the NHS in the United Kingdom). According to this view, infertile men or women deserve treatment at least as much as people who, for example, have made themselves ill by smoking or excessive drinking.
In 1995 IVF technologies made possible the birth of a child to Pauline Lyon at the age of 52 (having lied to the doctors about her age) and later had a second child at the age of 55. Since then, several other women in their late 50s and early to mid‐60s have had babies via IVF; indeed, there are private clinics in some countries, including the United Kingdom, that are happy to provide IVF services to older women. Cases of this type raise the question that although men can become fathers into old age, women who have beaten the menopause by artificial reproductive techniques come in for direct or implied criticism. For example, it is often said that the child will be socially disadvantaged by having ‘such an old mother’ who may indeed not survive long enough to see the child into adulthood. Such criticisms are not usually directed at older fathers, exemplified by the rock musician Mick Jagger who in 2016 became a father at the age of 73. He already had seven children aged between 17 and 45 and his first great‐grandchild was born in 2014.
Returning to the issue of the right of a child to a father, the 1997 case of Diane Blood raised a considerable ethical debate and protracted legal wrangle. Diane’s husband, Stephen, fell into a coma as a result of meningitis. Told that he was not likely to recover, Diane requested that sperm be collected from him. In spite of the fact that he was unable to give consent, this procedure was done, and the sperm frozen in storage. Stephen died without recovering consciousness. Arguing that they had agreed to start a family and that it would be Stephen’s wish that Diane should use his sperm to have a child, her hopes were dashed when the HFEA ruled that this was not permissible, because Stephen had not consented to using his sperm for fertility treatment. However, Diane was allowed, via a decision of the HFEA, to take the frozen sperm to Belgium, where she had ICSI treatment. Her son Liam was born in 2000, and Diane succeeded in having a second son, Joel, in 2002. Her final legal victory came in 2003 when she was allowed to register Stephen posthumously as their father.
3.3.5 Reception of Oocytes from Partner
In relation to assisted reproduction for same‐sex couples, a further variation on the IVF theme ROPA has recently become legally available in several countries, including the United Kingdom. This technique allows lesbian couples to become parents both legally and in part biologically by egg donation by one partner, IVF and transfer to the other partner for gestation and delivery. The legal mother is thus the woman who carries and delivers the baby, with her partner contributing 50% of the genetic material, the other 50% coming from a sperm donor.
3.4 Embryo Testing
Otherwise known as pre‐implantation embryo screening (PGS) or pre‐implantation genetic diagnosis (PGD), these options are available to couples who have an identifiable and serious genetic disease in their family history (see also Chapter 6). It is only available to those who are undergoing IVF or ICSI treatment and involves the removal of one or two cells (blastomeres) from the developing embryo before it is transferred into the woman’s uterus. In PGS the chromosomes of the blastomeres are then examined for abnormalities. PGD involves the same process, but a more specific analysis of the DNA is carried out in order to detect the presence or absence of the relevant mutation. Only those embryos without chromosomal abnormalities or without the disease‐causing mutation are transferred or stored for subsequent transfer. Occasionally, diagnosis is carried out at a later stage of embryo development. Embryos can be allowed to develop until the cells have differentiated into trophectoderm (the cells that will go on to form the placenta and extra‐embryonic membranes) and the inner cell mass (which will become the embryo itself after implantation). In a technique that is said to less traumatic for the embryo, known as trophectoderm biopsy, a number of cells are removed from the trophectoderm and examined to achieve what some embryologists to be a more reliable diagnosis. Nevertheless, in the majority of cases, PGD is performed at the eight‐cell stage of embryo development
3.5 Mitochondrial Donation
A small proportion of a person’s DNA complement resides in the mitochondria, organelles primarily responsible for cellular respiration that also have other functions including the regulation of some cellular processes. Human mitochondria carry 37 coding genes and in rare cases, mutation of the genes can cause serious genetic disorders, one example of which is Leigh syndrome, a condition of the nervous system that is fatal in the first year of life. Following fertilisation, it is only mitochondria from the egg cell that divide and are carried forward in the growing embryo. Mitochondrial genes, including mutated genes, are thus maternally inherited. For some women whose mitochondria carry mutations causing very serious disease, there is the heartbreak of seeing successive children die soon after birth. For others who carry less serious conditions, it may be that offspring develop symptoms such as muscle weakness or blindness as young adults.The seriousness of some of these mitochondrial diseases has led to the development of methods that allow the replacement of ‘faulty’ mitochondria with properly functioning mitochondria.
The basic method, called pronuclear transfer (PNT), involves fertilising both the mother’s egg (with the mitochondrial mutation) and a healthy donor egg with the father’s sperm (see Figure 3.1). Before the fertilised eggs start dividing, each nucleus is removed. The nucleus from the donor’s fertilised egg is discarded and replaced by that from the mother’s fertilised egg. The baby thus has DNA from the father’s sperm, the mother’s egg nucleus and the donor’s mitochondrial DNA. When the child grows to reproductive age himself/herself, only healthy mitochondrial DNA will be passed to the next generation, thus permanently eliminating the fatal mutation from the family.
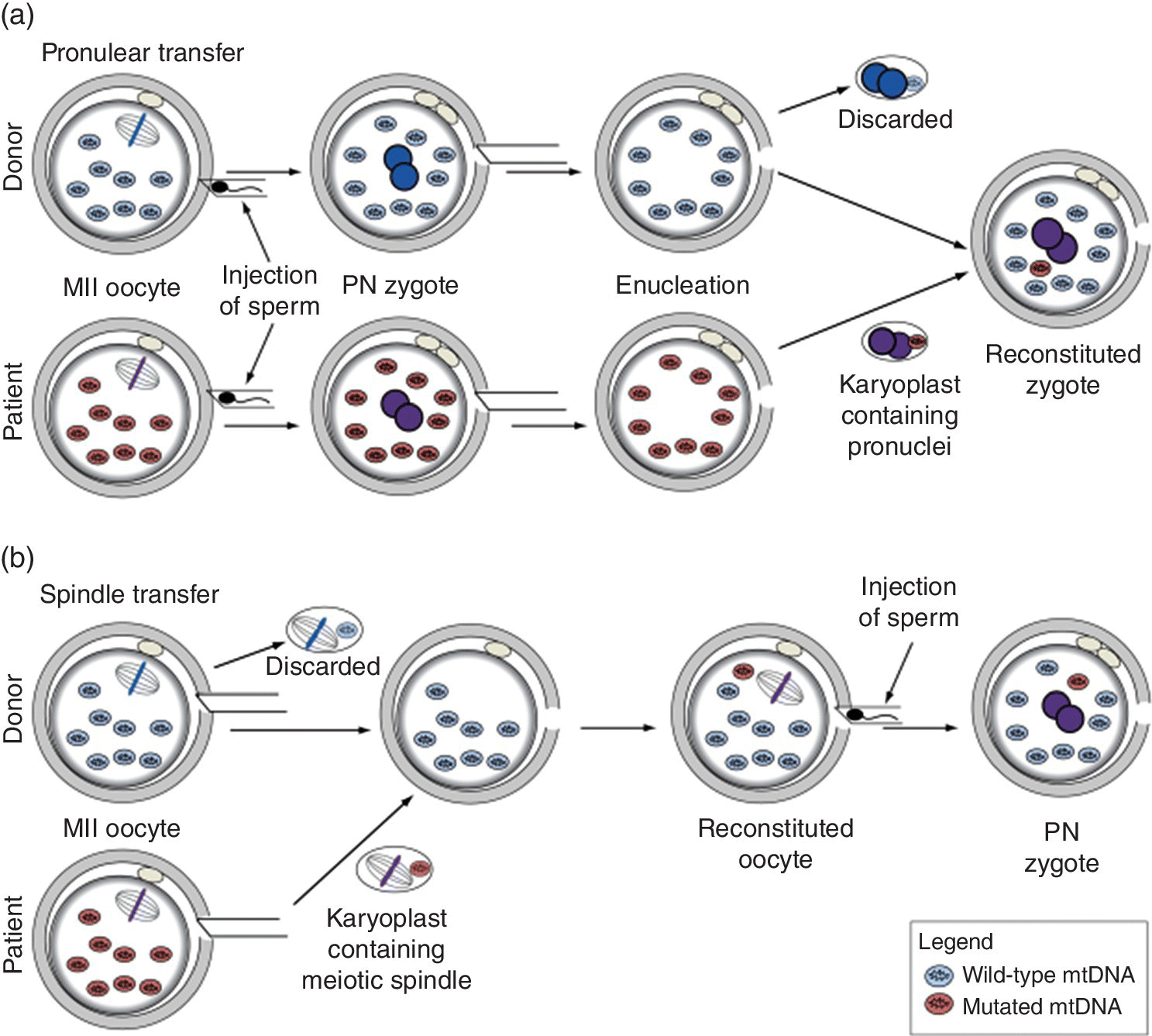
Figure 3.1 Methods for mitochondrial donation. (a) Pronuclear transfer. (b) Transfer of maternal spindle.
Source: Reproduced by permission from Richardson J et al. (2015) Stem Cells 33, 639–645. DOI: 10.1002/stem.1887. © Creative Commons.
In a variation on this technique, known as maternal spindle transfer (MST), the nucleus from one of the mother’s eggs is removed and inserted into an enucleated donor egg. The resulting egg, with maternal nuclear DNA and mitochondrial DNA from the donor, is then fertilised with the father’s sperm. Further work on PNT and MST was approved by the UK Parliament in 2015 (on the recommendation of the HFEA) and the techniques were declared safe for clinical use in late 2016. However, the first successful treatment was actually in a New York clinic in 2016, involving a Jordanian woman who had lost her first two babies to Leigh syndrome. In the United Kingdom, the HFEA granted a licence to the Newcastle fertility clinic in March 2017 to use mitochondrial donation in clinical treatment; the first babies born after use of the technique are likely to be born early in 2018.
The media constantly refer to these methods as ‘three‐parent IVF’ and thus babies born are referred to as ‘three‐parent babies’. This has led to considerable misunderstanding amongst members of the public who are not familiar with the details of the methods. But is it a fair description? The number of genes in the mitochondrion represents about 0.18% of the total. Further, those genes are responsible for specific, albeit essential, metabolic pathways but are not involved, for example, in regulation of growth, differentiation or development. Biologists and medical scientists in general reject the term ‘three‐parent IVF’ because it is misleading. Indeed, it has been said that using this term is like calling someone who has had an organ transplant a ‘four‐parent’ person.
Whatever one’s view of the term, the development of the technique and then its approval and legalisation in several countries evoked considerable opposition. Indeed, one of us has participated in number of debates and discussions about the ethics of mitochondrial transfer. Much of the opposition comes from those groups that oppose IVF totally (such as the Roman Catholic Church) plus other groups and persons who also attribute a high level of moral significance to the embryo (‘human life begins at fertilisation’; see Chapter 4). Some also question the safety of the technique – does it affect the growth and development of the child to be born? However, in general, the safety issue is raised by people who are already opposed to the technique for other reasons.
The possible ethical issues around mitochondrial donation that we have just presented in the question box arise from more general views about the ethics of IVF and the moral status of embryos. However, for this technique a further objection has been raised. In the provisions of the 1990 and 2008 HFE Acts, it is prohibited to try to start a pregnancy with genetically modified (GM) embryos. Here, we distinguish between PGD, in which embryos with particular genetic make‐ups are selected to start a pregnancy, and GM, in which the DNA of an embryo has been altered in some way (perhaps by adding a gene). Strictly speaking, the replacement of one complement of mitochondria (and hence mitochondrial genes) with another is a form of GM. However, although not explicitly stated in the provisions of the Act, the prohibition of starting a pregnancy with a GM embryo applies to the nuclear genes. Mitochondrial transfer was not envisaged. Nevertheless, opponents of mitochondrial donation suggest that it opens the door or places us on a slippery slope, leading to ‘designer babies’ (see Chapter 6). Now, as biologists we need to say that it is extremely unlikely that anyone would attempt to produce a designer baby via manipulation of mitochondrial DNA but we can ask whether mitochondrial donation begins to make it more acceptable to alter the nuclear genes of an embryo.
3.6 Embryo Research
In addition to providing hope for subfertile couples, IVF techniques also make available human embryos that may be used in research. Whether one thinks this is acceptable depends to a large extent on one’s view of the human embryo in relation to personhood (see next section and Chapter 4). It is certainly a sensitive subject on which there are a range of views. In the United Kingdom, research on embryos created outside the body is regulated under the provisions of the HFE Acts. Research licences are only granted by the HFEA when it can be demonstrated that the researchers will add to the body of knowledge in relation to particular problems (see below) and in doing so gain technical competence. The type of research that can be undertaken is strictly limited and very closely monitored by the HFEA as it proceeds.
The whole debate about whether or not embryo research is morally acceptable centres at least partly on the definition of an embryo, and this is again related to the question of when life begins. In this respect, the arguments against research using embryos are similar to those against IVF in general and against abortion. For those people who emphasise that a unique human life comes into being at the completion of fertilisation (‘conception’), any procedure that interferes with the normal development of the embryo is unacceptable because they see that embryo as having the same rights and interests as any other child or adult.
The genetic identity of a new individual is established at syngamy, about 30 hours after the initial encounter of the fertilising sperm cell with the egg membrane, and it is at this stage that cleavage or embryonic cell division begins. It has been known for some years that human eggs can undergo cleavage in the absence of fertilisation. This condition is known as parthenogenesis, and in an experimental situation, development beyond the primitive streak stage has been observed from unfertilised mouse eggs. This again calls into question the moral status of the embryo – is it based on genetic uniqueness? If so, then what about the parthenogenetically dividing embryo? What about identical twins? What about cloned embryos? Although this situation raises uncertainties over the moral status of the embryo, it is relevant to note that the embryo undoubtedly has a human genetically programmed uniqueness (except for identical twins), giving it the potentiality for a life within humankind. It is in this context that the moral status of the embryo should be considered.
Experimental procedures on human embryos are only permitted under the law for the first 14 days after the mixing of the gametes – in other words before the appearance of the primitive streak. This has led to the use by some of the term ‘pre‐embryo’. Under the law, only certain types of research on human embryos are allowed. These include research for the purposes of:
- Increasing knowledge about serious disease or other serious conditions
- Developing treatments
- Increasing knowledge about the causes of congenital conditions
- Advancing the treatments for infertility
- Increasing knowledge about the causes of miscarriage
- Developing more effective methods of contraception
- Developing methods for detecting genetic, chromosomal or mitochondrial abnormalities in pre‐implantation embryos
- Increasing knowledge about the development of embryos.
Three further limitations were placed on the research. Firstly, no embryo that had been the subject of research can be implanted into a woman’s uterus. Secondly, donors who provide gametes or embryos for research must do so with fully informed consent. Thirdly, no embryo must be allowed to develop in the laboratory for more than 14 days (at which point, in natural embryonic development, the primitive streak appears). Some commentators interpret this as meaning that in the United Kingdom, legally protected human life starts at 14 days after fertilisation (notwithstanding the fact that abortion is allowed up until the 24th week of pregnancy).
However, in respect of the purposes of the research, in the early years of this century, the HFEA started to grant licences for research on embryonic stem cells in connection with developments in regenerative medicine (as described more fully in Chapter 5). This lies outside the list presented above but the practice was regularised in the provisions of the new HFE Act in 2008. That Act also made legal the creation of cloned embryos for therapeutic purposes that, as we discuss in Chapter 5, proved controversial. Finally we note that the 14‐day limit is also being challenged with suggestions that it be extended to 21 or even 28 days, as we discuss more fully in the next chapter.
3.7 Rights of the Unborn Child
Within the usual understanding of the concept of ‘rights’ comes the acceptance that with rights come responsibilities. Clearly this cannot be the case for an unborn child, but in a biological sense, the struggle to maintain life, to survive, might be considered to be the responsibility, albeit an unconscious responsibility, of the child as a member of the human species. Humans, as far as we know, are the only extant species able to rationalise, and we have a basic instinctive drive to protect life. This has been termed the ‘presumption in favour of life’; its consequences are often keenly argued in medical ethics generally and more specifically in relation to the beginning and end of life. However, the status of the unborn is morally problematic, because decisions often hinge upon agreement about ‘when human life begins’. Throughout history passionate debate has raged on questions such as the following: at what stage do we have a duty to protect human life, what is entailed by or involved in our duty to protect human life, and when is it justifiable to interrupt the fulfilment of human development? The teaching authority of the Roman Catholic Church and the views of many conservative Protestant Christians currently hold that life begins at fertilisation and that a human life is distinguished from that of animals because humans are spiritual beings, a notion that is also discussed in Chapter 13. But if there is a spiritual element, when does it actually arise? Or to put it in terms more generally understood by those who have no religious faith, at what stage does the embryo or foetus become a person? Can we ascribe human personhood to eggs and sperm or even to their progenitor cells in the ovaries and testes?
The attribution of personhood in relation to stages of embryonic and foetal development is discussed in detail in Chapter 4. Here, we just need to state that in present‐day UK law, the foetus has no rights until it is born (notwithstanding the 14‐day rule mentioned above). Most societies put the rights of a pregnant woman above those of a foetus, the argument being that the mother is a person and has responsibilities, whereas the foetus has no legal personality until it is born. These matters are discussed more fully in Chapter 4. In the meantime we continue this chapter with a discussion of the ethical issues raised by an equally controversial topic.
3.8 Men and Women: Do We Need Both?
Societies in all countries throughout the history of humankind have developed for the major part because of the relationships between men and women, so the question heading this section might seem very strange. But reproductive technologies in theory make it possible to do without one or the other. Human sperm are the smallest and most highly specialised cells in the body and over the course of evolution have developed as they have to be perfectly fitted to fertilise an egg. The only actual part of the sperm that is necessary to produce an embryo is the nucleus, containing the genetic information. So if you artificially introduce that part into an egg, as, for example, in the process of ICSI, all the highly evolved specialist structures of the sperm – its midpiece, packed with mitochondria to power the flagellum and its acrosome, containing powerful enzymes to help the passage of the sperm genome into the cytoplasm of the egg – are rendered unnecessary, as are all the parts of the man’s reproductive tract that are specialised for the differentiation of the mature sperm cell from the stem cells from which they arise. The recently developed ability to develop sperm from stem cells (Chapter 5) adds another interesting twist to this discussion.
Eggs, by contrast, are the largest cells in the body and have important membranes and vestments around them, which play a vital role in the passage of the egg from the ovary down the fallopian tube and also in the process of fertilisation. Eggs contain special cytoplasm or ‘yolk’, which plays a vital role in the first few cell divisions of embryonic life. Just like sperm, these specialisms have evolved over time to ensure reproductive success, but the vital part is the genetic component of the egg. As ICSI and cloning technologies have shown, it is entirely possible to introduce genetic material into an egg and create an embryo that can develop into offspring. Animal experiments have shown that the genetic component of another egg can successfully be placed into an unfertilised egg and that viable offspring can result from this. These are all female. This suggests that sperm may not be needed at all, and since it is the sperm that determines the gender of mammalian offspring, these experiments suggest that reproduction is technically possible without males. It is perhaps reassuring to know that although it is quite common in frogs, this means of reproduction has an extremely low success rate and is not likely to be allowed in humans in the near future.
Although it is not at present possible to create an embryo without the membranes and cytoplasm of the egg, the rate of advance of the science is such that even this technical hurdle may in time be overcome. To date, both mouse and human sperm have been generated from stem cells and from reprogrammed skin cells (see Chapter 5) and mouse eggs have been made from stem cells. In mice, the ‘artificial’ sperm were used to fertilise normal eggs and the ‘artificial’ eggs were fertilised by normal sperm. Both experiments led to the birth of live mice. It has been suggested that the technology will eventually be used to help people who do not produce functional gametes to have children. Gametes could be produced from their own reprogrammed skin cells.
While some are excited about these possibilities, at least in respect of the scientific achievement, others regard them as deeply worrying. How far should we go in controlling human reproduction as we continue to manipulate and control natural reproductive systems, perhaps even to the extent of eliminating the need for uterine incubation of the embryo/foetus (see next chapter)? For some, this is a distinctly unpleasant and unethical scenario, raising parallels with Aldous Huxley’s dystopic novel Brave New World. We suspect that Cardinal Cormac Murphy‐O’Connor spoke for a wider constituency than just the Roman Catholic Church when he said (some years before the development of artificial gametes):
The issues which the new technologies have thrown up touch on the very source and mystery of life. We need an ethical rigour capable of meeting the challenge.
Key References and Suggestions for Further Reading
- Barton M, Walker K, Wiesner BP (1945) Artificial Insemination. The British Medical Journal 1, 40–43.
- Berkowitz JM (1995) Mummy was a fetus: motherhood and fetal ovarian transplantation. Journal of Medical Ethics 21, 298–304.
- BioNews (2003) In vitro maturation of ovarian follicles. BioNews 215. http://www.bionews.org.uk/page_11663.asp (accessed 18 September 2017).
- Breedenoord AL, Hyun I (2017) Ethics of stem cell‐derived gametes made in a dish: fertility for everyone? EMBO Molecular Medicine 9, 396–398.
- Donor Unknown (2011). http://www.donorunknown.com/debate‐home (accessed 18 September 2017).
- Gosden R (1992) Transplantation of fetal germ cells. Journal of Assisted Reproduction and Genetics 9, 118–123.
- Greely HT (2006) The End of Sex and the Future of Human Reproduction. Harvard University Press, Cambridge, MA.
- Harris J (2003) The Future of Human Reproduction: Ethics, Choice, and Regulation. Oxford University Press, Oxford.
- Lewin T (2017) Babies from skin cells? Prospect is unsettling to some experts. New York Times, 16 May 2017. https://www.nytimes.com/2017/05/16/health/ivg‐reproductive‐technology.html?_r=0 (accessed 18 September 2017).
- Mills C (2011) Futures of Reproduction: Bioethics and Biopolitics. Springer, Dordrecht, The Netherlands.
- Morrell P, Morrell S, Hunt A (2010) Misconception – One Couple’s Journey from Embryo Mix‐up to Miracle Baby. Howard Books, New York.
- Moreno I, Miguez‐Forjan JM, Simón C (2015) Artificial gametes from stem cells. Clinical and Experimental Reproductive Medicine 42, 38–44.
- Nordqvist P, Smart C (2014) Relative Strangers: Family Life, Genes and Donor Conception. Palgrave‐Macmillan, Basingstoke, UK.
- Sample I (2003) Prospect of babies from unborn mothers. The Guardian, 1 July 2003. https://www.theguardian.com/world/2003/jul/01/health.healthandwellbeing (accessed 18 September 2017).
- Sample I (2017) First UK licence to create three‐person baby granted by fertility regulator. The Guardian, 16 March 2017. https://www.theguardian.com/science/2017/mar/16/first‐licence‐to‐create‐three‐person‐baby‐granted‐by‐uk‐fertility‐regulator (accessed 18 September 2017).
- Snowden E, Snowden R (1993) The Gift of a Child, 2nd edition. Exeter University Press, Exeter, UK.
- Wilkinson S (2012) Choosing Tomorrow’s Children: The Ethics of Selective Reproduction. Oxford University Press, Oxford.